Abstract
Attachment of Streptococcus gordonii to the acquired pellicle of the tooth surface involves specific interactions between bacterial adhesins and adsorbed salivary components. To study saliva-regulated gene expression in S. gordonii, we used random arbitrarily primed PCR (RAP-PCR). Bacteria were incubated in either brain heart infusion medium or saliva. Total RNA from both conditions was purified and RAP fingerprinted and then PCR amplified with an arbitrary primer. The differentially displayed DNA fragments were cloned, sequenced, and analyzed using the BLAST search network service. Three DNA products were up-regulated. One was identified as that of the sspA and -B genes, which encode the salivary agglutinin glycoprotein-binding proteins SspA and SspB of S. gordonii; another had 79% identity with the Lactococcus lactis clpE gene, encoding a member of the Clp protease family; and the third product showed no significant homology to known genes. Five down-regulated genes were identified which encode proteins involved in bacterial metabolism. We have shown, for the first time, direct induction of sspA and -B in S. gordonii by human saliva.
Streptococcal adhesion to oral surfaces results partially from initial binding of cells to absorbed salivary components, which are tightly bound to the mineral matrix of human enamel and form the salivary pellicle. Bacterial binding to salivary proteins and glycoproteins is relevant to initial binding events for which genes have been identified by transposon mutagenesis (22), as well as to accumulation of bacteria in the complex biofilm of mature dental plaque. The streptococcal salivary adhesins that have been described in the most detail are the LraI family polypeptides and the antigen I/II family of polypeptides (15, 31). Antigen I/II polypeptides bind a mucin-like salivary component named salivary agglutinin glycoprotein (SAG) in a lectin-like interaction (4).
Although much progress has been made in defining the role of saliva in oral microbial ecology, little is known concerning the physiological response of bacteria following binding to a salivary component. The objective of this research was to identify the genes regulated by contact between oral bacteria and salivary molecules of the conditioning film or salivary pellicle on the enamel surface. We chose to study Streptococcus gordonii DL1 because it is genetically transformable, it binds salivary components, and it is an early colonizer of the clean enamel surface.
To study saliva-regulated gene expression in S. gordonii, we used random arbitrarily primed PCR (RAP-PCR), a method adapted from the differential-display (DD) PCR method (29). We identified eight saliva-regulated cDNA fragments, seven of which had sequence identity to known genes. Sequence analysis showed that five down-regulated products encode proteins involved in bacterial metabolism. Of the three up-regulated genes, one has been found to be 95% identical to the SAG-binding cell surface adhesin SspA and SspB (antigen I/II family)-encoding genes of S. gordonii.
S. gordonii DL1 (Challis) was cultured anaerobically (BBL GasPak system; Becton Dickinson Microbiology System, Cockeysville, Md.) at 37°C in brain heart infusion (BHI) medium (Difco Laboratories, Detroit, Mich.). Escherichia coli Epicurian Coli XL10-Gold Ultracompetent Cells (Stratagene, La Jolla, Calif.) were used to clone differentially expressed RAP-PCR products. Transformation and identification of bacterial colonies that contain recombinant plasmids were performed in accordance with standard protocols (27). Fresh stimulated whole saliva samples were collected from six or more healthy persons. The donors were not on medication or ill, nor had they eaten or drunk in the 60 min prior to saliva collection. Saliva secretion was stimulated by Parafilm chewing, and the saliva was collected on ice. Dithiothreitol (2.5 mM final concentration) was added to the collected saliva, and the mixture was stirred for 20 min at 