Abstract
Besides nasopharyngeal swabs, monkeypox virus (MPXV) DNA has been detected in a variety of samples such as saliva, semen, urine and fecal samples. Using the environmental surveillance network previously developed in Spain for the routine wastewater surveillance of SARS-CoV-2 (VATar COVID-19), we have analyzed the presence of MPXV DNA in wastewater from different areas of Spain. Samples (n = 312) from 24 different wastewater treatment plants were obtained between May 9 (week 19 of 2022) and August 4 (week 31 of 2022). Following concentration of viral particles by a validated aluminum adsorption-precipitation method, a qPCR procedure allowed us to detect MPXV DNA in 56 wastewater samples collected from May 16 to August 4, 2022, with values ranging between 2.2 × 103 to 8.7 × 104 genome copies (gc)/L. This study shows that MPXV DNA can be reproducibly detected by qPCR in longitudinal samples collected from different Spanish wastewater treatment plants. According to data from the National Epidemiological Surveillance Network (RENAVE) in Spain a total of 6,119 cases have been confirmed as of August 19, 2022. However, and based on the wastewater data, the reported clinical cases seem to be underestimated and asymptomatic infections may be more frequent than expected.
Keywords: Monkeypox, Wastewater, Epidemiology, WBE
Graphical abstract
1. Introduction
In early May 2022, a multi-country outbreak of Monkeypox virus (MPXV) started in non-endemic regions, and on 23 July WHO declared a Public Health emergency of international concern (WHO, 2022). In Europe, a total of 13,911 cases of MPX have been reported up to 19 August 2022, with Spain accounting for 6,119 cases, the second highest number of Monkeypox (MPX) cases worldwide, being present in most regions of the country (Spanish Ministry of Health, 2022).
Symptoms developed include the appearance of rash, fever, fatigue, muscle pain, vomiting, diarrhea, chills, sore throat or headache, and the hospitalization rate is around 8–13% (European Centre for Disease Prevention and Control (ECDC), 2022; Thornhill et al., 2022). Sadly, two deaths linked to this outbreak have occurred in Spain due to complications associated with encephalitis (Aguilera-Alonso et al., 2022). It is assumed that transmission occurs after close contact with skin lesions of an infected person, as well as through contact with respiratory droplets and fomites, and that infection is symptomatic in all patients (McCollum and Damon, 2014). However, antibodies have been found in exposed asymptomatic individuals, which can be linked to subclinical infections (Wilson et al., 2014), and positive MPXV PCR results from anal samples in asymptomatic men who have sex with men (MSM) have also been documented (Baetselier et al., 2022; Ferré et al., 2022). The virus is also excreted in fluids, and its detection in saliva, semen, urine and feces has been reported (Peiró-Mestres et al., 2022, Hernaez et al., 2023). This implies that routine wastewater surveillance can be applied as a tool for early detection of the disease expansion as very recently reported following a model-based theoretical evaluation (Chen and Bibby, 2022). According with this model, wastewater-based epidemiology (WBE) can detect on average 7 MPX cases out of 100,000 people. Currently, various studies detected MPXV DNA in wastewater worldwide (de Jonge et al., 2022; la Rosa et al., 2023; Wolfe et al., 2022), highlighting again wastewater analysis as a non-invasive tool for monitoring the status and trend of an emerging infection. The aim of the present study was to trace the community circulation of the MPXV from potentially symptomatic, asymptomatic, or presymptomatic individuals using the previous established Spanish National SARS-CoV-2 Wastewater Surveillance Network (VATar COVID-19).
