Abstract
BACKGROUND
Darolutamide is a potent androgen-receptor inhibitor that has been associated with increased overall survival among patients with nonmetastatic, castration-resistant prostate cancer. Whether a combination of darolutamide, androgen-deprivation therapy, and docetaxel would increase survival among patients with metastatic, hormone-sensitive prostate cancer is unknown.
METHODS
In this international, phase 3 trial, we randomly assigned patients with metastatic, hormone-sensitive prostate cancer in a 1:1 ratio to receive darolutamide (at a dose of 600 mg [two 300-mg tablets] twice daily) or matching placebo, both in combination with androgen-deprivation therapy and docetaxel. The primary end point was overall survival.
RESULTS
The primary analysis involved 1306 patients (651 in the darolutamide group and 655 in the placebo group); 86.1% of the patients had disease that was metastatic at the time of the initial diagnosis. At the data cutoff date for the primary analysis (October 25, 2021), the risk of death was significantly lower, by 32.5%, in the darolutamide group than in the placebo group (hazard ratio 0.68; 95% confidence interval, 0.57 to 0.80; P<0.001). Darolutamide was also associated with consistent benefits with respect to the secondary end points and prespecified subgroups. Adverse events were similar in the two groups, and the incidences of the most common adverse events (occurring in ≥10% of the patients) were highest during the overlapping docetaxel treatment period in both groups. The frequency of grade 3 or 4 adverse events was 66.1% in the darolutamide group and 63.5% in the placebo group; neutropenia was the most common grade 3 or 4 adverse event (in 33.7% and 34.2%, respectively).
CONCLUSIONS
In this trial involving patients with metastatic, hormone-sensitive prostate cancer, overall survival was significantly longer with the combination of darolutamide, androgen-deprivation therapy, and docetaxel than with placebo plus androgen-deprivation therapy and docetaxel, and the addition of darolutamide led to improvement in key secondary end points. The frequency of adverse events was similar in the two groups. (Funded by Bayer and Orion Pharma; ARASENS ClinicalTrials.gov number, NCT02799602.)
Standard treatment for patients with metastatic, hormone-sensitive prostate cancer includes the addition of either docetaxel or an androgen-receptor pathway inhibitor to androgen-deprivation therapy.1–4 In two randomized, phase 3 trials involving such patients, overall survival was longer among those who received docetaxel plus androgen-deprivation therapy than among those who received androgen-deprivation therapy alone.5–8 Subsequent randomized, phase 3 trials have shown that the addition of an androgen-receptor pathway inhibitor (abiraterone, enzalutamide, or apalutamide) to androgen-deprivation therapy has greater clinical benefit than the use of androgen-deprivation therapy alone.9–12
Phase 3 trials of combination therapy with an androgen-receptor pathway inhibitor, androgen-deprivation therapy, and docetaxel have shown conflicting results. In PEACE-1 (A Phase III Study for Patients with Metastatic Hormone-naïve Prostate Cancer), overall survival was longer among patients who received abiraterone in combination with androgen-deprivation therapy and docetaxel than among those who received androgen-deprivation therapy and docetaxel alone.13 In contrast, a subgroup analysis of the ENZAMET (Enzalutamide in First Line Androgen Deprivation Therapy for Metastatic Prostate Cancer) trial showed that survival was not longer with enzalutamide, androgen-deprivation therapy, and docetaxel than with androgen-deprivation therapy and docetaxel alone.12
Darolutamide is a structurally distinct androgen-receptor inhibitor with low blood–brain barrier penetration and limited potential for clinically relevant drug–drug interactions.14–17 Studies involving patients with prostate cancer, including the phase 3 ARAMIS (Androgen Receptor Antagonizing Agent for Metastasis-free Survival) trial involving patients with nonmetastatic, castration-resistant prostate cancer, have shown that darolutamide has potent antitumor efficacy.18–22 In the ARAMIS trial, the median metastasis-free survival was almost 2 years longer and the risk of death was 31% lower among patients who received darolutamide with androgen-deprivation therapy than among those who received placebo with androgen-deprivation therapy, and the incidence of adverse events was similar in the two groups.21,22 In the phase 3 ARASENS (ODM-201 in Addition to Standard ADT and Docetaxel in Metastatic Castration Sensitive Prostate Cancer) trial, we evaluated the efficacy and safety of darolutamide added to androgen-deprivation therapy and docetaxel in patients with metastatic, hormone-sensitive prostate cancer.
METHODS
TRIAL DESIGN AND CONDUCT
This international, randomized, double-blind, placebo-controlled trial was sponsored by Bayer and Orion Pharma. The trial was designed by Bayer and the first and last authors, with support from the protocol steering committee. The institutional review board at each participating institution approved the trial, which was conducted in accordance with the principles of the Declaration of Helsinki and the International Council for Harmonisation Good Clinical Practice guidelines. All the patients provided written informed consent.
