Abstract
Purpose
Sickle cell disease (SCD) is a significant burden for patients and healthcare systems due to multiple factors, including high readmission rates. This study aimed to determine the general characteristics, etiology of admissions, annual admission rate, length of stay, and readmission rate of patients with SCD.
Patients and Methods
This retrospective observational study included all adult patients with SCD admitted to the General Internal Medicine (GIM) unit between 2016 and 2021.
Results
There were 160 patients (mean age, 31.08 ± 9.06 years; 51.25% female) with SCD included in this study. Most originated from southern Saudi Arabia (45.62%). The average annual number of emergency department (ED) visits was 4, and approximately 19% of patients had ≥3 annual admissions. The mean length of stay was 6 days. The readmission rates at 7, 30, 60, and 90 days were 8%, 24.5%, 13.6%, and 10.8%, respectively.
Conclusion
SCD generates a significant economic burden on the Saudi society and the effects on the healthcare system and patients’ quality of life are evident in the high ED visits, readmission rates and prolonged hospitalization. Thereupon we advocate the implementation of sickle cell disease-specialized multidisciplinary clinics.
Keywords: complications, readmission, quality of life, middle east, hemoglobinopathy
Introduction
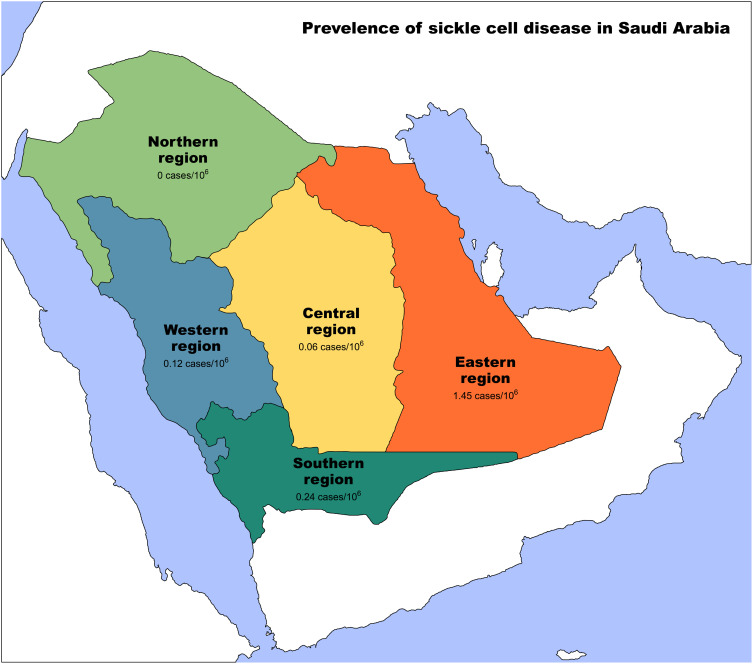
Sickle cell disease (SCD) is one of the most common hematological disorders, affecting millions of people worldwide.1 It is caused by a single gene mutation that leads to the production of abnormal hemoglobin, which can ultimately result in chronic manifestations affecting nearly every organ in the body.2 The prevalence of SCD varies globally. In the United States, the estimated prevalence is 329 cases per 1,000,000 of the population based on the National Newborn Screening Information System census and the National Population Projections Datasets.2,3 Whereas in the United Kingdom, the prevalence is approximately 217 per 1,000,000 of the population.4 However, the prevalence is much higher in the African and middle eastern populations, as sickle cell disease affects more than 20,000 per 1,000,000 of the Nigerian population.5 And more than 45,100 per 1,000,000 among adults in Saudi Arabia. Moreover, among Saudi children and adolescents, 2400 per 1,000,000 were estimated to have sickle cell disease. SCD is more dominant in the eastern and southwestern regions of the country6,7 (Figure 1).
Figure 1.
Prevalence of Sickle cell disease in Saudi Arabia. Yellow: Central region of Saudi Arabia, Orange: Eastern region of Saudi Arabia, Green: Southern region of Saudi Arabia, Blue: Western region of Saudi Arabia, Light green: Northern region of Saudi Arabia.
