Abstract
Background:
Diabetes reduces semen quality and increasingly occurs during reproductive years. Diabetes medications, such as metformin, have glucose-independent effects on the male reproductive system. Associations with birth defects in offspring are unknown.
Objective:
To evaluate whether the risk for birth defects in offspring varies with preconceptional pharmacologic treatment of fathers with diabetes.
Design:
Nationwide prospective registry-based cohort study.
Setting:
Denmark from 1997 to 2016.
Participants:
All liveborn singletons from mothers without histories of diabetes or essential hypertension.
Measurements:
Offspring were considered exposed if their father filled 1 or more prescriptions for a diabetes drug during the development of fertilizing sperm. Sex and frequencies of major birth defects were compared across drugs, times of exposure, and siblings.
Results:
Of 1116779 offspring included, 3.3% had 1 or more major birth defects (reference). Insulin-exposed offspring (n = 5298) had the reference birth defect frequency (adjusted odds ratio [aOR], 0.98 [95% CI, 0.85 to 1.14]). Metformin-exposed offspring (n = 1451) had an elevated birth defect frequency (aOR, 1.40 [CI, 1.08 to 1.82]). For sulfonylurea-exposed offspring (n = 647), the aOR was 1.34 (CI, 0.94 to 1.92). Offspring whose fathers filled a metformin prescription in the year before (n = 1751) or after (n = 2484) sperm development had reference birth defect frequencies (aORs, 0.88 [CI, 0.59 to 1.31] and 0.92 [CI, 0.68 to 1.26], respectively), as did unexposed siblings of exposed offspring (3.2%; exposed vs. unexposed OR, 1.54 [CI, 0.94 to 2.53]). Among metformin-exposed offspring, genital birth defects, all in boys, were more common (aOR, 3.39 [CI, 1.82 to 6.30]), while the proportion of male offspring was lower (49.4% vs. 51.4%, P = 0.073).
Limitation:
Information on underlying disease status was limited.
Conclusion:
Preconception paternal metformin treatment is associated with major birth defects, particularly genital birth defects in boys. Further research should replicate these findings and clarify the causation.
Primary Funding Source:
National Institutes of Health.
Diabetes mellitus is a growing threat to public health (1). Diabetes increasingly occurs in persons of reproductive age (2), compromises sperm quality (3–5), and is associated with impaired male fertility (3, 4, 6). Even more concerning for future generations would be an association with offspring birth defects, which has not been evaluated.
In pregnant women, poorly regulated diabetes is associated with adverse pregnancy outcomes, including major birth defects (7, 8). Hence, pregnant women are advised to aggressively control blood glucose levels (9). Although little attention is being given to men with diabetes who are aspiring to fatherhood, fathers contribute half of an offspring’s DNA, and evidence increasingly suggests a role for sperm beyond DNA alone (10).
Some diabetes drugs may also affect male reproductive health. Metformin, a first-line oral diabetes drug, improves semen parameters in obese men (11) but reduces serum testosterone levels independently of glycemic control (12). Given its nonneutral effect on male reproductive potential and its increasing use as a first-choice medication for type 2 diabetes, the effect of paternal exposure needs evaluation.
Hence, we aimed to evaluate whether the risk for birth defects in offspring varies with preconceptional pharmacologic treatment of fathers with diabetes. We did a nationwide prospective registry-based cohort study covering all 1 255 772 births in Denmark from 1997 to 2016. For each newborn, individual-level information on birth defects, parental drug prescriptions, and potential confounders was obtained from the relevant nationwide registries.
METHODS
Data and Exclusion Criteria
Newborns and parents were identified through the Medical Birth Registry (13) (1997 to 2016), which contains all registered pregnancies in Denmark from 20 weeks of gestation. It provides pregnancy characteristics like gestational age (and thus conception date as birth date minus gestational age) and maternal smoking status.
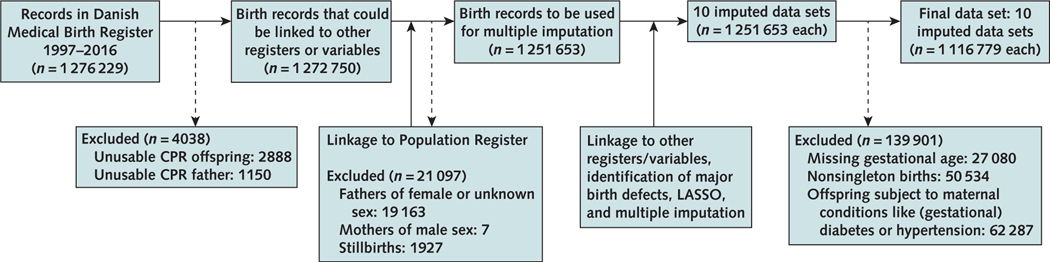
Each inhabitant of Denmark has an identification number (14) that links registries. Medical Birth Registry records with unusable identification numbers of offspring or either parent were deleted. We also removed registered fathers of female or unknown sex and registered mothers of male sex. Stillbirths were deleted because of differential birth defect ascertainment (Figure 1).
Figure 1.
Flow chart of the creation of the data set: registries merged and numbers of inclusion and exclusion.
CPR = Centrale Personregister; LASSO = least absolute shrinkage and selection operator.
The Patient Registry (15) (1995 to mid-2018) contains all individual-level diagnoses in the outpatient and inpatient (but not general practitioner) settings, including birth defects. One-year follow-up from birth was allowed to diagnose birth defects, which were then classified according to the EUROCAT (European Concerted Action on Congenital Anomalies and Twins) guidelines (16) (by International Classification of Diseases, 10th Revision codes). Birth defects classified by EUROCAT as minor were excluded. Organ-specific birth defect subgroups were made according to the same guidelines. Parental diagnoses were retrieved as needed.
The Prescription Registry (17) (1995 to mid-2018) contains all prescriptions redeemed but not the prescription reason. We created indicator variables for specified exposures (see Exposure subsection). We linked with socioeconomic variables held at Statistics Denmark, including highest education achieved (both parents) and disposable income (father), both by birth year.
All liveborn singletons were eligible for analysis, but we excluded births with mothers using diabetes medication (both oral and insulin) or receiving a diabetes mellitus (International Classification of Diseases, 10th Revision codes E10 through E14) or gestational diabetes (O24) diagnosis at any time before giving birth, mothers receiving a diagnosis of essential hypertension (I10 through I15) at any time before giving birth, and mothers prescribed cardiovascular drugs (Anatomical Therapeutic Chemical codes C01 through C04 and C06 through C10) during the 6 months up to conception (Figure 1).