An independent data and safety monitoring board reviewed unblinded safety and efficacy data throughout the trial. The data were collected by the investigators, analyzed by statisticians who were employed by Bayer, and interpreted by the authors, including employees of the sponsors. Bayer provided funding for medical writing assistance. All the authors reviewed and approved the manuscript that was submitted for publication. The authors assume responsibility for the completeness and accuracy of the data and for the fidelity of the trial to the protocol and the statistical analysis plan, available with the full text of this article at NEJM.org.
PATIENTS AND INTERVENTIONS
Patients were eligible for participation if they were 18 years of age or older and had an Eastern Cooperative Oncology Group (ECOG) performance-status score of 0 or 1 (scores range from 0 to 5, with higher scores indicating greater disability), histologically or cytologically confirmed prostate cancer, and metastases detected on bone scanning, contrast-enhanced computed tomography (CT), or magnetic resonance imaging (MRI). Eligible patients had to be candidates for androgen-deprivation therapy and docetaxel, in the investigator’s judgment. Patients were excluded if they had regional lymph-node involvement only (N1, below the aortic bifurcation) or if they had received androgen-deprivation therapy more than 12 weeks before randomization, second-generation androgen-receptor pathway inhibitors, chemotherapy, or immunotherapy for prostate cancer before randomization, or radiotherapy within 2 weeks before randomization. Full eligibility criteria are provided in the Supplementary Appendix, available at NEJM.org.
All the patients received androgen-deprivation therapy (a luteinizing hormone–releasing hormone [LHRH] agonist or an LHRH antagonist) or underwent orchiectomy within 12 weeks before randomization and received six cycles of docetaxel (75 mg per square meter of body-surface area on day 1 and every 21 days), with prednisone or prednisolone administered at the investigator’s discretion, initiated within 6 weeks after randomization. The recommended premedication to prevent docetaxel-related hypersensitivity reactions and fluid retention was oral dexamethasone, administered at a dose of 8 mg at 12 hours, 3 hours, and 1 hour before infusion. For patients receiving LHRH agonists, the use of these agonists in combination with a first-generation antiandrogen for at least 4 weeks before randomization was recommended. First-generation antiandrogen therapy was discontinued before randomization.
Patients were randomly assigned in a 1:1 ratio to receive either darolutamide (at a dose of 600 mg [two 300-mg tablets] twice daily with food) or matched placebo. Randomization was stratified according to the metastasis stage in the tumor–node–metastasis system (nonregional lymph-node metastases only [M1a], bone metastases with or without lymph-node metastases [M1b], or visceral metastases with or without lymph-node or bone metastases [M1c]) and according to whether the alkaline phosphatase level was below or at or above the upper limit of the normal range. Patients continued to receive darolutamide or placebo until symptomatic disease progression, a change in antineoplastic therapy, unacceptable toxic effects, patient or physician decision, death, or nonadherence.
END POINTS
The primary end point was overall survival, which was defined as the time from randomization until death from any cause. The secondary end points were time to castration-resistant prostate cancer, time to pain progression, symptomatic skeletal event–free survival, time to a first symptomatic skeletal event, time to initiation of subsequent systemic antineoplastic therapy, time to worsening of disease-related physical symptoms, time to initiation of opioid treatment for 7 or more consecutive days, and safety. Definitions of the secondary efficacy end points are provided in the Supplementary Appendix.
ASSESSMENTS
During the trial, patients were evaluated every 12 weeks for evidence of castration resistance, the initiation of subsequent antineoplastic therapy, symptomatic skeletal events, opioid use for 7 or more consecutive days, pain progression, the worsening of physical symptoms of disease, and adverse events and serious adverse events that occurred during treatment. Patients underwent contrast-enhanced chest, abdomen, and pelvic CT or MRI and bone scanning at baseline, within 30 days after the last cycle of docetaxel, and yearly thereafter during trial treatment. Pain was assessed with the use of the Brief Pain Inventory Short-Form questionnaire, and disease-related physical symptoms were assessed with the use of the Functional Assessment of Cancer Therapy–Prostate (National Comprehensive Cancer Network) symptom index 17-item questionnaire. In safety assessments, adverse events were graded with the use of the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03.
STATISTICAL ANALYSIS
The sample size was calculated on the basis of the primary end point, overall survival. We estimated that approximately 1300 patients would be required to observe approximately 509 deaths, allowing for a 5% dropout rate, which would provide the trial with 90% power to detect a 25% decrease in the risk of death in the darolutamide group versus the placebo group, at a two-sided alpha level of 0.05. The secondary end points were tested with a hierarchical gatekeeping procedure in the order described above only if the primary end point and each preceding secondary end point in the hierarchy were statistically significant. If the primary end point or a secondary end point did not reach significance, the hierarchical procedure was stopped, and subsequent analyses were considered to be exploratory.
