Abstract
Background
The prognostic value of preoperative systemic inflammatory markers, including the neutrophil‐to‐lymphocyte ratio (NLR), platelet‐to‐lymphocyte ratio (PLR), and lymphocyte‐to‐monocyte ratio (LMR), remains controversial in patients with intrahepatic cholangiocarcinoma (ICC). Therefore, this meta‐analysis aimed to investigate the prognostic value of preoperative NLR, PLR, and LMR in patients with ICC who underwent hepatic resection.
Methods
We conducted a comprehensive search of four electronic databases. Two researchers assessed the quality of the available data using the Newcastle–Ottawa Scale. We selected overall survival (OS) as the primary outcome and recurrence‐free survival (RFS) and disease‐free survival (DFS) as secondary outcomes. Hazard ratios (HRs) and 95% confidence intervals (CIs) were merged to evaluate the associations between inflammatory markers and ICC patient prognosis.
Results
Fifteen studies (18 cohorts) with 4123 cases were included in this meta‐analysis. The results revealed that a high preoperative NLR was associated with short OS and RFS (HR = 1.04, 95% CI: 1.01–1.07, and HR = 1.29, 95% CI: 1.04–1.60, respectively) in patients with ICC. However, the association between PLR or LMR and ICC prognosis was not statistically significant. In addition, the publication bias and sensitivity analyses demonstrated that the results were reliable and stable.
Conclusion
Our meta‐analysis revealed that preoperative NLR may be a useful prognostic predictor for patients with ICC.
Keywords: inflammatory biomarkers, intrahepatic cholangiocarcinoma, meta‐analysis, prognosis
This meta‐analysis aimed to investigate the prognostic value of preoperative inflammatory markers in patient with intrahepatic cholangiocarcinoma (ICC) who underwent hepatic resection. Our study revealed that high preoperative neutrophil‐lymphocyte ratio (NLR) is associated with worse prognosis in patients with ICC. The results from our meta‐analysis suggest that NLR may be used as a potential prognostic predictor for patients with ICC.
1. BACKGROUND
Intrahepatic cholangiocarcinoma (ICC) is one of the most common types of primary liver cancer, accounting for 10%–15% of all primary liver cancer cases. 1 In recent years, its incidence has increased significantly worldwide. 2 , 3 The most effective radical treatment is hepatectomy, but most patients with ICC are not fit to undergo surgery upon diagnosis with ICC. 4 For patients with operable ICC, the median survival after curative‐intent resection is 24–36 months, which is still unsatisfactory. 5 , 6 , 7 Although most patients with ICC receive the same treatment, their clinical outcomes may differ due to tumor heterogeneity and various systemic factors. Therefore, it is necessary to identify factors that can predict the prognosis and aid clinicians in selecting the most suitable therapeutic strategies for patients with ICC.
Inflammation is a hallmark of cancer. 8 Increasing evidence indicated that cancer‐associated inflammations are involved in numerous cancer‐related processes, including cancer initiation, progression, and metastasis. 9 Neutrophil‐lymphocyte ratio (NLR) is defined as the ratio of absolute neutrophil count to absolute lymphocyte count, platelet‐to‐lymphocyte ratio (PLR) is defined as the ratio of absolute thrombocyte count to absolute lymphocyte count, and lymphocyte‐to‐monocyte ratio (LMR) is defined as the ratio of absolute lymphocyte count to absolute monocyte count. These blood biomarkers reflect the inflammatory status 10 and have been proved to be valuable in predicting the prognosis of many cancer types, including colorectal cancer, 11 breast cancer, 12 urinary neoplasm, 13 and esophagogastric junction cancer. 14
The associations between NLR, LMR, PLR, and clinical outcomes in patients with ICC have been explored in various studies, but there is no consensus in results. 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 Two previous meta‐analyses evaluated the role of inflammatory factors in predicting the prognosis of patients with cholangiocarcinoma. 30 , 31 However, they focused on the whole cholangiocarcinoma cohort, not specifically on patients with ICC. In addition, they did not exclude the impact of preoperative treatment on the clinical outcomes of patients. Therefore, in this study, we assessed the predictive values of preoperative NLR, PLR, and LMR in patients with ICC who did not receive preoperative therapy by performing a meta‐analysis.
2. METHODS
The protocol for this meta‐analysis was registered in PROSPERO (CRD42021250132). We followed the Preferred Reporting Items for Systematic Reviews and Meta‐analysis (PRISMA) guidelines for this meta‐analysis. 32
2.1. Search strategy
We conducted a comprehensive search of PubMed, Cochrane Library, EMBASE, and Web of Science using a combination of relevant keywords and medical subject heading terms. The main keywords were as follows: biliary tract neoplasms, cholangiocarcinoma, neutrophil‐to‐lymphocyte ratio, PLR, LMR, NLR, PLR, LMR, prognosis, overall survival, recurrence‐free survival, disease‐free survival, OS, RFS, and DFS. File S1 describes the detailed methods used to search PubMed. All databases were searched from their inception to May 2021. We also manually searched the references of each relevant article to identify more suitable articles.