2. Material and methods
2.1. Sample concentration and DNA extraction
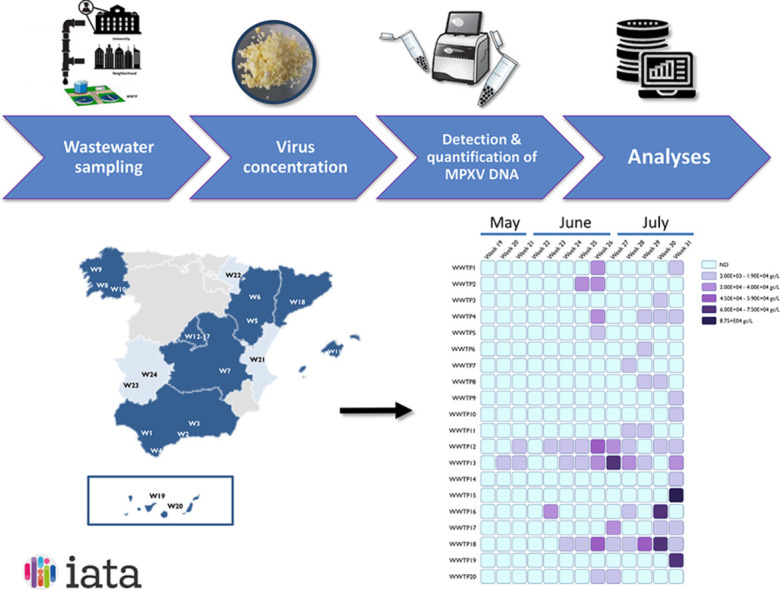
Grab sewage samples were weekly collected from 24 Spanish wastewater treatment plants (WWTPs) (Fig. 1 C) between May 9 (week 19 of 2022) and August 4 (week 31 of 2022) and kept at 4 °C until analysis. Concentration of viral fraction was performed by a previously validated method for SARS-CoV-2 using an aluminum-based adsorption precipitation procedure (Pérez-Cataluña et al., 2021). In order to evaluate the analytical performance of this concentration method, 200 ml of grab sewage samples (n = 4) that previously tested negative for MPXV were inoculated with 107 PFU of inactivated MPXV. MPXV suspension was obtained by infecting a clinical MPXV specimen obtained from a patient pustule in BSC-1 cells. MPXV stock was inactivated in the BSL-3 laboratory (Molecular Biology Center Severo Ochoa, CBM, Madrid) by limited cross-linking with a combination of psoralen (4–9-aminomethyl-Trioxsalen; Sigma) and long-wave UV light, as previously described for other poxviruses (Tsung et al., 1996). Briefly, virus stock was incubated with 2 µg/ml psoralen for 10 min at room-temperature followed by 10 min irradiation with long-wave ultra-violet light (365 nm). Virus inactivation was further confirmed by two consecutive plaque assays in BSC-1 cells to discard virus-induced effects.
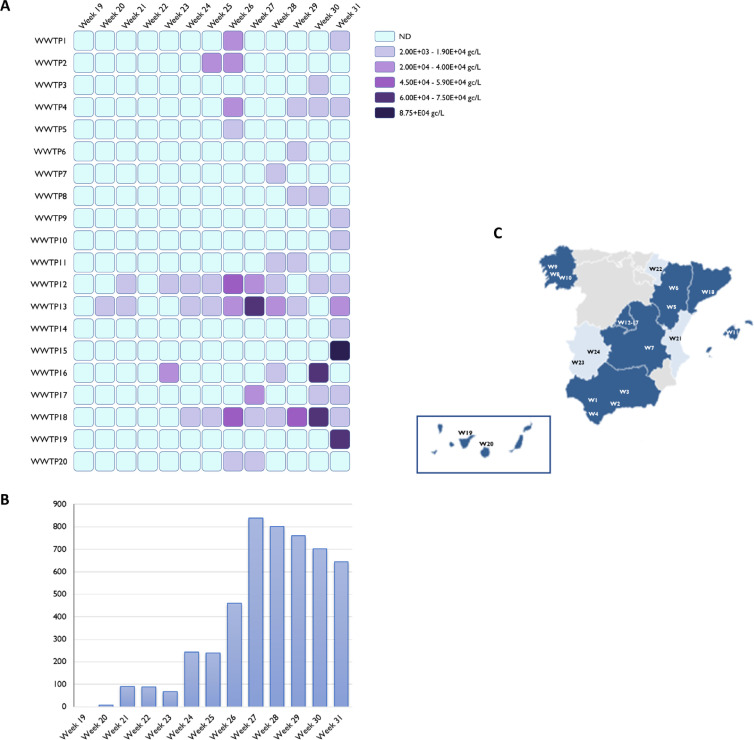
Fig. 1.
(A) Evolution of MPXV DNA prevalence over time, as measured by qPCR in wastewater samples from 20 wastewater treatment plants with positive detection (B) Number of cases of monkeypox per week (Spanish Ministry of Health) (C) Geographical localization of wastewater treatment plants, dark blue (Autonomous Community with positive detection in the analyzed wastewater samples), light blue (no detection in wastewater samples) and gray (regions not covered in the study). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Nucleic acids extraction (100 µl) of the concentrated samples (300 µl) was performed by the Maxwell® RSC Instrument (Promega) using the Maxwell RSC Pure Food GMO and authentication kit (Promega) and the “Maxwell RSC Viral total Nucleic Acid” program.
