Abstract
BACKGROUND
In a pilot study involving patients with cutaneous squamous-cell carcinoma, a high percentage of patients had a pathological complete response with the use of two doses of neoadjuvant cemiplimab before surgery. Data from a phase 2 study are needed to confirm these findings.
METHODS
We conducted a phase 2, confirmatory, multicenter, nonrandomized study to evaluate cemiplimab as neoadjuvant therapy in patients with resectable stage II, III, or IV (M0) cutaneous squamous-cell carcinoma. Patients received cemiplimab, administered at a dose of 350 mg every 3 weeks for up to four doses, before undergoing surgery with curative intent. The primary end point was a pathological complete response (the absence of viable tumor cells in the surgical specimen) on independent review at a central laboratory, with a null hypothesis that a pathological complete response would be observed in 25% of patients. Key secondary end points included a pathological major response (the presence of viable tumor cells that constitute ≤10% of the surgical specimen) on independent review, a pathological complete response and a pathological major response on investigator assessment at a local laboratory, an objective response on imaging, and adverse events.
RESULTS
A total of 79 patients were enrolled and received neoadjuvant cemiplimab. On independent review, a pathological complete response was observed in 40 patients (51%; 95% confidence interval [CI], 39 to 62) and a pathological major response in 10 patients (13%; 95% CI, 6 to 22). These results were consistent with the pathological responses determined on investigator assessment. An objective response on imaging was observed in 54 patients (68%; 95% CI, 57 to 78). Adverse events of any grade that occurred during the study period, regardless of whether they were attributed to the study treatment, were observed in 69 patients (87%). Grade 3 or higher adverse events that occurred during the study period were observed in 14 patients (18%).
CONCLUSIONS
Neoadjuvant therapy with cemiplimab was associated with a pathological complete response in a high percentage of patients with resectable cutaneous squamous-cell carcinoma. (Funded by Regeneron Pharmaceuticals and Sanofi; ClinicalTrials.gov number, NCT04154943.)
Cutaneous squamous-cell carcinoma is the second most common form of skin cancer worldwide, with approximately 2.4 million new cases and 56,000 deaths in 2019.1 From 1990 through 2019, the overall incidence increased by more than 200%.1
Most patients with cutaneous squamous-cell carcinoma present with early-stage disease that can be successfully treated with surgery alone.2 A small percentage of patients present with locoregionally advanced disease or disease with adverse histopathological features, which may be treated with adjuvant radiation therapy and possibly systemic therapy, in addition to surgery.3 Cutaneous squamous-cell carcinoma most commonly develops in sun-exposed areas, such as the head and neck, where surgical extirpation may lead to disfiguration.4-7 Thus, treatment can have a profound effect on psychosocial functioning and quality of life.8-10
Cemiplimab, an anti-programmed cell death 1 (PD-1) monoclonal antibody, has been approved for the treatment of metastatic or locally advanced cutaneous squamous-cell carcinoma for which no curative local treatment options are available. In studies involving patients with advanced cutaneous squamous-cell carcinoma, cemiplimab was associated with an objective response in 44 to 50% of patients, along with durable disease control and improved patient-reported quality of life.11-13 Other PD-1 inhibitors have also been shown to have activity in patients with advanced cutaneous squamous-cell carcinoma.14,15 A potential reason for the exceptional responsiveness of cutaneous squamous-cell carcinoma to immunotherapy is a high tumor mutational burden due to sun-related ultraviolet mutagenesis.16 Currently, no role has been established for any systemic therapy as a curative treatment option for cutaneous squamous-cell carcinoma.
The promising results associated with the use of immunotherapy for advanced cutaneous squamous-cell carcinoma prompted interest in assessing the use of neoadjuvant immunotherapy for resectable cutaneous squamous-cell carcinoma. In a single-institution pilot study involving the administration of two doses of neoadjuvant cemiplimab in 20 patients with resectable stage III or IV (M0) cutaneous squamous-cell carcinoma, a pathological complete response was observed in 55% of the patients.17 The current phase 2, multicenter study was designed to provide confirmatory data and formally assess the efficacy of up to four doses of neoadjuvant therapy with cemiplimab for resectable stage II, III, or IV (M0) cutaneous squamous-cell carcinoma.
METHODS
STUDY OVERSIGHT
The study protocol (available with the full text of this article at NEJM.org) was approved by the appropriate institutional review board or independent ethics committee at each participating study site. The study was conducted in accordance with the principles of the Declaration of Helsinki and the International Conference on Harmonisation Good Clinical Practice guidelines. All patients provided written informed consent.
The study sponsors were Regeneron Pharmaceuticals and Sanofi. The study was designed by employees of Regeneron Pharmaceuticals in collaboration with the authors. The data were collected by investigators, analyzed by statisticians who were employed by the sponsors, and interpreted by the authors, including employees of Regeneron Pharmaceuticals. The authors had unrestricted access to the data and were responsible for all content. The first draft of the manuscript was prepared by a medical writer who was paid by Regeneron Pharmaceuticals. Thereafter, the first draft was critically reviewed and extensively revised by the authors. The authors vouch for the accuracy and completeness of the data and the fidelity of the study to the protocol.
STUDY POPULATION
Patients 18 years of age or older were eligible for inclusion in the study if they had resectable stage II, III, or IV (M0) cutaneous squamous-cell carcinoma for which primary surgery would be recommended in routine clinical practice. Tumor–node–metastasis (TNM) staging of cutaneous squamous-cell carcinoma with involvement of the head and neck was based on the eighth edition of the American Joint Committee on Cancer Staging Manual,18 and TNM staging of cutaneous squamous-cell carcinoma without involvement of the head and neck was based on the ninth edition of the Union for International Cancer Control Manual of Clinical Oncology.19 The metastasis stage indicates the presence of distant metastasis (M1) or the absence of distant metastasis (M0). All patients included in this study had a metastasis stage of M0. Patients with stage II disease had a primary tumor that measured at least 3 cm in greatest diameter. All patients had adequate organ function; at least one measurable lesion according to Response Evaluation Criteria in Solid Tumors, version 1.1 (RECIST 1.1)20; and a score on the Eastern Cooperative Oncology Group (ECOG) performance-status scale of 0 or 1 (with scores ranging from 0 to 5 and higher scores indicating greater disability). Patients who had previously received radiation therapy for cutaneous squamous-cell carcinoma were ineligible. The full list of eligibility criteria is available in the protocol.
