INTRODUCTION
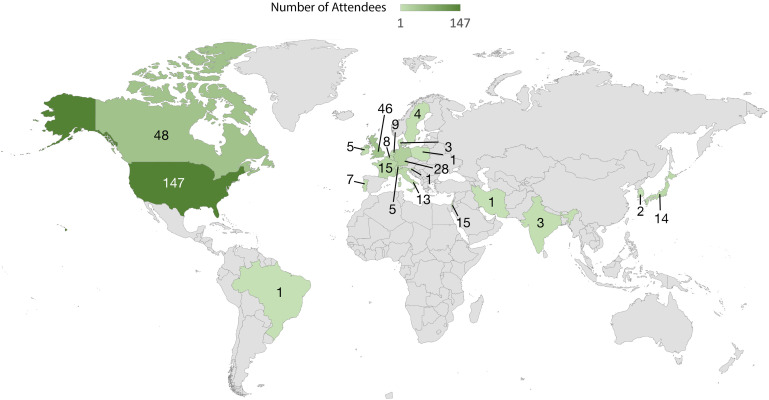
Individuals from all over the world recently gathered in Dublin, Ireland for the 31st Annual Meeting of the Neural Control of Movement (NCM) society. As the first in-person NCM meeting in two years due to the COVID-19 pandemic, the enthusiasm and excitement were tangible. NCM 2022 drew participants from over 20 different countries (Fig. 1), who conduct research using diverse sets of animal species, systems, and approaches. This transdisciplinary research shares the common goal of understanding how the brain achieves exquisite control over movement, allowing us to survive, explore, interact with our environment, and express ourselves artistically.
Figure 1.
Neural Control of Movement (NCM) 2022 attendance map based on country of primary affiliation.
Progress in any field benefits from multiple perspectives. Where gender inequities exist in the realm of academia (1), this NCM meeting provided a platform for over 20 women to give full research talks or panel presentations, making up ∼50% of the total number of talks. Notably, two women received career awards recognizing their contributions to the field. Emily Oby (University of Pittsburgh) was presented with the Early Career Award for her work on brain-computer interfaces, and Fay Horak (Oregon Health and Science University) received the Distinguished Career Award for her lifetime contributions to research on postural control in healthy and clinical populations. Greater than 40% of meeting participants were new attendees. Altogether, this NCM meeting delivered a message that resonated with aspiring young scientists: everyone’s perspective is welcome and essential for advancing this dynamic field.
As with previous years (2–5), this current article was written by a subset of the trainee scholarship award winners to spotlight innovative research presented at NCM 2022. We specifically aim to highlight research that falls into six themes that emerged from the program content (Fig. 2): 1) model organisms spanning the animal kingdom, 2) complex motor behaviors in naturalistic environments, i.e., the wild, 3) the neural control of eye movements, 4) the neural control of posture and gait, 5) circuit mechanisms and sensory factors involved in learning, and 6) large-scale neural population recording and analysis.
Figure 2.
Most common words found in talk and poster abstracts of the Neural Control of Movement (NCM) 2022 program. Bigger font size indicates higher frequency of occurrence. Font color is arbitrarily assigned (green or orange) from colors on the national flag of Ireland, the location of this year’s meeting.
MOTOR CONTROL ACROSS THE ANIMAL KINGDOM
The nervous systems of different species have evolved to maximize survival in their native environments. One of the keys to understanding the neural control of movement, in healthy individuals and in patients with movement disorders, may reside in understanding the unique adaptations found across species. This theme touches on the spectrum of animal models presented at NCM 2022, including insects, reptiles, birds, rodents, and primates, with a focus on the unique insights each species provides in the context of motor control.
Model Organisms with Genetic Tractability
A key feature of a model organism is genetic tractability, which enables researchers to understand neuronal cell types in relation to developmental origin and neurotransmitter identity. In this regard, three species were highlighted at NCM 2022, including Drosophila melanogaster (fly), Danio rerio (zebrafish), and Mus musculus (mouse). In Drosophila, studies performed in the Chiappe (Champalimaud Centre for the Unknown) and Ramdya (École Polytechnique Fédérale de Lausanne) laboratories highlighted the use of loss-of-function and gain-of-function perturbations to interfere with or promote specific aspects of behavior. For example, using genetically targeted alleles, these researchers could promote male courtship (Nuno Rito of the Chiappe laboratory), silence sensory feedback during walking (Adam Gosztolai of the Ramdya laboratory), and optogenetically induce antennal grooming (Pembe Gizem Ozdil of the Ramdya laboratory). These techniques can now be combined with rich kinematic analyses and neuromechanical models, including NeuroMechFly, a biomechanical model of Drosophila recently developed in the Ramdya laboratory (6).
In the zebrafish, Martha Bagnall (University of Washington) presented work dissecting the role of inhibitory ipsilaterally projecting neurons (V1, En1-lineage; V2b, Gata3-lineage) within zebrafish spinal motor networks (7, 8). Using patterned optogenetic stimulation combined with recording from postsynaptic neurons, Bagnall and coworkers mapped connectivity matrices of V1 and V2b neurons. In addition, Bagnall presented work on the organization of utricular projections in the larval zebrafish. Leveraging a recent whole brain library of ultrathin electron microscopy sections of the larval zebrafish brain (9), Bagnall and coworkers (10) imaged utricular circuits at a remarkably fine (4 × 4 nm) resolution and generated a detailed reconstruction of utricular afferent cell bodies, peripheral sensory endings, and central brainstem projections. These data together illustrate the powerful use of zebrafish as a model organism in motor control research.
Several laboratories presented work using genetic perturbations in mice, including the laboratories of Megan Carey (Champlimaud Centre for the Unknown), Court Hull (Duke University), Ole Kiehn (University of Copenhagen), and Holly Holman (University of Utah). Uniquely, mouse genetic alleles can be combined with viral targeting to impart spatially specific circuit manipulations. For example, Court Hull presented work that used optogenetic silencing of inferior olive neurons deep within the caudal medulla to examine how climbing fiber signals shape cerebellar output. Similarly, Ana Machado (Megan Carey’s laboratory) presented work on optogenetic stimulation of Purkinje cell terminals in each of the deep cerebellar nuclei (medial, interposed, and lateral), which uncovered specific functions for each nucleus during locomotion in freely moving mice.
Recording from the Reptilian Spinal Cord
The spinal cord is notoriously difficult to record from in freely moving mammals owing to the movement of the vertebra and spinal cord within the vertebral space. Uniquely, the turtle anatomy lends itself to stable recordings because the spinal cord rests tightly within the vertebral bodies, which are themselves locked within the shell. Rune Berg (University of Copenhagen) presented their laboratory’s latest work using this reptilian spinal cord preparation to examine lumbar neuronal population activity during rhythmic motor behaviors. Rune and coworkers (11) were able to record from hundreds of neurons during the hindlimb scratching reflex, and found that population activity exhibits rotational dynamics, meaning an orderly activation of neurons spanning all phases, with the radius of rotation correlated with muscle force.
Avian Models in Motor Control
Birds are well known for their ability to produce elaborate songs and accurately navigate over vast distances. Sam Sober’s laboratory (Emory University) studies vocal motor control and sensorimotor learning in songbirds, with a focus on the complex relationship between muscle activity and song production. To advance the ability to examine muscle activity in birds and other species, Sober’s laboratory has developed high-density flexible electrode arrays to record tens of well-isolated motor units from individual muscles. These electrode arrays have now been distributed to other laboratories and implemented across several species including mice, monkeys, and humans. Avian research was also highlighted by David Dickman (Baylor University) in the context of navigation. Dickman demonstrated that pigeons possess a remarkable ability to integrate signals from the earth’s magnetic field with vestibular information about self-motion. Here, Dickman showed that neurons in the vestibular nuclei vary their activity with both the direction and magnitude of the earth’s magnetic field. The specific population of neurons that was magnetic field sensitive was also primarily sensitive to anterior-posterior translations with a reference frame oriented to gravity (as opposed to head-referenced). Finally, Dickman demonstrated that cells in the hippocampus respond similarly to place-cells (i.e., cells that fire when an animal is in a specific location), but in magnetic space. Altogether, studying the unique skills that birds require for survival provides an intriguing perspective on motor control.
Predictive Sensing across Organisms and Sensory Systems
Sensory feedback can be generated by our own motor actions or by unexpected external events. In many cases, it is important to distinguish this self-generated (i.e., reafferent) sensory feedback from externally generated (i.e., exafferent) feedback. At NCM 2022, a panel discussed the similarities and differences in reafference cancellation across four species: mice, electric fish, monkeys, and humans, where these species are amenable to studying auditory, electrosensory, vestibular, or somatosensory feedback modalities, respectively.
David Schneider (New York University) presented on predicting the acoustic consequences of actions in the mouse auditory cortex. To address questions regarding the frequency and temporal specificity of reafference cancellation, Schneider studied a combination of natural sound-generating behaviors and engineered sounds. Schneider specifically examined mice after they learned the statistical relationship between movement and the sound it produced, and then violated their prediction by shifting the sound in frequency or time or omitting it altogether. Responses of neurons in the auditory cortex were suppressed during expected self-generated sounds (12); however, a lack of reafference cancellation was observed when the sound was unexpectedly shifted in frequency or time. Interestingly, omitting the sound altogether generated an increase in neural activity, as opposed to a “negative image” that has been described in other systems (e.g., vestibular and electrosensory, see below in this section).
The mormyrid weakly electric fish is uniquely suited to the study of sensory prediction due to its use of electrical pulses for sensing objects in the environment as well as for communicating with other fish. Avner Wallach (Columbia University) demonstrated in freely moving electric fish that neurons in the electrosensory lobe are able to account for (i.e., cancel) electrosensory feedback based on self-generated motion of their tail while swimming. When an expected electrosensory input is omitted, neural activity exhibits a “negative image,” suggesting the existence of precise prediction and cancellation signals.