2.2. Selection criteria
The inclusion criteria were as follows: (1) P: patients in the original studies were diagnosed with ICC; (2) I: ICC group with high preoperative NLR, PLR, and LMR; (3) C: ICC group with low preoperative NLR, PLR, and LMR; (4) O: studies that reported the association between NLR, PLR, LMR, and overall survival (OS), recurrence‐free survival (RFS), and disease‐free survival (DFS) of patients with ICC; and (5) S: prospective or retrospective cohort studies. Exclusion criteria were as follows: (1) patients who underwent preoperative therapies; (2) studies that provided insufficient information for extracting hazard ratios (HRs) and 95% confidence intervals (CIs); (3) reviews, conference abstracts, comments, case reports, and letters; (4) duplicated studies or publications; and (5) nonhuman studies
2.3. Data extraction
Two researchers selected eligible articles and extracted the following information: author, country, publication year, type of research, basic characteristics of patients, tumor type, treatment strategy, tumor stage, cutoff values of inflammatory markers, study endpoints, HRs, and 95% CIs for OS, RFS, and DFS. When HRs obtained from univariate and multivariate analyses were both reported, we selected the HRs obtained from multivariate analysis as multivariate analysis can exclude correlated confounding factors and is more accurate. If some original articles did not report HRs directly, Engauge Digitizer software (version 4.1) was used to extract the HRs from survival curves. The two researchers reached consensus after discussing all differences.
2.4. Quality assessment
The quality of each included study was evaluated using the Newcastle–Ottawa Scale (NOS). The NOS criteria included three items: (1) selection, (2) comparability, and (3) outcome (cohort study). 33 The highest NOS score was 9, and studies with a score of ≥6 were considered to be high‐quality studies.
2.5. Statistical analysis
We selected OS as the primary outcome and RFS and DFS as the secondary outcomes. We combined the HRs and 95% CIs of each included study to evaluate the influence of preoperative NLR, PLR, and LMR on the prognosis of patients with ICC. We tested heterogeneity among the enrolled studies using Cochrane's Q (Chi squared) and I2 statistic. If I 2 < 50% or p > 0.10, indicating that significant heterogeneity did not exist, then HRs and 95% CIs were merged with a fixed effects model; otherwise, a random‐effects model was selected. Subgroup analyses based on statistical methods (multivariate or univariate) and sample sizes (<200 or ≥200) were performed to identify the cause of heterogeneity. In addition, Egger's test and Begg's test were performed for the evaluation of publication bias, and the trim‐and‐fill method was used to adjust for publication bias. A sensitivity analysis was conducted to determine the robustness of the synthesized results. Statistical analyses were performed using Stata 14.0 (Stata Corp.).
3. RESULTS
3.1. Selection of studies
Based on the selection criteria, 5702 articles were retrieved. First, 947 duplicates were removed. We then browsed the titles and abstracts of these studies and excluded 4629 studies if they included reviews, conference abstracts, comments, case reports, letters, nonhuman studies, and irrelevant studies. From 126 studies selected for full‐text examination, 111 were excluded owing to the following selection criteria: duplicate studies (n = 5), no survival date (n = 6), and no focus on ICC (n = 100). Finally, 15 studies (18 cohorts) [15‐29]with 4123 patients were included in our meta‐analysis. Figure 1 is the process of literature screening.
FIGURE 1.

Procedure of literature screening
3.2. Characteristics of included studies
The main features of the selected 15 studies are listed in Table 1. Notably, among these 15 studies, three contained simultaneous training and validation cohorts. Therefore, we divided each of the three studies into two separate cohorts and finally conducted a comprehensive analysis of 18 cohorts. The included cohorts were published between 2015 and 2021, with 11 cohorts from China, three from international multi‐centers, two from Japan, one from America, and one from Singapore. The sample size of each study ranged from 44 to 688. All cohorts determined the prognostic value of NLR, seven cohorts studied the relationship between PLR and prognosis, and 12 cohorts explored the predictive value of LMR. All studies were retrospective in nature. The NOS scores of all cohorts ranged from 7 to 8, suggesting good quality.