2.2. MPXV real-time PCR assays
The MPXV West Africa (G2R_WA) assay (Li et al., 2010) was applied to quantify MPXV DNA using the qPCR Premix Ex Taq™ kit (Takara Bio Inc). Additionally, a subset of samples (Table S1) was tested for MPXV DNA using the MPXV generic (G2R_G) assay (Li et al., 2010). Both assays targeted the TNF receptor gene. Undiluted and ten-fold diluted DNA (2.5 µl) was tested in duplicate. Positive control consisted in the nucleic acid material extracted from a cell culture infected with a clinical MPXV specimen obtained from a patient pustule. For each qPCR, serial dilutions of standard curves were run in quintuplicates and the numbers of estimated genome copies were calculated (Table S2). Each run included negative controls (nuclease-free water and negative extraction controls). Depending on the laboratory, reactions were carried out in the QuantStudio™ 3 and QuantStudio™ 5 Real-Time PCR Systems (ThermoFisher Sci.). For each specific target, Cq values ≤ 40 were converted into genome copies (gc) per liter using the corresponding standard curve and volumes tested. Occurrence of inhibition was estimated by comparing average viral titers obtained from duplicate wells tested on undiluted DNA with duplicate wells tested on 10-fold diluted DNA. Inhibition was ascertained when difference in average viral titers was higher than 0.5 log10, and if that occurred, viral titers were inferred from the 10-fold DNA dilution.
2.3. Clinical epidemiological data
The number of declared active cases per week for the different Autonomous communities was obtained from the Spanish Ministry of Health (Spanish Ministry of Health, 2022).
3. Results
3.1. Estimated levels of Monkeypox virus DNA in wastewater samples
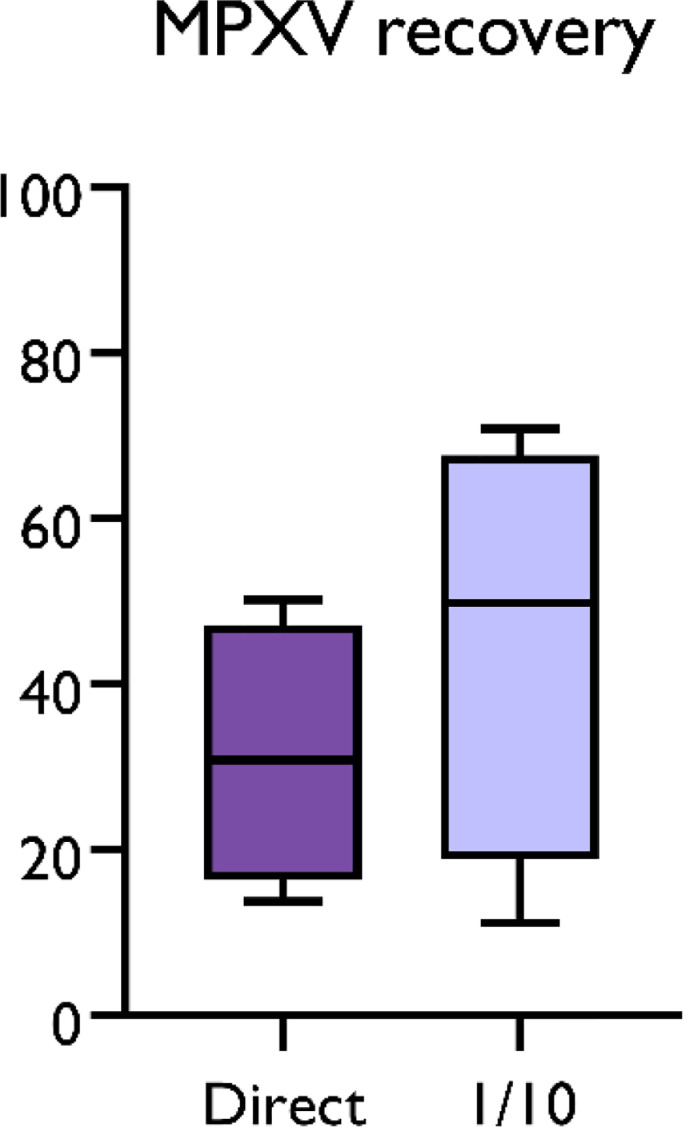
Here, we report the first detection of MPXV DNA in wastewater samples from different regions of Spain. 56 out of 312 samples showed positive results for MPXV DNA, corresponding to samples collected from week 20 to week 31 of 2022. Cycle threshold values ranged between 39.98 and 34.5, corresponding to values from 2.2 × 103 to 8.7 × 104 estimated gc per liter (Fig. 1A). The aluminum-based adsorption-precipitation method was tested by spiking negative wastewater samples with inactivated MPXV suspension. On average, MPXV was recovered at ranges of 31.5 ± 15.9% and 45.5 ± 25.7% in the undiluted and ten-fold diluted samples, respectively, thus validating the results (Fig. 2 ).