4°C. The saliva was centrifuged at 5,000 × g, and the supernatant fluid was filtered through a 0.22-μm-pore-size polyethersulfone filter. Resulting sterile saliva samples were kept frozen at −20°C until used. An overnight culture of S. gordonii was diluted 1:11 in fresh BHI medium or 1:11 in dilute saliva (whole saliva at 1:4 in sterile water). Bacteria were cultured for 2 h anaerobically (GasPak) at 37°C. After 2 h in either BHI medium or saliva, bacteria were harvested by centrifugation (2,500 × g, 10 min, 4°C). The pellet was washed with diethylpyrocarbonate-treated water, resuspended in Ultraspec RNA reagent solution (Biotecx Laboratories Inc., Houston, Tex.), and transferred to a Multimix Tube containing a commercially prepared mixture of 0.1-, 1.4-, and 4-mm-diameter silica-ceramic beads (Bio 101, Inc., Vista, Calif.). The tube was shaken in a FastPrep FP120 bead beater (Bio 101) at top speed for 45 s and placed on ice, and the lysate was clarified by centrifugation (2,500 × g, 2 min, 4°C). RNA was isolated from the supernatant by the procedure of Lunsford (24). Subsequently, the RNA samples were treated with DNase I for 15 min at 37°C using RQ1 DNase (Promega, Madison, Wis.). The integrity of the RNA was assessed by electrophoresis in accordance with standard protocols (27). Each reverse transcription reaction was performed as recommended by the manufacturer (Stratagene) with 2 μg of S. gordonii DL1 total RNA and the chosen arbitrary primer, A3 (5′-AATCTAGAGCTCTCCTGG-3′). Reactions for each condition were done in triplicate. As a negative control to identify products amplified in subsequent steps as a result of residual genomic DNA contamination, an identical reaction mixture without Moloney murine leukemia virus reverse transcriptase was done. The second DNA strand was synthesized and PCR amplified in the presence of [α-33P]dATP (DuPont NEN, Boston, Mass.) in a thermocycler (Perkin-Elmer Cetus, Norwalk, Conn.) as follows: 1 low-stringency cycle (94°C for 1 min, 36°C for 5 min, and 72°C for 5 min), 40 high-stringency cycles (94°C for 1 min, 50°C for 2 min, and 72°C for 2 min), and an elongation step (72°C for 10 min). Following PCR amplification, the reaction mixture was combined with stop buffer containing formamide, xylene cyanol, and bromophenol blue (USB Corp., Cleveland, Ohio) and heated at 80°C for 2 min. Each reaction was electrophoresed in adjacent lanes of a CastAway precast 4.5% acrylamide–7 M urea sequencing gel (Stratagene). The gel was dried and radioautographed using Kodak X-Omat AR film (Eastman Kodak Co., Rochester, N.Y.). The autoradiogram was aligned with the gel; bands of interest were cut from the gel and placed into a centrifuge tube. The DNA was eluted in 1× TE buffer (Digene, Beltsville, Md.) for 1 h at 60°C and then incubated overnight at room temperature. The sample was centrifuged, and 2 μl of the eluate was amplified using primer A3 and the 40-cycle high-stringency RAP-PCR program. No radioactive deoxynucleoside triphosphates were included. The products were analyzed on a 2% (wt/vol) agarose gel. The RAP-PCR products were extracted from the agarose gel with a QIAquick gel extraction kit (Qiagen, Santa Clarita, Calif.) and purified using a Bio 101 Geneclean Spin kit. The RAP-PCR products were blunt ended by mixing with Pfu DNA polymerase and deoxynucleoside triphosphates (Stratagene). The blunt-ended DNA products were cloned in cloning vector pPCR-Script Amp SK(+) (Stratagene). Cloned DNAs were sequenced using the Perkin-Elmer Applied Biosystems 377XL automated DNA sequencer. Sequence analysis was performed by the BLAST search algorithm (10). A Northern blot was prepared in accordance with standard protocols (27) following electrophoretic separation of 5 μg of total RNA isolated from each condition (BHI medium or saliva). The blot was probed with the DNA fragment purified from the sequencing gel, reamplified, and α-32P radiolabeled by random primer extension (Lofstrand Labs Limited, Gaithersburg, Md.).
