Abstract
In the pathogenesis of bacterial endocarditis (BE), the clotting system plays a cardinal role in the formation and maintenance of the endocardial vegetations. The extrinsic pathway is involved in the activation of the coagulation pathway with tissue factor (TF) as the key protein. Staphylococcus aureus is a frequently isolated bacterium from patients with BE. We therefore investigated whether S. aureus can induce TF activity (TFA) on fibrin-adherent monocytes, used as an in vitro model of BE. We also assessed in vivo in rabbits with catheter induced vegetations, the effect of S. aureus infection on vegetational TFA. In vitro experiments showed that adherent S. aureus induced TFA on fibrin-adherent monocytes which was optimal at a bacterium/monocyte ratio of 1 to 1. Monocyte damage occurred when this ratio exceeded 4 to 1 (visually) or 6 to 1 (propidium iodide influx) Consequently, TFA decreased. In vivo S. aureus led to very high bacterial numbers in the vegetations and a significant increase of their weight. However, TFA of infected vegetations was the same as of sterile ones. This may be due to the high bacteria to monocyte ratio as well as bacterium-induced monocyte damage. Teicoplanin treatment of infected rabbits reduced bacterial numbers in the blood and in the vegetations. Two-day treatment resulted in an increase of vegetational TFA, but after four-day treatment vegetational TFA dropped, most probably due to a suboptimal bacterium/monocyte ratio. S. aureus endocarditis in etoposide (Vepesid)-treated rabbits, leading to a selective monocytopenia, caused a rapid death of the animals. In these rabbits no vegetations were found at all. We conclude that, like Streptococcus sanguis and Staphylococcus epidermidis, S. aureus is able to induce TFA in fibrin-adherent blood monocytes. In addition, monocytes have a protective effect during the course of S. aureus endocarditis.
An inflammatory process resulting in the formation of so-called endocardial vegetations characterizes bacterial endocarditis (BE). These vegetations consist of a fibrin clot, which contains the infecting microorganisms embedded in a matrix of proteins and blood cells (17). For their formation the coagulation system has to be activated which, as shown earlier, occurs via the extrinsic pathway (4, 11). Vegetations have a procoagulant activity which is factor VII (FVII) dependent, indicating the involvement of tissue factor (TF), a transmembrane glycoprotein which serves as the central point in the extrinsic clotting pathway (1, 2, 5, 9, 11). In earlier studies we have shown that monocytes account for the TF activity (TFA) of vegetations infected with Streptococcus sanguis or Staphylococcus epidermidis (2, 3, 4).
Another frequently isolated bacterium from patients with BE is Staphylococcus aureus (10). It often causes an acute and massive valvular destruction in patients with previously intact heart valves (6, 14), in contrast to S. sanguis, which causes subacute endocarditis on natural valves, and S. epidermidis, which causes prosthetic valve endocarditis (6, 16). Because of these differences, we investigated whether S. aureus can also induce TFA on fibrin-adherent monocytes, used as an in vitro model of BE (1). We assessed in rabbits with catheter-induced vegetations the effect of S. aureus infection on vegetational TFA in vivo.
S. aureus 5558, the same strain as used in a previous study (21), was grown overnight in brain heart infusion (BHI) broth (Oxoid, London, England) at 37°C. Before use, bacteria were harvested by centrifugation, washed twice with phosphate-buffered saline (PBS; pH 7.45), and diluted to the concentration to be used. Staphylococcal numbers in the overnight cultures were measured by colony count in serial dilutions incubated overnight at 37°C on blood agar.