Fig. 2.
Monkeypox virus recovery (%) in wastewater samples using the aluminum-based adsorption-precipitation method. MPXV detection was performed using the West Africa (G2R_WA) assay (Li et al., 2010) of undiluted and ten-fold diluted DNA.
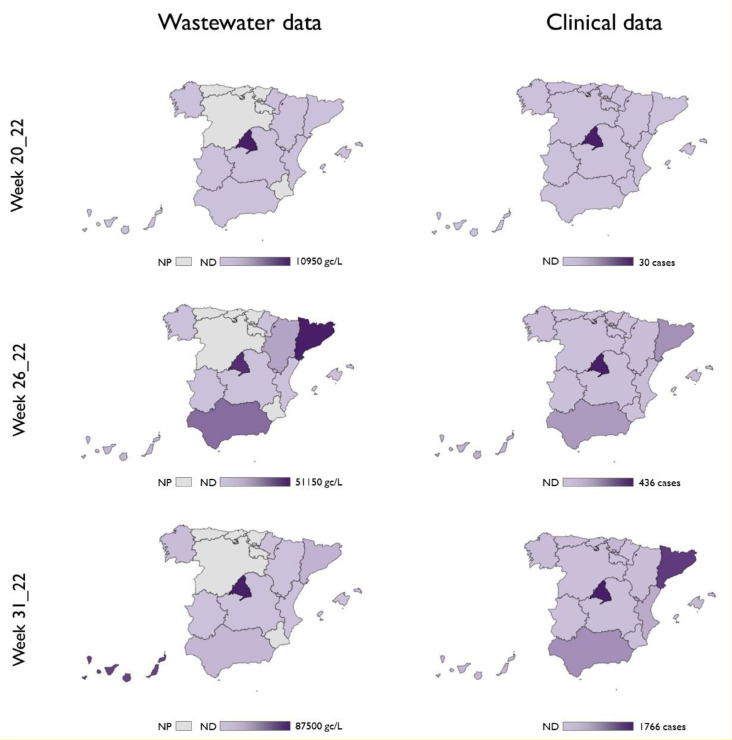
First detection of MPXV DNA in wastewater samples occurred in WWTP13 from the city of Madrid in week 20 of 2022 (Figs. 1A and 3 ), with positive detection using two different assays (Table S1). On that week, Madrid reported the first suspected cases of MPX which represented the first cases of MPX in Spain accounting for one of the largest outbreaks reported outside Africa (Martínez et al., 2022). Later on, several cases were reported in Madrid before the outbreak declaration on 17 May, most of them attending the same sauna in the city of Madrid or with travel history to Maspalomas Gay Pride festival that took place on 5–15 May in Gran Canaria.
Fig. 3.
Comparison of MPXV DNA estimates from wastewater testing (left panels) and confirmed cases of monkeypox by Autonomous Community reported by the Health Ministry authorities (right panels). For wastewater samples, highest level within the same Autonomous Community are depicted. ND: No MPXV detection (light purple); NP: regions without WWTP analyzed in the study (gray). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
In week 21 of 2022, MPXV DNA was detected in the nearest WWTPs of Madrid city (WWTP12 and WWTP13) and in WWTP20 of Gran Canaria (Canary Islands) (Fig. 1, Table S1). Interestingly, we consistently detected MPXV DNA in samples collected from week 23 of 2022 in WWTP12 and WWTP13 from Madrid, when only 275 cumulative cases were declared in the entire region (Martínez et al., 2022). Our data also showed percentages of WWTPs with MPXV DNA detection increased progressively, up to 15% by week 24 of 2022 and 40% by week 26 of 2022 (Figs. 1A and 3). In Barcelona, the second largest Spanish city, first detection occurred in week 24 of 2022 in WWTP18 with the first peak observed in week 26 of 2022 when 130 cumulative cases were detected in Catalonia (Fig. 3, Table S3). Intermittent detection (negative results after previous qPCR detection) was reported from some WWTPs where the number of confirmed clinical cases was low (Figs. 1 and 3). The regions of Murcia, Asturias, Cantabria, Basque country and Castilla Leon (Fig. 1C) were under reported as none WWTP were analyzed in this study. Furthermore, all weekly samples collected from Valencia (WWTP21), Extremadura (WWTP23 and WWTP24), and Navarra (WWTP22) regions tested negative for the presence of MPXV DNA (Fig. 3), with a total number of clinical cases of 331, 21 and 13 as August 9, respectively (Spanish Ministry of Health, 2022).