Analysis was done on a secure server at Statistics Denmark in R, version 3.6.3 (R Foundation for Statistical Computing) (18).
Exposure
The development of fully mature spermatozoa, including spermatogenesis from type A spermatogonia to type Sd spermatids and further maturation in the epididymis, takes approximately 3 months (19). Hence, offspring of fathers filling a prescription for the following specified diabetes drugs during the 3 months before conception (hereafter called “sperm development” [SDev]) were considered exposed: insulins (Anatomical Therapeutic Chemical code A10A); metformin (A10BA02, the only biguanide in the data); sulfonylureas (A10BB); and other diabetes drugs, primarily glucagon-like peptide-1 analogues (A10BJ) and dipeptidyl peptidase-4 inhibitors (A10BH).
Pharmacologic treatment varies by underlying disease status and prescription practices. Yet, all patients with (suspected) type 1 diabetes are seen by a specialist at least once (and usually more often); these have a diagnosis in the Patient Registry. Patients with (suspected) type 2 diabetes are not necessarily seen by a specialist, and so they may not have a diagnosis in the Patient Registry (which does not cover primary care). Thus, although exposure is primarily pharmacologic, which is nonrandom, for a subset of the data it is possible to ascertain whether birth defects, if elevated, follow diagnosis more than treatment or vice versa.
Outcome
Primary outcome was the diagnosis of 1 or more major birth defects in the first year of life (binary variable) following the EUROCAT guidelines (16). Secondary outcomes were offspring sex and the diagnosis of 1 or more major birth defects in the first year of life in a EUROCAT organ-specific category (binary variable) (16).
Missing Data
Approximately 15% of observations had 1 or more entries missing (usually few), mostly maternal smoking status (8%, other variables <3%). We used multiple imputation to create 10 complete data sets using the mice package in R, version 3.13.0 (R Foundation for Statistical Computing) (20), assuming missingness at random. Because of high dimensionality of the data, we used least absolute shrinkage and selection operator regression (R glmnet package, version 4.0–2 [R Foundation for Statistical Computing] [21]) to select informative predictors to be included in multiple imputation for each variable with missing data. Imputation used predictive mean matching for continuous variables and unrestricted polytomous regression for factors.
Statistical Analyses
We calculated crude birth defect frequencies and adjusted odds ratios (aORs) with 95% CIs, adjusting for birth year; paternal age, income, and education; and maternal age, smoking status, and education. These variables were selected a priori for possible associations with both exposure and outcome (7, 22–24). Results reported are from generalized additive logistic regression models with smoothing splines for continuous variables (R mgcv package, version 1.8–33 [R Foundation for Statistical Computing] [25], all default settings) and indicator variables without further assumptions for factors. The generalized additive model allows for adjustment of complex associations between potential confounders and birth defect risk. To account for correlations among births clustered by father, we estimated the aOR assuming working independence between births but calculated robust SEs by bootstrapping birth clusters sharing the same father (see the Statistical Appendix section in the Supplement, available at Annals.org).
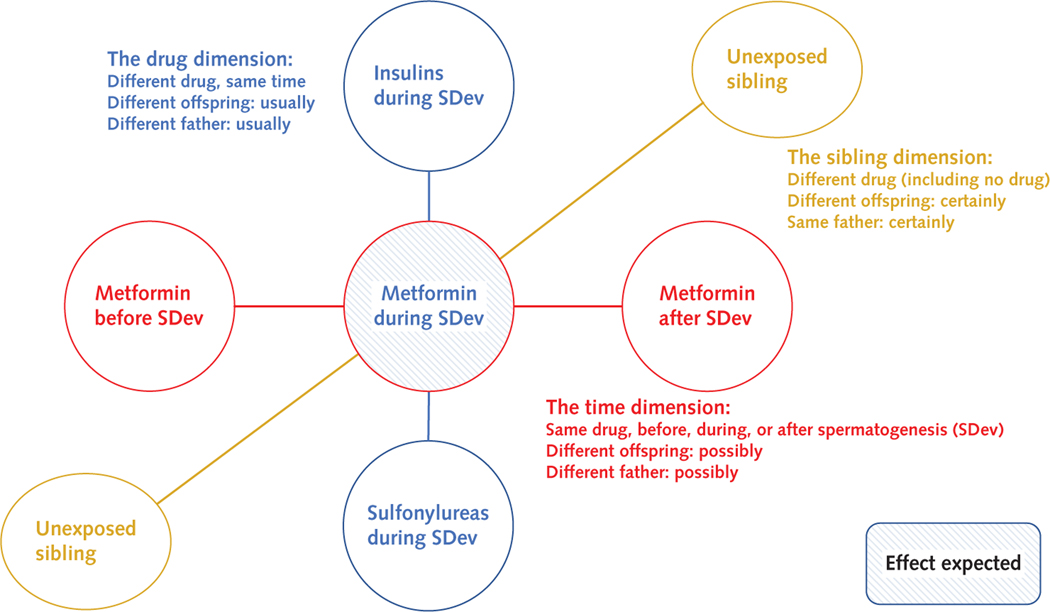
Our main analysis contrasted exposure during SDev for the identified drugs in 1 model (Figure 2). On the basis of this model, we did the following sensitivity analyses: restricted to mothers younger than 35 years, restricted to fathers younger than 40 years, restricted to those exposed to any diabetes drug, expanded to all liveborn singletons, and excluding offspring conceived by a father after birth of the first offspring with a defect (if any). We also assessed for association between the father’s ethnicity (“Danish,” “immigrant,” or “immigrant family”) with birth defects and drug exposure. To evaluate unmeasured confounding effects, we calculated E-values. These estimate the minimum strength of association (OR) that an unmeasured confounder would need to have with both the exposure and outcome to fully explain away a specific exposure–outcome association (www.evalue-calculator.com) (26, 27). We further assessed whether birth defects could be explained by a (type 1 or type 2) diabetes diagnosis independently of drug.
Figure 2.
Study design.
We made 3 comparisons: different drugs during SDev, the same drug at different time points, and exposed versus unexposed siblings. SDev = sperm development.
For drugs associated with birth defects in the main analysis, we did 3 further analyses (Figure 2). First, we assessed for association with birth defects of the same drug exposure in different time frames: more than 1 year before SDev, 1 year before SDev, during SDev, 1 year after SDev, and more than 1 year after SDev (all binary variables). A birth could have exposure to a drug in each of these 5 time frames. Drug exposures in the 4 additional time frames were added to the main regression model as independent variables.
