Abstract
Biochemical recurrence develops in almost one-third of men with prostate cancer after treatment with local therapy. There are numerous options for management, including surveillance, salvage radiation, androgen deprivation therapy (ADT), and clinical trials. This article reviews the current approaches to radiation therapy, ADT, and molecular imaging in men with biochemically recurrent prostate cancer. First, radiation therapy, including selection of field, dose, and use of concurrent antiandrogen therapy, is reviewed. Next, molecular imaging is addressed, including prostate-specific membrane antigen PET imaging and its increased sensitivity in identifying sites of disease. Finally, the factors associated with starting ADT are explored, and the data supporting intermittent over continuous ADT are reviewed. Lastly, the use of prostate-specific membrane antigen PET imaging and its potential role influencing therapy are discussed.
Despite undergoing definitive local therapy with radical prostatectomy (RP) or radiation for prostate cancer, many men will go on to develop prostate-specific antigen (PSA) recurrence with no evidence of disease on conventional imaging. This disease state is called biochemical recurrence (BCR). Estimates for the risk of developing BCR range from 20% to 40%.1,2 The Phoenix criteria3 are used to define BCR postradiation therapy, which requires an increase in PSA of at least 2 ng/mL above the postradiation PSA nadir, whereas BCR post-RP is defined as at least two PSA values that are 0.2 ng/mL or higher.4 Therapeutic options include salvage radiation therapy (SRT) for patients with post-RP PSA recurrence. For those men with postradiation recurrence, the best approach is controversial. Current options include surveillance, androgen deprivation therapy (ADT), and clinical trials. This article reviews the current diagnostic and therapeutic approaches for patients with BCR and discusses the increasing use of prostate-specific membrane antigen (PSMA) PET imaging in this disease state.
SALVAGE TREATMENT OF BIOCHEMICAL RECURRENCE AFTER RADICAL PROSTATECTOMY
Postoperative radiation therapy to the prostate bed has been widely used as a salvage treatment for PSA recurrence after RP. Although not supported by randomized trials, retrospective comparisons have found an association between the use of SRT and improved long-term outcomes. Trock et al reported the long-term outcomes of 635 men with BCR following RP.5 Patients were classified according to the choice of treatment: SRT with or without ADT (238 patients) or observation (397 patients). Median follow-up from the time of RP was 9 years, and the median follow-up time from recurrence was 6 years. Treatment with SRT was associated with a threefold reduction in prostate cancer–specific mortality compared with observation (HR, 0.32; p < .001). The outcome of salvage prostate bed radiation therapy was described in a pooled analysis of 2,460 patients from 10 centers.6 The median pre-SRT PSA was 0.5 ng/mL (interquartile range, 0.3–1.1). The 5-year biochemical control rate was 56%, and an association was observed between lower pre-SRT PSA values and improved biochemical control. This has contributed to the widely held belief that earlier SRT might be more effective than later SRT.
Retrospective studies have also found an association between dose to the prostate bed and biochemical control after SRT.6 However, such studies are subject to confounding, and results are now available from SAKK 09/10, a randomized trial of 350 men with BCR after RP comparing 64 Gy in 32 fractions versus 70 Gy in 35 fractions.7 Median PSA at randomization was 0.3 ng/mL. Dose escalation to 70 Gy increased late gastrointestinal toxicity, but no benefit was seen with regard to efficacy. The 6-year freedom from biochemical progression rates were 62% and 61% for 64 Gy versus 70 Gy, respectively. These data support the use of 64 Gy in 32 fractions, or equivalent shorter fractionation schedules, as standard. Even this dose may be more than necessary. EORTC 22911 used 60 Gy in 30 fractions in the adjuvant setting and showed a substantial improvement in biochemical control after RP.8
The pelvic lymph nodes are a common site of recurrence after SRT to the prostate bed.9 Therefore, there has been interest in pelvic nodal radiation therapy in addition to prostate bed treatment. This was studied in the RTOG 0534 trial that was reported at the American Society for Radiation Oncology Annual Meeting in 2019 but has not yet been published in full.10 This was a three-arm randomized trial of 1,792 patients undergoing SRT after RP, including a comparison between radiation therapy to the prostate bed alone versus treatment to the prostate bed plus pelvic nodes. All patients in this comparison received short-term ADT. Five-year freedom from progression was improved by the addition of pelvic nodal radiation therapy: 89.1% versus 82.7% (HR, 0.71; 95% CI, 0.51–0.98; p = .0063). There was also a trend in favor of pelvic nodal radiation therapy with respect to the development of metastatic disease (25 vs. 38 patients; HR, 0.64; 95% CI, 0.39–1.06). Pelvic nodal radiation therapy was well tolerated, with a modest increase in late-grade 3+ toxicity of 6% versus 4.9% for genitourinary events and 1.1% versus 0.4% for gastrointestinal events. Exploratory subgroup analysis generated the hypothesis that the benefit from nodal radiation therapy might be greater in those patients with a higher PSA level before radiation therapy. Although longer-term follow-up will be required to confirm the effect on distant metastases and prostate cancer mortality, this trial provides good evidence to support the use of radiation therapy to the pelvic nodes and the prostate bed.