STUDY DESIGN AND TREATMENT
This phase 2, multicenter, single-group, nonrandomized study enrolled patients in Australia, Germany, and the United States. The first part of the study evaluated cemiplimab as neoadjuvant therapy before surgery with curative intent in eligible patients with resectable cutaneous squamous-cell carcinoma (Fig. S1 in the Supplementary Appendix, available at NEJM.org). The second part allowed for optional adjuvant cemiplimab therapy, adjuvant radiation therapy, or observation only, with the approach determined according to investigator discretion. In this article, we report results of the first part of the study.
After a screening period of up to 28 days, patients received neoadjuvant cemiplimab, administered intravenously at a dose of 350 mg every 3 weeks for up to four doses over a 12-week period (study days 1, 22, 43, and 64) or until the occurrence of unacceptable toxic effects, disease progression, or withdrawal of consent. Patients underwent imaging assessments (computed tomography [CT], magnetic resonance imaging, or both) at baseline, at week 6 (before the third dose of neoadjuvant cemiplimab), and at week 12 (before surgery). Imaging of externally visible lesions was supplemented with digital medical photography. After the neoadjuvant period, the protocol-specified window for surgery was study days 75 to 100. If the patient met criteria for early discontinuation of cemiplimab during the neoadjuvant period, the treating physician could refer the patient for surgery at an earlier time.
END POINTS
The primary end point was a pathological complete response, defined as the absence of viable tumor cells in the surgical specimen obtained after treatment, determined on independent review. A key secondary end point was a pathological major response, defined as the presence of viable tumor cells that constitute up to 10% of the surgical specimen obtained after treatment, determined on independent review. The definitions for pathological complete response and pathological major response were specified according to immune-related pathological response criteria.21 On independent review, the surgical specimen was assessed at a central laboratory separately by two pathologists who were not investigators and were not aware of the results of other pathological assessments; the specimen was also reviewed by an adjudicator if needed (with 10 cases requiring adjudication). Other secondary end points included the following: a pathological complete response and a pathological major response determined on investigator assessment at a local laboratory; an objective response on imaging, defined according to RECIST 1.1 as a best overall response of complete or partial response and determined on investigator assessment; and adverse events to assess the safety and side-effect profile of cemiplimab.
EXPLORATORY ANALYSES
Exploratory analyses of potential associations of programmed cell death ligand 1 (PD-L1) expression and tumor mutational burden with treatment response were performed. Slides of formalin-fixed, paraffin-embedded tumor samples obtained before treatment were received at Q2 Solutions. The PD-L1 tumor proportion score was assessed by means of immunohistochemical staining with the use of the Ventana SP263 assay (Roche Diagnostics). The value was categorized as either PD-L1–negative (PD-L1 expression in <1% of tumor cells) or PD-L1–positive (PD-L1 expression in ≥1% of tumor cells). DNA was extracted from samples with at least 20% tumor content, and the tumor mutational burden was assessed with the use of the TruSight Oncology 500 assay (Illumina). The value was categorized as either high (equal to or higher than the median tumor mutational burden for the study population) or low (lower than the median tumor mutational burden). Details regarding the exploratory analyses are provided in the Supplementary Appendix.
STATISTICAL ANALYSIS
We estimated that a sample of 72 patients would provide the study with at least 90% power to reject the null hypothesis — that a pathological complete response would be observed in 25% of patients, a percentage that is not clinically meaningful — at a two-sided significance level of 0.05. This estimate was based on the assumption that a pathological complete response would be observed in 44% of the patients. The planned sample size was increased by 5%, to 76 patients, to account for the possibility that patients might prematurely withdraw from the study. The null hypothesis could be ruled out on the basis of the lower limit of the exact 95% confidence interval if a pathological complete response was observed in at least 37% of the patients. The percentages of patients with a pathological complete response, a pathological major response (with the definition excluding patients with a pathological complete response), and an objective response on imaging and associated exact 95% confidence intervals were calculated with the use of the Clopper–Pearson method.
The primary analysis was performed when all patients had completed surgery or were no longer deemed to be candidates for surgery after treatment with neoadjuvant cemiplimab. The efficacy and safety of cemiplimab were assessed in all patients who received at least one dose of the study treatment. All the reported data are based on a data-cutoff date of December 1, 2021, the date on which the final patient completed the 1-month follow-up visit after surgery.
RESULTS
PATIENTS
From March 20, 2020, to July 8, 2021, a total of 79 patients began to receive the study treatment (Fig. S2). Characteristics of the patients at baseline are summarized in Table 1. The median age was 73 years (range, 24 to 93), and 67 patients (85%) were men. The predominant primary anatomical site of the tumor was the head and neck (72 patients [91%]). Most patients had an ECOG performance-status score of 0 (60 patients [76%]) and had stage III disease (38 patients [48%]) or stage IV (M0) disease (36 patients [46%]); 47 patients (60%) presented with nodal metastases (Table S1). During the neoadjuvant period, 62 patients (78%) received all four doses of neoadjuvant cemiplimab (Table S2). The median duration of the follow-up period, between the administration of the first dose of cemiplimab and the data-cutoff date, was 9.7 months (range, 1.3 to 19.6).
Table 1.
Characteristics of the 79 Patients at Baseline.*
| Characteristic | Value |
|---|---|
| Median age (range) — yr | 73 (24–93) |
| Male sex — no. (%) | 67 (85) |
| Race — no. (%)† | |
| White | 69 (87) |
| Other | 2 (3) |
| Not reported | 8 (10) |
| Not Hispanic or Latinx — no. (%)† | 74 (94) |
| Primary tumor site — no. (%) | |
| Head and neck | 72 (91) |
| Trunk, arms, and legs | 7 (9) |
| Stage group — no. (%)‡ | |
| II | 5 (6) |
| III | 38 (48) |
| IV (M0) | 36 (46) |
| Tumor stage at screening — no. (%)‡ | |
| TX | 23 (29) |
| Tis | 1 (1) |
| T1 | 4 (5) |
| T2 | 10 (13) |
| T3 | 39 (49) |
| T4a | 2 (3) |
| Node stage at screening — no. (%)‡ | |
| NX | 1 (1) |
| N0 | 31 (39) |
| N1 | 13 (16) |
| N2§ | 11 (14) |
| N2b | 9 (11) |
| N2c | 1 (1) |
| N3¶ | 1 (1) |
| N3a | 1 (1) |
| N3b | 11 (14) |
| ECOG performance-status score — no. (%)∥ | |
| 0 | 60 (76) |
| 1 | 19 (24) |
Percentages may not total 100 because of rounding.