The full analysis set (all the patients who underwent randomization and were assessed according to the treatment to which they were assigned) was included in the analysis of the primary and secondary efficacy end points with the use of a stratified log-rank test, with randomization stratification factors. Hazard ratios for the comparison of darolutamide with placebo and 95% confidence intervals were calculated with the Cox proportional-hazards model stratified according to the randomization factors. A log–log survival plot, which was produced to assess whether application of the Cox proportional-hazards model was appropriate, confirmed that the proportional-hazards assumption was met for all time-to-event end points. Kaplan–Meier estimates for overall survival are presented for both treatment groups. For the analysis of overall survival, data on patients in whom death had not been confirmed were censored as of the last known date the patients were alive. For subgroup analyses, intervals were not adjusted for multiplicity and cannot be used to infer effects. Descriptive statistics were used to summarize baseline characteristics in the full analysis set, and safety end points were assessed in the safety analysis set (i.e., all the patients who received at least one dose of darolutamide or placebo and were assessed in accordance with the treatment that they actually received). For the primary efficacy end point, subgroup analyses were prespecified to assess the consistency of treatment effect. Statistical analyses were performed with the use of SAS software, version 9.4 (SAS Institute).
RESULTS
PATIENTS
Between November 2016 and June 2018, a total of 1306 patients underwent randomization at 286 centers in 23 countries. A total of 651 patients were assigned to receive darolutamide and 655 patients were assigned to receive placebo, both in combination with androgen-deprivation therapy and docetaxel (Fig. S1 and Table S1 in the Supplementary Appendix). Of these patients, 1305 patients (651 in the darolutamide group and 654 in the placebo group) were included in the full analysis set, and 1302 patients (652 in the darolutamide group and 650 in the placebo group) were included in the safety analysis set.
Demographic and baseline characteristics were well balanced in the two groups (Tables 1 and S2). The median age was 67 years in both groups, most patients (71.1%) had an ECOG performance-status score of 0, and 78.2% of the patients had a Gleason score of 8 or greater (scores range from 6 to 10, with higher scores indicating a more aggressive form of prostate cancer). All the patients had metastatic disease at baseline; 79.5% had bone metastases (metastasis stage M1b) and 17.5% had visceral metastases (metastasis stage M1c). Most patients (86.1%) had disease that was metastatic at the time of the initial diagnosis.
Table 1.
Patient Demographic and Clinical Characteristics at Baseline.*
| Characteristic | Darolutamide–ADT– Docetaxel (N = 651)† |
Placebo–ADT–Docetaxel (N = 654)† |
|---|---|---|
| Median age (range) — yr | 67 (41–89) | 67 (42–86) |
| Age group — no. (%) | ||
| <65 yr | 243 (37.3) | 234 (35.8) |
| 65–74yr | 303 (46.5) | 306 (46.8) |
| 75–84 yr | 102 (15.7) | 110 (16.8) |
| ≥85 yr | 3 (0.5) | 4 (0.6) |
| ECOG performance-status score — no. (%)‡ | ||
| 0 | 466 (71.6) | 462 (70.6) |
| 1 | 185 (28.4) | 190 (29.1) |
| Race — no. (%)§ | ||
| White | 345 (53.0) | 333 (50.9) |
| Asian | 230 (35.3) | 245 (37.5) |
| Black | 26 (4.0) | 28 (4.3) |
| Other | 7 (11) | 2 (0.3) |
| Not reported | 43 (6.6) | 46 (7.0) |
| Region — no. (%) | ||
| North America | 125 (19.2) | 119 (18.2) |
| Asia-Pacific | 229 (35.2) | 244 (37.3) |
| Rest of the world¶ | 297 (45.6) | 291 (44.5) |
| Gleason score at initial diagnosis — no. (%)∥ | ||
| <8 | 122 (18.7) | 118 (18.0) |
| ≥8 | 505 (77.6) | 516 (78.9) |
| Data missing | 24 (3.7) | 20 (3.1) |
| Metastasis stage at initial diagnosis — no. (%) | ||
| M1, distant metastasis | 558 (85.7) | 566 (86.5) |
| M0, no distant metastasis | 86 (13.2) | 82 (12.5) |
| MX, distant metastasis not assessed | 7 (11) | 6 (0.9) |
| Metastasis stage at screening — no. (%) | ||
| M1a, nonregional lymph-node metastases only | 23 (3.5) | 16 (2.4) |
| M1b, bone metastases with or without lymph-node metastases | 517 (79.4) | 520 (79.5) |
| M1c, visceral metastases with or without lymph-node or bone metastases | 111 (17.1) | 118 (18.0) |
| Median serum PSA level (range) — ng/ml** | 30.3 (0.0–9219.0) | 24.2 (0.0–11,947.0) |
| Median serum ALP level (range) — U/liter** | 148 (40–4885) | 140 (36–7680) |
| ALP category — no. (%)** | ||
| <ULN | 290 (44.5) | 291 (44.5) |
| ≥ULN | 361 (55.5) | 363 (55.5) |
Percentages may not total 100 because of rounding. ADT denotes androgen-deprivation therapy, ALP alkaline phosphatase, PSA prostate-specific antigen, and ULN upper limit of the normal range.
One patient who was randomly assigned to the placebo group but received darolutamide was included in the placebo group in the full analysis set.
Eastern Cooperative Oncology Group (ECOG) performance-status scores range from 0 to 5, with higher scores indicating greater disability.
Race was reported by the investigators.
The “rest of the world” geographic region was made up predominantly of European countries, Australia, Brazil, Israel, and Mexico.