The outcome of prostate bed radiation therapy might also be improved by the addition of ADT. There are two mature, randomized controlled trials using this approach.11 In the RTOG 9601 trial, 760 men with PSA recurrence after RP were randomly assigned to receive 2 years of bicalutamide or placebo in addition to SRT to the prostate bed. Overall survival at 12 years was 76.3% for bicalutamide versus 71.3% for placebo (HR, 0.77; 95% CI, 0.59–0.99; p = .04). Subsequent subgroup analyses generated the hypothesis that the benefit of bicalutamide may be greater in men with a higher PSA level before radiation therapy,12 as well as in those with a higher Decipher score.13
GETUG-16 tested 6 months of luteinizing hormone-releasing hormone analog treatment in the same setting.14 This trial included 743 patients who were randomly selected to receive prostate bed radiation therapy alone or radiation therapy plus 6 months of goserelin. Not surprisingly, 6 months of ADT led to an improvement in PSA control. There was also a significant improvement in metastasis-free survival (HR, 0.73; 95% CI, 0.54–0.98; p = .0339), but this was not a prespecified endpoint, and the magnitude of this difference is of dubious clinical significance. Furthermore, no difference was seen in overall survival at 12 years (86% for radiation therapy plus goserelin and 85% for radiation therapy alone; HR, 0.93; 95% CI, 0.63–1.39; two-sided p = .73).
In summary, there is good evidence that 2 years of bicalutamide improves overall survival in men having SRT to the prostate bed, but there is no proven advantage for 6 months of ADT. The results of the RADICALS-HD trial are expected this year. That trial recruited 3,000 men who received postoperative radiation therapy to the prostate bed and compared radiation therapy alone versus radiation therapy plus 6 months of ADT versus radiation therapy plus 24 months of ADT (NCT00541047).
MOLECULAR-BASED IMAGING REDEFINING THE DISEASE LANDSCAPE
Molecular imaging has been used to stage patients with prostate cancer, starting with bone scans, 18F-NaF PET, 18F-fluorodeoxyglucose PET, and, more recently, 18F-flucicovine PET. These imaging studies are used to determine the presence of local recurrence, regional nodal involvement, and distant metastases. The vast majority of patients being considered for SRT have no evidence of disease on these imaging modalities, and radiation therapy planning depends on previously defined consensus tumor volumes. This has changed with the introduction of radiopharmaceuticals that target PSMA, a transmembrane protein that is overexpressed on prostate cancer cells.
Two PSMA PET radiopharmaceuticals are currently approved by the U.S. Food and Drug Administration: DCFPyL (18F-piflufolostat) and 68Ga-PSMA-11 (68Ga-gozetotide). The compounds performed similarly in their respective phase III studies in the BCR setting, with positive predictive values ranging between 84% and 92%.15,16 These two imaging agents have similar biodistributions and are considered interchangeable by National Comprehensive Cancer Network and Society of Nuclear Medicine and Molecular Imaging guidelines.17,18
Additionally, a number of compounds, including 18F-PSMA-1007 (NCT04742361) and 18F-rh-PSMA-7.3 (NCT04186845 and NCT04186819) are in phase III clinical trials that should lead to additional approvals. In general, these compounds appear to perform similarly in the initial staging and biochemically recurrent settings and should be considered a class. One potentially important difference is that 18F-PSMA-1007 has predominantly hepatobiliary clearance and, therefore, little activity in the bladder.19 This might be a benefit for detection of local recurrence that may be obscured by high activity in the bladder.