4. Discussion
The COVID-19 pandemic has demonstrated that WBE is a cost-effective tool to anticipate the circulation of SARS-CoV-2 in a community and to closely track its incidence, evolution and geographic spread (Bivins et al., 2020). WBE has been implemented worldwide and most of the countries are ready to perform this monitoring as a routing basis for other emerging pathogens likely to be found in wastewater, due to their presence in feces and/or urine. In Spain, the National WBE Network, VATar COVID-19, has been successfully used to determine the extent of the COVID-19 disease along the country (Carcereny et al., 2021).
The increasing number of MPXV cases around the world continue to pose challenges to control its transmission with a total number of 41,358 cases as of 19 Aug 2022 (CDC, 2022). This underscores the urgent needs for simple and cost-effective tools to facilitate early detection, evolution and spatial distribution of cases. DNA of MPXV has been detected in urine and feces from symptomatic individuals (Antinori et al., 2022; Peiró-Mestres et al., 2022), and although limited data are available, viral shedding has been observed in stool in 63% of patients (Cts values from 17.8 to 31.4) and in urine in 56% (Cts values from 19.1 to 40.0) (Peiró-Mestres et al., 2022). It is not known whether MPXV present in stool and urine is infectious. Altogether, these findings warned the interest of assessing the presence of MPXV DNA in sewage samples (Chen and Bibby, 2022). In the current study, a qPCR assay designed for the West African clade (Li et al., 2010) was applied on wastewater samples collected from week 19 to week 31 of 2022, showing that MPXV DNA can be reproducibly detected by qPCR in longitudinal samples collected from several Spanish WWTPs. First detection of MPXV DNA was retrieved in a single sample from WWTP13 collected on May 17 (Week 20 of 2022) using the specific qPCR assay and confirmed by the MPXV generic assay, providing the earliest piece of evidence that the virus was circulating in the community of Madrid. Interestingly, we consistently detected MPXV DNA in samples collected in WWTP18 (Barcelona) since week 23 of 2022, when only 39 cumulative cases were declared in the entire Autonomous Community of Catalonia. In line, MPXV DNA was also detected in week 21 of 2022 on wastewater samples collected from Schiphol Airport and in different Dutch WWTPs from week 22 of 2022 onwards (de Jonge et al., 2022).
The viral concentration method used in this study has been validated for SARS-CoV-2 detection and quantification (Pérez-Cataluña et al., 2021) and it seems promising for MPXV monitoring in wastewater, too. However, in contrast to what has been reported for SARS-CoV-2 (Bivins et al., 2020; Medema et al., 2020; Randazzo et al., 2020), anticipation of clinical cases has not been observed for MPXV, for which the first wastewater detection occurred at the same time that MPX cases were declared (Fig. 3). This could be due to several factors, including differences in shedding levels and kinetics, proportion of asymptomatic cases, diagnosis of the disease and fast identification of cases, environmental factors affecting virus stability, a much larger scale of transmission of SARS-CoV-2 in the community, and low performance of the method to concentrate MPXV. This latter was further rule out as the performance characteristics of the methodology was carried out for MPXV (Fig. 2) showing similar mean recoveries compared with SARS-CoV-2 and its surrogates (Pérez-Cataluña et al., 2021). Moreover, it is important to highlight that wastewater positive samples have been found in areas with very low reported disease prevalence. For instance, in Castilla la Mancha, a region located at the middle-south of Spain, MPXV was detected in sewage with only 42 clinical cases being reported, indicating that probably, a higher number of people may be affected. As previously discussed by other authors, stigma and discrimination may be limiting the awareness or willingness of at-risk people to have their symptoms evaluated. In these situations, WBE may be even more useful, because the anonymous pooled samples can evidence the contributions of a community without divulging individual identities (Nelson, 2022).