Gleason scores for the histologic pattern of carcinoma range from 6 to 10, with higher scores indicating a more aggressive form of prostate cancer.
These values were centrally assessed. Samples were obtained while patients were receiving ADT.
At the data cutoff date for the primary analysis (October 25, 2021), the median treatment duration was longer in the darolutamide group (41.0 months) than in the placebo group (16.7 months), and a higher percentage of patients in the darolutamide group (45.9% [299 of 651 patients]) than in the placebo group (19.1% [125 of 654 patients]) were still receiving the assigned trial treatment. Six cycles of docetaxel were completed in 571 of 652 patients (87.6%) in the darolutamide group and in 556 of 650 patients (85.5%) in the placebo group. The reasons for discontinuation of the trial agent are provided in Figure S1. The median follow-up for overall survival was 43.7 months in the darolutamide group and 42.4 months in the placebo group.
PRIMARY END POINT
The primary analysis of overall survival was performed after 533 patients had died (229 patients in the darolutamide group and 304 patients in the placebo group). The risk of death was 32.5% lower in the darolutamide group than in the placebo group (hazard ratio, 0.68; 95% confidence interval [CI], 0.57 to 0.80; P<0.001) (Fig. 1). A significant improvement in overall survival was observed despite a high percentage of patients who received subsequent life-prolonging systemic therapies, primarily different androgen-receptor pathway inhibitors, among those who entered follow-up in the placebo group (374 of 495 patients [75.6%]) (Table S3). The overall survival at 4 years was 62.7% (95% CI, 58.7 to 66.7) in the darolutamide group and 50.4% (95% CI, 46.3 to 54.6) in the placebo group. The treatment effect of darolutamide with respect to overall survival was favorable across most subgroups (Figs. S2 and S3).
Figure 1. Overall Survival (Full Analysis Set).
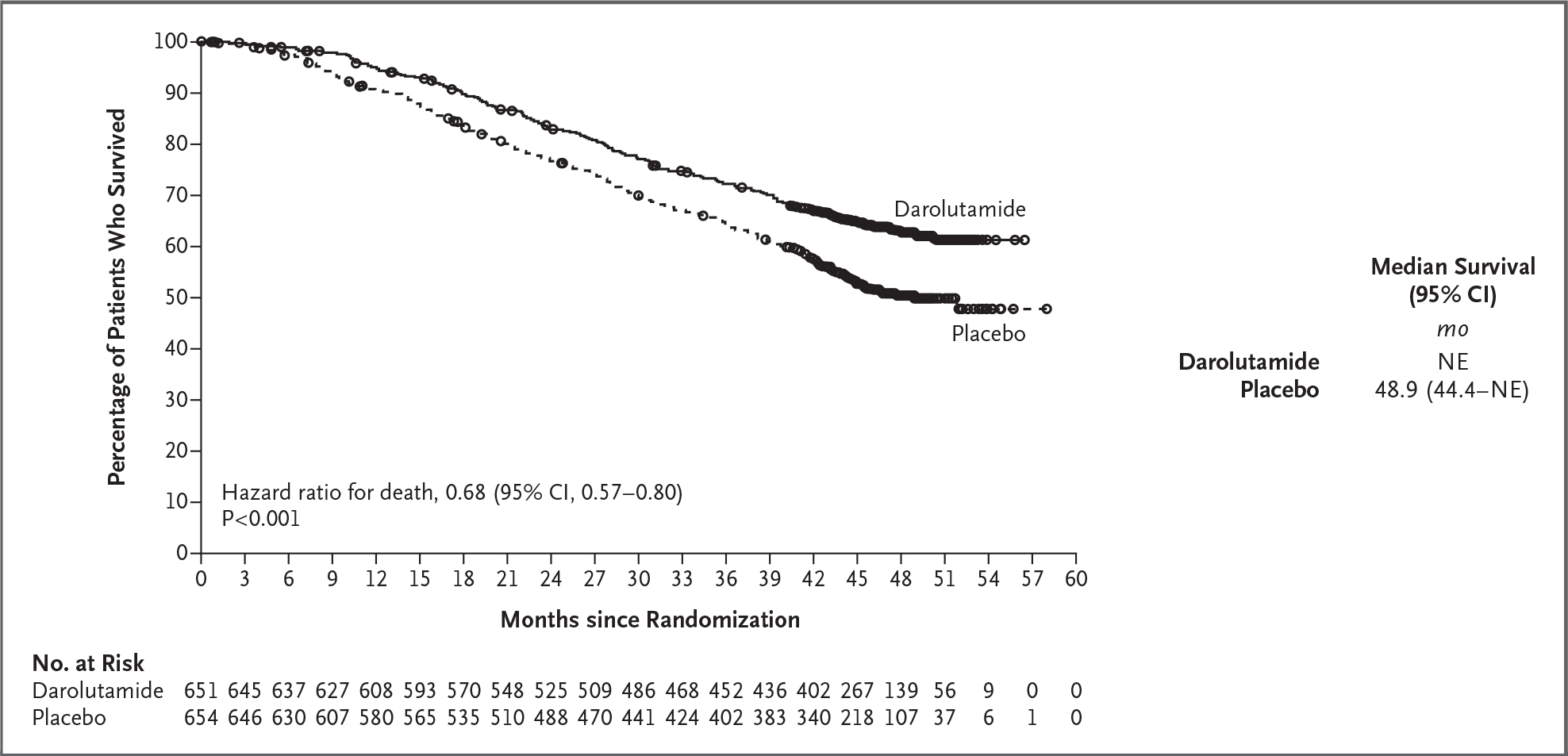
Kaplan−Meier estimates of overall survival are shown. For the analysis of overall survival, data were censored as of the last known date the patients were alive. One patient who was randomly assigned to the placebo group but received darolutamide was included in the placebo group in the full analysis set. CI denotes confidence interval, and NE not estimable.
