Abstract
Pseudomonas aeruginosa infection of cystic fibrosis patients causes lung damage that is substantially orchestrated by cytokines. In this study, multi-gene probe analysis was used to characterize the ability of the P. aeruginosa mitogen, exoenzyme S, to induce proinflammatory and immunoregulatory cytokines and chemokines. Exoenzyme S strongly induced transcription of proinflammatory cytokines and chemokines (tumor necrosis factor alpha, interleukin-1α [IL-1α], IL-1β, IL-6, IL-8, MIP-1α, MIP-1β, MCP-1, RANTES, and I-309), modest transcription of immunoregulatory cytokines (IL-10 and IL-12p40), and weak transcription of Th1 cytokines (IL-2 and gamma interferon). The response occurred early and subsided without evolving over time. These data suggest that cells responding to exoenzyme S would rapidly express proinflammatory cytokines and chemokines that may contribute to pulmonary inflammation in cystic fibrosis.
Virtually all cystic fibrosis (CF) patients are colonized by Pseudomonas aeruginosa, and 90% of those that are colonized die as a result of lung damage (11). One of the hallmarks of the lung damage in CF is an ineffective inflammatory response that results in severe neutrophil-mediated pulmonary damage and an inability to clear the organisms. P. aeruginosa contributes to the lung damage by the production of virulence factors; one of the most important virulence factors, exoenzyme S, has been shown to induce pulmonary damage in animal models (17, 29, 35), and increased levels of exoenzyme S correlate with human disease (18, 36). The cytotoxicity of exoenzyme S for epithelial cells follows both contact-dependent type III translocation into eukaryotic target cells and contact-independent type III secretion, suggesting two mechanisms of cellular activation: intracellular and extracellular (15, 34, 35). We have recently described an additional activity: extracellular exoenzyme S is mitogenic for T cells, inducing tremendous T-cell activation (5–7). Exoenzyme-S-induced activation of T cells is neither dependent upon nor inhibited by ADP-ribosyltransferase activity, and this activity is present in exoenzyme S from both purified and recombinant sources (7). Further, we have found that activation by exoenzyme S induces T-cell apoptosis (6). The current studies were performed to determine whether this T-cell activation culminates in the induction of cytokines that have the potential of influencing immunoinflammatory responses or whether apoptosis precludes cytokine transcription.
Immunoinflammatory responses are orchestrated by cytokines, and T-cell mitogens and superantigens are potent stimuli for cytokine production from T cells, monocytes, and macrophages (2, 3). In CF there is a misdirected or dysregulated response, since there is a chronic and exuberant immunoinflammatory response without clearance of the pathogen. This response may be a result of altered cytokine induction that includes decreased secretion of the anti-inflammatory cytokine interleukin-10 (IL-10) (13, 28) with concordant increases in proinflammatory cytokine production, including tumor necrosis factor alpha (TNF-α), IL-1α, IL-1β, IL-6, and IL-8 (4, 11, 22). T-cell mitogens induce the production of many cytokines and are capable of altering or polarizing the Th1 and Th2 cytokine profile, which can have a potential pathogenic role during infection (8, 9, 14, 16, 31). We hypothesized that exoenzyme S could contribute to a dysregulated immune response by the exaggerated production of both Th1 and Th2 cytokines and proinflammatory cytokines.
Cytokine effects and interactions are complex, with many cytokines influencing the production of others throughout the time course of the response. Because of this, multiparameter technology has been applied to cytokine work (24) with the goal of determining the kinetics of induction and the nature of the most important cytokine response. For this purpose, the RNase protection assay (RPA) has significant advantages; it is a sensitive and quantitative measure of cytokine and chemokine mRNA induction that allows for the simultaneous determination of multiple genes. Three gene panels were used to examine transcriptional expression of Th1 and Th2 cytokines, proinflammatory and immunoregulatory cytokines, and chemokines.
Exoenzyme S purification.
