Abstract
Understanding immunity in humans to Group A Streptococcus (Strep A) is critical for the development of successful vaccines to prevent the morbidity and mortality attributed to Strep A infections. Despite decades of effort, no licensed vaccine against Strep A exists and immune correlates of protection are lacking; a major impediment to vaccine development. In the absence of a vaccine, we can take cues from the development of natural immunity to Strep A in humans to identify immune correlates of protection. The age stratification of incidence of acute Strep A infections, peaking in young children and waning in early adulthood, coincides with the development of specific immune responses. Therefore, understanding the immune mechanisms involved in natural protection from acute Strep A infection is critical to identifying immune correlates to inform vaccine development. This perspective summarises the findings from natural infection studies, existing assays of immunity to Strep A, and highlights the gaps in knowledge to guide the development of Strep A vaccines and associated correlates of protection.
Subject terms: Translational research, Bacterial infection
Introduction
Group A Streptococcus (Strep A, S. pyogenes) is among the top 10 leading causes of global infection-related morbidity and mortality across a diverse clinical spectrum including acute infections such as pharyngitis and impetigo, invasive infections and immune-mediated sequelae including acute rheumatic fever, rheumatic heart disease (RHD) and acute glomerular nephritis1,2. Despite successful treatment with penicillin, Strep A disease control remains challenging, leaving a vaccine as the most effective option for disease prevention3. To date, no licensed vaccine against Strep A exists and there is a lack of understanding of the mechanisms of protective immunity. This is a significant impediment to vaccine development both in terms of identifying optimal antigenic targets of vaccination, and developing assays that act as correlates of protection (CoP), the latter being a major focus of current Strep A research4.
Importantly, once identified, CoP assays could replace the need for clinical endpoints in vaccine efficacy trials, reducing the requirement for lengthy and costly studies with the disease as an endpoint5,6. For Strep A, this could obviate the burden of quantifying Strep A pharyngitis in hard-to-reach populations and reduce the need to judge vaccine efficacy based on the incidence of RHD, an autoimmune sequela that may arise years after repeated Strep A infections. CoP assays that are readily transferable between laboratories and countries and use standardized reagents that do not require specialist culture conditions or know-how are more likely to be accepted by licensing authorities. Furthermore, such assays may also allow for ongoing surveillance of immunity in target populations. At present, there are several knowledge gaps hindering the development of such assays for Strep A7, but the gaps are closing.
Natural immunity against Strep A
The strongest evidence of protective immune responses against Strep A is the observed decreased susceptibility to infection with increasing age7. The frequency of acute Strep A infections peaks in childhood, with a much lower incidence of these diseases in adulthood. By contrast, invasive infections are seen in both the very young and very old populations8. This evidence provides a rationale to believe that an effective vaccine against Strep A is achievable.
The incidence of symptomatic throat infections, including scarlet fever, increases significantly around 4 years of age9. This could be due to the expansion of tonsil tissue allowing greater access for Strep A, increased exposure to other children at the start of school, or simply an artefact of school-aged children being able to articulate throat pain. Towards the end of childhood, the frequency of strep sore throats diminishes markedly. A similar peak and fall in incidence occur with Strep A skin infections, at a slightly earlier age than throat infections7. Invasive infections, seen in both the very young and very old populations10, may be associated with immune system naivety and immunosenescence respectively, along with the increased risk of skin injury and exposed portals of entry.
Immunity to primary infection
Non-invasive infections would be the ideal target of vaccination. The mechanisms that confer resistance to colonisation and primary infection of the oropharynx or skin are unknown, but likely include a combination of prevention of bacterial adhesion, innate defence mechanisms, opsonophagocytic killing of bacteria supported by antibody and complement, inhibition of directly acting virulence factors, inhibition of bacterial immune evasion strategies, and cellular immunity.
During streptococcal infections, there appears to be a temporal sequence of adherence and colonisation by the bacteria. The initial “pioneer” cells perform long-range adherence and form molecular bridges with host proteins11. The following “settler” cells have shorter-range adherence with higher affinity and specificity. As the bacterial “society” forms in biofilms, there is environmental sensing, extracellular polymeric substance formation and quorum sensing. Finally, a “community” is established with cell-to-cell signalling, coaggregation, metabolic synergy and genetic exchange11. It is unknown against which stage or stages of colonisation an effective immune response must act to inhibit the development of infection, or whether targeting one or more stages by vaccination will be required to prevent non-invasive colonisation or infection.
Animal models of nasopharyngeal infection have shown that whole bacteria, single and combinations of antigens, and passive immunisation can induce immunity12,13. The route of immunisation may also be important to induce an effective immune response against Strep A14. Intranasal vaccines can generate both secretory immunoglobulin A (IgA) at the mucosa and serum IgG, as well as cell-mediated forms of immunity that are less well characterised15. Mice are not naturally exposed to Strep A, so provide a naive experimental system. There are however several drawbacks to the use of mouse models in Strep A vaccine research, including the large inoculum required to establish infection, lack of tonsils, use of lethal intranasal models that can result in pneumonia, and use of adjuvants unsuitable for humans16.
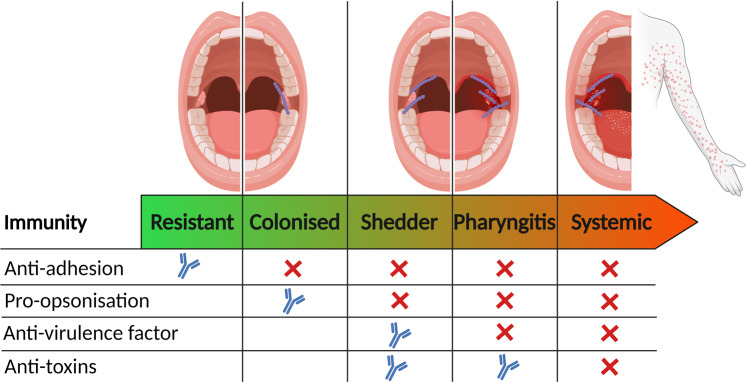
There are many unknowns relating to human nasopharyngeal infection. When studying outbreaks of pharyngitis and scarlet fever in children, it was shown that over 25% of children acquire the outbreak strain17. Of these children the majority carry the bacteria asymptomatically, and some become heavy shedders of the strain, yet only a small number of children develop scarlet fever or pharyngitis17. Others never acquire the outbreak strain despite the same level of exposure. This may indicate a spectrum of different levels of immunity to different virulence factors in the human nasopharynx (Fig. 1), even in children.