Adherence of the staphylococci to fibrin plates, prepared from fibrinogen (Sigma, St. Louis, Mo.) in 24-well culture plates (Costar, Cambridge, England) was measured as described earlier (3). In the in vitro study, human peripheral blood monocytes were incubated on staphylococcus-infected fibrin plates for 4 h, after which monocytic TFA was determined. These monocytes were isolated from a fresh buffy coat as described previously (4) and cultured overnight in RPMI 1640 (Gibco BRL, Paisley, Scotland) in Teflon bags (19) at 37°C in 5% CO2 before use. The TFA of monocytes was assessed as described by Bancsi et al. (1, 2). Briefly, each well was incubated with purified Factor VII (FVII) and CaCl2 (Merck, Darmstadt, Germany) for 15 min to allow formation of TF-FVII-Ca complex. Next, purified Factor X (FX) was added and, after 5 min, achieved FX formation (FXa) was stopped by the addition of EDTA (Boehringer, Mannheim, Germany). Then, PefachromeFXa (Kordia, Leiden, The Netherlands), a chromogenic substrate for FXa, was added. After 20 min, the addition of acetic acid (Merck) stopped the conversion of the substrate. The optical density at 405 nm was measured and converted to FXa concentrations. For this calculation a calibration curve was used from purified FX that was fully activated with Russel Viper Venom (Chromogenix, Mölndal, Sweden). Data are expressed as picomoles of FXa/106 monocytes.
The effect of the bacterium/monocyte ratio on the TFA of the monocytes was determined by incubating different numbers of adherent bacteria with a standard number of 1.5 × 106 monocytes, as described earlier (3). Because bacterial constituents such as peptidoglycan and lipoteichoic acid can activate endothelial cells and monocytes (8, 13, 20), we investigated whether such constituents, generated during overnight incubation of S. aureus in the presence of 10 μg of teicoplanin (Gist-brocades NV, Delft, The Netherlands) per ml, i.e., “the supernatant,” could activate monocytes to generate TFA. Supernatants were collected from S. aureus cultures grown for 5 h at 37°C in BHI broth, after which 10 μg of teicoplanin per ml was added to the medium, and then the bacteria were cultured for an additional 20 h. Monocytes were then incubated for 4 h on fibrin plates with these supernatants diluted 1 to 10 with RPMI 1640.
Cytotoxicity for monocytes of staphylococci or their breakdown products was determined by measuring cell permeability with propidium iodide (PI) influx. Monocytes were recovered from the fibrin plates after 4 h of incubation with bacteria or bacterial supernatant, incubated for 10 min with 1 μg of PI per ml, and then analyzed in a fluorescence-activated cell sorter (Becton Dickinson). Results are expressed as mean arbitrary units (AU) of fluorescence.
For the in vivo study, BE was induced in male New Zealand White rabbits as described elsewhere (4, 12). A polyethylene catheter (Portex, Hythe, England) was introduced into the left ventricle of the heart via the left carotid artery and left in situ during the experiment. Animals were sacrificed by intravenous injection of sodium pentobarbital (Euthesate; Apharmo, Arhnem, The Netherlands). Hearts were removed from which vegetations were aseptically isolated. Care was taken that vegetations were isolated without underlying endocardial tissue to avoid isolation of other potential sources of TF, such as adjacent endothelial cells. Isolated vegetations were handled as described previously (2). They were weighed and homogenized (5% [wt/vol]) in saline. Portions (100 μl) of serial dilutions of the homogenate were plated on blood agar to determine the number of CFU per gram of vegetation. The remainder of the homogenate was three times frozen in liquid nitrogen and thawed at 37°C in a water bath to lyse cells. To measure TFA, 25 μl of the homogenate with lysed cells was used. TFA was expressed as picomoles of FXa/gram of vegetation. The effect of S. aureus on vegetational TFA was assessed by comparing results of vegetations of noninfected control rabbits with those of infected rabbits either treated or not treated with teicoplanin. The MIC and minimal bactericidal concentration (MBC) of teicoplanin for S. aureus were determined as described earlier (3). The MIC was 0.25 μg/ml, while the MBC was 0.5 μg/ml. Serum concentrations of teicoplanin were determined at several time points with the Innufluor Reagent Set for the quantitative determination of teicoplanin (International Bioclinical, Inc., Portland, Oreg.). Two hours after administration of the first teicoplanin dose, serum concentrations were already 16 times the MBC value. At 8 h the serum levels of teicoplanin were maximal, after which they dropped but at the time of administration of the second dose they were still 20 times larger than the MBC. For determination of bacterial numbers in the circulation, blood was drawn immediately before sacrifice and collected in vials containing crystalline potassium EDTA (Sherwood Medical, S-H Hertogenbosch, The Netherlands). Bacterial numbers were measured by colony count in serial dilutions of 200 μl of blood plated on blood agar and incubated overnight at 37°C.
