Abstract
Systemic assessment is a pillar in the neurological, oncological, mechanical, and systemic (NOMS) decision-making framework for the treatment of patients with spinal metastatic disease. Despite this importance, emerging evidence relating systemic considerations to clinical outcomes following surgery for spinal metastatic disease has not been comprehensively summarised. We aimed to conduct a scoping literature review of this broad topic. We searched MEDLINE, Embase, Scopus, Cochrane Central Register of Controlled Trials, Web of Science, and CINAHL databases from Jan 1, 2000, to July 31, 2021. 61 articles were included, accounting for a total of 22 335 patients. Preoperative systemic variables negatively associated with postoperative clinical outcomes included demographics (eg, older age [>60 years], Black race, male sex, low or elevated body-mass index, and smoking status), medical comorbidities (eg, cardiac, pulmonary, hepatic, renal, endocrine, vascular, and rheumatological), biochemical abnormalities (eg, hypoalbuminaemia, atypical blood cell counts, and elevated C-reactive protein concentration), low muscle mass, generalised motor weakness (American Spinal Cord Injury Association Impairment Scale grade and Frankel grade) and poor ambulation, reduced performance status, and systemic disease burden. This is the first comprehensive scoping review to broadly summarise emerging evidence relevant to the systemic assessment component of the widely used NOMS framework for spinal metastatic disease decision making. Medical, surgical, and radiation oncologists can consider these findings when prognosticating spinal metastatic disease-related surgical outcomes on the basis of patients’ systemic condition. These factors might inform a shared decision-making approach with patients and their families.
Introduction
An estimated 10% of patients with cancer will develop symptomatic spinal metastatic disease; of the patients that do, up to 50% will require treatment and 5–10% will require surgical intervention.1 Surgery for spinal metastatic disease is indicated for decompression of the neural elements, restoration of biomechanical stability, and relief of intractable pain.2 Preservation of ambulatory status and decreasing mortality have been shown in large prospective trials.1
Patients undergoing surgery for spinal metastatic disease are increasingly old (>60 years) and frail, necessitating consideration of their physical reserve and their ability to tolerate surgery.2 Failure to do so could result in preventable morbidity and mortality.3 Oncological measures of physical reserve include the Kamofsky performance score (KPS) and Eastern Cooperative Oncology Group (ECOG) performance status. Scoring systems (eg, the Tokuhashi score) accounting for the burden of malignancy and neurological status have been developed to facilitate the surgical candidate selection process and estimate overall survival.4 Furthermore, decision frameworks provide a common language across disciplines to facilitate the development of multimodal treatment plans. Neurological, oncological, mechanical, and systemic considerations are pillars in the widely used neurological, oncological, mechanical, and systemic (NOMS) framework.2
Medical, surgical, and radiation oncologists routinely evaluate patients’ systemic condition when assessing their ability to tolerate palliative surgery.2 Despite the inherent importance of systemic assessment in the context of spinal metastatic disease, emerging evidence relating to systemic variables and postoperative outcomes has not been comprehensively summarised. We aimed to systematically conduct a scoping review of this broad topic. Identifying impactful systemic variables represents a first step towards the development of evidence-based tools for prognosticating spinal metastatic disease-related surgical outcomes on the basis of patients’ systemic condition.
Methods
Study design
The AO Spine Knowledge Forum Tumor group—an international group of spine oncology surgeons and oncologists seeking to advance the care of patients with spinal metastatic disease—systematically conducted a scoping review using a framework derived from Arksey and O’Malley, according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Reviews (PRISMA-ScR) checklist (appendix pp 1–4).5 A review protocol is not published before scoping reviews.5 A formal and transparent scoping review was deemed more suitable than a systematic review for several reasons in accordance with published6 methodological recommendations: (1) emerging evidence relating systemic variables and clinical outcomes following surgical treatment of spinal metastatic disease has not been comprehensively summarised and it was unclear what types of evidence were available regarding this broad topic; (2) given the substantial heterogeneity in study designs (eg, tumour histologies, treatment strategies, and outcome measures) used across the general spinal metastatic disease-related literature; (3) we did not intend to critically appraise or address the appropriateness or effectiveness of specific spinal metastatic disease-related practices or treatments; and (4) we aimed to broadly map and summarise the spinal metastatic disease literature pertaining to preoperative systemic variables influencing a wide range of postoperative clinical outcomes.
Research question
Among adults (≥18 years) surgically treated for spinal metastatic disease, what preoperative systemic variables influence postoperative clinical outcomes?
Study selection
A two-stage screening process was used to select studies. Abstracts were independently screened by two reviewers (MAM and CJT) using the inclusion and exclusion criteria detailed in table 1. Duplicates were removed manually. Full texts selected for citations were assessed for eligibility. In cases of disagreement, consensus was reached through open discussion and detailed review of the full text. Reference lists of included full-text articles were manually searched (by MAM).
Table 1:
Inclusion and exclusion criteria (PICO model)
| Inclusion criteria | Exclusion criteria | |
|---|---|---|
|
| ||
| Patients | Adult patients (≥18 years old) and first surgery for spinal metastasis (non-revision) | Primary spinal cancer, sarcomatous tumours, and paediatric patients (<18 years old) |
| Intervention | Any spinal surgical procedure open or minimally invasive surgery for one of the following indications related to spinal metastatic disease: intractable pain, spinal instability (neurological or mechanical), neurological deficit related to spinal metastatic disease, and oncological treatment or resection enabling adjuvant therapies (eg, separation surgery to allow for stereotactic radiosurgery or fractionated radiotherapy) | Patients who did not undergo spinal surgery for spinal metastasis; patients treated solely with radiotherapy, stereotactic radiosurgery, chemotherapy, or immunotherapy; or patients treated solely with kyphoplasty, vertebroplasty, radio-ablation, or stereotactic biopsies |
| Study design | Original series including at least 30 patients; published in any peer-reviewed journal between Jan 1, 2001, and july 31, 2021; English language; studies analysing established scoring systems if preoperative systemic variables are individually analysed as predictors of outcome; univariate or multivariate analysis of at least one independent systemic variable as a predictor of outcome | Animal studies, opinion papers, commentaries, editorials, reviews, meta-analyses; technical note or paper assessing only technique safety and efficacy; studies in which predictive systemic variables are not individually analysed (ie, where they are analysed concomitantly with other variables as a multifactorial score) |
PICO=population, intervention, comparison, and outcome.
Data collection and summary
Data collection was independently done by two reviewers (MAM and CJT) using a standardised extraction form that was reviewed and refined by all authors before use (appendix p 7). Study characteristics are detailed in table 2 and the appendix (pp 8–18). Clinical outcomes were not restricted or predefined given the exploratory nature of this scoping review. Independent systemic variables and respective clinical outcomes affected are described in table 3 and the appendix (pp 19–32). Institution and database of published studies were used to avoid inclusion of duplicate data. We did not include multiple studies using the same cohort; instead, only a single respective study with the largest sample and the longest follow-up was included.
Table 2:
Summary of characteristics of included studies
| Number of patients | Included studies (n=61) |
|---|---|
|
| |
| Cohort characteristics | |
| Number of patients | 22 335 |
| Sex (%) | |
| Male | 14 071 (63%) |
| Female | 8264 (37%) |
| Mean number of patients per study | 366 |
| Age at surgery (years) | |
| Weighted mean | 60·4 (SD 2·15) |
| Cohort geography | |
| Asian | 23 (38%) |
| North American | 28 (46%) |
| European | 10 (16%) |
| Study types | |
| Prospective cohorts | 4 (7%) |
| Retrospective cohorts | 57 (93%) |
| Surgical indications * | |
| Neurological dysfunction | 49 (80%) |
| Intractable pain | 20 (33%) |
| Spinal instability | 12 (20%) |
| General oncological treatment | 8 (13%) |
| Surgical approach (patients) | |
| Patients for which approach is reported | 9 834 (44·0%) |
| Anterior | 808 (8·3%) |
| Posterior | 8 090 (83·6%) |
| Combined | 934 (9·7%) |
| Surgical intervention (patients) * | |
| Patients for which intervention is reported | 19 945 (89·3%) |
| Decompression with or without fusion | 15 924 (79·8%) |
| Corpectomy | 1 583 (7·9%) |
| Spondylectomy | 299 (1·5%) |
| Tumour histology | |
| Number of studies reporting histology | 52 (85%) |
| Not reported | 9 (15%) |
| Single tumour | 24 (39%) |
| Multiple tumour | 28 (46%) |
| Tumour histology type (patients) | |
| Total patients for which histology is reported | 9 984 (44·7%) |
| Lung | 3 065 (30·7%) |
| Kidney | 1 764 (17·7%) |
| Breast | 1 582 (15·9%) |
| Prostate | 1 411 (14·1%) |
| Other (not specified) | 672 (6·7%) |
| Hepatobiliary | 362 (3·6%) |
| Thyroid | 337 (3·4%) |
| Colorectal | 160 (1·6%) |
| Outcomes examined * | |
| Survival | 40 (66%) |
| Complications (any) | 20 (33%) |
| Neurological function, ambulation, or mobility | 10 (16%) |
| Non-home discharge or length of stay | 4 (7%) |
| Health-related quality of life | 1 (2%) |
Data refer to studies and are given as n (%), unless otherwise stated.
Not mutually exclusive: patients could have more than one indication for surgery, surgical intervention, or outcome examined.
