Abstract
When tachyzoites were incubated with human peripheral blood leukocytes in vitro, more monocytes and dendritic cells than neutrophils or lymphocytes were infected. Although tachyzoites were able to divide in each of these cell types, monocytes and dendritic cells were more permissive to rapid tachyzoite division than neutrophils or lymphocytes.
Toxoplasma gondii is an obligate intracellular parasite that can infect any nucleated host cell by a process of active penetration (7). Acute toxoplasmosis is characterized by dissemination and intracellular growth of the tachyzoite in a variety of host organs. In the innate phase of the immune response, nonspecific inflammatory cells, including neutrophils, monocytes, and dendritic cells, are elicited to the site of infection. The ensuing response of these cells allows for the establishment of the specific cell-mediated immune response that provides for long-term protection in the host against recurrent infection (13). Previous studies have reported that human neutrophils and monocytes become infected by tachyzoites. However, for each cell type there are conflicting reports of the subsequent fate of these intracellular parasites; both parasite stasis (14, 16, 30) and parasite division (15, 19) are described. In a recent study it was shown that there was markedly less uptake and division of tachyzoites in adherent monocytes than in nonadherent monocytes (9). In the present study the response of human peripheral blood leukocytes to tachyzoites was evaluated.
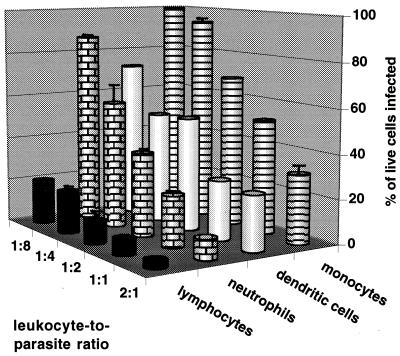
Infection of human leukocytes is parasite dose dependent.
Dendritic cells, monocytes, lymphocytes, and neutrophils were isolated from peripheral blood from toxoplasma-seronegative donors under endotoxin-free conditions. To obtain dendritic cells, mononuclear cells were isolated from blood by density gradient centrifugation (2:1 [vol/vol] ratio of blood to 1.07 g of Ficoll-Hypaque per ml; 500 × g for 20 min). Washed cells were plated for 2 h at 37°C in tissue culture flasks, and then nonadherent cells were removed. The adherent cells were cultured in medium supplemented with 2,000 U of recombinant human granulocyte-macrophage colony-stimulating factor (rhGM-CSF; generously supplied by Immunex, Seattle, Wash.) and 20 ng of recombinant human interleukin-4 (rhIL-4; PeproTech Inc., Rocky Hill, N.J.) per ml. Every 3 days the medium in these flasks was supplemented with rhGM-CSF and rhIL-4. At day 8, the cells demonstrating dendritic cell morphology (nonadherent with projections) were harvested, and their phenotype was characterized by flow cytometry as described previously (27).
Monocytes and lymphocytes were isolated from peripheral blood mononuclear cells obtained by cytophoresis carried out with volunteers as described previously (2). Briefly, monocytes were enriched to 85 to 95% by aggregation of washed cytophoresed cells at 4°C. Supernatants containing enriched lymphocytes were removed and purified further (see below). Platelets were removed from monocytes by washing cells twice in Versene buffer (0.2 g of EDTA per ml in phosphate-buffered saline). Under these conditions monocytes in 24-well tissue culture plates remain nonadherent. Lymphocytes were purified further by incubation in serum-free medium in large tissue culture flasks for 1 h at 37°C to adhere contaminating monocytes. Nonadherent cells were then washed twice in medium before use. Diff-Quik-stained cytospins of these nonadherent cells were 95% enriched for lymphocytes (the other cells were monocytes).