TABLE 1.
Major characteristics of eligible studies
| Author | Year | Country | Stage | No. of pts | Age (y) | Tumor type | Markers | Cutoff value | Outcome | Study design | Analysis method | Treatment | NOS score | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NLR | PLR | LMR | |||||||||||||
| Brustia 15 | 2019 | International multi‐centered | I–IV | 355 | 68 (60–75) a | ICC | NLR | NR | NR | NR | OS | Retrospective | MV | Surgery + adjuvant therapy | 8 |
| Chen 16 | 2015 | China | I–IVa | 322 | 57.8 ± 11.2 a | ICC | NLR, LMR | NR | 123 | NR | OS/RFS | Retrospective | MV | NR | 8 |
| Lin 29 | 2015 | China | I–IV | 102 | NR | ICC | NLR | 3 | NR | NR | OS/RFS | Retrospective | MV | Surgery | 8 |
| Lin(A) 17 TC | 2019 | China | I‐IV | 123 | 60 (31–85) a | ICC | NLR, PLR, LMR | 2.94 | 130.6 | 3.62 | OS/DFS | Retrospective | MV/UV | NR | 8 |
| Lin(B) 17 VC | 2019 | China | I‐IV | 95 | 61 (37–79) a | ICC | NLR, PLR, LMR | 2.94 | 130.6 | 3.62 | OS/DFS | Retrospective | UV | NR | 8 |
| Ma 18 | 2021 | China | I‐IV | 102 | 49 (28–77) a | ICC | NLR, PLR, LMR | 3 | 90 | 2.7 | OS/DFS | Retrospective | MV/UV | NR | 8 |
| Ohira 19 | 2019 | Singapore | I‐IV | 52 | NR | ICC | NLR, PLR, LMR | 1.93 | 98 | 4.36 | OS/DFS | Retrospective | MV/UV | Surgery + adjuvant therapy | 8 |
| Sasaki(A) 20 TC | 2017 | International multi‐centered | I–IV | 269 | 58 (51–66) a | ICC | NLR | NR | NR | NR | OS | Retrospective | MV | NR | 8 |
| Sasaki(B) 20 VC | 2017 | International multi‐centered | I–IV | 269 | 57 (49–64) a | ICC | NLR | NR | NR | NR | OS | Retrospective | MV | NR | 8 |
| Tsilimigras 21 | 2020 | America | I‐IV | 688 | 57 (49–65) a | ICC | NLR, PLR | 5 | 190 | NR | OS | Retrospective | MV | Surgery + adjuvant therapy | 8 |
| Wang 22 TC | 2020 | China | I‐III | 264 | 57.26 ± 10.71 b | ICC | NLR, LMR | 2.62 | 103 | NR | OS/RFS | Retrospective | MV | NR | 8 |
| Wang 22 VC | 2020 | China | I‐III | 263 | 57.26 ± 10.72 b | ICC | NLR, LMR | 2.62 | 103 | NR | OS/RFS | Retrospective | MV | NR | 8 |
| Watanabe 23 | 2019 | Japan | I‐IV | 44 | 46–88 b | ICC | NLR | 3 | NR | NR | RFS | Retrospective | UV | NR | 7 |
| Wu 24 | 2018 | China | I‐IV | 123 | 56.8 ± 10.67 b | ICC | NLR, LMR | 2.05 | NR | 3.42 | OS | Retrospective | MV | NR | 8 |
| Yoh 25 | 2017 | Japan | I‐IV | 134 | 65 (26–84) a | ICC | NLR, LMR | 5 | 120 | NR | OS | Retrospective | MV | Surgery + adjuvant therapy | 8 |
| Zhang 27 | 2020 | China | I–III | 128 | 56.19 ± 9.63 b | ICC | NLR, PLR, LMR | 3.3 | 156.8 | 3.2 | OS/RFS | Retrospective | MV/UV | Surgery | 8 |
| Zhang 26 | 2018 | China | I–IVa | 322 | 57.9 (27–81) b | ICC | NLR, LMR | NR | NR | 4.45 | OS | Retrospective | MV | NR | 8 |
| Zhao 28 | 2021 | China | I–IVa | 468 | 58 (51–65) a | ICC | NLR, PLR, LMR | NR | 143.5 | NR | OS | Retrospective | MV/UV | Surgery + adjuvant therapy | 8 |
Abbreviations: MV, multivariate analysis; NOS, Newcastle–Ottawa Scale; NR, not reported; TC, training cohort; UV, univariate analysis; VC, validation cohort.
Median.
Mean.
3.3. Prognostic value of NLR
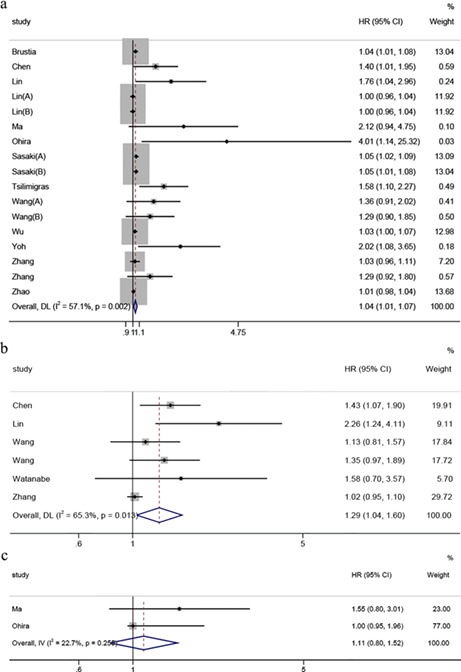
Seventeen cohorts reported the predictive value of NLR for OS. Due to the presence of moderate heterogeneity between studies (I 2 = 57.1%, p = 0.001), HRs were pooled using a random‐effects model. The pooled results revealed that a high preoperative NLR was significantly associated with poorer OS in patients with ICC (HR = 1.04, 95% CI: 1.01–1.07, Figure 2A). To further identify the potential factors for heterogeneity, we performed subgroup analyses stratified by statistical methods and sample size. Subgroup analyses showed that statistical methods could slightly reduce the heterogeneity. NLR was still significantly associated with OS in studies analyzed using the multivariate method (HR = 1.07, 95% CI: 1.03–1.11) and in studies with a sample size ≥200 (HR = 1.05, 95% CI: 1.01–1.08). In contrast, NLR did not have a significant prognostic effect on OS in studies analyzed by univariate method (HR = 1.00, 95% CI: 0.98–1.02), in studies with sample size <200 (HR = 1.03, 95% CI: 0.98–1.07). The details are presented in Table 2.
FIGURE 2.
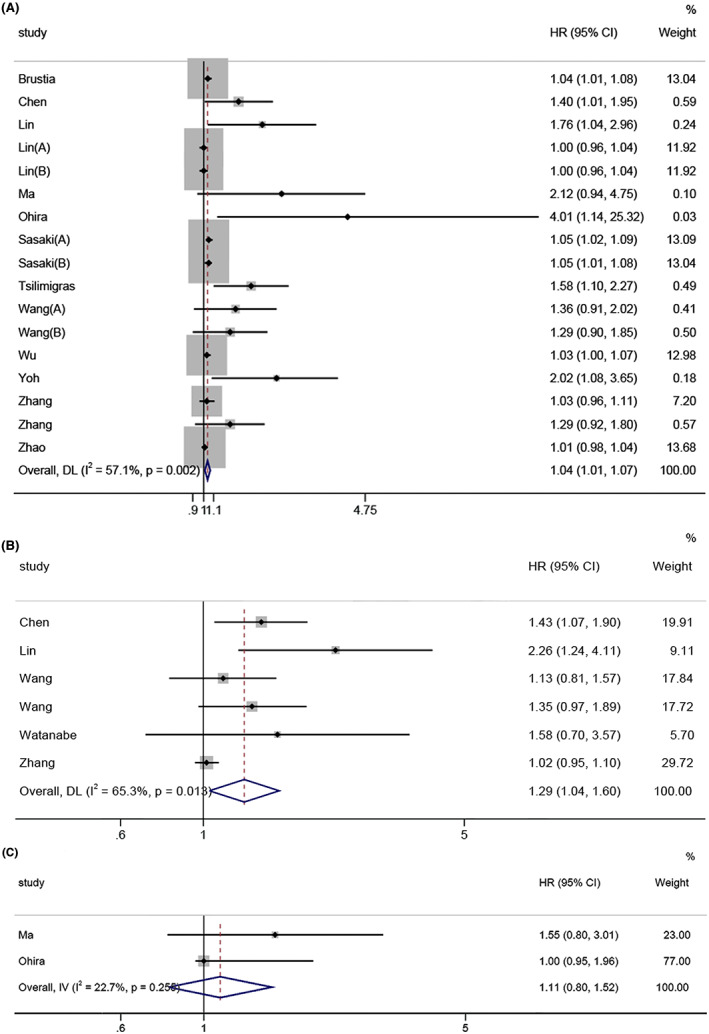
Forest plot for association between NLR and survival in ICC. (a) OS; (b) RFS; (c) DFS
TABLE 2.
