Abstract
In a recent Lancet Oncology article, Yau et al. report the CheckMate 459 trial results. This is the first phase III trial comparing the single-agent anti-programmed death protein 1 (PD-1) therapy nivolumab to the tyrosine kinase inhibitor sorafenib for treatment-naive patients with advanced hepatocellular carcinoma.
Liver cancer is the fourth leading cause of cancer-related mortality worldwide, and its incidence is rising in Western countries. Hepatocellular carcinoma (HCC) is the most common form of liver cancer, and it accounts for 85%–90% of all primary liver cancers (Ferlay et al., 2019). The majority of patients present with advanced-stage HCC, for which systemic therapy has been limited to a single agent—the antiangiogenic tyrosine kinase inhibitor (TKI) sorafenib—until 2017 (Llovet et al., 2008). Since then, we have witnessed substantial development of systemic therapies and the approval of three other multi-TKIs and immune checkpoint inhibitors (ICIs) used alone or in combinaton (Sangro et al., 2021) (Figure 1A).
Figure 1. Therapeutic options for hepatocellular carcinoma.
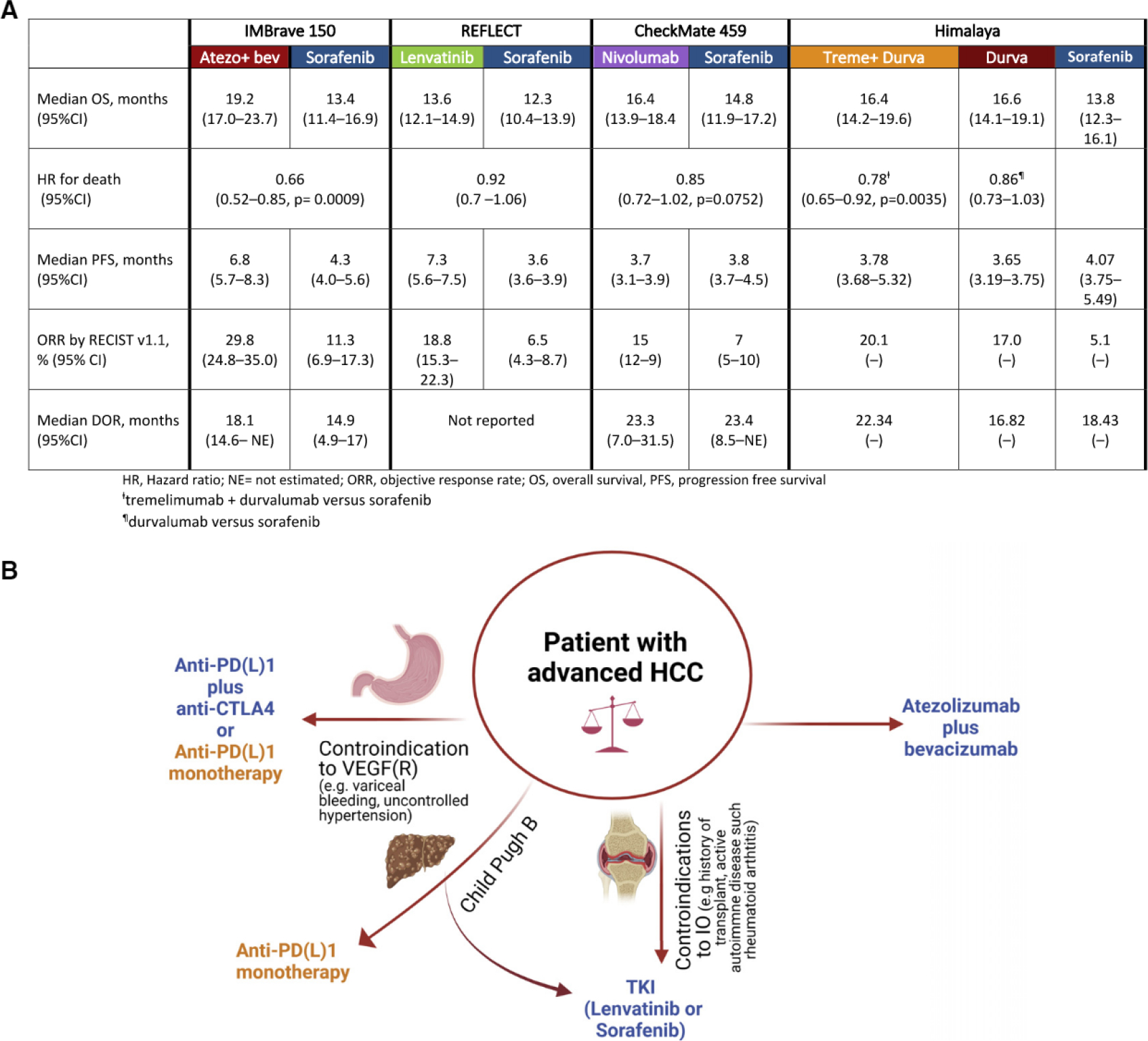
(A) Summary of the recent landmark trials of first-line therapies in hepatocellular carcinoma (HCC).
(B) Selection of first-line therapy in patients with advanced HCC.
In Lancet Oncology, Yau et al., (2022) recently reported on the CheckMate 459 trial, an open-label, phase III trial that randomized patients to nivolumab versus sorafenib for first-line therapy. CheckMate 459 builds on a previous phase 1 b/ll trial of nivolumab as monotherapy, mostly as second line, in patients with advanced HCC; that trial demonstrated an objective response rate (ORR) of 15% and a median duration of response of 17 months (95% confidence interval [Cl] 6–24) (El-Khoueiry et al., 2017). In the CheckMate 459 trial, 743 patients with histologically confirmed, advanced HCC, Child-Pugh score A, were randomly assigned to receive either nivolumab (240 mg IV every 2 weeks) or sorafenib (400 mg orally twice daily). Overall survival (OS) was the primary endpoint. Secondary endpoints were objective response (OR), progression-free survival (PFS), and efficacy based on baseline tumor cell programmed death ligand-1 (PD-L1) expression.