To isolate neutrophils, dextran (6% in saline; T500; Amersham Pharmacia Biotech Inc., Piscataway, N.J.) was added to blood at a ratio of 1:9 (vol/vol) to sediment erythrocytes (1 × g for 30 min) at room temperature. The leukocyte-rich plasma above the sedimented erythrocytes was removed and overlaid onto a two-step gradient comprised of 1.07 g of Ficoll-Hypaque (Winthrop Laboratories, New York, N.Y.) per ml underlaid with 1.095 g of OptiPrep (Accurate Chemical & Scientific Corp., Westbury, N.Y.) per ml in the ratio of 2:1:1 (vol/vol/vol). After centrifugation (500 × g for 20 min), the neutrophil layer was removed and washed twice in RPMI medium. Cells were 99% polymorphonuclear (3 to 5% eosinophils) and 1% mononuclear (lymphocytes and monocytes) as determined from stained cytospins.
Isolated leukocytes were resuspended in RPMI 1640 containing 25 mM HEPES buffer with l-glutamine (Gibco Laboratories), supplemented with gentamicin sulfate (50 μg/ml; United States Biochemical Corp., Cleveland, Ohio) and 10% (vol/vol) fetal bovine serum (with a low endotoxin concentration; HyClone Laboratories, Inc., Logan, Utah) which had been heat inactivated at 56°C for 30 min. T. gondii (PLK strain) was maintained in human foreskin fibroblasts and isolated as described previously (3). Parasites were added to each type of leukocyte, and cytospin preparations were made after 2 and 24 h of incubation. Infection of peripheral blood cells and dendritic cells by tachyzoites was parasite dose dependent, and at each parasite dose more monocytes than neutrophils and lymphocytes were infected (Fig. 1). Multiple infections of monocytes, dendritic cells, and neutrophils were observed for leukocyte-to-parasite ratios of ≥1:2. The percentage of leukocytes infected after 24 h was never less than the percentage of cells infected after 2 h (data not shown), with the exception that overnight incubation of neutrophils with tachyzoites at cell-to-parasite ratios of ≥1:2 led to increasing degeneration of neutrophils. Similar results were seen when monocytes, neutrophils, and lymphocytes were mixed in equal numbers and then infected with tachyzoites (data not shown).
FIG. 1.
Infection of host cells is parasite dose dependent. Tachyzoites were added to monocytes, neutrophils, and lymphocytes (5 × 105/500 μl in 24-well tissue culture plates) or in vitro-cultured dendritic cells (27) (1.4 × 105/140 μl in 96-well tissue culture plates) at various cell-to-tachyzoite ratios. Triplicate cytospin preparations (100,000 cells centrifuged for 5 min at 700 rpm using a Shandon Cytospin 3) of each infected cell type were made 2 and 24 h postinfection. Cells were fixed and stained with Diff-Quik, and 200 to 300 cells were counted per slide. For each cell the number of tachyzoites per vacuole was determined. Cells with multiple vacuoles were scored for the highest number of tachyzoites per single vacuole (i.e., as if they contained a single vacuole), and the cell-to-tachyzoite ratios where multiple vacuoles occurred were recorded. Results from 24 h postinfection are reported as the mean + standard deviations of triplicate samples for lymphocytes, neutrophils, and monocytes and as the mean of duplicate samples for dendritic cells. Results are representative of two donors.
Monocytes and dendritic cells are permissive to rapid parasite division.