During unexpected motion induced by balance perturbation, it is necessary for the vestibular system to generate stabilizing postural reflexes. In contrast, when motion is generated by one’s own actions, vestibular reflexes would be counterproductive to the intended movement. Kathleen Cullen (Johns Hopkins University) presented work in rhesus monkeys on how vestibular feedback arising from active self-motion is canceled when there is a match between predicted and actual sensory feedback. Previous work from the Cullen laboratory has demonstrated that neurons at the first stage of central processing, the vestibular nuclei (13), as well as the deep cerebellar nuclei (14), exhibit markedly suppressed responses to active relative to passive head motion. New work from the Cullen laboratory has helped elucidate reafference cancellation mechanisms. Specifically, when considering a population of cerebellar Purkinje cells, which exhibit heterogeneous responses to vestibular, neck proprioceptive, and motor inputs, activity could be linearly combined to predict the responses of target neurons in the deep cerebellar nuclei during active and passive motion. But what happens when perturbations are more subtle, and only influence movement within the natural range of variability? By performing high-density neural recordings in the cerebellum, recent work in Cullen’s laboratory by Omid Zobeiri and Robyn Mildren found that deep cerebellar nuclei (rostral fastigial) neurons exhibit an abrupt change in coding even with the smallest perturbations that fall within the range of natural movement variability. Meanwhile, Purkinje cells exhibited a more gradual change in sensitivity as a function of perturbation level, but the combined activity of >30 Purkinje cells was sufficient to predict responses of their target neurons in the deep cerebellar nuclei.
In humans, Konstantina Kilten (Karolinska Institute) discussed reafference suppression in the context of the somatosensory system. Work from Kilten’s laboratory demonstrated that the activity in the somatosensory cortex is attenuated during self-generated, relative to externally generated, stimuli. Similar to the auditory system (see above in this section), when self-generated touch is shifted in time, perceptual and cortical responses are less attenuated. Altogether, the powerful ability of the brain to predict the consequences of our actions to increase the salience of external inputs is essential for accurate motor control, learning, and threat detection. This requisite ability to predict and suppress self-generated sensory feedback is conserved across many species and sensory systems.
Translation of Work in Animal Models to Humans
Research at NCM 2022 focused on the translation of experimental work in animals directly to humans, and this theme was particularly apparent in the area of upper-limb reaching. Namely, Elizaveta Okorokova (Sliman Bensmaia’s laboratory, University of Chicago) characterized force patterns during grasping and linked them to a population of neurons in primary motor cortex whose activities tracked manual forces. This method was then applied to healthy macaques, and used to develop a manual force decoder for human participants with tetraplegia. The force decoder allowed participants to exert variable forces on a virtual object. Similarly, Catia Fortunato (Juan Gallego’s laboratory, University College London) compared the neural manifolds between the motor cortex and striatum in mice. They found that the degree of manifold nonlinearity was related to circuit connectivity. Subsequently, Fortunato used the same methods to record from the human motor cortex during a handwriting task, and also found that increased task complexity was associated with greater manifold nonlinearity. These two contributions highlight how knowledge generated from research on animal models can be translated to protocols for studying motor control in humans to inform the design of devices such as brain-computer interfaces (BCIs).
Research presented at NCM 2022 traversed the chordata phylum of the animal kingdom. Although a fundamental goal of motor control research is to better understand human physiology, researchers routinely exploit the unique features of model chordata organisms to study physiological mechanisms in detail. As species-specific knowledge becomes mature, integration of this knowledge will undoubtedly shed light into human physiology and illuminate possibilities for restoring or even enhancing function.
FROM THE LAB INTO THE WILD: REMOVING CONSTRAINTS ON STUDYING MOTOR CONTROL
An important endeavor in the field of motor control is to bridge the gap between traditional, rigorously constrained experimental tasks studied in the laboratory and real-life behavior. Recent advances in technology, combined with creative experimental designs, have enabled scientists to study motor control like never before. In humans, it is becoming increasingly important to fully integrate traditional laboratory-based techniques to study motor control in the home environment. Furthermore, animals exhibit a remarkable variety of skills when interacting with objects and exploring their native environments. In this theme, we highlight a selection of studies presented at NCM 2022 that implemented novel approaches for performing data collection remotely in humans or interrogating motor control in animal models under naturalistic conditions.
Human Tasks Reflective of Activities Performed in Daily Life
Several studies at NCM 2022 investigated human strategies in tasks reflective of daily life. For example, Kobayashi and colleagues (Daichi Nozaki’s laboratory, The University of Tokyo) studied human strategies for bimanual manipulation of a virtual stick, a task where multiple hand configurations could result in the same orientation. During the task, a perturbation in task-irrelevant space was introduced. According to the minimum intervention principle (15), subjects would not be expected to correct for perturbations in a task-irrelevant space. Surprisingly, subjects did correct for perturbations over trials, demonstrating that changes in task-irrelevant dimensions can affect motor learning. In another example, Rashida Nayeem (Dagmar Sternad’s laboratory, Northeastern University) studied manual control over a virtual cup with a sliding ball. The cup-ball system exhibited complex nonlinear dynamics, making it difficult to predict its behavior. Before manipulating the cup-ball system, subjects chose the initial state, which impacted the evolution of the system dynamics. With practice, subjects converged on initial states that reached predictable outcomes faster (16). A portable real-life version of the task was then developed to assess patients who had sustained a stroke (17). They found that moderately affected individuals exhibited a reduced ability to predict the cup-ball dynamics compared with able-bodied controls.
Fully Integrating Lab-Based Assessments into the Home
Integration of traditional laboratory-based techniques into the home environment is important for remote monitoring of disease progression as well as the efficacy of clinical trials. Fay Horak, the recipient of the NCM 2022 Distinguished Career Award, discussed the potential of wearable inertial sensors for providing more robust monitoring and assessment of postural control. Horak suggested that integrating these sensors and automated algorithms into daily life could facilitate sensitive passive monitoring (18, 19). Along similar lines, using a fully remote data collection approach, Clara Kuper (Martin Rolf's laboratory, Humbodt Universität zu Berlin) explored motor adaptation during a hand movement task performed on a smartphone or tablet. Kuper showed that only behaviorally relevant changes (positional changes of dots on the screen), but not behaviorally irrelevant changes (background screen flashes) had overt consequences for rapid hand movements. This revealed that sensory signals are filtered for task-relevance before they affect the motor plan.
The Neural Control of Movement in Freely Behaving Animals
In their native environment, animals display a rich set of motor behaviors. Documenting their movement in the wild requires innovative ways to unobtrusively record kinematic data. Novel methods have been developed that do not require the animal to be fitted with sensors or suits, and can estimate poses solely from video. Two approaches for video-based pose estimation were presented at this year’s NCM. Ilka Diester (University of Freiburg) presented FreiPose, a three-dimensional (3-D) posture-tracking framework that uses up to six cameras to estimate the position of an animal in space (20). This system is capable of isolating movements and positions of individual body parts, enabling researchers to study fine movements independent of posture. This approach was equated to virtual head fixation. Diester went on to show selective tuning of neural populations to fine paw movements that would have otherwise been masked by body posture. Jesse Marshall (Bence Ölveczky’s laboratory, Harvard University) introduced CAPTURE, a marker-based tool that enabled the team to efficiently code animal position by storing marker positions rather than full video streams (21). Position data from this marker-based system was then used as ground-truth information to instruct marker-free pose estimation—the DANNCE model (22). Such kinematic techniques will be instrumental for understanding the flexibility of motor control during a range of natural behaviors.
Approaches to studying neuronal activity in freely moving animals have become increasingly sophisticated. To interrogate complex naturalistic motor tasks, it is essential to provide an environment that allows for a wide range of motor behaviors. David Xing (Andrew Miri’s laboratory, Northwestern University) developed a novel mouse arena with interchangeable surface textures and walls. Mice were encouraged to search this rich arena for water ports, which allowed the researchers to probe a large contingent of the motor space, including dexterous reaching, climbing, walking on varied surfaces and grids, grooming, feeding, drinking, as well as more vigorous movements such as jumping. During these behaviors, Xing and colleagues recorded muscle and brain activity from the primary motor cortex (M1) and dorsolateral striatum using high-density electrodes (Neuropixels). Across the behavioral space, they found that muscles exhibited distinct coactivation patterns during different naturalistic motor tasks (e.g., climbing, crawling), with corresponding changes in the activity of corticostriatal networks. Similarly, Jared Cregg (Ole Kiehn’s laboratory, University of Copenhagen), combined a number of sophisticated techniques to examine the neural control of gait and turning in freely moving mice. Cregg and colleagues used optical (Ca2+) imaging in tandem with optogenetic activation or silencing of neural activity to investigate distributed neuronal networks. These experiments revealed how the basal ganglia coordinate with spinal locomotor networks, extending their previous work describing the regulation of gait asymmetries through a brainstem-spinal cord pathway (23, 24).
There is growing interest in understanding primate sensorimotor processing in diverse contexts. Ann Kennedy (Northwestern University) recorded the activity of muscles and neurons in primate M1 during active movement through an enclosure and during precision grasping. Furthermore, Isabelle Mackrous and Jerôme Carriot (Kathleen Cullen’s laboratory, Johns Hopkins University and Maurice Chacron’s laboratory, McGill University) recorded from vestibular pathways in rhesus macaques as they actively walked or ran. They observed that vestibular primary afferents encode motion similarly during passive stimulation as compared with walking and running, indicating the vestibular efferent system does not exert context-dependent modulation of afferent feedback (25).