For radiation oncologists using PSMA PET to plan for SRT, it is important to realize that there are many reasons for uptake that do not represent prostate cancer. Although PSMA PET has better interreader agreement than other imaging modalities, including fluciclovine,20,21 and although there are well-described criteria for positive disease,22 interpretation does take experience. The first issue is ganglia.23 Presacral and para-aortic ganglia can mimic nodal disease, and knowledge of normal anatomy is important so as to not irradiate normal ganglia. The second issue is bone lesions. In general, solitary rib lesions with low uptake are common and should be considered benign.24 Additionally, there are a number of other bone lesions with known uptake on PSMA PET, including hemangiomas, Paget disease, and fibrous dysplasia.25
In a cohort of patients eligible for SRT with a PSA less than 2.0 ng/dL, PSMA PET detected recurrence in 50% of patients, with 30% of patients having disease outside of the consensus tumor volumes.26 In a separate cohort with a PSA less than 1.0 ng/dL, PSMA PET detected disease outside of the consensus tumor volume in 19% of patients.27 The most common locations of recurrence outside of the consensus tumor volumes are the bones and perirectal lymph nodes. The results of studies like these led to the revision of the NRG Consensus Atlas for pelvic nodal volumes.28 The impact of the additional sites of disease detected by PSMA PET is being evaluated in several prospective trials, including the PSMA-SRT trial, in which postoperative patients were randomly selected to receive conventional imaging and PSMA PET.29 The EMPIRE-1 study was performed using 18F-fluciclovine, which demonstrated improved biochemical-free survival with molecular imaging compared with conventional imaging (63.0% vs. 75.5%; p = .0028) for radiation therapy planning.30 The ORIOLE trial demonstrated that patients who had PSMA-positive disease that was not included in the treatment plan experienced disease recurrence faster than did those whose disease was covered.31
TIMING AND INTENSITY OF ANDROGEN DEPRIVATION THERAPY IN BIOCHEMICAL RECURRENCE
For men with BCR who have received postoperative SRT or who are not candidates for SRT, ADT is an option. When to initiate ADT (early vs. delayed) and how to give ADT (intermittent vs. continuous) must be considered. Much of the controversy around ADT therapy stems from the fact that the benefits are unclear, BCR is generally an asymptomatic state, and the initiation of ADT has important effects upon quality of life and long-term health consequences.32
The question of when to initiate ADT was examined in a retrospective analysis of the CaPSURE registry, which compared immediate versus deferred ADT in the BCR population. Men who started ADT within 3 months of BCR were retrospectively assigned to the “immediate arm,” and those who started ADT when they presented with metastases were assigned to the “delayed arm.”33 This analysis demonstrated no difference in overall survival (HR, 0.91; 95% CI; 0.52–1.60) or prostate cancer–specific mortality (HR, 1.09; 95% CI, 0.31–3.78) between patients undergoing immediate versus delayed ADT.
The TOAD trial prospectively examined the impact of immediate versus delayed ADT treatment in a randomized phase III trial.34 Participants were eligible if they had an increasing PSA after prior curative therapy, including SRT, or if they were not suitable for curative therapy. Men in the immediate ADT group received ADT starting 8 weeks from randomization, whereas men in the delayed ADT group were encouraged to start at least 2 years after randomization unless they developed symptoms, evidence of metastatic disease, or a PSA doubling time of less than 6 months. For the entire cohort, the hazard ratio was 0.55 for the primary endpoint of overall survival comparing immediate ADT with delayed ADT (95% CI, 0.3–1.00; p = .05), and it was 0.58 for the PSA-relapse–only cohort (95% CI, 0.30–1.12; p = .10). There was also no difference for prostate cancer–specific mortality when comparing immediate with delayed ADT. However, there was a longer time to local and distant progression for men receiving immediate ADT. Unfortunately, this trial had several limitations, including underenrollment and the grouping of patients with BCR and those with locally advanced disease who were unable to pursue curative therapy. The findings from this trial indicate that immediate ADT delayed local and distant progression; however, there was no clear benefit for overall survival or prostate cancer–specific mortality in the PSA recurrent population.
Given the lack of a clear overall survival benefit in the general BCR population, multiple studies have tried to determine which patients with BCR are at highest risk for progression and death and, therefore, may benefit from ADT therapy. Antonarakis et al reported on the natural history of 450 men with BCR after RP to better understand the factors associated with metastasis-free survival.35 In their cohort, the median time from surgery to BCR was 3 years and the median metastasis-free survival after PSA recurrence was 10 years (95% CI, 8.0–14.0). They reported that PSA doubling time was the strongest predictor of metastasis, finding that men with short PSA doubling times were more likely to develop metastases (HR, 33.3; 95% CI, 16.4–67.4 for PSA doubling time less than 3 months and HR, 8.0; 95% CI, 4.5–14.1 for PSA doubling time of 3 to 9 months) compared with men with a PSA doubling time greater than 15 months.
In addition to PSA doubling time, other factors that have been identified for risk of disease progression and prostate cancer–specific mortality are initial PSA, Gleason score, pathologic findings at RP (i.e., seminal vesicle involvement, extraprostatic extension, and intraductal carcinoma), time to BCR, and PSA level at BCR.36–39 Current guidelines suggest that intermittent ADT therapy can be offered to patients with higher-risk BCR, with most definitions of high risk including PSA doubling time less than 10 to 12 months, Gleason score 8 or greater, or biochemical relapse interval of up to 18 months.40–42
The question of how to initiate ADT was addressed by Crook et al,43 who, in a noninferiority trial, compared intermittent ADT with continuous ADT in men with BCR after radiation therapy. Intermittent ADT consisted of ADT therapy for 8-month cycles, followed by observation, with ADT reinitiated when PSA reached more than 10 ng/mL. There was no difference in the primary endpoint of overall survival when comparing intermittent ADT versus continuous ADT (HR, 1.02; 95% CI, 0.86–1.21); no differences remained after stratifying based on Gleason score, PSA level, or time since completion of radiation therapy. Notably, those treated with intermittent ADT reported better quality-of-life scores. Based upon these findings and other data,44 intermittent dosing is the preferred strategy when initiating ADT for BCR.