Subgroup analysis of NLR for OS in ICC
| Factor | No. of studies | No. of pts | HR and 95%CI | Heterogeneity | |
|---|---|---|---|---|---|
| I 2 (%) | p‐value | ||||
| OS | 17 | 4079 | 1.04(1.01–1.07) | 57.1 | 0.002 |
| Statistical method | |||||
| Multivariate | 13 | 3265 | 1.07 (1.03–1.11) | 55.7 | 0.008 |
| Univariate | 4 | 814 | 1.00 (0.98–1.02) | 0 | 0.884 |
| Sample size | |||||
| <200 | 8 | 859 | 1.03 (0.98–1.07) | 60.3 | 0.014 |
| ≥200 | 9 | 3220 | 1.05 (1.01–1.08) | 56.0 | 0.020 |
Six cohorts reported data on the association between the NLR and RFS. 16 , 22 , 23 , 26 , 29 With observable heterogeneity in the six studies (I 2 = 65.3%, p = 0.013), we adopted a random‐effects model. The emerged HR was 1.29 (95% Cl: 1.04–1.60; Figure 2B), indicating that a high preoperative NLR significantly predicted shorter RFS in patients with ICC.
Only two cohorts studied the prognostic effect of NLR on DFS. We did not observe significant heterogeneity between the studies (I 2 = 22.7%, P = 0.255); therefore, a fixed‐effects model was employed. The combined results implied that NLR did not have a predictive value for DFS (HR = 1.11, 95% CI: 0.80–1.52; Figure 2c).
3.4. Prognostic value of PLR
Eleven studies assessed the relationship between the PLR and OS. Significant heterogeneity was detected (I 2 = 66.6%, p = 0.001); therefore, we pooled the HRs using a random‐effects model. The pooled results revealed that PLR was not related to OS (HR = 1.00, 95% CI: 0.99–1.01, Figure S1). To further identify the potential factors for heterogeneity, we performed subgroup analyses stratified by statistical methods and sample size. Subgroup analyses showed that the sample size could slightly reduce the heterogeneity. PLR was not related to OS, regardless of statistical methods or sample size. The results are summarized in Table 3.
TABLE 3.
Subgroup analysis of PLR for OS in ICC
| Subgroup | Number of studies | No. of pts | HR and 95% CI | Heterogeneity | |
|---|---|---|---|---|---|
| I 2 (%) | p‐value | ||||
| OS | 11 | 2833 | 1.00 (0.99–1.01) | 66.6 | 0.001 |
| Statistical method | |||||
| Multivariate | 7 | 372 | 1.00 (1.00–1.01) | 79.8 | 0.000 |
| Univariate | 4 | 2461 | 1.00 (0.98–1.02) | 0 | 0.987 |
| Sample size | |||||
| <200 | 6 | 506 | 1.00 (0.99–1.01) | 0 | 0.265 |
| ≥200 | 5 | 2327 | 1.00 (1.00–1.01) | 30.4 | 0.000 |
Four studies analyzed the predictive effect of PLR on RFS and only two studies supplied data on the association between PLR and DFS. We noted significant heterogeneity between studies for RFS (I 2 = 66.1%, p = 0.032) and DFS (I 2 = 72.8%, p = 0.055). Analysis with the random‐effects model suggested that PLR had no predictive effect on RFS (HR = 1.06, 95% CI: 0.87–1.29; Figure S2) or DFS (HR = 1.25, 95% CI: 0.70–2.24; Figure S3).
3.5. Prognostic value of LMR
Eight studies provided the association between the LMR and OS. Given the existence of heterogeneity (I 2 = 65.4%, p = 0.005), a random‐effects model was employed. No significant difference was observed in the association between LMR and OS (HR = 0.99, 95% CI: 0.93–1.05; Figure S4). In the subgroup analyses of statistical methods and sample size, we observed that the above two factors did not reduce heterogeneity. In addition, LMR was not related to OS, regardless of statistical methods or sample size. The results are summarized in Table 4.
TABLE 4.
Subgroup analysis of LMR for OS in ICC
| Subgroup | Number of studies | No. of pts | HR and 95%CI | Heterogeneity | |
|---|---|---|---|---|---|
| I 2 (%) | p‐value | ||||
| OS | 8 | 1413 | 0.99 (0.93–1.05) | 65.4 | 0.005 |
| Statistical method | |||||
| Multivariate | 5 | 722 | 0.82 (050–1.35) | 76.4 | 0.002 |
| Univariate | 3 | 691 | 1.00 (1.00–1.01) | 0 | 0.951 |
| Sample size | |||||
| <200 | 6 | 623 | 0.96 (0.81–1.14) | 74.9 | 0.001 |
| ≥200 | 2 | 790 | 1.00 (0.99–1.01) | 0 | 0.608 |
Only one study reported an association between LMR and RFS; therefore, we did not merge these data. Next, we included two studies that evaluated the association between LMR and DFS. No heterogeneity was found (I 2 = 0%, p = 0.534); therefore, we adopted the fixed‐effects model for analysis. The synthesized HR was 1.00 (95% Cl: 0.95–1.05; Figure S5), indicating that LMR had no predictive value for DFS in patients with ICC.