Sensory systems have adapted coding strategies specific to stimuli encountered during daily life. In the context of the vestibular system, head motion is complex and spans a large frequency bandwidth (26, 27). Thus, to understand vestibular processing in an ethologically valid manner, it is important to tailor vestibular stimulation paradigms to encompass this natural range. An example of this was presented by Robyn Mildren (Kathleen Cullen’s laboratory, Johns Hopkins University), who examined vestibular stimulation mimicking complex natural motion in rhesus monkeys. Specifically, when both low- and high-frequency motion stimuli were delivered concurrently, as is often the case during daily life, the vestibulospinal responses to low-frequency motion were attenuated. In addition, under natural conditions, both the visual and vestibular systems function synergistically. To parse the specific contributions of these two systems to postural control, Mildren and Cullen manipulated visual self-motion cues in normal and bilateral vestibular loss monkeys. Results indicated that the vestibular system plays an essential role in head postural control across the physiological range (up to 20 Hz) during yaw motion, whereas visual feedback has a minimal influence in naive monkeys and does not substitute for absent vestibular feedback following bilateral peripheral vestibular loss.
New approaches to examine motor control during tasks reflective of real-life challenges are beginning to shed light on the skilled motor control required to survive and thrive in our world. Bridging what we have learned from traditional rigorously controlled laboratory-based experiments with naturalistic behaviors will undoubtedly lead to breakthroughs in our knowledge of how the brain exerts exquisite control over movement.
THE NEURAL CONTROL OF EYE MOVEMENTS
The oculomotor system consists of a set of cortical areas and subcortical nuclei that coordinate the precise eye movements needed to explore our visual environment. The NCM 2022 Satellite Meeting on the vestibular and oculomotor systems offered a series of talks that focused on understanding the mechanisms underlying the triggering and execution of different types of eye movements.
Several talks highlighted complexity within the oculomotor system. For example, Wu Zhou (University of Mississippi Medical Center) presented findings that appear to contradict the original oculomotor plant hypothesis put forward by Robinson (28). Zhou recorded activity from the abducens nucleus in rhesus monkeys, which contains motor neurons that innervate the lateral rectus muscle. The oculomotor plant hypothesis would predict that abducens motor neurons fire during horizontal eye movements, including complex movements such as combined eye-head gaze shifts, pursuit, and sleep. Under each of these conditions, however, Zhou observed that motor neuron activity did not directly translate into changes in position and/or velocity of the eyes. Predictions of the oculomotor plant hypothesis may thus need revisiting in the context of more complex eye movements. The complexity of the oculomotor plant was also addressed by Paul May (University of Mississippi Medical Center), who presented work examining the distribution of premotor neurons that control lens accommodation in monkeys (29). Using two-color retrograde transsynaptic labeling initiated independently in the ciliary muscle of each eye, May demonstrated that premotor neurons are located in the supraoculomotor area, central mesencephalic reticular formation, and tectal longitudinal column, with each nucleus exhibiting bilaterally labeled populations of premotor neurons. May also observed that some premotor neurons were only labeled by one of the two viruses. Together, these data support hypotheses for both yoked and independent mechanisms of lens accommodation and vergence.
Shifting focus to initiation of eye movements, Mayu Takahashi (Tokyo Medical and Dental University) presented recordings from the brainstem circuits that trigger saccades (30). Original models put forward by Robinson (31, 32) described an executive system in which two parallel commands generate eye movements: one signal driving excitatory burst neurons that move the eyes, and another signal inhibiting the tonic activity of omnipause neurons (OPNs), which act as a “gate” for saccades. In the anesthetized cat, Takahashi found that the rostral superior colliculus excites OPNs monosynaptically, keeping the eyes steady. In contrast, disynaptic inhibition from the caudal superior colliculus (working via a population of inhibitory burst neurons) releases OPNs, triggering the saccade. Antimo Buonocore and coworkers (University of Tuebingen) also examined the activity of OPNs, this time in rhesus monkeys. Buonocore observed that OPNs exhibit fast transient responses to visual stimuli, and these responses are tuned to stimulus features such as spatial frequency, contrast, and orientation. OPN tuning to visual stimulus features was similar to visual responses in the superior colliculus (33), but with even shorter latencies, hinting at a source of input independent of the superior colliculus. The authors suggested that OPN tuning properties might thus be critical for facilitating or interrupting an eye movement when new visual information appears.
Dora Angelaki (New York University) presented a series of behavioral studies that described eye movement strategies during a navigation task based on optic flow. Both macaques and humans relied on early eye movements as a memory aid (34), whereas humans also exhibited rapid sweeping movements with only a small percentage of eye movements invested in places with no target or desired trajectory. Taken together, these results suggest that these eye movements reflect memory of target location.
To perform accurate eye movements, information from other senses must also be integrated. Jorge Otero-Millan (University of California Berkeley) tested the hypothesis that directional biases in saccades are introduced by both head and scene orientation. Using head tilt in virtual reality during free viewing of fractals, Otero-Millan noted that saccades largely followed the orientation of the head. When presenting natural images with tilt in both the frame of reference as well as head orientation, directional biases fell in between these tilts. Interestingly, however, microsaccades (i.e., small eye movements generated during periods of fixation) did not show any bias related to the orientation of the scene. They concluded that two frames of references bias saccade generation: an egocentric frame of reference associated with head position that affects microsaccades, and an allocentric frame related to scene orientation that biases large saccades.
Finally, Satellite keynote speaker Michael King (University of Michigan) presented work on the persistence of oscillopsia in patients with bilateral vestibular hypofunction. Oscillopsia refers to a sensation of jumpiness of the visual scene that is caused by a failure of the vestibulo-ocular reflex (VOR) to stabilize eye position in space during head motion. Although oscillopsia is resistant to rehabilitation, monkeys can learn to produce compensatory eye movements that replace the VOR. Compensatory eye movements are also observed in some patients with vestibular hypofunction, indicating that humans can learn to execute these responses—although this compensation does not fully eliminate oscillopsia. To probe mechanisms of VOR compensation, King and coworkers examined guinea pigs with bilateral lesions of the vestibular system. Although guinea pigs with bilateral vestibular loss exhibited no VOR in response to passive rotation of the head, a VOR-like response was present again during voluntary movement. These data suggest that an efference copy of the intended movement allows for compensation. Altogether, while guinea pigs, monkeys, and humans can execute eye movements that compensate for a loss of the VOR, the persistence of human oscillopsia remains enigmatic.
This year’s NCM Satellite meeting highlighted fundamental interactions between oculomotor control and integrated functions of the mammalian brain. From ocular motor neurons, to executive premotor networks, to higher levels of cognitive function, understanding the basic control of eye movements provides a unique vantage point with which to study the fundamental principles underlying motor control.
THE NEURAL CONTROL OF POSTURE AND GAIT
The ability to maintain control of posture and balance is mediated by the neural integration of multisensory and motor information. Using behavioral and neurophysiological approaches, several presentations at NCM 2022 addressed the contributions of sensory systems to the control of posture and gait in neurotypical humans, patient populations, and animal models.
Aging and Vestibular Disorders
The vestibular system is an essential sensory modality that contributes to stabilizing head and body posture as well as gaze. Understanding how neurological disorders and/or aging affect vestibular function is an essential area of research with implications for mitigating the risk of falls. The links between aging, vestibular function, and balance were addressed in the work by Daniel Merfeld (Harvard Medical School). They found that the signal-to-noise ratio of vestibular inputs, represented by vestibular thresholds of self-motion, declined linearly by ∼15%–83% per decade starting at the age of 40. After controlling for age, vestibular thresholds were still associated with clinical balance function (35). Merfeld further highlighted the value of re-analyzing previously published data with dimensionality reduction (principal component analysis), multivariate statistics, and mediation analyses (36, 37). Re-analyses of data in this manner revealed that differences in vestibular thresholds explain ∼50% of the age effects on balance. Moving from neurotypical aging to neurological disorders, Susan King (Jenks Vestibular Physiology Laboratory, Massachusetts Eye and Ear) examined deficits in vestibular pathways in patients with unilateral vestibular Schwannomas. Vestibular Schwannomas are tumors that damage the vestibular nerve and labyrinth, resulting in imbalance and dizziness. By assessing vestibular function using the VOR, King found that imbalance correlates with VOR metrics that reflect central signal-to-noise ratios. Dizziness, however, was not correlated with any dynamic VOR metrics. These data suggest that VOR-independent factors contribute to the perception of dizziness in patients with vestibular Schwannomas (38).
In addition to aging and neurological disorders, evidence suggests that noise exposure (an environmental factor) can damage the peripheral vestibular system. Courtney Stewart (Lieutenant Colonel Charles S. Kettles VA Medical Center) examined noise-vestibular interactions in rats by measuring vestibular short latency-evoked potentials (VsSEPs) before and after noise stimulation. VsSEPs were transiently abolished after noise stimulation, and although detectable VsSEPs did re-emerge, their amplitude continued to be attenuated for up to three weeks postexposure. Noise stimulation also slowed balance beam crossing times, demonstrating a behavioral correlate for altered vestibular function. These findings offer insight into how noise exposure can influence motor control in addition to causing hearing loss.
Restoring Function Using a Vestibular Prosthesis
Research aimed at restoring vestibular feedback using prostheses has the potential to mitigate deficits in vestibular function associated with aging and/or disease. Olivia Leavitt (Kathleen Cullen’s laboratory, Johns Hopkins University) presented work that aimed to optimally restore balance function using different mapping functions between head motion and prosthesis stimulation in a rhesus monkey model of complete bilateral peripheral vestibular loss. Leavitt implemented different biomimetic mapping functions that were developed based on recorded responses of primary vestibular afferents (regular and irregular) to head motion. Leavitt found that the biomimetic mapping function that better captured the dynamics of endogenous irregular primary vestibular afferents provided superior balance-correcting responses during transient support surface perturbations. As it is thought that the irregular vestibular afferents provide stronger input to pathways that stabilize head and body posture, this work highlights how implementing knowledge of natural neural encoding can inform prosthesis design.
