Abstract
Objective:
To define frequencies, pattern of progression (invasive vs noninvasive), and risk factors of progression of resected noninvasive intraductal papillary mucinous neoplasms (IPMNs).
Background:
There is a risk of progression in the remnant pancreas after resection of IPMNs.
Methods:
Four hundred forty-nine consecutive patients with resected IPMNs from 1995 to 2018 were included to the study. Patients with invasive carcinoma or with follow-up < 6 months were excluded. Noninvasive progression was defined as a new IPMN, increased main pancreatic duct size, and increased size of an existing lesion (5 mm compared with preoperative imaging). Invasive progression was defined as development of invasive cancer in the remnant pancreas or metastatic disease.
Results:
With a median follow-up of 48.9 months, progression was identified in 124 patients (27.6%); 108(24.1%) with noninvasive and 16 (3.6%) with invasive progression. Median progression follow-up was longer for invasive progression (85.4 vs 55.9 months; P = 0.001). Five-and 10-year estimates for a cumulative incidence of invasive progression were 6.4% and 12.9% versus 26.9% and 41.5% for noninvasive progression. After risk adjustment, multifocality (HR 4.53, 95% CI 1.34–15.26; P = 0.02) and high-grade dysplasia (HGD) in the original resection (HR 3.60, 95% CI 1.13–11.48; P = 0.03) were associated with invasive progression.
Conclusions:
Progression to invasive carcinoma can occur years after the surgical resection of a noninvasive IPMN. HGD in the original resection is a risk factor for invasive progression but some cases of low-grade dysplasia also progressed to cancer. Patients with high-risk features such as HGD and multifocal cysts should be considered for more intensive surveillance and represent an important cohort for future trials such as anti-inflammatory or prophylactic immunotherapy.
Keywords: intraductal papillary mucinous neoplasm, IPMN, noninvasive, progression
Pancreatic intraductal papillary mucinous neoplasms (IPMNs) are mucin-producing cystic lesions comprised of dysplastic ductal epithelium.1 Often incidentally found, these neoplasms have increased in incidence over the past few decades due to the increased usage of enhanced imaging modalities.2 Although the majority of IPMNs have a benign and indolent course, these neoplasms have demonstrated a well-established potential to progress to invasive carcinoma.1,3 As only a small fraction of IPMNs progress, a balance between surgery and conservative measures remains the standard of treatment.4,5
Although most IPMNs present as localized lesions, it has been shown that the entire gland is at risk of developing an invasive cancer when an IPMN is present. Thus, patients who undergo pancreatec-tomy for IPMN have an increased risk of developing a recurrent IPMN or even an invasive cancer in the pancreatic remnant.6 High-grade dysplasia and involvement of the main duct are both predictors of who is likely to progress in remnant pancreas.7–10 After resection, postoperative surveillance is strongly recommended as these patients are at risk for progression in the remnant pancreas.10,11 However, the frequency and duration of postoperative surveillance has become an area of controversy. Different follow-up paradigms have been recommended owing to discrepancies in the definition of progression within remnant pancreas and limited analyses that often combine invasive and noninvasive IPMNs.12–14 Recently, the American Gastroenterological Association recommended against routine surveillance after the resection of a noninvasive IPMN. In contrast, the International Association of Pancreatology and European guidelines recommend that all patients with IPMN, even those with noninvasive IPMN, should undergo routine surveillance after surgery as long as the patient remains fit for surgery.4,5,15
The risk of any progression after resection of noninvasive IPMN has been evaluated in multiple previous studies of various follow-up periods and has been estimated to range between 7% and 22%.9,13,14,16 These previous studies however have many limitations; the 2 most significant limitations are that varying definitions of IPMN ”progression” were employed and that many of these studies included patients who had undergone resection for an IPMN with an associated adenocarcinoma where the overwhelming risk is death from metastatic disease from the invasive component, rather than new lesions within the pancreatic remnant.9,13,14 In addition, due to the short duration of follow-up, the development of invasive cancer is subject to underestimation.9,13,14
To establish an effective treatment strategy and improve surveillance after surgery for patients with IPMN, we defined the frequencies, pattern of progression (invasive vs. noninvasive), and risk factors of progression after surgery for noninvasive IPMNs using a high-volume center data with long-term follow-up.
METHODS
Patient Selection
A review of the prospectively collected database at Johns Hopkins Hospital was performed, and 924 patients were identified who underwent pancreatectomy for diagnosis of IPMN, between January 1995 and January 2018. Patients with IPMN associated invasive adenocarcinoma, concomitant pancreatic cancer (invasive adenocarcinoma separate from the IPMN) or other cancer on final pathology, incipient IPMN (lesions between 0.5 and 1.0 cm in diameter with long finger-like papillae, villous intestinal or onco-cytic differentiation, or with a guanine nucleotide binding protein, alpha stimulating activity polypeptide mutation),1 previous history of pancreas cancer, or follow-up less than 6 months were excluded from the analysis (Fig. 1). The study was approved by the Johns Hopkins Institutional Review Board for Human Research and complied with the Health Insurance Portability and Accountability Act regulations.
FIGURE 1.
