Keywords: COVID-19, electromyography, nerve conduction studies, peripheral neuropathy
Abstract
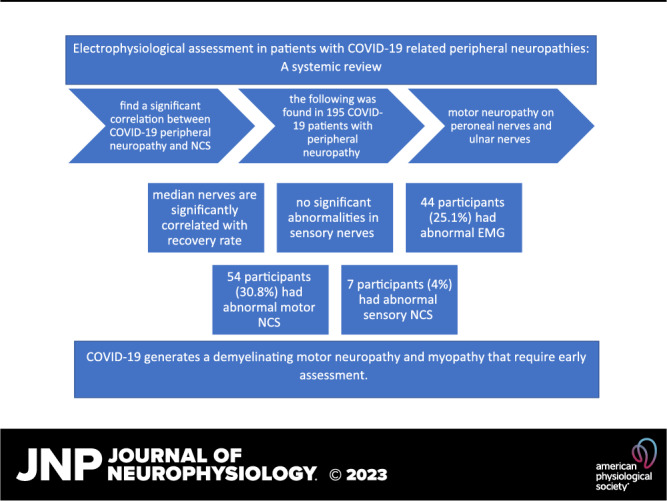
Neurological manifestations associated with Coronavirus Disease-2019 (COVID-19) are commonly reported, but patients were not referred to perform the electrophysiological assessment. We aimed to review the existing literature on clinical studies on COVID-19 peripheral neuropathy to correlate patients’ symptoms and characteristics with nerve conduction studies/electromyography (NCS/EMG) outcomes. This protocol is registered in the Open Science Framework (https://www.doi.org/10.17605/OSF.IO/ZF4PK). The systematic search included PubMed, ScienceDirect, and Google Scholar, for articles published from December 2019 to March 2022. A total of 727 articles were collected, and according to our inclusion and exclusion criteria, only 6 articles were included. Of 195 participants, only 175 underwent NCS/EMG assessment. Of these, 44 participants (25.1%) had abnormal EMG, 54 participants (30.8%) had abnormal motor NCS, and only 7 participants (4%) had abnormal sensory NCS. All cases presented with myopathy, while a limited number of cases presented with polyneuropathy. According to motor NCS and EMG, the most affected nerves were the tibial and peroneal in the lower extremities and the ulnar nerve in the upper extremities. Interestingly, the median nerve was reported to be associated with the severity and the rate of motor recovery of patients with COVID-19. COVID-19 generates a demyelinating motor neuropathy and myopathy. Clinicians are encouraged to refer patients with COVID-19 presenting with neurological symptoms to be assessed by electrophysiological methods to objectively determine the nature of their symptoms, follow their prognosis, and plan their rehabilitation.
INTRODUCTION
Neurological manifestations associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) or Coronavirus Disease-2019 (COVID-19) are common (1). These symptoms might be related to the central nervous system, causing dizziness, headache, seizures, ataxia, stroke, and altered consciousness, or the peripheral nervous system, such as loss of taste and smell, altered vision, and myalgia (2). Multiple pathogenesis of these neurological manifestations in COVID-19 were suggested, which are not necessarily caused by the direct effect of COVID-19 on the peripheral or central nervous system but might also occur secondary to a severe immunological reaction to viral infection (3, 4). Direct viral infection and inflammatory response of cytokines cause immune-induced complications, which have a central role in neuroinflammation and neural injury and the development of neurological manifestation in patients with COVID-19, but the evidence is still inconclusive (5).
Peripheral neuropathy symptoms such as nerve pain, myalgia, and paresthesia are reported in patients with COVID-19 (6). These symptoms were not electrodiagnostically confirmed but were mainly diagnosed through physical examinations such as deep tendon reflexes, muscle testing, sensory test, and laboratory tests. A limited number of patients with COVID-19 with peripheral neuropathy symptoms were referred to perform electrodiagnostic studies to evaluate their peripheral nerves. Physical examination and electrodiagnostic are integral approaches to diagnosing peripheral neuropathies (7). The various attributes and symptoms of peripheral nerve dysfunction in patients with COVID-19 make diagnosis challenging. Patients referred to electrophysiological testing presented with sensory symptoms (decreased sensation, pain, myalgia, and hyperalgesia), motor symptoms (quadriparesis and decreased muscle power), and difficulty in weaning intubation (8–12). Delayed diagnosis of peripheral neuropathy and myopathy leads to increased healthcare costs, increased rehabilitation period, higher mortality rate, and reduced patients’ quality of life (13).
Commonly used noninvasive electrophysiological assessments to evaluate peripheral nerve dysfunctions are nerve conduction studies (NCS) and electromyogram (EMG) (14). NCS is a noninvasive electrodiagnostic procedure routinely used at the neurophysiology clinic to evaluate peripheral nerve functions in many disorders, such as diabetes-related neuropathy and carpal tunnel syndrome. It is also time-efficient and provides information on the presence of peripheral neuropathy, severity, and prognosis of the disorder. But, it can only measure large fiber nerve function and cannot assess for small fiber neuropathy.
