Abstract
Objective
To investigate whether bipolar androgen therapy (BAT), involving rapid cyclic administration of high-dose testosterone, as a novel treatment for metastatic castration-resistant prostate cancer (mCRPC) promotes improvements in body composition and associated improvements in lipid profiles and quality of life.
Patients and Methods
Men from two completed trials with computed tomography imaging at baseline and after three cycles of BAT were included. Cross-sectional areas of psoas muscle, visceral and subcutaneous fat were measured at the L3 vertebral level. Functional Assessment of Chronic Illness Therapy – Fatigue questionnaire and 36-item short-form health survey were used to assess quality of life.
Results
The 60 included patients lost a mean (sd) of 7.8 (8.2)% of subcutaneous fat, 9.8 (18.2)% of visceral fat, and gained 12.2 (6.7)% muscle mass. Changes in subcutaneous and visceral fat were positively correlated with each other (Spearman’s correlation coefficient 0.58, 95% confidence interval 0.35–0.71) independent of the effects of age, body mass index, and duration of androgen-deprivation therapy. Energy, physical function, and measures of limitations due to physical health were all significantly improved at 3 months. The improvements in body composition were not correlated with decreases in lipid levels or observed improvements in quality of life.
Conclusions
In the present study, BAT was associated with significant improvements in body composition, lipid parameters, and quality of life. This has promising implications for the long-term health of men with mCRPC.
Keywords: prostate cancer, body composition, androgen-deprivation therapy, cancer survivorship, metastatic castration-resistant prostate cancer, bipolar androgen therapy, #PCSM, #ProstateCancer, #uroonc
Introduction
The primary treatment for metastatic prostate cancer is androgen-deprivation therapy (ADT), which is continued indefinitely once started [1]. The low testosterone state induced by ADT has negative impacts on body composition, causing an increase in body fat, a decrease in muscle mass, and alteration of lipid profiles [2–4]. ADT has been associated with a loss of ~5% of muscle mass and a gain of up to 10% of body fat, including both subcutaneous and visceral fat [2–4]. Furthermore, it has been associated with increases in total cholesterol, low- and high-density lipoprotein (LDL and HDL) levels, as well as triglyceride (TG) levels [5]. These changes are relevant because cardiovascular disease (CVD) is a major cause of morbidity and mortality in men with prostate cancer and these alterations in CVD risk factors may contribute to the possible link between CVD and ADT [6,7]. Furthermore, low muscle mass, i.e. sarcopenia, and high levels of subcutaneous fat both associated with ADT, have been associated with poor outcomes in metastatic castration-resistant prostate cancer (mCRPC), including lower overall survival among men with advanced prostate cancer [8–10].
Newer oral therapies in prostate cancer targeting the androgen axis such as abiraterone, a cytochrome P450 17A (CYP17A) inhibitor, and enzalutamide, an androgen receptor antagonist, have improved overall survival for men with mCRPC [11,12]. However, the addition of these agents to ADT is further associated with negative effects on body composition such as skeletal muscle loss and body fat increases, which may contribute to the increased risk of CVD in these patients [8,13–15].
Bipolar androgen therapy (BAT) is the cyclic administration of high-dose testosterone in men who remain on long-term LHRH agonists or antagonists [16]. The effect of this therapy is rapid cycling between the polar extremes of supraphysiological and near-castrate serum levels of testosterone [16]. This strategy has been associated with tumour responses in men with mCRPC [17,18]. We hypothesised that this rapid cycling would also promote improvements in body composition, lipid profiles, and quality of life that would be a counter to the effects of anti-androgen therapies.
Patients and methods
Patients
Data were pooled from two completed clinical trials at a single institution: TRANSFORMER (NCT02286921) and RESTORE (NCT02090114). The TRANSFORMER trial was a randomised trial in men with progressive mCRPC after treatment with abiraterone who received either BAT or enzalutamide, with the option for cross-over after progression [19]. Men treated with BAT on trial were screened for eligibility. In both trials, 400 mg testosterone cypionate was given intramuscularly and LHRH agonists were continued. The RESTORE trial was a single-arm trial of men who had progressive disease after either abiraterone or enzalutamide and who then were treated with BAT [17,20]. Patients who received BAT therapy prior to enrolment (n = 11), who were not previously treated with abiraterone or enzalutamide (n = 24), or did not have CT imaging performed within the Johns Hopkins medical system (n = 10) were excluded. Participants in both protocols had CT imaging at baseline (just prior to start of BAT) and after three 28-day cycles of BAT.