Clinical manifestations of SCD are diverse as the disease can impact any body organ. Acute and chronic complications intersect, and the primary process is either hypercoagulability causing vaso-occlusion and vascular damage or hemolysis leading to chronic anemia. Acute vaso-occlusive crisis (VOC) types include vaso-occlusive pain crisis (Bone, Abdomen, chest, …etc.), acute chest syndrome (ACS), infarction crisis (ie, strokes, Transient Ischemic Attack (TIA), Deep Vein Thrombosis (DVT). etc.), priapism, and pregnancy-related crises. Fatal acute sickle cell crises related to other pathophysiologies contribute greatly to the disease’s ramifications, including acute hepatic or splenic sequestration crisis, hyperhemolysis or aplastic anemia crisis, acute intrahepatic cholestasis, and bacterial infections.1,8
In the United States, Sickle cell disease-related emergency department (ED) visits and subsequent hospitalizations are estimated to cost approximately $2.4 million.9,10 Data on the economic outcomes of SCD in Saudi Arabia are insufficient. Nevertheless, it is estimated to cost an average of $13,700 per patient per year in Gulf countries.11
In addition, SCD negatively affects the quality of life, educational achievement, financial income, and mental health of the patients. A substantial proportion of patients with SCD experience a significant effect on their daily life activities, emotional well-being, employment, education, social life, and relationships.12
Depression and depressive symptoms are common in patients with SCD and are associated with an increased risk of poor outcome. Adam et al reported a significant association among depression, poor health-related quality of life, and mental and physical health; moreover, overall healthcare costs, including inpatient care, were substantially higher in patients with depression than in those without depression.13
Understanding and targeting the factors contributing to this enormous healthcare utilization is vital in creating quality improvement approaches for reducing avoidable healthcare expenditures. The national premarital screening program was a critical preventive strategy implemented and funded by the Saudi Arabian government in 2004. The program consists of mandatory screening for individuals who are married for common genetic and infectious disorders, including sickle cell anemia, thalassemia, human immunodeficiency virus, and hepatitis B and C viruses.14
The primary objective of this study was to establish the characteristics of patients with SCD admitted to the general internal medicine (GIM) unit at a tertiary hospital and determine the cause of admission, annual admission rate, and length of stay (LOS).
Materials and Methods
Study Design
This retrospective observational study was conducted at King Saud University Medical City (KSUMC), a tertiary care hospital in Riyadh, Saudi Arabia, from January 1, 2016, to October 31, 2021. In total, 324 patients were initially extracted via electronic medical records using the keywords “sickle cell disease”, “sickle cell anemia”, and “sickle cell trait.”
Inclusion Criteria
This study included all consecutive patients with SCD disease who were aged > 14 years at the time of recruitment and were admitted to the general internal medicine unit during the study period for any reason.
Exclusion Criteria
Patients who were younger than 14 years at the time of recruitment, with sickle cell trait only, or who were admitted to other units were excluded from this study.
Data Collection
Data were collected using electronic health medical records between December 15, 2021, and February 15, 2022. Some variables were obtained via phone call. Extracted data included the following patient demographic data: age, sex, nationality, patient origin in Saudi Arabia, marital status, educational qualification, employment status, monthly income, smoking status, and the average body mass index (BMI) of all admissions. Other data extracted included clinical characteristics, such as mortality, cause of mortality, hemoglobin genotype, Sickle cell disease-modifying therapy (including hydroxyurea, folic acid, penicillin, and L-glutamine), home pain medications, blood transfusion, and blood exchange frequency, past surgical history, past medical history, comorbidities, psychiatric disorders, complications, number of admissions, length of admissions, Emergency department visits, and whether patients were followed up in multiple hospitals.
Statistical Analyses
The data were collected and entered into a Microsoft Excel (Microsoft Corp., Redmond, WA, USA) spreadsheet. Data sorting was performed before analysis. Descriptive analyses were performed using frequencies, means, and standard deviations (SDs) for patient information. Continuous and categorical variables are expressed as mean ± SD and percentages.
Ethical Consideration
Participant anonymity was assured by assigning each participant a code number for analysis only. Informed consent was obtained from all participants before participation. No incentives or rewards were provided to participants. This study was approved by King Saud University’s College of Medicine Research Ethics Committee (IRB: E-21-6469).
Results
After an extensive review of our electronic health medical records, we identified 324 patients, of whom only 160 were recruited after applying our study’s inclusion and exclusion criteria.
The participants’ demographics are presented in Table 1. The mean age of the participants was 31.08 ± 9.06 years. More than half of the participants were female (n =82, 51.3%). The majority were from Saudi Arabia (n =156, 97.5%) and originally from the southern area (n =73, 45.6%). Regarding marital status, 50.6% (n =81), 42.5% (n =68), and 13.1% (n =5) were single, married, and divorced, respectively. More than half of the participants were unemployed (n =74, 57.5%). A large percentage had completed postgraduate education, including bachelor’s degrees, master’s degrees, and diplomas (n =69, 43.1%), whereas less than 9% (n =13) of the patients held a primary degree or were illiterate. The average monthly income of the participants was 8297 Saudi riyals (2211 USD). Moreover, 17.5% (n =28), 5% (n =8), and 67.5% (n = 108) of the participants were current smokers, ex-smokers, and had never smoked, respectively. More than half of the participants had a normal BMI (n =100, 62.5%), whereas 19.3% (n =31) were underweight.