Secondary Efficacy End Points
Darolutamide was associated with significantly greater benefits than placebo for the first five secondary efficacy end points tested hierarchically (Table 2 and Fig. 2). The time to development of castration-resistant disease was significantly longer in the darolutamide group (hazard ratio, 0.36; 95% CI, 0.30 to 0.42; P<0.001). The time to pain progression was also significantly longer in the darolutamide group (hazard ratio, 0.79; 95% CI, 0.66 to 0.95; P=0.01), as were symptomatic skeletal event–free survival (hazard ratio, 0.61; 95% CI, 0.52 to 0.72; P<0.001) and the time to a first symptomatic skeletal event (hazard ratio, 0.71; 95% CI, 0.54 to 0.94; P=0.02). The time to the initiation of subsequent systemic antineoplastic therapy was significantly longer in the darolutamide group (hazard ratio, 0.39; 95% CI, 0.33 to 0.46; P<0.001).
Table 2.
Secondary Efficacy End Points (Full Analysis Set).*
| End Point | Darolutamide–ADT–Docetaxel (N=651)† |
Placebo–ADT–Docetaxel (N = 654)† |
Hazard Ratio (95% CI) |
P Value | ||
|---|---|---|---|---|---|---|
| Median | Patients with Event | Median | Patients with Event | |||
| mo | no. (%) | mo | no. (%) | |||
| Time to castration-resistant prostate cancer | NR | 225 (35) | 19.1 | 391 (60) | 0.36 (0.30–0.42) | <0.001 |
| Time to pain progression | NR | 222 (34) | 27.5 | 248 (38) | 0.79 (0.66–0.95) | 0.01 |
| Symptomatic skeletal event–free survival | 51.2 | 257 (40) | 39.7 | 329 (50) | 0.61 (0.52–0.72) | <0.001 |
| Time to first symptomatic skeletal event | NR | 95 (15) | NR | 108 (17) | 0.71 (0.54–0.94) | 0.02 |
| Time to initiation of subsequent systemic antineoplastic therapy | NR | 219 (34) | 25.3 | 395 (60) | 0.39 (0.33–0.46) | <0.001 |
| Time to worsening of disease-related physical symptoms | 19.3 | 351 (54) | 19.4 | 308 (47) | 1.04 (0.89–1.22) | 0.59 |
| Time to initiation of opioid use for ≥7 consecutive days | NR | 92 (14) | NR | 117 (18) | 0.69 (0.52–0.91) | NA |
NA denotes not applicable, and NR not reached.
One patient who was randomly assigned to the placebo group but received darolutamide was included in the placebo group in the full analysis set.
Figure 2. Analyses of Secondary End Points (Full Analysis Set).

Panel A shows the time to castration-resistant prostate cancer, and Panel B shows the time to pain progression. The Kaplan−Meier method was used to estimate the time to events; data were censored at the date of the patients’ last assessment for that end point. One patient who was randomly assigned to the placebo group but received darolutamide was included in the placebo group in the full analysis set.
SAFETY
The incidences of adverse events of any grade, grade 3 to 5 adverse events, and serious adverse events were similar in the two groups (Table 3). The incidences of the most common adverse events (in ≥10% of the patients), many of which are known toxic effects related to docetaxel therapy, were highest in both groups during the period when the patients received both docetaxel and either darolutamide or placebo, and these effects progressively decreased thereafter, with grade 3 or 4 adverse events in 66.1% of the patients in the darolutamide group and 63.5% of those in the placebo group; neutropenia was the most common grade 3 or 4 event (in 33.7% and 34.2%, respectively). Serious adverse events occurred in 44.8% of the patients in the darolutamide group and in 42.3% of those in the placebo group. The frequency of death due to adverse events was low and similar in the two groups (27 of 652 patients in the darolutamide group [4.1%] and 26 of 650 patients in the placebo group [4.0%]) (Table S4). Few patients discontinued darolutamide or placebo as a result of adverse events (13.5% of the patients in the darolutamide group and 10.6% of those in the placebo group). The most frequently reported adverse events were alopecia (in 40.5% of the patients in the darolutamide group and 40.6% of the patients in the placebo group), neutropenia (in 39.3% and 38.8%, respectively), fatigue (in 33.1% and 32.9%), and anemia (in 27.8% and 25.1%) (Table S5).