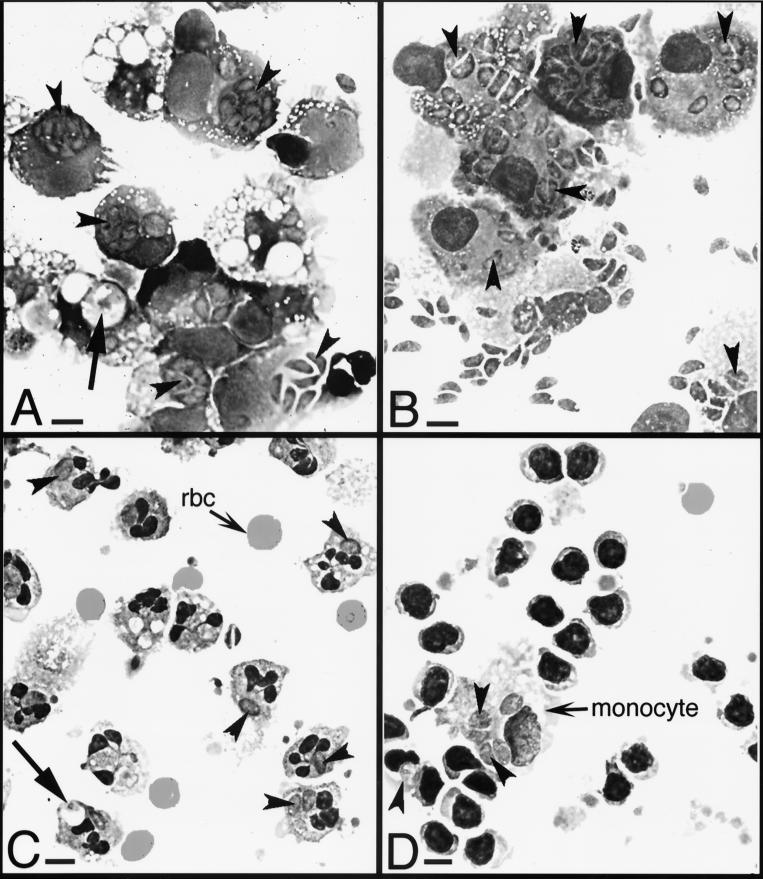
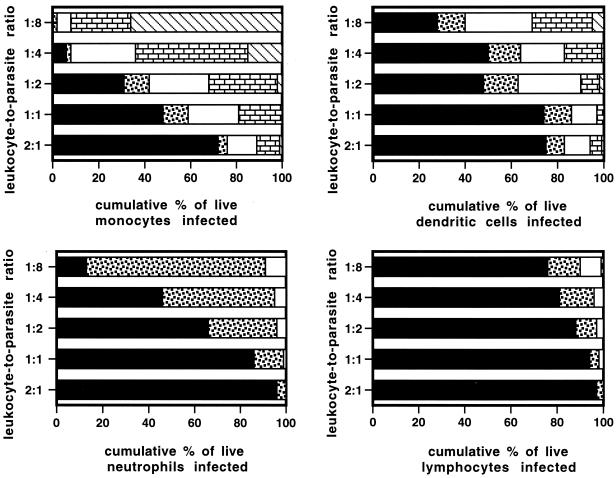
Tachyzoites divide through a type of binary fission termed endodygeny, and a division time of 5 to 9 h is characteristic of any toxoplasma strain (21). To determine whether leukocytes were permissive to intracellular parasite division, cells infected overnight were scored for numbers of parasites per vacuole. Photomicrographs of these infected leukocytes are presented in Fig. 2. Large vacuoles containing small pieces of parasites, consistent with phagocytosis of parasites lysed extracellularly, were seen for a minority of monocytes and neutrophils, particularly at higher multiplicities of infection (Fig. 2A and C). The infected leukocytes were quantified for numbers of intracellular parasites. Figure 3 shows parasite division for each type of leukocyte. There is a striking difference between the parasite division time in dendritic cells and monocytes and that in neutrophils and lymphocytes. While dendritic cells and monocytes support the characteristic rapid parasite division time (21) with four to eight parasites per vacuole, neutrophils and lymphocytes are less permissive for parasite division, harboring mainly single parasites. For example, at a cell-to-parasite ratio of 1:4, 92 and 36% of monocytes and dendritic cells, respectively, had vacuoles containing two, four, or eight parasites (Fig. 3). In contrast, 46% of neutrophils were uninfected, 49% had one tachyzoite per vacuole, and 5% had two tachyzoites per vacuole (Fig. 3). At the same multiplicity of infection 81% of lymphocytes were uninfected, 15% had one tachyzoite per vacuole, and 4% had two tachyzoites per vacuole (Fig. 3). Between 18 and 48 h of infection monocytes and dendritic cells lysed following the egress of viable intracellular tachyzoites. For monocytes, the number of tachyzoites per vacuole was dependent on the multiplicity of infection.