Clear guidance about when to initiate ADT for men with BCR remains elusive, highlighting the importance of discussing the pros and cons of early versus delayed ADT with patients. The side effects and long-term health consequences of ADT must be weighed against the potential for disease progression and patient preferences. Given the controversy surrounding the use of ADT, there has been interest in exploring non-ADT options for the treatment of BCR. Table 1 lists ongoing trials that are exploring ADT alternatives.
TABLE 1.
Ongoing Trials for Treatment of Biochemical Recurrence That Do Not Involve the Use of Androgen Deprivation Therapy
| Identifier | Name | Phase | Intervention | Length of Treatment |
|---|---|---|---|---|
| NCT04019964 | Nivolumab in Biochemically Recurrent dMMR Prostate Cancer | II | Nivolumab | Continuous |
| NCT03047135 | Olaparib in Men With High-Risk Biochemically-Recurrent Prostate Cancer Following Radical Prostatectomy, With Integrated Biomarker Analysis | II | Olaparib | Continuous |
| NCT04336943 | Durvalumab and Olaparib for the Treatment of Prostate Cancer in Men Predicted to Have a High Neoantigen Load | II | Durvalumab, with or without olaparib | 6 months |
| NCT04206319 | Radium-223 in Biochemically Recurrent Prostate Cancer | II | Radium-223 | 6 months |
| NCT02649439 | Prostvac in Patients With Biochemically Recurrent Prostate Cancer | II | Prostvac (PSA-targeted cancer vaccine) | 6 months |
| NCT03315871 | Combination Immunotherapy in Biochemically Recurrent Prostate Cancer | II | Prostvac, bintrafusp alfa, and CV301 (cancer vaccine targeting MUC1 and CEA) | 7 months |
| NCT03374254 | Nivolumab in Patients With High-Risk Biochemically Recurrent Prostate Cancer | II | Nivolumab | Continuous |
| NCT05077098 | Study of ADXS-504 Immunotherapy for Recurrent Prostate Cancer | I | ADXS-504 (Listeria-based multineoantigen vector) | 21 Weeks |
| NCT03513211 | Phase I/II Study of Hydroxychloroquine With Itraconazole With Biochemically Recurrent Prostate Cancer (HITMAN-PC) | I/II | Itraconazole and hydroxychloroquine | Continuous |
| NCT04519879 | White Button Mushroom Sup for the Reduction of PSA in Pts With Biochemically Recurent or Therapy Naive Fav Risk Prostate Cancer | II | White button mushroom supplement | 48 weeks |
| NCT03810105 | A Study of Olaparib and Durvalumab in Prostate Cancer | II | Durvalumab with olaparib | Continuous |
Abbreviations: dMMR, deficient mismatch repair; PSA, prostate-specific antigen.
INTEGRATING PSMA PET SCANS INTO THE TREATMENT OF BIOCHEMICAL RECURRENCE
As discussed above, the use of PSMA PET scan allows for earlier detection of disease on imaging, leading to stage migration. Patients who historically would be considered N0 and M0 are being recharacterized as N1- and M1-positive patients. With the increased use of PSMA PET, there is a large ongoing reclassification of patients, with resultant changes in treatment plans. This change in management is demonstrated by the results from the CONDOR trial, whose main endpoint was determining the diagnostic performance of PSMA PET; however, the investigators also reported changes in the treatment plan based upon PSMA PET.15 They found that 64% of patients had a change in their care plan based upon the results of the PSMA scan. Notably, not all of the changes in care included escalation to systemic therapy, with 28% adding systemic therapy to previously planned local salvage therapy, 21% transitioning from systemic therapy to local salvage therapy, 24% initiating therapy when observation had been planned, and 4% transitioning to observation from planned treatment. Fendler et al reported similar results when surveying physicians treating patients with BCR undergoing PSMA PET scans; PET results changed management in 260 of 382 patients (68%).45
Prior to the use of PSMA PET, SRT was normally given at the time of PSA recurrence, and it was believed that earlier treatment is better than later treatment. However, that practice has been questioned in light of using PSMA PET to detect the site of recurrence in men with BCR. Recurrence can often be detected at relatively low PSA levels, and it is striking that the site of recurrence is often outside the prostate bed (Fig. 1).27,46 This has led to the possibility of later image-directed SRT as an alternative to earlier prostate bed radiation therapy for PSA recurrence alone. Although delaying SRT until radiologic evidence of recurrence might detract from its efficacy, advantages of this delay include targeting of radiation therapy to the site of recurrence and omission of radiation therapy in patients with recurrent polymetastatic disease.