Second, we compared, by conditional logistic regression, exposed versus unexposed offspring of the same fathers (that is, a within-father analysis) and, separately, mothers (within-mother analysis). Seen from the father (mother), this is a self-controlled case series where the preconception period of a father (mother) for each of his (her) children (exposed vs. unexposed siblings) is compared (28). Third, we explored whether any EUROCAT organ-specific birth defect groups were elevated.
This study was exempted from institutional review board review because it was deidentified. No new data were collected for this study.
Role of the Funding Source
The funders had no role in the analysis, decision to publish, or writing of the manuscript.
RESULTS
Cohort
Of 1 276 229 records, excluding those with unusable identification numbers, births to fathers of unknown or female sex, mothers of male sex, and stillbirths left 1251653 records available for multiple imputation (Figure 1). Further exclusion by missing gestational age (n = 27080), nonsingleton births (n = 50534), and births to mothers with diabetes or essential hypertension (n = 62287) left 1116779 records for analysis (Figure 1).
Among these offspring, 3.3% (n = 36585) had 1 or more major birth defects (Table 1), and 51.4% were male. The median ages of mothers and fathers were 30 and 33 years, respectively. A total of 7029 offspring were exposed to paternal diabetes medications, including insulins (n = 5298), metformin (n = 1451), and sulfonylureas (n = 647). Few (n = 276) offspring were exposed to other diabetes drugs; we do not consider these drugs further.
Table 1.
Characteristics of the 1 116 779 Births in Denmark From 1997 to 2016 After Exclusion, by Diabetes Medication Group*
| Characteristic | No Diabetes Drug (n = 1 109 750) | Insulins (n = 5298) | Metformin (n = 1451) | Sulfonylureas (n = 647) |
|---|---|---|---|---|
|
| ||||
| Median age of mothers (Q1–Q3), y | 30.3 (27.1–33.6) | 30.5 (27.3–34.0) | 33.4 (30.0–37.5) | 33.6 (29.6–37.4) |
| Mothers with low education, % (n) | 19 (200 227) | 20 (1032) | 32 (413) | 39 (226) |
| Mother smoked during pregnancy, % (n) | 13 (135 996) | 14 (681) | 11 (159) | 13 (79) |
| Median age of fathers (Q1–Q3), y | 32.5 (29.1–36.3) | 33.2 (29.7–37.5) | 40.3 (35.6–46.3) | 41.8 (36.8–47.6) |
| Fathers with low education, % (n) | 20 (208 695) | 20 (1028) | 32 (429) | 30 (170) |
| Median paternal income (Q1–Q3), 1000 Danish crowns | 187 (137–246) | 186 (139–243) | 172 (123–236) | 146 (107–206) |
| Father receiving β-blocker, % (n)† | 0.3 (3602) | 1.5 (78) | 5.4 (78) | 5.9 (38) |
| Father receiving diuretic, % (n)‡ | 0.1 (1546) | 2.4 (125) | 4.5 (66) | 4.6 (30) |
| Father receiving calcium-channel blocker, % (n)§ | 0.2 (2600) | 2.7 (144) | 6.9 (100) | 4.5 (29) |
| Father receiving angiotensin-converting enzyme inhibitor or similar, % (n)|| |
0.5 (5000) | 11.8 (623) | 25.4 (369) | 18.7 (121) |
| Father receiving lipid-modifying agent, % (n)¶ | 0.3 (3100) | 7.2 (382) | 34.0 (493) | 24.3 (157) |
| Father receiving antidepressant, % (n)** | 1.5 (16 206) | 2.8 (150) | 6.7 (97) | 5.7 (37) |
| Median gestational age (Q1–Q3), d | 279 (273–287) | 279 (273–287) | 278 (273–286) | 279 (273–287) |
| Nulliparity, % (n) | 46 (508 982) | 43 (2292) | 31 (451) | 32 (205) |
| Median birthweight (Q1–Q3), kg | 3.5 (3.2–3.9) | 3.5 (3.2–3.9) | 3.5 (3.1–3.8) | 3.4 (3.1–3.8) |
| Median birth length (Q1–Q3), cm | 52 (50–54) | 52 (50–54) | 52 (50–53) | 52 (50–53) |
| Major birth defect, % (n) | 3.3 (36 326) | 3.3 (174) | 5.2 (75) | 5.1 (33) |
| Male sex, % (n) | 51.4 (569 913) | 51.3 (2716) | 49.4 (717) | 49.3 (319) |
ATC = Anatomical Therapeutic Chemical; Q1 = first quartile; Q3 = third quartile.
Other diabetes drugs (e.g., glucagon-like peptide-1 analogues and dipeptidyl peptidase-4 inhibitors) were rare (n = 276). The last 3 columns may overlap (44.2% of those exposed to sulfonylureas were also exposed to metformin).
All ATC code C07.
All ATC code C03.
All ATC code C08.
All ATC code C09.
All ATC code C10.
All ATC code N06A.
Diabetes Drugs During SDev
Parents of insulin-exposed offspring were generally similar to those of offspring unexposed to diabetes drugs (the reference group, Table 1). Offspring exposed to metformin and/or sulfonylureas had older parents with lower education, had fathers with lower income, and were less often firstborns (Table 1). Fathers receiving oral diabetes drugs used cardiovascular drugs more often than fathers receiving insulins, and these more often than fathers receiving no diabetes drug (Table 1).
Insulin-exposed offspring had the baseline birth defect frequency (3.3%) and sex ratio (51.3% male) (Table 1). Offspring exposed to metformin or sulfonylureas had 5.2% or 5.1% birth defects, respectively, and were less often male, 49.4% and 49.3%, respectively. Of fathers receiving sulfonylureas, 44.2% received metformin (Table 1).
In the main regression analysis, the aOR of having 1 or more major birth defects was 0.98 (95% CI, 0.85 to 1.14) for insulins, 1.40 (CI, 1.08 to 1.82) for metformin, and 1.34 (CI, 0.94 to 1.92) for sulfonylureas (Table 2). Point estimates were similar for all sensitivity analyses (Table 2). Metformin-exposed offspring were 49.4% male versus 51.4% in the reference group (P = 0.073).
Table 2.