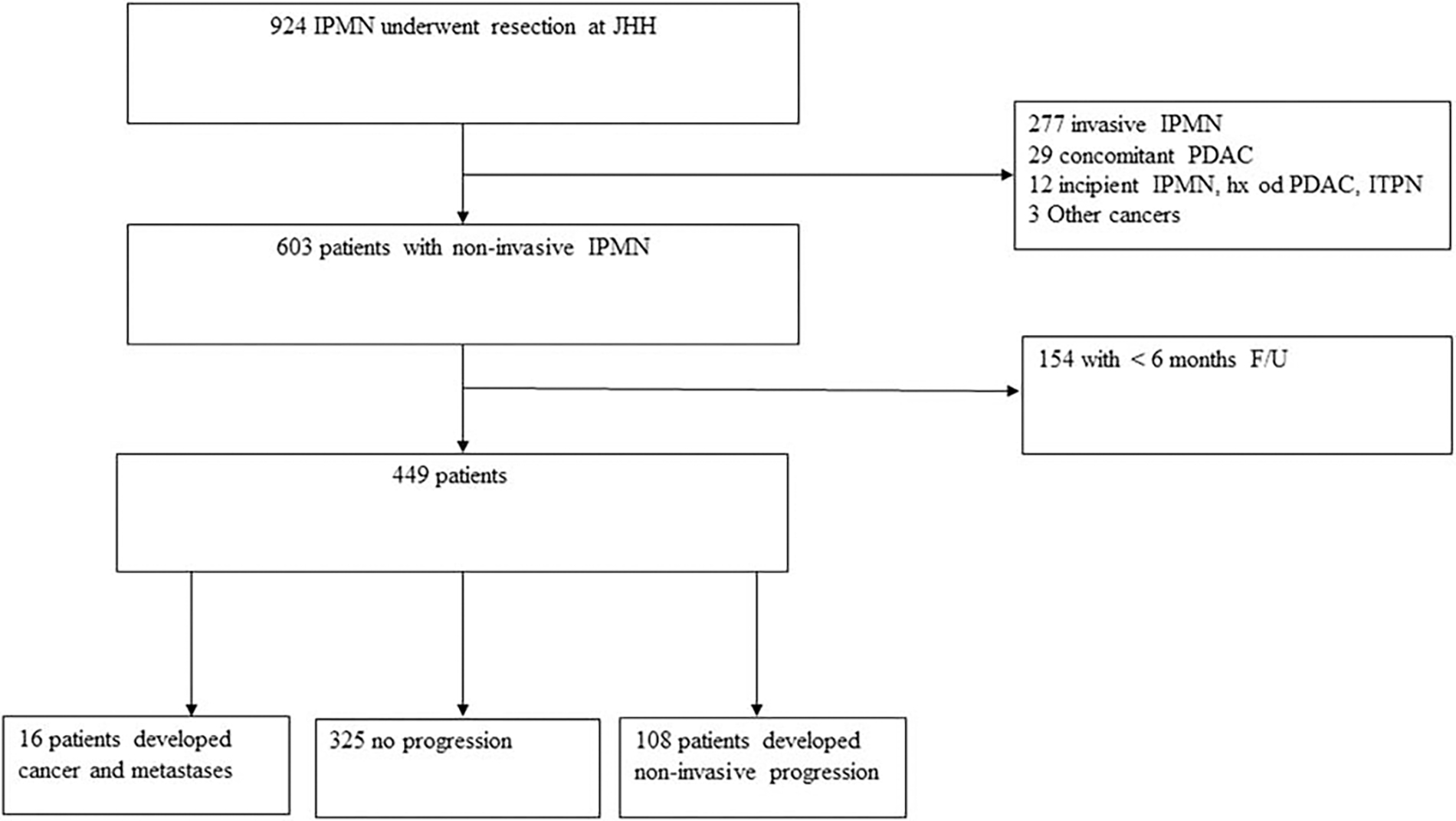
Flow chart on patient inclusion and exclusion criteria.
Involvement of the main or branch ducts, or both, was determined based on preoperative computerized tomography (CT)/mag-netic resonance imaging (MRI). IPMNs were classified as main duct type, if the main pancreatic duct was dilated (≥5 mm) without an associated cyst; as branch duct type, when a cystic parenchymal lesion was present but the main pancreatic duct was not dilated; or as mixed type, when a cystic parenchymal lesion and dilated main pancreatic duct coexisted.
Patient demographics and clinicopathologic data were collected. These included age, sex, presenting symptoms (acute pancreatitis, jaundice, and diabetes), family history of pancreatic cancer (first or second degree relative with pancreatic cancer), the time between diagnosis and operation, and smoking. The maximum cyst size, main pancreatic duct diameter, multifocal cysts (more than 1 cyst that are anatomically separate from one another for branch duct-IPMNs and diffuse dilation of main pancreatic duct for Main-Duct-IPMNs). The presence of a mural nodule was documented when it was present on either CT/MRI or endoscopic ultrasound (EUS) reports. All the images were reviewed by a dedicated pancreas radiologist. Level of preoperative serum carbohydrate antigen 199 was also documented. All pathologic specimens were reviewed by pathologists at the Johns Hopkins Hospital. IPMNs were graded based on the revised classification system and recommendations from the Baltimore consensus into low-grade dysplasia, high-grade dysplasia, or IPMN with an associated invasive carcinoma.1
Definition of Progression
All patients were followed postoperatively with imaging and clinic visits on a routine basis every 6 to 12 months based on established institutional guidelines. Imaging studies included CT scan, MRI, or EUS. The definition of progression was determined based on the new national institutes of health multicentric trail (NCT number: 04207944). Noninvasive progression was defined as a development of a new cystic lesion in the remnant pancreas (>5 mm), increase dilation of main pancreatic duct > 3 mm compared with preoperative imaging (tight stricture at the anastomosis was excluded. Main duct dilatation was considered as progression, if there was a diffuse dilatation of the main pancreatic duct or the dilatation size changed over the time without sign of postop pancreatic insufficiency), increased size of an existing lesion (> 5 mm compared with preoperative imaging), and reoperation for progression. Progression to invasive carcinoma was deemed present if the patient developed pancreatic cancer in the remnant pancreas or developed metastatic disease. All patients with progression to invasive carcinoma had biopsy-proven pancreatic ductal adenocarcinoma.
Statistical Analysis
Numerical variables described as totals and frequencies (n, %) whereas continuous variables were described by the median with interquartile range (IQR). Continuous variables were compared using Wilcoxon-Mann-Whitney or Kruskal-Wallis tests as appropriate. Chi-squared test or Fisher exact test (if needed) was used for categorical variables as appropriate. Progression-free survival (PFS) was calculated from the date of surgery using Kaplan-Meier survival analysis and compared using a log-rank test. Cox proportional-hazard-regression was performed to determine predictors of progression. Variables with significance in univariable (P < 0.05) analysis were included in the multivariable analysis. The proportional assumption of the Cox model was tested using the Schoenfeld residuals test. Statistical analysis was performed using Stata/MP version 13.1 (StataCorp, College Station, TX).
RESULTS
A total of 449 patients with a non-invasive IPMN were included (Fig. 1). The cohort included 216 (48.1%) males, and the median age was 69 years (IQR 61 −75). Two hundred ninety (64.6%) patients underwent pancreatoduodenectomy and 132 (29.4%) underwent distal pancreatectomy. The distribution of grade was as follows: low-grade in 319 patients (71.1%) and high-grade in 130 (28.9%). Initially, a positive margin existed in 87 (19.4%) of patients. Thirteen (14.9%) underwent reresection which led to negative margin in 7 of them. In the final specimen, a positive margin existed in 80 (17.8%) patients; 4 (5.0%) with high-grade dysplasia at the margin, 73 (91.3%) with low-grade, and 3 (3.8%) with unknown grade of dysplasia. Of the 449 IPMNs, 57 (12.7%) were main duct, 231(51.5%) were branch duct IPMN, and 161 (35.9%) were mixed type IPMN. The baseline characteristics of patients with noninvasive progression or invasive progression were compared with patients without progression (Table 1). Noninvasive progression was more common in males, in those with a positive family history of pancreas cancer, in smokers, and in those with a smaller main pancreatic duct when compared with patients without progression. On the other hand, patients with progression to invasive carcinoma had a shorter time between diagnosis and index operation, were more likely to have a family history of pancreas cancer, multifocal cysts, a smaller main pancreatic duct, and high-grade dysplasia in initial operation compared with patients without progression. A comparison between patients with noninvasive progression and progression to invasive carcinoma was also performed (Supplemental Table 1, http://links.lww.com/SLA/C633). Patients with progression to invasive carcinoma were more likely to be male (invasive progression 68.8% vs noninvasive progression 38.0%, P = 0.03), and had a higher percentage of high-grade dysplasia at initial operation (invasive progression 56.3% vs nonin-vasive progression 25%; P = 0.02).