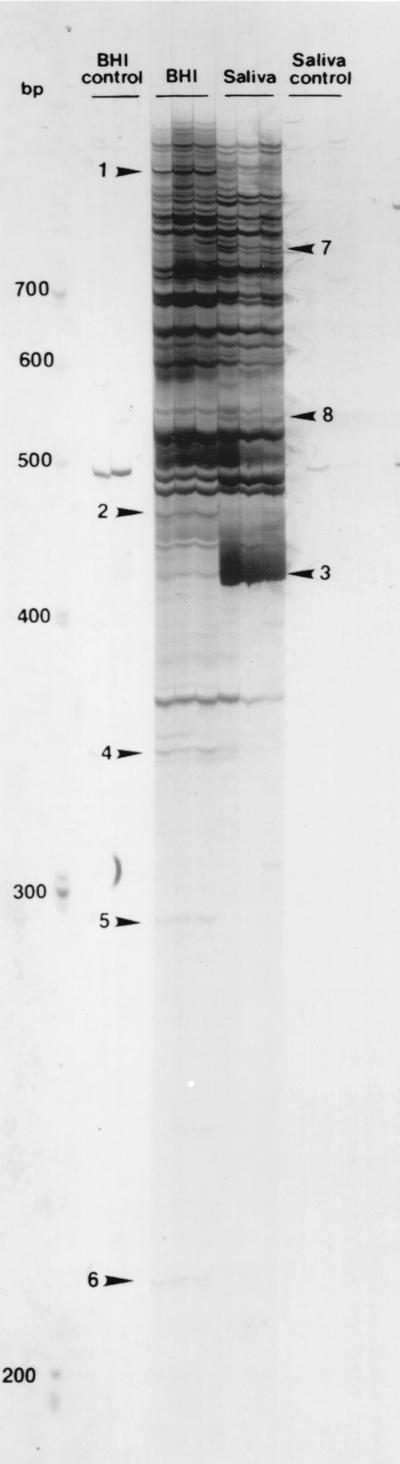
The RNA purification method used gave high-quality RNA with no detectable degradation of rRNA by gel electrophoresis (data not shown). A single primer, A3, was selected from a reverse transcription-tested set of five commercial primers (Stratagene) with RNA from S. gordonii DL1 under the BHI medium condition, although all five primers yielded PCR products of useful size for future differential display experiments (data not shown). The RAP-PCRs were done in triplicate on the same sample. This experiment with primer A3 was repeated twice with different RNA preparations, and similar results were obtained. Total RNA (represented by cDNA) of S. gordonii DL1 incubated with saliva was compared to the BHI medium condition. Eight bands appeared to be differentially expressed (Fig. 1). Five products were down-regulated, and three were up-regulated. Cloned inserts of the RAP-PCR products ranged in size from about 200 to 1,000 bp. The DNA sequence of the eight RAP-PCR products derived from differentially expressed genes was compared to that in the GenBank database by using the BLAST search algorithm to identify similarities to known sequences (Table 1). Sequence analysis showed that the down-regulated products have sequence similarity to proteins likely to be involved in bacterial metabolism in the shift from BHI medium to saliva. For example, dihydrodipicolinate synthase (bands 2 and 4) is involved in the pathway for the biosynthesis of diaminopimelate and lysine (2), glucose kinase (band 5) is involved in glucose metabolism (9), and the PhoH protein (band 6) is normally induced by phosphate starvation (30). Bacteria in saliva are in the presence of less freely metabolizable nutrient than when in BHI medium, which has much low-molecular-weight nutrient. For the up-regulated products, band 3 was particularly overexpressed and was 95% identical to a region common to both sspA and sspB, which encode the SAG-binding cell surface adhesin proteins SspA and SspB (antigen I/II family) of S. gordonii (6). These adhesins recognize multiple ligands, and they mediate a wide range of streptococcal adherence properties, including binding to salivary agglutinin glycoproteins (11, 25, 26), type 1 collagen (23, 28), and other microbial cells, including Actinomyces naeslundii (6, 16), Candida albicans (12), and Porphyromonas gingivalis (1, 21). The band 7 product was 79% identical to the Lactococcus lactis clpE gene that encodes a member of the Clp protease family (13). Band 8 showed no identity to any known gene.