ExoS/DG1 from P. aeruginosa strain DG1 was isolated as previously described using (NH4)2SO4 precipitation of culture supernatants, ion-exchange chromatography, and acetone precipitation, followed by gel filtration, and migrated as a 50-kDa band without ADP-ribosyltransferase activity (35). rExoS was isolated from Escherichia coli BL21(DE3) bearing a plasmid encoding histidine-tagged exoenzyme S cloned from P. aeruginosa 388(pETrHisExoS). rExoS was purified by Ni2+ affinity chromatography from cellular lysates and migrated as a 52-kDa band possessing ADP-ribosyltransferase activity (7, 21). Neutralizing antibodies generated against ExoS/DG1 were shown to neutralize T-cell activation induced by rExoS, indicating that both preparations share the mitogenic epitope (7).
Cell culture and RPA.
Peripheral blood mononuclear cell(s) (PBMC) were isolated from healthy adults by Ficoll-Hypaque density centrifugation (27). Cells (2 × 105 cells/well) were cultured in AIM V serum free medium (Gibco BRL, Burlington, Ontario, Canada) in 24-well nonadherent plates (Costar) in the presence of 10 μg of polymyxin B per ml in order to exclude cellular activation from lipopolysaccharides (LPS) (30). Resting PBMC were stimulated with 1 μg of either ExoS/DG1 or rExoS per ml.
At various times, PBMC were collected, and total RNA was extracted (Qiagen, Inc., Mississauga, Ontario, Canada). RNA probes (PharMingen, Mississauga, Ontario, Canada) to cytokines and chemokines were radiolabeled with 35S and hybridized overnight to 3.5 μg of RNA. Each panel of probes included L32 and GAPDH (glyceraldehyde-3-phosphate dehydrogenase), which are constitutively expressed and served as internal controls for RNA content. Following hybridization, single-stranded RNA was digested with RNase A and RNase T1. Double-stranded RNA was extracted and separated by electrophoresis in a 5% polyacrylamide gel. Protected fragments were visualized by autoradiography, and the intensity of each band was quantified by exposure to a Phosphorimager screen and calculated using ImageQuant software (Molecular Dynamics, Sunnyvake, Calif.).
Th1 and Th2 cytokines.
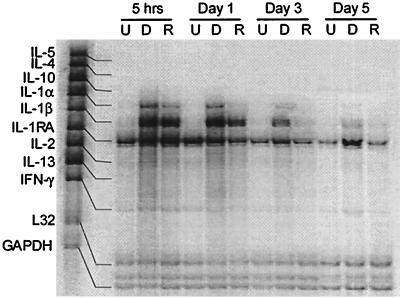
T-cell mitogens and superantigens are capable of altering or polarizing the Th1 or Th2 cytokine profile which can have a significant role in the pathogenesis of infection (23, 26, 31). Since exoenzyme S is a T-cell mitogen, we investigated whether T-cell cytokines were induced. A positive response was defined as a >2-fold increase in the intensity compared to unstimulated PBMC ([i.e., stimulated cytokine band intensity/stimulated L32 band intensity]/[unstimulated cytokine band intensity/unstimulated L32 band intensity]). Only those genes that were detected are displayed in tabular form (Tables 1 and 2). Neither ExoS/DG1 nor rExoS induced transcription of IL-4 or IL-5 (Fig. 1 and Table 1). Modest induction of Th1 type cytokines (IL-2 and gamma interferon [IFN-γ]) was seen (Fig. 1 and Table 1), which was confirmed by reverse transcription-PCR (RT-PCR) (data not shown). We were somewhat surprised by the relative lack of induction, since exoenzyme S causes rapid expression of high levels of CD69 on a large percentage of T cells, which reflects substantial activation (5, 7). By contrast, both ExoS/DG1 and rExoS strongly induced transcription of the proinflammatory cytokines IL-1α and IL-1β that peaked between 5 and 24 h and declined after 5 days. Since strong induction of proinflammatory cytokines and a vigorous inflammatory response characterize the response in CF patients infected with P. aeruginosa (11), this led us to hypothesize that exoenzyme S preferentially induces proinflammatory cytokines. Thus, we examined the ability of exoenzyme S to induce proinflammatory cytokines and chemokines.