Table 3.
Adverse Events.*
| Event | Darolutamide–ADT–Docetaxel (N = 652)† |
Placebo–ADT–Docetaxel (N = 650)† |
|---|---|---|
| number of patients (percent) | ||
| Any adverse event | 649 (99.5) | 643 (98.9) |
| Worst grade | ||
| Grade 1 | 28 (4.3) | 35 (5.4) |
| Grade 2 | 162 (24.8) | 169 (26.0) |
| Grade 3 | 248 (38.0) | 232 (35.7) |
| Grade 4 | 183 (28.1) | 181 (27.8) |
| Grade 5 | 27 (4.1) | 26 (4.0) |
| Serious adverse event | 292 (44.8) | 275 (42.3) |
| Adverse event leading to permanent discontinuation of trial agent | ||
| Darolutamide or placebo | 88 (13.5) | 69 (10.6) |
| Docetaxel | 52 (8.0) | 67 (10.3) |
| Selected grade 3 or 4 adverse events‡ | ||
| Neutropenia§ | 220 (33.7) | 222 (34.2) |
| Febrile neutropenia | 51 (7.8) | 48 (7.4) |
| Hypertension | 42 (6.4) | 21 (3.2) |
| Anemia | 31 (4.8) | 33 (5.1) |
| Pneumonia | 21 (3.2) | 20 (3.1) |
| Hyperglycemia | 18 (2.8) | 24 (3.7) |
| Increased ALT level | 18 (2.8) | 11 (17) |
| Increased AST level | 17 (2.6) | 7 (11) |
| Increased weight | 14 (2.1) | 8 (12) |
| Urinary tract infection | 13 (2.0) | 12 (1.8) |
ALT denotes alanine aminotransferase, and AST aspartate aminotransferase.
Three patients who underwent randomization never received the assigned trial treatment; all three patients were in the placebo group. One patient who was assigned to the placebo group but received darolutamide was included in the darolutamide group of the safety analysis set.
In the column of data for patients who received darolutamide, ADT, and docetaxel, listed are all grade 3 or 4 events that occurred in at least 2% of the patients.
The neutropenia category includes the preferred terms of leukopenia, neutropenia, decreased neutrophil count, and decreased white-cell count.
Certain adverse events are of special interest for patients receiving androgen-receptor pathway inhibitors. These events include fatigue, falls, fractures, mental impairment, rash, hypertension, and cardiovascular events. In this trial, the incidences of these events of interest were similar (with no more than a 2 percentage-point difference) in the two groups, with the exception of rash (in 16.6% of the patients in the darolutamide group and 13.5% of those in the placebo group) and hypertension (in 13.7% and 9.2%, respectively) (Table S6). Among the most frequently reported adverse events of interest, the incidences of vasodilation and flushing (in 20.4% of the patients in the darolutamide group and 21.7% of those in the placebo group) and diabetes mellitus and hyperglycemia (in 15.2% and 14.3%) were also similar in the two groups.
DISCUSSION
In this international, randomized, double-blind, placebo-controlled, phase 3 trial involving patients with metastatic, hormone-sensitive prostate cancer, overall survival was significantly longer among patients who received darolutamide plus androgen-deprivation therapy and docetaxel than among those who received androgen-deprivation therapy and docetaxel alone. This survival benefit was observed despite a high percentage of patients who received subsequent life-prolonging systemic therapy in the placebo group. The survival benefit of darolutamide was consistent across most subgroups. The time to the development of castration-resistant disease was significantly longer in the patients who received darolutamide, and improvements were observed with respect to the other key secondary end points. The incidence, severity, and nature of adverse events were consistent with the established safety profiles of androgen-deprivation therapy and docetaxel.
Our results are consistent with those in the PEACE-1 trial, which had a two-by-two factorial design. In that trial, 1173 patients with hormone-sensitive prostate cancer that was metastatic at the time of the initial diagnosis were randomly assigned to receive abiraterone, prostate radiotherapy, neither, or both. All the patients received standard of care (androgen-deprivation therapy with or without docetaxel). In a planned subgroup analysis involving 710 patients who received docetaxel, overall survival was significantly longer among those who received abiraterone than among those who did not (hazard ratio, 0.75; 95% CI, 0.59 to 0.96).13
All the patients in the ARASENS trial were prospectively assigned to receive androgen-deprivation therapy and docetaxel. This trial provided clear and compelling evidence that overall survival was significantly longer among patients who received combination therapy with darolutamide, androgen-deprivation therapy, and docetaxel than among those who received androgen-deprivation therapy and docetaxel alone. Darolutamide was associated with a 32.5% reduction in the risk of death. The combination did not result in more toxic effects than did the combination of androgen-deprivation therapy and docetaxel alone. In both the phase 3 ARAMIS trial involving patients with nonmetastatic, castration-resistant prostate cancer and the ARASENS trial, there was no more than a 2 percentage-point difference between the darolutamide and placebo groups with respect to the incidence of most adverse events that are commonly associated with androgen-receptor pathway inhibitors.22
This trial has several strengths and limitations. The large sample size enabled us to conduct a robust statistical analysis to assess the effect of the addition of darolutamide, an androgen-receptor pathway inhibitor, to androgen-deprivation therapy and docetaxel on overall survival and a number of key secondary end points. Most patients who were enrolled in our trial had metastatic disease, with bone metastases, visceral metastases, or both, at the time of the initial diagnosis. Thus, limited information is available on the benefit–risk considerations for patients with metastatic, hormone-sensitive prostate cancer and a better prognosis, including those with recurrent metastatic disease, node-only metastases, or both. In addition, the efficacy and safety of combination therapy are unknown in patients with a poor performance status, because the trial included only patients with an ECOG performance-status score of 0 or 1. Our trial was not designed to compare the efficacy of darolutamide plus androgen-deprivation therapy with that of docetaxel plus androgen-deprivation therapy.