Fig. 1. Proposed spectrum of antibody-derived immunity in oropharynx to explain the range of phenotypes observed in children17.
In humans resistant to Strep A pharyngeal acquisition (first column), antibodies may inhibit adhesion and encourage opsonophagocytosis of bacteria before colonisation is established. In this situation antibodies targeting bacterial virulence factors and toxins may or may not be present. Colonised humans likely do not block adhesion with antibodies but, at least for some time, can limit the proliferation of bacteria. Shedders often have heavy colonisation but may inhibit symptomatic infection by controlling bacterial virulence factors. In most cases, pharyngitis is superficial and self-limiting, but can also develop into systemic illness including Scarlet fever (final column), which has been linked to toxins including superantigens94. The antibody symbols (blue) indicate where antibodies inhibit bacterial function, the crosses (red) where antibodies are lacking and no symbol where there is no requirement for antibodies. Created with Biorender.com.
Immunity to invasive infection
Resistance to Strep A invasive infection is better understood, due to the uncommon ability of Strep A bacteria to grow in non-immune human blood. This forms the basis of the classic “Lancefield” assay18, which measures the opsonophagocytic capacity of donor plasma antibodies as a marker of resistance to invasive infection. Subcutaneous19–21, intramuscular22,23 and intranasal24,25 vaccination with adjuvanted protein vaccines appear effective at preventing even lethal invasive infections in mouse models of infection. Furthermore, passive transfer of immunity using antisera and pooled human intravenous immunoglobulin (IVIg) have all successfully induced immunity in animal models, particularly when highly concentrated13. IVIg contains high levels of anti-Strep A antibodies and is proposed as a clinical treatment for severe invasive Strep A disease26,27.
Anti-Strep A antibodies
Although cell-mediated immunity no doubt contributes to long-term protection against Strep A, circulating IgG antibodies are recognised to be protective and are the most characterised adaptive immune mechanism. Furthermore, vaccines typically elicit antibody production and all CoP assays to date have relied upon antibody detection in vaccinated individuals; a better understanding of antibody responses is therefore central to vaccine development.
Reports of serum-based immune reactions to Strep A during Scarlet fever predate the consensus discovery of Strep A as the cause of Scarlet fever28. Convalescent human sera was shown to detoxify Strep A cultures and changed Dick skin test results from positive (susceptible) to negative (protected) following passive transfer29. Data from animal studies30, human serology31 and vaccine trials32 has linked antibody responses to specific Strep A antigens with various functions, including neutralisation of virulence factors, adhesins, and bacterial opsonophagocytosis.
The natural immunity to Strep A observed in adulthood is thought to be primarily driven by an accumulation of anti-Strep A antibodies33,34. Serosurveys of antibodies against the clinical serology antigens, Streptolysin O (SLO) and DNAse B, in different age groups and in different countries and socioeconomic settings support an age-related increase in these antibodies which are believed to represent biomarkers of Strep A exposure35,36. There are different theories for how Strep A antibodies confer protection: (1) natural human immunity to Strep A infection is type-specific and directed against the M-protein37, so that repeated exposures to different M types (or emm-clusters38) builds a repertoire of type-specific responses responsible for protection39; and (2) responses accumulate with repeated exposures to conserved (non-M-protein) Strep A antigens, raising the threshold of protection against subsequent infection40,41. Measurements of antigen-specific antibodies following Strep A infections indicate antibodies against both type-specific and conserved antigens occur concurrently and both likely contribute to immunity42,43.
Strep A has evolved several strategies to inhibit antibody function including the IgG-specific proteases, Ides and EndoS, that cleave antibodies at the hinge44 or Fc region45. The streptococcal cysteine protease, SpeB, regulates virulence by cleaving both host and bacterial proteins. Although SpeB is another example of a Strep A IgG cleaving protease46, it also inactivates bacterial-derived EndoS47. M and M-like proteins are an important family of Strep A virulence factors48. Their immune evasion functions include non-immune binding of IgG by the Fc region rendering them non-functional, and likely involvement in immune masking49.
Longitudinal studies suggest naturally acquired M protein antibodies may convey protection against infection, but not colonisation, and this may be strain-specific. Because colonisation without clinical symptoms (“asymptomatic carriage”) can be both immunogenic and immunologically silent, occur in the throat and skin, and can be of variable duration before resolution or resultant infection, here we describe pharyngeal acquisition events. Wannamaker et al. in 1953 followed 131 adult men with known baseline type-specific antibody responses and found the presence of type-specific antibody reduced subsequent symptomatic infection but not asymptomatic pharyngeal acquisition with the same M type50. Guirguis et al. also showed that the presence of type-specific bactericidal antibodies did not protect individuals from secondary pharyngeal acquisition, except against M1 strains51. Another longitudinal study found that 11 of the 75 pharyngeal acquisitions occurred in children who had pre-existing type-specific antibodies and only 12.5% of children developed type-specific antibodies following infection43. Thus, type-specific M protein-based immunity following infection is not guaranteed. In a longitudinal cohort of Fijian children, there was no association between the time to next Strep A skin infection and total serum IgG antibody titres to the M protein semi-conserved C-repeat region J8 peptide following primary skin infection52. It is important to note that vaccine-induced and natural immunity may differ significantly23.
Existing assays of immunity to Strep A
Although protective immunity to Strep A is not well understood, the main mechanisms are likely to include the promotion of opsonophagocytosis, toxin or virulence factor neutralisation, and blocking of bacterial adhesion by B cell-derived antibodies and phagocytes, supported by T cells, cytokines, chemokines, and components of innate immunity. Several assays that mimic effector function in human immunity to Strep A are established as outlined below, although none would fulfil the specifications needed for a standardized, readily tranferrable assay of protection.