Table 3:
Expanded summary of evidence demonstrating statistically significant, negative associations between systemic variables and postoperative clinical outcomes
| Study design | Sample size | Age* | Men (%) | Primary cancer site (% by type) | Significance in univariate analysis | Significance in multivariate analyses | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Cheung et al (2019)7 | Retrospective | 2202 | NR | 63% | NR | Pulmonary complications: increased among patients with obesity (p=0·046); VTE: increased among patients with obesity (p=0·014); duration of hospital stay: decreased among patients with obesity (p=0·0010); and RBC transfusion: decreased among patients with obesity (p<0·001) | VTE: obesity, OR 1·75 (95% CI 1·2–2·6, p=0·0070); and UTI: obesity, 0·38 (0·15–0·95, p=0·038) |
| De la Garza Ramos et al (2016)8 | Retrospective | 4583 | 62 (54–70) | 59% | Lung (34%), breast (21%), prostate (21%), kidney (20%), and thyroid (4%) | More than one major complication: anaemia, CHF, chronic lung disease, coagulopathy, electrolyte imbalance, pulmonary circulation disorders, renal failure, malnutrition, and pathological fracture (all more likely, p<0·001); association between frailty (mild, moderate, or severe) and inpatient mortality: moderate (OR 5·15 [95% CI 2·4–10·9]) and severe (5·74 [2·7–12·2], both p<0·001); association between frailty (mild, moderate, or severe) and major inpatient complications: mild (1·88 [1·33–2·66]), moderate (3·83 [2·71–5·41]), and severe (6·97 [4·98–9·74], all p<0·001); and association between frailty (mild, moderate, or severe) and LOS: mild (mean 3·3 [SD 0·4] days, p<0·001), moderate (5·6 [0·4] days, p<0·001), and severe (6·4 [0·4] days, p<0·001) | More than one major complication: anaemia, OR 1·36 (95% CI 1·1–1·7, p=0·0010); CHF, 1·83 (1·2–2·7, p=0·0090); chronic lung disease, 1·64 (1·3–2·0); coagulopathy, 1·98 (1·5–2·6); electrolyte imbalance, 2·66 (2·2–3·2); pulmonary disease, 3·19 (2·3–4·4); renal failure, 1·79 (1·2–2·7); malnutrition, 2·11 (1·5–2·99, all six p<0·001); and pathological fracture, 1·23 (1·01–1·5, p=0·033) |
| De la Garza Ramos et al (2020)9 | Retrospective | 1601 | 61·2 | 62% | NR | RBC transfusions: men (p=0·034), coagulopathy (p=0·090), hypoalbuminaemia (p=0·0080), previous transfusion, preoperative anaemia, decreased preoperative haematocrit (all three p<0·001), increased preoperative international normalised ratio (p=0·065), and higher ASA physical status class (p=0·0010) | RBC transfusions: higher ASA physical status class, OR 1·5 (95% CI 1·2–2·1, p=0·0030); preoperative anaemia, 3·1 (2·1–4·56, p<0·001); any complications: red blood cell transfusion, 1·65 (1·1–2·6, p=0·022); and hypoalbuminaemia, 1·53 (1·01–2·3, p=0·042) |
| De la Garza Ramos et al (2021)10 | Retrospective | 1226 | Non-Hispanic White: 62 (SD 12); Black: 60 (SD 11) | Non-Hispanic White: 62%; Black: 68% | NR | Black race: overall complications, increased (p=0·013) and minor complications, increased (p=0·0080) | Minor complications: Black race, OR 1·87 (95% CI 1·2–3·01, p=0·010); older age: 1·02 (1·0–1·03, p=0·026); and dependent functional status (partly or fully dependent for ADLs), 1·84 (1·1–3·2, p=0·027); major complications: smoking, 2·56 (1·60–4·10); dependent functional status (partly or fully dependent for ADLs), 2·93 (1·7–5·1); and hypoalbuminaemia, 1–67 (1·1–2·6, all three p<0·001) |
| Dea et al (2014)11 | Prospective | 101 | 62 (33–85) | 49% | Lymphoma (5%), breast (22%), NSCLC (20%), kidney (17%), colorectal (11%), prostate (7%), and other (19%) | NR | Complications (any): increased age (p=0·035); pressure sores: motor score (p=0·031); increased LOS: preoperative motor score (p<0·0001); worse overall survival: Frankel grade (statistically significant; p value NR); and sex: male (statistically significant, p value NR)† |
| Dea et al (2020)12 | Retrospective | 253 | 57·5 (SD 11·2) | 49% | Breast (18%), lung (20%), prostate (6%), kidney (16%), and other (40%) | Less than 3-month survival for men (p<0·001): worse ECOG-PS score (p=0·038), worse AIS score (p<0·001), worse NRS score (p=0·044), and worse EQ-5D score (p=0·0010) | NR |
| Elsamadicy et al (2020)13 | Retrospective | 4423 | 30-day readmission: 60 (SD 15); 90-day readmission: 60 (SD 13); and no readmission: 62 (SD 14) | 30-day readmission: 61%; 90-day readmission: 60%; and no readmission: 61% | NR | NR | 30-day readmission: hypertension, OR 1·45 (95% CI 1·2–1·8, p=0·0020), and renal failure, 1·53 (1·1–2·2, p=0·025); 31–90-day readmission: rheumatoid arthritis or collagen vascular disease, 4·08 (1·5–11·5, p=0·0080) and coagulopathy, 0·50 (0·3–0·97, p=0·040) |
| Hussain et al (2019)14 | Retrospective | 1498 | Normoalbuminaemic (>3·5 g/dL): 41% ≥65 years of age; hypoalbuminaemic (<3·5 g/dL): 36% ≥65 years of age | Normoalbuminaemic (>3·5 g/dL): 65%; hypoalbuminaemic (<3·5 g/dL): 64% | NR | Association between hypoalbuminaemia and 30-day survival; any complications; sepsis; RBC transfusion; LOS ≥10 days; or non-home discharge (all p<0·001) | Association between hypoalbuminaemia and 30-day survival, OR 5·2 (95% CI 3·4–8·0, p<0·001); any complications, 3·2 (2·4–4·1, p<0·001); sepsis, 3·1 (1·9–4·9, p<0·001); RBC transfusion, 1·4 (1·1–1·8, p<0·001); LOS ≥10 days, 4·3 (3·3–5·6, p<0·001); or non-home discharge, 2·9 (2·3–3·7, p<0·001) |
| Karhade et al (2019)15 | Retrospective | 732 | 61 (53–69) | 58% | NR | Overall survival: BMI <18 (vs 18–30), HR 1·8 (95% CI 1·1–2·9, p=0·010); Charlson comorbidity other than metastases, 1·3 (1·1–1·5, p=0·0070); ECOG-PS 3–4, 2·7 (2·1–3·4, p<0·001); ASIA score (A-D), 1·5 (1·3–1·8, p<0·001); anaemia (haemoglobin <13 g/dL), 1·7 (1·4–2·0, p<0·001); thrombocytopenia (<150 × 103 cells/μL), 1·5 (1·1–1·9, p=0·0040); thrombocytosis (>450×103 cells/μL), 1·4 (1·1–2·0, p=0·020); absolute lymphocyte count (×103 cells/μL) <1, 1·6 (1·3–1·9, p<0·001); absolute neutrophil count (×103 cells/μL) >6, 1·3 (1·1–1·6, p=0·010); neutrophil-to-lymphocyte ratio ≥4·7, 1·8 (1·5–2·2, p<0·001); platelet-to-lymphocyte ratio ≥408, 1·7 (1·4–2·0, p<0·001); albumin <3·5 g/dL, 2·0 (1·6–2·4, p<0·001); ALP (IU/L) ≥100, 1·8 (1·5–2·2, p<0·001); calcium (mg/dL) ≥9, 0·7 (0·6–0·9, p<0·001); creatinine (mg/dL) ≥1, 0·8 (0·6–0·9, p=0·0090) | Overall survival, HR (95% CI): Charlson comorbidity other than metastases, 1·2 (1·02–1·4, p=0·030); ECOG-PS 3–4, 2·7 (2·1–3·3, p<0·001); anaemia (haemoglobin) <13 g/dL: 1·4 (1·1–1·7, p=0·0010); albumin <3·5 g/dL: 2·0 (1·7–2·5, p<0·001); ALP (IU/L) ≥100: 1·3 (1·1–1·5, p=0·0060) |
| Nater et al (2018)16 | Prospective | 142 | 59 (47–71) | 58% | Lung (34%), kidney (22%), breast (21%), and prostate (19%) | Overall survival: men, HR 1·59 (95% CI 1·0–2·5, p=0·038); elevated BMI, 0·945 (0·91–0·98, p=0·0062); ODI, 10·014 (1·0–1·0, p=0·012); EQ-5D, 0·300 (0·1–0·7, p=0·0049); SF-36 PCS, 0·95 (0·92–0·97, p<0·001); and metastases to other organs with or without extraspinal bone metastases, 2·2 (1·4–3·4, p<0·001) | Overall survival: SF-36 PCS, HR 0·95 (95% CI 0·92–0·97, p<0·0001); metastases to other organs: 1·9 (1·3–3·2, p<0·001) |
| Park et al (2016)17 | Prospective | 50 | 58 (26–79) | 54% | Lung (100%) | Postoperative non-ambulatory status: pre-operative non-ambulatory status, OR 17·7 (95% CI 1·6–203·1, p=0·021) | NR |
