Abstract
Berberine, a well-known isoquinoline alkaloid derivative, has a varied range of pharmacological effects. Herein, we notice the radio-modulatory outcome of berberine in cultured ovarian cancer (SKOV-3) cells exposed to γ-rays as radiotherapy (RT). Cells pre-treated with berberine were irradiated by γ-irradiation and the liberation of reactive oxygen species (ROS) was analyzed by flow cytometry. Apoptotic cell death along with the DNA damage associated with protein expressions was projected by flow cytometry and confocal microscopy. Experimental findings established that berberine might be a capable radiosensitizer for treating SKOV-3, because of oxidative DNA damage. Moreover, the in-silico study of the compound, berberine suggests free energy of binding (ΔG) −7.5 kcal/mol with SKOV-3 and −8.8 kcal/mol of PALB/BRCA2, which proves an effective and compact binding of the complex and is safe for future clinical trials. Thus, our approach is probably to widen the field of study of SKOV-3 and PALB/BRCA2 from the inhibition of these targets as a prospective nutraceutical for the anti-cancer theragnostic candidate.
Keywords: Radiotherapy, Berberine, SKOV-3, Cancer, Ovarian
1. Introduction
In the present scenario, ovarian cancer is an evolving cause of mortality among gynecological tumors (Ajjarapu et al., 2021, Chan et al., 2018, Teekaraman et al., 2019). It arises not only from the ovarian surface epithelial but also from the endometrium wall of fallopian tubes and metastasizes through the lymphatic routes (Kurman and Shih, 2010, Lim et al., 2016, Vafadar et al., 2020, Vetter and Hays, 2018). In Saudi Arabia, according to the National Cancer Registry, ovarian cancer is the seventh most common cancer representing 3.3 % of the total cancers affecting women. Ovarian cancer holds the 10th most lethal cancer among several other cancers as per the latest data from the International Agency for Research on Cancer. In 2020, Saudi Arabia reported a total of 444 new ovarian cancer cases constituting 1.6 % of the new cancer cases and 281 deaths (Aga et al., 2022). Since, a lack of early diagnosis and symptoms, ovarian cancer appeared at an advanced stage leads to extensive invasion and becoming a poor prognosis toward patient compliance (Teckie et al., 2013; J. Yan et al., 2011). Because of the intricate heterogeneity related with ovarian cancer, numerous treatment regimes (chemotherapy, radiotherapy, and immunotherapy) are available to control tumor growth in the localized areas (Bi et al., 2018, Goel and Aggarwal, 2010, Gong et al., 2021, Liskova et al., 2021). Among them, majority of ovarian cancer requires high intensity of ionizing radiations to destroy infected tissue (Medhat et al., 2017). Nevertheless, it has certain drawbacks, assaulting normal tissues, lower radiation supply to the infected cells, and develop resistance to the radiation (Jagetia and Venkatesha, 2005, Medhat et al., 2017). Thus, there is an imperative need to find the new window by limiting the doses of (RT) with a suitable radiosensitizers, which is capable of targeting ovarian cancer, leaving behind healthy cells unaffected or at minimal side effect (Medhat et al., 2017). During this course of radiology, high-energy gamma or X-rays are primarily employed to ionize cellular constituents. Radiosensitizers sensitize water molecules (a major cellular component) for radiolysis resulting in the discharge of free radicals species, and charged water molecules (Hogle, 2006, Mallick and Waldron, 2009). These radicals together with the ionizing radiations targeting DNA and other cellular membrane causes structural injuries resulting in the initiation of apoptosis followed by lipid peroxidation (Halliwell & Aruoma, 1991).