Table 1.
Participants’ Demographics (n = 160)
| Variable | Mean (SD, Range) or Frequency (%) |
|---|---|
| Age | 31.08 ± 9.06 |
| Sex | |
| Male | 78 (48.75%) |
| Female | 82 (51.25%) |
| Nationality | |
| Saudi | 156 (97.5%) |
| Non-Saudi | 4 (2.5%) |
| Area in Saudi (patient’s origin) | |
| South | 73 (45.62%) |
| North | 0 (0.0%) |
| West | 2 (1.25%) |
| East | 7 (4.37%) |
| Middle | 64 (40%) |
| Unknown | 14 (8.75%) |
| Marital status | |
| Single | 81(50.62%) |
| Married | 68 (42.5%) |
| Divorced | 5 (3.12%) |
| Widowed | 1 (0.62%) |
| Unknown | 5 (3.12%) |
| Educational qualification | |
| University and diploma | 69 (43.12%) |
| Intermediate and secondary | 59 (36.87%) |
| Illiterate and primary | 13 (8.12%) |
| Unknown | 19 (11.87%) |
| Occupation | |
| Employed | 68 (42.5%) |
| Unemployed | 74 (46.25%) |
| Unknown | 18 (11.25%) |
| Monthly income | |
| Above average | 56 (35%) |
| Below average | 104 (65%) |
| Unknown | 20 (12.5%) |
| Smoking status | |
| Non-smoker | 108 (67.5%) |
| Active smoker | 28 (17.5%) |
| Ex-smoker | 8 (5%) |
| Unknown | 16 (10%) |
| BMI | |
| <18.5 | 31 (19.3%) |
| 18.5–24.9 | 100 (62.5%) |
| 25–29.9 | 22 (13.75%) |
| 30–39.9 | 4 (2.5%) |
| ≥40 | 3 (1.87%) |
Abbreviations: BMI, body mass index; SD, standard deviation.
Over the 6-year study period, 3826 emergency department (ED) visits and 891 admissions were attributed to SCD complications, including 33 admissions to the intensive care unit. VOC was the main reason for admission in more than 90% of cases.
More than half of the patients (n= 93, 58%) were readmitted during the study period. Of the 891 admissions, 218 (24.5%) were within 30 days of discharge, of which 71 (32.5%) were within 7 days of discharge. In contrast, 121 (13.6%) and 95 (10.8%) readmissions occurred within 60 and 90 days, respectively.
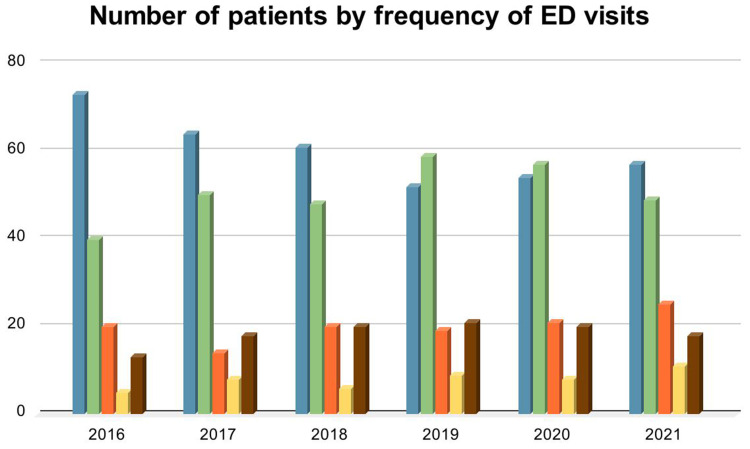
Moreover, 11.5% (n= 18) of the patients visited the emergency department more than six times per year (Figure 2), and 31.2% (n= 50) were admitted six times or more during the study period. The average length of stay (LOS) was 6.43 days per admission. Table 2 demonstrates the annual distribution of admissions, ED visits, and length of stay.
Figure 2.
Number of patients by frequency of ED visits. Blue: No ED visits, green: 1–2 ED visits, orange: 3–4 ED visits, yellow: 5–6 ED visits, brown ≥ 7 ED visits.
Table 2.