FIG. 1.
DD of S. gordonii DL1 total RNA samples from cells incubated for 2 h in BHI medium or saliva using the arbitrary primer A3. Reactions for each condition were done in triplicate. Arrows with numbers indicate the differentially amplified RAP-PCR products. Each experimental reaction mixture containing RNA and primer was split into two tubes. Moloney murine leukemia virus reverse transcriptase was added to one tube and omitted from the control (BHI and saliva controls). For example, the first BHI control lane is the control for the reaction of the first BHI lane.
TABLE 1.
Genetic identificationa of cloned, differentially expressed RAP-PCR products in S. gordonii DL1 in contact with saliva
| RAP-PCR product (band no.) | Saliva inductionb | Size (bp) | % DNA sequence identity | Accession no. | Reference |
|---|---|---|---|---|---|
| 1 | − | 1,100 | 97 (with S. gordonii oligopeptide-binding lipoprotein gene hppB) | L41358 | 14 |
| 2 | − | 474 | 87 (with Bacillus subtilis dihydrodipicolinate synthase gene) | L08471 | 2 |
| 3 | + | 438 | 95 (with S. gordonii M5 cell surface adhesin genes sspA and sspB) | U40025, U40026 | 6 |
| 4 | − | 347 | 89 (with Bacillus subtilis dihydrodipicolinate synthase gene) | L08471 | 2 |
| 5 | − | 300 | 81 (with S. mitis glucose kinase gene gki) | AJ232323 | 9 |
| 6 | − | 210 | 71 (with Bacillus subtilis PhoH protein)c | U29177 | 30 |
| 7 | + | 850 | 79 (with Lactococcus lactis clpE) | AF023421 | 13 |
| 8 | + | 542 | None with anything known |
Based on DNA homologies with DNA sequences in GenBank.
+, up-regulation; −, down-regulation.
No identity with any known gene at the nucleic acid level.
The up-regulated 438-bp RAP-PCR product corresponding to sspA and -B (Fig. 1, band 3; Table 1) was reamplified and used as a probe for the Northern blot (Fig. 2). The mRNA corresponding to sspA and -B (approximately 4.5 kb) was present in RNA purified from cells in contact with saliva (Fig. 2, lane 2) but was not detectable in RNA purified from cells suspended in BHI medium (Fig. 2, lane 1). The 4.5-kb transcript has the predicted size corresponding to the translation of the sspA and -B product of approximately 1,500 amino acid (aa) residues (6).
FIG. 2.
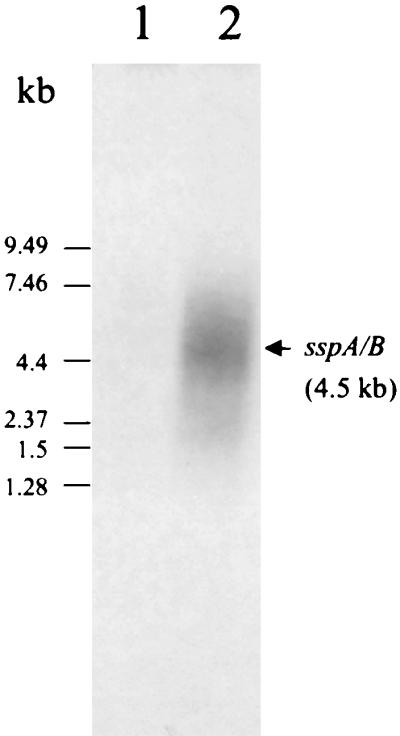
Northern blot confirming up-regulation of the sspA and -B genes in the presence of saliva. The hybridization probe was the α-32P-radiolabeled 438-bp product identified by DD (band 3) having 95% identity to the sspA and -B genes. It hybridized with a 4.5-kb mRNA transcript under the saliva condition (lane 2). Lanes: 1, total RNA from the BHI medium condition; 2, total RNA from the saliva condition.