Various clinical studies used different diagnostic methods to identify COVID-19-related sensorimotor neuropathy, but these studies have yet to propose a reliable method of diagnosis and whether the electrophysiological assessment has a significant role in the diagnostic and prognosis of patients with COVID-19. To accurately diagnose peripheral neuropathy, clinicians need to incorporate medical history, physical examination, and electrophysiological assessment into their diagnostic procedures (7). Since there is mounting evidence of the involvement of peripheral neuropathy in patients with COVID-19, it is crucial to have a clear guideline to diagnose COVID-19-induced neuropathy accurately. Clinical studies reported that the noninvasive diagnosis was based on sensory testing, such as vibration sense, touch, temperature, pain assessment, and motor assessment, such as muscle testing with limited NCS/EMG (9, 15). However, none of these methods can provide an accurate, objective diagnosis of the change in nerve function and cannot determine the progression of the nerve dysfunction. These patients would have difficulty accurately tracking their progress during the follow-up visits.
Electrophysiological testing provides an objective, reliable, and sensitive measure of nerve dysfunction that allows clinicians to determine its severity and follow their patients’ progression. This systematic review aims to find the effects of COVID-19 on the peripheral nerves determined by electrophysiological assessment (14).
Previous systematic reviews and meta-analyses were done to determine the neurological manifestation in patients with COVID-19, but none focused on electrophysiological assessment as a diagnostic tool. This is the first systematic review to determine electrophysiological assessment, NCS and EMG as assessment tools for nerve dysfunction in patients with COVID-19. Studies that reported abnormal nerve conduction due to an immune reaction to the virus or intensive care unit-acquired weakness (ICU-AW) were not considered COVID-19-related peripheral neuropathy.
Electrophysiological Assessment
Electrophysiological assessment is a supportive tool that objectively diagnoses peripheral nerve dysfunction. Choosing an electrodiagnostic test depends on the extent of symptoms and type of the disorder (14). Usually, peripheral and longer nerves are the first to be affected. Gold standards for electrodiagnosis in COVID-19-related peripheral neuropathy have yet to be established. Therefore, the studies included in this systematic review used different electrodiagnostic methods with different nerves selected for assessment.
Routine nerve conduction studies include sensory nerves assessment, which is presented as sensory nerve action potential (SNAP), and motor nerves assessment, which is represented as compound muscle action potential (CMAP) (16). They are determined by the conduction velocity duration and amplitude, which are affected by limb length and temperature. Previous studies on patients with COVID-19 tested the lower extremities nerves that included the peroneal and tibial for motor nerves and their F-wave and Sural for sensory nerves. In the upper limb, motor and sensory for the ulnar, median, and radial nerves and their F-waves were tested (14). Abnormal measurements indicate neuropathy. For example, in axonal injury, there is a reduction in both SNAP and CMAP amplitude, whereas in demyelination, which affects the nerve roots, there is a prolonged distal and F-wave latency, and the conduction velocity is slow (16).
Electromyography (EMG) assesses muscle action potential during rest and activity, called a motor unit action potential (MUAP). The presence of positive sharp waves and fibrillation at rest is an indicator of denervation. During muscle activity, MUAP duration has several phases (polyphasic) and several turns (polyturns) that are also known as complex MUAP (16). EMG was performed to assess the electrophysiological properties in muscles due to COVID-19. Changes in muscles were assessed during rest and activity, and amplitude and duration of motor unit action potentials (MUAPs) along with polyphasic potentials and recruitment patterns, in addition to spontaneous activity, were assessed in all studies.
METHODS
Protocol and Registration
A systematic review was conducted to study the effects of COVID-19 infection on the peripheral nerves determined by nerve conduction studies (NCS) following a preestablished protocol prospectively registered in the Open Science Framework (OSF) (https://www.doi.org/10.17605/OSF.IO/ZF4PK) (17). This systematic review was carried out according to Prisma guidelines for writing systematic reviews and meta-analyses (18).
Literature Search Strategy
The systematic search included the following electronic databases: PubMed, ScienceDirect, and Google Scholar, for articles published from December 2019 to March 2022. The search strategies contained a combination of Medical Subject Headings (MeSH) or their equivalent (where available), the keywords for the search strategy were as follows: for PubMed: (“Peripheral Nerves”) AND (“COVID-19” OR “SARS-CoV-2”), Science Direct: (“Nerve conduction”) AND (“COVID-19” OR “SARS-CoV-2”), and Google Scholar: (“Nerve conduction” OR “Peripheral Nerves”) AND (“COVID-19” OR “SARS-CoV-2”).
This systematic review includes cross-sectional and cohort studies with the following inclusion criteria: 1) published between December 2019 and March 2022; 2) included adults aged 18 and older of both sexes infected with COVID-19 and confirmed by reverse transcription-polymerase chain reaction (RT-PCR); 3) patients with symptoms of COVID-19-related peripheral neuropathy; and 4) a reported value of at least one of the electrophysiological parameters NCS and EMG.
As for the exclusion criteria, 1) studies with pediatric participants; 2) studies that did not measure nerve dysfunction by NCS; 3) participants with neurological symptoms not related to COVID-19 infection, such as ICU-acquired weakness; 4) studies that reported neurological symptoms as a result of an autoimmune reaction to the virus such as Guillain barre syndrome; and 5) other excluded studies are those reported as abstract-only, non-English studies, gray literature, letters to the editor, comments, animal studies, and case reports.
Selection of Studies and Data Extraction
Two reviewers first screened titles and abstracts retrieved from the initial database according to our literature search strategy. After searching, the articles were uploaded and managed through Mendeley, and any duplicates were removed. Abstracts were reviewed, and irrelevant articles were excluded. Then, the full text was reviewed according to our inclusion and exclusion criteria. Articles that did not meet the inclusion criteria were removed, and any disagreement was resolved through mutual consensus.