The role of monocytes on vegetational TFA in rabbits with S. aureus BE was investigated by induction of selective monocytopenia with the cytostatic drug etoposide (Vepesid; kindly donated by Bristol-Meyers Squibb B.V., Woerden, The Netherlands) as described previously (2, 3). Etoposide treatment results in a significant decrease in the numbers of blood monocytes and of vegetational monocytes (18) without affecting other white blood cells or platelets, as shown by Meddens et al. (15).
Unpaired Student's t test was used to calculate the significance of difference of the TFA of monocytes by the different bacterium/monocyte ratios. To calculate the significance of the differences in the vegetational TFA, weight, and infection, the Bonferroni test was used.
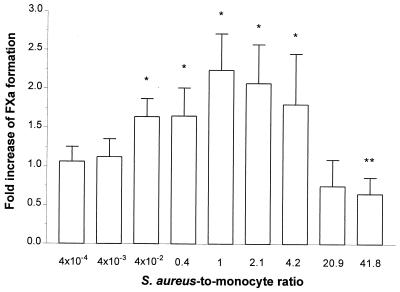
In vitro the adherence of staphylococci to fibrin plates with inocula ranging from 106 to 108 CFU/ml was approximately 10%. This was somewhat higher than the 5% of S. sanguis NCTC 7864 (2) and the 7% of S. epidermidis ATCC 149900 (3). Fibrin-bound S. aureus stimulated monocytes to generate TFA. At a bacterium/monocyte ratio of about 1:1, this stimulation was maximal (Fig. 1), with the TFA being twofold higher than that of monocytes cultured without bacteria. At lower as well as higher ratios, the TFA was lower (Fig. 1). These findings were different from those with S. sanguis (2) and S. epidermidis (3). With these microorganisms an increase in the TFA was found with an increasing ratio, reaching a maximum at bacterium-to-cell ratios of 7:1 and 9:1, respectively, while at levels above these ratios the TFA remained at the high level. Supernatants of S. aureus cultured in the presence of teicoplanin induced monocytes to express TFA at the same level as was found for monocytes incubated on S. aureus-infected fibrin matrixes (Table 1).
FIG. 1.
Influence of S. aureus-to-monocyte ratio on monocytic TFA. Approximately 1.5 × 106 monocytes were incubated with various numbers of fibrin-adherent S. aureus for 4 h. The TFA was then determined as described in the text. The results are mean ± the standard deviation (SD) of four experiments and are expressed as the fold increase of FXa formation over that of monocytes incubated on noninfected fibrin plates. These cells had a TFA of 33.6 ± 8.7 pmol of FXa/106 cells. ∗, P < 0.05; ∗∗, P < 0.01.
TABLE 1.
Effect of teicoplanin on the TFA of monocytes incubated with S. aureus or staphylococcal constituents
| Treatmenta | Mean fold increase in FXa formation ± SDb:
|
|
|---|---|---|
| Without teicoplanin | With teicoplaninc | |
| None | 1B | 1.25 ± 0.02D |
| S. aureus | 1.59 ± 0.36A | 1.65 ± 0.22C |
| Supernatantd | 1.13 ± 0.20 | 1.63 ± 0.35C |
1.5 × 106 monocytes were incubated for 4 h on noninfected or infected fibrin plates, and then the TFA was determined as described in the text.
That is, the fold increase of FXa formation over that of monocytes cultured on noninfected fibrin plates in the absence of teicoplanin. These cells had a TFA of 33.6 ± 8.7 pmol of FXa/106 monocytes. P values (superscript letters): A versus B, P < 0.05; C versus D, P < 0.05.
Supernatants were collected from S. aureus cultures grown for 5 h at 37°C in BHI broth, after which 10 μg of teicoplanin per ml was added to the medium, and bacteria were then cultured for an additional 20 h.
Supernatant of S. aureus, cultured in the presence or absence of 10 μg of teicoplanin per ml. Supernatants, diluted 1 to 10 with RPMI, were added to monocytes that were cultured for 4 h on fibrin plates.