From these viewpoints, it is significant to mention that redox balance is necessary to sustain the physiological activity for fitness and the immune system (Tang et al., 2018). Redox activities like phosphorylation and other kinds of cellular processes facilitated by kinases/phosphatases are playing a critical role in cell regulating systems (Sarkar et al., 2022). Of particular importance, RAC (Rho family)-alpha serine/threonine-protein kinase (AKT) is much expressed in ovarian cancers and it is capable of phosphorylating various downstream effectors, such as apoptotic proteins, transcription factors, and other oncogenes, including mammalian target of rapamycin (mTOR) (Ponte et al., 2021; Y.-H. Wu et al., 2020). Most of the anticancer candidates derived from natural products were found to decrease AKT activation as the main molecular mode of action (Gasparri et al., 2017; H. Wu et al., 2019). Based on the abundant anti-oxidant effects, natural products inhibited cancer progression by decreasing AKT/mTOR pathway, and suppressed AKT-mediated activation of mTOR and its effectors in a series of cancer cell line is found to be potential (Ponte et al., 2021). Berberine (2,3-methylenedioxy-9, 10-dimenthoxyprotoberberinechloride, BBR) (Fig. 1)- a water soluble alkaloid derived from Berberis species (Li et al., 2020). It may be extracted from many other sources viz., European barberry, goldenseal, goldthread, Oregon grape, phellodendron etc (Maria et al., 2018). It exhibited antibacterial, antifungal and shows anticancer activity against various cancers (Chen et al., 2015). It can inhibit tumor metastasis, and enhance radiosensitivity via the regulation of multiple pathways in ovarian cancer cells (Hou et al., 2017, Wang et al., 2017). Liu Z. et al. have observed the effect of berberine on multiple cancer cell lines at lower concentrations (15 µM) upon exposure of a certain frequency (2–6 Gy) X-ray irradiation. The results shown that the proliferation of cancer cells was inhibited without affecting non-malignant cells (Liu et al., 2009).
Fig. 1.
Molecular structure of Berberine.
Herein, we are presenting radiosensitizer effect of berberine against ovarian cancer cell line (SKOV-3), displaying promising effects in combination with radiotherapy. Therefore, the investigation could be beneficial for the finding of a potential radiosensitizer for ovarian cancer patients. Our outcomes may allow implications in planning cancer therapy approaches of drug with radiosensitizers.
2. Materials and methods
2.1. Cell line and chemicals
The human ovarian cancer (SKOV-3) and Hyman epithelial kidney (HEK 293) cell line was obtained from the ATCC (Manassas, VA, USA). Dulbecco's Modified Eagle's Medium (DMEM), 100 µg/ml penicillin streptomycin with 10 % fetal bovine serum (FBS), was used for SKOV-3 culture in 5 % CO2 at 37 °C. The cell culture media constituents viz.; FBS, DMEM, PSN antibiotic cocktail, EDTA, and trypsin were acquired from Gibco (Grand Island, NY, USA). Biochemical assay kits were purchased from Calbiochem (Massachusetts, USA). Antibodies were bought from eBioscience (San Diego, USA), Santa Cruz Biotechnology (Texas USA) and Abcam (U.K.).
2.2. MTT assay
A cell viability assay was performed using earlier described methods (Nandi et al., 2017), where viable cells metabolized by mitochondrial succinic dehydrogenase activity of proliferating cells to yield a purple formazan product. Dimethyl sulfoxide (DMSO) is used to solubilise the purple colour. The absorbance of developed formazan was taken spectrometricaly identified at 570 nm. Here, MTT assay used to quantify the cell viability post irradiation along with the berberine treatment.
The SKOV-3 cells were trypsinised and centrifuged. Population of 1.5 × 104 cells were calculated by haemocytometer before reseeding in 96 well plates. Staining and analysis were accomplished after 24 h of post-irradiation to permit enough time for apoptosis behind irradiation to examine the metabolic activity. 2 × 96 well plates were castoff for each liberation of cell lines. In each plate, five wells were seeded for each radiation dose (0, 1, 2, 3, 4 and 5 Gy), as well as five wells were taken for each concentration of berberine (0, 5, 10, 15, 20, 25 and 30 μM). Furthermore, five wells were seeded for each set of berberine and irradiation combination (berberine- 0 μM + irradiation- 0 Gy, berberine- 0 μM + irradiation- 2 Gy, berberine- 5 μM + irradiation- 2 Gy and berberine- 10 μM + irradiation- 2 Gy); berberine- 0 μM + irradiation- 0 Gy has been taken as control. The cell medium was removed from the wells after 24 h of incubation. 100 µl of tetrazolium salt (0.2 mg/ml) was augmented to each well and incubated at 37 °C for 2 h under humidified atmosphere. The supernatant was discarded from the wells and formazan crystals were dissolved in 200 µl of isopropanol. The absorbance was measured after 30 min. ELISA reader read the blank, which was left as empty wells having 200 µl of isopropanol. MTT assay was also performed in the HEK-293 cell line to check the cytotoxicity of berberine in a healthy cell.