TABLE 1.
Patient Demographic and Clinicopathologic Stratified by Type of Progression
| Total | No Progression | Noninvasive Progression | P * | Invasive Progression | P † | |
|---|---|---|---|---|---|---|
|
| ||||||
| n | 449 | 326 | 108 | 16 | ||
| Age at surgery (yrs), median (IQR) | 69 (61–75) | 69 (61–75) | 69 (62–76) | 0.89 | 72 (62–74) | 0.87 |
| Male sex, N (%) | 216 (48.1) | 164 (50.5) | 41 (38.0) | 0.02 | 11 (68.8) | 0.20 |
| Symptoms, N (%) | ||||||
| Acute pancreatitis | 73 (16.5) | 55 (17.2) | 15 (13.9) | 0.42 | 3 (18.8) | 0.74 |
| Jaundice | 12 (2.7) | 11 (3.4) | 1 (0.9) | 0.31 | 0 | NA |
| Diabetes | 80 (17.8) | 62 (19.1) | 14 (13.0) | 0.15 | 4 (25.0) | 0.52 |
| Family history of pancreatic cancer | 66 (14.7) | 36 (11.1) | 25 (23.2) | 0.002 | 5 (31.3) | 0.03 |
| Time since diagnosis of IPMN to operation (mo) (median) | 2.5 (0.7–9.7) | 2.4 (0.7–9) | 3.1 (0.9–13.7) | 0.54 | 0.5 (0.2–3.5) | 0.009 |
| Delayed surgery (more than 6 mo) | 135 (30.2) | 95 (29.3) | 38 (35.5) | 0.23 | 2 (12.5) | 0.25 |
| Smoking | ||||||
| Never | 253 (56.4) | 194 (59.7) | 131 (40.3) | 0.04 | 7 (43.8) | 0.30 |
| Current/past | 196 (43.7) | 131 (40.3) | 56 (51.9) | 9 (56.3) | ||
| Cyst ≥ 3 cm, N (%) | 194 (44.2) | 137 (43.4) | 46 (43.0) | 0.95 | 11 (68.8) | 0.07 |
| Cyst size (cm), median (IQR) | 2.6 (1.8–3.6) | 2.6 (1.7–3.7) | 2.5 (1.8–3.4) | 0.92 | 3.1 (2.5–3.5) | 0.09 |
| Type of operation | ||||||
| Pancreatoduodenectomy | 290 (64.6) | 204 (62.8) | 75 (69.5) | 0.44 | 11 (68.8) | 0.92 |
| Distal pancreatectomy | 132 (29.4) | 100 (30.8) | 28 (25.9) | 4 (25.0) | ||
| Others | 27 (6.0) | 21 (6.4) | 5 (4.6) | 1 (6.2) | ||
| Type of cyst, N (%) | ||||||
| Branch duct | 231 (51.5) | 160 (49.2) | 62 (57.4) | 0.33 | 9 (56.3) | 0.33 |
| Main duct | 57 (12.7) | 45 (13.9) | 12 (11.1) | 0 | ||
| Mixed duct | 161 (35.9) | 120 (36.9) | 34 (31.5) | 7 (43.7) | ||
| Multifocal, N (%) (n = 401) | 164 (40.9) | 109 (37.5) | 46 (47.4) | 0.08 | 9 (69.2) | 0.04 |
| Dilated main pancreatic duct | 240 (53.5) | 180 (55.4) | 55 (50.9) | 0.42 | 5 (31.3) | 0.07 |
| ≥ 5 mm (n = 442) | 188 (42.5) | 150 (46.9) | 35 (33.0) | 0.01 | 3 (18.8) | 0.04 |
| ≥ 10 mm (n = 442) | 62 (14.0) | 52 (16.3) | 8 (7.6) | 0.03 | 2 (12.5) | 1.00 |
| Mural nodule/solid component, N (%) | 93 (20.7) | 66 (20.3) | 25 (23.2) | 0.53 | 2 (12.5) | 0.75 |
| Ca19-9 > 37, N (%) | 20 (4.5) | 14 (4.3) | 5 (4.6) | 1.0 | 1 (6.7) | 0.52 |
| Positive margin, N (%) | 80 (17.8) | 50 (15.4) | 25 (23.2) | 0.07 | 5 (31.3) | 0.15 |
| Low-grade margin | 73 (91.3) | 49 (98.0) | 22 (88.0) | 0.04 | 2 (40.0) | < 0.001 |
| High-grade margin | 4 (5.0) | 0 | 3 (12.0) | 1 (20.0) | ||
| Unknown | 3 (3.8) | 1 (2.0) | 0 | 2 (40.0) | ||
| Grade of dysplasia/invasive cancer, N (%) | ||||||
| Low-intermediate | 319 (71.1) | 231 (71.1) | 81 (75.0) | 0.43 | 7 (43.8) | 0.03 |
| High | 130 (28.9) | 94 (28.9) | 27 (25.0) | 9 (56.3) | ||
P between noninvasive progression and no progression.
Between invasive progression and no progression.
CA indicates 19-9 Carbohydrate antigen 19-9; IPMN, intraductal papillary mucinous neoplasm; IQR, interquartile range.
Pattern of Progression Following Pancreatectomy for IPMN
Median follow-up was 48.9 months and a total of 124 (27.6%) patients progressed after surgery with 108 (24.1%) developing noninvasive progression and 16 (3.6%) progressed to invasive carcinoma. The median follow-up time was significantly longer in patients progressing to invasive carcinoma (85.4 mo, IQR 56.1118) compared with patients who progressed to noninvasive IPMN (55.9 mo, IQR 34.2–97.2) and patient without progression (44.0 mo, IQR 22.4–71.4) (P = 0.001) (Table 2) (Supplementary Figure 2, http://links.lww.com/SLA/C632).