TABLE 1.
Exoenzyme S induces Th1 and proinflammatory cytokines
| Cytokine | Ratio of stimulated/unstimulateda (stimulated/L32)b band intensity after stimulation with ExoS/DG1 for various periods:
|
|||
|---|---|---|---|---|
| 5 h | Day 1 | Day 3 | Day 5 | |
| IFN-γ | 1.2 (0.06) | 0.9 (0.1) | 0.7 (0.04) | 2.8 (0.3) |
| IL-2 | 1.8 (1.1) | 2.5 (0.6) | 3.2 (0.3) | 2.4 (0.13) |
| TNF-α | 18 (2.1) | 5.2 (0.56) | 3.3 (0.3) | 2.5 (0.22) |
| IL-1α | 9.6 (4.5) | 107 (2.8) | 9.9 (0.8) | 3.4 (0.13) |
| IL-1β | 21 (20) | 67 (25) | 28 (5) | 12 (1.2) |
| IL-1RA | 2.0 (18) | 1.1 (9.4) | 2.3 (8) | 3.7 (6) |
| IL-6 | – (5.54) | 73 (3.1) | 6.3 (0.15) | – (0) |
| IL-10 | 4.1 (0.27) | 1.2 (0.11) | 0.5 (0.12) | 0.9 (0.17) |
| TGF-β1 | 1.0 (0.43) | 1.2 (0.87) | 0.7 (0.44) | 2.2 (0.98) |
| TGF-β3 | 1.2 (0.53) | 2.0 (0.69) | 1.1 (0.27) | 3.0 (0.37) |
| IL-12p40 | 11 (0.19) | 6.0 (0.08) | 1.3 (0.03) | 1.2 (0.56) |
Densitometry corresponds to the bands in Fig. 1 and 2A. Cytokine intensity was quantified by phosphorimager analysis. Cytokine intensity is displayed as the fold increase over unstimulated values, i.e., (stimulated band intensity/L32 band intensity)/(unstimulated band intensity/L32).
Within the brackets cytokine mRNA was normalized to L32 (cytokine band intensity/L32 band intensity). The experiment was repeated twice with similar results. Only genes that were detected are shown. –, No band was observed in the unstimulated sample, and only the cytokine/L32 ratio is given.
TABLE 2.
Exoenzyme S induces chemokines
| Chemokine | Ratio of stimulated/unstimulateda (stimulated/L32)b band intensity after simulation with ExoS/DG1 for various periods:
|
|||
|---|---|---|---|---|
| 5 h | Day 1 | Day 3 | Day 5 | |
| Ltn | 4.0 (0.43) | 0 (0) | 2.4 (1.6) | 1.5 (0.27) |
| RANTES | 17.4 (11) | 22 (11) | 16 (18) | 5.7 (3.9) |
| IP-10 | 173 (20) | 3.6 (0.7) | 2.2 (0.3) | – (0.1) |
| MIP-1β | 81 (79.4) | 13 (13.1) | 7.8 (4.2) | 2.4 (1.0) |
| MIP-1α | 14 (21.5) | 12 (20.6) | 7.0 (8.2) | 4.8 (3.7) |
| MCP-1 | 4.2 (5.4) | 16 (35.5) | 12 (13) | 0.6 (0.46) |
| IL-8 | 53 (32.6) | 86 (35.9) | 39 (16) | 9.5 (3.7) |
| I-309 | 9.0 (1.43) | – (4.0) | – (2.1) | 1.6 (0.24) |
Densitometry corresponding to the bands in Fig. 2B. Cytokine intensity was quantified by phosphorimager analysis. Cytokine intensity is displayed as the fold increase over unstimulated values, i.e., (stimulated band intensity/L32 band intensity)/(unstimulated band intensity/L32).