FIGURE 1. Example of a Patient With No Detectable Disease on Conventional Imaging and Extensive Nodal Disease Detected on PSMA PET.
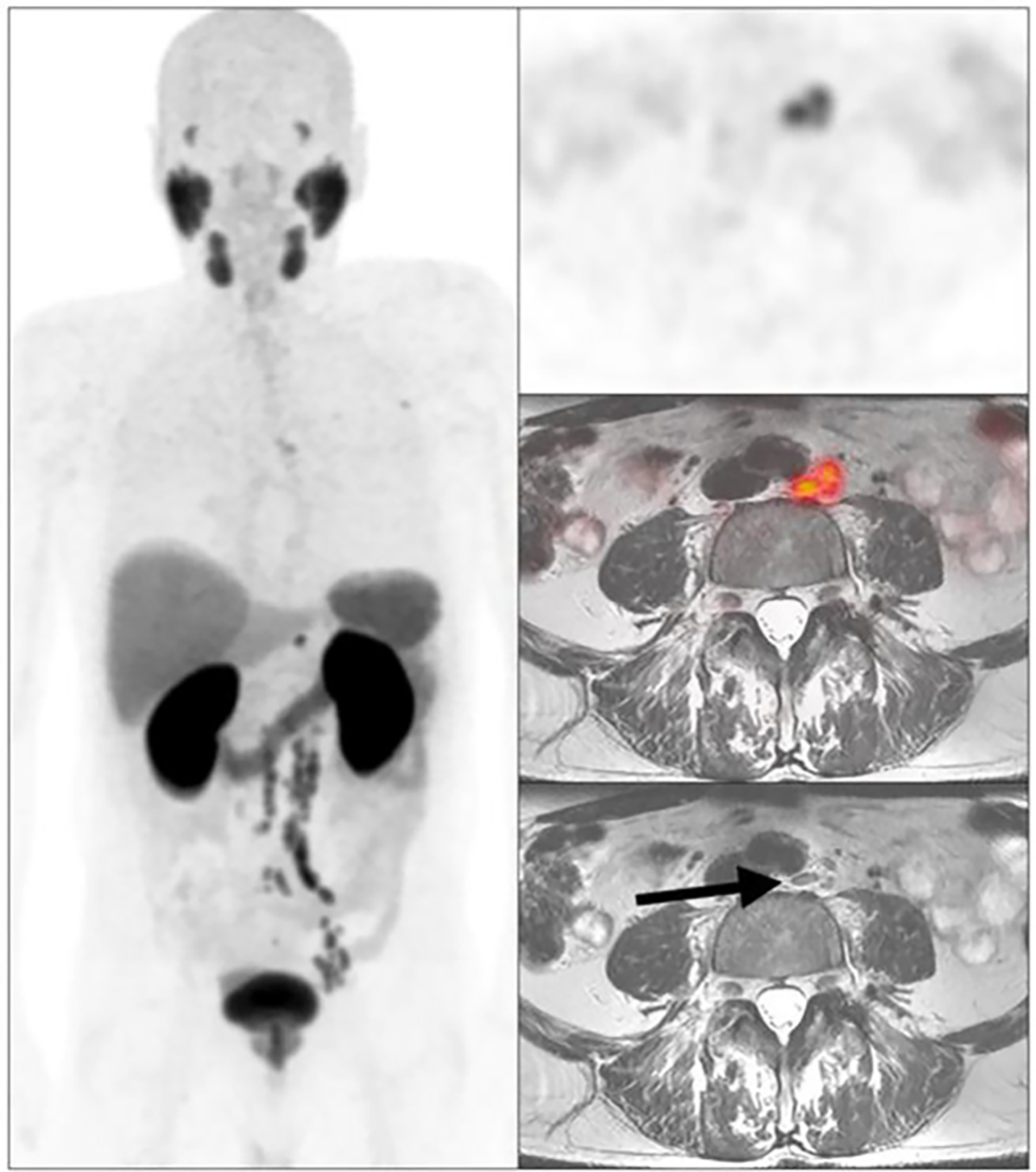
Arrow denotes nonpathologically enlarged lymph node.
Evidence for PET imaging–directed therapy comes from the STOMP trial, which randomly assigned patients with BCR to observation or metastasis-directed therapy based upon choline PET imaging.47 Using ADT-free survival as a primary endpoint, they reported a median ADT-free survival of 21 months for the metastasis-directed therapy group and 13 months for the surveillance group (HR, 0.6; 95% CI, 0.4–0.9). This led to the practice of including PSMA PET–positive disease in radiation plans. However, PSMA PET positivity is not the only factor to be considered. Emmett et al provide evidence supporting the use of radiation therapy with a negative PSMA PET.48 They reported 3-year freedom from progression in a prospective nonrandomized study of men with BCR post-RP who underwent PSMA PET scans. They found that, in men with negative PSMA PET scans, the 3-year freedom from progression was higher if they received radiation therapy (82.5%) than if they were observed without treatment (66%), indicating that there may be a freedom from progression benefit in treating PSMA PET–negative patients with BCR.