TABLE 2.
Progression Survival Stratifies by Progression Type
| Total | No Progression | Noninvasive Progression | Invasive Progression | P | |
|---|---|---|---|---|---|
|
| |||||
| Follow-up, mo, median (IQR) | 48.9 (24.9–82.3) | 44.0 (22.4–71.4) | 55.9 (34.2–97.2) | 85.4 (56.1–118) | 0.001 |
| 1-yr cumulative HR | 4.7% | NA | 4.9% | 0% | |
| 5-yr cumulative HR | 29.8% | NA | 26.9% | 6.4% | |
| 10-yr cumulative HR | 46.8% | NA | 41.5% | 12.9% | |
HR indicates hazard ratio; IQR, interquartile range.
Amongst patients with progression to noninvasive IPMN, 41.7% (n = 45) developed a new cyst in pancreas remnant, 23.2% (n = 25) developed dilation of main pancreatic duct > 3 mm, 18.5% (n = 20) had an increase in the size of a previous lesions, 11.1% (n = 12) underwent reoperation for progression, and 5.5% (n = 5) had a combination of discovery of new cyst and dilation of main pancreatic duct.
Amongst the 16 patients who progressed to invasive carcinoma, 7 (43.8%) underwent reoperation with pancreas cancer confirmed on the final pathology of the second operation, whereas 9 (43.8%) developed metastatic pancreatic cancer. Interestingly, 7 (43.8%) patients who progressed to invasive carcinoma had only low-grade dysplasia on pathologic review of their original IPMN resection.
Factors Associated With Progression to Invasive Carcinoma
After pancreatectomy, 1-, 5-, and 10-year estimates for cumulative progression to invasive carcinoma were 0%, 6.4%, and 12.9%, respectively (Fig. 2A). After univariable cox regression, a family history of pancreas cancer, multifocal cyst, and high-grade dysplasia at initial operation were associated with progression to invasive carcinoma (Table 3). Subsequent multivariable analysis, after adjusting for competing risk factors, multifocality (HR4.53, 95% CI 1.34–15.26; P = 0.02), and high-grade dysplasia (HR 3.60, 95% CI 1.13–11.48; P = 0.03) remained statistically significant. Interestingly, factors such as type of cyst (main duct vs branch duct) and positive margin for any grade at the time of initial operation were not associated with progression to invasive carcinoma (Supplementary Figure 1, http://links.lww.com/SLA/C631).
FIGURE 2.
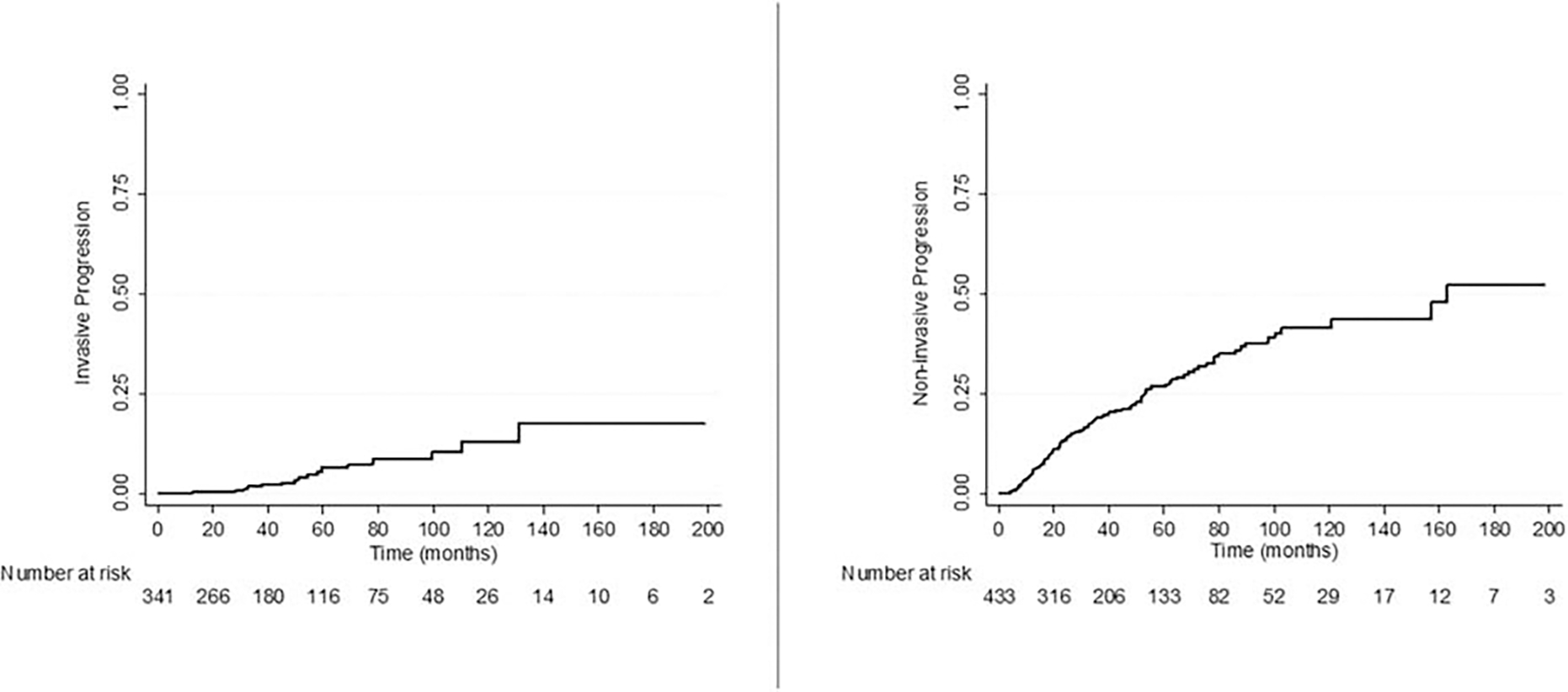
A, Cumulative incidence of progression to invasive carcinoma. B, Cumulative incidence of noninvasive progression.
TABLE 3.