This decline in monocytic TFA at a ratio exceeding 1:1 is probably due to progressive cell damage caused by S. aureus, which was apparent from less-adherent monocytes and many irregularly shaped cells in the supernatants when ratios exceeded 4:1. Moreover, PI influx in monocytes increased with increasing bacterium/monocyte ratios. The mean fluorescence intensity of monocytes adherent to noninfected fibrin matrix and of monocytes adherent to infected fibrin matrix at a ratio of 1:1 was approximately 34 AU. This fluorescence intensity increased approximately 14-fold at a bacterium/monocyte ratio of 6:1 and 35-fold when this ratio was increased to 60:1, indicating an increase in cell permeability, which is a measure of cell damage. This increase of PI influx coincided with a decrease of monocyte TF antigen expression (data not shown). As with intact bacteria, the supernatant of S. aureus cultures exposed to teicoplanin induced an increase of PI influx in a concentration-dependent manner. In contrast to the findings for S. aureus, cell damage was not observed with either S. sanguis or S. epidermidis, even at the highest ratio tested (2, 3).
Control rabbits infected with S. aureus had bacterial numbers in the blood ranging from 8 × 102 to 7 × 103 CFU/ml. Infected vegetations were different in texture compared to sterile vegetations. They were easy to remove and were loose and flabby. The weight of the infected vegetations was significantly higher than that of sterile vegetations (44.26 ± 14.21 and 11.19 ± 5.07 mg, respectively; P = 0.002, n = 4). Bacterial titers in the infected vegetations were more than 1010 CFU/g of vegetation. This was markedly higher than found with S. sanguis (5 × 108 CFU/g) and S. epidermidis (107 CFU/g) (2, 3). However, the TFA of the S. aureus-infected vegetations was similar to that of sterile vegetations (Table 2).
TABLE 2.
Effect of S. aureus 5558 infection and teicoplanin treatment on TFA, infection, and weight of rabbit endocardial vegetationsa
| Treatment | Infection (log CFU/g of vegetation) | TFA (pmol of FXa/g of vegetation) | Wt (mg) |
|---|---|---|---|
| Sterile | 116 ± 23D | 11.19 ± 5.07H | |
| S. aureus | >10A | 119 ± 6E | 44.26 ± 14.21J |
| S. aureus plus 2× teicoplaninb | 8.63 ± 0.59B | 157 ± 17F | 30.80 ± 11.07K |
| S. aureus plus 4× teicoplaninb | 7.27 ± 0.50C | 85 ± 4G | 36.63 ± 7.90L |
Endocarditis was induced as described in the text. All values represent the mean ± the SD of four rabbits. P values (superscript letters): A versus B, P = 0.004; A versus C, P < 0.001; B versus C, P = 0.005; D versus E, P > 0.05; E versus F, P = 0.032; F versus G, P < 0.001; H versus J, P = 0.002; J versus K, P > 0.05; J versus L, P > 0.05.
Teicoplanin treatment started 24 h after injection of staphylococci and was given at 24-h intervals.
To investigate in vivo the importance of the bacterium/monocyte ratio on the vegetational TFA, catheterized infected rabbits were treated with teicoplanin. This treatment resulted in reduction of the bacterial numbers in the blood from up to 7 × 103 CFU/ml to a maximum of 30 CFU/ml. Moreover, bacterial numbers were reduced in vegetations to 8.63 ± 0.59 log CFU/g of vegetation after two doses and to 7.27 ± 0.5 log CFU/g of vegetation after four doses (Table 2). The TFA of vegetations from infected rabbits after two doses of teicoplanin was higher than that of vegetations from infected non-teicoplanin-treated rabbits. After four doses of teicoplanin, the vegetational TFA dropped below that of infected non-teicoplanin-treated rabbits, although the difference was not significant (Table 2). Teicoplanin treatment had no effect on vegetational weight (Table 2).
During etoposide treatment, blood monocyte numbers fell to 5 to 10% of the initial values within 2 days. At day 6 of etoposide treatment, 106 CFU of staphylococci were injected. Six of eight monocytopenic rabbits died within 20 h. The two remaining rabbits were in a very poor condition and were consequently sacrificed. Bacterial counts in the blood were 5 × 106 CFU/ml. Most surprisingly, S. aureus-infected etoposide-treated rabbits had no vegetations at all neither on the valves nor the mural endocard. Valves were completely destroyed. Most probably the vegetations were released as septic emboli into the circulation as a result of the massive destruction of the valvular tissue. These findings were markedly different from those in etoposide-treated rabbits infected with S. sanguis or S. epidermidis (2, 3). These rabbits always had valvular vegetations. In rabbits with S. sanguis BE, monocytopenia led to a reduction of vegetational weight and a decrease of vegetational TFA, whereas with S. epidermidis neither vegetational weight nor TFA were affected. With both microorganisms, monocytopenia had no effect on the bacterial counts in the vegetation (2, 3).