Association of Paternal Preconception Diabetic Medication and Offspring Birth Defects
| Main Model and Sensitivity Analyses | aOR (95% CI)* | ||
|---|---|---|---|
|
|
|||
| Insulins | Metformin | Sulfonylureas | |
|
| |||
| Main model (n = 1 116 779) | 0.98 (0.85–1.14) | 1.40 (1.08–1.82) | 1.34 (0.94–1.92) |
| Main model, mothers <35 y (n = 923 587) | 0.92 (0.77–1.08) | 1.40 (1.02–1.91) | 1.33 (0.83–2.12) |
| Main model, fathers <40 y (n = 995 857) | 0.96 (0.82–1.13) | 1.37 (0.97–1.94) | 1.42 (0.82–2.46) |
| Main model, any diabetes mellitus drug (n = 7029) | 1 (reference) | 1.41 (1.02–1.96) | 1.42 (0.95–2.11) |
| Main model, all liveborn singletons (n = 1 174 727) | 1.00 (0.87–1.15) | 1.47 (1.18–1.84) | 1.21 (0.85–1.71) |
| Main model, without births after defect† (n = 1 097 228) | 0.98 (0.85–1.14) | 1.41 (1.09–1.83) | 1.34 (0.93–1.93) |
aOR = adjusted odds ratio.
Based on robust SEs and adjusted for birth year; paternal age, education, and disposable income; and maternal age, education, and smoking status during pregnancy.
Excluded are all offspring conceived by a father after the birth of the first (if any) offspring sired by that father with a defect.
In total, 13% of offspring had “immigrant” fathers, and 1% had “descendant of immigrant” fathers. The remaining 86% had “Danish” fathers. Metformin use did not differ across these groups (all 0.13%); birth defects affected slightly more births of immigrant fathers (3.5%).
The E-value for the metformin association was 2.15 to reduce the point estimate (1.40) to the null (that is, 1) and 1.37 to reduce the lower limit of the CI to the null.
A documented preconception diagnosis of type 2 diabetes (n = 2382) gave the baseline birth defect frequency when the father was not receiving metformin (3.1%, n = 1594) but gave an elevated birth defect frequency when the father was receiving metformin (4.6%, n = 788). Joint modeling of diabetes drugs and preconception diabetes diagnoses assigned a slight, nonsignificant negative association to diagnosis while slightly increasing the point estimates for oral drugs (Supplement Table 1, available at Annals.org). The insulin group, 84% of which received no prior oral treatment but had a preconception diagnosis of type 1 diabetes, had baseline birth defect frequencies regardless of prior oral treatment or type 1 diabetes diagnosis (Supplement Table 1).
Populating the model with potential confounders showed a small effect for birth year but not for other variables; in particular, adding in major defects for parents and/or nulliparity did not change the results (Supplement Table 1). A metformin–sulfonylureas interaction term did not improve the model (P = 0.70; χ2 test, not shown). Excluding births subject to preeclampsia did not change the results (not shown). Birth year, parental age, and socioeconomic status did not differ between metformin-exposed offspring with a birth defect and those without (not shown). Including cardiovascular drugs in the main model did not change the regression results (Supplement Table 1). A modest but statistically significant elevation in the OR for birth defects was found for β-blockers (1.25 [CI, 1.06 to 1.47]) (Supplement Table 2, available at Annals. org). These birth defects were particularly heart defects; various other categories were also elevated (not shown).
Diabetes Drugs Before or After SDev
In terms of parental and pregnancy characteristics, offspring of fathers filling a metformin prescription before or after SDev were similar to those of fathers filling a prescription of the same drug during SDev (Supplement Table 3, available at Annals.org), with the exception of metformin prescriptions filled more than 1 year after SDev, which was a large and heterogeneous group. These births occurred at the relative beginning of the 1997 to 2016 period, and fathers were younger when conceiving. Cardiovascular drug use was slightly higher for those fathers filling a metformin prescription during spermatogenesis than for those in other categories (Supplement Table 3).
Before SDev, the crude birth defect frequency increased steadily as metformin exposure approached the biologically sensitive period of SDev (Table 3). After SDev, the crude birth defect frequency declined, down to normal levels for fathers redeeming a prescription more than 1 year after SDev. In a joint model, birth defects were attributed to metformin prescriptions during SDev, but not during other time frames (Table 3). In particular, offspring whose fathers filled a metformin prescription in the year before (n = 1751) or after (n = 2484) SDev had reference birth defect frequencies (aOR, 0.88 [CI, 0.59 to 1.31] and 0.92 [CI, 0.68 to 1.26], respectively).
Table 3.
Time Frame Analysis for Metformin Exposure*
| Crude and Adjusted Result | >1 y Before SDev (n = 1861) | 1 y Before SDev (n = 1751) | SDev (n = 1451) | 1 y After SDev (n = 2484) | >1 y After SDev (n = 28 112) |
|---|---|---|---|---|---|
|
| |||||
| Major birth defect, % (n) | 4.0 (74) | 4.4 (77) | 5.2 (75) | 4.3 (107) | 3.3 (921) |
| aOR (95% CI) | 0.86 (0.61–1.21) | 0.88 (0.59–1.31) | 1.75 (1.18–2.61) | 0.92 (0.68–1.26) | 1.05 (0.98–1.12) |
aOR = adjusted odds ratio; SDev = sperm development.
Compared are metformin more than 1 y before SDev, 1 y before SDev, during SDev, the first year after SDev, and more than 1 y after SDev. One birth could have exposure to metformin use during more than one of these time frames. Reference group is offspring of fathers who had no exposure at any point in time. The crude birth defect frequency increases as SDev, the biologically vulnerable period, is approached, and decreases again as metformin exposure happens after SDev. When put into a single regression model, the birth defects are attributed to metformin exposure during SDev, but not at other moments. Results are from robust SEs from a bootstrapped approach to account for clustering by father. Group profiles are given in Supplement Table 2 (available at Annals.org).
Unexposed Siblings
Unexposed offspring (n = 1268) of fathers with metformin-exposed offspring had 3.2% birth defects; unexposed offspring (n = 1227) from mothers with offspring exposed to paternal metformin had 3.0% birth defects (that is, baseline). Conditional logistic regression gave ORs for paternal metformin during SDev of 1.54 (CI, 0.94 to 2.53) comparing exposed versus unexposed offspring of the same father and 1.66 (CI, 1.00 to 2.75) comparing exposed versus unexposed offspring of the same mother (Table 4).
Table 4.