Univariable and Multivariable Associated With Invasive Progression
| Univariable HR (95% CI) | P | Multivariable HR (95% CI) | P | |
|---|---|---|---|---|
|
| ||||
| Age at surgery | 1.01 (0.96–1.06) | 0.60 | – | |
| Male sex | 2.41 (0.84–6.95) | 0.10 | – | |
| Acute pancreatitis | 1.67 (0.47–5.94) | 0.43 | – | |
| Diabetes | 2.01 (0.65–6.23) | 0.23 | – | |
| Family history of pancreatic cancer | 3.49 (1.21–10.07) | 0.02 | 2.74 (0.79–9.58) | 0.11 |
| Time since diagnosis to operation | 0.99 (0.97–1.02) | 0.74 | – | |
| Delayed surgery ≥ 6 mo | 0.55 (0.12–2.42) | 0.43 | – | |
| Smoking | ||||
| Never | Ref | |||
| Current/past | 1.77 (0.66–4.75) | 0.26 | – | |
| Cyst ≥ 3 cm | 2.76 (0.95–7.99) | 0.06 | – | |
| Type of cyst | ||||
| Branch duct | Ref | |||
| Main duct | – | – | ||
| Mixed duct | 1.36 (0.50–3.69) | 0.55 | – | |
| Multifocal cysts | 4.48 (1.38–14.59) | 0.01 | 4.53 (1.34–15.26) | 0.02 |
| Dilated main pancreatic duct | 0.55 (0.19–1.61) | 0.28 | – | |
| MPD ≥ 5 mm | 0.39 (0.11–1.38) | 0.14 | – | |
| MPD ≥ 10 mm | 1.48 (0.33–6.61) | 0.60 | – | |
| Mural nodule/solid component | 0.54 (0.12–2.40) | 0.42 | – | |
| Ca19-9 > 37 | 2.19 (0.29–16.88) | 0.45 | – | |
| Positive margin | 2.19 (0.76–6.31) | 0.15 | – | |
| Grade of dysplasia/invasive cancer | ||||
| Low-intermediate | Ref | Ref | ||
| High | 2.96 (1.10–8.00) | 0.03 | 3.60 (1.13–11.48) | 0.03 |
CA 19-9 indicates carbohydrate antigen 19-9; MPD, main pancreatic duct.
In a subgroup analysis, after exclusion of patients without progression, a comparison was performed between noninvasive progression and progression to invasive carcinoma (patients without progression censored), history of diabetes at initial diagnosis (HR 5.92, 95% CI 1.63–21.62; P = 0.007), and high-grade dysplasia (HR 4.63; 95% CI 1.45–14.72; P = 0.009) were associated with risk of progression to invasive carcinoma in multivariable analysis (Supple– mental Table 2, http://links.lww.com/SLA/C634). Of note, all these patients had history of diabetes before initial operation. The median time between diagnosis of diabetes and progression was 5 years for invasive progression versus 11 years for noninvasive progression (P = 0.36).
Factors Associated With Progression to Invasive Carcinoma Among Patients With Low-grade Dysplasia Versus High-grade Dysplasia
A subgroup analysis was performed amongst patient with progression to invasive carcinoma based on the highest degree of dysplasia in their initial resection; within these subcohorts, the 5-and 10-year PFS for low-grade dysplasia were 4.0% and 8.8% versus 12.4% and 26.7% for high-grade dysplasia (P = 0.02). Amongst patients with low-grade dysplasia on initial resection, only family history of pancreatic cancer was associated with progression to invasive carcinoma (HR 4.76, 95% CI 1.06–21.36; P = 0.04). However, in patients with high-grade dysplasia in the initial resection, a positive margin (any grade) was associated with progression to invasive carcinoma (HR 4.11, 95% CI 1.10–15.46; P = 0.04).
Factors Associated With Progression to Noninvasive IPMN
The median PFS for progression to noninvasive IPMN was 162.9 months and 1-, 5-, and 10-year estimates for cumulative progression to noninvasive IPMN were 4.9%, 26.9%, and 41.5%, respectively (Fig. 2B). Family history of pancreas cancer, delayed surgery for more than 6 months in patients who did not initially meet criteria for resection, smoking, and multifocal cysts were associated with progression to noninvasive IPMN in univariable Cox regression (Table 4). In multivariable analysis, family history ofpancreas cancer (HR 1.75, 95% CI 1.05–2.91; P = 0.03) and multifocal cysts (HR 1.54, 95% CI 1.02–2.34; P = 0.04) remained statistically significant. Of note, factors such as high-grade dysplasia in initial resection, positive margin for any grade, type of cysts (main duct vs. branch duct) were not associated with progression to noninvasive IPMN.
TABLE 4.
Univariable and Multivariable Associated With Noninvasive Progression
| Univariable HR (95% CI) |
P | Multivariable HR (95% CI) |
P | |
|---|---|---|---|---|
|
| ||||
| Age at surgery | 1.01 (0.99–1.03) | 0.14 | – | |
| Male sex | 0.73 (0.50–1.08) | 0.13 | – | |
| Acute pancreatitis | 1.11 (0.64–1.91) | 0.72 | – | |
| Jaundice | 0.33 (0.05–2.37) | 0.27 | – | |
| Diabetes | 0.86 (0.49–1.52) | 0.61 | – | |
| Family history of pancreatic cancer | 2.43 (1.55–3.82) | < 0.001 | 1.75 (1.05–2.91) | 0.03 |
| Delayed surgery ≥6 mo | 1.70 (1.14–2.54) | 0.009 | 1.44 (0.94–2.22) | 0.09 |
| Smoking | ||||
| Never | Ref | Ref | ||
| Current/past | 1.51 (1.03–2.20) | 0.04 | 1.51 (1.00–2.33) | 0.05 |
| Cyst > 3 cm | 0.98 (0.67–1.45) | 0.94 | – | |
| Type of cyst | ||||
| Branch duct | Ref | |||
| Main duct | 0.70 (0.37–1.35) | 0.29 | – | |
| Mixed duct | 0.85 (0.56–1.30) | 0.46 | – | |
| Multifocal cysts | 1.68 (1.13 –2.52) | 0.01 | 1.54 (1.02–2.34) | 0.04 |
| Dilated main pancreatic duct | 0.97 (0.66–1.42) | 0.87 | – | |
| MPD ≥ 5 mm | 0.68 (0.45–1.02) | 0.06 | – | |
| MPD ≥ 10 mm | 0.70 (0.34–1.46) | 0.34 | – | |
| Mural nodule/solid component | 1.11 (0.71–1.73) | 0.66 | – | |
| CA19-9 > 37 | 1.27 (0.52–3.14) | 0.60 | – | |
| Positive margin | 1.57 (1.00–2.34) | 0.05 | – | |
| Grade of dysplasia/invasive cancer | ||||
| Low-intermediate | Ref | |||
| High | 0.89 (0.57–1.38) | 0.60 | – | |
CA 19-9 indicates carbohydrate antigen 19-9; MPD, main pancreatic duct.