The following data were extracted from eligible articles by two independent reviewers based on the JBI extraction tool: author, year, country, study design, setting, participant and their characteristics, outcome measured, and summary of findings. In addition, diagnostic parameters for NCS, including velocity and amplitude as follows: motor nerve conduction velocity (MNCV) of (the peroneal, tibial, median, radial, and ulnar nerves) and sensory nerve conduction velocity (SNCV) of (peroneal, tibial, sural, median, radial and ulnar nerves). Also, EMG parameters include amplitude and duration of motor unit action potentials (MUAPs) along with polyphasic potentials.
Two authors independently assessed the quality of the article and its risk of bias using the National Institutes of Health (NIH) quality assessment tool, where the article was considered poor if it scored between 0 to 4, fair, if it scored between 5–10, and good quality if it scored between 11–14. All authors were involved in the result analysis and manuscript preparation. Any inconsistencies between reviewers were resolved by consultation.
RESULTS
Study Selection
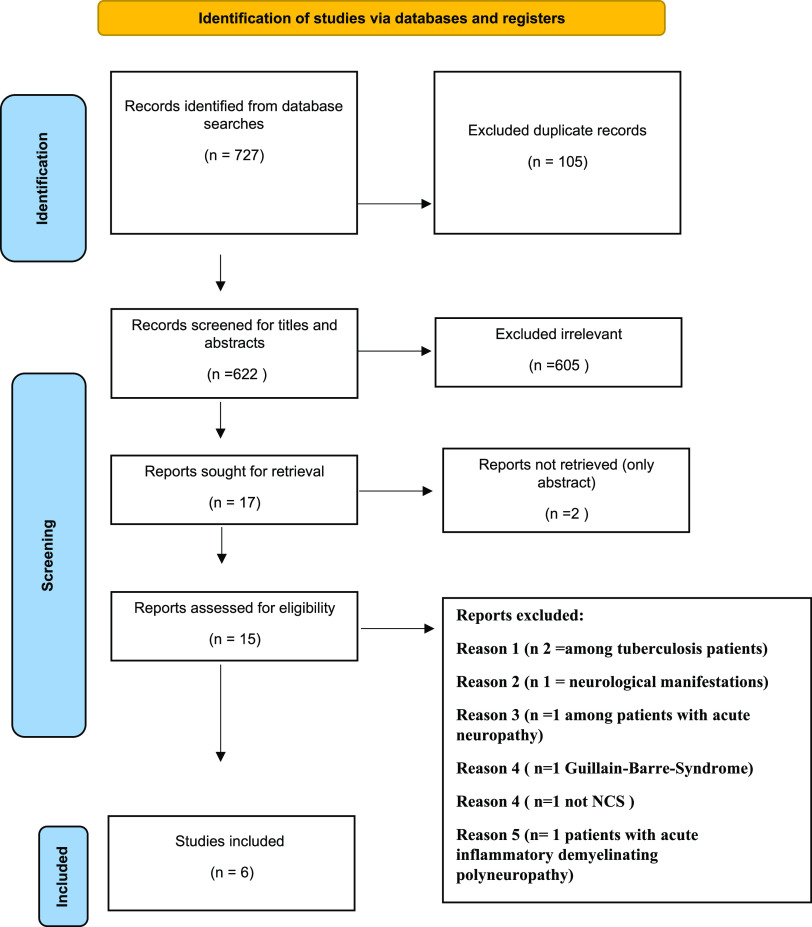
A total of 727 articles were initially collected according to the keywords searched from the 3 databases. After removing 105 duplicates, 622 articles were screened for title and abstract. After that, 605 were excluded, and only 17 articles met our criteria. However, two of these studies were published as abstracts, and their full text was not retrieved. Then, the full text of these 15 articles was evaluated. According to our inclusion and exclusion criteria, nine articles were excluded because two of these articles were conducted among patients with tuberculosis, one reported neurological manifestation without electrophysiological findings, one was conducted among patients with a history of neuropathy, one reported Guillain-Barre-Syndrome as an outcome, one study did not conduct a nerve conduction study as a diagnostic tool and one study with acute inflammatory demyelinating polyneuropathy. Finally, only six articles were qualified for the systematic review and data extraction. Figure 1 illustrates the PRISMA systematic review flow diagram. All included studies performed NCS/EMG for participants (9, 19–23).
Figure 1.
PRISMA systematic review flow diagram.
Characteristic of the Participants
Table 1 summarizes the participants’ characteristics in the included studies. The 6 studies had a total sample size of 239. Of these participants, 124 were females, and 111 were males, divided into two experimental groups (175 cases and 64 control). The mean age of participants was 52.2 ± 9.47. The severity of the symptoms, hospitalization, and time from neurological symptoms to NCS/EMG application vary dramatically between studies and sometimes within the same study (Table 1). Three were cohort studies, and two were retrospective cross-sectional studies. All studies lacked randomization were participants with COVID-19 infection who showed neurological manifestations were selectively invited to participate.
Table 1.