FIG. 2.
Photomicrographs of cytospin preparations of tachyzoite-infected host cells. Host cells were incubated for 24 h with tachyzoites at a cell-to-parasite ratio of 1:4. Arrowheads indicate parasites. Arrows indicate vacuoles containing digesting tachyzoites. Magnification, ×630. Bar = 2 μm. (A) Monocytes showing rapid tachyzoite division, as evidenced by up to eight tachyzoites per vacuole (see arrowheads). (B) Dendritic cells showing rapid tachyzoite division. (C) Neutrophils showing slow tachyzoite division, as seen by fewer than two tachyzoites per vacuole. rbc, red blood cells. (D) Lymphocytes showing slow tachyzoite division, even though tachyzoites in the contaminating monocyte were dividing rapidly. Photomicrographs were made using Kodak Elite chrome ASA 100 slide film, and 35-mm slides were scanned into Adobe Photoshop files using a SprintScan 35 (Polaroid Corp., Cambridge, Mass.).
FIG. 3.
Intracellular tachyzoites divide at a rapid rate in monocytes and dendritic cells but not in neutrophils or lymphocytes. Host cells were incubated with parasites at various multiplicities of infection for 24 h and the number of tachyzoites per host intracellular vacuole was determined by light microscopy of cytospin preparations. Results are reported as the cumulative percentage of cells that are uninfected (■), or have one ( ), two (□), four (
), two (□), four ( ), or eight (▧) tachyzoites per vacuole. Results are representative of two donors.
), or eight (▧) tachyzoites per vacuole. Results are representative of two donors.
Parasites in infected neutrophils have a longer division time.
The majority of infected neutrophils contained one whole parasite per vacuole (Fig. 2C) and the intracellular parasite was often distended. To determine whether distended parasites could have been dividing, cytospins of infected neutrophils were fixed in 2% formaldehyde for 30 min and permeabilized in ice-cold acetone for 15 min. Parasites were stained with a tachyzoite-specific antibody (30 min of incubation with 10 μg of fluorescein isothiocyanate-conjugated anti-SAG-1 rabbit polyclonal immunoglobulin G, made in our laboratory, per ml), and cell nuclei were stained with propidium iodide (5 min of incubation with 2.5 μg/ml in phosphate-buffered saline). Cells were examined using a Zeiss Axiophot microscope equipped with a fluorescein isothiocyanate-tetramethyl rhodamine isocyanate filter set. For 24-h-infected neutrophils, all distended tachyzoites contained two daughter nuclei, consistent with endodyogeny (21). Moreover, when the samples were examined 48 h postinfection, the frequency of infected neutrophils containing two tachyzoites per vacuole was found to be increased (data not shown). These results suggest that parasite division time in neutrophils is markedly longer than the typical 5 to 9 h seen for nonadherent monocytes and dendritic cells. Similar results were seen for intracellular tachyzoites of lymphocytes (data not shown).
Parasites in infected neutrophils can reinfect fibroblasts, where they show a rapid division time.