| Prost et al (2020)18 | Prospective | 264 | 64 (SD 19) | 56% | Lung (21%), breast (19%), kidney (13%), prostate (10%), gastrointestinal (4%), head and neck (3%), thyroid (3%), hepatic (3%), melanoma (2%), gynaecological (2%), bladder (2%), and other (20%) | NR | 12-month survival: CRP >10 mg/L, 2·7 (p<0·01); albumin (>35 g/L), 0·5 (p<0·001); hypercalcaemia (>2·6 nmol/L): 2·3 (p<0·001); any complications: CRP >10 mg/L, 1·7 (p<0·01)‡ |
| Schoenfeld et al (2016)19 | Retrospective | 318 | 60·2 (SD 13·2) | 58% | Lung (16%), breast (13%), lymphoma or multiple myeloma (12%), and other (58%) | 30-day survival: ambulatory preoperative, OR 9·6 (95% CI 2·2–41·1, p value NR); albuminaemia ≥3·5 g/dL, 8·0 (3·0–21·7, p value NR); BMI <18·5, 0·2 (0·1–0·7, p value NR); 90-day survival: ambulatory status, 3·0 (1·7–5·4, p value NR); albuminaemia ≥3·5 g/dL, 3·5 (2·1–5·9, p value NR); BMI <18·5, 0·6 (0·2–1·8, p value NR)§ | 30-day survival, OR (95% CI): albuminaemia ≥3·5 g/dL, 9·0 (3·1–26·6, p<0·001); normal ambulatory status at the time of surgery, 6·8 (1·5–30·7, p=0·010); BMI <18·5, 0·18 (0·04–0·8, p=0·020, decreased odds of survival); 90-day survival, OR (95% CI): albuminaemia ≥3·5 g/dL: 3·9 (2·2–6·8, p<0·001); ambulatory status, 2·4 (1·3–4·5, p=0·0060) |
| Sebaaly et al (2018)20 | Retrospective | 297 | 61 (SD 10·9) | 54% | NR | NR | SSI: ASA ≥3, OR 1·1 (95% CI 1·03–1·2, p=0·020) and smoking, 2·4 (1·06–7·2, p=0·040) |
| Tatsui et al (2014)21 | Retrospective | 267 | 59·2 (17–86) | 77% | Renal (100%) | Overall survival: progressive systemic disease, HR 4·1 (95% CI 3·1–5·4, p<0·001) and preoperative neurological deficit, 2·3 (1·6–3·1, p<0·001) | Overall survival: progressive systemic disease, HR 4·1 (95% CI 2·9–5·8, p<0·001) and preoperative neurological deficit 1·8 (1·2–2·7, p<0·002) |
| Zairi et al (2016)22 | Retrospective | 271 | Smallest psoas tertile (n=90): 61 (SD 13); middle psoas tertile (n=90): 61 (SD 9·3); largest psoas tertile (n=91): 57·4 (SD 12) | Smallest psoas tertile (n=90): 59%; middle psoas tertile (n=90): 58%; largest psoas tertile (n=91): 57% | Lung (23%), prostate (15%), renal (14%), breast (13%), haematological (8%), gastrointestinal (7%), nasopharynx (6%), thyroid (4%), hepatic (2%), skin (2%), and other (7%) | Ambulatory status: KPS (p<0·001), Frankel grade (p=0·022); non-discharge home: KPS <80 (p=0·016), Frankel grade (p=0·0087), Tokuhashi score (p=0·039) | NR |
| Zakaria et al (2020); 30-day survival23 | Retrospective | 247 | 60 (25–87) | 52% | Lung (23%), prostate (15%), kidney (14%), breast (13%), haematopoietic (8%), gastrointestinal (7%), nasopharynx (6%), thyroid (4%), liver (2%), skin (2%), and other (7%) | Stroke: OR 4·8 (95% CI 1·4–16·4, p=0·013); liver disease: 5·8 (1·6–20·3; p=0·0060); middle versus smallest psoas tertile: 0·6 (0·4–0·8; p<0·001); largest vs smallest psoas tertile: 0·4 (0·3–0·6; p<0·001); Tokuhashi score: 0·8 (0·6–0·9, p=0·0010); and KPS: 0·96 (0·94–0·99; p=0·0010) | Liver disease: OR 7·6 (95% CI 1·2–47·0, p=0·029);Tokuhashi score: 0·7 (0·5–0·98, p=0·039) |
| Zakaria et al (2020); 90-day survival23 | Retrospective | 247 | 60 (25–87) | 52% | Lung (23%), prostate (15%), kidney (14%), breast (13%), hematopoietic (8%), gastrointestinal (7%), nasopharynx (6%), thyroid (4%), liver (2%), skin (2%), and other (7%) | Older age: OR 1·3 (95% CI 1·1–1·7, p=0·014); diabetes: 2·7 (1×·1, p=0·0030); middle versus smallest psoas tertile: 0·3 (0·2–0·6, p=0·0010); largest versus smallest psoas tertile: 0·2 (0·1–0·4, p<0·001); Tokuhashi score: 0·7 (0·7–0·8, p<0·001); Tomita score: 1·3 (1·1–1·4, p<0·001); KPS: 0·97 (0·96–0·99, p<0·001) | Diabetes: OR 2·8 (95% CI 1·01–7·8, p=0·046); middle versus smallest psoas tertile: 0·2 (0·09–0·6, p=0·0030); largest versus smallest psoas tertile: 0·16 (0·05–0·4, p<0·001); Tokuhashi score: 0·7 (0·6–0·9, p=0·0020) |
| Zakaria et al (2020); overall survival23 | Retrospective | 247 | 60 (25–87) | 52% | Lung (23%), prostate (15%), kidney (14%), breast (13%), hematopoietic (8%), gastrointestinal (7%), nasopharynx (6%), thyroid (4%), liver (2%), skin (2%), and other (7%) | Myocardial infarction or CAD: 1·7 (1·2–2·5, p=0·0040); COPD: 1·7 (1·2–2·5, p=0·0050); low BMI: 0·97 (0·94–0·99, p=0·012); middle versus smallest psoas tertile: 0·6 (0·4–0·8, p<0·001); largest versus smallest psoas tertile: 0·4 (0·3–0·6, p<0·001), Tokuhashi score: 0·9 (0·86–0·93, p<0·001); Tomita score: 1·2 (1·1–1·2, p<0·001) | COPD: 1·7 (1·1–2·7, p=0·19); middle versus smallest psoas tertile: 0·5 (0·4–0·7, p<0·001); largest versus smallest psoas tertile: 0·5 (0·3–0·7, p<0·001); Tokuhashi score: 0·9 (0·85–0·97, p=0·010) |
| Zakariaetal (2020); any complications and postoperative neurological function23 | Retrospective | 247 | 60 (25–87) | 52% | Lung (23%), prostate (15%), kidney (14%), breast (13%), hematopoietic (8%), gastrointestinal (7%), nasopharynx (6%), thyroid (4%), liver (2%), skin (2%), and other (7%) | Any complications: middle versus smallest psoas fertile, OR 0·5 (95% CI 0·3–0·9, p=0·030); Tokuhashi score, 1·1 (1·0–1·3, p=0·024); KPS: 1·0 (1·0–1·1, p=0·0010) | Postoperative neurological function: previous stroke, OR 0·2 (95% CI 0·04–0·95, p=0·044); largest versus smallest psoas fertile, 4·1 (1·3–13·3, p=0·018); any complications: myocardial infarction or CAD, 2·9 (1·2–7·4, p=0·021); chronic renal disease, 3·0 (1·1–8·8, p=0·040) |
| Zhang et al (2021)24 | Retrospective | 411 | 58 (SD 11) | 56% | NR | VTE: Frankel grade (A–C vs D–E), OR 5·6 (95% CI 3·0–11·1, p=0·0010); CCI >7 (vs 7 or less), 5·8 (1·4–24·8, p=0·017) | VTE: Frankel grade (A–C vs D–E), OR 5·6 (95% CI 3·0–11·1, p=0·0010); CCI >7 (vs 7 or less), 2·3 (1·3–4·2, p=0·017) |
The table lists prospective studies (any sample size) and retrospective studies (>200 patients). BMI reported as kg/m2. AIS=ASIA Impairment Scale. ADL=activities of daily living. ALP=alkaline phosphatase. ASA=American Society of Anesthesiologists. ASIA=American Spinal Injury Association. BMI=body-mass index. CAD=coronary artery disease. CHF=congestive heart failure. CCI=Charlson Comorbidity Index. COPD=chronic obstructive pulmonary disease. CHF=congestive heart failure. CRP=C-reactive protein. ECOG-PS=Eastern Cooperative Oncology Group Performance Status. EQ-5D=EuroQoL-5 dimensions. HR=hazard ratio. KPS=Karnofsky performance score. LOS=length of stay. NSCLC=non-small-cell lung cancer. NR=not reported. NRS=Numeric Rating Scale. ODI=Oswestry Disability Index. OR=odds ratio. RBC=red blood cell. SF-36 PCS=Short-Form 36 physical component summary. SSI=surgical site infection. UTI=urinary tract infection. VTE=venous thromboembolism.
Median or mean age in years (range, or SD if no range).
In their paper, Dea and colleagues state that statistically significant associations were found. However, they do not report p values.
HR, OR, and associated CIs were not reported for any of these data.
Schoenfeld and colleagues do not cite p values in this paper, but state in their methodology that only variables that maintained p<0·02 in initial bivariate testing were subsequently included into a multivariate logistic regression analysis.