Sensory Reweighting
When sensory feedback is altered, the nervous system can compensate using substitution or reweighting by other sensory modalities (39). Pieter Medendorp (Radboud University) examined sensory reweighting in individuals with DFNA9, a vestibulo-cochlear disorder. Under different tilt conditions, patients were tasked with judging the orientation of a rod within a static square frame. Patients with DFNA9 exhibited larger biases and greater variability in the perceived direction of gravity relative to healthy control subjects. Using Bayesian inference, the authors found that while both patients with DFNA9 and control subjects exhibited visual reweighting under different tilts, patients with DFNA9 exhibited a larger reliance on visual weight than controls (40). Another model to study sensory reweighting is exposure to microgravity. When re-introduced to gravity after spaceflight, astronauts exhibit pronounced postflight changes in mobility and balance, beyond what can be explained by muscle atrophy alone. At the level of the cortex, changes in vestibular areas have previously been found postflight (41). At this meeting, Heather McGregor (Rachael Seidler’s laboratory, University of Florida) discussed how preflight resting state functional connectivity may be able to predict individual differences in balance function assessed with the Sensory Orientation Tests. McGregor found that individuals with weaker connectivity between cortical sensory areas including the left insula, left primary somatosensory cortex, and left lateral occipital cortex had larger balance deficits following spaceflight. These individual differences in brain connectivity and behavior may be useful for the development of individualized preflight training.
Cortical Contributions to Balance
Individual differences in cortical sensory integration in balance and gait function were also highlighted by Jasmine Mirdamadi (Michael Borich’s and Lena Ting’s laboratories, Emory University) in a perspective session with Sue Peters (University of Western Ontario), and Sam Stuart (Northumbria University). Although balance and gait primarily involve subcortical circuitry, this panel emphasized the involvement of the cerebral cortex, which can be influenced by individual ability, task difficulty, aging, and neurological disease. Similar to the link between vestibular thresholds and balance discussed in the Satellite meeting, Mirdamadi demonstrated that individuals with worse whole body motion perception exhibited worse balance. Furthermore, the state of sensory cortical processing before perturbations, indexed by β rhythm over the supplementary motor area, was associated with individual differences in whole body motion perceptual ability. Peters demonstrated how deficits in cortical sensory processing have functional relevance for balance and mobility in individuals with and without stroke. In neurotypical individuals, attention during motor planning of ankle movements gated sensory information, indexed by suppression of somatosensory-evoked potentials (SEPs) elicited by tibial nerve stimulation. This attention-mediated gating of sensory information was absent in individuals with stroke and was associated with worse balance and mobility. Alterations in attention-related changes in SEPs poststroke could not be explained by somatosensory cortex alone, as participants did not have lesions within somatosensory cortex (42). Thus, these data indicate that cortical sensory processing likely involves the interplay of multiple areas including prefrontal cortex and higher-order sensory areas. Finally, Sam Stuart shared work on how different sensory cueing interventions (e.g., visual lines on the floor or vibration applied to soles of feet) impact gait quality and cortical activity in older adults and patients with Parkinson’s disease (PD). Visual and tactile cues enhanced gait characteristics compared with no cues; however, there were distinct differences in the magnitude of cortical activation across groups and individuals. Compared with older adults, visual cueing enhanced activation in the parietal cortex in individuals with PD, particularly in those individuals who exhibited more severe impairments including freezing of gait (43). Together, this panel highlighted the value of quantifying cortical activity during movement, which will further enhance our understanding of sensory contributions to balance and gait and assist with the development of personalized interventions.
In their keynote talk, Fay Horak emphasized that postural control is not mediated by a single brain area, and illustrated how objective measures of brain structure and function can offer mechanistic insight into unique subdomains underlying control of balance and gait. For instance, resting-state functional network connectivity (rsFC) of distinct subcortical and cortical networks predicted different subdomains of postural impairments in PD: frontoparietal and ventral attention rsFC predicted anticipatory postural adjustments, cerebellar-subcortical and visual rsFC predicted automatic postural adjustments, and ventral attention and ventral multimodal rsFC predicted postural sway (44). The specificity of these networks to different subdomains of postural control suggests the possibility of personalized interventions depending on the nature of the individual’s postural impairments. Structural neuroimaging of the brain may also offer biomarkers of balance and gait impairments. Similar to their findings with rsFC, brain volumes of the ventricles, brainstem, and gray matter predicted distinct balance and gait metrics (45). Advances in neuroimaging now allow for the quantification of spinal cord structure, which will complement existing metrics of subcortical and cortical volumes toward a more complete neuroimaging approach for understanding balance and gait.
Vivian Weerdesteyn (Radboud University Medical Center) examined the contribution of different subcortical pathways involved in rapid goal-directed stepping (46). They assessed express visuomotor responses (EVRs), which are directionally tuned bursts of muscle activity known to enable rapid reaching (47, 48). They asked whether EVRs are also present in the lower limb to enable rapid stepping, and if so, whether these EVRs are influenced by postural demands. The presence of EVRs was compared with anticipatory postural adjustments, previously known to occur in more challenging postural tasks before stepping. EVRs were present in a low postural demand task and facilitated rapid stepping, but were largely suppressed in a high postural demand task, potentially through higher-order cortical mechanisms. Lack of EVR suppression was associated with larger anticipatory postural adjustments and slower stepping. Together, these NCM talks highlight the complex interplay between subcortical and cortical mechanisms that are differentially recruited depending upon postural demands.
Complexity of Quantifying Balance and Gait
The ability of humans to maintain postural control while efficiently traversing their environment requires complex neuromuscular control. A number of methods are available for characterizing specific aspects of gait for the purpose of diagnosis, monitoring, and/or treatment of mobility impairments; however, focusing on correcting one of a few aspects of posture and gait severely limits the scope in which individuals may operate. Fay Horak provided several examples of how posture and gait represent different domains of mobility. For instance, levodopa, the gold standard medication for PD, improves gait pace but actually worsens postural sway, and thus may increase fall risk (49). Different subdomains of posture and gait may also reflect different subdomains of cognitive function. For example, performance on a visuospatial cognitive task predicts postural sway, whereas performance on the Stroop task predicts gait pace and turning (50). The sensitivity and specificity of different measures used to characterize mobility impairments may depend on the specific neurological disorder (51). Although there are several disease-specific clinical assessments, such as the Unified Parkinson’s Disease Rating Scale (UPDRS) for Parkinson’s severity, other measures obtained through wearable sensor data may be more appropriate for detecting mobility impairments. Although turning is not incorporated in the UPDRS, nor objectively quantified by neurologists, turning speed is actually a more sensitive measure for discriminating fallers from nonfallers (18). A final consideration is the use of at-home community assessments versus assessments conducted in a clinic or a laboratory setting. Continual daily monitoring using wearable sensors at home may provide progress toward understanding the multifactorial nature of mobility impairments in complex environments (19).
With recent emphasis on holistic assessments of movement, Elizabeth Carlisle (Arthur Kuo’s laboratory, University of Calgary) highlighted the importance of both energy and time expenditure during goal-directed movements. They developed an optimization principle to examine how speed during point-to-point walking bouts is influenced by task urgency or movement vigor. The relationship between energy and time costs provides an objective measure that can predict walking speed trajectories. Similarly, holistic assessments of movement can be captured using artificial neural networks. Taniel Winner (Lena Ting’s laboratory, Georgia Institute of Technology and Emory University) leveraged a recurrent neural network model to extract and analyze gait dynamics in able-bodied subjects and individuals that had sustained hemiplegic stroke. The resulting individual-specific, low-dimensional representations of gait dynamics were used to define “gait signatures” in these two populations. Interestingly, the gait signatures metric revealed stereotypy among able-bodied controls and heterogeneity among individuals in the stroke-survivor cohort. Together, advances in quantifying posture and gait in the laboratory and home environments may improve diagnosis and treatment of mobility impairments.
LEARNING: CIRCUIT MECHANISMS AND SENSORY FEEDBACK
Motor learning enables us to execute new movement patterns and adapt to changes in the environment. Presentations at NCM 2022 highlighted the complexity of interacting circuits and learning mechanisms, as well as sensory contributions to motor learning.
Reward-Based Learning in the Basal Ganglia and Cerebellum
The basal ganglia and cerebellum both play critical roles in motor learning, but have historically been studied in isolation. For instance, the basal ganglia have been traditionally ascribed a role in reward-based learning, whereas the cerebellum has been linked to error-based learning. A series of talks highlighted new insights into circuit interactions within and between these two subcortical areas, as well as the cortex, suggesting complex interplay among mechanisms for learning.
The diversity of basal ganglia functions reflects parallel basal ganglia-cortical loops traditionally thought to converge with cerebellar-cortical loops at the cortical level. However, Andreea Bostan (University of Pittsburgh) shared evidence that these two areas also communicate at a subcortical level, with dense reciprocal disynaptic projections between regions (52). These anatomical studies motivate new research on exploring how the basal ganglia, cerebellum, and cortex form a functionally integrated network for motor learning.
Dopamine is known to signal reward prediction error in the basal ganglia (53), a basic mechanism for motor learning. However, recent work from Court Hull’s laboratory (Duke University) suggests that the cerebellum may also employ learning rules that resemble reward prediction. Indeed, Hull’s laboratory has demonstrated, using mesoscale calcium imaging in the cerebellar cortex, that climbing fiber input to the cerebellum preferentially signals correctly executed movements (54, 55). In the laboratory’s latest work presented at NCM 2022, Hull and coworkers used optogenetic silencing of climbing fiber input to the cerebellum during an appetitive classical conditioning task. They found that climbing fiber input can flexibly signal task-specific rules depending on reward context, and silencing climbing fiber-related reward signals impaired learning. This contrasts with a strict view that the cerebellum mediates error-based learning whereas the basal ganglia mediate reward-based learning.