Data extraction and characteristics of included studies
| Author/Year | Study Design | Setting | Diagnostic Method | Group | n | Age, yr | Sex (M/F) | Time to NCS/EMG,days | ICU Admission | Outcome and Finding |
|---|---|---|---|---|---|---|---|---|---|---|
| Hamed et al. (2021) (9) | Retrospective (cross sectional) | Hospital setting | NCS/EMG | Cases | 18 | 55 ± 12 | 12/6 | 41 days | Only 11 patients (mean 18.8 days) |
Clinical assessment: nine of the participants had a normal sensory exam and four had a decreased sensation Intubated: Nine (82%) patients had a myopathy and five of them also had a concurrent axonal neuropathy Not intubated: 43% had myopathy |
| Abenza-Abildúa et al. (2021) (18) | Retrospective observational study | Hospital setting (inpatient) | NCS/EMG | Cases | 30 | 57.41 ± 11.61 | 21/9 | 2 days after ICU discharge | Yes (mean 18.8 days) |
15 with signs of neuromuscular myopathy Only six patients had NCS Five of them had myopathy (EMG) but normal NCS and one had polyneuropathy |
| Cabañes-Martínez et al. (2020) (19) | Retrospective (cross sectional) | Hospital setting (ICU) | NCS/EMG | Cases | 12 | 64.41 ± 8.24 | 10/2 | 24 days | Yes (mean 24 days) |
One case had normal NCS/EMG Four patients had decreased amplitude of both motor and sensory NCS five patients had short motor unit potentials, with decreased amplitude and duration |
| Koskderelioglu et al. (2022) (21) | Retrospective cohort study | Hospital setting among health care workers | NCS | Cases Control |
76 44 |
37.5 ± 9.6 38.8 ± 9.5 |
29/47 19/25 |
133.8 days | No | No significant difference was found between patients with past COVID-19 infection and healthy controls in term of nerve conduction velocity, amplitude, and latency values |
| Agergaard et al. (2021) (20) | Retrospective cohort study | Hospital setting | NCS/EMG | Cases Control |
20 20 |
53.05 ± 1.86 52.6 ± 1.86 |
4/16 4/16 |
77 to 255 days | No | No significant difference in motor conduction velocity, compound muscle action potential amplitudes, peroneal and tibial nerves between healthy controls and patients. 11 patients (55%) motor unit potential duration was shorter in patients compared to healthy controls in biceps brachii, vastus medialis, and anterior tibial muscle |
| Shabat et al. (2021) (22) | Retrospective cohort study | Hospital setting | NCS/EMG | Cases | 19 | 59.1 ± 10.4 | 16/3 | 176.4 days | No | NCS/EMG for 15 patients (78.9%) were abnormal EMG results of 9 patients (47.4%) had myopathy |
EMG, electromyography; ICU, intensive care unit; n, number of patients; NCS, nerve conduction study.
Reason of Referral
Although many studies and systematic reviews reported neurological manifestations in patients with COVID-19, few studies used electrophysiology to confirm and find the extent of peripheral nerve dysfunctions (3, 24). Difficulty in weaning off the ventilator support was the main reason for the referral of intensive care unit (ICU) patients, followed by muscle weakness. The muscle weakness in ICU patients was flaccid quadriparesis and weakness in the upper and lower limbs (9, 20). Other patients were referred for neurological symptoms, mainly confusional syndrome associated with neuromuscular disorder (19). One study recruited patients with COVID-19 from healthcare staff, mainly those with myalgia, who voluntarily participated in their study (22). Others were referred from the long-term COVID-19 clinic (21). Another study recruited post-COVID-19 patients referred to the rehabilitation department due to reduced endurance, motor impairment, functional impairment, cognitive impairment, or mental impairment (23).
Outcomes
Electrophysiological assessment.
In the studies included in our systematic review, the electrophysiological assessments were by NCS alone or both NCS and EMG.
Electromyography.
In the studies included in this systematic review, the studied nerves were according to the patient’s symptoms and the severity of COVID-19 illness. In general, distal motor latency, motor and sensory conduction velocities, compound muscle action potential (CMAP), and sensory nerve action potential (SNAP) amplitude and duration were measured. F-waves (the conduction along the motor nerves to the root) of the posterior tibial nerve were also performed (20, 22). Findings of EMG of the 175 patients referred for electrophysiological assessment were mainly consistent with myopathy (25.1%). All studies assessed muscles of the lower extremity, three studies assessed both upper and lower extremities (9, 21, 23), one assessed only the median nerve in the upper extremities along with the lower extremities (20), one assessed only the lower extremities (19), and one study assessed both upper and lower extremities by NCS without EMG (22).
The main findings were short motor unit action potentials with low amplitude and duration. Spontaneous activity and positive sharp waves were seen in a limited number of patients, mainly in severe COVID-19 cases. The EMG outcomes for each patient or the mean outcomes for all cases were unavailable in all studies. Only one study reported that gastrocnemius was affected in five out of six patients of their studied population with confirmed myopathy (19).
Nerve conduction studies.
Nerve conduction studies used in patients with COVID-19 with peripheral neurological symptoms were limited. In 6 studies, 64 healthy control and 175 patients with COVID-19 with COVID-19-related peripheral neuropathy underwent NCS. Motor and sensory NCS were done for the upper extremities’ nerves (median, ulnar, and radial) and the lower extremities nerves (sural, peroneal, and posterior tibial nerves).
Sensory nerve conduction.