S. gordonii is the only oral streptococcal species so far identified that expresses two antigen I/II proteins. Mature SspA (1,542 aa residues) and SspB (1,462 aa residues) are the products of tandemly arranged chromosomal genes that are independently transcribed (6). In previous studies, SspA and -B were detected in BHI medium-grown cells, suggesting that sspA and sspB are constitutively expressed (5, 8, 16). In our experiments with BHI medium, the RAP-PCR product corresponding to sspA and -B was weak even after 40 cycles of PCR amplification (Fig. 1, band 3). In addition, the Northern blot did not show any detectable level of sspA or -B mRNA from BHI medium-grown cells, suggesting that the level of expression is low under these conditions.
The present study is the first to show up-regulation of sspA and -B transcription in the presence of saliva. The 438-bp RAP-PCR up-regulated product detected in DL1 corresponds to a region with a sequence present in both sspA and sspB in S. gordonii M5. Structural and transcriptional start site differences between the promoters of sspA and sspB in S. gordonii M5 indicate that sspA is regulated differently from sspB in S. gordonii M5 (7). It would be interesting to study the differential expression of sspA and sspB in S. gordonii DL1, but only a 2,347-bp region containing the 3′ end of sspA, the intergenic region, and the 5′ end of sspB has been sequenced in this strain (accession no. U40027) (6). The 438-bp RAP-PCR product is not homologous to this region. Sequencing of these two genes from S. gordonii DL1 is necessary to further analyze potential differential regulation of sspA and sspB in this streptococcus.
Survival of streptococci in the oral cavity may be dependent on their ability to adhere tightly to host tissue surfaces and to evade the host defenses in this open-flow system. We show that the transcription of the SAG-binding adhesin sspA and -B genes is directly induced when S. gordonii DL1 is placed in contact with saliva; this behavior may influence binding and colonization of the tooth surface. Early colonization can occur not only by direct binding of S. gordonii to saliva-coated surfaces (3, 19) but also by coadhesion of S. gordonii and other saliva-coated cells (3, 17), as well as by coaggregation of S. gordonii and other saliva-coated partners, including streptococci (18) and actinomyces (20). Our findings support the consideration of S. gordonii as a primary colonizer and as an anchor during biofilm development of dental plaque.
Acknowledgments
This research was supported by the Conseil Regional de Bretagne (France).
We thank M. Gilmore for extensive advice on DD. Thanks also to D. Demuth, H. Jenkinson, and D. Wall for helpful suggestions and R. Andersen for technical assistance.
REFERENCES
- 1.Brooks W, Demuth D R, Gil S, Lamont R J. Identification of a Streptococcus gordonii SspB domain that mediates adhesion to Porphyromonas gingivalis. Infect Immun. 1997;65:3753–3758. doi: 10.1128/iai.65.9.3753-3758.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen N Y, Jiang S Q, Klein D A, Paulus H. Organization and nucleotide sequence of the Bacillus subtilis diaminopimelate operon, a cluster of genes encoding the first three enzymes of diaminopimelate synthesis and dipicolinate synthase. J Biol Chem. 1993;268:9448–9465. [PubMed] [Google Scholar]