Annual Distribution of Admissions, Emergency Department Visits, and Length of Stay
| Year | ED Visits | Admissions | Average LOS (Days) | Range LOS (Days) | Hospital Days |
|---|---|---|---|---|---|
| 2016 | 329 | 42 | 7.89 | 32–1 | 315 |
| 2017 | 564 | 107 | 5.08 | 31–1 | 630 |
| 2018 | 865 | 141 | 5.71 | 24–1 | 794 |
| 2019 | 846 | 192 | 6.90 | 36–1 | 1739 |
| 2020 | 592 | 194 | 5.83 | 22–1 | 1037 |
| 2021 | 632 | 239 | 6.72 | 57.5–1 | 1609 |
| Total | 3826 | 891 | 6.43 | 90–1 | 2646 |
Abbreviations: ED, emergency department; LOS, length of stay.
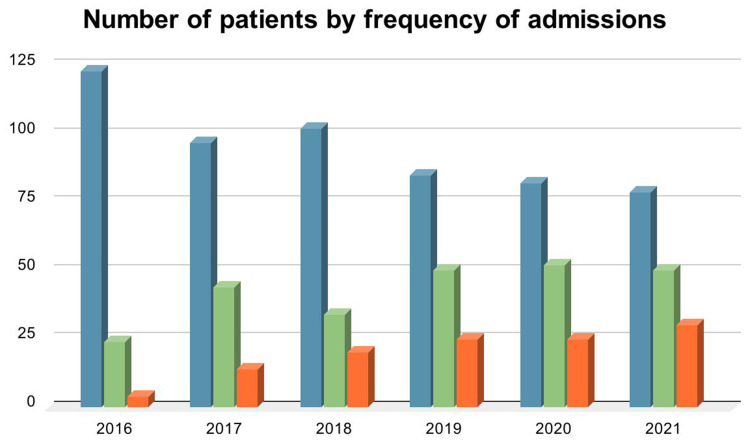
As illustrated in Figure 3, 12.3% (n= 20) of the participants had an average annual admission rate of three or more. Moreover, the annual admission rate of the patients increased over the study period, starting at 17.5% (n= 28) in 2016 and reaching 50% (n= 80) by 2021.
Figure 3.
Number of patients by frequency of admissions. Blue: No admissions, green: 1–2 admissions, orange: ≥ 3 admissions.
The clinical characteristics of participants are shown in Table 3. The mortality rate was 1.9% (n= 3) during the study period, with causes including gastrointestinal bleeding, pulmonary embolism, and multiorgan failure. The genotype of the majority was Hb SS (n= 124, 77.5%), followed by Hb S-thalassemia (n= 33, 22.6%). Most patients received daily doses of hydroxyurea and folic acid (n= 133, 83.1%), and only half of the patients (n= 81, 50%) were compliant with their medications (medication compliance was evaluated through an extensive review of the charts and confirmed via phone calls). Acetaminophen with codeine was the most commonly used home analgesic (n= 67, 41.9%). Most patients (n= 144, 90%) received blood transfusions and exchange transfusions, as required. Moreover, 43.1% (n= 69) visited different hospitals, whereas 45.6% (n= 73) were followed up at a single center.
Table 3.
Participants’ Clinical Characteristics (n = 160)
| Variable | Number (%) |
|---|---|
| Mortality | |
| No | 146 (91.25%) |
| Yes, cause of death (%) | 3 (1.87%) |
| Unknown | 11 (6.87%) |
| Genotype | |
| HbSS | 124 (77.5%) |
| HbS thalassemia | 33 (20.6%) |
| HbS G6PD | 3 (1.87%) |
| SCD-modifying therapy | |
| Hydroxyurea | 4 (2.5%) |
| Folic acid | 17 (10.6%) |
| Hydroxyurea and folic acid | 133 (83.12%) |
| Penicillin | 4 (2.5%) |
| L glutamine | 2 (1.25%) |
| Nill | 5 (3.12%) |
| Medication compliance. | |
| Well compliant | 81 (50%) |
| Non-compliant | 76 (47.5%) |
| Unknown | 3 (1.87%) |
| Home pain medications | |
| None | 15 (9.37%) |
| Paracetamol/codeine | 67 (41.87%) |
| NSAIDs | 30 (18.75%) |
| Opioid | 58 (36.25%) |
| Paracetamol | 30 (18.75%) |
| GABA analog (gabapentin/pregabalin) | 15 (9.37%) |
| Blood transfusion | |
| On-demand transfusion | 144 (90%) |
| Regular blood transfusion | 3 (1.87%) |
| Regular exchange transfusion | 13 (8.12%) |
| Visiting multiple hospitals | |
| Yes | 69 (43.12%) |
| No | 73 (45.62%) |
| Unknown | 18 (11.25%) |
| Surgeries | |
| None | 83 (51.8%) |
| Cholecystectomy | 63 (39.37%) |
| Splenectomy | 22 (13.75%) |
| Orthopedic surgeries | 13 (8.12%) |
Abbreviations: G6PD, glucose-6-phosphate dehydrogenase; GABA, gamma-aminobutyric acid; NSAIDs, non-steroidal anti-inflammatory drugs; SCD, sickle cell disease.