Although the predefined threshold of statistical significance for OS was not met, patients receiving nivolumab survived longer (median survival 16.4 versus 14.7 months, hazard ratio [HR] 0.85; p = 0.07) with 1 -year and 2-year survival rates of 60% and 37% compared with 55% and 33% for sorafenib, respectively. Median PFS was comparable: 3.8 months for nivolumab and 3.9 months for sorafenib (HR 0.98).
Notably, the authors show that nivolumab leads to significant improvements in ORR (15% versus 7%) compared to sorafenib, regardless of the underlying liver disease etiology. Other endpoints in the CheckMate 459 trial favored nivolumab over sorafenib: nivolumab showed longer-lasting disease control (median 7.5 months versus 5.7 months), a better safety profile with fewer grade 33 treatment-related adverse events (TRAEs) (22% versus 49%), and fewer events leading to treatment discontinuation as compared to the sorafenib arm.
The results of this trial have significant implications, arising both from a clinical and a translational standpoint, for the management of HCC patients. First, a tendency toward favorable outcomes with ICI monotherapy was observed. These results mirror the findings from KEYNOTE-240, a phase III trial that randomized patients with advanced HCC, previously treated with sorafenib, to pembrolizumab plus best supportive care (BSC) versus placebo plus BSC; the results of this trial showed a trend toward improved OS, although it was not statistically significant (OS HR 0.78; 95% Cl 0.61–0.99; p = 0.0238). In both studies, a higher proportion of patients in the sorafenib group in CheckMate 459 (31 %) and in the BSC arm in KEYNOTE 240 (47%) went on to receive ICI or an investigational agent at the time of progression, and these therapies probably affected the OS analysis (Finn et al., 2020b; Yau et al., 2022).