Race and ethnic group were reported by the patient.
Tumor–node–metastasis (TNM) staging of cutaneous squamous-cell carcinoma with involvement of the head and neck was based on the eighth edition of the American Joint Committee on Cancer Staging Manual, and TNM staging of cutaneous squamous-cell carcinoma without involvement of the head and neck was based on the ninth edition of the Union for International Cancer Control Manual of Clinical Oncology.
These values were not further specified as N2a, N2b, or N2c.
These values were not further specified as N3a or N3b.
Scores on the Eastern Cooperative Oncology Group (ECOG) performance-status scale range from 0 to 5, with higher scores indicating greater disability.
CLINICAL EFFICACY
After treatment with neoadjuvant cemiplimab, a pathological complete response was observed in 40 patients (51%; 95% confidence interval [CI], 39 to 62) on independent review at a central laboratory (Table 2). These results ruled out the null hypothesis that a pathological complete response would be observed in 25% of patients. A pathological major response was observed in 10 patients (13%; 95% CI, 6 to 22) on independent review. In addition, 20 patients (25%) were found to have no pathological complete response or pathological major response, defined as the presence of viable tumor cells that constitute more than 10% of the surgical specimen. The results on independent review were similar to the pathological responses determined on investigator assessment at a local laboratory. On investigator assessment, a pathological complete response was observed in 42 patients (53%; 95% CI, 42 to 65) and a pathological major response in 10 patients (13%; 95% CI, 6 to 22).
Table 2.
Tumor Response to Neoadjuvant Cemiplimab in the 79 Patients According to Pathological and Imaging-Based Response Assessment.*
| Tumor Response | Value | |||
|---|---|---|---|---|
| Independent Review | Investigator Assessment | |||
| no. (%) | 95% CI | no. (%) | 95% CI | |
| Pathological response | ||||
| Pathological complete response: absence of viable tumor cells in surgical specimen | 40 (51) | 39–62 | 42 (53) | 42–65 |
| Pathological major response: presence of viable tumor cells that constitute ≤10% of surgical specimen | 10 (13) | 6–22 | 10 (13) | 6–22 |
| No pathological complete response or pathological major response: presence of viable tumor cells that constitute >10% of surgical specimen† | 20 (25)† | — | NA | — |
| No pathological evaluation‡ | 9 (11) | — | 9 (11) | — |
| Response on imaging § | ||||
| Objective response: complete or partial response | — | — | 54 (68) | 57–78 |
| Best overall response¶ | ||||
| Complete response | — | — | 5 (6) | — |
| Partial response | — | — | 49 (62) | — |
| Stable disease | — | — | 16 (20) | — |
| Progressive disease | — | — | 8 (10) | — |
| No imaging-based evaluation | — | — | 1 (1) | — |
| Disease control∥ | — | — | 70 (89) | 80–95 |
Patients with resectable cutaneous squamous-cell carcinoma received cemiplimab, administered at a dose of 350 mg every 3 weeks for up to four doses, before undergoing surgery with curative intent. In the analysis of tumor response to neoadjuvant cemiplimab, exact 95% confidence intervals were calculated with the use of the Clopper–Pearson method. NA denotes not available.
Among patients with no pathological complete response or pathological major response, viable tumor cells constituted 11 to 30% of the surgical specimen in 5 patients, 31 to 60% of the specimen in 5 patients, and more than 60% of the specimen in 10 patients. On investigator assessment performed at a local laboratory, the tumor response was reported as a pathological complete response, a pathological major response, or other; therefore, data regarding no pathological complete response or pathological major response are not available for this assessment.
The 9 patients who did not undergo surgery were included in the pathological response analysis in accordance with the statistical analysis plan.
Responses on imaging were defined according to Response Evaluation Criteria in Solid Tumors, version 1.1.
Shown is the best overall response on prespecified imaging assessments performed after two or four planned doses of cemiplimab had been administered. Confirmation of complete response or partial response on imaging was not required because surgery was planned.
Disease control was defined as a complete response, a partial response, or stable disease.
Among the 17 patients (22%) who did not receive all four doses of neoadjuvant cemiplimab (Table S3), the most common reason was disease progression, a finding that was based on either imaging (8 patients) or clinical evaluation (3 patients). Overall, 9 patients (11%) did not undergo surgery in the protocol-specified window (study days 75 to 100) (Table S4) and therefore had no pathological evaluation. Of these patients, 5 had a partial response on imaging after treatment with neoadjuvant cemiplimab, including 3 who declined surgery, 1 who was lost to follow-up, and 1 who died from an adverse event that occurred before surgery (myocardial infarction in an 85-year-old man). Of the remaining 4 patients who did not undergo surgery, 2 had presented with bulky disease at baseline, which progressed to inoperable disease (after one dose of cemiplimab in 1 patient and after two doses of cemiplimab in the other patient). One patient who did not have an imaging-based evaluation died from an adverse event that occurred before surgery (exacerbation of congestive heart failure in a 93-year-old woman). One patient had disease progression after two doses of cemiplimab and did not attend protocol-specified follow-up visits.
An objective response on imaging after treatment with neoadjuvant cemiplimab, defined according to RECIST 1.1 and determined on investigator assessment, was observed in 54 patients (68%; 95% CI, 57 to 78) (Table 2). On imaging-based response assessment, 5 patients had a complete response, 49 had a partial response, 16 had stable disease, and 8 had progressive disease; 1 patient had no imaging-based evaluation. The percentage of patients with a complete response on imaging (6%) was much lower than the percentage of patients with a pathological complete response on independent review (51%).
Figure 1 shows both the pathological response determined on independent review and the response on imaging, defined according to RECIST 1.1 as the best percentage change from baseline in the sum of target-lesion diameters, for each patient with at least one cross-sectional imaging-based response assessment during the study period. Most patients who had a pathological complete response were not classified as having a complete response on preoperative imaging.