2.3. Irradiation
Irradiation was executed at room temperature from a Cobalt-60 source at a dose rate of 2.5 Gy/min.
2.4. Quantification of apoptosis using flow cytometry
To determine the mechanistic approach of cell death, using the Annexin-V FITC/DAPI apoptosis kit (Calbiochem, CA, USA) (Bhanja et al., 2017) was employed. Briefly, γ-rays were introduced into berberine pre-treated SKOV-3 cells. Berberine treatment was given for 2 h prior to the irradiation. After the 2 h of berberine treatment, treated cells were washed by PBS twice for irradiation. After irradiation, cells were incubated for 24 h. Then the incubated cells were washed and stained with Annexin-V-FITC as per company’s protocols. The measurement of FITC appearance was carried out by Flow cytometer (BD LSRFortessa TM San Jose, CA, USA) to ensure the apoptosis.
2.5. Determination of intracellular ROS
iROS production displays a vital part in the stimulation of apoptosis at cellular level (Mishra et al., 2020). To calculate the iROS, we incubated the treated cells with 10 μM (2′, 7′-dichlorofluorescein diacetate prior the analysis by flow cytometer (BD LSRFortessa, USA). The increase of DCF fluorescence directly exposes the produced ROS.
2.6. Determination of reduced glutathione (GSH) activity
The cell lysate was subjected in 0.1 ml of 25 % TCA to analyse the GSH level, followed by centrifugation. The endogenous R − SH group was deliberated in a mixture of 1 ml (20 µl of 0.5 mM DTNB set in 0.2 M PBS, with 25 µl of cell supernatant and 955 µl of reaction buffer). A yellow complex of DTNB produced after reduction of -SH group of GSH. The absorbance was recorded at 412 nm and the assay was executed three independent times (n = 3) (Ognjanović et al., 2012).
2.7. Determination of lipid peroxidation
Thiobarbituric acid reactive substance (TBARS) level in the cell lysate was measured as per the adapted protocol of Beuege and Aust (Buege & Aust, 1978). The homogenate mixture containing TCA (15 %), TBA (0.375 %), and HCl (5 N) was boiled at 95 °C for 15 min. The absorbance at 535 nm was noted against a suitable blank. The lipid peroxidation was revealed as the amount of TBARS formed, in nmol/mg protein (Olszewska-Słonina et al., 2011).
2.8. Assessment of protein expression
Cells were fixed in 4 % paraformaldehyde in PBS (pH 7.4) followed by permeabilization (0.1 % Triton X-100 in PBS) with 0.1 % FBS for 5 min. Before incubation with respective primary antibody, the washing of intracellular cells were carried out using PBS with 3 % FBS. After that, the primary antibody’s labelled cells were raised with FITC conjugated goat anti rabbit/mouse IgG (secondary antibody). Stained cells were exposed to flow cytometry (BD LSRFortessa flow cytometer, USA) (Mukherjee et al., 2019).
2.9. Immunofluorescence
Concisely, control/treated SKOV-3 cells were washed in PBS (0.01 M) for 10 min followed by incubation in blocking solution having 2 % bovine serum and 0.3 % Triton X-100 in PBS for 1 h. Further incubation of cells were takes place with the individual primary antibody (p-AKT and RAD50), monitored by washing and staining with respective fluorophore-conjugated secondary antibody. Prior to mounting with the ProLong antifade reagent (Molecular Probe, Eugene, OR, USA), slides were counterstained with 6-diamidino2-phenylindole for 10 min and inspect under confocal laser-scanning microscope (FV 10i, Olympus, Japan) (Manna et al., 2019).
2.10. Molecular docking
The ligand of interest was undertaken from the PubChem database (Bonvino et al., 2018), and retrieved in SDF format (Kim et al., 2019). Open Babel software was used for the PDB format conversion (O’Boyle et al., 2011). The energy minimization was performed by means of UCSF Chimera (Pettersen et al., 2004) and Amber ff 14 sb force field. Ovarian cancer receptor SKOV-3 (Pdb: 5IHJ) and PALB2/BRCA2 (Pdb: 3EU7) were obtained from a protein data bank (Berman, 2000). The preparation of protein before docking was followed by previous protocol employing AutoDock Tools (v.1.5.6) of the MGL software package (Forli et al., 2016).