Among the 160 patients with SCD, 150 had comorbidities or disease-related complications. The most common complications were avascular osteonecrosis (n= 50, 31.3%), acute chest syndrome (n= 30, 18.8%), iron overload (n= 27, 16.9%), pulmonary embolism (n= 18, 11.3%), cholelithiasis (n= 12, 7.5%), deep vein thrombosis (n= 11, 6.8%), bronchial asthma (n= 10, 6.3%), heart failure (n= 9, 5.6%), and cerebrovascular accident (n= 8, 5%). Moreover, a few participants were diagnosed with psychiatric disorders, including major depressive disorder (n= 10, 6.3%), anxiety (n= 8, 5%), and substance use disorder (n= 6, 3.8%). Nearly half of the participants (n= 77, 48.2%) underwent at least one surgical intervention, most commonly cholecystectomy, splenectomy, and orthopedic surgery, including joint replacement, amputation, and spinal surgery (Table 4).
Table 4.
Complications/Comorbidities of Patients with SCD
| Category | Frequency (N) | Percentage % |
|---|---|---|
| CNS | Migraine headache (2) | 12 (7.5%) |
| Seizure disorder (2) | ||
| Stroke/TIA (8) | ||
| CVS | Heart failure (9) | 18 (11.25%) |
| Cardiomyopathy (0) | ||
| Valvular heart disease (2) | ||
| Arrhythmia (1) | ||
| Hypertension (6) | ||
| Pulmonary | Bronchial asthma (10) | 46 (28.75%) |
| Acute chest syndrome (30) | ||
| Pulmonary hypertension (6) | ||
| Genitourinary | Priapism (6) | 7 (4.37%) |
| Neurological bladder (1) | ||
| Renal | CKD (7) | 12 (7.5%) |
| Sickle cell nephropathy (5) | ||
| Gastrointestinal | Liver cirrhosis (1) | 27 (16.87%) |
| Cholelithiasis (12) | ||
| Cholechodolithiasis (6) | ||
| Sickle cell hepatopathy (4) | ||
| Peptic ulcer disease (1) | ||
| Chronic/acute intrahepatic cholestasis (1) | ||
| Hepatic/splenic sequestration (1) | ||
| Acute cholecystitis (1) | ||
| Endocrine | Diabetes (2) | 11 (6.87%) |
| Osteopenia (1) | ||
| Hypothyroidism (4) | ||
| Pseudohypoparathyroidism (1) | ||
| Hyperparathyroidism (1) | ||
| Adrenal insufficiency (1) | ||
| Prolactinoma (1) | ||
| Hematological | Iron overload (27) | 61 (38.12%) |
| PE (18) | ||
| DVT (11) | ||
| Bilateral renal infarction (1) | ||
| Peripheral arterial disease (1) | ||
| Essential thrombocytosis (1) | ||
| Thrombophlebitis (2) | ||
| Musculoskeletal | AON (50) | 50 (31.25%) |
| Hip (45) | ||
| Shoulder (8) | ||
| Knee (1) | ||
| Rheumatological disease | RA (1) | 3 (1.87%) |
| SLE (2) | ||
| Infectious disease | IE (1) | 14 (4.33%) |
| OM (5) | ||
| TB (2) | ||
| HepB/C(6) | ||
| Psychiatric disorders | MDD (10) | 25 (15.62%) |
| Anxiety (8) | ||
| Borderline personality (1) | ||
| Substance use disorder (6) |
Abbreviations: AON, avascular osteonecrosis; CKD, chronic kidney disease; CNS, central nervous system; CVS, cardiovascular system; DVT, deep vein thrombosis; HepB/C, hepatitis B/C; IE, infective endocarditis; MDD, major depressive disorder; OM, otitis media; PE, pulmonary embolism; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus; TB, tuberculosis; TIA, transient ischemic attack.
We also examined the rate of coronavirus disease 2019 (COVID-19) infection in our patients and found that 36.2% (n= 58) had a positive polymerase chain reaction test result (regardless of symptomatic status). Only three participants among the whole population were unvaccinated, whereas the vast majority had received a second dose of the COVID-19 vaccine (n= 82, 51.3%) and were considered fully vaccinated according to the Ministry of Health requirements at the time of this study.