To determine whether parasites that exhibit a longer division time in neutrophils also have a longer division time in a more permissive cell type, infected neutrophils (sorted free of extracellular parasites using flow cytometry) were added to human fibroblasts. For these experiments neutrophils were infected overnight with parasites at a cell-to-parasite ratio of 1:1 and then stained for cell surface expression of Fcγ receptor III (murine monoclonal antibody 3G8 supernatant; generous gift of M. Fanger) and examined by flow cytometry. Fcγ receptor III-stained neutrophils fell into two populations, a brightly stained viable population and a dimly stained nonviable population. A gate was set on the brightly stained cells, and cells were sorted using a FacStar flow cytometer (Becton Dickinson) under sterile conditions. Cytospin preparations of sorted brightly stained cells showed them to be 100% viable, 12% tachyzoite infected, and free of extracellular tachyzoites, whereas dimly stained cells were 100% nonviable and uninfected. Serial dilutions of brightly stained neutrophils were added to small flasks of confluent human fibroblasts and incubated at 37°C. After 4 days, fibroblasts were examined for plaques caused by tachyzoite infection and division (24). Incubation of fibroblasts with 120,000 infected neutrophils (106 total neutrophils) resulted in the formation of 25,000 plaques. The efficiency of plating, i.e., the ability of extracellular PLK strain tachyzoites to make plaques, was 50%. Hence, at least 40% of the intracellular tachyzoites within neutrophils were infective. Moreover, the majority of infected fibroblasts contained four to eight parasites per vacuole.
We have demonstrated that tachyzoites differentially infect and replicate in human monocytes, neutrophils, dendritic cells, and lymphocytes. Although tachyzoites are able to infect each of these cell types, they do not infect them equally. At low multiplicities of infection more monocytes and dendritic cells than neutrophils or lymphocytes are infected. At higher multiplicities of infection many more neutrophils, but not lymphocytes, become infected. Monocytes, dendritic cells, and neutrophils are all phagocytes. Both monocytes and neutrophils have been reported to mediate significant lysis of extracellular tachyzoites (8). Since the percentage of cells infected after overnight incubation was never less than the percentage of cells infected after 2 h of incubation, it is unlikely that phagocytosis of whole parasites plays a role in eliminating tachyzoites. Rather, the majority of these infected cells contained dividing parasites. For a host cell to contain dividing tachyzoites, the tachyzoites must have entered by active penetration rather than by being phagocytosed, since fusion of tachyzoite-containing phagosomes with endosomes would result in phagosome acidification, an event known to cause parasite death (28).
In vivo, tachyzoites are disseminated from the gut to a variety of organs during an acute infection. The survival of both the host and parasite is dependent on some of these tachyzoites becoming encysted as slowly dividing bradyzoites, while the remaining tachyzoites are eliminated (13). The mechanisms that control tachyzoite elimination and tachyzoite-to-bradyzoite interconversion in host cells in vivo are not known, although a role for the host's specific immunity, in particular for gamma interferon (IFN-γ), has been suggested (29). Both hemopoietic and nonhemopoietic cell types have been shown to play a critical role in IFN-γ-mediated immunity to T. gondii (33). From our studies and those of others, hemopoietic and nonhemopoietic human cell types infected with tachyzoites in vitro appear to fall into two categories: permissive cells in which tachyzoites undergo rapid division and nonpermissive cells in which tachyzoite division time is longer (Table 1). Moreover, preincubation of most of these permissive cells with IFN-γ before infection switches them to a nonpermissive phenotype (Table 1). Reactive oxygen and nitrogen metabolites and a lack of tryptophan have been implicated in restricting tachyzoite division (Table 1).
TABLE 1.
Division rate of intracellular tachyzoites in primary human cells in vitroa
| Cell type | Parasite division rate
|
Mechanism | Reference(s) | |
|---|---|---|---|---|
| Unprimed | IFN-γ primed | |||
| Hemopoietic | ||||
| Lymphocyte | S | ND | This study | |
| Neutrophil | S | ND | This study, 19, 30 | |
| Adherent monocyte | S | ROM; not TS | 5, 9, 15–17, 30 | |
| Nonadherent monocyte | R | R | This study, 9 | |
| Dendritic cell | R | ND | This study | |
| Alveolar macrophage | R | S | Partly TS | 17 |
| Peritoneal macrophage | R | S | ND | 31 |
| Monocyte-derived macrophage | R | S | ROM; not RNM | 1, 5, 16, 17, 31 |
| Nonhemopoietic | ||||
| Neuron | S | ND | 10 | |
| Foreskin fibroblast | R | S | TS | 22, 23 |
| Umbilical vein endothelial cell | R | S | Not RNM, TS, or ROM | 16, 32 |
| Retinal pigment epithelial cell | R | S | TS | 18 |
| Fetal astrocyte | R | S | RNM | 10, 20 |
| Fetal microglial cell | R | R | 4 | |
S, slow; R, rapid; ND, not determined; ROM, reactive oxygen metabolites; TS, tryptophan starvation; RNM, reactive nitrogen metabolites.