In the non-etoposide-treated rabbits, infection with S. aureus had no effect on vegetational TFA. This result was different from the increase of vegetational TFA caused by S. sanguis and S. epidermidis. However, with the latter microorganisms in vitro monocytic TFA remained maximal above given bacterium/cell ratio, whereas for S. aureus monocytic TFA was maximal at a bacterium/cell ratio of 1:1 and decreased at higher ratios. This decrease can be accounted for by monocyte damage caused by S. aureus. Although infection of vegetations results in monocyte recruitment (18), the overwhelming bacterial numbers in the S. aureus-infected vegetations resulted in bacterium/monocyte ratios most probably exceeding the optimal ratio for induction of monocytic TFA. Also, monocyte damage, caused by S. aureus as shown by PI influx, could be a factor contributing to the lower vegetational TFA. Although not specifically considered here, an effect of the overwhelming bacteremia on platelet numbers could be an additional factor in the defective vegetation formation. This might also explain the difference in texture of the vegetations compared to those found with S. sanguis (2) or S. epidermidis (3) infection. Teicoplanin treatment reduced bacterial numbers in the vegetations and led to an increase of vegetational TFA after 2 days, but after 4 days of treatment the TFA again dropped. Apparently, reduction of bacterial titers initially lead to a more effective bacterium/monocyte ratio with respect to the TFA, but with a further decrease of the bacterial numbers in the vegetation this ratio again became suboptimal. Moreover, supernatants of S. aureus, incubated in the presence of teicoplanin, induced monocytes to express TFA. Thus, due to the decline in bacterial numbers induced by teicoplanin, not only the bacterium/monocyte ratio may have become more effective with respect to TFA but also the bacterial breakdown products, such as peptidoglycan or lipoteichoic acid, may have stimulated monocyte TFA. However, further reduction of the bacterial numbers after prolonged teicoplanin treatment apparently was more important than accumulation of bacterial breakdown products with regard to the TFA.
In conclusion, S. aureus can induce TFA on fibrin-adherent monocytes. Furthermore, monocytes play a protective role in S. aureus BE, which is a finding comparable to the findings with S. epidermidis BE made by Meddens et al. (15).
REFERENCES
- 1.Bancsi M J L M F, Thompson J, Bertina R M. Stimulation of monocyte tissue factor expression in an in vitro model of bacterial endocarditis. Infect Immun. 1994;62:5669–5672. doi: 10.1128/iai.62.12.5669-5672.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bancsi M J L M F, Veltrop M H A M, Bertina R M, Thompson J. Influence of monocytes and antibiotic treatment on tissue factor activity of endocardial vegetations in rabbits infected with Streptococcus sanguis. Infect Immun. 1996;64:448–451. doi: 10.1128/iai.64.2.448-451.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bancsi M J L M F, Veltrop M H A M, Bertina R M, Thompson J. Role of monocytes and bacteria in Staphylococcus epidermidis endocarditis. Infect Immun. 1998;66:448–450. doi: 10.1128/iai.66.2.448-450.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buiting A G M, Thompson J, van de Keur D, Schmall-Bauer W C, Bertina R M. Procoagulant activity of endocardial vegetations in rabbits with Streptococcus sanguis endocarditis. Thromb Haemost. 1989;62:1029–1033. [PubMed] [Google Scholar]
- 5.Camerer E, Kolsto A-B, Prydz H. Cell biology of tissue factor, the principal initiation of blood coagulation. Thromb Res. 1996;81:1–41. doi: 10.1016/0049-3848(95)00209-x. [DOI] [PubMed] [Google Scholar]