Survival
Five- and 10-year overall survival for the entire cohort were 87.2% and 65.6% respectively. The 5-year overall survival for patients with progression to noninvasive IPMN was 96% versus 75% for progression to invasive carcinoma. Of note, risk of death was not increased for patients with progression to noninvasive IPMN compared with patients without progression (HR 0.32, 95% CI 0.15–0.68; P = 0.003). This likely reflects the older overall age of this patient population (median of the entire cohort was 69 years). However, patients with progression to invasive carcinoma had an almost 2-fold increase risk of death HR 2.40, 95% CI 1.27–4.51; P = 0.007) compared with those with no progression (Fig. 3).
FIGURE 3.
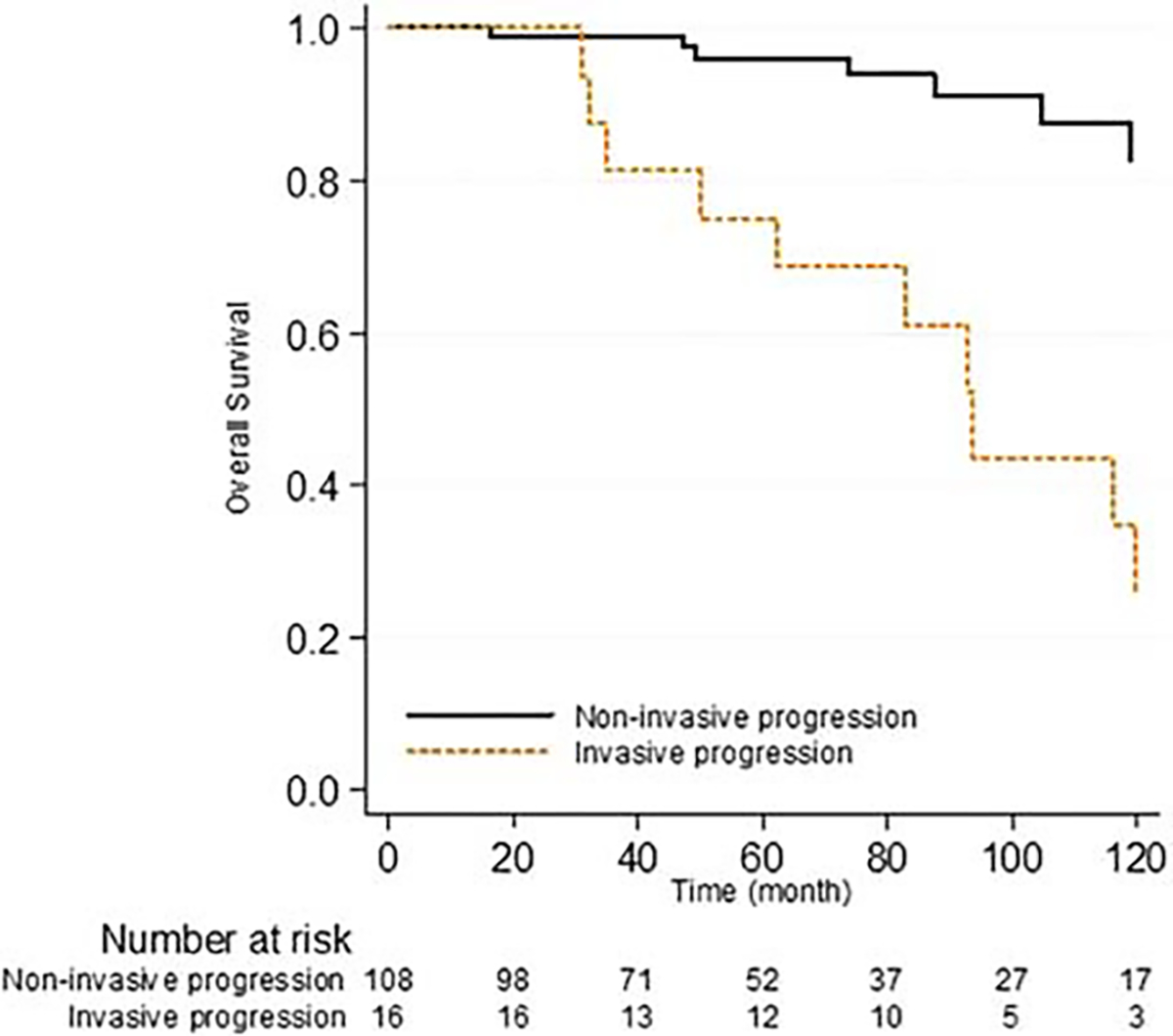
Overall survival stratified by type of progression.
DISCUSSION
In this high-volume institutional analysis of 449 patients with long-term follow-up who underwent surgical resection for a nonin-vasive IPMN, we found 27.6% of patients developed progression after resection; 3.5% of patients progressed to invasive carcinoma while 24.1% progressed to noninvasive IPMN. Remarkably, progression to invasive carcinoma occurred at a median of 7 years after surgery.
The overall rate of progression in our study was 27.6%, which was comparable with studies from Indiana University and Memorial Sloan Kettering, where the overall progression rates were 20% and 22% with rates of progression to invasive cancer of 2% and 3% respectively.13,16 The former investigated solely noninvasive IPMNs while the latter included patients with microinvasive disease as well. Interestingly, Marchegiani et al14 studied 381 patients with both invasive and noninvasive IPMN and found that 17% of patients progressed and further sensitivity analysis showed that only 9% of the noninvasive IPMNs had progression. However, the overall median follow-up was only 17 months, which was significantly shorter than our study (median 48.9 mo; mean 59.1 mo).
We found, as have others, that multifocal IPMNs have a higher propensity toward developing subsequent disease in the remnant pancreas.17–19 The mechanism for this multifocality is not clear, but molecular analysis of multifocal IPMNs has found that the separate cysts of multifocal IPMNs arise independently and display clonal heterogeneity.20 The risk of multifocal disease in the remnant pancreas emphasizes the importance of long-term and closer follow-up after initial resection in patients with multifocal disease.21 We also found that high-grade dysplasia is significantly associated with the risk of progressing to invasive carcinoma. This result is supported by previous studies in the literature.9,10,14 As a result, we suggest patients with high-grade dysplasia in the original resection or multifocal cysts, should be followed with more intense surveillance such as a CT or MRI every 6 months with a subsequent EUS for any significant changes.