Anthropometry based on CT Image Analyses
The CT scans taken at baseline and 3 months were analysed by three readers of varying training levels: attending radiologist (R.G.), 2 years postgraduate level (J.T.) and second year medical student (V.D.). All readers were blinded to patient identity, characteristics, and study group, and to the measurements made by the other readers. OsiriX v10.0.4 (Pixmeo Sàrl, Bernex, Switzerland) software was utilised to perform the image analysis. A slice at the L3 vertebral level, determined by the attending radiologist, was used by all readers to make measurements, consistent with previous studies and because analyses here are associated with estimates of whole body composition measurements [21]. Readers first generated a closed polygon between the visceral adipose layer and abdominal muscle layer and then generated regions of interest (ROIs) using attenuation values for the various tissue types (muscle: −30 to 150 Hounsfield units [HU], subcutaneous adipose tissue: −190 to −30 HU, and visceral adipose tissue: −150 to −50 HU) [22]. The psoas muscle was individually segmented using a closed polygon around the bilateral psoas. Cross-sectional areas (cm2) of psoas muscle, visceral adipose tissue, and subcutaneous adipose tissue were measured using the newly created ROIs. Inter-reader variability was calculated and the measurements obtained by the three readers were averaged.
Measures
Lipid panels were obtained as part of the trial protocol at start of testosterone and at 3 months after BAT treatment. Optimal lipid parameters were defined based on the National Cholesterol Education Program/Adult Treatment Panel III Guidelines [23]. This categorises the thresholds for optimal LDL levels as <100 mg/dL, HDL levels >60 mg/dL, and TG levels <150 mg/dL. HDL levels <40 mg/dL are considered low. Sarcopenia was defined as having a Skeletal Muscle Index (SMI), based on the area of the psoas muscle at L3, divided by the height in meters (cm2/m2) of <55 cm2/m2, which is consistent with consensus definitions on cachexia in patients with cancer [24]. The Functional Assessment of Chronic Illness Therapy – Fatigue (FACIT-F) scale and the 36-item short-form health survey (SF-36) questionnaires were used to measure fatigue, energy, physical functioning levels, emotional well-being and limitations due to physical health at baseline and at 3 months [25–27].
Statistical Analysis
Patient characteristics were summarised via descriptive statistics (mean/SD or median/range for continuous variables, and frequency/percentage for categorical variables). Consistencies of body composition measures from three different readers at each time point were first assessed via intraclass correlation coefficients (ICCs). As a result of the high yield ICC (>0.96), the mean value of body composition measures among the three readers was utilised for each individual patient at each time point. Changes in body composition were summarised by the percentage change from baseline as a way of individual normalisation. The means of the baseline and 3-month values for the outcomes were reported and the distributions of differences, from baseline to 3 months, were tested via paired Wilcoxon tests. Associations between changes of body composition measures, and the associations between body composition changes and lipid level changes or changes of quality-of-life outcomes were assessed via Spearman’s correlation coefficients. In addition, linear regression models were used for testing the associations between changes of outcomes (changes of body composition outcomes, changes of lipid outcomes, and changes of quality-of-life outcomes) and patient characteristics (pre-specified for patient age at baseline, body mass index [BMI], and duration of prior ADT). The associations between changes of body composition measures and changes of lipid outcomes with adjustment of patient characteristics were examined via multivariable linear regression models. The McNemar test was used for assessing the differences in the percentage of sarcopenic patients before and after treatment. Statistical analyses were conducted using R, version 3.6.0 (R Foundation for Statistical Computing, Vienna, Austria). All P values were calculated using two-sided tests with a threshold of 0.05 significance level.
Results
A total of 60 patients were included in the final cohort. Baseline patient characteristics are shown in Table 1. The median age of the cohort was 73 years and 85% of the patients were White. Two-thirds of the population had localised disease at initial diagnosis, while one-third had de novo metastatic disease. Additionally, 30% had Gleason Grade ≤7, 27% had Gleason 8 disease and 43% had Gleason 9 or 10 disease. The mean (sd) time on ADT was 4 (5.7) years. All patients had received prior abiraterone or enzalutamide and only three patients (5%) had received any prior chemotherapy. The mean (sd) BMI at baseline was 29.3 (4.6) kg/m2, with 17% classified as having a normal BMI, 43% as overweight and 40% as obese. Bone metastases were the most common site of metastatic disease (48 patients, 80%), followed by lymph node metastases (23, 38%) and visceral disease (three, 5%), with 13 patients having more than one site of disease (22%). The median (interquartile range) baseline PSA was 28.6 (9.6–68.2) ng/mL and 27 of the patients had PSA responses (45%) during these 3 months. There was excellent interclass concordance between the readers for each of the measures of body composition (Table 2 with all ICCs >0.96).
Table 1.