If parasite dissemination from the gut to other organs occurs via the bloodstream, peripheral blood monocytes, dendritic cells, and neutrophils would be excellent candidates to transport tachyzoites to other host tissues. Inflammation would elicit neutrophils (12, 25, 26), monocytes, and dendritic cells to the site of infection. Neutrophils are the first cells to be elicited during an inflammatory response and appear within minutes of chemokine release from the site of tachyzoite infection (reference 6 and unpublished observations). Our studies suggest that neutrophils play a critical dual role in restricting tachyzoite growth; they lyse extracellular tachyzoites and, when infected, they retard intracellular tachyzoite division. Monocytes and dendritic cells represent 4% and less than 0.1%, respectively, of the peripheral blood leukocytes (11). Monocytes are elicited to the site of infection a few hours later than neutrophils. These cells would encounter lysed tachyzoites, infected neutrophils, and perhaps viable extracellular tachyzoites at the site of infection. Since monocytes and dendritic cells are professional antigen-presenting cells, lysed or damaged extracellular parasites would provide a pool of extracellular antigen that could be presented in the context of either major histocompatibility complex class I or II. This antigen presentation is critical for establishing specific immunity leading to the release of IFN-γ and the protection of tissue macrophages and nonhemopoietic cells from rapid parasite division, overt stimulation of the specific immune response, and consequent host pathology.
Acknowledgments
This work was supported by grants AI19613 and AI30000 from the National Institutes of Health. Flow cytometry and fluorescence microscopy were carried out at Dartmouth Medical School in the Herbert C. Englert Cell Analysis Laboratory, which was established by a grant from the Fannie E. Rippel Foundation and is supported in part by the Core Grant of the Norris Cotton Cancer Center (CA 23108).
REFERENCES
- 1.Anderson S E, Bautista S, Remington J S. Induction of resistance to Toxoplasma gondii in human macrophages by soluble lymphocyte products. J Immunol. 1976;117:381–387. [PubMed] [Google Scholar]
- 2.Channon J Y, Kasper L H. Toxoplasma gondii-induced immune suppression by human peripheral blood monocytes: role of gamma interferon. Infect Immun. 1996;64:1181–1189. doi: 10.1128/iai.64.4.1181-1189.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Channon J Y, Suh E I, Seguin R M, Kasper L H. Attachment ligands of viable Toxoplasma gondii induce soluble immunosuppressive factors in human monocytes. Infect Immun. 1999;67:2547–2551. doi: 10.1128/iai.67.5.2547-2551.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chao C C, Gekke R G, Hu S, Peterson P K. Human microglial cell defense against Toxoplasma gondii. The role of cytokines. J Immunol. 1994;152:1246–1252. [PubMed] [Google Scholar]
- 5.Delemarre F G, Stevenhagen A, Kroon F P, Van Eer M Y, Meenhorst P L, Van Furth R. Effect of IFN-gamma on the proliferation of Toxoplasma gondii in monocytes and monocyte-derived macrophages from AIDS patients. Immunology. 1994;83:646–650. [PMC free article] [PubMed] [Google Scholar]