For the purpose of summarisation, table 3 and appendix (pp 19–32) group results together according to dichotomised independent systemic variables, as presented in the respective articles. For example, studies that dichotomised the independent variable of age (eg, comparing the influence of age greater or less than 60 years on a given clinical outcome) and found that the older age group negatively predicted outcomes (eg, the group >60 years of age) would be included under the grouping of older age. Similarly, this grouping was done for elevated body-mass index (BMI), low BMI, elevated Charlson Comorbidity Index, higher American Society of Anesthesiologists physical status class, worse ECOG performance status, worse health-related quality of life (HRQOL), worse Oswestry Disability Index, and for most biochemical parameters. This grouping was performed for two reasons: (1) given the substantial variability in exact cutoff values used to dichotomise independent systemic variables from study to study; and (2) it allowed the data to be summarised concisely. Specific cutoff values for all systemic variables are fully described in the data extraction tables (appendix pp 8–32).
Comparative meta-analyses are not typically performed as part of scoping reviews.5 Specifically, a meta-analysis would not be indicated given the broad topic, unclear evidence types available, and substantial heterogeneity in study designs (eg, surgical indication and approach, tumour histology, and oncological treatment regimens).
Evidence appraisal
Evidence quality was assessed using the Oxford Centre for Evidence-Based Medicine Levels of Evidence tool.25 Quality assessment was performed by two independent reviewers (MAM and CJT). In cases of disagreement, consensus was reached through open discussion and a detailed review of the full text. Article bias is not typically assessed according to PRISMA-ScR guidelines.5
Results
Literature search and data extraction
Our initial database search yielded 4295 articles after duplicates were removed. Titles and abstracts were screened, and 4025 articles were excluded. 270 full-text articles were obtained and assessed for eligibility. 209 full-text articles were excluded. Data were extracted from 61 full-text articles.3,4,12,8–24,26–67 The PRISMA flow diagram summarises the selection process (figure 1).
Figure 1: Study selection.

Study characteristics
Study characteristics are detailed in table 2 and the appendix (pp 8–18). The 61 included studies had a combined total of 22 335 patients. The mean study cohorts contained 366 patients (867· 6) and 63% (16· 1) were men. The mean age (weighted average) at time of surgery was 60–4 years (SD 2·15). Data included in this Review are mainly from North American (28 [46%] of 61 studies), Asian (23 [38%]), and European (10 [16%]) cohorts. Prospective cohort studies represent 7% (n=4) of the data,11,16–18 whereas retrospective cohort studies account for 93% (n=57).
Cohort characteristics
Cohort characteristics are detailed in table 2 and the appendix (pp 8–18). All patients were diagnosed with spinal metastatic disease. Surgical indications were not mutually exclusive (ie, patients could have more than one indication for surgery) and included progressive neurological dysfunction (49 [80%] of 61 studies), intractable pain (20 [33%] studies), spinal instability (12 [20%] studies), and general oncological resection (eight [13%] studies). Surgical approach was reported for 9834 (44·0%) of 22335 patients and included anterior, posterior, or combined antero-posterior approaches (table 2). Surgical intervention data were reported for 19945 (89·3%) patients. Intervention types were not mutually exclusive and included decompression with or without fusion, corpectomy, and spondylectomy (table 2). Most studies pooled both cervical and thoracolumbar spinal metastases and did not control for previous radiotherapy or chemotherapeutic regimens. 52 (85%) of 61 studies included either multiple tumour histologies (46%) or a single tumour histology (39%). Nine (15%) studies did not report tumour histology.7,9,10,13,14,15,24,28,53 Histology data were available for 9984 patients, with lung, kidney, breast, and prostate tumours being the most frequently reported (table 2, appendix pp 8–18).
Clinical outcomes
Survival (eg, overall survival, 30-day survival, and 90-day survival) was the most common postoperative clinical outcome investigated (40 [66%] of 61 studies), followed by complications (20 [33%] studies),7–11,14,18,20,23,24,26,28,30,34,45,50,53,55,60,62 neurological function and ambulation (ten [16%] studies),17,22,23,32,42,48,52,58,61,65 and HRQOL (one [2%] study; table 3, appendix pp 19–32).3 Complications were commonly reported as “any”, “minor” (Clavien-Dindo grade I–II),68 “major” (Clavien-Dindo grade III—IV)or more specifically (eg, venous thromboembolism, urinary tract infection, blood transfusions, hardware failure, surgical site infections, or by organ system; table 3, appendix pp 21–32). Clinical outcomes were stratified by time course (survival) and severity (complications) in the appendix (pp 19–22).
Systemic variables influencing postoperative clinical outcomes
Study characteristics
We identified studies reporting statistically significant (p<0·05), negative associations between survival (eg, overall survival, progression-free survival, and 30-day survival) and systemic variables including demographics (17 studies);11,12,15,16,19,23,29,31,38,43,51,56,57,59,64,66,67 medical comorbidities (four studies);15,23,31,51 biochemical abnormalities (six studies);14,15,18,19,31,46 generalised motor weakness or poor ambulation (ie, American Spinal Cord Injury Association Impairment Scale grade, and Frankel grade; 18 studies);11,12,15,19,21,31,36,41,42,46,47,51,56,57,59,64,67 reduced overall level of function or performance status (16 studies);12,15,16,18,23,30,31,36,38,41–43,46,56,64,66 and increased systemic disease burden (12 studies; table 3, appendix pp 19–32).4,16,21,30,31,35,36,38,39,42,51,54 Demographics (ten studies),7,20,23,28,53,60,62,9–11 medical comorbidities (nine studies),8,13,23,24,26,28,30,53,60 and biochemical abnormalities (nine studies)8–10,14,15,18,28,53,60 also negatively affected complication rates (table 3, appendix pp 21–32).
Demographics
Older age (ie, >60 years) negatively influenced survival in ten studies.23,29,31,38,43,51,56,59,64,67 Older age was also associated with an increased rate of any complications in four studies.10,11,60,62 Kanda and colleagues3 reported that older age was associated with worse HRQOL on the EuroQol-5D. Schuss and colleagues53 reported an association between younger age (<60 years) and an increased complication rate.
Low BMI, weight loss, and low muscle mass (eg, psoas size) were associated with worse survival (overall, 30 day, and 90 day),19,23,29,15 worse neurological function,23 and an increased rate of complications.23 Elevated BMI negatively influenced overall survival in a single study.16 Cheung and colleagues7 reported that elevated BMI negatively influenced the rates of pulmonary complications, venous thromboembolism, and urinary tract infections. They reported that an elevated BMI was favourably associated with reduced length of hospital stay and red blood cell transfusions.7
Male sex was associated with worse overall and 30-day survival (five studies),11,12,16,31,66 any complications,28 reduced rate of red blood cell transfusions,9 a KPS of 70 or lower,37 and with an ambulatory status at the time of discharge from hospital.65
De la Garza Ramos and colleagues10 examined racial disparities in spinal metastatic disease-related oncological morbidity and found that Black race was associated with an increased rate of any and minor complications.
Smoking was negatively associated with overall survival31,57 and any complications,10 including surgical site infections.20
Generalised motor weakness and ambulation
Worse American Spinal Cord Injury Association Impairment Scale grade, Frankel grade, or generalised motor weakness negatively affected survival.11,12,15,21,31,36,46,47,54,56,57,59,64,67 These variables were also significantly associated with an increased 30-day complication rate,53 pressure sores,11 and venous thromboembolism.24 Worse preoperative strength and ambulation, as reported descriptively in the studies, was significantly associated with worse postoperative ambulatory status (four studies),48,52,65,22 worse functional status,3 increased length of hospital stay,11 and decreased likelihood of being discharged from the hospital.22 Preoperative non-ambulatory status was significantly associated with postoperative non-ambulatory status (four studies)17,48,52,65 and worse motor function.42
Performance status and physical status
Higher American Society of Anesthesiologists physical status class was significantly associated with increased complication rates,28,53 including surgical site infections,20 red blood cell transfusion,9 and length of hospital stay.28
Lower KPS (typically <80) and partial or full dependence on another individual for activities of daily living were associated with worse survival (six studies).23,31,33,43,46,47 These variables were significantly associated with increased rates of postoperative complications (four studies),10,23,53,60 including wound-related re-operations.34 Lower KPS was associated with worse postoperative ambulatory status (three studies)22,58,65 and decreased likelihood of being discharged from the hospital.22
Worse performance status, measured using ECOG performance status, was associated with worse survival (11 studies).12,15,30,36,38,41,42,46,56,64,66 Longo and colleagues45 reported an association between worse preoperative ECOG performance status and hardware failure (eg, screw pullout or loosening, cage migration, progressive kyphosis, or an otherwise noticeable instrumentation deficit detectable on radiographic imaging). He and colleagues36 found increased preoperative ECOG performance status was associated with worse progression-free survival.