In summary, PSMA PET imaging has clearly demonstrated an increased sensitivity for detecting disease earlier and at lower PSAs. It remains to be seen whether survival is improved when treatment plans are changed based upon PSMA PET findings when conventional imaging is negative. Questions remain regarding how the presence of low-volume nodal and metastatic PSMA-detectable disease should impact the use of SRT, the duration of ADT, and the use of androgen receptor–targeted therapies. Ongoing trials are underway to address this question, examining how PSMA-guided SRT compares with conventional SRT, as well as how PSMA-guided SRT, with or without ADT, impacts survival and quality of life (NCT05053152, NCT03582774, NCT03525288, NCT04423211).
PRACTICAL APPLICATIONS.
Salvage radiation to the prostate bed and pelvic lymph nodes is the standard approach to treating biochemical recurrence.
The concurrent use of antiandrogen therapy with radiation therapy has also demonstrated improved overall survival.
Prostate-specific membrane antigen PET scans have the ability to detect recurrent disease at lower prostate-specific antigen levels and improve progression-free survival when these lesions are covered in the radiation treatment plan.
The decision to initiate androgen deprivation factors depends on multiple factors, including Gleason score, initial prostate-specific antigen, prostate-specific antigen doubling time, and patient preference.
If androgen deprivation therapy is initiated, intermittent therapy is preferable to continuous therapy.
Footnotes
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST AND DATA AVAILABILITY STATEMENT
Disclosures provided by the authors and data availability statement (if applicable) are available with this article at DOI https://doi.org/10.1200/EDBK_351033.
REFERENCES
- 1.Suardi N, Porter CR, Reuther AM, et al. A nomogram predicting long-term biochemical recurrence after radical prostatectomy. Cancer.2008;112:1254–1263. [DOI] [PubMed] [Google Scholar]
- 2.Chun FK, Graefen M, Zacharias M, et al. Anatomic radical retropubic prostatectomy-long-term recurrence-free survival rates for localized prostate cancer. World J Urol. 2006;24:273–280. [DOI] [PubMed] [Google Scholar]
- 3.Roach M, Hanks G, Thames H, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: Recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65:965–974. [DOI] [PubMed] [Google Scholar]
- 4.Cookson MS, Aus G, Burnett AL, et al. Variation in the definition of biochemical recurrence in patients treated for localized prostate cancer: the American Urological Association Prostate Guidelines for Localized Prostate Cancer Update Panel report and recommendations for a standard in the reporting of surgical outcomes. J Urol. 2007;177:540–545. [DOI] [PubMed] [Google Scholar]
- 5.Trock BJ, Han M, Freedland SJ, et al. Prostate cancer-specific survival following salvage radiotherapy vs observation in men with biochemical recurrence after radical prostatectomy. JAMA. 2008;299:2760–2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tendulkar RD, Agrawal S, Gao T, et al. Contemporary update of a multi-Institutional predictive nomogram for salvage radiotherapy after radical prostatectomy. J Clin Oncol. 2016;34:3648–3654. [DOI] [PubMed] [Google Scholar]
- 7.Ghadjar P, Hayoz S, Bernhard J, et al. Swiss Group for Clinical Cancer Research (SAKK). Dose-intensified versus conventional-dose salvage radiotherapy for biochemically recurrent prostate cancer after prostatectomy: The SAKK 09/10 randomized phase 3 trial. Eur Urol. 2021;80:306–315. [DOI] [PubMed] [Google Scholar]
- 8.Bolla M, van Poppel H, Tombal B, et al. ; European Organisation for Research and Treatment of Cancer, Radiation Oncology and Genito-Urinary Groups. Postoperative radiotherapy after radical prostatectomy for high-risk prostate cancer: long-term results of a randomised controlled trial (EORTC trial 22911). Lancet. 2012;380:2018–2027. [DOI] [PubMed] [Google Scholar]
- 9.Brand DH, Parker JI, Dearnaley DP, et al. Patterns of recurrence after prostate bed radiotherapy. Radiother Oncol. 2019;141:174–180. [DOI] [PubMed] [Google Scholar]
- 10.Pollack A, Karrison T, Balogh A, et al. Short term androgen deprivation therapy without or with pelvic lymph node treatment added to prostate bed only salvage radiotherapy: The NRG Oncology/RTOG 0534 SPPORT trial. Int J Radiat Oncol Biol Phys. 2018;102:1605. [Google Scholar]