By-Father and By-Mother (Sibling) Analyses*
| Parent Shared | Offspring, n | Discordant Sets, n | Birth Defect Frequency in Unexposed Siblings (All), % | OR† (95% CI) |
|---|---|---|---|---|
|
| ||||
| Father | 1268 | 171 | 3.2 | 1.54 (0.94–2.53) |
| Mother | 1227 | 143 | 3.0 | 1.66 (1.00–2.75) |
OR = odds ratio.
By conditional logistic regression, after exclusion of offspring exposed to other diabetes drugs.
The OR given is for exposed vs. unexposed siblings. Unadjusted analysis; correcting for birth year, maternal age, and nulliparity, results were similar. Birth defect frequency of unexposed offspring is for all offspring, regardless of whether they were in a discordant set and hence contributed to the analysis.
Birth Defect Categories
For metformin-exposed offspring, genital birth defects were elevated compared with the cohort (0.90% vs. 0.24%; aOR, 3.39 [CI, 1.82 to 6.30]) (Table 5). All of these genital birth defects occurred in boys (cohort: >99%). All of these boys were from different fathers. There was no sex bias in other categories. Genital birth defects were not elevated in sulfonylurea-exposed offspring (aOR, 0.96).
Table 5.
Frequencies of Having ≥1 Major Birth Defect in Each of the Respective EUROCAT Categories, by Metformin Exposure
| EUROCAT Birth Defect Category | Metformin, % (n) | aOR (95% CI)* | |
|---|---|---|---|
|
|
|||
| Yes (n = 1451) | No (n = 1 115 328) | ||
|
| |||
| Digestive | 0.35 (5) | 0.21 (2343) | 1.25 (0.45–3.46) |
| Urinary | 0.35 (5) | 0.26 (2878) | 1.38 (0.53–3.60) |
| Heart | 1.24 (18) | 0.68 (7616) | 1.58 (0.94–2.66) |
| Chromosomal | 0.41 (6) | 0.11 (1234) | 2.16 (0.83–5.64) |
| Limb | 0.90 (13) | 0.92 (10 301) | 1.01 (0.55–1.84) |
| Genital | 0.90 (13) | 0.24 (2691) | 3.39 (1.82–6.30) |
| Other | 1.10 (16) | 0.63 (6996) | 1.23 (0.70–2.16) |
aOR = adjusted odds ratio; EUROCAT = European Concerted Action on Congenital Anomalies and Twins.
Adjusted for birth year; paternal age, education, and income; and maternal age, education, and smoking status during pregnancy. The aORs are not corrected for multiple testing. We are unable to report on the following categories because of low numbers (<5): nervous, eye, ear–face–neck, abdominal wall, respiratory birth defects, and orofacial clefts.
DISCUSSION
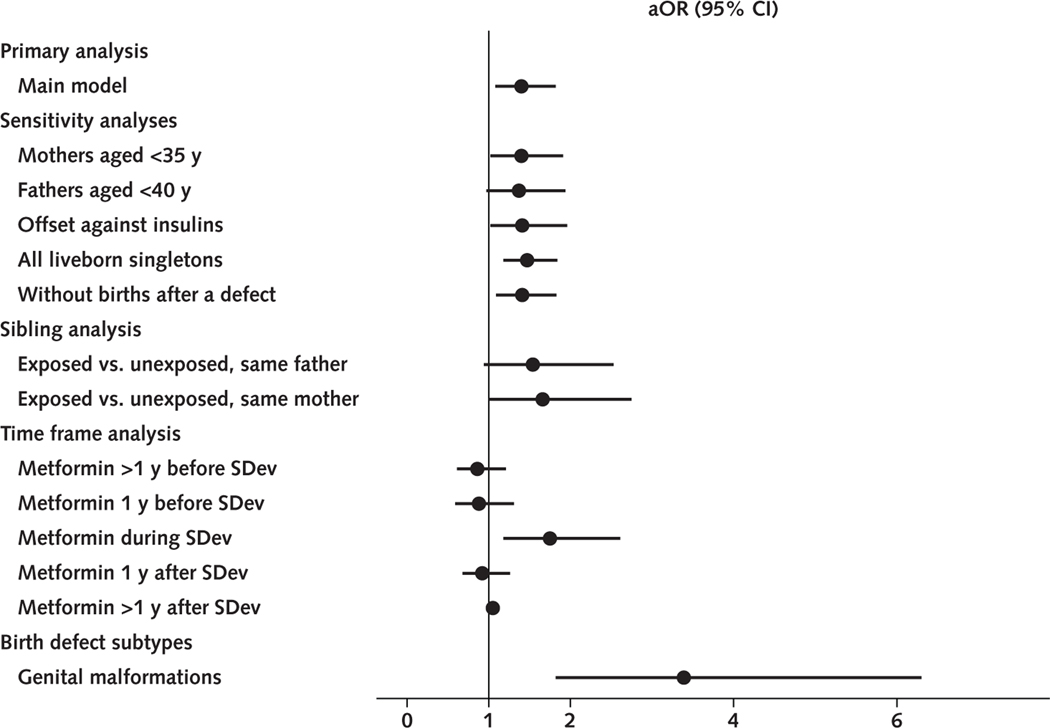
The current report found elevated birth defect frequencies among metformin-exposed offspring (aOR, 1.40 [CI, 1.08 to 1.82]; P = 0.012) but not among insulin-exposed offspring, unexposed siblings, or offspring of fathers filling a metformin prescription before or after SDev (Figure 3). Metformin-exposed offspring more often had a genital birth defect, all in boys, while the proportion of boys was lower (49.4% vs. 51.4%, P = 0.073) (Figure 3). Sulfonylureas showed a similar association (aOR, 1.34 [CI, 0.94 to 1.92]) but lacked statistical significance (P = 0.107) and specificity to a birth defect category.
Figure 3.
Summary of results.
The figure displays aORs (points) with their 95% CIs (bars) for the main analysis, sensitivity analyses, sibling analysis, time frame analysis, and birth defect categories. aOR = adjusted odds ratio; SDev = sperm development.
The population-based approach using high-quality registry data limited scope for selection and information bias. The approach contrasting different drug exposures during SDev, identical drugs at different times relative to SDev, and exposed versus unexposed siblings, all of which supported the result, greatly limits potential for confounding, as did the specificity of the finding to male genital birth defects.
The E-value of 2.15 for the metformin association suggests that an unmeasured confounder would need to have an aOR of 2.17 or greater with both exposure and outcome to fully explain away the observed association. Because this is a strong association, it seems difficult to attribute the observed association entirely to unobserved confounding. The more moderate E-value of 1.37 for the lower bound of the CI suggests that the statistical significance may disappear after adjustment for unobserved confounding.