Figure 1. Tumor Response to Neoadjuvant Cemiplimab in Each Patient According to Pathological and Imaging-Based Response Assessment.
Patients with resectable cutaneous squamous-cell carcinoma received cemiplimab, administered at a dose of 350 mg every 3 weeks for up to four doses, before undergoing surgery with curative intent. For each patient, the pathological response to neoadjuvant cemiplimab determined on independent review at a central laboratory is indicated by color coding. The response to neoadjuvant cemiplimab detected on imaging is indicated by the plot, which shows the best percentage change from baseline in the sum of target-lesion diameters on imaging after treatment with neoadjuvant cemiplimab; responses on cross-sectional imaging were defined according to Response Evaluation Criteria in Solid Tumors, version 1.1 (RECIST 1.1). Data are not shown for the two patients who did not undergo cross-sectional imaging after baseline. The dashed lines indicate imaging-based criteria for partial response (≥30% decrease in the sum of target-lesion diameters) and progressive disease (≥20% increase in the sum of target-lesion diameters). Lesion measurements obtained after disease progression or surgery were excluded. An increase in the sum of target-lesion diameters of more than 100% is reported as 100%. According to RECIST 1.1, regression of 100% on imaging is not required for a complete response in patients with nodal target lesions.
Of the 70 patients who proceeded to surgery,5 had a complete response, 44 had a partial response, and 16 had stable disease on imaging. The 5 patients with a complete response on imaging were also found to have a pathological complete response (Table S5 and Fig. S3). Of the 44 patients with a partial response on imaging, 30 (68%) were found to have a pathological complete response, 8 (18%) were found to have a pathological major response, and 6 (14%) were found to have no pathological complete response or pathological major response. In these 6 patients, residual viable tumor cells constituted 15%, 20%, 40%, 65%, 89%, and 90% of the surgical specimen (Table S5). Of the 16 patients who had stable disease on imaging, 5 (31%) had a pathological complete response and 2 (12%) had a pathological major response. As of the time of data cutoff, none of the patients had had disease recurrence after surgery.
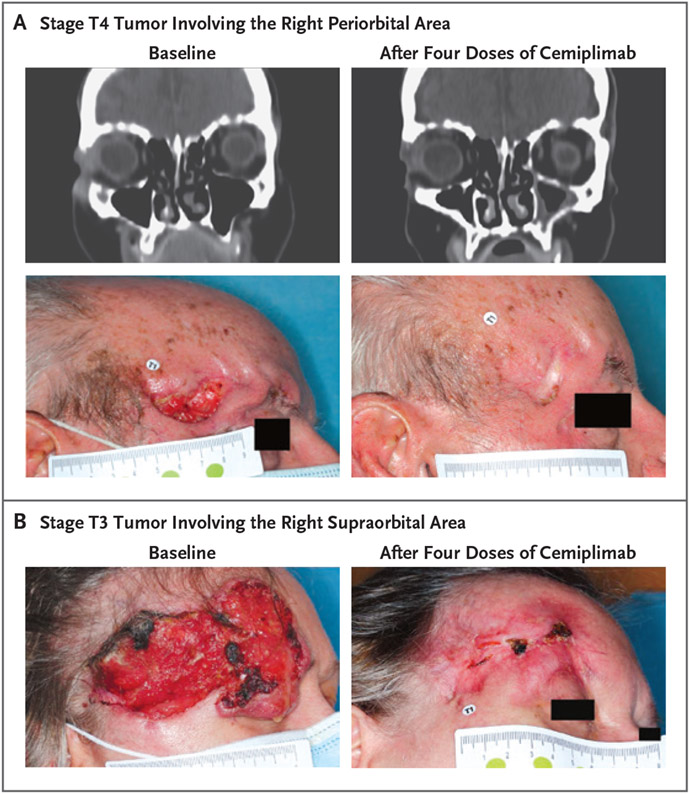
Figure 2 shows photographs and CT images of representative patients’ target lesions at baseline and after preoperative treatment with four doses of cemiplimab. These two patients were found to have a pathological complete response to cemiplimab, as well as a partial response on imaging. In both patients, the response to cemiplimab detected on imaging allowed them to be spared from orbital exenteration.
Figure 2. Clinical Responses in Patients with a Pathological Complete Response to Neoadjuvant Cemiplimab.
Panels A and B show photographs and contrast-enhanced computed tomographic (CT) images obtained from representative patients at baseline and after treatment with four doses of neoadjuvant cemiplimab, administered at a dose of 350 mg every 3 weeks. In Panel A, the patient is an 86-year-old man who presented with stage T4 cutaneous squamous-cell carcinoma involving the right periorbital area. In Panel B, the patient is a 59-year-old woman who presented with stage T3 cutaneous squamous-cell carcinoma involving the right supraorbital area. Neither patient underwent orbital exenteration, because the patients had a partial response on imaging, defined according to RECIST 1.1 and determined on investigator assessment, which allowed for less-extensive surgery.
SAFETY
Adverse events of any grade that occurred during the study period, regardless of whether they were attributed to the study treatment, were observed in 69 patients (87%) who received neoadjuvant cemiplimab (Table 3). The most common adverse events of any grade were fatigue (occurring in 24 patients [30%]), as well as diarrhea, nausea, and maculopapular rash (each occurring in 11 patients [14%]). Grade 3, 4, and 5 adverse events, regardless of attribution, were observed in 8 patients (10%), 2 patients (3%), and 4 patients (5%), respectively.
Table 3.