Studies showed normal NCV (9, 19) or no significant difference in SNCV between healthy participants and patients with COVID-19 (21, 22). Some abnormalities were found in severe cases of COVID-19, such as axonal neuropathy, polyneuropathy, and mononeuropathy, mainly involving the ulnar and peroneal nerves (9, 20). One study reported neuropathies in most of the studied population (78.9%) (23). Although electrophysiological abnormalities were found in almost all the studied population (11 out of 12 cases), only 3 cases had abnormal SNCV characterized by decreased amplitude and average velocity and duration predominantly in the lower extremities (20). A study showed a significant decrease in ulnar nerves SNCV in patients with severe COVID-19 with pneumonia compared with patients with COVID-19 without pneumonia, but this correlation was not significant compared with the healthy control group (22). Age was significantly correlated with neurophysiological symptoms in some studies (20, 22) but not others (21). One study reported the correlation between SNCV and the patients’ motor function, which reported that only the median nerve was correlated with the motor function (the higher the SNCV amplitude, the better the functional level) (23).
Motor nerve conduction.
Of 175 patients who underwent MNCS in all six included studies, 54 patients (30.8%) showed motor neuropathy. One study showed that most of the population (82%) had myopathy, which was worse in intubated patients, but the authors were not specific if the diagnosis was made by MNCV or needle EMG (9). Another study reported normal MNCV in all the studied populations, although they were diagnosed with myopathy by EMG (19, 21). The same findings of normal MNCV were also reported in all studied populations when comparing healthy participants to patients with COVID-19, but there was a significant decrease in ulnar and tibial MNCV. Only median nerve F-wave was correlated to the severity of the illness (22). Median nerve MNCV was also positively correlated with the severity of the illness and the patient’s functional level and rehabilitation outcomes (23). Lower extremities myopathy (tibial and peroneal nerves) was characterized by decreased amplitude, but normal duration with absent F-wave found in the posterior tibial nerves (20, 23).
Main Findings
Out of 195 participants, only 175 underwent NCS/EMG assessment. Of these, 44 participants (25.1%) had abnormal EMG, 54 participants (30.8%) had abnormal MNCS, and only 7 participants (4%) had abnormal SNCS. The remaining patients had normal electrophysiological assessments.
Risk of Bias Assessment
In this systematic review, the NIH assessment tool was used to assess the quality of the studies (Supplemental data; see https://doi.org/10.6084/m9.figshare.21391059.v2). All studies had a fair quality score, ranging from 5 to 10. This score was given because all studies lacked sample size calculation and randomness, as most of the studies included patients who already had neurological symptoms. Also, due to the nature of the studies and COVID-19-related peripheral dysfunction is still inconclusive, all studies lack follow-up NCS assessment, and none were blinded. Three studies (9, 19, 23) included patients with comorbidities and one study included patients who had steroid treatment while they were infected with COVID-19 (23).
DISCUSSION
Many patients with COVID-19 have presented with neurological manifestations, especially severe cases which required intubation and hospitalization. These neurological manifestations have attracted the attention of neurologists in some medical centers to suggest a specific protocol for patients with COVID-19 (19). Clinical neurological assessment for peripheral neuropathy was done for patients with COVID-19 using different assessment and diagnostic methods, but their accuracy remains questionable. Combining clinical assessment, patient history, and validated objective clinical assessment, including electrophysiological measurements obtained via NCS/EMG, was suggested as a gold standard in evaluating peripheral neuropathy (7). Therefore, this work aimed to review the existing literature on clinical studies on COVID-19 peripheral neuropathy to correlate patients’ symptoms, characteristics, and medical history with the electrophysiological outcomes of these patients. We also aimed to find a significant correlation among different electrophysiological outcomes in patients with COVID-19.
This review focused on two electrophysiological methods for diagnosing COVID-19 peripheral neuropathy: NCS and EMG. Some studies compared NCS/EMG outcomes with healthy participants, and others correlated the severity of a patient’s illness with NCS/EMG outcomes. All the included studies used NCV to diagnose neuropathy, and five studies used both NCV/EMG for the upper and lower extremities to diagnose myopathy.
The most common peripheral symptoms are loss of smell and taste, but other neurological symptoms were also reported, including decreased peripheral sensation, decreased muscle power, and myalgia. In the reviewed studies, most cases presented with myopathy, whereas a limited number of cases presented with polyneuropathy. Also, parameters of MNCV and EMG of tibial and peroneal were most affected in the lower extremities in the studied population. Studies that assessed the upper extremities mainly reported abnormalities in most parameters of MNCV and EMG of the ulnar nerve. Interestingly, the median nerve was reported to be associated with the severity and the rate of motor recovery of patients with COVID-19. These results were similar to those reported in case studies and case series that also reported that only motor nerves of the tibial and peroneal in the lower extremities and ulnar and median in the upper extremities were abnormal (25). Case studies on patients with COVID-19 reported that patients’ electrophysiological outcomes were primarily myopathic (10, 26–28). Similar findings were previously reported in similar respiratory diseases known as Middle East Respiratory disease (MERS) and severe acute respiratory syndrome (SARS), which reported abnormal NCV/EMG where motor nerves were most affected, and the peroneal nerve was significantly affected by the disease (29, 30). Although electrodiagnostic tools are valuable for diagnosing and following the progress of patients with neuropathy, their assessment is limited to large nerve fibers and cannot determine the etiology of neuropathy (31).