3.6. Publication bias
Regarding the publication bias of NLR for OS, the p‐value of Begg's test was 0.007, and the p‐value of Egger's test was 0.000, suggesting the presence of publication bias (Begg's test, Figure 3A). Subsequently, we evaluated the stability of the combined HR value using the trim‐and‐fill method. The recombined result confirmed that NLR was still a useful predictive marker for OS (HR = 1.03, 95% CI: 1.02–1.04; Figure 3B), which did not differ from the initial result. Additionally, we found no significant publication bias of PLR for OS (p = 0.917 for Begg's test, Figure 3c; and p = 0.218 for Egger's test) or LMR for OS (p = 0.536 for Begg's test, Figure 3d; and p = 0.447 for Egger's test), indicating the robustness of our meta‐analysis.
FIGURE 3.
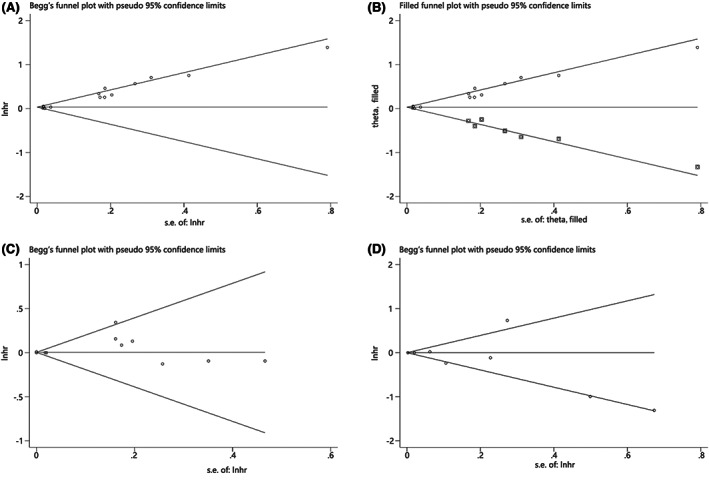
Funnel plot for publication bias. (a) Begg's funnel plot for OS of NLR; (b) Trim‐and‐fill funnel plot for OS of NLR; (c) Begg's funnel plot for OS of PLR; (d) Begg's funnel plot for OS of LMR.
3.7. Sensitivity analysis
We sequentially removed each study to assess its impact on the combined result. Sensitivity analysis revealed that the associations of OS with NLR, PLR, and LMR were not significantly changed in any independent study (Figure 4A–C), which supports the results of our meta‐analysis.
FIGURE 4.

Sensitivity analysis. (a) NLR and OS; (b) of PLR and OS; (c) LMR and OS
4. DISCUSSION
Cancer‐related inflammation is associated with tumor growth, progression, and metastasis. 8 , 9 , 34 NLR, PLR, and LMR are of great significance in determining the patient prognosis in various tumors. 35 , 36 , 37 Although there are many studies based on the association between these indices and ICC patient prognosis, the results are not uniform. In our meta‐analysis, we summarized the results of 15 studies (18 cohorts) to systematically assess the prognostic value of preoperative NLR in patients with ICC. Our results showed that a high NLR was associated with short OS and RFS, but PLR and LMR were not associated with OS, RFS, and DFS. Furthermore, subgroup analyses suggested that patients with a high NLR had a short OS than those with a low NLR in studies analyzed using the multivariate method and in studies with a sample size ≥200. Moreover, the publication bias and sensitivity analyses demonstrated the reliability and stability of our study. Collectively, preoperative NLR can be used as an important prognostic predictor for patients with ICC. As NLR can be easily determined via routine blood tests, this parameter may have great clinical application potential to guide decision‐making in clinical settings.
Although the NLR can serve as a prognostic biomarker of ICC, the specific mechanism remains unclear. Considering that NLR is the ratio of neutrophils to lymphocytes, we speculate that the potential mechanism may be related to the functions of neutrophils and lymphocytes. In general, neutrophils are involved in every step of carcinogenesis, including tumor initiation, growth, and metastasis. Specifically, during tumor initiation, neutrophils can produce reactive oxygen species (ROS), matrix metalloprotein (MMP9), and reactive nitrogen species (RNS), which further promote tumor initiation. 38 Neutrophils can promote tumor growth by inducing angiogenesis or by compromising immunity via amino acid consumption or release of specific cytokines. 39 At the site of metastasis, neutrophils can restrict the antitumor function of CD8+ T cells by generating inducible nitric oxide synthase (iNOS). 40 Moreover, regulatory B cells instruct neutrophils to restrict the response of natural killer cells (NK cells) and T cells to metastatic lesions. 41 , 42 In contrast, lymphocytes, which mainly generate T lymphocytes, NK cells, and B cells, can induce tumor cell death and inhibit tumor proliferation and migration. 43 More specifically, the antitumor response of lymphocytes is mainly mediated by the interaction between CD8+ and CD4+ T cells. 44 CD8+ T cells, which directly kill tumor cells upon contact by expressing death ligands, can secrete cytotoxic mediators (perforin) and cytokines (interferon‐γ and tumor necrosis factor‐α). 45 CD4+ T cells can release interleukin (IL)‐2, IL‐4, and IL‐5, which can activate B cells, cytotoxic T cells, and macrophages. 46 In addition, accumulating evidence has also demonstrated that the greater the number of tumor‐infiltrating lymphocytes, the better is the prognosis of patients. 47 , 48 According to the above mechanism, NLR is obtained by dividing the number of neutrophils with that of lymphocytes, and an increase in NLR can reflect an increase in the neutrophil‐dependent inflammatory response or a decrease in the lymphocyte‐mediated antitumor immune response, resulting in the poor prognosis of patients.