Adverse Events That Occurred during the Study Period in the 79 Patients Who Received Neoadjuvant Cemiplimab.*
| Adverse Event | Value | |
|---|---|---|
| Any Grade | Grade ≥3 | |
| no. of patients (%) | ||
| Any event | 69 (87) | 14 (18) |
| Serious event | 13 (16) | 10 (13) |
| Event that led to discontinuation of treatment | 1 (1) | 1 (1) |
| Event that led to death | 4 (5) | 4 (5) |
| Event of any grade that occurred in >5% of patients or grade ≥3 event that occurred in ≥1 patient† | ||
| Fatigue | 24 (30) | 1 (1)‡ |
| Diarrhea | 11 (14) | 1 (1)‡ |
| Nausea | 11 (14) | 0 |
| Maculopapular rash | 11 (14) | 0 |
| Constipation | 9 (11) | 0 |
| Pruritus | 8 (10) | 0 |
| Dizziness | 6 (8) | 0 |
| Anemia | 5 (6) | 1 (1)‡ |
| Decreased appetite | 5 (6) | 0 |
| Hypothyroidism | 5 (6) | 0 |
| Rash | 5 (6) | 0 |
| Arthralgia | 4 (5) | 0 |
| Headache | 4 (5) | 0 |
| Hypoesthesia | 4 (5) | 0 |
| Hyponatremia | 3 (4) | 2 (3)‡ |
| Insomnia | 3 (4) | 1 (1)‡ |
| Confusional state | 2 (3) | 2 (3)‡ |
| Myocardial infarction | 2 (3) | 1 (1)§ |
| Acute myocardial infarction | 1 (1) | 1 (1)§ |
| Agitation | 1 (1) | 1 (1)¶ |
| Cellulitis | 1 (1) | 1 (1)‡ |
| Congestive heart failure | 1 (1) | 1 (1)§ |
| Cholelithiasis | 1 (1) | 1 (1)‡ |
| Coronavirus disease 2019 pneumonia | 1 (1) | 1 (1)§ |
| Delusion | 1 (1) | 1 (1)¶ |
| Bullous dermatitis | 1 (1) | 1 (1)‡ |
| Impaired glucose tolerance | 1 (1) | 1 (1)‡ |
| Increased hepatic enzyme | 1 (1) | 1 (1)‡ |
| Immune-mediated hepatitis | 1 (1) | 1 (1)‡ |
| Hypertension | 1 (1) | 1 (1)‡ |
| Procedural hemorrhage | 1 (1) | 1 (1)¶ |
| Pulmonary embolism | 1 (1) | 1 (1)‡ |
Safety was assessed in all patients who received at least one dose of neoadjuvant cemiplimab. Adverse events were coded according to the preferred terms of the Medical Dictionary for Regulatory Activities, version 24.1. The severity of adverse events was graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 5.0.
The adverse events of any grade that occurred in more than 5% of patients are listed in descending order of frequency.
Grade 3 adverse events that occurred during the study period were observed in 8 patients (10%) who received neoadjuvant cemiplimab. A patient may have had more than one grade 3 adverse event.
Grade 5 adverse events that occurred during the study period were observed in 4 patients (5%) who received neoadjuvant cemiplimab.
Grade 4 adverse events that occurred during the study period were observed in 2 patients (3%) who received neoadjuvant cemiplimab: agitation and delusion both occurred in the same patient, and procedural hemorrhage occurred in 1 patient. The grade 4 hemorrhage occurred during a rotation-flap procedure 10 days after resection of the primary tumor; the patient had been taking clopidogrel until 3 days before the rotation-flap procedure and aspirin until the day of the procedure.
Adverse events of any grade that were considered by the investigator to be related to treatment occurred in 57 patients (72%), with the most common being fatigue (22 patients [28%]), maculopapular rash (11 patients [14%]), and diarrhea (9 patients [11%]) (Table S6). Immune-related adverse events occurred in 12 patients (15%) who received neoadjuvant cemiplimab, including grade 3 immune-related adverse events in 3 patients (4%) (Table S7).
Four adverse events that occurred during the study period were fatal. A fatal exacerbation of congestive heart failure occurred in a 93-year-old woman after two doses of cemiplimab. In that patient, coexisting medical conditions at baseline included congestive heart failure (grade 2), paroxysmal atrial fibrillation (grade 2), coronary artery disease, bilateral carotid-artery stenosis, hypercholesterolemia, and hypertension. The exacerbation of congestive heart failure was fatal despite treatment with glucocorticoids. This death was considered by the investigator to be possibly related to treatment.
The three other fatal adverse events were considered by the investigator to be unrelated to treatment. First, an 85-year-old man had a fatal acute myocardial infarction after three doses of cemiplimab. Coexisting medical conditions at baseline included peripheral vascular disease and coronary artery disease. Second, a 73-year-old man had disease progression after one dose of cemiplimab and had a fatal myocardial infarction approximately 7 weeks after that dose. Coexisting medical conditions at baseline included heart failure, atrial fibrillation, and type 2 diabetes. Third, an 82-year-old man died from pneumonia associated with coronavirus disease 2019 in the postoperative period.
BIOMARKER ANALYSES
Of the 79 patients included in the study, 56 had samples that could be assessed for the PD-L1 tumor proportion score at baseline. Of these patients, 15 had PD-L1 expression in less than 1% of tumor cells (PD-L1–negative) and 41 had PD-L1 expression in 1% of tumor cells or more (PD-L1–positive). Pathological responses were observed in both PD-L1–negative and PD-L1–positive patients, but the percentage of patients who had a pathological complete response was lower among patients with PD-L1 expression in less than 1% of tumor cells (20%; 95% CI, 4 to 48) than among those with PD-L1 expression in 1% of tumor cells or more (54%; 95% CI, 37 to 69) (Table S8). The percentage of patients who had an objective response on imaging, defined according to RECIST 1.1 and determined on investigator assessment, was 47% (95% CI, 21 to 73) among patients with PD-L1 expression in less than 1% of tumor cells, as compared with 76% (95% CI, 60 to 88) among those with PD-L1 expression in 1% of tumor cells or more (Table S9).
Among the 50 patients with samples that could be assessed for tumor mutational burden at baseline, the median tumor mutational burden was 58.5 mutations per megabase (interquartile range, 31.3 to 92.2) (Table S10). The median tumor mutational burden was 61.1 mutations per megabase among the 28 patients who had a pathological response (19 patients with a pathological complete response and 9 patients with a pathological major response), as compared with 46.2 mutations per megabase among the 22 patients who had no pathological complete response or pathological major response. However, given the wide range of values observed among both patients who had a pathological response and those who did not, no correlations can be made between efficacy and tumor mutational burden (Fig. S4). The percentage of patients who had a pathological complete response was 56% (95% CI, 35 to 76) among patients with a high tumor mutational burden, as compared with 20% (95% CI, 7 to 41) among those with a low tumor mutational burden (Table S11). The percentage of patients who had an objective response on imaging was 80% (95% CI, 59 to 93) among patients with a high tumor mutational burden, as compared with 56% (95% CI, 35 to 76) among those with a low tumor mutational burden (Table S12).