All studies included in this review reported that NCS, specifically sensory NCV, was not significantly affected when comparing healthy control to patients with COVID-19, which agrees with other studies that showed no or mild abnormalities in NCS (32, 33). Also, patients initially referred for electrophysiological assessments due to sensory symptoms had abnormal MNCV, while the SNCV was generally normal (21). Studies that compared the severity of COVID-19 illness, intubation, weaning, and pneumonia, showed a significant difference in NCV\EMG in severe cases compared with less severe cases (28).
Many case studies and clinical studies used NCS/EMG to diagnose COVID-19 peripheral neuropathy and myopathy and whether it is due to the viral infection directly on nerves and muscles or secondary to intensive care unit-acquired weakness. However, these studies had different results, and the outcomes were inconclusive. This might be because the parameters used in these studies were not standardized. Another primary reason is the small sample size in case studies and limited clinical studies that objectively determined nerve dysfunction in patients with COVID-19. Also, patients’ characteristics, such as age, the severity of the disease, comorbidities, type of drug treatment, time from infection to electrophysiological assessment, and type of examination used to confirm the diagnosis are factors that cause bias in the results. None of the studies provided a follow up for the patients to assess the progression or regression of the symptoms using electrophysiological methods.
Given that COVID-19 is a recent viral disease and patients present with different symptoms, we also expect that NCS/EMG outcomes are differently presented in patients with COVID-19 as observed in the reviewed studies. Also, we found that early screening of patients with COVID-19 with electrophysiological assessment is crucial to determine the severity of nerve and muscle damage, which is essential to determine the recovery and rehabilitation of affected patients (34).
In the reviewed studies, only some of them have reported the parameters of NCS/EMG in healthy or patients with COVID-19, which prevented us from conducting a meta-analysis. We recommend that more clinical studies be conducted on patients with COVID-19 (recovered or active) using NCV\EMG to understand the changes in neuromuscular activity due to COVID-19 infection.
The large variation in NCS/EMG among patients with COVID-19 suggests the importance of early electrodiagnostic in patients with COVID-19, especially those with severe illnesses. Previous studies of Severe Acute Respiratory Syndrome (SARS) reported that although the physical function is increased within 6 mo following infection, patients experienced physical impairments up to 2 years after the infection (35). These novel findings suggest the importance of increasing awareness of the association between electrophysiological parameters and rehabilitation outcomes in viral respiratory diseases to design a treatment plan for each patient, producing a better long-term prognosis. Also, clinicians should be aware of this association between the severity of the COVID-19 illness and electrophysiological abnormalities so that patients with a neurological manifestation of peripheral neuropathy and myopathy can receive early examinations and rehabilitation (23).
LIMITATION AND DIAGNOSIS BIAS
There was a great heterogeneity with respect to defining illness severity, patients’ ages, comorbidities, drug treatment, and neurological diagnosis assessments, which might affect NCV/EMG outcomes (9). In some studies, most cases were men (19, 23) or healthcare workers who presented with mild to moderate COVID-19 symptoms (22). Most of the included studies had less than 20 cases, except for one study (22), and one study included limited cases of their participants who underwent NCV and reported normal values (19). Also, recruiting patients who reported muscular symptoms may create outcomes bias (21, 23). One of the major limitations of this review is that it included only six studies found in the literature that have considered electrophysiological methods for assessing neuropathy or myopathy and determining a patient’s prognosis. These studies only reported some electrophysiological parameters, which makes a meta-analysis challenging to be conducted. Additional studies must be conducted to assess the effect of COVID-19 and the severity of illness on peripheral nerves.
CONCLUSIONS
Patients presented with motor neuropathy, but no significant abnormalities were observed in sensory nerves. We can conclude from the reviewed studies and literature that COVID-19 generates a demyelinating motor neuropathy and myopathy. Clinicians are encouraged to refer patients with COVID-19 presenting with neurological symptoms to be assessed by electrophysiological methods to objectively determine the nature of their symptoms, follow their prognosis, and plan their rehabilitation.
DATA AVAILABILITY
This protocol is registered in the Open Science Framework (https://www.doi.org/10.17605/OSF.IO/ZF4PK).
SUPPLEMENTAL DATA
Supplemental Data: https://doi.org/10.6084/m9.figshare.21391059.v2.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.A-M. and L.A-D. conceived and designed research; L.A-D. analyzed data; S.A-M. interpreted results of experiments; L.A-D. prepared figures; S.A-M. drafted manuscript; S.A-M. edited and revised manuscript; S.A-M. approved final version of manuscript.