Subgroup analysis of NLR and OS showed that the statistical methods only slightly reduced the heterogeneity, and several sources of heterogeneity were speculated. First, heterogeneity may be caused by differences in the study regions. The included cohorts were from different regions (14 from Asia, one from America, three from multi‐centers involving America, Europe, Asia, and Oceania). The multicenter studies included different regions, such as America and Asia, and did not provide information on the specific number of people from each region. In addition, only one American study was available; therefore, a subgroup analysis based on regions could not be carried out.
Second, different types of postoperative treatment may be a potential source of heterogeneity. Although all the original studies excluded patients who had received preoperative treatment, the postoperative treatment strategies may have been different, resulting in heterogeneity in the meta‐analysis. Few studies have provided a postoperative treatment scheme; only five studies reported that some patients had received postoperative adjuvant chemotherapy, so subgroup analysis based on postoperative treatment methods cannot be completed. We suggest that detailed postoperative treatment methods should be provided in future studies.
Third, the macroscopic types of ICC may cause heterogeneity. Uchiyama et al. 49 reported that the macroscopic types of ICC were associated with prognosis; however, the original 15 articles (18 cohorts) rarely considered macroscopic types of ICC. To decrease the heterogeneity from different macroscopic types, we suggest that future studies can limit the subjects to patients with a certain macroscopic type or provide sufficient subgroup data of each macroscopic type.
The strength of our meta‐analysis was that we excluded the interference of confounding factors. Patients may be exposed to corticosteroids or antibiotics after surgery, which may affect the levels of neutrophils and lymphocytes. 50 In addition, stress during operation may also affect systemic inflammation. 51 All studies enrolled in this meta‐analysis focused on preoperative blood samples, which excluded the effect of surgery on systemic inflammatory indicators. Moreover, all patients in the original studies did not receive any preoperative treatment, which eliminated the impact of drugs on the results of routine blood examinations. Finally, to our knowledge, previous meta‐analyses reported the prognostic role of one or two inflammatory factors in cholangiocarcinoma, while our meta‐analysis systematically summarized the potential clinical value of three inflammatory markers in ICC.
Several limitations for this meta‐analysis should be considered. First, our meta‐analysis was preliminary as only a small number of articles were included in this study; therefore, more high‐quality studies are needed to further evaluate the prognostic role of preoperative inflammatory markers in patients with ICC. Second, although the types of studies were not a part of the selection criteria, all the original studies included here were retrospective in nature, which may have caused a selection bias. Third, because the HR values were not reported in some original studies, we indirectly extracted the relevant data from the survival curves, which may have resulted in some bias. Fourth, most studies included in the present meta‐analysis were conducted in Asia. Owing to the differences in the genetic background, environment, and lifestyles of patients from various regions, the limited regions may have affected the reliability of our findings. Finally, the selected original studies did not have uniform cutoff values for NLR, PLR, or LMR; therefore, more large‐scale prospective studies are necessary to establish optimal cutoff values for these indicators.
5. CONCLUSION
In conclusion, our study revealed that high preoperative NLR is associated with worse prognosis in patients with ICC. Our results suggest that NLR may be used as a potential prognostic predictor for patients with ICC. Moreover, clinicians can combine the information on inflammatory markers with that on the TNM stage and histological subtype to predict the prognosis of patients with ICC.
AUTHOR CONTRIBUTIONS
HC and YL designed the study and wrote the main manuscript text. HC, YL, and SL selected publications and analyzed the data. HC and YL evaluated study quality. SL and GL revised manuscript. All authors approved the final manuscript.
CONFLICT OF INTEREST
The authors have declared no conflict of interest.
Supporting information
Figure S1
Figure S2
Figure S3
Figure S4
Figure S5
File S1
ACKNOWLEDGMENTS
The authors would like to thank all the people who helped us in this study.