- 6.Denney C F, Eckmann L, Reed S L. Chemokine secretion of human cells in response to Toxoplasma gondii infection. Infect Immun. 1999;67:1547–1552. doi: 10.1128/iai.67.4.1547-1552.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dobrowolski J M, Sibley L D. Toxoplasma invasion of mammalian cells is powered by the actin cytoskeleton of the parasite. Cell. 1996;84:933–939. doi: 10.1016/s0092-8674(00)81071-5. [DOI] [PubMed] [Google Scholar]
- 8.Erbe D V, Pfefferkorn E R, Fanger M W. Functions of the various IgG Fc receptors in mediating killing of Toxoplasma gondii. J Immunol. 1991;146:3145–3151. [PubMed] [Google Scholar]
- 9.Fadul C E, Channon J Y, Kasper L H. Survival of immunoglobulin G-opsonized Toxoplasma gondii in nonadherent human monocytes. Infect Immun. 1995;63:4290–4294. doi: 10.1128/iai.63.11.4290-4294.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Halonen S K, Lyman W D, Chiu F C. Growth and development of Toxoplasma gondii in human neurons and astrocytes. J Neuropathol Exp Neurol. 1996;55:1150–1156. doi: 10.1097/00005072-199611000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Haynes B F, Fauci A S. Introduction to the immune system. In: Fauci A, Braunwald E, Isselbacher K, Wilson J, Martin J, Kasper D, Hauser S, Longo D, editors. Harrison's principles of internal medicine. 14th ed. New York, N.Y: McGraw-Hill; 1998. pp. 1753–1776. [Google Scholar]
- 12.Jebbari H, Roberts C W, Ferguson D J, Bluethmann H, Alexander J. A protective role for IL-6 during early infection with Toxoplasma gondii. Parasite Immunol. 1999;20:231–239. doi: 10.1046/j.1365-3024.1998.00152.x. [DOI] [PubMed] [Google Scholar]
- 13.Kasper L H. Toxoplasma infection. In: Fauci A, Braunwald E, Isselbacher K, Wilson J, Martin J, Kasper D, Hauser S, Longo D, editors. Harrison's principles of internal medicine. 14th ed. New York, N.Y: McGraw-Hill; 1998. pp. 1197–1202. [Google Scholar]
- 14.Locksley R M, Nelson C S, Fankhauser J E, Klebanoff S J. Loss of granule myeloperoxidase during in vitro culture of human monocytes correlates with decay in antiprotozoa activity. Am J Trop Med Hyg. 1987;36:541–548. doi: 10.4269/ajtmh.1987.36.541. [DOI] [PubMed] [Google Scholar]
- 15.McLeod R, Bensch K G, Smith S M, Remington J S. Effects of human peripheral blood monocytes, monocyte-derived macrophages, and spleen mononuclear phagocytes on Toxoplasma gondii. Cell Immunol. 1980;54:330–350. doi: 10.1016/0008-8749(80)90214-2. [DOI] [PubMed] [Google Scholar]
- 16.Murray H W, Rubin B Y, Carriero S M, Harris A M, Jaffee E A. Human mononuclear phagocyte antiprotozoal mechanisms: oxygen-dependent vs oxygen-independent activity against intracellular Toxoplasma gondii. J Immunol. 1985;134:1982–1988. [PubMed] [Google Scholar]
- 17.Murray H W, Szuro-Sudol A, Wellner D, Oca M J, Granger A M, Libby D M, Rothermel C D, Rubin B Y. Role of tryptophan degradation in respiratory burst-independent antimicrobial activity of gamma interferon-stimulated human macrophages. Infect Immun. 1989;57:845–849. doi: 10.1128/iai.57.3.845-849.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagineni C N, Pardhasaradhi K, Martins M C, Detrick B, Hooks J J. Mechanisms of interferon-induced inhibition of Toxoplasma gondii replication in human retinal pigment epithelial cells. Infect Immun. 1996;64:4188–4196. doi: 10.1128/iai.64.10.4188-4196.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakao M, Konishi E. Proliferation of Toxoplasma gondii in human neutrophils in vitro. Parasitology. 1991;103:23–27. doi: 10.1017/s0031182000059242. [DOI] [PubMed] [Google Scholar]