Baseline characteristics of patients with mCRPC who were treated with at least 3 months of BAT as second-line and later therapy on RESTORE (n = 45) or TRANSFORMER (n = 15) (cohorts A/B).
| Variable | Value |
|---|---|
| Age at start of BAT, years, median (range) | 73, (50–87) |
| Race, n (%) | |
| White | 51 (85) |
| Black | 5 (8) |
| Other | 4 (7) |
| Stage at diagnosis of prostate cancer | |
| Localised | 40 (67) |
| metastatic | 20 (33) |
| Gleason score | |
| 6 | 2 (3) |
| 7 | 16 (27) |
| 8 | 16 (27) |
| 9–10 | 26 (43) |
| Duration of prior ADT, years, mean (sd) | 4 (5.7) |
| Year of BAT treatment, n (%) | |
| 2014–2016 | 44 (73) |
| 2017–2018 | 16 (27) |
| Sites of metastatic disease, n (%) | |
| Any bone metastases | 48 (80) |
| Any lymph node metastasis | 23 (38) |
| Visceral | 3 (5) |
| Prior chemotherapy, n (%) | 3 (5) |
| Prior abiraterone or enzalutamide, n (%) | 60 (100) |
| BMI at baseline (start of BAT), kg/m2, mean (sd) | 29.3 (4.6) |
| BMI (kg/m2) category at baseline (start of BAT), n (%) | |
| <25 | 10 (17) |
| 25–30 | 26 (43) |
| ≥30 | 24 (40) |
| PSA level, ng/mL | |
| Baseline PSA, mean (sd) | 51 (67) |
| Baseline PSA, median (interquartile range) | 29 (10–68) |
| PSA responses, n (%) | |
| Any response (decline in PSA from baseline) | 27 (45) |
| No response | 33 (55) |
| Bone protective agents, n (%) | |
| Yes | 29 (48) |
| No | 31 (52) |
Table 2.
Concordance between readers In outcomes of subcutaneous (SQ) fat, visceral (VC) fat, and muscle mass.
| Outcomes | N | ICC |
|---|---|---|
|
| ||
| Baseline | ||
| Subcutaneous fat | 19 | 0.998 |
| Visceral fat | 19 | 0.999 |
| Muscle mass | 20 | 0.963 |
| 3-month follow up | ||
| Subcutaneous fat | 17 | 0.998 |
| Visceral fat | 17 | 0.999 |
| Muscle mass | 17 | 0.991 |
Body Composition
The patients lost a mean (sd) of 7.8 (8.2)% of subcutaneous fat and 9.8 (18.2)% of visceral fat, with 83% of patients having at least some loss of subcutaneous fat and 68% of patients with some visceral fat loss (Fig. 1A). The mean subcutaneous fat area was 265 cm2 at baseline, which decreased to 245 cm2 at follow up (P value for difference < 0.01). Mean visceral fat decreased from 233 to 213 cm2 (P value for difference < 0.01). Neither