Opsonophagocytosis assays
The Lancefield bactericidal assay, first described in 1927 by Todd, capitalised on the uncommon ability of Strep A bacteria to grow in blood18. The addition of test sera, heat-treated to remove endogenous complement proteins, to non-immune donor whole blood that is co-incubated with actively growing Strep A, measures the ability of test serum to inhibit the growth of the bacteria through promoting opsonophagocytic killing. The assay assumes that any reduction in Strep A growth is due to antibodies in the sera, which allows for a measure of functional immunity in sera53. However, the requirement to start with pre-screened, non-immune freshly drawn whole blood and freshly inoculated bacterial cultures, coupled with the overnight culture steps and colony counting make the assay laborious, complex and often poorly reproducible. Furthermore, the assay measures the net change over time in Strep A quantity, which is the consequence of both bacterial growth and opsonophagocytic killing. As such, it is not a pure measure of bacterial killing, and results are strain-specific.
There have been several attempts to provide a more reproducible opsonophagocytosis assay. A recent update to the assay protocol incorporates the Strep A virulence factor IdeS to digest endogenous antibodies in whole blood, removing the requirement of pre-screening for non-immune donors54. Other variations have utilised purified single donor human neutrophils co-incubated with different heat-treated donor sera, with flow cytometric analysis of bacterial uptake. Although such assays often do not quantify bacterial killing, the ability to measure the deposition of complement on the bacterial surface directly demonstrates the mechanism of opsonisation55 but is again limited by a need for freshly cultured bacteria, fresh donor blood, and neutrophil purification. A key recent refinement of these assays resulted in an HL-60 opsonophagocytic killing assay56,57, based on the established S. pneumoniae assay, using a cell line as the source of neutrophils, frozen bacterial stocks, and an endpoint that measures bacterial killing58. Although successfully applied to a few strain types, there remain several unknowns relating to the mechanism of uptake and subsequent killing in these assays, including the subtype of antibody required to promote bacterial killing, the failure of some Strep A strains to perform in such assays, and the variable importance of M protein and other Strep A antigens.
Opsonophagocytic assays may be a sound and plausible predictor of systemic protection in blood; however, such assays have not been definitively linked to protection against non-invasive infection in humans. In Wannamaker’s 1953 longitudinal study of the pharyngeal acquisition of Strep A, baseline bactericidal assays were able to predict the probability of developing a symptomatic infection, but they did not correlate with susceptibility to colonisation50. In studies where antibody functionality and titre data are available, any correlations appear to be individual, strain and/or disease specific. For example, in the recent phase 1 trial of a 30-valent Strep A vaccine, there was a good correlation between antibody titre against the M5 peptide and opsonophagocytic killing of this strain. By contrast, there was little to no correlation between antibody titre and the killing of three other strains studied (Fig. 2)32.
Fig. 2. Correlation between titres of type-specific antibodies and opsonophagocytic killing (OPK) for four different Strep A strains following vaccination with the 30-valent vaccine candidate StreptAnovaTM that included M1, M3, M5, M12.
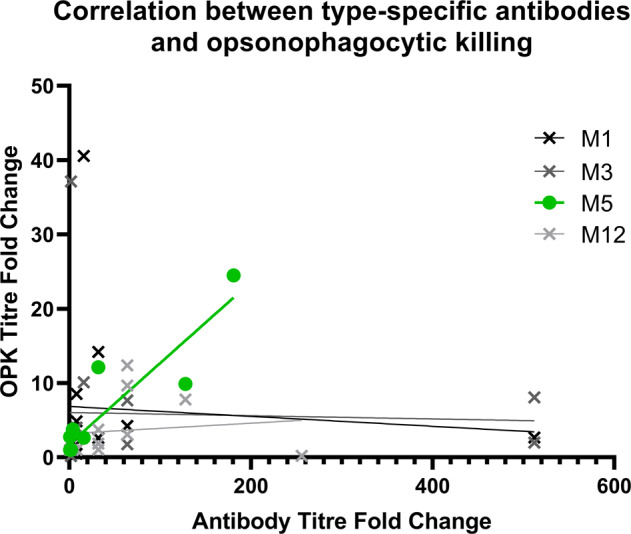
A clear correlation is seen between OPK titre and antibody fold changes for M5 (green; R2 = 0.82), which is not apparent for the other strains. Data are from Pastural et al.32.
Virulence factor neutralisation
The “Dick Test”, named after its inventors Dr. George and Dr. Gladys Dick, was a skin test for susceptibility to scarlet fever popular in the 1920s to 1950s29. The test involved intracutaneous injection of a small volume of filtered Strep A culture, which produced inflammatory reactions in susceptible individuals, and no reaction in those considered protected. The test represented a CoP assay of neutralising anti-superantigen activity which was also responsible for an alternative test using the “Shultz–Charlton phenomenon” whereby immune sera caused blanching of the scarlet fever-associated rash59. Unfortunately, the toxin-based vaccines assessed using these assays caused considerable inflammatory reactions and appeared to have reduced efficacy against other Strep A clinical syndromes60–62.
In recent decades, neutralising immunity to the superantigens associated with scarlet fever and toxic shock syndrome has been re-explored, partly to determine if the use of therapeutic IVIg might be of benefit. Building on the easily measured T-cell mitogenic effects of this group of toxins in vitro, titres of neutralising anti-toxin antibodies present in donor serum can be measured ex vivo. This demonstrated seroconversion and development of anti-SMEZ (Streptococcal mitogenic exotoxin) antibodies over a 7-day period in a patient who recovered from Streptococcal Toxic Shock Syndrome (STSS)63, and has also demonstrated the presence of anti-toxin antibodies in pooled IVIg64,65.
Established assays of immune responses to Strep A, often used to confirm a recent infection, include the measurement of functional neutralising anti-SLO and anti-DNAse B antibodies. Anti-SLO titres are calculated by the lysis of erythrocytes, as neutralising antibodies against SLO inhibit this function of the lysin. Likewise, anti-DNaseB titres are assessed based on inhibition of the degradation of DNA66. Most adults have some level of neutralising activity against these proteins indicative of previous Strep A exposure, but these antibodies are not known to be specifically protective. A rise in titres can indicate recent infection, although, in the case of SLO, it is now recognised that several strain types do not produce SLO owing to a variation in the promoter region67.