- 20.Peterson P K, Gekker G, Hu S, Chao C C. Human astrocytes inhibit intracellular multiplication of Toxoplasma gondii by a nitric oxide-mediated mechanism. J Infect Dis. 1995;171:516–518. doi: 10.1093/infdis/171.2.516. [DOI] [PubMed] [Google Scholar]
- 21.Pfefferkorn E R. Cell biology of Toxoplasma gondii. In: Wyler D J, editor. Modern parasite biology. Cellular, immunological, and molecular aspects. New York, N.Y: W. H. Freeman & Co.; 1990. pp. 26–50. [Google Scholar]
- 22.Pfefferkorn E R. Interferon-gamma blocks the growth of Toxoplasma gondii in human fibroblasts by inducing the host cells to degrade tryptophan. Proc Natl Acad Sci USA. 1984;81:908–912. doi: 10.1073/pnas.81.3.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pfefferkorn E R, Guyre P M. Inhibition of growth of Toxoplasma gondii in cultured fibroblasts by human recombinant gamma interferon. Infect Immun. 1984;44:211–216. doi: 10.1128/iai.44.2.211-216.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pfefferkorn E R, Pfefferkorn L C. Toxoplasma gondii: isolation and preliminary characterization of temperature-sensitive mutants. Exp Parasitol. 1976;39:365–376. doi: 10.1016/0014-4894(76)90040-0. [DOI] [PubMed] [Google Scholar]
- 25.Sayles P, Johnson L. –1997. Exacerbation of toxoplasmosis in neutrophil-depleted mice. Nat Immun. 1996;15:249–258. [PubMed] [Google Scholar]
- 26.Scharton-Kersten T M, Yap G, Magram J, Sher A. Inducible nitric oxide is essential for host control of persistent but not acute infection with the intracellular pathogen Toxoplasma gondii. J Exp Med. 1997;185:1261–1273. doi: 10.1084/jem.185.7.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seguin R M, Kasper L H. Sensitized lymphocytes and CD40 ligation augment interleukin-12 production by human dendritic cells in response to Toxoplasma gondii. J Infect Dis. 1999;179:467–474. doi: 10.1086/314601. [DOI] [PubMed] [Google Scholar]
- 28.Sibley L D, Weidner E, Krahenbuhl J L. Phagosome acidification blocked by intracellular Toxoplasma gondii. Nature. 1985;315:416–419. doi: 10.1038/315416a0. [DOI] [PubMed] [Google Scholar]
- 29.Suzuki Y, Orellana M A, Schreiber R D, Remington J S. Interferon-γ: the major mediator of resistance against Toxoplasma gondii. Science. 1988;240:516–518. doi: 10.1126/science.3128869. [DOI] [PubMed] [Google Scholar]
- 30.Wilson C B, Remington J S. Activity of human blood leukocytes against Toxoplasma gondii. J Infect Dis. 1979;140:890–895. doi: 10.1093/infdis/140.6.890. [DOI] [PubMed] [Google Scholar]
- 31.Wilson C B, Westall J. Activation of neonatal and human macrophages by alpha, beta, and gamma interferons. Infect Immun. 1985;49:351–356. doi: 10.1128/iai.49.2.351-356.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Woodman J P, Dimier I H, Bout D T. Human endothelial cells are activated by IFN-γ to inhibit Toxoplasma gondii replication. Inhibition is due to a different mechanism from that existing in mouse macrophages and human fibroblasts. J Immunol. 1991;147:2019–2023. [PubMed] [Google Scholar]
- 33.Yap G S, Sher A. Effector cells of both nonhemopoietic and hemopoietic origin are required for interferon (IFN)-gamma- and tumor necrosis factor (TNF)-alpha-dependent host resistance to the intracellular pathogen, Toxoplasma gondii. J Exp Med. 1999;189:1083–1092. doi: 10.1084/jem.189.7.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]