Worse physical functioning on the Short-Form 36 questionnaire (a 36-item survey that is routinely used to assess quality of life) was associated with worse overall survival in a single study.16
Medical comorbidities
Elevated Charlson Comorbidity Index scores were negatively associated with overall survival (three studies),15,31,51 any complications (three studies),28,30,60 and venous thromboembolism.24 Clinical outcomes were negatively affected by the following medical comorbidities: ambulation by urinary and bowel dysfunction;65 hospital readmission by inflammatory conditions, hypertension, and renal dysfunction;13 30-day survival of liver disease,23 overall survival of cardiac disease,23 30-day survival of stroke,23 90-day survival of diabetes,23 overall survival of pathological fractures,31 and overall survival of pulmonary disease;23 development of at least one major peri-operative complication by pathological fractures,8 pulmonary disease,8 cardiac disease,8,23 and diabetes;26,53 length of hospital stay by cardiac disease;28 neurological dysfunction by stroke;23 and poor outcome (KPS <70) at discharge by urinary dysfunction.37
Biochemical abnormalities
Hypoalbuminaemia was negatively associated with survival (five studies)14,15,18,19,46 and complications (six studies),8–10,14,28,60 including red blood cell transfusion (two studies),14,9 sepsis,14 length of hospital stay (two studies),14,28 and decreased likelihood of being discharged from hospital.14 Overall survival was negatively affected by elevated serum monocyte count,31 serum neutrophil count,15 neutrophil-to-lymphocyte ratio,15 platelet-to-lymphocyte ratio,15 and serum globulin,31 thrombocytopenia,15 thrombocytosis,15 and anaemia.15 Complication rates were negatively associated with anaemia,8 coagulopathy,8 electrolyte imbalance,8,15 and elevated C-reactive protein concentrations.18,60
Systemic disease burden
Visceral metastases and uncontrolled systemic disease were negatively associated with overall survival (11 Studies)16,21,30,31,35,36,38,39,42,51,54 and progression-free survival.36
HRQOL and disability
A single study reported worse HRQOL, measured on the EuroQoL-5D, was associated with worse overall and less than 3-month survival.12,16 Worse functional disability, as measured on the Oswestry Disability Index, was negatively associated with overall survival.16
Evidence appraisal
Most (60 [98%]) of the 61 included studies constitute level 4 evidence according to the Oxford Centre for Evidence- Based Medicine Levels of Evidence tool, as they were either retrospective series or included multiple tumour histologies, surgical approaches, or surgical interventions. A single cohort study provided level 2b evidence.21 Retrospective data related to studies of larger sample size (>1000 patients) were frequently obtained through national registry databases, without specific details pertaining to treatment regimen, surgical approach, or tumour histology (eg, American College of Surgeons National Surgical Quality Improvement Program,7,9,10,14 and Healthcare Cost and Utilization Project Nationwide Readmission Database13).
Discussion
Systemic assessment is a pillar in the widely used NOMS framework for spinal metastatic disease-related decision making.2 Here, to our knowledge, we present the first scoping review to comprehensively summarise emerging literature on systemic variables associated with postoperative spinal metastatic disease-related clinical outcomes (figure 2, table 3, appendix pp 8–32). 61 full-text articles were included, accounting for 22 335 patients. Overall survival7,9–11,14,18,20,24,26,28,30,34,45,50,53,55,60,62 and complications16,21,22,31,42,48,65 were the most frequently analysed outcomes. Systemic variables negatively associated with clinical outcomes included older age, low or elevated BMI, low muscle mass, male sex, Black race, smoking status (smoker), generalised motor weakness, non-ambulatory status, higher American Society of Anesthesiologists physical status class, worse KPS, worse ECOG performance status, medical comorbidities (eg, Charlson Comorbidity Index scores; urinary and bowel dysfunction; inflammatory conditions; pathological fractures; previous stroke; diabetes; and cardiac, renal, vascular, hepatic, and pulmonary disease), and biochemical abnormalities (eg, anaemia, coagulopathy, electrolyte imbalance, hypoalbuminaemia, and atypical blood cell counts). Medical, surgical, and radiation oncologists might consider these findings when prognosticating spinal metastatic disease-related surgical outcomes on the basis of a patient’s systemic condition. These factors might also guide a shared decision-making approach with affected individuals and their families.
Figure 2: An adapted NOMS decision-making framework for spinal metastatic disease: preoperative systemic considerations and negatively affected clinical outcomes.
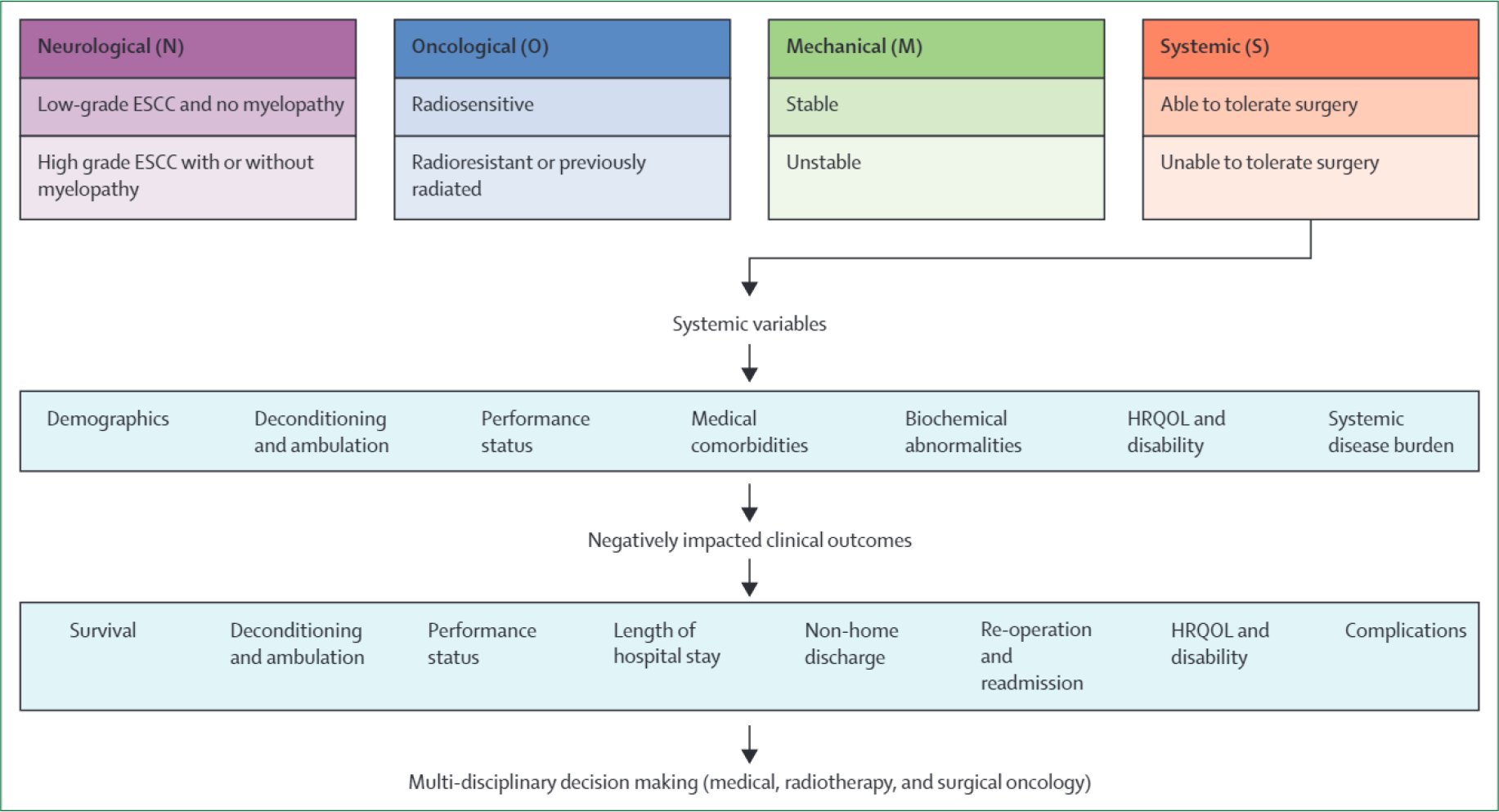
ESCC=epidural spinal cord compression. HRQOL=health-related quality of life. NOMS=neurological, oncological, mechanical, and systemic.
Surgery for spinal metastatic disease
Surgery for spinal metastatic disease can improve pain, neurological function, HRQOL, and survival.1 Compared with non-operative management, surgery decreases the likelihood of the patient losing ambulatory function.69 Surgery combined with radiotherapy improves ambulatory function and survival when compared with radiotherapy alone.1 Our AO Spine Knowledge Forum Tumor group provides a strong recommendation for urgent surgical decompression for patients who have neurological deficits from solid spinal metastatic disease resulting in loss of ambulation, in the absence of medical and oncological contraindications.70
Prognosticating clinical outcomes
Existing prognostic survival-related scoring systems have been evaluated for predictive value and are becoming out of date.71 New scoring tools should improve the accuracy of risk stratification and outcome prognostication for patients undergoing spinal metastatic disease-related surgery. Shortcomings of these systems include poor accuracy, failure to incorporate systemic assessment, and construction using retrospective, non-contemporary data without validation in a large prospective dataset.16,71 Eight commonly cited prognostic scoring systems were externally validated for clinical accuracy, revealing that all have low concordance between predicted and actual survival.16 In 2019, a 12-variable risk calculator was developed by the Global Spine Tumor Study Group and has been likened to well-established calculators for assessing the risk of stroke and cardiovascular disease.72
Currently, there is no widely accepted tool for prognosticating surgical outcomes on the basis of the systemic condition of patients with spinal metastatic disease; the variables identified herein might be considered during the development of such a tool. Although previous literature has focused on postoperative survival, we have summarised emerging evidence pertaining to postoperative HRQOL, complication rates, adverse event avoidance, length of hospital stay, and likelihood of hospital discharge. Future studies could consider examining additional clinically relevant outcomes of interest, such as the time between surgery and patients’ non-surgical oncological care (ie, exploring the effect of delays in receipt of further care).