baseline age at start of BAT treatment nor baseline BMI influenced the degree of change of body composition measures (all P > 0.05). The duration of prior ADT did not impact the degree of subcutaneous or visceral fat lost (P = 0.51 and P = 0.07). Changes observed in subcutaneous fat were positively correlated with the changes in visceral fat (Spearman’s correlation coefficient 0.58, 95% CI 0.35–0.71) and this was independent of effects of age, BMI, and duration of ADT.
Fig. 1.
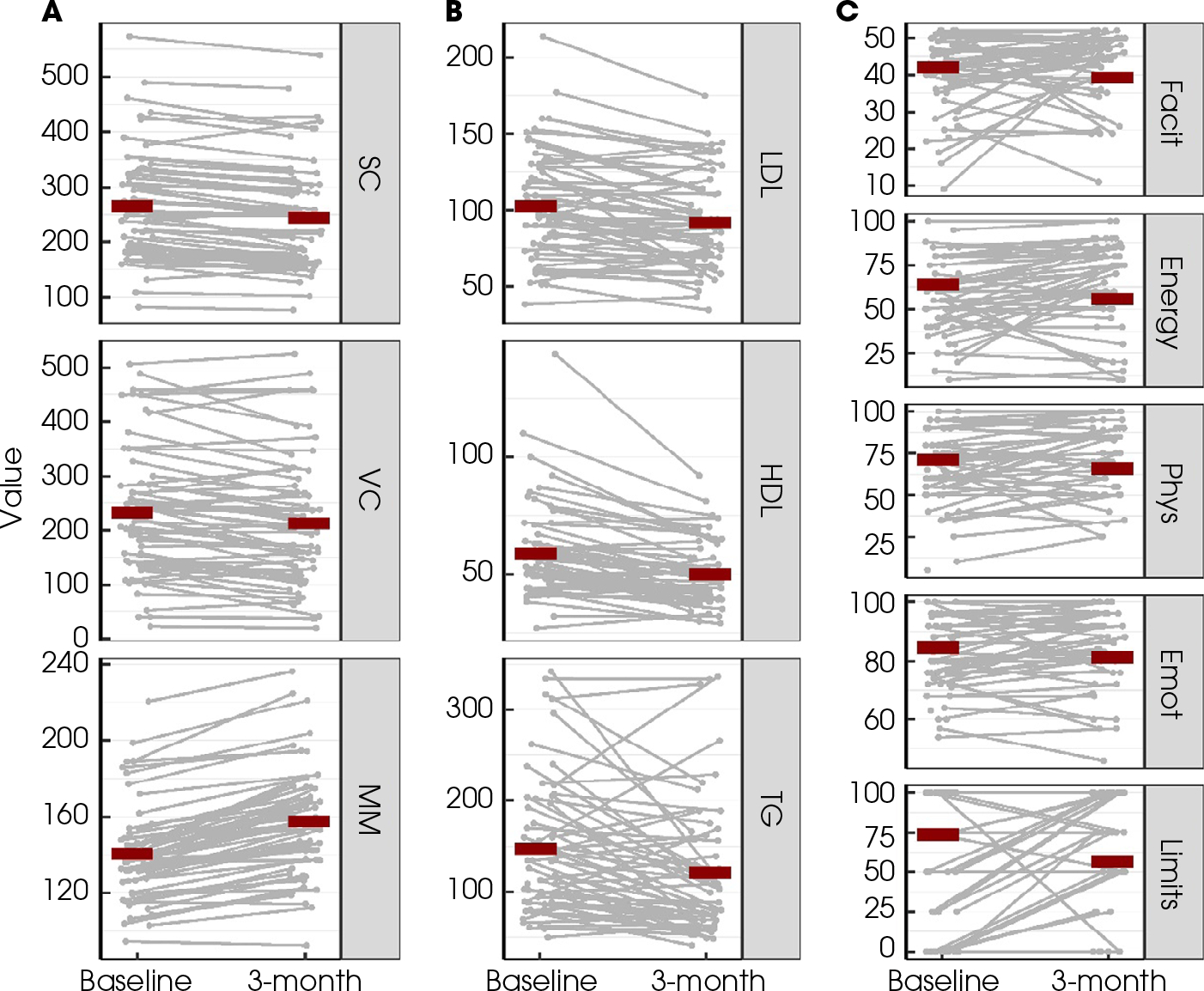
Overview of measurements at baseline and at 3-month follow-up after BAT for (A) body composition outcomes: subcutaneous (SC) fat, visceral (VC) fat, and muscle mass (MM); (B) lipid outcomes: LDL, HDL and TGs; (C) quality-of-life outcomes: FACIT-Fatigue scale (Facit), Energy, Physical Functioning (Phys), Emotional well-being (Emot), and Limitations due to physical health (Limits). Red lines indicated the corresponding mean values. Measurements from the same individual were connected with grey lines.
The patients gained a mean (sd) of 12.2 (6.7)% muscle mass in 3 months, with 97% of patients having some gain of muscle mass. Figure 2 shows a representative analysis of the increase in muscle mass seen with 3 months of BAT treatment. The mean muscle area increased from 140 to 158 cm2 (P value for difference < 0.001). The duration of ADT did have a significant impact on the degree of muscle mass gained (P = 0.02). Patients who had a prior duration of ADT of <4 years gained a mean (sd) of 9.7 (6.1)% muscle mass compared to a 14.7 (6.4)% gain of muscle mass among men who were on ADT for ≥4 years (P for difference = 0.003). A summary of the changes is shown in Table 3. In total, 55 (92%) patients were sarcopenic at baseline and the proportion of sarcopenia was significantly lower (75%, P = 0.004 for difference between baseline and follow-up) at the 3-month follow-up after BAT treatment.
Fig. 2.
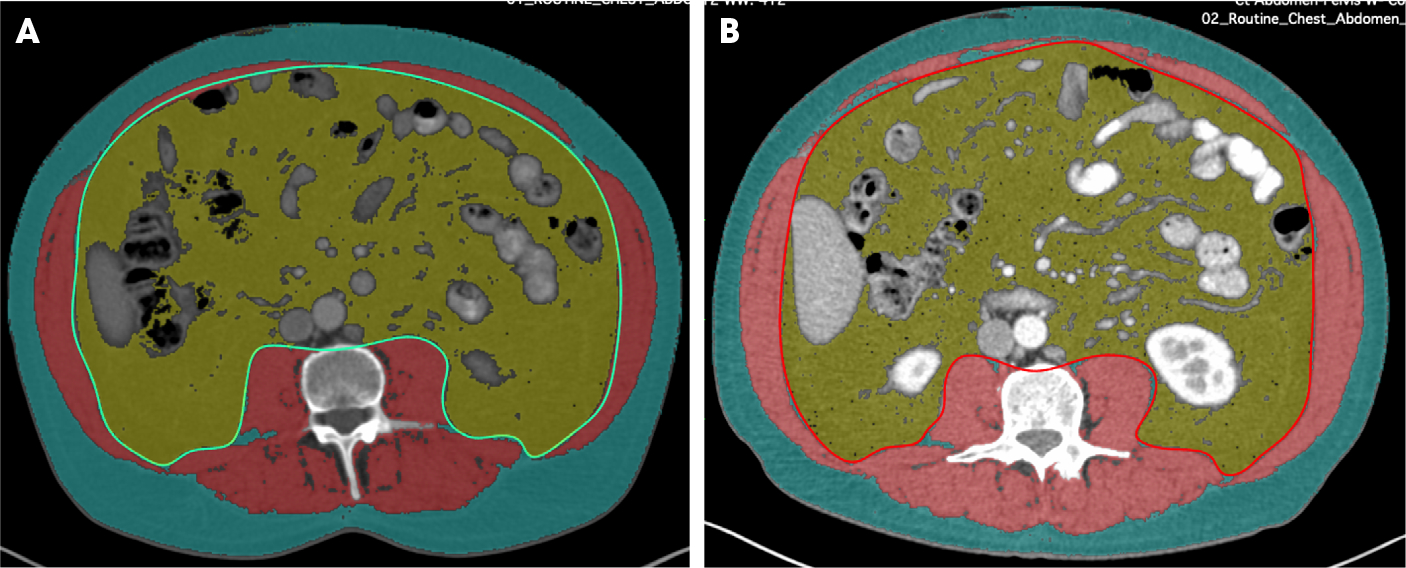
Image of CT abdomen at L3 vertebral level with segmentation of visceral fat (yellow area), subcutaneous fat (blue area) and abdominal wall musculature (red area) performed manually with OsiriX at 3-month interval between the two scans (A,B).