DISCUSSION
Neoadjuvant cemiplimab, administered at a dose of 350 mg every 3 weeks for up to four doses before surgery with curative intent, was associated with a pathological complete response in 51% of patients with stage II, III, or IV (M0) cutaneous squamous-cell carcinoma. On imaging-based response assessment, with responses defined according to RECIST 1.1 and determined on investigator assessment, an objective response was observed in 68% of patients. Overall, the percentage of patients with a pathological response was similar to the percentage of patients with a response on imaging, and the vast majority of patients who had a partial response on imaging were found to have a pathological complete response on examination of the surgical specimen. The percentage of patients who had a pathological complete response (51%) was substantially greater than the percentage of patients who had a complete response on imaging (6%); the reason for this discrepancy is unclear. These results suggest that neoadjuvant cemiplimab has high therapeutic activity in patients with resectable cutaneous squamous-cell carcinoma.
In patients with melanoma and non–small-cell lung cancer, a pathological response to neoadjuvant immunotherapy has been associated with a high incidence of disease-free survival after surgery.22-24 The durable clinical benefit of two doses of neoadjuvant cemiplimab in patients with cutaneous squamous-cell carcinoma was shown in the pilot study of this approach, in which all 15 patients with stage III or IV (M0) cutaneous squamous-cell carcinoma who had a pathological response (pathological complete response or pathological major response) remained disease-free at a median follow-up of 37.4 months, most of whom did not receive adjuvant radiation therapy.17,25
The assessment of pathological response in this study was rigorous. Independent review involved assessment of each specimen at a central laboratory separately by two pathologists who were not investigators and were not aware of the results of other pathological assessments, with adjudication as needed. In this study, only 10 cases required adjudication; the pathological findings were unambiguous. The pathological responses determined on independent review were highly consistent with the pathological responses determined on investigator assessments at local laboratories. Preservation of important functional structures, such as the eye, became possible in some patients who had a response on imaging, but the surgeries for curative intent performed in this study were required to involve attempted oncologic resection (R0) with removal of adequate material for comprehensive pathological response assessments.
No new safety concerns were identified for cemiplimab in this study. However, four fatal adverse events were observed. Three of these deaths were deemed to be most likely related to underlying cardiac disease. Cutaneous squamous-cell carcinoma occurs predominantly in the geriatric population, which has a higher likelihood of coexisting conditions than younger populations. Thus, careful attention to patient screening and selection before treatment is important, as is close monitoring of patients during treatment with checkpoint blockade. Analyses of tumor mutational burden and PD-L1 expression at baseline showed no clear correlation of these molecular features with efficacy outcomes, which suggests that these are not adequate biomarkers to guide decisions regarding neoadjuvant therapy with cemiplimab; these findings are similar to those observed in studies involving patients with advanced cutaneous squamous-cell carcinoma.12
Like the pilot study,17 the current study enrolled patients with high-risk, advanced-stage disease; 91% of the patients had a primary tumor of the head and neck, 49% had a stage T3 tumor, and 60% had nodal metastases. However, notable aspects of the current study that were different from the pilot study were the extension of neoadjuvant therapy with cemiplimab from two doses to four doses, the independent review of pathological response, and the multicenter study design. Most patients in the current study were able to receive all four doses of cemiplimab, but the appropriate duration of neoadjuvant therapy with cemiplimab is yet to be defined and may vary according to patient. In the pilot study, all patients with a response on imaging underwent surgery, whereas in the current study, 5 patients with a partial response on imaging did not undergo surgery and therefore were classified as having no pathological response.
These data provide a rationale for the use of neoadjuvant cemiplimab in patients with resectable cutaneous squamous-cell carcinoma, but several important questions remain unanswered. Limitations to this study include the absence of a control group; without randomization, the possibility of selection bias cannot be ruled out. A high percentage of White male participants were enrolled (Table S13). Furthermore, the relatively short median follow-up at the time of this report means that mature data regarding disease-free survival after surgery are not yet available. Although the end point of pathological major response has not yet definitively identified a distinct subgroup with an improved disease course in an independent series of patients with cutaneous squamous-cell carcinoma, the results of the current study are encouraging. Ongoing follow-up may provide clarity as to whether long-term outcomes among patients who have a pathological complete response differ from such outcomes among patients who have a pathological major response. Although optional adjuvant therapy was provided according to investigator discretion, this study was not designed to assess postsurgical management. The efficacy of neoadjuvant immunotherapy as compared with standard-of-care treatment or with adjuvant immunotherapy strategies currently under investigation is unknown. Finally, the discordance between the percentage of patients with a complete response on imaging (6%) and the percentage of patients with a pathological complete response (51%) may show the inherent technical limitation of contemporary cross-sectional imaging, underscoring the importance of tissue confirmation and highlighting the need for alternative methods of response assessment.
This multicenter study, with independent pathological review at a central laboratory, showed that cemiplimab was associated with a pathological complete response in a high percentage of patients with resectable stage II, III, or IV (M0) cutaneous squamous-cell carcinoma, and no new safety signals for cemiplimab were identified. The potential for function-preserving surgery, together with the high frequency of a pathological complete response, supports the use of neoadjuvant therapy with cemiplimab in this patient population.
Supplementary Material
Acknowledgments
Supported by Regeneron Pharmaceuticals and Sanofi.
We thank the patients, their families, and all staff members at the study sites who were involved in this study; and Atif Riaz, Ph.D., of Prime (Knutsford, United Kingdom) for medical-writing support, funded by Regeneron Pharmaceuticals.