Within the brackets cytokine mRNA was normalized to L32 (cytokine band intensity/L32 band intensity). The experiment was repeated twice with similar results. Only genes that were detected are shown. –, No band was observed in the unstimulated sample and only the cytokine/L32 ratio is given.
FIG. 1.
Preferential induction of Th1 cytokines from PBMC by stimulation with exoenzyme S. Resting PBMC were either unstimulated (U), stimulated with 1 μg of ExoS/DG1 per ml (D), or stimulated with 1 μg of rExoS per ml (R) for various times. Undigested fragments were used as markers to determine the identity of protected fragments. Densitometric values are given in Table 1. The experiment was repeated twice with similar results.
Proinflammatory cytokine induction by exoenzyme S.
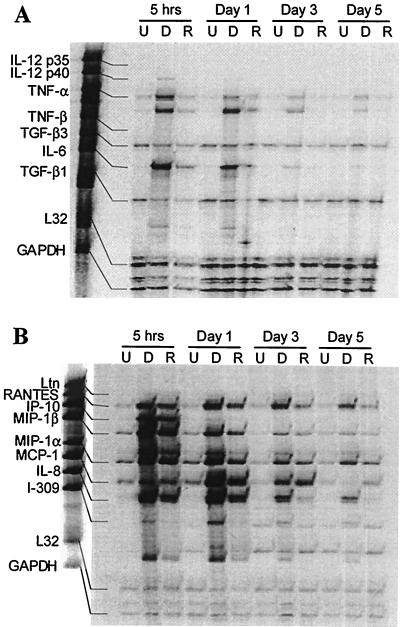
Together with IL-1α and IL-1β, ExoS/DG1 and rExoS induced rapid transcription of additional proinflammatory cytokines including TNF-α and IL-6 (Fig. 2A and Table 1). While both TNF-α and IL-6 peaked at 5 h, transcription of IL-6 decreased significantly by day 1 and transcription of TNF-α decreased gradually until day 5. The ExoS/DG1- and rExoS-induced cytokine profile was indicative of a proinflammatory stimulus more so than a polarized Th1-Th2 response with the induction of proinflammatory cytokines such as TNF-α, IL-1α, IL-1β, and IL-6. These cytokines mediate inflammatory responses through a variety of mechanisms, including the activation and recruitment of numerous cell types (9, 19).
FIG. 2.
Exoenzyme S induces transcription of proinflammatory cytokine (A) and chemokine (B) mRNA. Resting PBMC were either unstimulated (U), stimulated with 1 μg of ExoS/DG1 per ml (D), or with 1 μg of rExoS per ml (R) for various times. Undigested fragments were used as markers to determine the identity of protected fragments. Densitometric values are given in Tables 1 and 2. The experiment was repeated twice with similar results.
Both ExoS/DG1 and rExoS induced transcription of all of the chemokines tested (Fig. 2B). Transcription of IP-10, I-309, MIP-1β, and MIP-1α peaked at 5 h, and transcription of RANTES, MCP-1, and IL-8 peaked after 1 day of stimulation (Fig. 2B and Table 2). Ltn induction by both ExoS/DG1 and rExoS was weak (Fig. 2B and Table 2). Chemokine responses declined to near baseline levels by day 5. Based on mRNA expression, it would be predicted that neutrophils, monocytes, and T cells would be recruited to the lung. IL-8 and MIP-1α induce neutrophil chemotaxis, and neutrophil infiltration is believed to be responsible for neutrophil-mediated pulmonary tissue damage in CF (4, 11, 12). In addition to neutrophil recruitment, exoenzyme-S-induced MCP-1, IP-10, MIP-1α, MIP-1β, and RANTES are capable of recruiting monocytes to the lung (9, 38). The time course of transcription of all of the cytokines and chemokines was similar. There was no evidence of late transcription of some cytokines and therefore no suggestion that the response would evolve with time, with early recruitment of some cell types and late recruitment of other cells, or with a change in the activation state of these cells. This may be because apoptosis intercedes and abrogates late cytokine responses.