The results of our trial support the use of darolutamide in combination with androgen-deprivation therapy and docetaxel in patients with metastatic, hormone-sensitive prostate cancer. The addition of darolutamide to androgen-deprivation therapy and docetaxel increased overall survival, and improvements were observed with respect to key secondary end points, with no increase in adverse events.
Supplementary Material
Acknowledgments
Supported by Bayer and Orion Pharma.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
We thank the patients and their families and Sara Black, B.A., and Lauren Gallagher, R.Ph., Ph.D., both of OPEN Health Communications, London, for medical writing and editorial assistance with an earlier version of the manuscript.
Footnotes
A full list of investigators in the ARASENS trial is provided in the Supplementary Appendix, available at NEJM.org.
Contributor Information
Matthew R. Smith, Massachusetts General Hospital Cancer Center and Harvard Medical School, Boston
Maha Hussain, Robert H. Lurie Comprehensive Cancer Center, Northwestern University Feinberg School of Medicine, Chicago
Fred Saad, University of Montreal Hospital Center, Montreal
Karim Fizazi, Institut Gustave Roussy, University of Paris–Saclay, Villejuif, France
Cora N. Sternberg, Englander Institute for Precision Medicine, Weill Cornell Medicine, Meyer Cancer Center, New York–Presbyterian Hospital, New York
E. David Crawford, University of California San Diego School of Medicine, La Jolla
Evgeny Kopyltsov, Clinical Oncologic Dispensary of Omsk Region, Omsk, Russia
Chandler H. Park, Norton Cancer Institute, Louisville, KY
Boris Alekseev, P. Hertsen Moscow Oncology Research Institute, Moscow, Russia
Álvaro Montesa-Pino, La Unidad de Gestión Clínica Intercentros de Oncología Médica, Hospitales Universitarios Regional y Virgen Victoria, Instituto de Investigación Biomédica de Málaga, Malaga, Spain
Dingwei Ye, Fudan University Shanghai Cancer Center, Shanghai, China
Francis Parnis, Ashford Cancer Centre Research, Kurralta Park, SA, Australia, Finland
Felipe Cruz, Núcleo de Pesquisa e Ensino da Rede São Camilo, São Paulo, Finland
Teuvo L.J. Tammela, Tampere University Hospital and Tampere University, Tampere, Finland
Hiroyoshi Suzuki, Toho University Sakura Medical Center, Chiba, Japan
Tapio Utriainen, Helsinki University Central Hospital, Comprehensive Cancer Center, Helsinki, Finland
Cheng Fu, Liaoning Cancer Hospital and Institute, Shenyang, China
Motohide Uemura, Osaka University Hospital, Osaka, Japan
María J. Méndez-Vidal, Maimonides Institute for Biomedical Research of Córdoba, Reina Sofía University Hospital, Cordoba, Spain
Benjamin L. Maughan, Huntsman Cancer Institute, Salt Lake City
Heikki Joensuu, Orion Pharma, Espoo, Finland
Silke Thiele, Bayer, Berlin
Rui Li, Bayer HealthCare, Whippany, NJ
Iris Kuss, Bayer, Berlin
Bertrand Tombal, Division of Urology, Institut de Recherche Clinique, Cliniques Universitaires Saint Luc, Université Catholique de Louvain, Brussels
References
- 1.Gillessen S, Attard G, Beer TM, et al. Management of patients with advanced prostate cancer: report of the Advanced Prostate Cancer Consensus Conference 2019. Eur Urol 2020;77:508–47. [DOI] [PubMed] [Google Scholar]
- 2.Lowrance W, Breau R, Chou R, et al. Advanced prostate cancer: AUA/ASTRO/SUO guideline. American Urological Association, 2020. (https://www.auanet.org/documents/Guidelines/PDF/Advanced%20Prostate%20Cancer%20Guideline.pdf). [Google Scholar]
- 3.Mottet N, Cornford P, van den Bergh RCN, et al. Guidelines on prostate cancer. European Associated of Urology, 2021. (https://uroweb.org/guideline/prostate-cancer/). [DOI] [PubMed] [Google Scholar]
- 4.Parker C, Castro E, Fizazi K, et al. Prostate cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2020;31:1119–34. [DOI] [PubMed] [Google Scholar]