APPENDIX
The authors’ full names and academic degrees are as follows: Neil D. Gross, M.D., David M. Miller, M.D., Ph.D., Nikhil I. Khushalani, M.D., Vasu Divi, M.D., Emily S. Ruiz, M.D., M.P.H., Evan J. Lipson, M.D., Friedegund Meier, M.D., Yungpo B. Su, M.D., Paul L. Swiecicki, M.D., Jennifer Atlas, M.D., Jessica L. Geiger, M.D., Axel Hauschild, M.D., Jennifer H. Choe, M.D., Ph.D., Brett G.M. Hughes, M.D., Dirk Schadendorf, M.D., Vishal A. Patel, M.D., Jade Homsi, M.D., Janis M. Taube, M.D., Annette M. Lim, M.D., Ph.D., Renata Ferrarotto, M.D., Howard L. Kaufman, M.D., Frank Seebach, M.D., Israel Lowy, M.D., Ph.D., Suk-Young Yoo, Ph.D., Melissa Mathias, M.D., Keilah Fenech, B.Sc., Hyunsil Han, Ph.D., Matthew G. Fury, M.D., Ph.D., and Danny Rischin, M.D.
The authors’ affiliations are as follows: the Department of Head and Neck Surgery (N.D.G.) and the Department of Thoracic and Head and Neck Medical Oncology (R.F.), M.D. Anderson Cancer Center, Houston, and the Harold C. Simmons Cancer Center, University of Texas Southwestern Medical Center, Dallas (J.H.); the Department of Medicine, Division of Hematology and Oncology (D.M.M.), the Department of Dermatology (D.M.M.), and the Department of Surgery (H.L.K.), Massachusetts General Hospital and Harvard Medical School, and the Department of Dermatology, Brigham and Women’s Hospital and Harvard Medical School (E.S.R.) — all in Boston; the Department of Cutaneous Oncology, Moffitt Cancer Center, Tampa, FL (N.I.K.); the Department of Otolaryngology–Head and Neck Surgery, Stanford Cancer Institute, Institute for Stem Cell Biology and Regenerative Medicine, Stanford University School of Medicine, Stanford, CA (V.D.); Bloomberg–Kimmel Institute for Cancer Immunotherapy and Sidney Kimmel Comprehensive Cancer Center (E.J.L., J.M.T.) and the Department of Dermatology, School of Medicine (J.M.T.), Johns Hopkins University, Baltimore; the Skin Cancer Center at the University Cancer Center and the National Center for Tumor Diseases Dresden, Department of Dermatology, University Hospital Carl Gustav Carus and Technische Universität Dresden, Dresden (F.M.), the Department of Dermatology, Schleswig-Holstein University Hospital, Kiel (A.H.), and the Department of Dermatology, University Hospital of Essen and German Cancer Consortium, Partner Site Essen, Essen (D.S.) — all in Germany; Head and Neck Medical Oncology, Nebraska Cancer Specialists, Omaha (Y.B.S.); Rogel Comprehensive Cancer Center, University of Michigan, Ann Arbor (P.L.S.); Levine Cancer Institute, Atrium Health, Charlotte (J.A.), and Duke Cancer Institute, Durham (J.H.C.) — both in North Carolina; Taussig Cancer Institute, Department of Hematology and Medical Oncology, Cleveland Clinic, Cleveland (J.L.G.); the Department of Cancer Care Services, Royal Brisbane and Women’s Hospital and University of Queensland, Brisbane (B.G.M.H.), and the Department of Medical Oncology, Peter MacCallum Cancer Centre and University of Melbourne, Melbourne, VIC (A.M.L., D.R.) — both in Australia; the Departments of Dermatology, Medicine, and Oncology, George Washington University School of Medicine and Health Sciences, Washington, DC (V.A.P.); and Regeneron Pharmaceuticals, Tarrytown, NY (F.S., I.L., S.-Y.Y., M.M., K.F., H.H., M.G.F.).
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
The authors’ full names, academic degrees, and affiliations are listed in the Appendix.
Contributor Information
N.D. Gross, Department of Head and Neck Surgery, Boston
D.M. Miller, Department of Medicine, Division of Hematology and Oncology, Department of Dermatology, Boston
N.I. Khushalani, Department of Cutaneous Oncology, Moffitt Cancer Center, Tampa, FL, Germany
V. Divi, Department of Otolaryngology–Head, and Neck Surgery, Stanford Cancer Institute, Institute for Stem Cell Biology and Regenerative Medicine, Stanford University School of Medicine, Stanford, CA, Germany
E.S. Ruiz, Massachusetts General Hospital and Harvard Medical School, and the Department of Dermatology, Brigham and Women’s Hospital and Harvard Medical School, Boston
E.J. Lipson, Bloomberg–Kimmel Institute for Cancer Immunotherapy and Sidney Kimmel Comprehensive Cancer Center, Germany
F. Meier, Johns Hopkins University, Baltimore; the Skin Cancer Center at the University Cancer Center and the National Center for Tumor Diseases Dresden, Department of Dermatology, University Hospital Carl Gustav Carus and Technische Universität Dresden, Dresden, Germany
Y.B. Su, Head and Neck Medical Oncology, Nebraska Cancer Specialists, Omaha
P.L. Swiecicki, Rogel Comprehensive Cancer Center, University of Michigan, Ann Arbor
J. Atlas, Levine Cancer Institute, Atrium Health, Charlotte, North Carolina
J.L. Geiger, Taussig Cancer Institute, Department of Hematology and Medical Oncology, Cleveland Clinic, Cleveland
A. Hauschild, Department of Dermatology, Schleswig-Holstein University Hospital, Kiel, Germany
J.H. Choe, Duke Cancer Institute, Durham, North Carolina
B.G.M. Hughes, Department of Cancer Care Services, Royal Brisbane and Women’s Hospital and University of Queensland, Brisbane
D. Schadendorf, Department of Dermatology, University Hospital of Essen and German Cancer Consortium, Partner Site Essen, Essen, Germany
V.A. Patel, Departments of Dermatology, Medicine, and Oncology, George Washington University School of Medicine and Health Sciences, Washington, DC
J. Homsi, M.D. Anderson Cancer Center, Houston, and the Harold C. Simmons Cancer Center, University of Texas Southwestern Medical Center, Dallas, Boston
J.M. Taube, Bloomberg–Kimmel Institute for Cancer Immunotherapy and Sidney Kimmel Comprehensive Cancer Center; Department of Dermatology, School of Medicine, Germany
A.M. Lim, Department of Medical Oncology, Peter MacCallum Cancer Centre and University of Melbourne, Melbourne, VIC, Australia
R. Ferrarotto, Department of Thoracic and Head and Neck Medical Oncology, Boston
H.L. Kaufman, Department of Surgery, Boston
F. Seebach, Regeneron Pharmaceuticals, Tarrytown, NY
I. Lowy, Regeneron Pharmaceuticals, Tarrytown, NY
S.-Y. Yoo, Regeneron Pharmaceuticals, Tarrytown, NY
M. Mathias, Regeneron Pharmaceuticals, Tarrytown, NY
K. Fenech, Regeneron Pharmaceuticals, Tarrytown, NY
H. Han, Regeneron Pharmaceuticals, Tarrytown, NY
M.G. Fury, Regeneron Pharmaceuticals, Tarrytown, NY
D. Rischin, Department of Medical Oncology, Peter MacCallum Cancer Centre and University of Melbourne, Melbourne, VIC, Australia
REFERENCES
- 1.Institute for Health Metrics and Evaluation. Global Burden of Disease 2019 results (http://ghdx.healthdata.org/gbd-results-tool).