Cytokine and chemokine induction by exoenzyme S may play a key role in the deficient cell-mediated immunity observed in CF patients (32). I-309, RANTES, IL-8, MIP-1α, MIP-1β, and Ltn are chemotactic for T cells (9). The ability of exoenzyme-S-recruited T cells to mount a protective immune response may be altered by both direct and indirect mechanisms. Exoenzyme S induces apoptosis of T cells and, although the mechanism of this apoptosis has not yet been determined (6), it is possible that activated monocytes induce the death of T cells through a TNF-α-dependent mechanism (1), which would be an important mechanism of T-cell immunosuppression.
Immunoregulatory cytokines may also contribute to T-cell suppression. IL-12p40 was induced by both ExoS/DG1 (11-fold induction) and rExoS (4-fold induction), as was IL-10, which are capable of modulating T-cell function (Fig. 1 and 2A and Table 1) (9). Biologically active IL-12p70 is composed of the IL-12p35/p40 heterodimer, which induces IFN-γ secretion from T cells and NK cells and promotes differentiation of Th1 cells (9, 10). Transcription of IL-12p40 mRNA was detected, although transcription of IL-12p35 mRNA was not (Fig. 2A). Induction of IL-12p40 without IL-12p35 is a potential mechanism of altering the host response to P. aeruginosa since excess production of IL-12p40 can inhibit IL-12p70 function, resulting in decreased T-cell effector functions (25). This may be a potential explanation for the minimal transcription of IFN-γ despite marked T-cell activation by exoenzyme S. Furthermore, IL-12p40 may also contribute to the inflammatory response by directly recruiting monocytes (20). It is also noteworthy that, despite the transcription of IL-10, which regulates cytokines primarily at the level of transcription, substantial induction of proinflammatory cytokines and chemokines was present.
Polymyxin B was added to the cultures to eliminate any potential effect of LPS. Although it was unlikely that LPS was contributing when polymyxin B was present, additional experiments were performed to ensure that polymyxin B was capable of blocking the effect of any LPS contamination. A more sensitive RT-PCR-based assay (33) was used to amplify TNF-α mRNA, which is highly responsive to minute mounts of LPS (37). P. aeruginosa LPS (10 μg/ml) induced strong TNF-α induction (Fig. 3). In the presence of polymyxin B (10 μg/ml), LPS-induced TNF-α mRNA was abrogated, indicating that polymyxin B neutralized LPS. TNF-α mRNA that was induced by ExoS/DG1 was not inhibited by polymyxin B, indicating that the observed cytokine induction was not due to contaminating LPS.
FIG. 3.
LPS contamination is not responsible for cytokine upregulation. PBMC were stimulated in RPMI culture media for 3 h. PBMC were either unstimulated (Unst), stimulated with 10 μg of P. aeruginosa per ml (LPS), or stimulated with 1 μg of ExoS/DG1 per ml (DG1) with or without 10 μg of polymyxin B per ml. PBMC were collected and lysed, and the RNA was extracted. RT was performed using random hexamer primers. PCR was performed for 26 cycles using TNF-α-specific and GAPDH-specific primers. The experiment was repeated twice with similar results.
Increased levels of TNF-α, IL-8, IL-1α, IL-1β, and IL-6 and relatively unchanged levels of IL-1RA in response to exoenzyme S (Fig. 1 and 2) is a cytokine profile that is reminiscent of that found in the lungs of CF patients infected with P. aeruginosa compared to healthy individuals (4, 11, 22). It is likely that other proinflammatory cytokines also contribute to the inflammation, and certainly other cytokines are induced by exoenzyme S, although we are not aware of studies measuring these cytokines in the CF lung. We have observed that exoenzyme S isolated from two different strains of P. aeruginosa possess the ability to induce the transcription of proinflammatory cytokines and chemokines from PBMC, and this effect is not dependent on ADP-ribosyltransferase activity. Through the characterization of the cytokine-chemokine response of PBMC to exoenzyme S, we have identified a number of mechanisms by which exoenzyme S may contribute to ongoing pulmonary inflammation in CF. Understanding the mechanisms of an exoenzyme-S-induced inflammatory response will lead to a greater understanding of host-pathogen interactions and may help us devise immunotherapeutic strategies designed to alleviate P. aeruginosa-mediated inflammation in CF patients.