- 3.Ciardi J E, McCray G F A, Kolenbrander P E, Lau A. Cell-to-cell interaction of Streptococcus sanguis and Propionibacterium acnes on saliva-coated hydroxyapatite. Infect Immun. 1987;55:1441–1446. doi: 10.1128/iai.55.6.1441-1446.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Demuth D R, Berthold P, Leboy P S, Golub E E, Davis C A, Malamud D. Saliva-mediated aggregation of Enterococcus faecalis transformed with a Streptococcus sanguis gene encoding the SSP-5 surface antigen. Infect Immun. 1989;57:1470–1475. doi: 10.1128/iai.57.5.1470-1475.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Demuth D R, Davis C A, Corner A M, Lamont R J, Leboy P S, Malamud D. Cloning and expression of a Streptococcus sanguis surface antigen that interacts with a human salivary agglutinin. Infect Immun. 1988;56:2484–2490. doi: 10.1128/iai.56.9.2484-2490.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Demuth D R, Duan Y, Brooks W, Holmes A R, McNab R, Jenkinson H F. Tandem genes encode cell-surface polypeptides SspA and SspB which mediate adhesion of the oral bacterium Streptococcus gordonii to human and bacterial receptors. Mol Microbiol. 1996;20:403–413. doi: 10.1111/j.1365-2958.1996.tb02627.x. [DOI] [PubMed] [Google Scholar]
- 7.Demuth D R, Duan Y, Jenkinson H F, McNab R, Gil S, Lamont R J. Interruption of the Streptococcus gordonii M5 sspA/sspB intergenic region by an insertion sequence related to IS1167 of Streptococcus pneumoniae. Microbiology. 1997;143:2047–2055. doi: 10.1099/00221287-143-6-2047. [DOI] [PubMed] [Google Scholar]
- 8.Demuth D R, Lammey M S, Huck M, Lally E T, Malamud D. Comparison of Streptococcus mutans and Streptococcus sanguis receptors for human salivary agglutinin. Microb Pathog. 1990;9:199–211. doi: 10.1016/0882-4010(90)90022-i. [DOI] [PubMed] [Google Scholar]
- 9.Enright M C, Spratt B G. A multilocus sequence typing scheme for Streptococcus pneumoniae: identification of clones associated with serious invasive disease. Microbiology. 1998;144:3049–3060. doi: 10.1099/00221287-144-11-3049. [DOI] [PubMed] [Google Scholar]
- 10.Gish W, States D J. Identification of protein coding regions by database similarity search. Nat Genet. 1993;3:266–272. doi: 10.1038/ng0393-266. [DOI] [PubMed] [Google Scholar]
- 11.Hajishengallis G, Koga T, Russell M W. Affinity and specificity of the interactions between Streptococcus mutans antigen I/II and salivary components. J Dent Res. 1994;73:1493–1502. doi: 10.1177/00220345940730090301. [DOI] [PubMed] [Google Scholar]
- 12.Holmes A R, McNab R, Jenkinson H F. Candida albicans binding to the oral bacterium Streptococcus gordonii involves multiple adhesin-receptor interactions. Infect Immun. 1996;64:4680–4685. doi: 10.1128/iai.64.11.4680-4685.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ingmer H, Vogensen F K, Hammer K, Kilstrup M. Disruption and analysis of the clpB, clpC, and clpE genes in Lactococcus lactis: ClpE, a new Clp family in gram-positive bacteria. J Bacteriol. 1999;181:2075–2083. doi: 10.1128/jb.181.7.2075-2083.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jenkinson H F, Baker R A, Tannock G W. A binding-lipoprotein-dependent oligopeptide transport system in Streptococcus gordonii essential for uptake of hexa- and heptapeptides. J Bacteriol. 1996;178:68–77. doi: 10.1128/jb.178.1.68-77.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jenkinson H F, Demuth D R. Structure, function and immunogenicity of streptococcal antigen I/II polypeptides. Mol Microbiol. 1997;23:183–190. doi: 10.1046/j.1365-2958.1997.2021577.x. [DOI] [PubMed] [Google Scholar]
- 16.Jenkinson H F, Terry S D, McNab R, Tannock G W. Inactivation of the gene encoding surface protein SspA in Streptococcus gordonii DL1 affects cell interactions with human salivary agglutinin and oral actinomyces. Infect Immun. 1993;61:3199–3208. doi: 10.1128/iai.61.8.3199-3208.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kolenbrander, P. E., R. N. Andersen, K. M. Kazmerzak, and R. J. Palmer, Jr. Coaggregation and coadhesion in oral biofilms. In D. Allison, P. Gilbert, H. Lappin-Scott, and M. Wilson (ed.), Community structure and co-operation in biofilms, in press. Cambridge University Press, Cambridge, United Kingdom.