- 2.Nehal KS, Bichakjian CK. Update on keratinocyte carcinomas. N Engl J Med 2018;379:363–74. [DOI] [PubMed] [Google Scholar]
- 3.Que SKT, Zwald FO, Schmults CD. Cutaneous squamous cell carcinoma: management of advanced and high-stage tumors. J Am Acad Dermatol 2018;78:249–61. [DOI] [PubMed] [Google Scholar]
- 4.Jubran J, Sengelmann RD. High-risk squamous cell carcinoma and its impact on a 62-year-old male surgeon. BMJ Case Rep 2019;12(8):e229940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sweeny L, Zimmerman T, Carroll WR, Schmalbach CE, Day KE, Rosenthal EL. Head and neck cutaneous squamous cell carcinoma requiring parotidectomy: prognostic indicators and treatment selection. Otolaryngol Head Neck Surg 2014;150:610–7. [DOI] [PubMed] [Google Scholar]
- 6.Chabrillac E, Talawdekar A, Garikipati S, et al. A single centre’s experience of 23 cases of total rhinectomy for the treatment of squamous cell carcinoma involving the nasal vestibule. Eur Arch Otorhinolaryngol 2022;279:2069–75. [DOI] [PubMed] [Google Scholar]
- 7.Gerring RC, Ott CT, Curry JM, Sargi ZB, Wester ST. Orbital exenteration for advanced periorbital non-melanoma skin cancer: prognostic factors and survival. Eye (Lond) 2017;31:379–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mukoyama N, Nishio N, Kimura H, et al. Prospective evaluation of health-related quality of life in patients undergoing anterolateral craniofacial resection with orbital exenteration. J Neurol Surg B Skull Base 2020;81:585–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D’heygere V, Mattheis S, Stähr K, et al. Epithetic nasal reconstruction after total rhinectomy: oncologic outcomes, immediate and long-term adverse effects, and quality of life. J Plast Reconstr Aesthet Surg 2021;74:625–31. [DOI] [PubMed] [Google Scholar]
- 10.Xu V, Gill KS, Goldfarb J, et al. First bite syndrome after parotidectomy: a case series and review of literature. Ear Nose Throat J 2020. December 14 (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 11.Migden MR, Rischin D, Schmults CD, et al. PD-1 blockade with cemiplimab in advanced cutaneous squamous-cell carcinoma. N Engl J Med 2018;379:341–51. [DOI] [PubMed] [Google Scholar]
- 12.Migden MR, Khushalani NI, Chang ALS, et al. Cemiplimab in locally advanced cutaneous squamous cell carcinoma: results from an open-label, phase 2, single-arm trial. Lancet Oncol 2020;21:294–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rischin D, Khushalani NI, Schmults CD, et al. Integrated analysis of a phase 2 study of cemiplimab in advanced cutaneous squamous cell carcinoma: extended follow-up of outcomes and quality of life analysis. J Immunother Cancer 2021;9(8):e002757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grob J-J, Gonzalez R, Basset-Seguin N, et al. Pembrolizumab monotherapy for recurrent or metastatic cutaneous squamous cell carcinoma: a single-arm phase II trial (KEYNOTE-629). J Clin Oncol 2020;38:2916–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shalhout SZ, Emerick KS, Kaufman HL, Miller DM. Immunotherapy for non-melanoma skin cancer. Curr Oncol Rep 2021;23:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chalmers ZR, Connelly CF, Fabrizio D, et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med 2017;9:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferrarotto R, Amit M, Nagarajan P, et al. Pilot phase II trial of neoadjuvant immunotherapy in locoregionally advanced, resectable cutaneous squamous cell carcinoma of the head and neck. Clin Cancer Res 2021;27:4557–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amin MB, Edge SB, Greene FL, et al. , eds. AJCC cancer staging manual. 8th ed. New York: Springer, 2017. [Google Scholar]
- 19.O’Sullivan B, Brierley JD, D’Cruz A, et al. , eds. UICC manual of clinical oncology. 9th ed. Geneva: Union for International Cancer Control, 2015. [Google Scholar]
- 20.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. [DOI] [PubMed] [Google Scholar]
- 21.Cottrell TR, Thompson ED, Forde PM, et al. Pathologic features of response to neoadjuvant anti-PD-1 in resected non-small-cell lung carcinoma: a proposal for quantitative immune-related pathologic response criteria (irPRC). Ann Oncol 2018;29:1853–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Menzies AM, Amaria RN, Rozeman EA, et al. Pathological response and survival with neoadjuvant therapy in melanoma: a pooled analysis from the International Neoadjuvant Melanoma Consortium (INMC). Nat Med 2021;27:301–9. [DOI] [PubMed] [Google Scholar]
- 23.Uprety D, Mandrekar SJ, Wigle D, Roden AC, Adjei AA. Neoadjuvant immunotherapy for NSCLC: current concepts and future approaches. J Thorac Oncol 2020;15:1281–97. [DOI] [PubMed] [Google Scholar]
- 24.Forde PM, Spicer J, Lu S, et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N Engl J Med 2022;386:1973–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gross ND, Ferrarotto R, Amit M, et al. Long-term outcomes of a phase II trial of neoadjuvant immunotherapy for advanced, resectable cutaneous squamous cell carcinoma of the head and neck (CSCC-HN). J Clin Oncol 2022;40:Suppl:9519. abstract. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.