In light of the recent findings of cerebellar involvement in reward signaling, Mati Joshua (The Hebrew University of Jerusalem) directly compared neural activity from the basal ganglia and cerebellum in monkeys during eye-movement tasks. To examine whether these regions exhibit hierarchical organization in processing reward versus movement-related cues, they analyzed neural activity from input (caudate) or output (substantia nigra pars reticulata) regions of the basal ganglia as well as Purkinje cells or local neurons of the cerebellum. Similar to previous work, cerebellar neurons responded to both reward and movement signals. However, reward-related responses were strongest from basal ganglia output compared with either population of cerebellar neurons. Based on these results, the authors suggest that the basal ganglia and cerebellum have unique computational functions (56). Further insight into the integration of basal ganglia and cerebellar processes was elucidated by Vikram Chib (Johns Hopkins University) using fMRI in humans. Their results suggest there is some dissociation between striatal and cerebellar functions, with the striatum primarily encoding reward signals associated with value, and the cerebellum encoding motivation signals associated with reward salience. Altogether, the combined activity of the basal ganglia and cerebellum appears to be necessary to generate motivated behavior.
Clinical Implications of Basal Ganglia-Cerebellar Interactions
Cross talk between basal ganglia and cerebellar circuitry has important clinical implications. Wolf-Julian Neumann and Roxanne Lofredi (Charité—Universitätsmedizin Berlin) discussed mechanisms of how deep brain stimulation (DBS) of the subthalamic nucleus and globus pallidus may alleviate bradykinesia and enhance motor learning in Parkinson’s Disease (PD) (57–59). fMRI data suggest that DBS also influences resting-state cerebellar connectivity. Patients with PD with stronger cerebellar connectivity also exhibited enhanced learning (59). In patients with cerebellar degeneration, Amanda Therrien (Moss Rehabilitation Research Institute) examined how reward may influence cerebellar-based computations for learning. Although patients with cerebellar degeneration had impaired error-based learning, indicative of poor state estimation, they were still able to learn a simple reaching task with binary reinforcement feedback that involved reward processing (60, 61). The variability in the magnitude of reinforcement learning could be attributed to the degree of internal model impairment, suggesting that these patients have deficits in processing both reward information and state estimates that guide learning. In the Iowa Gambling Task, which is a nonmotor task that depends on processing reward and punishment-related information, patients with cerebellar degeneration exhibited slower and inefficient learning, further highlighting the importance of the cerebellum in processing reward signals at a behavioral level.
Several hypotheses have been put forward for the overall function of the basal ganglia, including action selection, initiation and termination of movements, procedural memory storage, and flexible parameterization of movement kinematics (i.e., vigor). David Robbe (Inserm Aix-Marseille University) sought to delineate distinct aspects of basal ganglia function by examining locomotor performance across a range of reward-oriented tasks with different requirements for speed and memory (62). Rats with lesions to the dorsal striatum were able to execute previously learned locomotor routines and exhibited an intact ability to execute a new routine that required little effort. However, rats exhibited reduced speed and initiation of reward-oriented movements, suggesting that the dorsal striatum may modulate kinematics through alterations in effort sensitivity. Therefore, the basal ganglia are likely not storing memories, but invigorating movement of reward-oriented actions.
The Transition from Learned Skills to Habits
After a skill is learned, actions become habitual over time. What happens, however, when stimulus-response associations change? Christopher Yang (Adrian Haith’s laboratory, Johns Hopkins University) presented the example of riding a “backwards bicycle,” where mapping between the handlebar and effective steering direction is reversed. Because riding a bike is habitual, people are not able to simply adapt, but instead require several weeks of practice to proficiently ride the backward bicycle. Inspired by this example, Yang developed a similar laboratory-based task where participants learned a new bimanual mapping that controlled movement of an on-screen cursor (63). After two, five, or ten days of practice, they altered the mapping to determine if participants could adjust their behavior or if their behavior had become habitual. Results showed that behavior was habitual after only two days of practice, and individuals could further increase their skill level with practice (64).
Sensory Contributions to Motor Learning
Similar to previous NCM meetings, there was an emphasis at NCM 2022 on the role of proprioception in sensorimotor control and learning (2, 4). In one panel, speakers looked at perceptual and motor changes after sensorimotor perturbations during upper-limb reaching and walking. Despite traditional models of visuomotor adaptation that suggest implicit adaptation is driven by minimizing visual errors (i.e., a visuocentric view), Jonathan Tsay (Rich Ivry’s Lab, University of California Berkeley) proposed a proprioceptive-focused perspective called the proprioceptive realignment model (PReMO). In PreMO, implicit adaptation is driven by minimizing proprioceptive errors. Tsay illustrated how the upper bound of implicit adaptation occurs when the perceived hand position aligns with the movement goal, i.e., is felt at the target. PReMO, but not visuocentric views, explained how adaptation increased with proprioceptive uncertainty and proprioceptive shifts (65, 66). This model has important implications for understanding adaptation in patients with cerebellar degeneration, where impaired adaptation may be a function of noisy sensory predictions.
Similarly, Hannah Block (Indiana University) described how visuo-proprioceptive misalignment, in the absence of motor adaptation, influences the perception of hand position and sensorimotor processing (67, 68). Individuals compensated for a visuo-proprioceptive misalignment by either realigning proprioceptive estimates or visual estimates of their fingertip position. Interestingly, proprioceptive but not visual realignment was observed even after individuals were provided with vision of the hand, and this realignment was retained 24 h after the misaligned reaching task (69). Increased visuo-proprioceptive realignment was associated with decreased motor cortex excitability probed by transcranial magnetic stimulation. This decrease in motor cortex excitability appeared to be mediated by projections from the somatosensory cortex, as suppression of the somatosensory cortex influenced proprioceptive realignment.
Proprioceptive realignment can occur in the absence of any sensory mismatch, as shown by Cristina Rossi (Amy Bastian’s laboratory, Johns Hopkins University) in a split-belt adaptation task. They examined the perception of leg speed before and after split-belt adaptation. In terms of biomechanics, individuals initially exhibited a limp when the belts were moving at different speeds, but rapidly adapted to walk symmetrically. When the belts returned to equal speeds, participants exhibited a negative aftereffect (i.e., a limp in the opposite direction) but also shifted their perception of leg speed. Since this perceptual change occurred in the absence of sensory mismatch, recalibration may be driven by a motor mechanism (70).
Chris Miall (University of Birmingham) examined how loss of proprioception through acquired versus congenital deafferentation influences motor control and adaptation (71). Patient IW, who exhibits acquired deafferentation, accomplishes tasks by heavily relying on vision and cognitive control. In contrast, patient KW, who exhibits congenital deafferentation, could accomplish tasks more automatically, potentially through subcortical mechanisms. The stark differences between these two patients offer insight into how development and experience reorganize sensorimotor pathways in the absence of a crucial sensory system.
Finally, Chen Avraham (Ben-Gurion University) described how tactile feedback may augment force field adaptation during upper-limb reaching. Tactile information was added in force field trials in which either a velocity-dependent force was applied, or where virtual walls resisted any lateral forces (thereby imposing a straight movement while canceling the visual error). The results showed no effect of the tactile augmentation on movement kinematics, but a significant effect on manipulation and grip force control (72). Interestingly, although the tactile modality naturally provides force information, the observed effects suggest that augmented tactile information was used as a haptic cue that affected motor output during adaptation.
Motor Learning in Human-Machine Interfaces
Understanding the control policies that underlie motor learning may benefit from studying human-machine interfaces. In their talk, Ali Shafti (Aldo Faisal’s laboratory, Imperial College London) presented a study in which humans and artificial intelligence (AI) were required to collaborate to achieve task success (73). The AI implementation involved data-efficient reinforcement learning, which was updated according to the user’s performances. Shafti found that humans and AI could develop corresponding control strategies with practice. Further investigating human-robot interactions, Steafan Khan (Florida International University) used a task where human subjects operated a robotic arm with five degrees of freedom. They showed that this interaction was primarily dependent on individuals’ optimization of feedback strategies and learning environment, and not on the mapping that was implemented to transfer the users’ performances to the robot. Overall, these studies emphasize the use of human-machine collaboration for understanding control policies in motor learning.
POWER OF THE MASSES: LARGE SCALE POPULATION RECORDING AND ANALYSIS
Even the simplest of movements requires large-scale coordination of neurons across the brain. A number of studies at NCM 2022 focused on understanding the dynamics of neural populations, as opposed to single neurons, and highlighted the expansion of methods and tools used for studying the nervous system at mesoscale resolution.
Several presentations at NCM 2022 described novel implementations of recording technologies. In particular, several laboratories have now implemented a nonhuman primate version of high-density neuropixels electrodes. Three groups present at NCM—Mark Churchland (Columbia University), Andrew Pruszynski (University of Western Ontario), and Kathleen Cullen (Johns Hopkins University)—engineered unique setups to acutely record from the brain of awake behaving macaques using primate neuropixels. One new finding is that systems previously thought to be low dimensional were likely undersampled. For example, Elom Amematsro (Mark Churchland’s laboratory, Columbia University) found that macaque M1 activity during an isometric force task was very high dimensional, with 80 first components explaining only 90% of the variance in the data. These findings are in line with the hypothesis that high dimensionality of motor areas might facilitate a large repertoire of distinct motor skills (Eric Trautmann, Mark Churchland’s laboratory, Columbia University). Beyond the motor cortex, researchers from Kathleen Cullen’s laboratory recorded from deeper structures, the brainstem and cerebellum, using prototype read-write neuropixels that can both record and deliver electrical stimulation. Cullen presented new data from Omid Zobeiri and Robyn Mildren that showed how populations of cerebellar Purkinje cells and deep cerebellar nuclei neurons perform internal-model based prediction and suppression of self-generated motion, and how these populations respond during small perturbations to voluntary movement. In addition to academic presentations, several neurotechnology companies, including Blackrock Microsystems and Ripple Neuro, showcased their most recent products that emphasized increased channel count, device portability (e.g., wireless headstages), and multi-functionality (e.g., simultaneous recording and stimulation).