Diabetes drugs may be confounded with glycemic control or other aspects of diabetes like obesity, on which we had no data. Patients receiving diabetes drugs generally have higher mean glucose levels than healthy persons. Hence, a direct role of mean glucose levels seems unlikely given the null result in the largest group, insulins, and among those with a preconception diagnosis of type 2 diabetes and not receiving metformin. In Denmark, metformin has been the first-choice diabetes drug for the entire study period regardless of weight, although patients previously receiving sulfonylureas may have continued this drug. The prevalence of men receiving glucose-lowering drugs has increased over time and thereby also metformin (29). The aOR for metformin was similar in both halves of the period (not shown). Glucagon-like peptide-1 analogues and dipeptidyl peptidase-4 inhibitors are increasingly replacing sulfonylureas as add-on therapy (30), making sulfonylureas increasingly obsolete. Of note, birth defects followed drug rather than diagnosis, yet those type 2 diabetics seen by a specialist may be more severe cases, receive better treatment, or both, giving an unclear net result. Importantly, any confounding by paternal factors would still imply a paternal pathway.
A patient actually redeeming a prescription strongly suggests but does not guarantee therapy adherence, and physicians may change treatment after prescription. This would have biased the results to the null.
We had no data on geographic areas, yet Denmark is a relatively homogeneous country, with universal health care and standardized protocols for diabetes and newborn checkups. Moreover, the time frame analysis and by-father analysis, which should account for geographic variability, supported the results, as did the observation that birth defects were elevated for those fathers with a preconception diagnosis of type 2 diabetes if and only if fathers received metformin.
The by-father analysis, when seen from the father, is a self-controlled case series (28). Although this can account for genetic, socioeconomic, and lifestyle factors that are unlikely to vary between births, it can be susceptible to time-varying effects like age.
Parents of metformin-exposed offspring were generally older and had lower socioeconomic status, raising the question of whether modeling adjustment was adequate. For metformin before or after SDev, parental socioeconomic status and age profiles were similar to those for metformin during SDev. Yet, birth defect frequencies were not elevated in these groups. Unexposed siblings of exposed offspring also had baseline birth defect frequencies. Birth year, parental age, and socioeconomic status did not differ between metformin-exposed offspring with a birth defect and those without. Hence, it is not obvious how correlated maternal or paternal factors like genetics, lifestyle, or medical conditions (31–33) beyond those closely linked to metformin prescriptions could explain the results.
We excluded offspring of mothers with histories of diabetes or essential hypertension, and extending the analysis to all liveborn singletons moved the point estimates only minimally. Indeed, the results were remarkably robust to all sensitivity analyses. Maternal pregestational diabetes, a possible maternal correlate if undiagnosed, is not known to predispose for genital birth defects specifically (34). Together with the time frame and by-mother analysis, which supported the result, these observations make confounding by maternal factors an unlikely explanation.
The finding that sulfonylurea-exposed offspring also tended to have a higher birth defect frequency complicates the interpretation because metformin and sulfonylureas have different pharmacologic mechanisms. Possibilities may be glycemic control beyond mean glucose levels, specificity of both of these drugs to spermatozoa, or, given that the sulfonylureas result lacked statistical significance (P = 0.107) and specificity to genital defects, chance variation. It is unlikely that the observation for sulfonylureas could be explained by patients using prior metformin prescriptions, given the null result for metformin exposure before SDev. Preclinical data support associations between metformin, a chemical of emerging concern with antiandrogenic properties (35), and male reproductive health. Metformin can affect stem cell functions (36) and adhesion factors (37) and has been associated with reduced apoptosis in rat testicular germ cells (38). Male rat offspring undergo alterations in reproductive behavior when exposed to metformin in utero or during lactation (39). In vivo administration of metformin in pregnant mice reduces fetal and neonatal testicular size, whereas in vitro metformin decreases testosterone secretion (40). In sum, the preclinical data on metformin and the male reproductive tract; the specificity of metformin, but not sulfonylureas, to genital birth defects; and the comparative statistical significance of the results suggest that metformin, but not sulfonylureas, is part of the observed association.
The lower proportion of male offspring in the metformin group may be explained by a scarring versus selection model, where the less severe cases are born (with a defect), whereas the more severe cases are aborted (41). More generally, it has been suggested that an excess of girls may indicate male reproductive impairment, as in the cases of the dibromochloropropane pesticide and dioxin (42). Furthermore, low semen quality has been linked with an inability of males to sire male offspring (43).
In Denmark between 1996 and 2016, the prevalence of type 2 diabetes at reproductive ages increased. By age 40 years, prevalence increased from near 0% to almost 2% during this time (2). Our own data show that insulins and metformin are among the fastest-rising drugs received by prospective fathers (44). As a result, approximately 120 offspring per year have fathers filling a metformin prescription during SDev. Given an approximate 1.5% absolute difference in birth defect frequency, this translates to approximately 2 defects per year. This finding may not translate 1- to-1 to other populations, yet the concern is clearly more general in light of the diabetes pandemic. Some of these defects may be lifelong conditions imposing important emotional, social, and economic costs. Even birth defects that could themselves be corrected with a single operation, such as hypospadias, may correlate with more severe illnesses through an association with genital dysgenesis, infertility, and testicular cancer (42).
The observed effect size is similar to that of maternal age greater than 45 years, a recognized risk factor, with 4.8% birth defects among liveborn singletons in our data. The sheer size of the diabetes pandemic suggests that treatment of prospective fathers with diabetes, including pharmacologic management and counseling on diet, physical exercise, and weight loss, should be subject to further study. Further research should replicate the findings while accounting for glycemic control and other metabolic features, and expose the underlying pathway.
Supplementary Material
Acknowledgment:
The authors thank Matthew Keys for comments.
Grant Support:
By grants HD096468 (MJW, YL, SR, TKJ, NES, RL, and MLE) and 1UL1TR003142 (LT and YL) from the National Institutes of Health and grant U01DD001226 (GMS) from the Centers for Disease Control and Prevention.
Footnotes
Disclosures: Disclosures can be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M21-4389.
Reproducible Research Statement: Study protocol: Not available. Statistical code: Available on request from Dr. Wensink (e-mail, mwensink@health.sdu.dk). Data set: Available by application to Statistics Denmark. Fee payable.