Table 3.
Summary Statistics for body composition measures and lipid parameters at baseline and at 3-month follow-up after initiation of BAT.
| Baseline, mean (sd) | 3-month follow-up, mean (sd) | % of ↑/↓ from baseline to 3-month follow-up | % change from baseline to 3-month follow-up, mean (sd) | P value for difference* | |
|---|---|---|---|---|---|
|
| |||||
| Subcutaneous fat area, cm2 | 265.1 (98.3) | 245.0 (96.0) | 83 ↓ | ↓ 7.8 (8.2) | <0.001 |
| Visceral fat area, cm2 | 233.1 (119.1) | 212.5 (121.8) | 68 ↓ | ↓ 9.8 (18.2) | 0.001 |
| Muscle area, cm2 | 140.4 (24.4)) | 157.5 (27.8) | 97 ↑ | ↑ 12.2 (6.7) | <0.001 |
| LDL, mg/dL | 102.4 (35.5) | 91.6 (31.2) | 74 ↓ | ↓ 12.4 (19.1) | <0.001 |
| HDL, mg/dL | 59.0 (20.1) | 49.7 (13.5) | 84 ↓ | ↓ 9.1 (11.4) | <0.001 |
| TGs, mg/dL | 146.9 (73.8) | 121.3 (71.9) | 69 ↓ | ↓ 26.9 (59.2) | 0.0005 |
| FACIT-F scale | 39.3 (10.5) | 42 (9.6) | 52 ↑ | ↑ 2.11 (12.6) | 0.33 |
| Energy | 56.1 (21.1) | 64.1 (24.7) | 63 ↑ | ↑ 7.8 (19.5) | 0.004 |
| Physical functioning | 66.0 (22.3) | 70.9 (21.8) | 54 ↑ | ↑ 4.2 (15.5) | 0.05 |
| Emotional well being | 81.2 (12.3) | 84.4 (13.2) | 54 ↑ | ↑ 1.8 (8.6) | 0.09 |
| Limitations due to physical health | 56.5 (43.7) | 73.4 (36.3) | 38 ↑ | ↑ 13.9 (32.2) | <0.001 |
P values were testing the differences of outcome’s distribution between baseline and 3-month follow-up after BAT therapy via Wilcoxon signed-rank test.
Lipids
The LDL levels decreased significantly by a mean (sd) of 12.4 (19.1) mg/dL, with 74% of patients having a decrease in LDL levels (P for difference < 0.001; Fig. 1B). Among the 32 patients who had baseline LDL levels above the optimal level (≥100 mg/dL), 14 (44%) had LDL levels at follow-up that were <100 mg/dL and in the optimal range.
The HDL levels decreased by a mean (sd) of 9.1 (11.4) mg/dL (P for difference < 0.001; Table 3) and16% of patients had an increase in their HDL levels (Fig. 1B). There were only three patients who had HDL levels of <40 mg/dL at baseline, which would be considered low. None of these three patients had follow-up levels that were >40 mg/dL at 3 months.
The TG levels significantly decreased by a mean (sd) of 26.9 (59.2) mg/dL (P for difference = 0.005; Table 3) with 69% of patients having some decrease in TG levels (Fig. 1B). Of the 23 patients who had TG levels of >150 mg/dL at baseline, 48% were within goal range, <150 mg/dL, at follow-up.
No associations were observed between changes of lipid measures and patient characteristics (baseline age at start of BAT treatment, baseline BMI and duration of ADT; all P > 0.05). Associations between changes in visceral fat and LDL or TG levels did not approach statistical significance (P = 0.09 and P = 0.92, respectively). Similarly, there was no significant association between changes in muscle mass and changes in HDL (P = 0.61).