Recent advances in large-scale recording have motivated the development of statistical and modeling tools to analyze how neural populations perform the computations necessary to plan and execute movements. Previous studies have demonstrated that although hundreds of neurons can be recorded at the same time, their activity is usually correlated, and can be described by a few patterns of activity confined to a low-dimensional space—the so-called neural manifold. This framework has allowed researchers to discover key features regarding how population activity generates movement. This continues to be the case, as several talks and posters made use of the manifold framework to explain how neural activity relates to behavior.
One emergent theme of NCM 2022 in the context of population dynamics was the use of Brain-Computer Interfaces (BCIs) to understand how the brain generates behaviors, with results that could improve the use of BCIs in clinical settings. The work of Early career award winner, Emily Oby (Aaron Batista’s laboratory, University of Pittsburgh) deserves honorable mention. Oby presented a series of experiments exploring how certain tasks are more difficult to learn than others (74). When monkeys used a BCI to control cursor velocity, Oby forced decoder mapping to fall outside the manifold. Performance improved through training, which was strongly correlated with an increase in new patterns of activity outside the initial repertoire. Oby further showed that the neural trajectories were not flexible, but rather, were temporally constrained. In addition to the insights that this work could provide to clinical BCIs, it could also open the door to further explore how BCIs might generalize across different skilled behaviors.
In a related task, Patrick Marino (Aaron Batista’s laboratory, University of Pittsburgh) explored interactions between volitional signals that encode movement goals and sensory signals that encode arm posture. Monkeys were trained to use a BCI to control cursor movement while their arm was placed in different positions. Marino observed that M1 population activity was not influenced by postural input. Furthermore, when changing the posture and performing an isometric force task, Marino demonstrated that posture and volitional information were encoded in separate neural dimensions, with limited reshaping across different postures. Importantly, this work sheds light into how M1 performs sensorimotor integration and can potentially help in the design of BCI decoders that generalize across postures.
Eric Trautmann (Mark Churchland’s laboratory, Columbia University) shared work describing how M1 implements context-specific feedback control. Monkeys were trained in a Pacman-style task, where they had to produce force in two different contexts in which the output was matched, but sensory feedback was opposing. Using demixed principal component analysis (75), Trautman showed that these two contexts are associated with very different patterns of activity in M1, suggesting that skills are generated from skill-specific rather than output-specific neural trajectories. These neural trajectories allowed for flexible relationships between sensory input and motor output. Importantly, this work predicts that M1 leverages high dimensionality to store various motor skills. In a similar task, Elom Amematsro (Mark Churchland’s laboratory, Columbia University) also showed that M1 can be separated into three almost orthogonal subspaces with distinct dynamics, corresponding to three movement motifs (slowly changing forces, ramps, and sinusoids).
Neural trajectories related to isometric force production were also investigated by Elizaveta Okorokova (Sliman Bensmaia’s laboratory, University of Chicago), who trained monkeys to grasp instrumented objects with different force levels. Okorokova showed that neural population responses contain weak but significant force signals that exhibit nonlinear dynamics. To accommodate for nonlinearities, Okorokova trained recurrent neural networks (RNNs) to continuously decode force profiles from neural population activity. The success of this approach suggested its suitability for decoding intended grasp force in a BCI setting. Using motor cortical activity from a tetraplegic subject attempting to grasp objects with varying amounts of force in a virtual environment, Okorokova showed that RNN-based decoders significantly outperform other known decoders of force in terms of accuracy and speed in a real-time setting.
In another BCI study, Brian Dekleva (Jennifer Collinger’s laboratory, University of Pittsburgh) investigated neural population responses in the human motor cortex during control of individual fingers and finger combinations. A tetraplegic subject was instructed to attempt to flex their fingers to perform virtual presses of five on-screen buttons (one button for each finger), either using one finger at a time or multi-finger combinations. Dekleva found that the neural manifold corresponding to multi-digit trials contained strong combination-specific dimensions that could not be explained by dimensions constructed from single-digit trials. These results indicate nonlinearities in the cortical control of the hand and fingers.
Although previous studies have largely focused on linear methods for identifying neural manifolds, Cátia Fortunato (Juan Gallego’s laboratory, Imperial College London) offered an alternative perspective. Fortunato described nonlinearity of neural manifolds on datasets from mice, monkeys, and humans performing a variety of reaching, grasping, and attempted writing tasks. Using linear (PCA) and nonlinear (Isomap) methods for dimensionality reduction, it appeared that even in a simple center-out reaching task, the estimated neural manifold is nonlinear. This nonlinearity is also network-dependent, where striatum exhibits a higher degree of nonlinearity than M1. Furthermore, nonlinearity of neural manifolds increased with task complexity. This work showed that consideration of nonlinearity might become crucial as the field evolves to incorporate multi-region recording during complex and naturalistic tasks.
Several groups presented new decoding approaches that make use of neural/kinematic manifold structures that could improve future integration of BCIs in the clinical setting. In particular, Sean Perkins (Mark Churchland’s laboratory, Columbia University) demonstrated a new kinematic decoder called MINT (Mesh of Idealized Neural Trajectories) that was inspired by the observation that neural trajectories in the motor cortex are stereotyped and directed. The decoder learns a manifold of neural states by learning condition-specific canonical neural trajectories and using interpolation to estimate states between trajectories. Perkins showed that MINT decoder achieves high performance yet remains computationally efficient, which makes it suitable for real-time applications. Indeed, Blackrock Microsystems is working to integrate MINT into its decoding libraries.
Another manifold-based decoding approach was demonstrated by Andres Agudelo-Toro (Hansjörg Scherberger’s laboratory, University of Göttingen). Decoder ReMAP (Recalibrated Map to Attempted Path) was designed for grasping prostheses and leverages the observation that kinematic trajectories during grip type execution follow a curved state-space manifold. The algorithm, inspired by variational autoencoders, projects attempted kinematic transitions onto the intended kinematic trajectory and uses the projection as the training objective function. The decoder was validated in nonhuman primates using neural data recorded from the grasping circuit (AIP, hand M1, and F5). Monkeys were first trained to perform an actual grasping task and then transitioned to an equivalent BCI task with no movement of the native arm. ReMAP decoder outperformed traditional training methods in a number of metrics, including success rate and accuracy of the BCI grasps.
The emergence of new neural recording technologies opens up the possibility to collect large-scale datasets across a range of brain structures involved in behavior. Such massive amounts of neural data will inevitably lead to the expansion of population-based analysis techniques, which will be exciting to follow in future meetings.
CONCLUSIONS
Altogether, NCM 2022 in Dublin, Ireland brought together a diverse group of researchers for in person scientific dialogue that had been missing during the Covid-19 pandemic. As highlighted in this article, NCM 2022 was crucial for assembling researchers working with various model species and humans, and from different experimental, theoretical, and analytical perspectives. Together, we can act as a unified front to address how the brain controls movement.
The implications of our work are clear: a variety of human diseases and conditions find root in aberrant activity of the sensorimotor system, and targeted interventions could offer significant benefits to patients clinically. Studying unconstrained, naturalistic movements will be essential for understanding normal versus pathological brain activity, and shedding light on the flexibility of motor circuits in everyday life. To this end, a major effort is underway to adapt our technologies to record and manipulate neural activity in freely moving animals and in clinical populations in the home environment. New genetic toolboxes are now enabling the dissection of neural circuits in unprecedented molecular and projection-specific detail, giving hope that solutions to longstanding questions in neurophysiology—oculomotor control, posture and gait, mechanisms of motor learning—are within our reach. In addition, new recording technologies that capture the activity of hundreds or thousands of neurons at a time are bringing to the forefront questions that were previously intractable. Sophisticated theoretical frameworks will be critical for bringing these pieces together to build an integrated understanding of motor control across echelons of the nervous system. Although the puzzle is complex and multifaceted, the exquisite beauty of movement continues to fascinate our innate curiosity and motivate our efforts moving forward.
GRANTS
Jared M. Cregg was supported by a postdoctoral fellowship from the Lundbeck Foundation under Grant No. R347-2020-2393. Jasmine L. Mirdamadi was supported by National Institutes of Health Eunice Kennedy Shriver National Institutes of Child Health & Human Development under Grant F32HD105458. Clara Kuper was supported by a doctoral fellowship from the Berlin School of Mind and Brain, Humboldt-Universität zu Berlin, Germany. Chen Avraham was supported by the Besor Fellowship and the Planning and Budgeting Committee Fellowship. Antimo Buonocore was supported by the Deutsche Forschungsgemeinschaft under Grant No. BU4031/1-1. Taniel S. Winner was supported by the National Science Foundation Graduate Research Fellowship Program 1937971, National Institutes of Health Eunice Kennedy Shriver National Institutes of Child Health & Human Development under Grant No. F31HD107968, and Alfred P. Sloan Foundation’s Minority Ph.D. (MPHD) program under Grant No. G-2019-11435. Robyn L. Mildren was supported by a Natural Sciences and Engineering Research Council of Canada postdoctoral fellowship and a Kavli Neuroscience Discovery Institute distinguished postdoctoral fellowship.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.F. and E.V.O. prepared figures; J.M.C., J.L.M., C.F., E.V.O., C.K., R.N., A.J.B., C.A., A.B., T.S.W., and R.L.M. drafted manuscript; J.M.C., J.L.M., and R.L.M. and edited and revised manuscript; J.M.C., J.L.M., C.F., E.V.O., C.K., R.N., A.J.B., C.A., A.B., T.S.W., and R.L.M. approved final version of manuscript.