Several other virulence factors are known to influence Strep A pathogenesis and assays of immunity to these have been developed. One example is SpyCEP, a Strep A protease that can cleave CXC chemokines, particularly CXCL8 (IL-8)68. SpyCEP is a vaccine candidate and protection is believed to result from vaccine-induced antibodies that neutralise protease activity69; as such vaccine efficacy could not be evaluated using an opsonophagocytosis assay. Although the protein is very small (8 kDa) it is possible to demonstrate CXCL8 cleavage in vitro by purified SpyCEP, by gel electrophoresis or ELISA68, and to therefore inhibit this process using an immune serum. Such an assay could readily be adapted to detect vaccine-induced immunity to SpyCEP.
Blocking adhesion
A sterilising immune response against Strep A must block the early stages of adhesion which lead to colonisation. Host-cell adhesion assays use monolayers of human cell lines, typically Detroit 562 to model pharyngeal colonisation and HaCat cells to model adhesion to the skin, and measure adherent live Strep A. Test serum containing antibodies against adhesins on the surface of Strep A can inhibit adhesion to these cells70. Animal studies have shown salivary IgA, but not serum IgG, against M protein reduces bacterial adherence to pharyngeal cells71. Rabbit antibodies against Strep A pili proteins inhibited bacterial adherence to a skin cell line72. As with antibodies that promote opsonophagocytosis and virulence factor neutralisation, there is little known about the characteristics of antibodies that inhibit adhesion, including isotype, glycosylation pattern, specific epitopes and affinity. As such, assays of immunity to combat adhesion are in their infancy yet may be of crucial functional importance.
More tractable correlates of immunity
Finding a simpler, more reproducible method of detecting protective anti-Strep A antibodies would accelerate vaccine development. There are a number of Strep A antigens targeted by antibodies that either promote opsonophagocytosis26,73,74 or inhibit virulence factors41,75–77. Some of these antigens are included in candidate Strep A vaccines19,78–80. Detecting antibodies against these antigens is essential for vaccine clinical trials to demonstrate the magnitude and breadth of the antibody response in vaccinees. Measuring antibodies against additional non-vaccine antigens could differentiate vaccine-induced immunity from natural immunity. Such antibodies may be a marker of recent infection, and might have a further use diagnostically and in surveillance. Recently, bead-based assays that simultaneously measure antibody titres against three81, four82 and eight83 Strep A antigens from low-volume serum samples have been established. Such assays are agnostic to the function of the antibodies they detect; the purpose is to enable large scale screening using a readily transferrable assay that can be done in a few hours. The advantage of bead assays is the requirement for just a few microlitres of serum that can be tested against multiple antigens, which is of particular importance given the target Strep A vaccine population is children. Indeed, it is possible to adapt these assays to use antibody eluted from finger prick samples83, and potentially from mucosal fluids. Alternate technologies that deliver a similar outcome include mesoscale discovery (MSD) assays such as those employed during SARS-CoV-2 vaccine evaluation, which have been accepted as satisfactory and tractable immunogenicity assays by licensing authorities84.
Future studies to develop CoP assays
Having developed reproducible, cell-free assays as above, in addition to the fundamental understanding provided by functional assays, it is now important to establish the best use of these assays and consider inclusion of standardised positive and negative controls that will be readily available to all those using the assays to facilitate comparison and correlation. At present, in the absence of any commercial vaccine, the best positive control is human pooled IVIg, while common negative controls include IgG-depleted serum and naive animal sera. Naive human sera, such as infant sera or cord blood, would be difficult to find and may be confounded by maternal antibodies.
Secondly, we need to understand whether the antibodies detected by such assays are functional or whether the antibodies simply correlate with functional immune activity. If either is found to correlate with clinical immunity this point may become less important. While both mechanistic and non-mechanistic CoPs are useful85, understanding mechanisms of immunity will have greater utility to vaccine development more broadly. Recent advances in systems serology have greatly improved the ability to link antigen-specific antibodies to effector functions86 and identify vaccine CoPs87. At present there is no gold standard for evaluation of immunity to Strep A, particularly if we accept that the classical Lancefield assay is a measure of immunity in blood but not the oropharynx. Unlike some viral vaccines, bacterial vaccines may employ several immune mechanisms, and it may be that no single functional assay can replicate the protection seen in humans5.
Finally, we need to optimise the assays to detect antibodies from different types of samples, including oral fluids, soft tissues and finger prick blood. Despite the oropharynx being a major site of Strep A infection, remarkably little is known about Strep A specific immunity in this mucosal tissue.
To understand how natural immunity develops we can look for signals in prospective cohort studies of children with active surveillance for Strep A infections17,88. Assays that measure antibody levels against Strep A antigens in oral fluid, specifically IgA, will be required to understand mucosal immunity. Studying adults as well as children, and conducting longitudinal studies, will provide further insight into what happens when a human encounters Strep A for the first time and how immunity evolves through adulthood. The CHIVAS-M75 human challenge model, where healthy adults were infected with an M75 Strep A, provides important information for the acute stage of colonisation and infection; most participants developed sore throat, but some did not89. The experiment paves the way to future studies that compare antibody profiles following vaccination, and in those who were resistant to the challenge and those who were susceptible. Similarly, the finding that young children, exposed to the same dose of Strep A exhibit a range of clinical phenotypes provides a natural opportunity to identify immunological differences between these phenotypes17. The roles of cellular immunity90, including T and B lymphocytes and their effectors, and cells in the tonsils91 warrant further investigation, as do genetic determinants of susceptibility and the differences between intranasal92 and intramuscular93 vaccine-induced immunity.
Conclusions
Natural immunity to Strep A exists and it is likely that several immune mechanisms contribute to the protection against Strep A infection afforded to adults. With prospective surveillance of Strep A infections and high-quality sample collections it will be possible to identify immune correlates of natural protection. However, it would be exceedingly difficult to define a CoP for a vaccine without first demonstrating clinical protection in a reproducible, verifiable manner from vaccine trials. Nonetheless, with multiple vaccines planning to enter clinical trials and vaccine-challenge trials in the next few years, the potential to demonstrate protective efficacy seems closer. By combining known features of natural immunity, currently available assays of immunity and emerging findings from human trials, a CoP for Strep A is highly plausible.