- 6.Chastre J, Trouillet J L. Early infective endocarditis on prosthetic valves. Eur Heart J. 1995;16(Suppl. B):32–38. doi: 10.1093/eurheartj/16.suppl_b.32. [DOI] [PubMed] [Google Scholar]
- 7.Cunha B A, Gill V, Lazar J M. Acute infective endocarditis. Diagnostic and therapeutic approach. Infect Dis Clin N Am. 1996;10:811–834. doi: 10.1016/s0891-5520(05)70328-7. [DOI] [PubMed] [Google Scholar]
- 8.Cuzzola M, Mancuso G, Beninati C, Biondo C, von Hunolstein C, Orefici G, Espevik T, Flo T H, Teti G. Human monocyte receptors involved in tumor necrosis factor responses to group B streptococcal products. Infect Immun. 2000;68:994–998. doi: 10.1128/iai.68.2.994-998.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davie E W, Fujikawa K, Kisiel W. The coagulation cascade: initiation, maintenance, and regulation. Biochemistry. 1991;30:10363–10370. doi: 10.1021/bi00107a001. [DOI] [PubMed] [Google Scholar]
- 10.Drake T A, Pang M. Staphylococcus aureus induces tissue factor expression in cultured valve endothelium. J Infect Dis. 1988;157:749–756. doi: 10.1093/infdis/157.4.749. [DOI] [PubMed] [Google Scholar]
- 11.Drake T A, Rodgers G M, Sande M A. Tissue factor is a major stimulus for vegetation formation in enterococcal endocarditis in rabbits. J Clin Investig. 1984;73:1750–1753. doi: 10.1172/JCI111383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Durack D T, Beeson P B. Experimental bacterial endocarditis. I. Colonization of a sterile vegetation. J Clin Investig. 1972;87:50–57. [PMC free article] [PubMed] [Google Scholar]
- 13.Kern W V, Engel A, Schieffer S, Prümmer O, Kern P. Circulating tumor necrosis factor alpha (TNF), soluble TNF receptors, and interleukin-6 in human subacute bacterial endocarditis. Infect Immun. 1993;61:5413–5416. doi: 10.1128/iai.61.12.5413-5416.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lowy F D. Staphylococcus aureus infections. N Engl J Med. 1998;339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 15.Meddens M J M, Thompson J, Bauer W C, Hermans J, van Furth R. Role of granulocytes and monocytes in experimental Staphylococcus epidermidis endocarditis. Infect Immun. 1983;41:145–153. doi: 10.1128/iai.41.1.145-153.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roberts R B. Streptococcal endocarditis. In: Kaye D, editor. Infective endocarditis. New York, N.Y: Raven Press; 1992. pp. 191–208. [Google Scholar]
- 17.Scheld W M, Sande M A. Endocarditis and intravascular infections. In: Mandell G L M, Douglas R G, Bennet J E, editors. Principals and practices of infection diseases. 3rd ed. New York, N.Y: John Wiley and Sons; 1990. pp. 670–706. [Google Scholar]
- 18.Thörig L, Thompson J, Eulderink F, Emeiss J J, van Furth R. Effects of monocytopenia and anticoagulation in experimental Streptococcus sanguis endocarditis. Br J Pathol. 1980;61:108–116. [PMC free article] [PubMed] [Google Scholar]
- 19.van der Meer J W M, van de Gevel J S, Elzenga-Claesen J, van Furth R. Suspension cultures of mononuclear phagocytes in the Teflon bag. Cell Immunol. 1979;42:208–212. doi: 10.1016/0008-8749(79)90236-3. [DOI] [PubMed] [Google Scholar]
- 20.van Langevelde P, Ravensbergen E, Grashoff P, Beekhuizen H, Groeneveld P H P, van Dissel J T. Antibiotic-induced cell wall fragments of Staphylococcus aureus increase endothelial chemokine secretion and adhesiveness for granulocytes. Antimicrob Agents Chemother. 1999;43:2984–2989. doi: 10.1128/aac.43.12.2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Voorn G P, Thompson J, Goessens W H F, Schmal-Bauer W C, Broeders P H M, Michel M. In vitro stability of tolerance to cloxacillin and vancomycin in Staphylococcus aureus. Eur J Clin Microbiol Infect Dis. 1994;13:741–746. doi: 10.1007/BF02276057. [DOI] [PubMed] [Google Scholar]