Quality of Life
Energy levels and physical functioning levels significantly improved from baseline to 3 months (P = 0.004 and P = 0.05 for change, respectively), as did limitations due to physical health (P < 0.01) (Table 3, Fig. 1C). However, none of these improvements were correlated with a change in muscle mass, visceral body fat, or subcutaneous body fat. There was no significant change in the absolute change from baseline to 3 months on fatigue (measured by FACIT-F; P = 0.33) or emotional well-being (P = 0.09; Table 3). While most patients reported improvements in energy, physical functioning, and emotional well-being, 46% (95% CI 31–61), 39% (95% CI 25–55), 28% (95% CI 16–43) of patients respectively, reported improvements that would be considered clinically meaningful [28]. On average, the changes seen in limitations due to physical health are considered clinically meaningful [28]. No statistically significant associations were observed between changes in quality of life and patient characteristics (baseline age at start of BAT treatment, baseline BMI and duration of ADT; all P > 0.05).
Discussion
The present study demonstrates that three cycles of BAT therapy in patients with mCRPC is associated with significant positive changes in body composition, lipid profiles, and measures of quality of life. Subcutaneous and visceral fat decreased by 7.8% and 9.8% respectively, while muscle mass increased by 12.2% on average. Additionally, LDL, HDL and TG levels decreased with BAT therapy by 12.4, 9.1, and 26.9 mg/dL, respectively. In studies with BAT performed to date, the duration of BAT has ranged from 1 to 60 months. Longer-term treatment with BAT may produce an even greater effect on body composition, although this must be confirmed in larger studies. While there did not seem to be a significant difference in the rate of cardiovascular thrombotic events in the context of the clinical trials, the long-term effect on cardiovascular health given these changes will need to be studied.
Although patients were analysed after only 3 months of BAT, these findings are consistent with data from previous studies of BAT in men with metastatic hormone-sensitive prostate cancer, where there was an improvement in quality-of-life scores [29]. It is also consistent with findings on supplementation of testosterone in the non-cancer patient population. In a meta-analysis of testosterone supplementation, it was found that testosterone was associated with a decrease in fat and an increase in muscle mass [30]. Among men who were hypogonadal at baseline, there was also an improvement seen in lipid profiles with decreases in total cholesterol and TGs [30]. There have been varying results of testosterone supplementation on older men in the literature. In one study of 24 men with a mean (sd) age of 57.5 (4.8) years with type 2 diabetes and visceral obesity, oral testosterone supplementation decreased visceral fat by 5.7% after 3 months [31]. Another study of men aged 65–85 years did not show changes in body composition with testosterone supplementation after 12 weeks; however, the doses used in the study (testosterone patch that delivered 5 mg testosterone over 24 h) were lower than used in other studies and in our present study. Testosterone supplementation in hypogonadal men has been associated with small decreases in cholesterol, up to 5 mg/dL, with no significant changes in TGs [32]. In older obese men (aged 62–78 years), testosterone supplementation was associated with a significant decrease in LDL (13.5 mg/dL), HDL (5.4 mg/dL) and TGs (35 mg/dL), consistent with our present study [33]. Meta-analyses of randomised studies on the effect of testosterone supplementation in hypogonadal men do show consistent improvements in quality of life, in particular energy levels, consistent with our present study [34].
There are no other therapies for prostate cancer that have produced such results in terms of body composition and lipid profiles, although some, including bone protective agents, may improve quality of life [35]. Exercise, both resistance and aerobic training, have had varying success in improving body composition parameters with estimates of up to 16% increase in muscle mass and as much as 29% loss of body fat, although the results are quite variable [36]. Pooled analyses have not shown a benefit of exercise on lipid profiles in men with prostate cancer treated with ADT [37,38].
Conclusion
BAT, a novel treatment strategy being studied for mCRPC, is associated with significant improvements in body composition, lipid parameters, and a decrease in the proportion of sarcopenic patients after 3 months of treatment. This is the only therapy to date for the treatment of advanced prostate cancer that is associated with these improvements and has promising implications for the long-term health of men with mCRPC.
Funding Information
This work was partly supported by National Institutes of Health Cancer Center Support Grants P30 CA006973, Department of Defense Grant W81XWH-14-2-0189 and National Institute of Health R01CA184012.
Abbreviations:
- ADT
androgen-deprivation therapy
- BAT
bipolar androgen therapy
- BMI
body mass index
- CVD
cardiovascular disease
- HDL
high-density lipoprotein
- HU
Hounsfield units
- ICC
intraclass correlation coefficient
- LDL
low-density lipoprotein
- mCRPC
metastatic castration-resistant prostate cancer
- ROI
region of interest
- TG
triglyceride
Footnotes
Disclosure of Interests
Catherine H. Marshall: Consulting with Bayer Pharmaceuticals, Dendreon pharmaceuticals, McGraw-Hill Publishing Company. Other authors report no significant conflicts of interest. Dr. Clifford R. Weiss reports and Grant Support: Siemens Healthcare, Boston Scientific, Guerbet, Medtronic. Consultant: Siemens Healthcare, Boston Scientific, Medtronic.