Acknowledgements
The authors would like to thank the Strep A Vaccine Global Consortium (SAVAC), the NIHR Imperial College London Biomedical Research Centre (UK), Telethon Kids Institute (Australia), Murdoch Children’s Research Institute (Australia) and the International Vaccine Institute (Republic of Korea).
Author contributions
All authors contributed to the preparation, review and editing of the manuscript. All authors read and approved the contents of the manuscript.
Funding information
This work was supported through a grant from the Wellcome Trust awarded to the International Vaccine Institute (IVI) (215490/Z/19/Z) and Wellcome Trust Collaborative Award (215539/Z/19/Z) to Imperial College London.
Data availability
No new data are presented in this report. Data used to create Fig. 2 are from Pastural et al.32.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Shiranee Sriskandan, Alma Fulurija.
Contributor Information
Shiranee Sriskandan, Email: s.sriskandan@imperial.ac.uk.
Alma Fulurija, Email: Alma.Fulurija@telethonkids.org.au.
References
- 1.Carapetis JR, Steer AC, Mulholland EK, Weber M. The global burden of group A streptococcal diseases. Lancet Infect. Dis. 2005;5:685–694. doi: 10.1016/S1473-3099(05)70267-X. [DOI] [PubMed] [Google Scholar]
- 2.Beaton A, et al. The American Heart Association’s call to action for reducing the global burden of rheumatic heart disease: a Policy Statement from the American Heart Association. Circulation. 2020;142:e358–e368. doi: 10.1161/CIR.0000000000000922. [DOI] [PubMed] [Google Scholar]
- 3.Excler JL, Kim JH. Accelerating the development of a Group A Streptococcus vaccine: an urgent public health need. Clin. Exp. Vaccin. Res. 2016;5:101–107. doi: 10.7774/cevr.2016.5.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vekemans J, et al. The path to Group A Streptococcus vaccines: World Health Organization research and development technology roadmap and preferred product characteristics. Clin. Infect. Dis. 2019;69:877–883. doi: 10.1093/cid/ciy1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Plotkin SA. Complex correlates of protection after vaccination. Clin. Infect. Dis. 2013;56:1458–1465. doi: 10.1093/cid/cit048. [DOI] [PubMed] [Google Scholar]
- 6.Plotkin SA. Correlates of protection induced by vaccination. Clin. Vaccin. Immunol. 2010;17:1055–1065. doi: 10.1128/CVI.00131-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsoi SK, Smeesters PR, Frost HR, Licciardi P, Steer AC. Correlates of protection for M protein-based vaccines against Group A Streptococcus. J. Immunol. Res. 2015;2015:167089. doi: 10.1155/2015/167089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steer AC, Lamagni T, Curtis N, Carapetis JR. Invasive group A streptococcal disease: epidemiology, pathogenesis and management. Drugs. 2012;72:1213–1227. doi: 10.2165/11634180-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lamagni T, et al. Resurgence of scarlet fever in England, 2014-16: a population-based surveillance study. Lancet Infect. Dis. 2018;18:180–187. doi: 10.1016/S1473-3099(17)30693-X. [DOI] [PubMed] [Google Scholar]
- 10.Lamagni TL, et al. Epidemiology of severe Streptococcus pyogenes disease in Europe. J. Clin. Microbiol. 2008;46:2359–2367. doi: 10.1128/JCM.00422-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nobbs AH, Lamont RJ, Jenkinson HF. Streptococcus adherence and colonization. Microbiol. Mol. Biol. Rev. 2009;73:407. doi: 10.1128/MMBR.00014-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moreland NJ, et al. Working towards a group A streptococcal vaccine: report of a collaborative Trans-Tasman workshop. Vaccine. 2014;32:3713–3720. doi: 10.1016/j.vaccine.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 13.Reglinski M, Gierula M, Lynskey NN, Edwards RJ, Sriskandan S. Identification of the Streptococcus pyogenes surface antigens recognised by pooled human immunoglobulin. Sci. Rep. 2015;5:15825. doi: 10.1038/srep15825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ozberk, V. et al. Prime-pull immunization with a bivalent M-protein and Spy-CEP peptide vaccine adjuvanted with CAF®01 liposomes induces both mucosal and peripheral protection from covR/S mutant Streptococcus pyogenes. mBio12, e03537–20 (2021). [DOI] [PMC free article] [PubMed]
- 15.Mortensen R, et al. Local Th17/IgA immunity correlate with protection against intranasal infection with Streptococcus pyogenes. PLoS ONE. 2017;12:e0175707. doi: 10.1371/journal.pone.0175707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watson, M. E. Jr., Neely, M. N. & Caparon, M. G. in Streptococcus pyogenes: Basic Biology to Clinical Manifestations (eds. Ferretti J. J., Stevens, D. L. & Fischetti, V. A.) (The University of Oklahoma Health Sciences Center, 2016). https://www.ncbi.nlm.nih.gov/books/NBK333421. Pages 497–522. [PubMed]
- 17.Cordery R, et al. Frequency of transmission, asymptomatic shedding, and airborne spread of Streptococcus pyogenes in schoolchildren exposed to scarlet fever: a prospective, longitudinal, multicohort, molecular epidemiological, contact-tracing study in England, UK. Lancet Microbe. 2022;3:e366–e375. doi: 10.1016/S2666-5247(21)00332-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Todd EW. A method of measuring the increase or decrease of the population of hæmolytic Streptococci in blood. Br. J. Exp. Pathol. 1927;8:1–5. [Google Scholar]
- 19.Batzloff, M., Yan, H., Davies, M., Hartas, J. & Good, M. Preclinical evaluation of a vaccine based on conserved region of M protein that prevents group A streptococcal infection. Indian J. Med. Res. 119(Suppl), 104–107 (2004). [PubMed]
- 20.Postol E, et al. StreptInCor: a candidate vaccine epitope against S. pyogenes infections induces protection in outbred mice. PLoS ONE. 2013;8:e60969. doi: 10.1371/journal.pone.0060969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henningham A, et al. Conserved anchorless surface proteins as group A streptococcal vaccine candidates. J. Mol. Med. 2012;90:1197–1207. doi: 10.1007/s00109-012-0897-9. [DOI] [PubMed] [Google Scholar]