Predicting postoperative HRQOL
HRQOL is an inherently important consideration in spinal metastatic disease-related decision making.69 Fehlings and colleagues69 published the prospective AO Spine North America Metastatic Epidural Spinal Cord Compression study, which represents the highest quality available data showing improved HRQOL after surgery for focal, symptomatic spinal metastatic disease. Although preserved neurological function might improve HRQOL following surgery, few studies have examined the ability of other preoperative factors to predict postoperative HRQOL. Kanda and colleagues3 reported that older age (>70 years) and elevated Katagiri score predicted worse HRQOL following surgery for spinal metastatic disease. An international AO Spine study revealed Frankel or American Spinal Cord Injury Association Impairment Scale grades; ambulatory function; healthy bowel, bladder, and sexual function; KPS; and EuroQoL-5D scores each predicted postoperative HRQOL.73 Nater and colleagues74 developed and validated the first clinical prediction model of survival and HRQOL for patients 3 months after surgery for spinal metastatic disease with epidural compression. Given the heterogeneity of clinical presentation and outcomes, such prognostic models might assist in tailoring a personalised medicine approach to surgical decision making.74 Together, these findings highlight the need for research pertaining to systemic variables that predict postoperative HRQOL.
Spinal metastatic disease and frailty
Older patients (aged >60 years) with multiple comorbidities constitute a substantial portion of patients with cancer. Advanced age and comorbidities are considered by surgeons when determining whether patients might tolerate invasive procedures. These variables might increase susceptibility to complications.11 Despite this concern, a role for surgery in older patients with spinal metastatic disease has been suggested. Among patients older than 60 years of age undergoing surgery for spinal metastatic disease, improved neurological function and performance status have been reported.3 In this scoping review, we identified ten studies reporting that older age negatively influenced postoperative survival.23,29,31,38,43,51,56,59,64,67 We identified additional demographics (eg, male sex, race, elevated or low BMI, and being a smoker) that negatively affected survival in ten studies.11,12,15,16,19,23,29,31,57,66
Frailty can be considered a state of susceptibility to decline after experiencing a stressor, or as an index of accumulating deficits.11 Such indices are calculated by dividing the number of health deficits present in an individual by the number of health deficits measured.75 Health deficits might broadly include, but are not limited to, medical comorbidities, biochemical abnormalities, overall level of function, and systemic disease burden. Frailty was not objectively defined across the studies included in this Review. At the time of this Review, the only objective, composite measure of frailty, designed specifically for use among patients with spinal metastatic disease, is the Metastatic Spinal Tumor Frailty Index.8 By definition, however, this index is not a frailty index as it includes surgical and pathological variables.
Frailty has performed well as an outcome predictor in spinal surgery;76 however, that has not been the case in spinal metastatic disease. Bourassa-Moreau and colleagues75 reported that a modified frailty index did not accurately predict postoperative adverse events in patients undergoing surgery for spinal metastatic disease. We propose that this issue might relate to the use of a modified frailty index, which does not capture patients’ systemic condition and functional status—variables known to be of crucial importance in oncology populations. Our Review highlights systemic variables and patient factors that affect clinical outcomes and could be particularly useful towards deriving a frailty measure that is specific to spinal metastatic disease.
Advances in personalised therapies for spinal metastatic disease
Selecting appropriate therapy for the individual patient is essential in the era of personalised medicine. With the advent of personalised oncology, prospective studies with homogeneous cohorts should be done to improve the quality of evidence available for decision making.71 Evolving personalised therapies have been derived from advances in genetic subtyping, immunotherapy, radiation techniques, and separation surgery.71 Novel scoring systems should stratify risk, accounting for genetic subtypes influencing prognosis (eg, BRAF mutation and melanoma).71 Frail patients with poor prognoses, who might not be candidates for standard surgical therapy, could benefit from the reduced morbidity associated with minimally invasive techniques, such as percutaneous cement augmentation or pedicle screw insertion, vertebral body stenting or support, and radiofrequency ablation.1
Limitations
This scoping review has several limitations, many of which are inherent to the nature of such a review. The broad, exploratory nature of this Review was purposeful; we included studies that varied in design, methods, and outcome. We have, however, captured emerging evidence relevant to the systemic assessment component of the widely used NOMS framework for spinal metastatic disease decision making. Given that the objective of this Review was to summarise systemic variables and their influence on postoperative outcomes, we did not intend to draw comparison with the relative effect of oncology-related variables (eg, tumour histology, staging, and number of metastases) on postoperative clinical outcomes. It is possible that oncology-related variables influence outcomes to a greater extent than do systemic variables, as has been previously suggested.16 Lastly, this Review does not discern the effect size of systemic variables on outcomes, or the comparative effectiveness of many possible treatment options.
Conclusions
To our knowledge, this is the first comprehensive scoping review to broadly summarise emerging evidence relevant to the systemic assessment component of the widely used NO MS framework for spinal metastatic disease decision making. Independent systemic variables negatively influencing postoperative outcomes included various demographics, medical comorbidities, biochemical abnormalities, generalised motor weakness, poor ambulation, reduced performance status, and increased systemic disease burden. This Review could represent a first step towards the development of an evidence-based tool for prognosticating spinal metastatic disease-related surgical outcomes on the basis of patients’ systemic condition. Moreover, the literature synthesis presented in this scoping review can guide clinical management and inform a shared decision-making process with patients and their families.
Supplementary Material
Search strategy and selection criteria.
References for this scoping Review were identified through searches on MEDLINE, Embase, Scopus, Cochrane Central Register of Controlled Trials, Web of Science, and CINAHL databases. We searched the terms “surgery”, “spinal”, “metastasis”, “systemic”, “disease”, and “frailty”. We included terms related to medical comorbidities, biochemical abnormalities performance status, physical function, and frailty synonyms (full search strategy: appendix pp 5–6). Only papers in English were reviewed. To survey this broad topic, capture emerging evidence, and account for the evolution of surgical techniques, the search included papers published between Jan 1,2000, and July 31,2021. Inclusion and exclusion criteria are described in table 1.
Acknowledgments
This study was organised by the AO Spine Knowledge Forum Tumor, a group of international spine oncology experts. AO Spine is a clinical division of the AO Foundation, which is an independent medically guided not-for-profit organisation.
Footnotes
Declaration of interests
We declare no competing interests.
Contributor Information
Mark A MacLean, Division of Neurosurgery, Department of Surgery, Dalhousie University, Halifax, NS, Canada.
Charles J Touchette, Division of Neurosurgery, Université de Sherbrooke, Sherbrooke, QC, Canada.
Miltiadis Georgiopoulos, Spine Surgery Program, Department of Surgery, Montreal General Hospital, McGill University Health Center, Montreal, QC, Canada.
Tristan Brunette-Clément, Faculty of Medicine, Université de Montréal, Montreal, QC, Canada.
Fahad H Abduljabbar, Department of Orthopedic Surgery, Faculty of Medicine, King Abdulaziz University, Jeddah, Saudi Arabia.
Christopher P Ames, Department of Neurological Surgery, University of California San Francisco, San Francisco, CA, USA.
Chetan Bettegowda, Department of Neurosurgery, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Raphaele Charest-Morin, Spine Surgery Institute, Vancouver General Hospital, University of British Columbia, Vancouver, BC, Canada.
Nicolas Dea, Spine Surgery Institute, Vancouver General Hospital, University of British Columbia, Vancouver, BC, Canada.
Michael G Fehlings, Department of Surgery, Division of Neurosurgery and Spine Program, University Health Network, University of Toronto, Toronto, ON, Canada.
Ziya L Gokaslan, Department of Neurosurgery, The Warren Alpert Medical School of Brown University, Providence, Rl, USA.
C Rory Goodwin, Department of Neurosurgery, Spine Division, Duke University, Durham, NC, USA.
Ilya Laufer, Department of Neurosurgery, New York University Langone Health, New York, NY, USA.
Cordula Netzer, Department of Spine Surgery, University Hospital of Basel, Basel, Switzerland.
Laurence D Rhines, Department of Neurosurgery, Division of Surgery, The University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Arjun Sahgal, Department of Radiation Oncology, Sunnybrook Health Sciences Centre, University of Toronto, Toronto, ON, Canada.
John H Shin, Department of Neurosurgery, Massachusetts General Hospital, Harvard University, Boston, MA, USA.
Daniel M Sciubba, Department of Neurosurgery, Zucker School of Medicine at Hofstra, Long Island Jewish Medical Center and North Shore University Hospital, Northwell Health, Manhasset, New York, NY, USA.
Byron F Stephens, Department of Orthopaedic Surgery, Vanderbilt University Medical Center, Nashville, TN, USA.
Daryl R Fourney, Division of Neurosurgery, Department of Surgery, College of Medicine, University of Saskatchewan, Saskatoon, SK, Canada.
Michael H Weber, Spine Surgery Program, Department of Surgery, Montreal General Hospital, McGill University Health Center, Montreal, QC, Canada.