5. Conclusions
Using an environmental surveillance tool previously developed for SARS-CoV-2, we have been able to detect MPXV DNA in wastewater samples from different regions of Spain when communicated clinical cases in that region were only incipient. We also found that the wastewater viral DNA detection increased rapidly and anticipated the subsequent ascent in the number of declared cases showing, once again, that WBE is a sensitive and cost-effective strategy for the surveillance of emerging viral threats. In those cases where stigma and blame might undermine the capacity to effectively respond during outbreaks, i.e., driving people away from health services, the implementation of WBE may represent a most valuable tool.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This research was supported by the European Commission NextGenerationEU fund, through CSIC's Global Health Platform (PTI Salud Global), project CEX2021-001189-S MCIN/AEI / 10.13039/501100011033 and Fundación Séneca (Region of Murcia). Samples were obtained from the COVID-19 wastewater surveillance project (VATar COVID-19) funded by the Spanish Ministry for the Ecological Transition and the Demographic Challenge and the Spanish Ministry of Health. IGG is recipient of a predoctoral contract from the Generalitat Valenciana (ACIF/2021/181) and AP-C was supported by a postdoctoral fellowship (APOSTD/2021/292). PT is holding a Ramón y Cajal contract from the Ministerio de Ciencia e Innovación and AC is recipient of a predoctoral contract FI-SDUR from the Generalitat de Catalunya.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.watres.2023.119621.
Appendix. Supplementary materials
Data availability
Data will be made available on request.
References
- Aguilera-Alonso D., Alonso-Cadenas J.A., Roguera-Sopena M., Lorusso N., Miguel L.G.S., Calvo C. Monkeypox virus infections in children in Spain during the first months of the 2022 outbreak. Lancet Child Adolesc. Health. 2022 doi: 10.1016/S2352-4642(22)00250-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antinori A., Mazzotta V., Vita S., Carletti F., Tacconi D., Lapini L.E., D'Abramo A., Cicalini S., Lapa D., Pittalis S., Puro V., Rivano Capparuccia M., Giombini E., Gruber C.E.M., Garbuglia A.R., Marani A., Vairo F., Girardi E., Vaia F., Nicastri E. Epidemiological, clinical and virological characteristics of four cases of Monkeypox support transmission through sexual contact, Italy, May 2022. Eurosurveillance. 2022;27 doi: 10.2807/1560-7917.ES.2022.27.22.2200421/CITE/PLAINTEXT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baetselier, I.de, Dijck, C.van, Kenyon, C., Coppens, J., van den Bossche, D., Smet, H., Liesenborghs, L., Vanroye, F., Block, T.de, Rezende, A., Florence, E., Vercauteren, K., Esbroeck, M.van, group, the M. study, 2022. Retrospective detection of asymptomatic monkeypox virus infections among male sexual health clinic attendees in Belgium. Nature Medicine 28, 2288–2292 10.1038/s41591-022-02004-w. [DOI] [PMC free article] [PubMed]
- Bivins A., North D., Ahmad A., Ahmed W., Alm E., Been F., Bhattacharya P., Bijlsma L., Boehm A.B., Brown J., Buttiglieri G., Calabro V., Carducci A., Castiglioni S., Cetecioglu Gurol Z., Chakraborty S., Costa F., Curcio S., de Los Reyes F.L., Delgado Vela J., Farkas K., Fernandez-Casi X., Gerba C., Gerrity D., Girones R., Gonzalez R., Haramoto E., Harris A., Holden P.A., Islam M.T., Jones D.L., Kasprzyk-Hordern B., Kitajima M., Kotlarz N., Kumar M., Kuroda K., la Rosa G., Malpei F., Mautus M., McLellan S.L., Medema G., Meschke J.S., Mueller J., Newton R.J., Nilsson D., Noble R.T., van Nuijs A., Peccia J., Perkins T.A., Pickering A.J., Rose J., Sanchez G., Smith A., Stadler L., Stauber C., Thomas K., van der Voorn T., Wigginton K., Zhu K., Bibby K. Wastewater-based epidemiology: global collaborative to maximize contributions in the fight against COVID-19. Environ. Sci. Technol. 2020;54:754–7757. doi: 10.1021/acs.est.0c02388. [DOI] [PubMed] [Google Scholar]
- Carcereny A., Martínez-Velázquez A., Bosch A., Allende A., Truchado P., Cascales J., Romalde J.L., Lois M., Polo D., Sánchez G., Pérez-Cataluña A., Díaz-Reolid A., Antón A., Gregori J., Garcia-Cehic D., Quer J., Palau M., Ruano C.G., Pintó R.M., Guix S. Monitoring Emergence of the SARS-CoV-2 B1.1.7 variant through the Spanish national SARS-CoV-2 wastewater surveillance system (VATar COVID-19) Environ. Sci. Technol. 2021;55:11756–11766. doi: 10.1021/ACS.EST.1C03589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC, 2022. 2022 Monkeypox Outbreak Global Map | Monkeypox | Poxvirus | CDC [W.W.W. Document]. URL https://www.cdc.gov/poxvirus/monkeypox/response/2022/world-map.html (accessed 8.3.22).