- 22.Rivera-Hernandez, T. et al. Differing efficacies of lead group A streptococcal vaccine candidates and full-length M protein in cutaneous and invasive disease models. mBio10.1128/mBio.00618-16 (2016). [DOI] [PMC free article] [PubMed]
- 23.Tan LKK, et al. Vaccine-induced, but not natural immunity, against the streptococcal inhibitor of complement protects against invasive disease. npj Vaccines. 2021;6:62. doi: 10.1038/s41541-021-00326-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park HS, Cleary PP. Active and passive intranasal immunizations with streptococcal surface protein C5a peptidase prevent infection of murine nasal mucosa-associated lymphoid tissue, a functional homologue of human tonsils. Infect. Immun. 2005;73:7878–7886. doi: 10.1128/IAI.73.12.7878-7886.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wozniak A, et al. Protective immunity induced by an intranasal multivalent vaccine comprising 10 Lactococcus lactis strains expressing highly prevalent M-protein antigens derived from Group A Streptococcus. Microbiol. Immunol. 2018;62:395–404. doi: 10.1111/1348-0421.12595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Basma H, et al. Opsonic antibodies to the surface M protein of group A streptococci in pooled normal immunoglobulins (IVIG): potential impact on the clinical efficacy of IVIG therapy for severe invasive group A streptococcal infections. Infect. Immun. 1998;66:2279–2283. doi: 10.1128/IAI.66.5.2279-2283.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parks T, Wilson C, Curtis N, Norrby-Teglund A, Sriskandan S. Polyspecific intravenous immunoglobulin in clindamycin-treated patients with streptococcal toxic shock syndrome: a systematic review and meta-analysis. Clin. Infect. Dis. 2018;67:1434–1436. doi: 10.1093/cid/ciy401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith RM. Scarlet fever prophylaxis with Streptococcus vaccine. Boston Med. Surg. J. 1910;162:242–245. doi: 10.1056/NEJM191002241620803. [DOI] [Google Scholar]
- 29.Dick GF, Dick GH. A skin test for susceptibility to scarlet fever. J. Am. Med. Assoc. 1924;82:265–266. doi: 10.1001/jama.1924.02650300011003. [DOI] [Google Scholar]
- 30.Okamoto S, Tamura Y, Terao Y, Hamada S, Kawabata S. Systemic immunization with streptococcal immunoglobulin-binding protein Sib 35 induces protective immunity against group: a Streptococcus challenge in mice. Vaccine. 2005;23:4852–4859. doi: 10.1016/j.vaccine.2005.02.035. [DOI] [PubMed] [Google Scholar]
- 31.Beachey EH, Stollerman GH, Johnson RH, Ofek I, Bisno AL. Human immune response to immunization with a structurally defined polypeptide fragment of streptococcal M protein. J. Exp. Med. 1979;150:862–877. doi: 10.1084/jem.150.4.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pastural É, et al. Safety and immunogenicity of a 30-valent M protein-based group a streptococcal vaccine in healthy adult volunteers: a randomized, controlled phase I study. Vaccine. 2020;38:1384–1392. doi: 10.1016/j.vaccine.2019.12.005. [DOI] [PubMed] [Google Scholar]
- 33.Sabharwal H, et al. Group A Streptococcus (GAS) carbohydrate as an immunogen for protection against GAS infection. J. Infect. Dis. 2006;193:129–135. doi: 10.1086/498618. [DOI] [PubMed] [Google Scholar]
- 34.O’Connor SP, et al. The human antibody response to streptococcal C5a peptidase. J. Infect. Dis. 1991;163:109–116. doi: 10.1093/infdis/163.1.109. [DOI] [PubMed] [Google Scholar]
- 35.Steer AC, et al. Normal ranges of streptococcal antibody titers are similar whether streptococci are endemic to the setting or not. Clin. Vaccin. Immunol. 2009;16:172–175. doi: 10.1128/CVI.00291-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Okello E, et al. Cross-sectional study of population-specific streptococcal antibody titres in Uganda. Arch. Dis. Child. 2020;105:825–829. doi: 10.1136/archdischild-2020-318859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lancefield RC. Persistence of type-specific antibodies in man following infection with Group A streptococci. J. Exp. Med. 1959;110:271–292. doi: 10.1084/jem.110.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frost HR, et al. Immune cross-opsonization within emm clusters following Group A Streptococcus skin infection: broadening the scope of type-specific immunity. Clin. Infect. Dis. 2017;65:1523–1531. doi: 10.1093/cid/cix599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jaggi P, et al. Age-associated differences in prevalence of group A streptococcal type-specific M antibodies in children. Eur. J. Pediatr. 2009;168:679–683. doi: 10.1007/s00431-008-0819-6. [DOI] [PubMed] [Google Scholar]
- 40.Pandey M, et al. Streptococcal immunity is constrained by lack of immunological memory following a single episode of pyoderma. PLoS Pathog. 2016;12:e1006122. doi: 10.1371/journal.ppat.1006122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mortensen R, et al. Adaptive immunity against Streptococcus pyogenes in adults involves increased IFN-γ and IgG3 responses compared with children. J. Immunol. 2015;195:1657–1664. doi: 10.4049/jimmunol.1500804. [DOI] [PubMed] [Google Scholar]
- 42.Hysmith ND, et al. Prospective longitudinal analysis of immune responses in pediatric subjects after pharyngeal acquisition of Group A Streptococci. J. Pediatr. Infect. Dis. Soc. 2017;6:187–196. doi: 10.1093/jpids/piw070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Quinn RW, Vander Zwaag R, Lowry PN. Acquisition of group A streptococcal M protein antibodies. Pediatr. Infect. Dis. 1985;4:374–378. doi: 10.1097/00006454-198507000-00008. [DOI] [PubMed] [Google Scholar]