Ethics Approval and Consent to Participate
All participants signed informed consent to participate in the clinical trials. The study was performed in accordance with the Declaration of Helsinki.
Consent for Publication
Not applicable.
Data Availability Statement
Data are from a clinical trial and individual patient data are not available publicly.
References
- 1.Denmeade SR, Isaacs JT. A history of prostate cancer treatment. Nat Rev Cancer 2002; 2: 389–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saylor PJ, Smith MR. Metabolic complications of androgen deprivation therapy for prostate cancer. JUrol. 2013; 189(Suppl): S34–42 [DOI] [PubMed] [Google Scholar]
- 3.Smith MR, Saad F, Egerdie B et al. Sarcopenia during androgen-deprivation therapy for prostate cancer. J Clin Oncol 2012; 30: 3271–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamilton EJ, Gianatti E, Strauss BJ et al. Increase in visceral and subcutaneous abdominal fat in men with prostate cancer treated with androgen deprivation therapy. Clin Endocrinol 2011; 74: 377–83 [DOI] [PubMed] [Google Scholar]
- 5.Levine GN, D’Amico AV, Berger P et al. Androgen-deprivation therapy in prostate cancer and cardiovascular risk: a science advisory from the American Heart Association, American Cancer Society, and American Urological Association: endorsed by the American Society for Radiation Oncology. Circulation 2010; 121: 833–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Epstein MM, Edgren G, Rider JR, Mucci LA, Adami HO. Temporal trends in cause of death among Swedish and US men with prostate cancer. J Natl Cancer Inst 2012; 104: 1335–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhatia N, Santos M, Jones LW et al. Cardiovascular effects of androgen deprivation therapy for the treatment of prostate cancer. Circulation 2016; 133: 537–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Antoun S, Bayar A, Ileana E et al. High subcutaneous adipose tissue predicts the prognosis in metastatic castration-resistant prostate cancer patients in post chemotherapy setting. Eur J Cancer 2015; 51: 2570–7 [DOI] [PubMed] [Google Scholar]
- 9.Ohtaka A, Aoki H, Nagata M et al. Sarcopenia is a poor prognostic factor of castration-resistant prostate cancer treated with docetaxel therapy. Prostate Int 2019; 7: 9–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stangl-Kremser J, Suarez-Ibarrola R, Andrea DD et al. Assessment of body composition in the advanced stage of castration-resistant prostate cancer: special focus on sarcopenia. Prostate Cancer Prostatic Dis 2020; 23: 309–15 [DOI] [PubMed] [Google Scholar]
- 11.Ryan CJ, Smith MR, Fizazi K et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol 2015; 16: 152–60 [DOI] [PubMed] [Google Scholar]
- 12.Beer TM, Armstrong AJ, Rathkopf D et al. Enzalutamide in men with chemotherapy-naïve metastatic castration-resistant prostate cancer: extended analysis of the phase 3 PREVAIL study. Eur Urol 2017; 71: 151–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pezaro C, Mukherji D, Tunariu N et al. Sarcopenia and change in body composition following maximal androgen suppression with abiraterone in men with castration-resistant prostate cancer. Br J Cancer 2013; 109: 325–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu JR, Duncan MS, Morgans AK et al. Cardiovascular effects of androgen deprivation therapy in prostate cancer: contemporary meta-analyses. Arterioscler Thromb Vasc Biol 2020; 40: e55–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fischer S, Clements S, Green A et al. Influence of treatment with abiraterone and enzalutamide on development of sarcopenia in patients with metastatic castration resistant prostate cancer. Eur Urol Open Sci 2020; 19: e879. [DOI] [PubMed] [Google Scholar]
- 16.Denmeade SR. Bipolar androgen therapy in the treatment of prostate cancer. Clin Adv Hematol Oncol 2018; 16: 408–11 [PubMed] [Google Scholar]
- 17.Teply BA, Wang H, Luber B et al. Bipolar androgen therapy in men with metastatic castration-resistant prostate cancer after progression on enzalutamide: an open-label, phase 2, multicohort study. Lancet Oncol 2018; 19: 76–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schweizer MT, Antonarakis ES, Wang H et al. Effect of bipolar androgen therapy for asymptomatic men with castration-resistant prostate cancer: results from a pilot clinical study. Sci Transl Med 2015; 7: 269ra2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Denmeade SR, Wang H, Agarwal N et al. TRANSFORMER: a randomized phase II study comparing bipolar androgen therapy versus enzalutamide in asymptomatic men with castration-resistant metastatic prostate cancer. J Clin Oncol 2021. [Online ahead of print]. 10.1200/JCO.20.02759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Markowski MC, Wang H, Sullivan R et al. A Multicohort open-label phase II trial of bipolar androgen therapy in men with metastatic castration-resistant prostate cancer (RESTORE): a comparison of post-abiraterone versus post-enzalutamide cohorts. Eur Urol 2020. [Online ahead of print]. 10.1016/j.eururo.2020.06.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab 2008; 33: 997–1006 [DOI] [PubMed] [Google Scholar]