REFERENCES
- 1. Beidas RS, Hannon PA, James AS, Emmons KM. Advancing gender equity in the academy. Sci Adv 8: eabq0430, 2022. doi: 10.1126/sciadv.abq0430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gallego JA, Hardwick RM, Oby ER. Highlights from the 2017 meeting of the Society for Neural Control of Movement (Dublin, Ireland). Eur J Neurosci 46: 2141–2148, 2017. doi: 10.1111/ejn.13670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mathis A, Pack AR, Maeda RS, McDougle SD. Highlights from the 29th annual meeting of the society for the neural control of movement. J Neurophysiol 122: 1777–1783, 2019. doi: 10.1152/jn.00484.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mazurek KA, Berger M, Bollu T, Chowdhury RH, Elangovan N, Kuling IA, Sohn MH. Highlights from the 28th annual meeting of the society for the neural control of movement. J Neurophysiol 120: 1671–1679, 2018. doi: 10.1152/jn.00475.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Russo M, Ozeri-Engelhard N, Hupfeld K, Nettekoven C, Thibault S, Sedaghat-Nejad E, Buchwald D, Xing D, Zobeiri O, Kilteni K, Albert ST, Ariani G. Highlights from the 30th annual meeting of the Society for the Neural Control of Movement. J Neurophysiol 126: 967–975, 2021. doi: 10.1152/jn.00334.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lobato-Rios V, Ramalingasetty ST, Özdil PG, Arreguit J, Ijspeert AJ, Ramdya P. NeuroMechFly, a neuromechanical model of adult Drosophila melanogaster. Nat Methods 19: 620–627, 2022. doi: 10.1038/s41592-022-01466-7. [DOI] [PubMed] [Google Scholar]
- 7. Sengupta M, Daliparthi V, Roussel Y, Bui TV, Bagnall MW. Spinal V1 neurons inhibit motor targets locally and sensory targets distally. Curr Biol 31: 3820–3833.e4, 2021. doi: 10.1016/j.cub.2021.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sengupta M, Bagnall MW. V2b neurons act via multiple targets in spinal motor networks (Preprint). bioRxiv, 2022. doi: 10.1101/2022.08.01.502410. [DOI]
- 9. Hildebrand DGC, Cicconet M, Torres RM, Choi W, Quan TM, Moon J, Wetzel AW, Scott Champion A, Graham BJ, Randlett O, Plummer GS, Portugues R, Bianco IH, Saalfeld S, Baden AD, Lillaney K, Burns R, Vogelstein JT, Schier AF, Lee W-CA, Jeong W-K, Lichtman JW, Engert F. Whole-brain serial-section electron microscopy in larval zebrafish. Nature 545: 345–349, 2017. doi: 10.1038/nature22356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu Z, Hildebrand DGC, Morgan JL, Jia Y, Slimmon N, Bagnall MW. Organization of the gravity-sensing system in zebrafish. Nat Commun 13: 5060, 2022. doi: 10.1038/s41467-022-32824-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lindén H, Petersen PC, Vestergaard M, Berg RW. Movement is governed by rotational neural dynamics in spinal motor networks. Nature 610: 526–531, 2022. doi: 10.1038/s41586-022-05293-w. [DOI] [PubMed] [Google Scholar]
- 12. Schneider DM, Sundararajan J, Mooney R. A cortical filter that learns to suppress the acoustic consequences of movement. Nature 561: 391–395, 2018. doi: 10.1038/s41586-018-0520-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Roy JE, Cullen KE. Dissociating self-generated from passively applied head motion: neural mechanisms in the vestibular nuclei. J Neurosci 24: 2102–2111, 2004. doi: 10.1523/JNEUROSCI.3988-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brooks JX, Cullen KE. The primate cerebellum selectively encodes unexpected self-motion. Curr Biol 23: 947–955, 2013. doi: 10.1016/j.cub.2013.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Todorov E, Jordan MI. A minimal intervention principle for coordinated movement. In: Proceedings of the 15th International Conference on Neural Information Processing Systems. Cambridge, MA: MIT Press, 2002, p. 27–34. [Google Scholar]
- 16. Nayeem R, Bazzi S, Sadeghi M, Hogan N, Sternad D. Preparing to move: setting initial conditions to simplify interactions with complex objects. PLoS Comput Biol 17: e1009597, 2021. doi: 10.1371/journal.pcbi.1009597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sohn WJ, Sipahi R, Sanger TD, Sternad D. Portable motion-analysis device for upper-limb research, assessment, and rehabilitation in non-laboratory settings. IEEE J Transl Eng Health Med 7: 2800314, 2019. doi: 10.1109/JTEHM.2019.2953257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mancini M, Schlueter H, El-Gohary M, Mattek N, Duncan C, Kaye J, Horak FB. Continuous monitoring of turning mobility and its association to falls and cognitive function: a pilot study. J Gerontol A Biol Sci Med Sci 71: 1102–1108, 2016. doi: 10.1093/gerona/glw019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shah VV, McNames J, Mancini M, Carlson-Kuhta P, Nutt JG, El-Gohary M, Lapidus JA, Horak FB, Curtze C. Digital biomarkers of mobility in Parkinson’s disease during daily living. J Parkinsons Dis 10: 1099–1111, 2020. doi: 10.3233/JPD-201914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zimmermann C, Schneider A, Alyahyay M, Brox T, Diester I. FreiPose: a deep learning framework for precise animal motion capture in 3D spaces (Preprint). bioRxiv, 2020. doi: 10.1101/2020.02.27.967620. [DOI]
- 21. Marshall JD, Aldarondo DE, Dunn TW, Wang WL, Berman GJ, Ölveczky BP. Continuous whole-body 3D kinematic recordings across the rodent behavioral repertoire. Neuron 109: 420–437.e8, 2021. doi: 10.1016/j.neuron.2020.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dunn TW, Marshall JD, Severson KS, Aldarondo DE, Hildebrand DGC, Chettih SN, Wang WL, Gellis AJ, Carlson DE, Aronov D, Freiwald WA, Wang F, Ölveczky BP. Geometric deep learning enables 3D kinematic profiling across species and environments. Nat Methods 18: 564–573, 2021. doi: 10.1038/s41592-021-01106-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cregg JM, Leiras R, Montalant A, Wanken P, Wickersham IR, Kiehn O. Brainstem neurons that command mammalian locomotor asymmetries. Nat Neurosci 23: 730–740, 2020. doi: 10.1038/s41593-020-0633-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Leiras R, Cregg JM, Kiehn O. Brainstem circuits for locomotion. Annu Rev Neurosci 45: 63–85, 2022. doi: 10.1146/annurev-neuro-082321-025137. [DOI] [PubMed] [Google Scholar]
- 25. Mackrous I, Carriot J, Cullen KE. Context-independent encoding of passive and active self-motion in vestibular afferent fibers during locomotion in primates. Nat Commun 13: 120, 2022. doi: 10.1038/s41467-021-27753-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Carriot J, Jamali M, Chacron MJ, Cullen KE. Statistics of the vestibular input experienced during natural self-motion: implications for neural processing. J Neurosci 34: 8347–8357, 2014. doi: 10.1523/JNEUROSCI.0692-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Carriot J, Jamali M, Chacron MJ, Cullen KE. The statistics of the vestibular input experienced during natural self-motion differ between rodents and primates. J Physiol 595: 2751–2766, 2017. doi: 10.1113/JP273734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Robinson DA. Models of oculomotor neural organization. In: The Control of Eye Movements, edited by Bach-y-Rita P, Collins CC, Hyde JE. New York: Academic Press, 1971, p. 519–538. [Google Scholar]
- 29. May PJ, Gamlin PD, Warren S. A novel tectal/pretectal population of premotor lens accommodation neurons. Invest Ophthalmol Vis Sci 63: 35, 2022. doi: 10.1167/iovs.63.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Takahashi M, Sugiuchi Y, Na J, Shinoda Y. Brainstem circuits triggering saccades and fixation. J Neurosci 42: 789–803, 2022. doi: 10.1523/JNEUROSCI.1731-21.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Robinson DA. Models of the saccadic eye movement control system. Kybernetik 14: 71–83, 1973. doi: 10.1007/BF00288906. [DOI] [PubMed] [Google Scholar]
- 32. Robinson DA. A quantitative analysis of extraocular muscle cooperation and squint. Invest Ophthalmol 14: 801–825, 1975. [PubMed] [Google Scholar]
- 33. Chen C-Y, Sonnenberg L, Weller S, Witschel T, Hafed ZM. Spatial frequency sensitivity in macaque midbrain. Nat Commun 9: 2852, 2018. doi: 10.1038/s41467-018-05302-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Alefantis P, Lakshminarasimhan K, Avila E, Noel J-P, Pitkow X, Angelaki DE. Sensory evidence accumulation using optic flow in a naturalistic navigation task. J Neurosci 42: 5451–5462, 2022. doi: 10.1523/JNEUROSCI.2203-21.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bermúdez Rey MC, Clark TK, Wang W, Leeder T, Bian Y, Merfeld DM. Vestibular perceptual thresholds increase above the age of 40. Front Neurol 7: 162, 2016. doi: 10.3389/fneur.2016.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Karmali F, Bermúdez Rey MC, Clark TK, Wang W, Merfeld DM. Multivariate analyses of balance test performance, vestibular thresholds, and age. Front Neurol 8: 578, 2017. [Erratum in Front Neurol 11: 556797, 2020]. doi: 10.3389/fneur.2017.00578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Beylergil SB, Karmali F, Wang W, Bermúdez Rey MC, Merfeld DM. Vestibular roll tilt thresholds partially mediate age-related effects on balance. Prog Brain Res 248: 249–267, 2019. doi: 10.1016/bs.pbr.2019.04.019. [DOI] [PubMed] [Google Scholar]