- 44.von Pawel-Rammingen U, Johansson BP, Björck L. IdeS, a novel streptococcal cysteine proteinase with unique specificity for immunoglobulin G. EMBO J. 2002;21:1607–1615. doi: 10.1093/emboj/21.7.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Collin M, Olsen A. EndoS, a novel secreted protein from Streptococcus pyogenes with endoglycosidase activity on human IgG. EMBO J. 2001;20:3046–3055. doi: 10.1093/emboj/20.12.3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Collin M, Olsen A. Effect of SpeB and EndoS from Streptococcus pyogenes on human immunoglobulins. Infect. Immun. 2001;69:7187–7189. doi: 10.1128/IAI.69.11.7187-7189.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Allhorn, M., Olsén, A. & Collin, M. EndoS from Streptococcus pyogenes is hydrolyzed by the cysteine proteinase SpeB and requires glutamic acid 235 and tryptophans for IgG glycan-hydrolyzing activity. BMC Microbiol.8, 3 (2008). [DOI] [PMC free article] [PubMed]
- 48.Frost HR, Sanderson-Smith M, Walker M, Botteaux A, Smeesters PR. Group A streptococcal M-like proteins: from pathogenesis to vaccine potential. FEMS Microbiol. Rev. 2018;42:193–204. doi: 10.1093/femsre/fux057. [DOI] [PubMed] [Google Scholar]
- 49.Mills JO, Ghosh P. Nonimmune antibody interactions of Group A Streptococcus M and M-like proteins. PLoS Pathog. 2021;17:e1009248. doi: 10.1371/journal.ppat.1009248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wannamaker LW, Denny FW, Perry WD, Siegel AC, Rammelkamp CH., Jr. Studies on immunity to streptococcal infections in man. AMA Am. J. Dis. Child. 1953;86:347–348. [PubMed] [Google Scholar]
- 51.Guirguis N, Fraser DW, Facklam RR, El Kholy A, Wannamaker LW. Type-specific immunity and pharyngeal acquisition of Group A Streptococcus. Am. J. Epidemiol. 1982;116:933–939. doi: 10.1093/oxfordjournals.aje.a113495. [DOI] [PubMed] [Google Scholar]
- 52.Campbell PT, et al. Investigation of Group A Streptococcus immune responses in an endemic setting, with a particular focus on J8. Vaccine. 2018;36:7618–7624. doi: 10.1016/j.vaccine.2018.10.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reglinski M. Lancefield whole blood killing assay to evaluate vaccine efficacy. Methods Mol. Biol. 2020;2136:317–322. doi: 10.1007/978-1-0716-0467-0_25. [DOI] [PubMed] [Google Scholar]
- 54.Reglinski M, Lynskey NN, Sriskandan S. Modification of the classical Lancefield assay of group A streptococcal killing to reduce inter-donor variation. J. Microbiol. Methods. 2016;124:69–71. doi: 10.1016/j.mimet.2016.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lynskey NN. Flow cytometry-based assays to quantify complement deposition and neutrophil uptake of Group A Streptococcus. Methods Mol. Biol. 2020;2136:233–241. doi: 10.1007/978-1-0716-0467-0_17. [DOI] [PubMed] [Google Scholar]
- 56.Salehi S, Hohn CM, Penfound TA, Dale JB. Development of an opsonophagocytic killing assay using HL-60 cells for detection of functional antibodies against Streptococcus pyogenes. mSphere. 2018;3:1–11. doi: 10.1128/mSphere.00617-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jones S, et al. Development of an opsonophagocytic killing assay for group a streptococcus. Vaccine. 2018;36:3756–3763. doi: 10.1016/j.vaccine.2018.05.056. [DOI] [PubMed] [Google Scholar]
- 58.Romero-Steiner S, et al. Multilaboratory evaluation of a viability assay for measurement of opsonophagocytic antibodies specific to the capsular polysaccharides of Streptococcus pneumoniae. Clin. Diagn. Lab. Immunol. 2003;10:1019–1024. doi: 10.1128/CDLI.10.6.1019-1024.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Futagi Y. Improvement of the prophylactic immunization against scarlet fever by means of anatoxin after Ramon’s method. J. Immunol. 1930;19:451–456. doi: 10.4049/jimmunol.19.5.451. [DOI] [Google Scholar]
- 60.Young, D. C. Failure of type specific Streptococcus pyogenes vaccine to prevent respiratory infections. US Nav. Med. Bull.46, 709–718 (1945). [PubMed]
- 61.Jackson RT. Active immunisation against the streptococcus scarlatinÆ. Ir. J. Med. Sci. 1941;16:260–266. doi: 10.1007/BF02951109. [DOI] [Google Scholar]
- 62.Place EH. Result of active immunization of nurses against scarlet fever. Am. J. Public Health Nations Health. 1938;28:137–142. doi: 10.2105/AJPH.28.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Proft T, Sriskandan S, Yang L, Fraser JD. Superantigens and streptococcal toxic shock syndrome. Emerg. Infect. Dis. 2003;9:1211–1218. doi: 10.3201/eid0910.030042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schrage B, Duan G, Yang LP, Fraser JD, Proft T. Different preparations of intravenous immunoglobulin vary in their efficacy to neutralize streptococcal superantigens: implications for treatment of streptococcal toxic shock syndrome. Clin. Infect. Dis. 2006;43:743–746. doi: 10.1086/507037. [DOI] [PubMed] [Google Scholar]
- 65.Darenberg J, Soderquist B, Normark BH, Norrby-Teglund A. Differences in potency of intravenous polyspecific immunoglobulin G against streptococcal and staphylococcal superantigens: implications for therapy of toxic shock syndrome. Clin. Infect. Dis. 2004;38:836–842. doi: 10.1086/381979. [DOI] [PubMed] [Google Scholar]
- 66.Johnson, D. R. et al. Laboratory Diagnosis of Group A Streptococcal Infections (World Health Organisation, 1996).
- 67.Turner, C. E. et al. The emergence of successful Streptococcus pyogenes lineages through convergent pathways of capsule loss and recombination directing high toxin expression. mBio10, e02521–19 (2019). [DOI] [PMC free article] [PubMed]
- 68.Edwards RJ, et al. Specific C-terminal cleavage and inactivation of interleukin-8 by invasive disease isolates of Streptococcus pyogenes. J. Infect. Dis. 2005;192:783–790. doi: 10.1086/432485. [DOI] [PubMed] [Google Scholar]
- 69.McKenna S, et al. The role of streptococcal cell-envelope proteases in bacterial evasion of the innate immune system. J. Innate Immun. 2022;14:69–88. doi: 10.1159/000516956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Khemlani, A. H. J., Proft, T. & Loh, J. M. S. In Group A Streptococcus: Methods and Protocols (eds. Proft, T. & Loh, J. M. S.) 271–278 (Springer US, 2020).