Cui H, Li Y, Li S, Liu G. Prognostic utility of preoperative inflammatory markers in patients with intrahepatic cholangiocarcinoma after hepatic resection: A systematic review and meta‐analysis. Cancer Med. 2023;12:99‐110. doi: 10.1002/cam4.4935
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Sung H, Ferlay J, Siegel RL: Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. 2021, 71(3):209–249. [DOI] [PubMed] [Google Scholar]
- 2. Wu L, Tsilimigras DI, Paredes AZ, et al. Trends in the incidence, treatment and outcomes of patients with intrahepatic cholangiocarcinoma in the USA: facility type is associated with margin status, use of lymphadenectomy and overall survival. World J Surg. 2019;43(7):1777‐1787. [DOI] [PubMed] [Google Scholar]
- 3. Singal AK, Vauthey JN, Grady JJ, Stroehlein JR. Intra‐hepatic cholangiocarcinoma—frequency and demographic patterns: thirty‐year data from the M.D Anderson Cancer Center, J Cancer Res Clin Oncol. 2011;137(7):1071‐1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Moeini A, Sia D, Bardeesy N, Mazzaferro V, Llovet JM. Molecular pathogenesis and targeted therapies for intrahepatic cholangiocarcinoma. Clin Cancer Res. 2016;22(2):291‐300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Konstantinidis IT, Groot Koerkamp B, Do RK, et al. Unresectable intrahepatic cholangiocarcinoma: systemic plus hepatic arterial infusion chemotherapy is associated with longer survival in comparison with systemic chemotherapy alone. Cancer. 2016;122(5):758‐765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Endo I, Gonen M, Yopp AC, et al. Intrahepatic cholangiocarcinoma: rising frequency, improved survival, and determinants of outcome after resection. Ann Surg. 2008;248(1):84‐96. [DOI] [PubMed] [Google Scholar]
- 7. Amini N, Ejaz A, Spolverato G, Kim Y, Herman JM, Pawlik TM. Temporal trends in liver‐directed therapy of patients with intrahepatic cholangiocarcinoma in the United States: a population‐based analysis. J Surg Oncol. 2014;110(2):163‐170. [DOI] [PubMed] [Google Scholar]
- 8. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646‐674. [DOI] [PubMed] [Google Scholar]
- 9. DeNardo DG, Johansson M, Coussens LM. Immune cells as mediators of solid tumor metastasis. Cancer Metastasis Rev. 2008;27(1):11‐18. [DOI] [PubMed] [Google Scholar]
- 10. Peng H, Luo X. Prognostic significance of elevated pretreatment systemic inflammatory markers for patients with prostate cancer: a meta‐analysis. Cancer Cell Int. 2019;19:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kwon HC, Kim SH, Oh SY, et al. Clinical significance of preoperative neutrophil‐lymphocyte versus platelet‐lymphocyte ratio in patients with operable colorectal cancer. Biomarkers. 2012;17(3):216‐222. [DOI] [PubMed] [Google Scholar]
- 12. Dirican A, Kucukzeybek BB, Alacacioglu A, et al. Do the derived neutrophil to lymphocyte ratio and the neutrophil to lymphocyte ratio predict prognosis in breast cancer? Int J Clin Oncol. 2015;20(1):70‐81. [DOI] [PubMed] [Google Scholar]
- 13. Ohno Y, Nakashima J, Ohori M, et al. Clinical variables for predicting metastatic renal cell carcinoma patients who might not benefit from cytoreductive nephrectomy: neutrophil‐to‐lymphocyte ratio and performance status. Int J Clin Oncol. 2014;19(1):139‐145. [DOI] [PubMed] [Google Scholar]
- 14. Liu XB, Gao ZY, Zhang QH, et al. Preoperative neutrophil lymphocyte ratio can be used as a predictor of prognosis in patients with adenocarcinoma of the Esophagogastric junction: a systematic review and meta analysis. Front Oncol. 2020;10:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brustia R, Langella S, Kawai T, et al. Preoperative risk score for prediction of long‐term outcomes after hepatectomy for intrahepatic cholangiocarcinoma: Report of a collaborative, international‐based, external validation study. Eur J Surg Oncol. 2020;46(4 Pt A):560‐571. [DOI] [PubMed] [Google Scholar]
- 16. Chen Q, Dai Z, Yin D, et al. Negative impact of preoperative platelet‐lymphocyte ratio on outcome after hepatic resection for intrahepatic cholangiocarcinoma. Medicine. 2015;94(13):e574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lin J, Fang T, Zhu M, et al. Comparative performance of inflammation‐based prognostic scores in patients operated for intrahepatic cholangiocarcinoma. Cancer Manag Res. 2019;11:9107‐9119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ma B, Meng H, Shen A, et al. Prognostic value of inflammatory and tumour markers in small‐duct subtype intrahepatic Cholangiocarcinoma after curative‐intent resection. Gastroenterol Res Pract. 2021;2021:6616062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ohira M, Yoshizumi T, Yugawa K, et al. Association of inflammatory biomarkers with long‐term outcomes after curative surgery for mass‐forming intrahepatic cholangiocarcinoma. Surg Today. 2020;50(4):379‐388. [DOI] [PubMed] [Google Scholar]
- 20. Sasaki K, Margonis GA, Andreatos N, et al. Preoperative risk score and prediction of Long‐term outcomes after hepatectomy for intrahepatic Cholangiocarcinoma. J Am Coll Surg. 2018;226(4):393‐403. [DOI] [PubMed] [Google Scholar]
- 21. Tsilimigras DI, Moris D, Mehta R, et al. The systemic immune‐inflammation index predicts prognosis in intrahepatic cholangiocarcinoma: an international multi‐institutional analysis. HPB. 2020;22(12):1667‐1674. [DOI] [PubMed] [Google Scholar]
- 22. Wang JJ, Li H, Li JX, Xu L, Wu H, Zeng Y. Preoperative gamma‐glutamyltransferase to lymphocyte ratio predicts long‐term outcomes in intrahepatic cholangiocarcinoma patients following hepatic resection. World J Gastroenterol. 2020;26(13):1501‐1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Watanabe A, Harimoto N, Araki K, et al. Absolute neutrophil count predicts postoperative prognosis in mass‐forming intrahepatic Cholangiocarcinoma. Anticancer Res. 2019;39(2):941‐947. [DOI] [PubMed] [Google Scholar]
- 24. Wu Y, Ren F, Chai Y, et al. Prognostic value of inflammation‐based indexes for intrahepatic cholangiocarcinoma following curative resection. Oncol Lett. 2019;17(1):165‐174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yoh T, Seo S, Hatano E, et al. A novel biomarker‐based preoperative prognostic grading system for predicting survival after surgery for intrahepatic Cholangiocarcinoma. Ann Surg Oncol. 2017;24(5):1351‐1357. [DOI] [PubMed] [Google Scholar]