REFERENCES
- 1. Tsang HF, Chan LWC, Cho WCS, Yu ACS, Yim AKY, Chan AKC, Ng LPW, Wong YKE, Pei XM, Li MJW, Wong S-CC. An update on COVID-19 pandemic: the epidemiology, pathogenesis, prevention and treatment strategies. Expert Rev Anti Infect Ther 19: 877–888, 2021. doi: 10.1080/14787210.2021.1863146. [DOI] [PubMed] [Google Scholar]
- 2. D V, Sharma A, Kumar A, Flora SJS. Neurological manifestations in COVID-19 patients: a meta-analysis. ACS Chem Neurosci 12: 2776–2797, 2021. doi: 10.1021/acschemneuro.1c00353. [DOI] [PubMed] [Google Scholar]
- 3. Chen X, Laurent S, Onur OA, Kleineberg NN, Fink GR, Schweitzer F, Warnke C. A systematic review of neurological symptoms and complications of COVID-19. J Neurol 268: 392–402, 2021. doi: 10.1007/s00415-020-10067-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Leven Y, Bösel J. Neurological manifestations of COVID-19 - an approach to categories of pathology. Neurol Res Pract 3: 39, 2021. doi: 10.1186/s42466-021-00138-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Almutairi MM, Sivandzade F, Albekairi TH, Alqahtani F, Cucullo L. Neuroinflammation and its impact on the pathogenesis of COVID-19. Front Med (Lausanne) 8: 745789, 2021. doi: 10.3389/fmed.2021.745789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Andalib S, Biller J, Di Napoli M, Moghimi N, McCullough LD, Rubinos CA, O'Hana Nobleza C, Azarpazhooh MR, Catanese L, Elicer I, Jafari M, Liberati F, Camejo C, Torbey M, Divani AA. Peripheral nervous system manifestations associated with COVID-19. Curr Neurol Neurosci Rep 21: 9, 2021. doi: 10.1007/s11910-021-01102-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Haque F, Reaz MBI, Ali SHM, Arsad N, Chowdhury MEH. Performance analysis of noninvasive electrophysiological methods for the assessment of diabetic sensorimotor polyneuropathy in clinical research: a systematic review and meta-analysis with trial sequential analysis. Sci Rep 10: 21770, 2020. doi: 10.1038/s41598-020-78787-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Terlemez R, Özekli Mısırlıoğlu T, Palamar D, Okutan D, Akgün K. Bilateral foot drop after COVID-19-related acute respiratory distress syndrome: a case report. Turk J Phys Med Rehabil 67: 378–381, 2021. doi: 10.5606/tftrd.2021.8194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hameed S, Khan AF, Khan S. Electrodiagnostic findings in COVID-19 patients: a single center experience. Clin Neurophysiol 132: 3019–3024, 2021. doi: 10.1016/j.clinph.2021.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Daia C, Scheau C, Neagu G, Andone I, Spanu A, Popescu C, Stoica SI, Verenca MC, Onose G. Nerve conduction study and electromyography findings in patients recovering from Covid-19 - case report. Int J Infect Dis 103: 420–422, 2021. doi: 10.1016/j.ijid.2020.11.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Becker J, Papagianni A, Herrmann E, Nöller F, Sommer C, Rittner HL. Transient hypoalgesia after COVID-19 infection. Pain Rep 7: e990, 2022. doi: 10.1097/PR9.0000000000000990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kayim Yildiz O, Yildiz B, Avci O, Hasbek M, Kanat S. Clinical, neurophysiological and neuroimaging findings of critical illness myopathy after COVID-19. Cureus 13: e13807, 2021. doi: 10.7759/cureus.13807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fekadu G, Bekele F, Tolossa T, Fetensa G, Turi E, Getachew M, Abdisa E, Assefa L, Afeta M, Demisew W, Dugassa D, Diriba DC, Labata BG. Impact of COVID-19 pandemic on chronic diseases care follow-up and current perspectives in low resource settings: a narrative review. Int J Physiol Pathophysiol Pharmacol 13: 86–93, 2021. [PMC free article] [PubMed] [Google Scholar]
- 14. Novello BJ, Pobre T. Electrodiagnostic evaluation of peripheral neuropathy. In StatPearls. Treasure Island, FL: StatPearls Publishing LLC, 2022. [PubMed] [Google Scholar]
- 15. Paterson RW, Brown RL, Benjamin L, Nortley R, Wiethoff S, Bharucha T, et al. The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain 143: 3104–3120, 2020. doi: 10.1093/brain/awaa240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bromberg MB. An electrodiagnostic approach to the evaluation of peripheral neuropathies. Phys Med Rehabil Clin N Am 24: 153–168, 2013. doi: 10.1016/j.pmr.2012.08.020. [DOI] [PubMed] [Google Scholar]
- 17. Al-Mazidi S, Aladkhil L. Nerve conduction studies in patients with COVID-19 related peripheral neuropathies: A systemic review and meta-analysis. 10.17605/OSF.IO/ZF4PK. [DOI]