Discussion
SCD is a genetically inherited hemoglobinopathy with various adverse consequences. The nature of the disease with recurrent VOC, complications, admissions and lengthy hospital stays has a tremendous impact on patients living with SCD. This burden affects financial, social, and psychological aspects and extends beyond to involve families and people caring for patients with SCD. We found substantial observations regarding the catastrophic effect of SCD on patients’ lives with significant financial strains and academic underachievement. In Saudi Arabia, the overall unemployment rate among citizens is 9.7%.15 In our study, more than half of the patients were unemployed, and the monthly income of the majority of patients was below average compared to the general population.16 Furthermore, despite the free and accessible education in the country, more than one-third of the patients failed to progress beyond the intermediate or secondary levels.
The high frequency of non-compliance to hydroxyurea therapy observed among SCD patients is in line with previous studies reporting high rates of non-adherence among SCD patients.17,18 This can be classified under intentional and unintentional non-adherence but is usually attributed to multiple factors, including inadequate education, lack of medication supply, polypharmacy, pregnancy, and side effects, such as infertility and cytopenia.19 One of the rarely explored reasons for non-compliance is drug formulation. Hydroxyurea in our center is usually supplied in a capsule form that some patients find hard to swallow and prefer syrup formulation, which is usually unavailable. This is supported by a cohort study in Italy, which concluded that providing different formulations and proper education would result in less toxicity and better compliance.20
From a nutritional point of view, 19.3% of the participants in this study had a lower BMI (ie, <18) compared to 2.5% of the general population of Saudi Arabia.21 Multiple factors, such as low socioeconomic status, educational background, comorbidities, and recurrent and prolonged hospitalization, can affect nutrition.
Despite being immunocompromised due to hyposplenism and poor nutritional status, the COVID-19 pandemic affected patients with SCD just as it affected the general population. Among the fully vaccinated majority, 5.625% developed Vaso-occlusive crisis post-vaccination, compared to 3.2% in another study.22 Moreover, the ED visits showed some fluctuation over the study period. In 2020/2021, a drop was observed in ED visits, mainly attributed to the restrictions that the COVID-19 pandemic had imposed on ordinary activities, including seeking health care for Vaso-occlusive crisis and managing it with over-the-counter medications instead. Overall, the average ED visits were close to four per patient per year, matching most patients enrolled in the well-known Cooperative Study of Sickle Cell Disease.23,24
Acute chest syndrome (ACS) is a well-known complication and the major cause of death in SCD. ACS diagnosis was established based on clinical and radiological findings in 43 (5%) patients, with more than 88% of ACS events being diagnosed later on during their hospital stay. The incidence of ACS in our study is lower compared to other studies.25,26 This can be attributed to differences in age group and SCD genotype as our study involved much older adults with no pediatric group and less HbSS genotype. More importantly, patients with ACS on presentation in our facility are admitted under the hematology service instead of the GIM unit.
Regarding other complications, the most frequently observed was Avascular Osteonecrosis (AON), affecting 31.3% of the patients based on Magnetic Resonance Imaging (MRI) of at least one joint. Other similar studies have reported a much lower prevalence of osteonecrosis. The study by Milner et al enrolled 2804 patients aged 5 years and older and reported an overall prevalence of osteonecrosis of 9.8% affecting one or both femoral heads.27 On the other hand, Akinyoola et al observed a prevalence of 15.9% among patients aged 15 years or older.28
We observed significant rates of acute care utilization in the form of high numbers of ED visits, recurrent admissions, and length of stay. More than half of the patients were rehospitalized at least once during the study period. Approximately 25% of readmissions occurred within 30 days, which is comparable to that reported in previous studies.1,2,10,12 Despite some controversy, the 30-day readmission interval is widely used as a benchmark for assessing the quality of inpatient care by healthcare providers and policymakers.10 Given the nature and progression of SCD, preventing readmissions within 30 days may not be feasible. Many studies have reported readmission rates within 30 days of hospital discharge of between 19% and 50%, and the majority are related to unmodifiable community and patient-related factors. In contrast, readmissions within shorter intervals (eg, 7 days) are more strongly associated with elements within hospital control. This includes modifiable factors, such as deficiencies in the quality of inpatient care, medication-related errors, premature discharge, suboptimal discharge planning, and inadequate post-discharge care. We found that 32.5% of 30-day readmissions occurred within the first week after discharge. Such a short interval could reflect a period of high vulnerability, with risk factors leading to preventable unplanned readmissions. A vaso-occlusive crisis was the reason for admission in 90.68% of the reviewed 891 admissions. This is in line with a census conducted on Sickle cell disease hospitalizations in the United States from 1994 to 2004, which found that more than 90% of admissions were caused by VOC.23 A patient’s length of stay may vary depending on the reason for admission and evolving complications, and it might reflect the quality of ambulatory care and pain management in patients with SCD. Overall, the mean Length of stay was 6.4 days, with no significant annual trend. This is compared to 7 days, as reported by Shah et al in a study that enrolled 8521 patients from 14 different states in the United States.24
Although the average number of admissions per year in our sample was almost equal to one, approximately 20% of the patients had reached ≥3 admissions per year in 2021. This gradual increase in the annual admission rate was also observed in a study conducted in England in which SCD admissions were reviewed from 2001 to 2010. This consensus may follow the natural history of a progressive disease.29 Another theory that explains the increasing annual admission rate is the growing cumulative number of patients with SCD at our institute.