Acknowledgments
This work was supported by a grant from the Canadian Cystic Fibrosis Foundation.
We would like to thank Joseph Barbieri for his kind gift of the pETrHisExoS vector and Howard Wong for his expertise in RT-PCR.
REFERENCES
- 1.Badley A D, Dockrell D, Simpson M, Schut R, Lynch D H, Leibson P, Paya C V. Macrophage-dependent apoptosis of CD4+ T lymphocytes from HIV-infected individuals is mediated by FasL and tumor necrosis factor. J Exp Med. 1997;185:55–64. doi: 10.1084/jem.185.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beharka A A, Iandolo J J, Chapes S K. Staphylococcal enterotoxins bind H-2Db molecules on macrophages. Proc Natl Acad Sci USA. 1995;92:6294–6298. doi: 10.1073/pnas.92.14.6294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bjork L, Andersson J, Ceska M, Andersson U. Endotoxin and Staphylococcus aureus enterotoxin A induce different patterns of cytokines. Cytokine. 1992;4:513–519. doi: 10.1016/1043-4666(92)90013-h. [DOI] [PubMed] [Google Scholar]
- 4.Bonfield T L, Panuska J R, Konstan M W, Hilliard K A, Hilliard J B, Ghnaim H, Berger M. Inflammatory cytokines in cystic fibrosis lungs. Am J Respir Crit Care Med. 1995;152:2111–2118. doi: 10.1164/ajrccm.152.6.8520783. [DOI] [PubMed] [Google Scholar]
- 5.Bruno T F, Buser D E, Syme R M, Woods D E, Mody C H. Pseudomonas aeruginosa exoenzyme S is a mitogen but not a superantigen for human T lymphocytes. Infect Immun. 1998;66:3072–3079. doi: 10.1128/iai.66.7.3072-3079.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruno, T. F., D. E. Woods, and C. H. Mody. Exoenzyme S induces apoptosis of T lymphocytes. J. Leukoc. Biol., in press. [DOI] [PubMed]
- 7.Bruno T F, Woods D E, Storey D G, Mody C H. Recombinant Pseudomonas exoenzyme S and exoenzyme S from Pseudomonas aeruginosa strain DG1 share the ability to stimulate T lymphocyte proliferation. Can J Microbiol. 1999;45:607–611. [PubMed] [Google Scholar]
- 8.Carding S R, West J, Woods A, Bottomly K. Differential activation of cytokine genes in normal CD4-bearing T cells is stimulus dependent. Eur J Immunol. 1989;19:231–238. doi: 10.1002/eji.1830190203. [DOI] [PubMed] [Google Scholar]
- 9.Curfs J H A J, Meis J F G M, Hoogkamp-Korstanje J A A. A primer on cytokines: sources, receptors, effects, and inducers. Clin Microbiol Rev. 1997;10:742–780. doi: 10.1128/cmr.10.4.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D'Andrea A, Rengaraju M, Valiante N M, Chemimi J, Kubin M, Aste M, Chan S H, Kobayashi M, Young D, Nickbarg E, Chizzonite R, Wolf S F, Trinchieri G. Production of natural killer cell stimulatory factor (interleukin 12) by peripheral blood mononuclear cells. J Exp Med. 1992;176:1387–1398. doi: 10.1084/jem.176.5.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis P B, Drumm M, Konstan M W. Cystic fibrosis. Am J Respir Crit Care Med. 1996;154:1229–1256. doi: 10.1164/ajrccm.154.5.8912731. [DOI] [PubMed] [Google Scholar]
- 12.Dayer Pastore F, Schlegel-Haueter S E, Belli D C, Rochat T, Dudez T S, Suter S. Chemotactic factors in bronchial secretions of cystic fibrosis patients. J Infect Dis. 1998;177:1413–1417. doi: 10.1086/517827. [DOI] [PubMed] [Google Scholar]