- 38. King S, Dahlem K, Karmali F, Stankovic KM, Welling DB, Lewis RF. Imbalance and dizziness caused by unilateral vestibular schwannomas correlate with vestibulo-ocular reflex precision and bias. J Neurophysiol 127: 596–606, 2022. doi: 10.1152/jn.00725.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sadeghi SG, Minor LB, Cullen KE. Neural correlates of sensory substitution in vestibular pathways following complete vestibular loss. J Neurosci 32: 14685–14695, 2012. doi: 10.1523/JNEUROSCI.2493-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Alberts BBGT, Selen LPJ, Verhagen WIM, Pennings RJE, Medendorp WP. Bayesian quantification of sensory reweighting in a familial bilateral vestibular disorder (DFNA9). J Neurophysiol 119: 1209–1221, 2018. doi: 10.1152/jn.00082.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hupfeld KE, McGregor HR, Koppelmans V, Beltran NE, Kofman IS, De Dios YE, Riascos RF, Reuter-Lorenz PA, Wood SJ, Bloomberg JJ, Mulavara AP, Seidler RD. Brain and behavioral evidence for reweighting of vestibular inputs with long-duration spaceflight. Cereb Cortex 32: 755–769, 2022. doi: 10.1093/cercor/bhab239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Peters S, Brown KE, Garland SJ, Staines WR, Handy TC, Boyd LA. Suppression of somatosensory stimuli during motor planning may explain levels of balance and mobility after stroke. Eur J Neurosci 48: 3534–3551, 2018. doi: 10.1111/ejn.14136. [DOI] [PubMed] [Google Scholar]
- 43. Stuart S, Wagner J, Makeig S, Mancini M. Brain activity response to visual cues for gait impairment in Parkinson’s disease: an EEG study. Neurorehabil Neural Repair 35: 996–1009, 2021. doi: 10.1177/15459683211041317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ragothaman A, Mancini M, Nutt JG, Fair DA, Miranda-Dominguez O, Horak FB. Resting state functional networks predict different aspects of postural control in Parkinson’s disease. Gait Posture 97: 122–129, 2022. doi: 10.1016/j.gaitpost.2022.07.003. [DOI] [PubMed] [Google Scholar]
- 45. Ragothaman A, Miranda-Dominguez O, Brumbach BH, Giritharan A, Fair DA, Nutt JG, Mancini M, Horak FB. Relationship between brain volumes and objective balance and gait measures in Parkinson’s disease. J Parkinsons Dis 12: 283–294, 2022. doi: 10.3233/JPD-202403. [DOI] [PubMed] [Google Scholar]
- 46. Billen LS, Corneil BD, Weerdesteyn V. Evidence for an intricate relationship between express visuomotor responses, postural control and rapid step initiation in the lower limbs (Preprint). bioRxiv, 2022. doi: 10.1101/2022.10.21.513067. [DOI] [PubMed]
- 47. Pruszynski JA, King GL, Boisse L, Scott SH, Flanagan JR, Munoz DP. Stimulus-locked responses on human arm muscles reveal a rapid neural pathway linking visual input to arm motor output. Eur J Neurosci 32: 1049–1057, 2010. doi: 10.1111/j.1460-9568.2010.07380.x. [DOI] [PubMed] [Google Scholar]
- 48. Gu C, Wood DK, Gribble PL, Corneil BD. A trial-by-trial window into sensorimotor transformations in the human motor periphery. J Neurosci 36: 8273–8282, 2016. doi: 10.1523/JNEUROSCI.0899-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Curtze C, Nutt JG, Carlson-Kuhta P, Mancini M, Horak FB. Levodopa is a double-edged sword for balance and gait in people with Parkinson’s disease. Mov Disord 30: 1361–1370, 2015. doi: 10.1002/mds.26269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Morris R, Martini DN, Smulders K, Kelly VE, Zabetian CP, Poston K, Hiller A, Chung KA, Yang L, Hu S-C, Edwards KL, Cholerton B, Grabowski TJ, Montine TJ, Quinn JF, Horak F. Cognitive associations with comprehensive gait and static balance measures in Parkinson’s disease. Parkinsonism Relat Disord 69: 104–110, 2019. doi: 10.1016/j.parkreldis.2019.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Shah VV, McNames J, Mancini M, Carlson-Kuhta P, Spain RI, Nutt JG, El-Gohary M, Curtze C, Horak FB. Quantity and quality of gait and turning in people with multiple sclerosis, Parkinson’s disease and matched controls during daily living. J Neurol 267: 1188–1196, 2020. doi: 10.1007/s00415-020-09696-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bostan AC, Strick PL. The basal ganglia and the cerebellum: nodes in an integrated network. Nat Rev Neurosci 19: 338–350, 2018. doi: 10.1038/s41583-018-0002-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science 275: 1593–1599, 1997. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- 54. Heffley W, Song EY, Xu Z, Taylor BN, Hughes MA, McKinney A, Joshua M, Hull C. Coordinated cerebellar climbing fiber activity signals learned sensorimotor predictions. Nat Neurosci 21: 1431–1441, 2018. doi: 10.1038/s41593-018-0228-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Heffley W, Hull C. Classical conditioning drives learned reward prediction signals in climbing fibers across the lateral cerebellum. eLife 8: e46764, 2019. doi: 10.7554/eLife.46764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Larry N, Zur G, Joshua M. The basal ganglia achieve a higher signal-to-noise ratio than the cerebellum by sharpening reward and movement signals (Preprint). bioRxiv, 2022. doi: 10.1101/2022.10.01.510246. [DOI]
- 57. Lofredi R, Neumann W-J, Bock A, Horn A, Huebl J, Siegert S, Schneider G-H, Krauss JK, Kühn AA. Dopamine-dependent scaling of subthalamic gamma bursts with movement velocity in patients with Parkinson’s disease. eLife 7: e31895, 2018. doi: 10.7554/eLife.31895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lofredi R, Tan H, Neumann W-J, Yeh C-H, Schneider G-H, Kühn AA, Brown P. Beta bursts during continuous movements accompany the velocity decrement in Parkinson’s disease patients. Neurobiol Dis 127: 462–471, 2019. doi: 10.1016/j.nbd.2019.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. de Almeida Marcelino AL, Horn A, Krause P, Kühn AA, Neumann W-J. Subthalamic neuromodulation improves short-term motor learning in Parkinson’s disease. Brain 142: 2198–2206, 2019. doi: 10.1093/brain/awz152. [DOI] [PubMed] [Google Scholar]
- 60. Therrien AS, Statton MA, Bastian AJ. Reinforcement signaling can be used to reduce elements of cerebellar reaching ataxia. Cerebellum 20: 62–73, 2021. doi: 10.1007/s12311-020-01183-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Therrien AS, Wolpert DM, Bastian AJ. Effective reinforcement learning following cerebellar damage requires a balance between exploration and motor noise. Brain 139: 101–114, 2016. doi: 10.1093/brain/awv329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Jurado-Parras M-T, Safaie M, Sarno S, Louis J, Karoutchi C, Berret B, Robbe D. The dorsal striatum energizes motor routines. Curr Biol 30: 4362–4372.e6, 2020. doi: 10.1016/j.cub.2020.08.049. [DOI] [PubMed] [Google Scholar]
- 63. Haith AM, Yang CS, Pakpoor J, Kita K. De novo motor learning of a bimanual control task over multiple days of practice. J Neurophysiol 128: 982–993, 2022. doi: 10.1152/jn.00474.2021. [DOI] [PubMed] [Google Scholar]
- 64. Yang CS, Cowan NJ, Haith AM. Control becomes habitual early on when learning a novel motor skill. J Neurophysiol 128: 1278–1291, 2022. doi: 10.1152/jn.00273.2022. [DOI] [PubMed] [Google Scholar]
- 65. Tsay JS, Kim H, Haith AM, Ivry RB. Understanding implicit sensorimotor adaptation as a process of proprioceptive re-alignment. eLife 11: e76639, 2022. doi: 10.7554/eLife.76639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ruttle JE, ’t Hart BM, Henriques DYP. Implicit motor learning within three trials. Sci Rep 11: 1627, 2021. [Erratum in Sci Rep 11: 9051, 2021]. doi: 10.1038/s41598-021-81031-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Munoz-Rubke F, Mirdamadi JL, Lynch AK, Block HJ. Modality-specific changes in motor cortex excitability after visuo-proprioceptive realignment. J Cogn Neurosci 29: 2054–2067, 2017. doi: 10.1162/jocn_a_01171. [DOI] [PubMed] [Google Scholar]
- 68. Mirdamadi JL, Block HJ. Somatosensory versus cerebellar contributions to proprioceptive changes associated with motor skill learning: a theta burst stimulation study. Cortex 140: 98–109, 2021. doi: 10.1016/j.cortex.2021.03.019. [DOI] [PubMed] [Google Scholar]
- 69. Wali M, Lee-Miller T, Babu R, Block HJ. Retention of visuo-proprioceptive recalibration in estimating hand position (Preprint). bioRxiv, 2022. doi: 10.1101/2022.11.28.517441. [DOI] [PMC free article] [PubMed]
- 70. Rossi C, Bastian AJ, Therrien AS. Mechanisms of proprioceptive realignment in human motor learning. Curr Opin Physiol 20: 186–197, 2021. doi: 10.1016/j.cophys.2021.01.011. [DOI] [Google Scholar]
- 71. Miall RC, Afanasyeva D, Cole JD, Mason P. The role of somatosensation in automatic visuo-motor control: a comparison of congenital and acquired sensory loss. Exp Brain Res 239: 2043–2061, 2021. doi: 10.1007/s00221-021-06110-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Avraham C, Nisky I. The effect of tactile augmentation on manipulation and grip force control during force-field adaptation. J Neuroeng Rehabil 17: 17, 2020. doi: 10.1186/s12984-020-0649-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Shafti A, Tjomsland J, Dudley W, Faisal AA. Real-world human-robot collaborative reinforcement learning (Preprint). arXiv, 2020. doi: 10.48550/arXiv.2003.01156. [DOI]
- 74. Oby ER, Golub MD, Hennig JA, Degenhart AD, Tyler-Kabara EC, Yu BM, Chase SM, Batista AP. New neural activity patterns emerge with long-term learning. Proc Natl Acad Sci USA 116: 15210–15215, 2019. doi: 10.1073/pnas.1820296116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kobak D, Brendel W, Constantinidis C, Feierstein CE, Kepecs A, Mainen ZF, Qi X-L, Romo R, Uchida N, Machens CK. Demixed principal component analysis of neural population data. eLife 5: e10989, 2016. doi: 10.7554/eLife.10989. [DOI] [PMC free article] [PubMed] [Google Scholar]