Study Limitations
This study was conducted at a single tertiary hospital located in the central region of Saudi Arabia. This study was affected by the distribution of cases between the GIM unit and hematology, where only GIM cases were enrolled; however, complicated cases in which patients were on regular exchange transfusion or those initially presenting with Acute chest syndrome or stroke were admitted under hematology and were not included. This study was also limited by the inability to access patients’ medical records during admission to other hospitals. In addition, there were missing data that could not be obtained owing to the nonresponse of some participants.
Conclusion
SCD is a chronic, debilitating disease characterized by recurrent Veno-occlusive Crises leading to frequent ED visits, high rates of hospitalizations, and recurrent re-admissions. The average number of admissions and ED visits per patient is one admission and four ED visits. The average Length of stay during the study period was 6.4 days. The readmission rates at 7, 30, 60, and 90 days were 8%, 24.5%, 13.6%, and 10.8%, respectively. The monthly income for most of the patients was below average, with more than half of the patients unemployed. These findings were not significantly distinct when compared to the literature. However, it demonstrates the effects of SCD on patients’ quality of life and the Saudi healthcare system.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author, AB, upon request.
Disclosure
The authors report no financial or non-financial competing interests in this work.
References
- 1.Mburu J, Odame I. Sickle cell disease: reducing the global disease burden. Int J Lab Hematol. 2019;41(Suppl 1):82–88. doi: 10.1111/ijlh.13023 [DOI] [PubMed] [Google Scholar]
- 2.Piel FB, Steinberg MH, Rees DC, Longo DL. Sickle cell disease. N Engl J Med. 2017;376(16):1561–1573. doi: 10.1056/NEJMra1510865 [DOI] [PubMed] [Google Scholar]
- 3.United States Census Bureau. 2008 national population projections datasets. Table 1, Projected population by age, sex, race, and Hispanic origin: July 1, 2000 to July 1, 2050. U.S. Census bureau; 2022. Available frrom: https://www.census.gov/data/datasets/2008/demo/popproj/2008-popproj.html. Accessed December 27, 2022.
- 4.Dormandy E, James J, Inusa B, et al. How many people have sickle cell disease in the UK? J Public Health. 2018;40(3):e291–e295. doi: 10.1093/pubmed/fdx172 [DOI] [PubMed] [Google Scholar]
- 5.Adewoyin AS. Management of sickle cell disease: a review for physician education in Nigeria (Sub-Saharan Africa). Anemia. 2015;2015:791498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Qurashi MM, El-Mouzan MI, Al-Herbish AS, et al. The prevalence of sickle cell disease in Saudi children and adolescents. A Community-Based Survey. Saudi Med J. 2008;29:1480–1483. [PubMed] [Google Scholar]
- 7.Memish ZA, Saeedi MY. Six-year outcome of the national premarital screening and genetic counseling program for sickle cell disease and β-thalassemia in Saudi Arabia. Ann Saudi Med. 2011;31:229–235. doi: 10.4103/0256-4947.81527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vichinsky EP. Overview of the clinical manifestations of sickle cell disease. In: Post TW, editor. UpToDate. Waltham, MA: UpToDate Inc; 2022. Available from: https://www.uptodate.com/contents/overview-of-the-clinical-manifestations-of-sickle-cell-disease. Accessed December 27, 2022. [Google Scholar]
- 9.Lanzkron S, Carroll CP, Haywood CJ. The burden of emergency department use for sickle-cell disease: an analysis of the national emergency department sample database. Am J Hematol. 2010;85:797–799. doi: 10.1002/ajh.21807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cronin RM, Hankins JS, Byrd J, et al. Risk factors for hospitalizations and readmissions among individuals with sickle cell disease: results of a U.S. survey study. Hematology. 2019;24:189–198. doi: 10.1080/16078454.2018.1549801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alkindi S, Altooq J, Dewedar H, et al. S113: cost Of Illness, Epidemiology And Healthcare Resource Utilization For Sickle Cell Disease In Gulf Countries (Cescgu). Hemasphere. 2022;6(Suppl):7. doi: 10.1097/01.HS9.0000821420.25455.37 [DOI] [Google Scholar]
- 12.Osunkwo I, Andemariam B, Minniti CP, et al. Impact of sickle cell disease on patients’ daily lives, symptoms reported, and disease management strategies: results from the international sickle cell world Assessment Survey (SWAY). Am J Hematol. 2021;96:404–417. doi: 10.1002/ajh.26063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adam SS, Flahiff CM, Kamble S, et al. Depression, quality of life, and medical resource utilization in sickle cell disease. Blood Adv. 2017;1:1983–1992. doi: 10.1182/bloodadvances.2017006940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gosadi IM. National screening programs in Saudi Arabia: overview, outcomes, and effectiveness. J Infect Public Health. 2019;12:608–614. doi: 10.1016/j.jiph.2019.06.001 [DOI] [PubMed] [Google Scholar]
- 15.General Authority for Statistics (GASTAT). The Labor Market Publication for the second quarter of 2022. Available from https://www.stats.gov.sa/en/6800. Accessed December 27, 2022.