Second, this encourages more studies of combinations of programmed death protein 1 (PD-1) and/or PD-L1 inhibitors with other target therapies or additional checkpoint inhibitors. Phase III trials investigating the efficacy of these combinations have been completed or are ongoing, and they will further change the armamentarium of HCC management. The combination of atezolizumab (an anti-PD-L1 antibody) and bevacizumab (an anti-vascular endothelial growth factor [VEGF] antibody) was the first regimen to improve OS compared with sorafenib (HR 0.58; 95% Cl 0.42–0.79; p < 0.001), and it has become the standard of care as a first-line therapy for HCC (Finn et al., 2020a). In addition, an expansion arm in the CheckMate 040 study evaluated the combination of nivolumab plus ipilimumab (an anti-cytotoxic T lymphocyte-associated antigen-4 [CTLA-4] antibody) in a three-arm randomized study in patients who progressed on prior treatment with sorafenib. The combination achieved an outstanding objective response of 31 % with a median duration of 17 months and a median OS of 23 months; and this led to FDA approval in the second-line setting. More recently, a phase III trial that used a single dose of tremelimumab combined with a continuous dose of the PD-L1 inhibitor durvalumab (Himalaya) presented a significant improvement in OS against sorafenib in the first-line setting (HR 0.78; 96% Cl 0.65–0.92; p = 0.0035) and had better safety profile. Additionally, the study showed that durvalumab monotherapy was not inferior to sorafenib (HR 0.86; 96% Cl 0.73–1.03) (Abou-Alfa et al., 2022).
Third, the long tail of the survival curves and the higher and longer-lasting radiological responses in the nivolumab arm suggest that a subset of patients may derive a meaningful benefit from a single-agent anti-PD-1 antibody. This also underscores the importance of identifying reliable predictive biomarkers to allow a proper selection of patients and minimize financial toxicities. In CheckMate 459, a higher response rate was observed in patients with positive tumor PD-L1 expression (defined as 31%). Other studies of nivolumab and pembrolizumab mirrored these findings, and this suggests higher rates of ORR and prolonged PFS in patients with positive PD-L1 expression scores. However, these data did not translate into better OS in randomized trials of ICIs in monotherapies and in combination regimens, and this highlights the limitation of the PD-L1 score (Llovet et al., 2021). The lack of consensus for cutoff values and methods to assess PD-L1 expression, and its intrinsic heterogeneity, contribute to these limitations. Other putative biomarkers such as tumor mutation burden, tumor lymphocytic infiltration, immune class gene signature, Wnt and/or β-catenin aberrations, and CTNNB1 mutation are under investigation. Post hoc subgroup analysis can also provide putative biomarkers that warrant further evaluation in patient selection.
As new therapies evolve the HCC treatment landscape, the choice of treatment will depend substantially on patient characteristics that best match the safety and toxicity profiles of the treatment options (Figure 1B). Indeed, HCC is a unique type of cancer that often arises in the background of advanced liver disease, and this per se can limit patients’ life expectancy and quality of life. Thus, single-agent ICI is certainly a useful option for patients with contraindications to the use of anti-angiogenics (such as bevacizumab or TKIs) due to cardiovascular comorbidities and thrombotic or bleeding risk. Anti-PD-1 monotherapy could be useful in patients who cannot undergo a timely endoscopic evaluation of variceal bleeding, a procedure which is recommended before treatment with bevacizumab. Furthermore, although the majority of the current available therapies have been tested only in patients with well-preserved liver function (Child pugh score A), nivolumab has shown clinical activity and an acceptable safety profile in patients with more advanced liver dysfunction (Child Pugh score B) who otherwise have limited therapeutic options (Llovet et al., 2021). In CheckMate 459, the authors performed an extensive HCC-specific quality of life assessment survey which demonstrated some subscales which favored nivolumab; these included more delayed time to deterioration in physical functioning, and this suggests a favorable benefit-to-risk ratio for ICI in advanced HCC (Yau et al., 2022).