- 13.Dosanjh A K, Elashoff D, Robbins R C. The bronchoalveolar lavage fluid of cystic fibrosis lung transplant recipients demonstrates increased interleukin-8 and elastase and decreased IL-10. J Interferon Cytokine Res. 1998;18:851–854. doi: 10.1089/jir.1998.18.851. [DOI] [PubMed] [Google Scholar]
- 14.Firestein G S, Roeder W D, Laxer J A, Townsend K S, Weaver C T, Hom J T, Linton J, Torbett B E, Glasebrook A L. A new murine CD4+ T cell subset with an unrestricted cytokine profile. J Immunol. 1989;143:518–525. [PubMed] [Google Scholar]
- 15.Frank D W. The exoenzyme S regulon of Pseudomonas aeruginosa. Mol Microbiol. 1997;26:621–629. doi: 10.1046/j.1365-2958.1997.6251991.x. [DOI] [PubMed] [Google Scholar]
- 16.Gantner F, Leist M, Kusters S, Vogt K, Volk H D, Tiegs G. T cell stimulus-induced crosstalk between lymphocytes and liver macrophages results in augmented cytokine release. Exp Cell Res. 1996;229:137–146. doi: 10.1006/excr.1996.0351. [DOI] [PubMed] [Google Scholar]
- 17.Grimwood K, To M, Rabin H R, Woods D E. Subinhibitory antibiotics reduce Pseudomonas aeruginosa tissue injury in the rat lung model. J Antimicrob Chemother. 1989;24:937–945. doi: 10.1093/jac/24.6.937. [DOI] [PubMed] [Google Scholar]
- 18.Grimwood K, To M, Semple R A, Rabin H R, Sokol P A, Woods D E. Elevated exoenzyme expression by Pseudomonas aeruginosa is correlated with exacerbations of lung disease in cystic fibrosis. Pediatr Pulmonol. 1993;15:135–139. doi: 10.1002/ppul.1950150302. [DOI] [PubMed] [Google Scholar]
- 19.Guidot D M, Hybertson B M, Kitlowski R P, Repine J E. Inhaled NO prevents IL-1-induced neutrophil accumulation and associated acute edema in isolated rat lungs. Am J Physiol. 1996;271:L225–L229. doi: 10.1152/ajplung.1996.271.2.L225. [DOI] [PubMed] [Google Scholar]
- 20.Ha S J, Lee C H, Lee S B, Kim C M, Jang K L, Shin H S, Sung Y C. A novel function of IL-12p40 as a chemotactic molecule for macrophages. J Immunol. 1999;163:2902–2908. [PubMed] [Google Scholar]
- 21.Knight D A, Finck-Barbancon V, Kulich S M, Barbieri J T. Functional domains of Pseudomonas aeruginosa exoenzyme S. Infect Immun. 1995;63:3182–3186. doi: 10.1128/iai.63.8.3182-3186.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kronborg G, Hansen M B, Svenson M, Fomsgaard A, Hoiby N, Bendtzen K. Cytokines in sputum and serum from patients with cystic fibrosis and chronic Pseudomonas aeruginosa infection as markers of destructive inflammation in the lungs. Pediatr Pulmonol. 1993;15:292–297. doi: 10.1002/ppul.1950150506. [DOI] [PubMed] [Google Scholar]
- 23.Kuge S, Miura Y, Nakamura Y, Mitomi T, Habu S, Nishimura T. Superantigen-induced human CD4+ helper/killer T cell phenomenon. Selective induction of Th1 helper/killer T cells and application to tumor immunotherapy. J Immunol. 1995;154:1777–1785. [PubMed] [Google Scholar]
- 24.Luo Y, Lloyd C, Gutierrez-Ramos J C, Dorf M E. Chemokine amplification in mesangial cells. J Immunol. 1999;163:3985–3992. [PubMed] [Google Scholar]
- 25.Mattner F, Fischer S, Guckes S, Jin S, Kaulen H, Schmitt E, Rude E, Germann T. The interleukin-12 subunit p40 specifically inhibits effects of the interleukin-12 heterodimer. Eur J Immunol. 1993;23:2202–2208. doi: 10.1002/eji.1830230923. [DOI] [PubMed] [Google Scholar]