- 5.Sweeney CJ, Chen YH, Carducci M, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med 2015;373:737–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.James ND, de Bono JS, Spears MR, et al. Abiraterone for prostate cancer not previously treated with hormone therapy. N Engl J Med 2017;377:338–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kyriakopoulos CE, Chen YH, Carducci MA, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer: long-term survival analysis of the randomized phase III E3805 CHAARTED trial. J Clin Oncol 2018;36:1080–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clarke NW, Ali A, Ingleby FC, et al. Addition of docetaxel to hormonal therapy in low- and high-burden metastatic hormone sensitive prostate cancer: long-term survival results from the STAMPEDE trial. Ann Oncol 2019;30:1992–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fizazi K, Tran N, Fein L, et al. Abiraterone acetate plus prednisone in patients with newly diagnosed high-risk metastatic castration-sensitive prostate cancer (LATITUDE): final overall survival analysis of a randomised, double-blind, phase 3 trial. Lancet Oncol 2019;20:686–700. [DOI] [PubMed] [Google Scholar]
- 10.Chi KN, Chowdhury S, Bjartell A, et al. Apalutamide in patients with metastatic castration-sensitive prostate cancer: final survival analysis of the randomized, double-blind, phase III TITAN study. J Clin Oncol 2021;39:2294–303. [DOI] [PubMed] [Google Scholar]
- 11.Armstrong AJ, Szmulewitz RZ, Petry-lak DP, et al. ARCHES: a randomized, phase III study of androgen deprivation therapy with enzalutamide or placebo in men with metastatic hormone-sensitive prostate cancer. J Clin Oncol 2019;37:2974–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis ID, Martin AJ, Stockler MR, et al. Enzalutamide with standard first-line therapy in metastatic prostate cancer. N Engl J Med 2019;381:121–31. [DOI] [PubMed] [Google Scholar]
- 13.Fizazi K, Carles Galceran J, Foulon S, et al. A phase III trial with a 2×2 factorial design in men with de novo metastatic castration-sensitive prostate cancer: overall survival with abiraterone acetate plus prednisone in PEACE-1. Ann Oncol 2021;32:Suppl 5:S1299. abstract (https://www.annalsofoncology.org/article/S0923-7534(21)04403-3/fulltext). [Google Scholar]
- 14.Zurth C, Koskinen M, Fricke R, et al. Drug-drug interaction potential of darolutamide: In vitro and clinical studies. Eur J Drug Metab Pharmacokinet 2019;44:747–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shore N, Zurth C, Fricke R, et al. Eval-uation of clinically relevant drug-drug interactions and population pharmacokinetics of darolutamide in patients with nonmetastatic castration-resistant prostate cancer: results of pre-specified and post hoc analyses of the phase III ARAMIS trial. Target Oncol 2019;14:527–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williams S, Mazibuko N, O’Daly O, et al. Significant localized reduction in cerebral blood flow (CBF) in regions relevant to cognitive function with enzalutamide (ENZA) compared to darolutamide (DARO) and placebo (PBO) in healthy volunteers. J Clin Oncol 2020;38: Suppl:326. abstract ( 10.1200/JCO.2020.38.6_suppl.326). [DOI] [Google Scholar]
- 17.Zurth C, Sandman S, Trummel D, Darolutamide in Metastatic Prostate Cancer Seidel D, Nubbemeyer R, Gieschen H. Higher blood–brain barrier penetration of [14C]apalutamide and [14C]enzalutamide compared to [14C]darolutamide in rats using whole-body autoradiography. J Clin Oncol 2019;37:Suppl:156. abstract ( 10.1200/JCO.2019.37.7_suppl.156). [DOI] [Google Scholar]
- 18.Fizazi K, Massard C, Bono P, et al. Activity and safety of ODM-201 in patients with progressive metastatic castration-resistant prostate cancer (ARADES): an open-label phase 1 dose-escalation and randomised phase 2 dose expansion trial. Lancet Oncol 2014;15:975–85. [DOI] [PubMed] [Google Scholar]
- 19.Massard C, Penttinen HM, Vjaters E, et al. Pharmacokinetics, antitumor activity, and safety of ODM-201 in patients with chemotherapy-naive metastatic castration-resistant prostate cancer: an open-label phase 1 study. Eur Urol 2016;69:834–40. [DOI] [PubMed] [Google Scholar]
- 20.Fizazi K, Massard C, Bono P, et al. Safety and antitumour activity of ODM201 (BAY-1841788) in castration-resistant, CYP17 inhibitor-naïve prostate cancer: results from extended follow-up of the ARADES trial. Eur Urol Focus 2017;3:606–14. [DOI] [PubMed] [Google Scholar]
- 21.Fizazi K, Shore N, Tammela TL, et al. Darolutamide in nonmetastatic, castration-resistant prostate cancer. N Engl J Med 2019;380:1235–46. [DOI] [PubMed] [Google Scholar]
- 22.Fizazi K, Shore N, Tammela TL, et al. Nonmetastatic, castration-resistant prostate cancer and survival with darolutamide. N Engl J Med 2020;383:1040–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