- Chen W., Bibby K. Model-based theoretical evaluation of the feasibility of using wastewater-based epidemiology to monitor monkeypox. Environ. Sci. Technol. Lett. 9, 772–778. 2022 doi: 10.1021/ACS.ESTLETT.2C00496. [DOI] [Google Scholar]
- de Jonge E.F., Peterse C.M., Koelewijn J.M., van der Drift A.-M.R., van der Beek R.F.H.J., Nagelkerke E., Lodder W.J. The detection of Monkeypox virus DNA in wastewater samples in the Netherlands. Sci. Total Environ. 2022;852 doi: 10.1016/J.SCITOTENV.2022.158265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Centre for Disease Prevention and Control (ECDC), n.d. Factsheet for health professionals on monkeypox [W.W.W. Document]. URL https://www.ecdc.europa.eu/en/all-topics-z/monkeypox/factsheet-health-professionals (accessed 8.3.22). 2022.
- Ferré V.M., Bachelard A., Zaidi M., Armand-Lefevre L., Descamps D., Charpentier C., Ghosn J. Detection of Monkeypox virus in anorectal swabs from asymptomatic men who have sex with men in a sexually transmitted infection screening program in Paris, France. Ann. Intern. Med. 2022;175:1491–1492. doi: 10.7326/M22-2183. [DOI] [PubMed] [Google Scholar]
- Hernaez, B., Muñoz-Gómez, A., Sanchiz, A., Orviz, E. Valls-Carbo, A., Sagastagoitia, I., Ayerdi, O., artín, R., Puerta, T., Vera, M., Cabello, N., Vergas, J., Prieto, C., Pardo-Figuerez, M., Negredo, A., Lagarón, J.M., del Romero, J., Estrada, V., Alcamí, A., 2023. Monitoring monkeypox virus in saliva and air samples inSpain: a cross-sectional study. The Lancet 4, e21-e28 10.1016/S2666-5247(22)00291-9. [DOI] [PMC free article] [PubMed]
- la Rosa G., Mancini P., Veneri C., Ferraro G.B., Lucentini L., Iaconelli M., Suffredini E. Detection of Monkeypox Virus DNA in Airport Wastewater, Rome, Italy. Emerg. Infect. Dis. 29, 193-196. doi: 10.3201/eid2901.221311. 2023 doi: 10.3201/eid2901.221311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Zhao H., Wilkins K., Hughes C., Damon I.K. Real-time PCR assays for the specific detection of Monkeypox virus West African and Congo Basin strain DNA. J. Virol. Methods. 2010;169:223–227. doi: 10.1016/J.JVIROMET.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez J.I., Gil Montalbán E., Jiménez Bueno S., Martín Martínez F., Nieto Juliá A., Sánchez Díaz J., García Marín N., Córdoba Deorador E., Nunziata Forte A., Alonso García M., Humanes Navarro A.M., Montero Morales L., Domínguez Rodríguez M.J., Carbajo Ariza M., Díaz García L.M., Mata Pariente N., Rumayor Zarzuelo M., Velasco Rodríguez M.J., Aragón Peña A., Rodríguez Baena E., Miguel Benito Á., Pérez Meixeira A., Ordobás Gavín M., Lopaz Pérez M.Á., Arce Arnáez A. Monkeypox outbreak predominantly affecting men who have sex with men, Madrid, Spain, 26 April to 16 June 2022. Eurosurveillance. 2022;27 doi: 10.2807/1560-7917.ES.2022.27.27.2200471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCollum A.M., Damon I.K. Human monkeypox. Clin. Infect. Dis. 2014;58:260–267. doi: 10.1093/CID/CIT703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medema G., Heijnen L., Elsinga G., Italiaander R., Brouwer A. Presence of SARS-Coronavirus-2 RNA in sewage and correlation with reported COVID-19 prevalence in the early stage of the epidemic in The Netherlands. Environ. Sci. Technol. Lett. 2020;7:511–516. doi: 10.1021/acs.estlett.0c00357. [DOI] [PubMed] [Google Scholar]