- 26.Miethke T, Wahl C, Heeg K, Wagner H. T cell-mediated lethal shock triggered in mice by the superantigen staphylococcal enterotoxin B: critical role of tumor necrosis factor. J Exp Med. 1992;175:91–98. doi: 10.1084/jem.175.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mody C H, Buser D E, Syme R M, Woods D E. Pseudomonas aeruginosa exoenzyme S induces proliferation of human T lymphocytes. Infect Immun. 1995;63:1800–1805. doi: 10.1128/iai.63.5.1800-1805.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moss R B, Bocian R C, Hsu Y P, Dong Y J, Kemna M, Wei T, Gardner P. Reduced IL-10 secretion by CD4+ T lymphocytes expressing mutant cystic fibrosis transmembrane conductance regulator (CFTR) Clin Exp Immunol. 1996;106:374–388. doi: 10.1046/j.1365-2249.1996.d01-826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nicas T I, Frank D W, Lile J D, Iglewski B H. Role of exoenzyme S in chronic Pseudomonas aeruginosa lung infections. Eur J Clin Microbiol. 1985;4:175–179. doi: 10.1007/BF02013593. [DOI] [PubMed] [Google Scholar]
- 30.Ofek I, Cohen S, Rahmani R, Kabha K, Tamarkin D, Herzig Y, Rubinstein E. Antibacterial synergism of polymyxin B nonapeptide and hydrophobic antibiotics in experimental gram-negative infections in mice. Antimicrob Agents Chemother. 1994;38:374–377. doi: 10.1128/aac.38.2.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rink L, Luhm J, Koester M, Kirchner H. Induction of a cytokine network by superantigens with parallel Th1 and Th2 stimulation. J Interferon Cytokine Res. 1996;16:41–47. doi: 10.1089/jir.1996.16.41. [DOI] [PubMed] [Google Scholar]
- 32.Sorensen R U, Stern R C, Polmar S H. Cellular immunity to bacteria: impairment of in vitro lymphocyte responses to Pseudomonas aeruginosa in cystic fibrosis patients. Infect Immun. 1977;18:735–740. doi: 10.1128/iai.18.3.735-740.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Syme R M, Wood C J, Wong H, Mody C H. Both CD4+ and CD8+ human lymphocytes are activated and proliferate in response to Cryptococcus neoformans. Immunology. 1997;92:194–200. doi: 10.1046/j.1365-2567.1997.00345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vallis A J, Yahr T L, Barbieri J T, Frank D W. Regulation of ExoS production and secretion by Pseudomonas aeruginosa in response to tissue culture conditions. Infect Immun. 1999;67:914–920. doi: 10.1128/iai.67.2.914-920.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Woods D E, Que J U. Purification of Pseudomonas aeruginosa exoenzyme S. Infect Immun. 1987;55:579–586. doi: 10.1128/iai.55.3.579-586.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Woods D E, Schaffer M S, Rabin H R, Campbell G D, Sokol P A. Phenotypic comparison of Pseudomonas aeruginosa strains isolated from a variety of clinical isolates. J Clin Microbiol. 1986;24:260–264. doi: 10.1128/jcm.24.2.260-264.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wright S D, Ramos R A, Tobias P S, Ulevitch R J, Mathison J C. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- 38.Wuyts A, Proost P, Put W, Lenaerts J P, Van Damme J. Leukocyte recruitment by monocyte chemotactic proteins (MCPs) secreted by human phagocytes. J Immunol Rev. 1994;174:237–247. doi: 10.1016/0022-1759(94)90028-0. [DOI] [PubMed] [Google Scholar]