- 26. Zhang Y, Shi SM, Yang H, et al. Systemic inflammation score predicts survival in patients with intrahepatic cholangiocarcinoma undergoing curative resection. J Cancer. 2019;10(2):494‐503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang Z, Zhou Y, Hu K, Huang Y. Investigating effects of preoperative inflammatory biomarkers on predicting survival outcomes of intrahepatic cholangiocarcinoma after curative resection. World J Surg Oncol. 2020;18(1):272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhao J, Chen Y, Wang J, et al. Preoperative risk grade predicts the long‐term prognosis of intrahepatic cholangiocarcinoma: a retrospective cohort analysis. BMC Surg. 2021;21(1):113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lin G, Liu Y, Li S, et al. Elevated neutrophil‐to‐lymphocyte ratio is an independent poor prognostic factor in patients with intrahepatic cholangiocarcinoma. Oncotarget. 2016;7(32):50963‐50971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tan DW, Fu Y, Su Q, et al. Prognostic significance of neutrophil to lymphocyte ratio in oncologic outcomes of Cholangiocarcinoma: a meta‐analysis. Sci Rep. 2016;6:33789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hu G, Liu Q, Ma JY, Liu CY. Prognostic significance of platelet‐to‐lymphocyte ratio in cholangiocarcinoma: a meta‐analysis. Biomed Res Int. 2018;2018:7375169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ (Clinical Research Ed). 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stang A. Critical evaluation of the Newcastle‐Ottawa scale for the assessment of the quality of nonrandomized studies in meta‐analyses. Eur J Epidemiol. 2010;25(9):603‐605. [DOI] [PubMed] [Google Scholar]
- 34. Khandia R, Munjal A. Interplay between inflammation and cancer. Adv Protein Chem Struct Biol. 2020;119:199‐245. [DOI] [PubMed] [Google Scholar]
- 35. Guo W, Lu X, Liu Q, et al. Prognostic value of neutrophil‐to‐lymphocyte ratio and platelet‐to‐lymphocyte ratio for breast cancer patients: an updated meta‐analysis of 17079 individuals. Cancer Med. 2019;8(9):4135‐4148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shao Y, Li W, Wang D, Wu B. Prognostic value of preoperative lymphocyte‐related systemic inflammatory biomarkers in upper tract urothelial carcinoma patients treated with radical nephroureterectomy: a systematic review and meta‐analysis. World J Surg Oncol. 2020;18(1):273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ni L, Tao J, Xu J, et al. Prognostic values of pretreatment neutrophil‐to‐lymphocyte and platelet‐to‐lymphocyte ratios in endometrial cancer: a systematic review and meta‐analysis. Arch Gynecol Obstet. 2020;301(1):251‐261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Deryugina EI, Zajac E, Juncker‐Jensen A, Kupriyanova TA, Welter L, Quigley JP. Tissue‐infiltrating neutrophils constitute the major in vivo source of angiogenesis‐inducing MMP‐9 in the tumor microenvironment. Neoplasia (New York, NY). 2014;16(10):771‐788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gabrilovich DI, Ostrand‐Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12(4):253‐268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Coffelt SB, Kersten K, Doornebal CW, et al. IL‐17‐producing γδ T cells and neutrophils conspire to promote breast cancer metastasis. Nature. 2015;522(7556):345‐348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bodogai M, Moritoh K, Lee‐Chang C, et al. Immunosuppressive and Prometastatic functions of myeloid‐derived suppressive cells rely upon education from tumor‐associated B cells. Cancer Res. 2015;75(17):3456‐3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Coffelt SB, Wellenstein MD, de Visser KE. Neutrophils in cancer: neutral no more. Nat Rev Cancer. 2016;16(7):431‐446. [DOI] [PubMed] [Google Scholar]
- 43. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer‐related inflammation. Nature. 2008;454(7203):436‐444. [DOI] [PubMed] [Google Scholar]
- 44. Pagès F, Galon J, Dieu‐Nosjean MC, Tartour E, Sautès‐Fridman C, Fridman WH. Immune infiltration in human tumors: a prognostic factor that should not be ignored. Oncogene. 2010;29(8):1093‐1102. [DOI] [PubMed] [Google Scholar]
- 45. Maher J, Davies ET. Targeting cytotoxic T lymphocytes for cancer immunotherapy. Br J Cancer. 2004;91(5):817‐821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Curtis LT, Sebens S, Frieboes HB. Modeling of tumor response to macrophage and T lymphocyte interactions in the liver metastatic microenvironment. Cancer Immunol Immunother. 2021;70(5):1475‐1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Denkert C, Loibl S, Noske A, et al. Tumor‐associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2010;28(1):105‐113. [DOI] [PubMed] [Google Scholar]
- 48. Loi S, Sirtaine N, Piette F, et al. Prognostic and predictive value of tumor‐infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node‐positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin‐based chemotherapy: BIG 02‐98. J Clin Oncol. 2013;31(7):860‐867. [DOI] [PubMed] [Google Scholar]
- 49. Uchiyama K, Yamamoto M, Yamaue H, et al. Impact of nodal involvement on surgical outcomes of intrahepatic cholangiocarcinoma: a multicenter analysis by the study Group for Hepatic Surgery of the Japanese Society of Hepato‐Biliary‐Pancreatic Surgery. J Hepatobiliary Pancreat Sci. 2011;18(3):443‐452. [DOI] [PubMed] [Google Scholar]
- 50. Yang C, Wen HB, Zhao YH, Huang WH, Wang ZF, Li ZQ. Systemic inflammatory indicators as prognosticators in glioblastoma patients: a comprehensive meta‐analysis. Front Neurol. 2020;11:580101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Han S, Liu Y, Li Q, Li Z, Hou H, Wu A. Pre‐treatment neutrophil‐to‐lymphocyte ratio is associated with neutrophil and T‐cell infiltration and predicts clinical outcome in patients with glioblastoma. BMC Cancer. 2015;15:617. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Figure S2
Figure S3
Figure S4
Figure S5
File S1
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