Our findings contradict the American Gastroenterological Association recommendation that recommend surveillance of only patients who undergo surgery for a high-grade IPMN, as we report here that a subcohort analysis showed that 7 of 16 (43.8%) of the patients with progression to invasive carcinoma, in fact, had low-grade IPMN at the time of resection.15 Although high-grade dyspla-sia at resection does significantly increase the risk for progression to invasive carcinoma, it is not a definite prerequisite to invasive progression and therefore, bring into question the recommendations to not surveil patients with low-grade dysplasia. This suggests that low-grade dysplasia progress to invasive carcinoma at a slower rate than does high-grade and hence, patients with a resected IPMN with low-grade dysplasia may even benefit from longer follow-up. Collectively, these data support the International Association of Pan-creatology and European recommendations for lifelong follow-up regardless of tumor grade as long as the patient remains surgically fit.4,5
Interestingly, in our series, main duct IPMN at the time of resection was not found to be a statistically significant risk factor for the progression of disease. This finding is in agreement with Mar-chegiani et al14 who reported that in multivariable analysis accounting for IPMN type, high-grade dysplasia at margin was in fact the significant driver for progression. Furthermore, Hirono et al and Al Efishat et al report that main duct IPMN was not associated with risk of progression after pancreatectomy for IPMN patients.10,13 Thus, our study, in agreement, concludes that both main duct IPMN and branch duct IPMN have a similar risk for progression to invasive carcinoma and should receive comparable surveillance strategies. This study does not allow us to further comment on or explain the reasoning behind this finding. We hypothesize that it may be due to more aggressive follow-up and closer surveillance in main duct compared with branch duct IPMNs which could, in turn, identify progression earlier and before the development of invasive carcinoma. However, this requires further studies to investigate and validate these results.
The risk of IPMN progression after a positive resection margin is a controversial topic. In our series, we found that margin status (any grade) was not an independent predictor for progression. This is consistent with a number of previous studies.10,13,16,22–24 On the other hand, some studies have found that positive margin status was associated with increased risk of progression.25,26 However, these studies were smaller and had shorter follow-up periods. In addition, in subgroup analysis based on grade of dysplasia at margin, we showed low-grade dysplasia at margin was not a significant factor for progression in patients with a low-grade IPMN (index lesion). In contrast, low-grade dysplasia at the margin was a factor for progression to invasive carcinoma for patients who had high-grade dysplasia in their index lesion. This finding supports the notion that the highest grade of dysplasia in an IPMN and margin status may be 2 distinct biomarkers for disease aggressiveness and diffuseness, respectively. This finding suggests low-grade dysplasia at margin may warrant reresection in the setting of high-grade dysplasia in the index lesion. However, this study was not able to prove this perception and future studies should investigate this.
We also found that family history of pancreatic cancer was a risk factor for the development of both noninvasive and invasive progression (in univariable analysis). In the updated recommendation from the international cancer of the pancreas screening consortium, experts agreed that the preferred surveillance tests for patients with familial pancreatic cancer are EUS and MRI/MRCP. However, no consensus was reached on how to alternate these modalities.27 We recommend that patients with a family history of pancreatic cancer should undergo closer surveillance (6–12 months) in the absence of concerning lesions and at a high-volume center led by a multidisci-plinary care team.
In regard to overall survival, patients with progression to invasive carcinoma had significantly poorer survival than those with progression to IPMN or no progression (75% vs 96% and 84.7% 5-year overall survival, respectively). These findings are consistent with the literature regarding patient survival following the resection of noninvasive IPMNs.16 These findings further underscore the importance of aggressive follow-up and early detection in patients at risk for progression to invasive carcinoma.
One of the important strengths of this study is the long-term follow-up, ranging from 6.2 months to 262.1 months, which allowed us to detect progression years after resection. Progression is a gradual process, and early termination of surveillance may result in missed progression, particularly progression to invasive carcinoma. This point is further emphasized when comparing this updated series (1995–2018) to our previous series (1995–2010) where with less follow-up we reported that 17% of patients, as opposed to 27.6%, developed disease progression.19 This further accentuates the importance of long-term follow-up for patients who undergo resection for an IPMN. Another strength of our study is the homogeneity of the study population (all patients had noninvasive IPMNs) compared with previous studies that combined invasive (or microinvasive) and noninvasive IPMNs. This allowed us to better elucidate the factors associated with invasive progression in a group of patients who did not have any component of invasiveness in the initial pathology. Furthermore, this study was able to distinctly identify separate factors associated with invasive versus noninvasive progression. This is important due to the significant differences in survival and management of these patients. Categorization of IPMN progression into invasive and noninvasive groups and defining factors associated with these progressions separately is one of the novel aspects of our study.
Limitations within this study first include its retrospective design which confers an element of selection bias. Additionally, since the study cohort spanned over 2 decades, the selection criteria for surgical intervention based on recognized guidelines varied which, in turn, leads to a heterogeneous final study population. Differentiation between IPMN related dilatation and postsurgical dilatation is challenging, however, we selected these patients carefully after rereviewing imaging using the criteria detailed in the methods section. We also did not have access to data on molecular cyst fluid markers and did not analyze histological IPMN subtypes due to high subjectivity in pathologic interpretation. Previous studies have showed had an association of these factors with progression after pancreatectomy for IPMN.28–30
In conclusion, our large series with the long-term follow-up on progression after the surgical resection of noninvasive IPMN demonstrated that these patients are at a substantial risk for developing a new IPMN in their remnant pancreas, as well as progression to invasive carcinoma. Particularly, patients with multifocal disease and high-grade dysplasia in the resected lesion are at an increased risk for progression to invasive carcinoma. Remarkably the invasive carcinomas developed at a mean of 7 years after initial surgery. Based on these findings, we recommend long-term surveillance for patients with resected noninvasive IPMN, even for those with an IPMN with low-grade dysplasia. The high-risk group of patients with multifocal disease and high-grade dysplasia represent an important cohort for future interventional trials such as anti-inflammatory or prophylactic immunotherapy treatment.
Supplementary Material
Acknowledgments
This work was supported by the Nikki Mitchell Foundation.
Footnotes
The authors report no conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.annalsofsurgery.com).