- 18.Kolenbrander P E, Andersen R N, Moore L V H. Intrageneric coaggregation among strains of human oral bacteria: potential role in primary colonization of the tooth surface. Appl Environ Microbiol. 1990;56:3890–3894. doi: 10.1128/aem.56.12.3890-3894.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kolenbrander, P. E., L. Dû, M. Aspiras, S. Li, K. Kazmerzak, R. Wu, and R. Andersen. Spatial organization and contact-induced gene expression in biofilms of Streptococcus gordonii Challis. In D. Martin and J. Tagg (ed.), Streptococci and streptococcal diseases—entering the new millennium, in press.
- 20.Kolenbrander P E, Phucas C S. Effect of saliva on coaggregation of oral Actinomyces and Streptococcus species. Infect Immun. 1984;44:228–233. doi: 10.1128/iai.44.2.228-233.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lamont R J, Gil S, Demuth D R, Malamud D, Rosan B. Molecules of Streptococcus gordonii that bind to Porphyromonas gingivalis. Microbiology. 1994;140:867–872. doi: 10.1099/00221287-140-4-867. [DOI] [PubMed] [Google Scholar]
- 22.Loo C Y, Corliss D A, Ganeshkumar N. Streptococcus gordonii biofilm formation: identification of genes that code for biofilm phenotypes. J Bacteriol. 2000;182:1374–1382. doi: 10.1128/jb.182.5.1374-1382.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Love R M, McMillan M D, Jenkinson H F. Invasion of dentinal tubules by oral streptococci is associated with collagen recognition mediated by the antigen I/II family of polypeptides. Infect Immun. 1997;65:5157–5164. doi: 10.1128/iai.65.12.5157-5164.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lunsford R D. Recovery of RNA from oral streptococci. BioTechniques. 1995;18:412–413. [PubMed] [Google Scholar]
- 25.Moisset A, Schatz N, Lepoivre Y, Amadio S, Wachsmann D, Schöller M, Klein J-P. Conservation of salivary glycoprotein-interacting and human immunoglobulin G-cross-reactive domains of antigen I/II in oral streptococci. Infect Immun. 1994;62:184–193. doi: 10.1128/iai.62.1.184-193.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakai M, Okahashi N, Ohta H, Koga T. Saliva-binding region of Streptococcus mutans surface protein antigen. Infect Immun. 1993;61:4344–4349. doi: 10.1128/iai.61.10.4344-4349.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 28.Sciotti M A, Yamodo I, Klein J P, Ogier J A. The N-terminal half part of the oral streptococcal antigen I/IIf contains two distinct binding domains. FEMS Microbiol Lett. 1997;153:439–445. doi: 10.1111/j.1574-6968.1997.tb12608.x. [DOI] [PubMed] [Google Scholar]
- 29.Shepard B D, Gilmore M S. Identification of aerobically and anaerobically induced genes in Enterococcus faecalis by random arbitrarily primed PCR. Appl Environ Microbiol. 1999;65:1470–1476. doi: 10.1128/aem.65.4.1470-1476.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Song B H, Neuhard J. Chromosomal location, cloning and nucleotide sequence of the Bacillus subtilis cdd gene encoding cytidine/deoxycytidine deaminase. Mol Gen Genet. 1989;216:462–468. doi: 10.1007/BF00334391. [DOI] [PubMed] [Google Scholar]
- 31.Whittaker C J, Klier C M, Kolenbrander P E. Mechanisms of adhesion by oral bacteria. Annu Rev Microbiol. 1996;50:513–552. doi: 10.1146/annurev.micro.50.1.513. [DOI] [PubMed] [Google Scholar]