In summary, PD-1 and PD-L1 inhibitors have shown unequivocal signs of activity in HCC. They are currently the backbone of systemic therapies in clinical practice and in ongoing clinical trials for HCC. These ongoing trials will provide much-needed tissues and mechanistic data for deep immune profiling of the HCC tumor microenvironment (Ho et al., 2021) and development of better biomarkers for optimization of future therapies in HCC.
Footnotes
DECLARATION OF INTERESTS
M.B. declares no competing interests. A.K.K. has received research funding from AstraZeneca and served on advisory boards for Exelixis and Eisai. R.A.A. receives research support from Bristol Myers Squibb, RAPT therapeutics, Stand up to Cancer, and the National Institutes of Health and serves on the advisory boards for Bristol Myers Squibb, Merck SD, and AstraZeneca.
REFERENCES
- Abou-Alfa GK, Lam Chan S, Kudo M, Lau G, Kelley RK, Furuse J, Sukeepaisarnjaroen W, Kang YK, Dao TV, De Toni EN, et al. (2022). Phase 3 randomized, open-label, multicenter study of tremelimumab (T) and durvalumab (D) as first-line therapy in patients (pts) with unresectable hepatocellular carcinoma (uHCC): HIMALAYA. J. Clin. Oncol. 40. [Google Scholar]
- El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M „ Hsu C„ Kim TY, Choo SP, Trojan J, Welling TH, et al. (2017). Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 389, 2492–2502. 10.1016/S0140-6736(17)31046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Pineros M, Znaor A, and Bray F (2019). Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 144, 1941–1953. 10.1002/ijc.31937. [DOI] [PubMed] [Google Scholar]
- Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, et al. ; IMbrave150 Investigators (2020a). Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 382, 1894–1905. 10.1056/NEJMOA1915745. [DOI] [PubMed] [Google Scholar]
- Finn RS, Ryoo BY, Merle P, Kudo M, Bouattour M, Lim HY, Breder V, Edeline J, Chao Y, Ogasawara S, et al. ; KEYNOTE-240 investigators (2020b). Pembrolizumab As SecondLine Therapy in Patients With Advanced Hepatocellular Carcinoma in KEYNOTE-240: A Randomized, Double-Blind, Phase III Trial. J. Clin. Oncol. 38, 193–202. 10.1200/JCO.19.01307. [DOI] [PubMed] [Google Scholar]
- Ho WJ, Zhu Q, Durham J, Popovic A, Xavier S, Leatherman J, Mohan A, Mo G, Zhang S, Gross N, et al. (2021). Neoadjuvant Cabozantinib and Nivolumab Converts Locally Advanced HCC into Resectable Disease with Enhanced Antitumor Immunity. Nat. Can. 2, 891–903. 10.1038/S43018-021-00234-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, et al. ; SHARP Investigators Study Group (2008). Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 359, 378–390. 10.1056/NEJMOA0708857/SUPPL_FILE/NEJM_LLOVET_378SA1.PDF. [DOI] [PubMed] [Google Scholar]
- Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, Lencioni R, Koike K, Zucman-Rossi J, and Finn RS (2021). Hepatocellular carcinoma. Nat. Rev. Dis. Primers 7, 6. 10.1038/S41572-020-00240-3. [DOI] [PubMed] [Google Scholar]
- Sangro B, Sarobe P, Hervás-Stubbs S, and Melero I (2021). Advances in immunotherapy for hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 18, 525–543. 10.1038/S41575-021-00438-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau T, Park JW, Finn RS, Cheng AL, Mathurin P, Edeline J, Kudo M, Harding JJ, Merle P, Rosmorduc O, et al. (2022). Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 23, 77–90. 10.1016/S1470-2045(21)00604-5/ATTACHMENT/9C28674D-C96B-4E35-B1E8-E008833FD446/MMC1.PDF. [DOI] [PubMed] [Google Scholar]