Contributor Information
Maarten J. Wensink, Department of Epidemiology, Biostatistics and Biodemography, and Interdisciplinary Center on Population Dynamics, University of Southern Denmark, Odense C, Denmark.
Ying Lu, Department of Biomedical Data Science, Stanford University School of Medicine, Stanford, California.
Lu Tian, Department of Biomedical Data Science, Stanford University School of Medicine, Stanford, California.
Gary M. Shaw, Department of Pediatrics, Stanford University School of Medicine, Palo Alto, California.
Silvia Rizzi, Interdisciplinary Center on Population Dynamics and Department of Epidemiology, Biostatistics and Biodemography, University of Southern Denmark, Odense M, Denmark.
Tina Kold Jensen, Department of Environmental Medicine, University of Southern Denmark, Odense C, Denmark.
Elisabeth R. Mathiesen, Centre for Pregnant Women with Diabetes, Rigshospitalet, Copenhagen University, Copenhagen, Denmark.
Niels E. Skakkebæk, Juliane Marie Centre, Department of Growth and Reproduction, Rigshospitalet, Copenhagen University Hospital, Copenhagen, Denmark.
Rune Lindahl-Jacobsen, Department of Epidemiology, Biostatistics and Biodemography, and Interdisciplinary Center on Population Dynamics, University of Southern Denmark, Odense C, Denmark.
Michael L. Eisenberg, Male Reproductive Medicine and Surgery, Department of Urology, Department of Obstetrics and Gynecology, Stanford University School of Medicine, Stanford, California.
References
- 1.GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1211–1259. [PMID: 28919117] doi: 10.1016/S0140-6736(17)32154-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carstensen B, Rønn PF, Jørgensen ME. Prevalence, incidence and mortality of type 1 and type 2 diabetes in Denmark 1996–2016. BMJ Open Diabetes Res Care. 2020;8. [PMID: 32475839] doi: 10.1136/bmjdrc-2019-001071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Temidayo SO, du Plessis Stefan S. Diabetes mellitus and male infertility. Asian Pacific Journal of Reproduction. 2018;7:6–14. doi: 10.4103/2305-0500.220978 [DOI] [Google Scholar]
- 4.Agbaje IM, Rogers DA, McVicar CM, et al. Insulin dependant diabetes mellitus: implications for male reproductive function. Hum Reprod. 2007;22:1871–7. [PMID: 17478459] [DOI] [PubMed] [Google Scholar]
- 5.Condorelli RA, La Vignera S, Mongioì LM, et al. Diabetes mellitus and infertility: different pathophysiological effects in type 1 and type 2 on sperm function. Front Endocrinol (Lausanne). 2018;9:268. [PMID: 29887834] doi: 10.3389/fendo.2018.00268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eisenberg ML, Sundaram R, Maisog J, et al. Diabetes, medical comorbidities and couple fecundity. Hum Reprod. 2016;31:2369–76. [PMID: 27591240] doi: 10.1093/humrep/dew200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harris BS, Bishop KC, Kemeny HR, et al. Risk factors for birth defects. Obstet Gynecol Surv. 2017;72:123–135. [PMID: 28218773] doi: 10.1097/OGX.0000000000000405 [DOI] [PubMed] [Google Scholar]
- 8.Ludvigsson JF, Neovius M, Söderling J, et al. Periconception glycaemic control in women with type 1 diabetes and risk of major birth defects: population based cohort study in Sweden. BMJ. 2018;362:k2638. [PMID: 29976596] doi: 10.1136/bmj.k2638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hod M, Kapur A, Sacks DA, et al. The International Federation of Gynecology and Obstetrics (FIGO) Initiative on gestational diabetes mellitus: a pragmatic guide for diagnosis, management, and care. Int J Gynaecol Obstet. 2015;131 Suppl 3:S173–211. [PMID: 26433807] doi: 10.1016/S0020-7292(15)30007-2 [DOI] [PubMed] [Google Scholar]
- 10.Immler S. The sperm factor: paternal impact beyond genes. Heredity (Edinb). 2018;121:239–247. [PMID: 29959427] doi: 10.1038/s41437-018-0111-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faure M, Bertoldo MJ, Khoueiry R, et al. Metformin in reproductive biology. Front Endocrinol (Lausanne). 2018;9:675. [PMID: 30524372] doi: 10.3389/fendo.2018.00675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu Y, Ding B, Shen Y, et al. Rapid changes in serum testosterone in men with newly diagnosed type 2 diabetes with intensive insulin and metformin. Diabetes Care. 2021;44:1059–1061. [PMID: 33536253] doi: 10.2337/dc20-1558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bliddal M, Broe A, Pottegård A, et al. The Danish Medical Birth Register. Eur J Epidemiol. 2018;33:27–36. [PMID: 29349587] doi: 10.1007/s10654-018-0356-1 [DOI] [PubMed] [Google Scholar]
- 14.Schmidt M, Pedersen L, Sørensen HT. The Danish Civil Registration System as a tool in epidemiology. Eur J Epidemiol. 2014;29:541–9. [PMID: 24965263] doi: 10.1007/s10654-014-9930-3 [DOI] [PubMed] [Google Scholar]
- 15.Lynge E, Sandegaard JL, Rebolj M. The Danish National Patient Register. Scand J Public Health. 2011;39:30–3. [PMID: 21775347] doi: 10.1177/1403494811401482 [DOI] [PubMed] [Google Scholar]
- 16.EUROCAT Central Registry, European Surveillance of Congenital Abnormalities. EUROCAT guide 1.4 and reference documents. Accessed at https://eu-rd-platform.jrc.ec.europa.eu/sites/default/files/Full_Guide_1_4_version_28_DEC2018.pdf on 12 December 2019.