- 22.van Vugt JL, Levolger S, Gharbharan A et al. A comparative study of software programmes for cross-sectional skeletal muscle and adipose tissue measurements on abdominal computed tomography scans of rectal cancer patients. J Cachexia Sarcopenia Muscle 2017; 8: 285–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001; 285: 2486–97 [DOI] [PubMed] [Google Scholar]
- 24.Fearon K, Strasser F, Anker SD et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011; 12: 489–95 [DOI] [PubMed] [Google Scholar]
- 25.Hewlett S, Dures E, Almeida C. Measures of fatigue: Bristol Rheumatoid Arthritis Fatigue Multi-Dimensional Questionnaire (BRAF MDQ), Bristol Rheumatoid Arthritis Fatigue Numerical Rating Scales (BRAF NRS) for Severity, Effect, and Coping, Chalder Fatigue Questionnaire (CFQ), Checklist Individual Strength (CIS20R and CIS8R), Fatigue Severity Scale (FSS), Functional Assessment Chronic Illness Therapy (Fatigue) (FACIT-F), Multi-Dimensional Assessment of Fatigue (MAF), Multi-Dimensional Fatigue Inventory (MFI), Pediatric Quality Of Life (PedsQL) Multi-Dimensional Fatigue Scale, Profile of Fatigue (ProF), Short Form 36 Vitality Subscale (SF-36 VT), and Visual Analog Scales (VAS). Arthritis Care Res 2011; 63: S263–86 [DOI] [PubMed] [Google Scholar]
- 26.Yellen SB, Cella DF, Webster K, Blendowski C, Kaplan E. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manage 1997; 13: 63–74 [DOI] [PubMed] [Google Scholar]
- 27.Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992; 30: 473–83 [PubMed] [Google Scholar]
- 28.Jayadevappa R, Malkowicz SB, Wittink M, Wein AJ, Chhatre S. Comparison of distribution- and anchor-based approaches to infer changes in health-related quality of life of prostate cancer survivors. Health Serv Res 2012; 47: 1902–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schweizer MT, Wang H, Luber B et al. Bipolar androgen therapy for men with androgen ablation naïve prostate cancer: results from the phase II BATMAN study. Prostate 2016; 76: 1218–26 [DOI] [PubMed] [Google Scholar]
- 30.Corona G, Giagulli VA, Maseroli E et al. Testosterone supplementation and body composition: results from a meta-analysis of observational studies. J Endocrinol Invest 2016; 39: 967–81 [DOI] [PubMed] [Google Scholar]
- 31.Boyanov MA, Boneva Z, Christov VG. Testosterone supplementation in men with type 2 diabetes, visceral obesity and partial androgen deficiency. Aging Male 2003; 6: 1–7 [PubMed] [Google Scholar]
- 32.Whitsel EA, Boyko EJ, Matsumoto AM, Anawalt BD, Siscovick DS. Intramuscular testosterone esters and plasma lipids in hypogonadal men: a meta-analysis. Am J Med 2001; 111: 261–9 [DOI] [PubMed] [Google Scholar]
- 33.Sattler F, He J, Chukwuneke J et al. Testosterone supplementation improves carbohydrate and lipid metabolism in some older men with abdominal obesity. J Gerontol Geriatr Res 2014; 3: 1000159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nian Y, Ding M, Hu S et al. Testosterone replacement therapy improves health-related quality of life for patients with late-onset hypogonadism: a meta-analysis of randomized controlled trials. Andrologia 2017; 49: e12630. [DOI] [PubMed] [Google Scholar]
- 35.von Moos R, Body JJ, Egerdie B et al. Pain and health-related quality of life in patients with advanced solid tumours and bone metastases: integrated results from three randomized, double-blind studies of denosumab and zoledronic acid. Support Care Cancer 2013; 21: 3497–507 [DOI] [PubMed] [Google Scholar]
- 36.Keogh JW, MacLeod RD. Body composition, physical fitness, functional performance, quality of life, and fatigue benefits of exercise for prostate cancer patients: a systematic review. J Pain Symptom Manage 2012; 43: 96–110 [DOI] [PubMed] [Google Scholar]
- 37.Yunfeng G, Weiyang H, Xueyang H, Yilong H, Xin G. Exercise overcome adverse effects among prostate cancer patients receiving androgen deprivation therapy. Medicine 2017; 96: e7368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Galvao DA, Taaffe DR, Spry N, Joseph D, Newton RU. Combined resistance and aerobic exercise program reverses muscle loss in men undergoing androgen suppression therapy for prostate cancer without bone metastases: a randomized controlled trial. J Clin Oncol 2010; 28: 340–7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are from a clinical trial and individual patient data are not available publicly.