- 11.Shipley WU, Seiferheld W, Lukka HR, et al. ; NRG Oncology RTOG. Radiation with or without antiandrogen therapy in recurrent prostate cancer. N Engl J Med. 2017;376:417–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dess RT, Sun Y, Jackson WC, et al. Association of presalvage radiotherapy PSA levels after prostatectomy with outcomes of long-term antiandrogen therapy in men with prostate cancer. JAMA Oncol. 2020;6:735–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng FY, Huang HC, Spratt DE, et al. Validation of a 22-gene genomic classifier in patients with recurrent prostate cancer: an ancillary study of the NRG/RTOG 9601 randomized clinical trial. JAMA Oncol. 2021;7:544–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carrie C, Magné N, Burban-Provost P, et al. Short-term androgen deprivation therapy combined with radiotherapy as salvage treatment after radical prostatectomy for prostate cancer (GETUG-AFU 16): a 112-month follow-up of a phase 3, randomised trial. Lancet Oncol. 2019;20:1740–1749. [DOI] [PubMed] [Google Scholar]
- 15.Morris MJ, Rowe SP, Gorin MA, et al. ; CONDOR Study Group. Diagnostic performance of 18F-DCFPyL-PET/CT in men with biochemically recurrent prostate cancer: results from the CONDOR phase III, multicenter study. Clin Cancer Res. 2021;27:3674–3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fendler WP, Calais J, Eiber M, et al. Assessment of 68Ga-PSMA-11 PET accuracy in localizing recurrent prostate cancer: a prospective single-arm clinical trial. JAMA Oncol. 2019;5:856–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Prostate Cancer (Version 3.2022). https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Accessed March 2, 2022.
- 18.Jadvar H, Calais J, Fanti S, et al. Appropriate use criteria for prostate-specific membrane antigen PET imaging. J Nucl Med. 2022;63:59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giesel FL, Knorr K, Spohn F, et al. Detection efficacy of 18F-PSMA-1007 PET/CT in 251 patients with biochemical recurrence of prostate cancer after radical prostatectomy. J Nucl Med. 2019;60:362–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Calais J, Ceci F, Eiber M, et al. 18F-fluciclovine PET-CT and 68Ga-PSMA-11 PET-CT in patients with early biochemical recurrence after prostatectomy: a prospective, single-centre, single-arm, comparative imaging trial. Lancet Oncol. 2019;20:1286–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fendler WP, Calais J, Allen-Auerbach M, et al. 68Ga-PSMA-11 PET/CT interobserver agreement for prostate cancer assessments: an international multicenter prospective study. J Nucl Med. 2017;58:1617–1623. [DOI] [PubMed] [Google Scholar]
- 22.Eiber M, Herrmann K, Calais J, et al. Prostate Cancer Molecular Imaging Standardized Evaluation (PROMISE): Proposed miTNM classification for the interpretation of PSMA-ligand PET/CT. J Nucl Med. 2018;59:469–478. [DOI] [PubMed] [Google Scholar]
- 23.Rischpler C, Beck TI, Okamoto S, et al. 68Ga-PSMA-HBED-CC uptake in cervical, celiac, and sacral ganglia as an important pitfall in prostate cancer PET imaging. J Nucl Med. 2018;59:1406–1411. [DOI] [PubMed] [Google Scholar]
- 24.Chen MY, Franklin A, Yaxley J, et al. Solitary rib lesions showing prostate-specific membrane antigen (PSMA) uptake in pre-treatment staging 68Ga-PSMA-11 positron emission tomography scans for men with prostate cancer: benign or malignant? BJU Int. 2020;126:396–401. [DOI] [PubMed] [Google Scholar]
- 25.de Galiza Barbosa F, Queiroz MA, Nunes RF, et al. Nonprostatic diseases on PSMA PET imaging: a spectrum of benign and malignant findings. Cancer Imaging. 2020;20:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boreta L, Gadzinski AJ, Wu SY, et al. Location of recurrence by gallium-68 PSMA-11 PET scan in prostate cancer patients eligible for salvage radiotherapy. Urology. 2019; 129:165–171. [DOI] [PubMed] [Google Scholar]
- 27.Calais J, Czernin J, Cao M, et al. 68Ga-PSMA-11 PET/CT mapping of prostate cancer biochemical recurrence after radical prostatectomy in 270 patients with a PSA level of less than 1.0 ng/mL: impact on salvage radiotherapy planning. J Nucl Med. 2018;59:230–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hall WA, Paulson E, Davis BJ, et al. NRG Oncology updated international consensus atlas on pelvic lymph node volumes for intact and postoperative prostate cancer. Int J Radiat Oncol Biol Phys. 2021;109:174–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Calais J, Czernin J, Fendler WP, et al. Randomized prospective phase III trial of 68Ga-PSMA-11 PET/CT molecular imaging for prostate cancer salvage radiotherapy planning [PSMA-SRT]. BMC Cancer. 2019;19:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jani AB, Schreibmann E, Goyal S, et al. 18F-fluciclovine-PET/CT imaging versus conventional imging alone to guide postprostatectomy salvage radiotherapy for prostate cancer (EMPIRE-1): a single centre, open-label, phase 2/3 randomised controlled trial. Lancet. 2021;397:1895–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Phillips R, Shi WY, Deek M, et al. Outcomes of observation vs stereotactic ablative radiation for oligometastatic prostate cancer: the ORIOLE phase 2 randomized clinical trial. JAMA Oncol. 2020;6:650–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Basaria S, Lieb J II, Tang AM, et al. Long-term effects of androgen deprivation therapy in prostate cancer patients. Clin Endocrinol (Oxf). 2002;56:779–786. [DOI] [PubMed] [Google Scholar]