- 71.Fluckiger U, Jones KF, Fischetti VA. Immunoglobulins to group A streptococcal surface molecules decrease adherence to and invasion of human pharyngeal cells. Infect. Immun. 1998;66:974–979. doi: 10.1128/IAI.66.3.974-979.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Loh JMS, Lorenz N, Tsai CJY, Khemlani AHJ, Proft T. Mucosal vaccination with pili from Group A Streptococcus expressed on Lactococcus lactis generates protective immune responses. Sci. Rep. 2017;7:7174. doi: 10.1038/s41598-017-07602-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Salvadori LG, Blake MS, McCarty M, Tai JY, Zabriskie JB. Group A Streptococcus-liposome ELISA antibody titers to group A polysaccharide and opsonophagocytic capabilities of the antibodies. J. Infect. Dis. 1995;171:593–600. doi: 10.1093/infdis/171.3.593. [DOI] [PubMed] [Google Scholar]
- 74.Podbielski A, Schnitzler N, Beyhs P, Boyle MD. M-related protein (Mrp) contributes to group A streptococcal resistance to phagocytosis by human granulocytes. Mol. Microbiol. 1996;19:429–441. doi: 10.1046/j.1365-2958.1996.377910.x. [DOI] [PubMed] [Google Scholar]
- 75.Chiappini N, et al. Streptococcus pyogenes SpyCEP influences host-pathogen interactions during infection in a murine air pouch model. PLoS ONE. 2012;7:e40411. doi: 10.1371/journal.pone.0040411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Reglinski M, Sriskandan S. The contribution of group A streptococcal virulence determinants to the pathogenesis of sepsis. Virulence. 2014;5:127–136. doi: 10.4161/viru.26400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Matsumura T, et al. An anti-perfringolysin O monoclonal antibody cross-reactive with streptolysin O protects against streptococcal toxic shock syndrome. BMC Res. Notes. 2020;13:419. doi: 10.1186/s13104-020-05264-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rivera-Hernandez, T. et al. An experimental Group A Streptococcus vaccine that reduces pharyngitis and tonsillitis in a nonhuman primate model. MBio10, e00693-19 (2019). [DOI] [PMC free article] [PubMed]
- 79.Bensi G, et al. Multi high-throughput approach for highly selective identification of vaccine candidates: the Group A Streptococcus case. Mol. Cell Proteomics. 2012;11:M111 015693. doi: 10.1074/mcp.M111.015693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dale JB, et al. Protective immunogenicity of group A streptococcal M-related proteins. Clin. Vaccin. Immunol. 2015;22:344–350. doi: 10.1128/CVI.00795-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Whitcombe, A. L. et al. Development and evaluation of a new triplex immunoassay that detects Group A Streptococcus antibodies for the diagnosis of rheumatic fever. J. Clin. Microbiol. 58, e00300–20 (2020). [DOI] [PMC free article] [PubMed]
- 82.Keeley, A. J. et al. Development and characterisation of a four-plex assay to measure Streptococcus pyogenes antigen-specific IgG in human sera. Methods Protoc.5, 55 (2022). [DOI] [PMC free article] [PubMed]
- 83.Whitcombe AL, et al. An eight-plex immunoassay for Group A Streptococcus serology and vaccine development. J. Immunol. Methods. 2022;500:113194. doi: 10.1016/j.jim.2021.113194. [DOI] [PubMed] [Google Scholar]
- 84.Johnson M, et al. Evaluation of a novel multiplexed assay for determining IgG levels and functional activity to SARS-CoV-2. J. Clin. Virol. 2020;130:104572. doi: 10.1016/j.jcv.2020.104572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Plotkin SA, Gilbert PB. Nomenclature for immune correlates of protection after vaccination. Clin. Infect. Dis. 2012;54:1615–1617. doi: 10.1093/cid/cis238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Arnold KB, Chung AW. Prospects from systems serology research. Immunology. 2018;153:279–289. doi: 10.1111/imm.12861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jin, C. et al. Vi-specific serological correlates of protection for typhoid fever. J. Exp. Med.218, e20201116 (2020). [DOI] [PMC free article] [PubMed]
- 88.Moore HC, Miller KM, Carapetis JR, Van Beneden CA. Harmonizing surveillance methodologies for group A streptococcal diseases. Open Forum Infect. Dis. 2022;9:S1–S4. doi: 10.1093/ofid/ofac210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Osowicki J, et al. A controlled human infection model of Streptococcus pyogenes pharyngitis (CHIVAS-M75): an observational, dose-finding study. Lancet Microbe. 2021;2:e291–e299. doi: 10.1016/S2666-5247(20)30240-8. [DOI] [PubMed] [Google Scholar]
- 90.Anderson J, et al. Immune signature of acute pharyngitis in a Streptococcus pyogenes human challenge trial. Nat. Commun. 2022;13:769. doi: 10.1038/s41467-022-28335-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dan, J. M. et al. Recurrent Group A Streptococcus tonsillitis is an immunosusceptibility disease involving antibody deficiency and aberrant T(FH) cells. Sci. Transl. Med.11, eaau3776 (2019). [DOI] [PMC free article] [PubMed]
- 92.Polly SM, Waldman RH, High P, Wittner MK, Dorfman A. Protective studies with a group A streptococcal M protein vaccine. II. Challange of volenteers after local immunization in the upper respiratory tract. J. Infect. Dis. 1975;131:217–224. doi: 10.1093/infdis/131.3.217. [DOI] [PubMed] [Google Scholar]
- 93.Fox EN, Waldman RH, Wittner MK, Mauceri AA, Dorfman A. Protective study with a group A streptococcal M protein vaccine. Infectivity challenge of human volunteers. J. Clin. Investig. 1973;52:1885–1892. doi: 10.1172/JCI107372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Johansson L, Thulin P, Low DE, Norrby-Teglund A. Getting under the skin: the immunopathogenesis of Streptococcus pyogenes deep tissue infections. Clin. Infect. Dis. 2010;51:58–65. doi: 10.1086/653116. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data are presented in this report. Data used to create Fig. 2 are from Pastural et al.32.