- 17.Pottegård A, Schmidt SAJ, Wallach-Kildemoes H, et al. Data resource profile: the Danish National Prescription Registry. Int J Epidemiol. 2017;46:798–798f. [PMID: 27789670] doi: 10.1093/ije/dyw213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.R Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing; 2020. [Google Scholar]
- 19.Neto FT, Bach PV, Najari BB, et al. Spermatogenesis in humans and its affecting factors. Semin Cell Dev Biol. 2016;59:10–26. [PMID: 27143445] doi: 10.1016/j.semcdb.2016.04.009 [DOI] [PubMed] [Google Scholar]
- 20.van Buuren S, Groothuis-Oudshoorn K. mice: multivariate imputation by chained equations in R. J Stat Softw. 2011;45:1–67. doi: 10.18637/jss.v045.i03 [DOI] [Google Scholar]
- 21.Friedman J, Hastie T, Tibshirani R. Regularization paths for generalized linear models via coordinate descent. J Stat Softw. 2010;33:1–22. [PMID: 20808728] [PMC free article] [PubMed] [Google Scholar]
- 22.Rynn L, Cragan J, Correa A. Update on overall prevalence of major birth defects—Atlanta, Georgia, 1978–2005 (Reprinted from MMWR. 2008;57:1–5.). JAMA. 2008;299:756–758. doi: 10.1001/jama.299.7.756 [DOI] [PubMed] [Google Scholar]
- 23.Hackshaw A, Rodeck C, Boniface S. Maternal smoking in pregnancy and birth defects: a systematic review based on 173 687 malformed cases and 11.7 million controls. Hum Reprod Update. 2011;17:589–604. [PMID: 21747128] doi: 10.1093/humupd/dmr022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wasserman CR, Shaw GM, Selvin S, et al. Socioeconomic status, neighborhood social conditions, and neural tube defects. Am J Public Health. 1998;88:1674–80. [PMID: 9807535] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wood SN. Generalized Additive Models: An Introduction With R. 2nd ed. Chapman and Hall/CRC; 2017. [Google Scholar]
- 26.VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med. 2017;167:268–274. [PMID: 28693043] doi: 10.7326/M16-2607 [DOI] [PubMed] [Google Scholar]
- 27.Mathur MB, Ding P, Riddell CA, et al. Web site and R package for computing E-values. Epidemiology. 2018;29:e45–e47. [PMID: 29912013] doi: 10.1097/EDE.0000000000000864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cadarette SM, Maclure M, Delaney JAC, et al. Control yourself: ISPE-endorsed guidance in the application of self-controlled study designs in pharmacoepidemiology. Pharmacoepidemiol Drug Saf. 2021;30:671–684. [PMID: 33715267] doi: 10.1002/pds.5227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Christensen DH, Rungby J, Thomsen RW. Nationwide trends in glucose-lowering drug use, Denmark, 1999–2014. Clin Epidemiol. 2016;8:381–387. [PMID: 27789974] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Persson F, Bodegard J, Lahtela JT, et al. Different patterns of second-line treatment in type 2 diabetes after metformin monotherapy in Denmark, Finland, Norway and Sweden (D360 Nordic): a multinational observational study. Endocrinol Diabetes Metab. 2018;1:e00036. [PMID: 30815564] doi: 10.1002/edm2.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eisenberg ML, Chen Z, Ye A, et al. Relationship between physical occupational exposures and health on semen quality: data from the Longitudinal Investigation of Fertility and the Environment (LIFE) Study. Fertil Steril. 2015;103:1271–7. [PMID: 25765658] doi: 10.1016/j.fertnstert.2015.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eisenberg ML, Li S, Behr B, et al. Relationship between semen production and medical comorbidity. Fertil Steril. 2015;103:66–71. [PMID: 25497466] doi: 10.1016/j.fertnstert.2014.10.017 [DOI] [PubMed] [Google Scholar]
- 33.Guo D, Li S, Behr B, et al. Hypertension and male fertility. World J Mens Health. 2017;35:59–64. [PMID: 28868816] doi: 10.5534/wjmh.2017.35.2.59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gabbay-Benziv R, Reece EA, Wang F, et al. Birth defects in pregestational diabetes: defect range, glycemic threshold and pathogenesis. World J Diabetes. 2015;6:481–8. [PMID: 25897357] doi: 10.4239/wjd.v6.i3.481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alla LNR, Monshi M, Siddiqua Z, et al. Detection of endocrine disrupting chemicals in Danio rerio and Daphnia pulex: step-one, behavioral screen. Chemosphere. 2021;271:129442. [PMID: 33476875] doi: 10.1016/j.chemosphere.2020.129442 [DOI] [PubMed] [Google Scholar]
- 36.Bednar F, Simeone DM. Metformin and cancer stem cells: old drug, new targets. Cancer Prev Res (Phila). 2012;5:351–4. [PMID: 22389436] doi: 10.1158/1940-6207.CAPR-12-0026 [DOI] [PubMed] [Google Scholar]
- 37.Romero R, Erez O, Hüttemann M, et al. Metformin, the aspirin of the 21st century: its role in gestational diabetes mellitus, prevention of preeclampsia and cancer, and the promotion of longevity. Am J Obstet Gynecol. 2017;217:282–302. [PMID: 28619690] doi: 10.1016/j.ajog.2017.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ghasemnejad-Berenji M, Ghazi-Khansari M, Yazdani I, et al. Effect of metformin on germ cell-specific apoptosis, oxidative stress and epididymal sperm quality after testicular torsion/detorsion in rats. Andrologia. 2018;50. [PMID: 28730645] doi: 10.1111/and.12846 [DOI] [PubMed] [Google Scholar]
- 39.Forcato S, Novi DRBDS, Costa NO, et al. In utero and lactational exposure to metformin induces reproductive alterations in male rat offspring. Reprod Toxicol. 2017;74:48–58. [PMID: 28867217] doi: 10.1016/j.reprotox.2017.08.023 [DOI] [PubMed] [Google Scholar]
- 40.Tartarin P, Moison D, Guibert E, et al. Metformin exposure affects human and mouse fetal testicular cells. Hum Reprod. 2012;27:3304–14. [PMID: 22811314] doi: 10.1093/humrep/des264 [DOI] [PubMed] [Google Scholar]
- 41.Bruckner TA, Catalano R. Selection in utero and population health: theory and typology of research. SSM Popul Health. 2018;5:101–113. [PMID: 29928686] doi: 10.1016/j.ssmph.2018.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Skakkebaek NE, Rajpert-De Meyts E, Buck Louis GM, et al. Male reproductive disorders and fertility trends: influences of environment and genetic susceptibility. Physiol Rev. 2016;96:55–97. [PMID: 26582516] doi: 10.1152/physrev.00017.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eisenberg ML, Murthy L, Hwang K, et al. Sperm counts and sperm sex ratio in male infertility patients. Asian J Androl. 2012;14:683–6. [PMID: 22842703] doi: 10.1038/aja.2012.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wensink MJ, Rizzi S, Jensen TK, et al. Paternal prescription medication before conception: a retrospective cohort study of all births in Denmark 1997–2017. Scand J Public Health. 2021;49:884–890. [PMID: 33615897] doi: 10.1177/1403494820987468 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.