- 33.Garcia-Albeniz X, Chan JM, Paciorek A, et al. Immediate versus deferred initiation of androgen deprivation therapy in prostate cancer patients with PSA-only relapse. An observational follow-up study. Eur J Cancer. 2015;51:817–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duchesne GM, Woo HH, Bassett JK, et al. Timing of androgen-deprivation therapy in patients with prostate cancer with a rising PSA (TROG 03.06 and VCOG PR 01–03 [TOAD]): a randomised, multicentre, non-blinded, phase 3 trial. Lancet Oncol. 2016;17:727–737. [DOI] [PubMed] [Google Scholar]
- 35.Antonarakis ES, Feng Z, Trock BJ, et al. The natural history of metastatic progression in men with prostate-specific antigen recurrence after radical prostatectomy: long-term follow-up. BJU Int. 2012;109:32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Antonarakis ES, Chen Y, Elsamanoudi SI, et al. Long-term overall survival and metastasis-free survival for men with prostate-specific antigen-recurrent prostate cancer after prostatectomy: analysis of the Center for Prostate Disease Research National Database. BJU Int. 2011;108:378–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brockman JA, Alanee S, Vickers AJ, et al. Nomogram predicting prostate cancer-specific mortality for men with biochemical recurrence after radical prostatectomy. Eur Urol. 2015;67:1160–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.D’Amico AV, Moul JW, Carroll PR, et al. Surrogate end point for prostate cancer-specific mortality after radical prostatectomy or radiation therapy. J Natl Cancer Inst. 2003;95:1376–1383. [DOI] [PubMed] [Google Scholar]
- 39.Kimura K, Tsuzuki T, Kato M, et al. Prognostic value of intraductal carcinoma of the prostate in radical prostatectomy specimens. Prostate. 2014;74:680–687. [DOI] [PubMed] [Google Scholar]
- 40.Virgo KS, Rumble RB, de Wit R, et al. Initial management of noncastrate advanced, recurrent, or metastatic prostate cancer: ASCO guideline update. J Clin Oncol. 2021;39:1274–1305. [DOI] [PubMed] [Google Scholar]
- 41.Lowrance WT, Breau RH, Chou R, et al. Advanced prostate cancer: AUA/ASTRO/SUO guideline, PART I. J Urol. 2021;205:14–21. [DOI] [PubMed] [Google Scholar]
- 42.Van den Broeck T, van den Bergh RCN, Arfi N, et al. Prognostic value of biochemical recurrence following treatment with curative intent for prostate cancer: A systematic review. Eur Urol. 2019;75:967–987. [DOI] [PubMed] [Google Scholar]
- 43.Crook JM, O’Callaghan CJ, Duncan G, et al. Intermittent androgen suppression for rising PSA level after radiotherapy. N Engl J Med. 2012;367:895–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Botrel TE, Clark O, dos Reis RB, et al. Intermittent versus continuous androgen deprivation for locally advanced, recurrent or metastatic prostate cancer: a systematic review and meta-analysis. BMC Urol. 2014;14:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fendler WP, Ferdinandus J, Czernin J, et al. Impact of 68Ga-PSMA-11 PET on the management of recurrent prostate cancer in a prospective single-arm clinical trial. J Nucl Med. 2020;61:1793–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abghari-Gerst M, Armstrong WR, Nguyen K, et al. A comprehensive assessment of 68Ga-PSMA-11 PET in biochemically recurrent prostate cancer: results from a prospective multi-center study in 2005 patients. J Nucl Med. 2022;63:567–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ost P, Reynders D, Decaestecker K, et al. Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence: a prospective, randomized, multicenter phase II trial. J Clin Oncol. 2018;36:446–453. [DOI] [PubMed] [Google Scholar]
- 48.Emmett L, Tang R, Nandurkar R, et al. 3-Year freedom from progression after 68Ga-PSMA PET/CT-triaged management in men with biochemical recurrence after radical prostatectomy: results of a prospective multicenter trial. J Nucl Med. 2020;61:866–872. [DOI] [PubMed] [Google Scholar]