- 16.General Authority for Statistics (GASTAT). Household income and expenditure survey; 2018. Available from: https://www.stats.gov.sa/en/37. Accessed September 21, 2022.
- 17.Candrilli SD, O’Brien SH, Ware RE, et al. Hydroxyurea adherence and associated outcomes among Medicaid enrollees with sickle cell disease. Am J Hematol. 2011;86(3):273–277. doi: 10.1002/ajh.21968 [DOI] [PubMed] [Google Scholar]
- 18.Badawy SM, Thompson AA, Lai J-S, et al. Adherence to hydroxyurea, health-related quality of life domains, and patients’ perceptions of sickle cell disease and hydroxyurea: a cross-sectional study in adolescents and young adults. Health Qual Life Outcomes. 2017;15(1):136. doi: 10.1186/s12955-017-0713-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agrawal RK, Patel RK, Shah V, et al. Hydroxyurea in sickle cell disease: drug review. Indian J Hematol Blood Transfus. 2014;30(2):91–96. doi: 10.1007/s12288-013-0261-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Colombatti R, Palazzi G, Masera N, et al. Hydroxyurea prescription, availability and use for children with sickle cell disease in Italy: results of a National Multicenter survey. Pediatr Blood Cancer. 2018;65(2):10. doi: 10.1002/pbc.26774 [DOI] [PubMed] [Google Scholar]
- 21.Althumiri NA, Basyouni MH, AlMousa N, et al. Obesity in Saudi Arabia in 2020: prevalence, distribution, and its current association with various health conditions. Healthcare. 2021;9:311. doi: 10.3390/healthcare9030311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jan H, Waheeb A, AlAhwal H, et al. COVID-19 vaccine perception and hesitancy among patients with sickle cell disease in the western region of Saudi Arabia. Cureus. 2022;14:e21026. doi: 10.7759/cureus.21026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steiner CA, Miller JL. Sickle cell disease patients in U.S. Hospitals. 2004. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2006. [PubMed] [Google Scholar]
- 24.Shah N, Bhor M, Xie L, et al. Sickle cell disease complications: prevalence and resource utilization. PLoS One. 2019;14:e0214355. doi: 10.1371/journal.pone.0214355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vichinsky EP, Neumayr LD, Earles AN, et al. Causes and outcomes of the acute chest syndrome in sickle cell disease. National Acute Chest Syndrome Study Group. N Engl J Med. 2000;342(25):1855–1865. [DOI] [PubMed] [Google Scholar]
- 26.Bartolucci P, Habibi A, Khellaf M, et al. Score predicting acute chest syndrome during vaso-occlusive crises in adult sickle-cell disease patients. EBiomedicine. 2016;10:305–311. doi: 10.1016/j.ebiom.2016.06.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Milner PF, Kraus AP, Sebes JI, et al. Sickle cell disease as a cause of osteonecrosis of the femoral head. N Engl J Med. 1991;325:1476–1481. doi: 10.1056/NEJM199111213252104 [DOI] [PubMed] [Google Scholar]
- 28.Akinyoola AL, Adediran IA, Asaleye CM. Avascular necrosis of the femoral head in sickle cell disease in Nigeria: a retrospective study. Niger Postgrad Med J. 2007;14:217–220. [PubMed] [Google Scholar]
- 29.Aljuburi G, Phekoo KJ, Okoye NO, et al. Patients’ views on improving sickle cell disease management in primary care: focus group discussion. JRSM Short Rep. 2012;3:84. doi: 10.1258/shorts.2012.011153 [DOI] [PMC free article] [PubMed] [Google Scholar]