References
- 1.Sciubba DM, Petteys RJ, Dekutoski MB, et al. Diagnosis and management of metastatic spine disease. A review. J Neurosurg Spine 2010; 13: 94–108. [DOI] [PubMed] [Google Scholar]
- 2.Laufer I, Rubin DG, Lis E, et al. The NOMS framework: approach to the treatment of spinal metastatic tumors. Oncologist 2013; 18: 744–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kanda Y, Kakutani K, Sakai Y, et al. Prospective cohort study of surgical outcome for spinal metastases in patients aged 70 years or older. Bone Joint J 2020; 102-B: 1709–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cai Z, Tang X, Yang R, Yan T, Guo W. Modified score based on revised Tokuhashi score is needed for the determination of surgical intervention in patients with lung cancer metastases to the spine. World J Surg Oncol 2019; 17: 194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med 2018; 169: 467–73. [DOI] [PubMed] [Google Scholar]
- 6.Munn Z, Peters MDJ, Stern C, Tufanaru C, McArthur A, Aromataris E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Methodol 2018; 18: 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheung ZB, Vig KS, White SJW, et al. Impact of obesity on surgical outcomes following laminectomy for spinal metastases. Global Spine J 2019; 9: 254–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De la Garza Ramos R, Goodwin CR, Jain A, et al. Development of a metastatic spinal tumor frailty index (MSTFI) using a nationwide database and its association with inpatient morbidity, mortality, and length of stay after spine surgery. World Neurosurg 2016; 95: 548–555. [DOI] [PubMed] [Google Scholar]
- 9.De la Garza Ramos R, Gelfand Y, Benton JA, et al. Rates, risk factors, and complications of red blood cell transfusion in metastatic spinal tumor surgery: an analysis of a prospective multicenter surgical database. World Neurosurg 2020; 139: e308–15. [DOI] [PubMed] [Google Scholar]
- 10.De la Garza Ramos R, Choi JH, Naidu I, et al. Racial disparities in perioperative morbidity following oncological spine surgery. Glob Spine J 2021; published online June 14. 10.1177/21925682211022290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dea N, Versteeg A, Fisher C, et al. Adverse events in emergency oncological spine surgery, a prospective analysis. J Neurosurg Spine 2014; 21: 698–703. [DOI] [PubMed] [Google Scholar]
- 12.Dea N, Versteeg AL, Sahgal A, et al. Metastatic spine disease: should patients with short life expectancy be denied surgical care? An international retrospective cohort study. Neurosurgery 2020; 87: 303–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elsamadicy AA, Koo AB, David WB, et al. Thirty- and 90-day readmissions after spinal surgery for spine metastases: a national trend analysis of 4423 patients. Spine 2021; 46: 828–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hussain AK, Cheung ZB, Vig KS, et al. Hypoalbuminemia as an independent risk factor for perioperative complications following surgical decompression of spinal metastases. Global Spine J 2019; 9: 321–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karhade AV, Thio QCBS, Kuverji M, Ogink PT, Ferrone ML, Schwab JH. Prognostic value of serum alkaline phosphatase in spinal metastatic disease. Br J Cancer 2019; 120: 640–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nater A, Tetreault LA, Kopjar B, et al. Predictive factors of survival in a surgical series of metastatic epidural spinal cord compression and complete external validation of 8 multivariate models of survival in a prospective North American multicenter study. Cancer 2018; 124: 3536–50. [DOI] [PubMed] [Google Scholar]
- 17.Park SJ, Lee CS, Chung SS. Surgical results of metastatic spinal cord compression (MSCC) from non-small cell lung cancer (NSCLC): analysis of functional outcome, survival time, and complication. Spine J 2016; 16: 322–28. [DOI] [PubMed] [Google Scholar]
- 18.Prost S, Bouthors C, Fuentes S, et al. Influence of preoperative biological parameters on postoperative complications and survival in spinal bone metastasis. A multicenter prospective study. Orthop Traumatol Surg Res 2020; 106: 1033–38. [DOI] [PubMed] [Google Scholar]
- 19.Schoenfeld AJ, Leonard DA, Saadat E, Bono CM, Harris MB, Ferrone ML. Predictors of 30- and 90-day survival following surgical intervention for spinal metastases: a prognostic study conducted at four academic centers. Spine 2016; 41: e503–09. [DOI] [PubMed] [Google Scholar]
- 20.Sebaaly A, Shedid D, Boubez G, et al. Surgical site infection in spinal metastasis: incidence and risk factors. Spine J 2018; 18: 1382–87. [DOI] [PubMed] [Google Scholar]
- 21.Tatsui CE, Suki D, Rao G, et al. Factors affecting survival in 267 consecutive patients undergoing surgery for spinal metastasis from renal cell carcinoma. J Neurosurg Spine 2014; 20: 108–16. [DOI] [PubMed] [Google Scholar]
- 22.Zairi F, Karnoub M-A, Vieillard M-H, et al. Evaluation of the relevance of surgery in a retrospective case series of patients who underwent the surgical treatment of a symptomatic spine metastasis from lung cancer. Eur Spine J 2016; 25: 4052–59. [DOI] [PubMed] [Google Scholar]
- 23.Zakaria HM, Wilkinson BM, Pennington Z, et al. Sarcopenia as a prognostic factor for 90-day and overall mortality in patients undergoing spine surgery for metastatic tumors: a multicenter retrospective cohort study. Neurosurgery 2020; 87:1025–36. [DOI] [PubMed] [Google Scholar]
- 24.Zhang HR, Xu MY, Yang XG, et al. Nomogram for predicting the postoperative venous thromboembolism in spinal metastasis tumor a multicenter retrospective study. Front Oncol 2021; 11: 629823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Howiek J, Chalmers I, Glasziou P, et al. Oxford Centre for Evidence-Based Medicine: Levels of Evidence. March, 2009. https://www.cebm.ox.ac.uk/resources/levels-of-evidence/oxford-centre-for-evidence-based-medicine-levels-of-evidence-march-2009 (accessed March 22, 2022).
- 26.Demura S, Kawahara N, Murakami H, et al. Surgical site infection in spinal metastasis: risk factors and countermeasures. Spine 2009; 34: 635–39. [DOI] [PubMed] [Google Scholar]
- 27.Dobran M, Iacoangeli M, Brunozzi D, et al. Surgical treatments of spinal metastases: analysis of prognostic factors during a seven-year experience. J Neurosurg Sci 2018; 62: 94–97. [DOI] [PubMed] [Google Scholar]
- 28.Ehresman J, Ahmed AK, Schilling A, et al. Preoperative nutrition consults associated with decreased postoperative complication rate and decreased length of hospital stay after spine metastasis surgery. World Neurosurg 2020; 133: el73–79. [DOI] [PubMed] [Google Scholar]
- 29.Gakhar H, Dhillon A, Blackwell J, et al. Study investigating the role of skeletal muscle mass estimation in metastatic spinal cord compression. Eur Spine J 2015; 24: 2150–55. [DOI] [PubMed] [Google Scholar]
- 30.Gao X, Wu Z, Wang T, et al. A discussion on the criteria for surgical decision-making in elderly patients with metastatic spinal cord compression. Glob Spine J 2021; published online Feb 2. 10.1177/2192568221991107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao Z-Y, Zhang T, Zhang H, Pang C-G, Jiang W-X. Establishment and validation of nomogram model for survival predicting in patients with spinal metastases secondary to lung cancer. Neurol Res 2021; 43: 327–35. [DOI] [PubMed] [Google Scholar]
- 32.Gazzeri R, Telera S, Galarza M, Callovini GM, Sperduti I, Alfieri A. Surgical treatment of solitary intradural extramedullary spinal cord metastases from solid cancers of non-neurogenic origin. A multicenter study. J Neurooncol 2021; 154: 101–12. [DOI] [PubMed] [Google Scholar]
- 33.Ha K-Y, Kim YH, Ahn J-H, Park H-Y. Factors affecting survival in patients undergoing palliative spine surgery for metastatic lung and hepatocellular cancer, dose the type of surgery influence the surgical results for metastatic spine disease? Clin Orthop Surg 2015; 7: 344–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carl HM, Ahmed AK, Abu-Bonsrah N, et al. Risk factors for wound-related reoperations in patients with metastatic spine tumor. J Neurosurg Spine 2018; 28: 663–68. [DOI] [PubMed] [Google Scholar]
- 35.Han S, Wang T, Jiang D, et al. Surgery and survival outcomes of 30 patients with neurological deficit due to clear cell renal cell carcinoma spinal metastases. Eur Spine J 2015; 24:1786–91. [DOI] [PubMed] [Google Scholar]
- 36.He S, Wei H, Ma Y, Zhao J, Xu W, Xiao J. Outcomes of metastatic spinal cord compression secondary to primary hepatocellular carcinoma with multidisciplinary treatments. Oncotarget 2017; 8: 43439–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hohenberger C, Schmidt C, Höhne J, Brawanski A, Zeman F, Schebesch K-M. Effect of surgical decompression of spinal metastases in acute treatment—predictors of neurological outcome. J Clin Neurosci 2018; 52: 74–79. [DOI] [PubMed] [Google Scholar]
- 38.Jiang W, Lei M, Liu Y, et al. Postoperative survival and functional outcome of palliative decompression and stabilization for thoracic metastatic spinal cord compression: prognostic factor analysis. Int J Clin Exp Pathol 2016; 9:10536–42. [Google Scholar]
- 39.Kato S, Murakami H, Demura S, et al. Spinal metastasectomy of renal cell carcinoma: a 16-year single center experience with a minimum 3-year follow-up. J Surg Oncol 2016; 113: 587–92. [DOI] [PubMed] [Google Scholar]