REFERENCES
- 1.Basturk O, Hong SM, Wood LD, et al. A revised classification system and recommendations from the Baltimore consensus meeting for neoplastic precursor lesions in the pancreas. Am J Surg Pathol. 2015;39:1730–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klibansky DA, Reid-Lombardo KM, Gordon SR, et al. The clinical relevance of the increasing incidence of intraductal papillary mucinous neoplasm. Clin Gastroenterol Hepatol. 2012;10:555–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tanaka M Clinical management and surgical decision-making of IPMN of the pancreas. Methods Mol Biol. 2019;1882:9–22. [DOI] [PubMed] [Google Scholar]
- 4.European Study Group on Cystic Tumours of the Pancreas. European evidence-based guidelines on pancreatic cystic neoplasms. Gut. 2018;67:789–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tanaka M, Fernandez-Del Castillo C, Kamisawa T, et al. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology. 2017;17:738–753. [DOI] [PubMed] [Google Scholar]
- 6.Farrell JJ. Prevalence, diagnosis and management of pancreatic cystic neoplasms: current status and future directions. Gut Liver. 2015;9:571–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kang MJ, Jang JY, Lee KB, et al. Long-term prospective cohort study of patients undergoing pancreatectomy for intraductal papillary mucinous neoplasm of the pancreas: implications for postoperative surveillance. Ann Surg. 2014;260:356–363. [DOI] [PubMed] [Google Scholar]
- 8.Majumder S, Philip NA, Singh Nagpal SJ, et al. High-grade dysplasia in resected main-duct intraductal papillary mucinous neoplasm (MD-IPMN) is associated with an increased risk of subsequent pancreatic cancer. Am J Gastroenterol. 2019;114:524–529. [DOI] [PubMed] [Google Scholar]
- 9.Rezaee N, Barbon C, Zaki A, et al. Intraductal papillary mucinous neoplasm (IPMN) with high-grade dysplasia is a risk factor for the subsequent development of pancreatic ductal adenocarcinoma. HPB (Oxford). 2016;18:236–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirono S, Shimizu Y, Ohtsuka T, et al. Recurrence patterns after surgical resection of intraductal papillary mucinous neoplasm (IPMN) of the pancreas; a multicenter, retrospective study of 1074 IPMN patients by the Japan Pancreas Society. J Gastroenterol. 2020;55:86–99. [DOI] [PubMed] [Google Scholar]
- 11.Sohn TA, Yeo CJ, Cameron JL, et al. Intraductal papillary mucinous neoplasms of the pancreas: an updated experience. Ann Surg. 2004;239:788–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Huijgevoort NCM, Del Chiaro M, Wolfgang CL, et al. Diagnosis and management of pancreatic cystic neoplasms: current evidence and guidelines. Nat Rev Gastroenterol Hepatol. 2019;16:676–689. [DOI] [PubMed] [Google Scholar]
- 13.Al Efishat M, Attiyeh MA, Eaton AA, et al. Progression patterns in the remnant pancreas after resection of non-invasive or micro-invasive intraductal papillary mucinous neoplasms (IPMN). Ann Surg Oncol. 2018;25:1752–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marchegiani G, Mino-Kenudson M, Ferrone CR, et al. Patterns of recurrence after resection of IPMN: who, when, and how? Ann Surg. 2015;262:1108–1114. [DOI] [PubMed] [Google Scholar]
- 15.Vege SS, Ziring B, Jain R, et al. American gastroenterological association institute guideline on the diagnosis and management of asymptomatic neo-plastic pancreatic cysts. Gastroenterology. 2015;148:819–822. [DOI] [PubMed] [Google Scholar]
- 16.Miller JR, Meyer JE, Waters JA, et al. Outcome of the pancreatic remnant following segmental pancreatectomy for non-invasive intraductal papillary mucinous neoplasm. HPB (Oxford). 2011;13:759–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cauley CE, Waters JA, Dumas RP, et al. Outcomes of primary surveillance for intraductal papillary mucinous neoplasm. J Gastrointest Surg. 2012;16:258–267. [DOI] [PubMed] [Google Scholar]
- 18.Griffin JF, Poruk KE, Wolfgang CL. Is it time to expand the role of total pancreatectomy for IPMN? Dig Surg. 2016;33:335–342. [DOI] [PubMed] [Google Scholar]
- 19.He J, Cameron JL, Ahuja N, et al. Is it necessary to follow patients after resection of a benign pancreatic intraductal papillary mucinous neoplasm? J Am Coll Surg. 2013;216:657–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matthaei H, Norris AL, Tsiatis AC, et al. Clinicopathological characteristics and molecular analyses of multifocal intraductal papillary mucinous neoplasms of the pancreas. Ann Surg. 2012;255:326–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raman SP, Kawamoto S, Blackford A, et al. Histopathologic findings of multifocal pancreatic intraductal papillary mucinous neoplasms on CT. AJR Am J Roentgenol. 2013;200:563–569. [DOI] [PubMed] [Google Scholar]
- 22.Bhardwaj N, Dennison AR, Maddern GJ, et al. Management implications of resection margin histology in patients undergoing resection for IPMN: a meta-analysis. Pancreatology. 2016;16:309–317. [DOI] [PubMed] [Google Scholar]
- 23.Winner M, Epelboym I, Remotti H, et al. Predictors of recurrence in intra-ductal papillary mucinous neoplasm: experience with 183 pancreatic resections. J Gastrointest Surg. 2013;17:1618–1626. [DOI] [PubMed] [Google Scholar]
- 24.Yogi T, Hijioka S, Imaoka H, et al. Risk factors for postoperative recurrence of intraductal papillary mucinous neoplasms of the pancreas based on a long-term follow-up study: proposals for follow-up strategies. J Hepatobiliary Pancreat Sci. 2015;22:757–765. [DOI] [PubMed] [Google Scholar]
- 25.Frankel TL, LaFemina J, Bamboat ZM, et al. Dysplasia at the surgical margin is associated with recurrence after resection of non-invasive intraductal papillary mucinous neoplasms. HPB (Oxford). 2013;15:814–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.White R D’Angelica M, Katabi N, et al. Fate of the remnant pancreas after resection of noninvasive intraductal papillary mucinous neoplasm. J Am Coll Surg. 2007;204:987–993. [DOI] [PubMed] [Google Scholar]
- 27.Goggins M, Overbeek KA, Brand R, et al. Management of patients with increased risk for familial pancreatic cancer: updated recommendations from the International Cancer of the Pancreas Screening (CAPS) Consortium. Gut. 2020;69:7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Al Efishat MA, Attiyeh MA, Eaton AA, et al. Multi-institutional validation study of pancreatic cyst fluid protein analysis for prediction of high-risk intraductal papillary mucinous neoplasms of the pancreas. Ann Surg. 2018;268:340–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Das KK, Geng X, Brown JW, et al. Cross validation of the monoclonal antibody das-1 in identification of high-risk mucinous pancreatic cystic lesions. Gastroenterology. 2019;157:720.e2–730.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maker AV, Katabi N, Qin LX, et al. Cyst fluid interleukin-1beta (IL1beta) levels predict the risk of carcinoma in intraductal papillary mucinous neoplasms of the pancreas. Clin Cancer Res. 2011;17:1502–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