- 18. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372: n71, 2021. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Abenza-Abildúa MJ, Ramírez-Prieto MT, Moreno-Zabaleta R, Arenas-Valls N, Salvador-Maya MA, Algarra-Lucas C, Rojo Moreno-Arrones B, Sánchez-Cordón B, Ojeda-Ruíz de Luna J, Jimeno-Montero C, Navacerrada-Barrero FJ, Borrue-Fernández C, Malmierca-Corral E, Ruíz-Seco P, González-Ruano P, Palmí-Cortés I, Fernández-Travieso J, Mata-Álvarez de Santullano M, Almarcha-Menargues ML, Gutierrez-Gutierrez G, Palacios-Castaño JA, Alonso-Esteban R, Gonzalo-García N, Pérez-López C. Neurological complications in critical patients with COVID-19. Neurologia (Engl Ed) 35: 621–627, 2020. doi: 10.1016/j.nrleng.2020.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cabañes-Martínez L, Villadóniga M, González-Rodríguez L, Araque L, Díaz-Cid A, Ruz-Caracuel I, Pian H, Sánchez-Alonso S, Fanjul S, Del Álamo M, Regidor I. Neuromuscular involvement in COVID-19 critically ill patients. Clin Neurophysiol 131: 2809–2816, 2020. doi: 10.1016/j.clinph.2020.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Agergaard J, Leth S, Pedersen TH, Harbo T, Blicher JU, Karlsson P, Østergaard L, Andersen H, Tankisi H. Myopathic changes in patients with long-term fatigue after COVID-19. Clin Neurophysiol 132: 1974–1981, 2021. doi: 10.1016/j.clinph.2021.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Koskderelioglu A, Eskut N, Ortan P, Ozdemir HO, Tosun S. Visual evoked potential and nerve conduction study findings in patients recovered from COVID-19. Neurol Sci 43: 2285–2293, 2022. doi: 10.1007/s10072-021-05816-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shabat S, Meiner Z, Tsenter J, Schwartz I, Portnoy S. Correlations between electro-diagnostic findings, the severity of initial infection, and the rehabilitation outcomes among COVID-19 patients. Biology (Basel) 11: 277, 2022. doi: 10.3390/biology11020277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mahdizade Ari M, Mohamadi MH, Shadab Mehr N, Abbasimoghaddam S, Shekartabar A, Heidary M, Khoshnood S. Neurological manifestations in patients with COVID-19: a systematic review and meta-analysis. J Clin Lab Anal 36: e24403, 2022. doi: 10.1002/jcla.24403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Daia C, Toader C, Scheau C, Onose G. Motor demyelinating tibial neuropathy in COVID-19. J Formos Med Assoc 120: 2032–2036, 2021. doi: 10.1016/j.jfma.2021.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Needham E, Newcombe V, Michell A, Thornton R, Grainger A, Anwar F, Warburton E, Menon D, Trivedi M, Sawcer S. Mononeuritis multiplex: an unexpectedly frequent feature of severe COVID-19. J Neurol 268: 2685–2689, 2021. doi: 10.1007/s00415-020-10321-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Villa D, Ardolino G, Borellini L, Cogiamanian F, Vergari M, Savojardo V, Peyvandi F, Barbieri S. Subclinical myopathic changes in COVID-19. Neurol Sci 42: 3973–3979, 2021. doi: 10.1007/s10072-021-05469-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Uncini A, Foresti C, Frigeni B, Storti B, Servalli MC, Gazzina S, Cosentino G, Bianchi F, Del Carro U, Alfonsi E, Piccinelli SC, De Maria G, Padovani A, Filosto M, Ippoliti L. Electrophysiological features of acute inflammatory demyelinating polyneuropathy associated with SARS-CoV-2 infection. Neurophysiol Clin 51: 183–191, 2021. doi: 10.1016/j.neucli.2021.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chao CC, Tsai LK, Chiou YH, Tseng MT, Hsieh ST, Chang SC, Chang Y-C. Peripheral nerve disease in SARS: report of a case. Neurology 61: 1820–1821, 2003. doi: 10.1212/01.WNL.0000099171.26943.D0. [DOI] [PubMed] [Google Scholar]
- 30. Kim JE, Heo JH, Kim HO, Song SH, Park SS, Park TH, Ahn JY, Kim MK, Choi JP. Neurological complications during treatment of Middle East Respiratory syndrome. J Clin Neurol 13: 227–233, 2017. doi: 10.3988/jcn.2017.13.3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Buxton WG, Dominick JE. Electromyography and nerve conduction studies of the lower extremity: uses and limitations. Clin Podiatr Med Surg 23: 531–543, 2006. doi: 10.1016/j.cpm.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 32. Ser MH, Çalıkuşu FZ, Tanrıverdi U, Abbaszade H, Hakyemez S, Balkan İİ, Karaali R, Gündüz A. Autonomic and neuropathic complaints of long-COVID objectified: an investigation from electrophysiological perspective. Neurol Sci 43: 6167–6177, 2022. doi: 10.1007/s10072-022-06350-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fleischer M, Szepanowski F, Tovar M, Herchert K, Dinse H, Schweda A, Mausberg AK, Holle-Lee D, Köhrmann M, Stögbauer J, Jokisch D, Jokisch M, Deuschl C, Skoda E-M, Teufel M, Stettner M, Kleinschnitz C. Post-COVID-19 syndrome is rarely associated with damage of the nervous system: findings from a prospective observational cohort study in 171 patients. Neurol Ther 11: 1637–1657, 2022. doi: 10.1007/s40120-022-00395-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Avenali M, Martinelli D, Todisco M, Canavero I, Valentino F, Micieli G, Alfonsi E, Tassorelli C, Cosentino G. Clinical and electrophysiological outcome measures of patients with post-infectious neurological syndromes related to COVID-19 treated with intensive neurorehabilitation. Front Neurol 12: 643713, 2021. doi: 10.3389/fneur.2021.643713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rooney S, Webster A, Paul L. Systematic review of changes and recovery in physical function and fitness after Severe Acute Respiratory Syndrome-related Coronavirus infection: implications for COVID-19 rehabilitation. Phys Ther 100: 1717–1729, 2020. doi: 10.1093/ptj/pzaa129. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Data: https://doi.org/10.6084/m9.figshare.21391059.v2.
Data Availability Statement
This protocol is registered in the Open Science Framework (https://www.doi.org/10.17605/OSF.IO/ZF4PK).