- 40.Lee MH, Lee S-H, Kim E-S, Eoh W, Chung S-S, Lee C-S. Survival-related factors of spinal metastasis with hepatocellular carcinoma in current surgical treatment modalities: a single institute experience. J Korean Neurosurg Soc 2015; 58: 448–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lei M, Liu Y, Tang C, Yang S, Liu S, Zhou S. Prediction of survival prognosis after surgery in patients with symptomatic metastatic spinal cord compression from non-small cell lung cancer. BMC Cancer 2015; 15: 853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lei M, Liu Y, Yan L, Tang C, Liu S, Zhou S. Posterior decompression and spine stabilization for metastatic spinal cord compression in the cervical spine. A matched pair analysis. Eur J Surg Oncol 2015; 41: 1691–98. [DOI] [PubMed] [Google Scholar]
- 43.Chen Y-J, Chen H-T, Hsu H-C. Preoperative palsy score has no significant association with survival in non-small-cell lung cancer patients with spinal metastases who undergo spinal surgery. J Orthop Surg Res 2015; 10: 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li Z, Long H, Guo R, et al. Surgical treatment indications and outcomes in patients with spinal metastases in the cervicothoracic junction (CTJ). J Orthop Surg Res 2018; 13: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Longo M, De la Garza Ramos R, Gelfand Y, Echt M, Kinon MD, Yassari R. Incidence and predictors of hardware failure after instrumentation for spine metastasis: a single-institutional series. World Neurosurg 2019; 125: e1170–75. [DOI] [PubMed] [Google Scholar]
- 46.Massaad E, Hadzipasic M, Alvarez-Breckenridge C, et al. Predicting tumor-specific survival in patients with spinal metastatic renal cell carcinoma: which scoring system is most accurate? J Neurosurg Spine 2020; 33: 1–11. [DOI] [PubMed] [Google Scholar]
- 47.Park J-H, Lee D-G, Hwang J, Lee S-H, Eoh W, Kim E-S. The impact of surgical treatment on survival in patients with cervical spine metastases. Neurospine 2018; 15:144–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Park JH, Rhim SC, Jeon SR. Efficacy of decompression and fixation for metastatic spinal cord compression: analysis of factors prognostic for survival and postoperative ambulation. J Korean Neurosurg Soc 2011; 50: 434–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Park BJ, Seaman SC, Noeller JL, et al. Metastatic renal cell carcinoma to the spine: outcomes and morbidity: single-center experience. World Neurosurg 2021; 154: e398–405. [DOI] [PubMed] [Google Scholar]
- 50.Pedreira R, Abu-Bonsrah N, Karim Ahmed A, et al. Hardware failure in patients with metastatic cancer to the spine. J Clin Neurosci 2017; 45: 166–71. [DOI] [PubMed] [Google Scholar]
- 51.Petteys RJ, Spitz SM, Goodwin CR, et al. Factors associated with improved survival following surgery for renal cell carcinoma spinal metastases. Neurosurg Focus 2016; 41: e13. [DOI] [PubMed] [Google Scholar]
- 52.Putz C, Gantz S, Bruckner T, et al. Preoperative scoring and limits of prognostication: functional outcome after surgical decompression in metastatic spinal cord compression. Oncology 2014; 86:177–84. [DOI] [PubMed] [Google Scholar]
- 53.Schuss P, Güresir Á, Schneider M, Velten M, Vatter H, Güresir E. Factors influencing early postoperative complications following surgery for symptomatic spinal metastasis: a single-center series and multivariate analysis. Neurosurg Rev 2020; 43: 211–16. [DOI] [PubMed] [Google Scholar]
- 54.Sellin JN, Suki D, Harsh V, et al. Factors affecting survival in 43 consecutive patients after surgery for spinal metastases from thyroid carcinoma. J Neurosurg Spine 2015; 23: 419–28. [DOI] [PubMed] [Google Scholar]
- 55.Shehadi JA, Sciubba DM, Suk I, et al. Surgical treatment strategies and outcome in patients with breast cancer metastatic to the spine: a review of 87 patients. Eur Spine J 2007; 16: 1179–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tang Y, Qu J, Wu J, Li S, Zhou Y, Xiao J. Metastatic spinal cord compression from non-small-cell lung cancer treated with surgery and adjuvant therapies: a retrospective analysis of outcomes and prognostic factors in 116 patients. J Bone Joint Surg Am 2015; 97: 1418–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Truong VT, Shedid D, Al-Shakfa F, et al. Surgical intervention for patients with spinal metastasis from lung cancer: a retrospective study of 87 cases. Clin Spine Surg 2021; 34: e133–40. [DOI] [PubMed] [Google Scholar]
- 58.Crnalic S, Hildingsson C, Bergh A, Widmark A, Svensson O, Löfvenberg R. Early diagnosis and treatment is crucial for neurological recovery after surgery for metastatic spinal cord compression in prostate cancer. Acta Oncol 2013; 52: 809–15. [DOI] [PubMed] [Google Scholar]
- 59.Vanek P, Bradac O, Trebicky F, Saur K, de Lacy P, Benes V. Influence of the preoperative neurological status on survival after the surgical treatment of symptomatic spinal metastases with spinal cord compression. Spine 2015; 40: 1824–30. [DOI] [PubMed] [Google Scholar]
- 60.Wang S, Wang Y, Yu Z, et al. Surgical results and clinical risks of postoperative complications in patients with painful malignant spinal cord compression after decompressive surgery. J Pain Res 2018; 11: 1679–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang S-X, Wang H-L, Lin K-Y, Bian C, Sun C, Dong J. Surgical outcomes and prognostic factors for metastatic spine hepatocellular carcinoma. World Neurosurg 2019; 122: e1052–58. [DOI] [PubMed] [Google Scholar]
- 62.Williams BJ, Fox BD, Sciubba DM, et al. Surgical management of prostate cancer metastatic to the spine. J Neurosurg Spine 2009; 10: 414–22. [DOI] [PubMed] [Google Scholar]
- 63.Xu K, Liu Y, Li B, et al. Prognostic factors of patients with malignant epithelioid vascular tumors in the spine: retrospective analysis of 46 patients in a single center. Spine 2018; 43: e1218–24. [DOI] [PubMed] [Google Scholar]
- 64.Yang S-Z, Tang Y, Zhang Y, Chen W-G, Sun J, Chu T-W. Prognostic factors and comparison of conservative treatment, percutaneous vertebroplasty, and open surgery in the treatment of spinal metastases from lung cancer. World Neurosurg 2017; 108: 163–75. [DOI] [PubMed] [Google Scholar]
- 65.Younsi A, Riemann L, Scherer M, Unterberg A, Zweckberger K. Impact of decompressive laminectomy on the functional outcome of patients with metastatic spinal cord compression and neurological impairment. Clin Exp Metastasis 2020; 37: 377–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zaw AS, Kantharajanna SB, Maharajan K, Tan B, Vellayappan B, Kumar N. Perioperative blood transfusion: does it influence survival and cancer progression in metastatic spine tumor surgery? Transjusion 2017; 57: 440–50. [DOI] [PubMed] [Google Scholar]
- 67.Zhang D, Gong H, Shen M, et al. Surgical management and factors affecting the prognosis for patients with thyroid cancer spinal metastases: a retrospective analysis of 52 consecutive patients from a single center. World Neurosurg 2019; 129: e330–36. [DOI] [PubMed] [Google Scholar]
- 68.Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 2009; 250:187–96. [DOI] [PubMed] [Google Scholar]
- 69.Fehlings MG, Nater A, Tetreault L, et al. Survival and clinical outcomes in surgically treated patients with metastatic epidural spinal cord compression: results of the prospective multicenter AOSpine study. J Clin Oncol 2016; 34: 268–76. [DOI] [PubMed] [Google Scholar]
- 70.Laufer I, Zuckerman SL, Bird JE, et al. Predicting neurologic recovery after surgery in patients with deficits secondary to MESCC: systematic review. Spine 2016; 41 (suppl 20): S224–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Choi D, Bilsky M, Fehlings M, Fisher C, Gokaslan Z. Spine oncology-metastatic spine tumors. Neurosurgery 2017; 80: S131–37. [DOI] [PubMed] [Google Scholar]
- 72.Choi D, Pavlou M, Omar R, et al. A novel risk calculator to predict outcome after surgery for symptomatic spinal metastases; use of a large prospective patient database to personalise surgical management. Eur J Cancer 2019; 107: 28–36. [DOI] [PubMed] [Google Scholar]
- 73.Nater A, Tetreault LL, Davis AM, Sahgal AA, Kulkarni AV, Fehlings MG. Key preoperative clinical factors predicting outcome in surgically treated patients with metastatic epidural spinal cord compression: results from a survey of438 AOSpine international members. World Neurosurg 2016; 93: 436–48.e15. [DOI] [PubMed] [Google Scholar]
- 74.Nater A, Chuang J, Liu K, et al. A personalized medicine approach for the management of spinal metastases with cord compression: development of a novel clinical prediction model for postoperative survival and quality of life. World Neurosurg 2020; 140: 654–63.e13. [DOI] [PubMed] [Google Scholar]
- 75.Bourassa-Moreau É, Versteeg A, Moskven E, et al. Sarcopenia, but not frailty, predicts early mortality and adverse events after emergent surgery for metastatic disease of the spine. Spine J 2020; 20: 22–31. [DOI] [PubMed] [Google Scholar]
- 76.Moskven E, Bourassa-Moreau E, Charest-Morin R, Flexman A, Street J. The impact of frailty and sarcopenia on postoperative outcomes in adult spine surgery. A systematic review of the literature. Spine J 2018; 18: 2354–69. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