- Nelson B. What poo tells us: wastewater surveillance comes of age amid covid, monkeypox, and polio. BMJ. 2022;378:o1869. doi: 10.1136/BMJ.O1869. [DOI] [PubMed] [Google Scholar]
- Pérez-Cataluña A., Cuevas-Ferrando E., Randazzo W., Falcó I., Allende A., Sánchez G. Comparing analytical methods to detect SARS-CoV-2 in wastewater. Sci. Total Environ. 2021;758, 143870 doi: 10.1016/J.SCITOTENV.2020.143870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiró-Mestres A., Fuertes I., Camprubí-Ferrer D., Marcos M.Á., Vilella A., Navarro M., Rodriguez-Elena L., Riera J., Català A., Martínez M.J., Blanco J.L., Hospital Clinic de Barcelona Monkeypox Study Group Frequent detection of Monkeypox virus DNA in saliva, semen, and other clinical samples from 12 patients, Barcelona, Spain, May to June 2022. Eurosurveillance. 2022;27 doi: 10.2807/1560-7917.ES.2022.27.28.2200503/CITE/PLAINTEXT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randazzo W., Truchado P., Cuevas-Ferrando E., Simón P., Allende A., Sánchez G. SARS-CoV-2 RNA in wastewater anticipated COVID-19 occurrence in a low prevalence area. Water Res. 2020;181 doi: 10.1016/j.watres.2020.115942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanish Ministry of Health . Spanish Ministry of Health; 2022. Alert On Monkeypox Infection in Spain and Other Non-Endemic Countries.https://www.sanidad.gob.es/profesionales/saludPublica/ccayes/alertasActual/alertaMonkeypox/home.htm [W.W.W. Document]. URL. (accessed 8.19.22) [Google Scholar]
- Thornhill, J.P., Barkati, S., Walmsley, S., Rockstroh, J., Antinori, A., Harrison, L.B., Palich, R., Nori, A., Reeves, I., Habibi, M.S., Apea, V., Boesecke, C., Vandekerckhove, L., Yakubovsky, M., Sendagorta, E., Blanco, J.L., Florence, E., Moschese, D., Maltez, F.M., Goorhuis, A., Pourcher, V., Migaud, P., Noe, S., Pintado, C., Maggi, F., Hansen, A.-B.E., Hoffmann, C., Lezama, J.I., Mussini, C., Cattelan, A., Makofane, K., Tan, D., Nozza, S., Nemeth, J., Klein, M.B., Orkin, C.M., SHARE-net Clinical Group, 2022. Monkeypox virus infection in humans across 16 countries - April-June 2022. N. Engl. J. Med. 387, 679-691doi:10.1056/NEJMoa2207323. [DOI] [PubMed]
- Tsung K., Yim J.H., Marti W., Mark R., Buller L., Norton J.A. Gene expression and cytopathic effect of vaccinia virus inactivated by psoralen and long-wave UV light. J. Virol. 1996;70(1):165–171. doi: 10.1128/JVI.70.1.165-171.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . WHO; 2022. Director-General Declares the Ongoing Monkeypox Outbreak a Public Health Emergency of International Concern.https://www.who.int/europe/news/item/23-07-2022-who-director-general-declares-the-ongoing-monkeypox-outbreak-a-public-health-event-of-international-concern [W.W.W. Document]. URL. (accessed 9.6.22) [Google Scholar]
- Wilson M.E., Hughes J.M., McCollum A.M., Damon I.K. Human monkeypox. Clin. Infect. Dis. 2014;58:260–267. doi: 10.1093/cid/cit703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe M.K., Duong D., Hughes B., Chan-Herur V., White B.J., Boehm A.B., Wolfe M.K., Boehm A. Detection of Monkeypox viral DNA in a routine wastewater monitoring program. medRxiv. 2022 doi: 10.1101/2022.07.25.22278043. 2022.07.25.22278043. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.