2.11. Statistical analysis
Data of each experiment were statistically analyzed using Origin Pro followed by mean ± standard deviation (SD) using Student’s t-test, at the significance level of P < 0.05.
3. Results
3.1. Cytotoxicity measurement of berberine with and without gamma irradiation
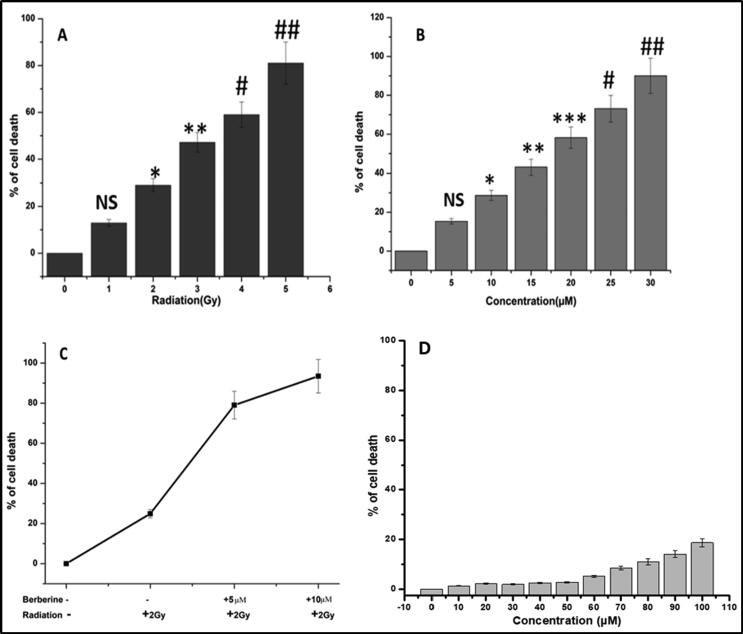
Initially we have performed the cytotoxicity of gamma irradiation in SKOV-3 cell line by MTT assay (Fig. 2A). From the MTT assay data, it has been detected that at 5 Gy of irradiation, cells viability decreased by nearly 80 % and we have also witnessed that at 2 Gy of irradiation almost 30 % cells death occurred. Simultaneously the cytotoxicity of berberine was assessed (Fig. 2B), and it has been recognized that at 30 µM almost 90 % of cells were not viable whereas at 5 and 10 µM of berberine almost 20 and 30 % cells death were observed during 24 h. Then the radio-sensitization property of berberine (5 and 10 µM) was estimated under gamma irradiation of frequency 2 Gy (Fig. 2C).
Fig. 2.
MTT assay data of (A) only Gamma irradiated (0–5 Gy) SKOV-3 cells, (B) Only Berberine (0–30 µM) treated SKOV-3 cells, (C) with berberine pretreated Gamma irradiated SKOV-3 cells, and (D) with berberine in HEK 293 cell line. The significant results presenting P value < 0.05 were labeled as ∗, # (In A; * denoted control versus 2 Gy, ** denoted control versus 3 Gy, # denoted control versus 4 Gy, ## denoted control versus 5 Gy, and in B; * denoted control versus 10 µM, ** denoted control versus 15 µM, *** denoted control versus 20 µM, # denoted control versus 25 µM, ## denoted control versus 30 µM).
The MTT result showed that pretreated by 5 µM of berberine treatment followed by 2 Gy irradiation has resulted in more than 80 % SKOV-3 cell death. In the case of 10 µM of berberine treatment followed by 2 Gy irradiation has resulted in more than 90 % SKOV-3 cell death. This initial information suggested that in the case of berberine (5 and 10 µM) treated gamma irradiated well the cell death is significantly higher compared to only 2 Gy of gamma irradiation or only berberine treatment of 5 and 10 µM. This study suggested to evaluate further confirmation of detail pathway for berberine mediated radiosensitization in SKOV-3 cell line. We have also checked the cytotoxicity of berberine (Fig. 2D) by MTT assay against HEK-293 cell line. From the MTT data it has been established that there is no significant cytotoxicity upto 100 μM concentration of berberine.
3.2. Measurement of reactive oxygen species
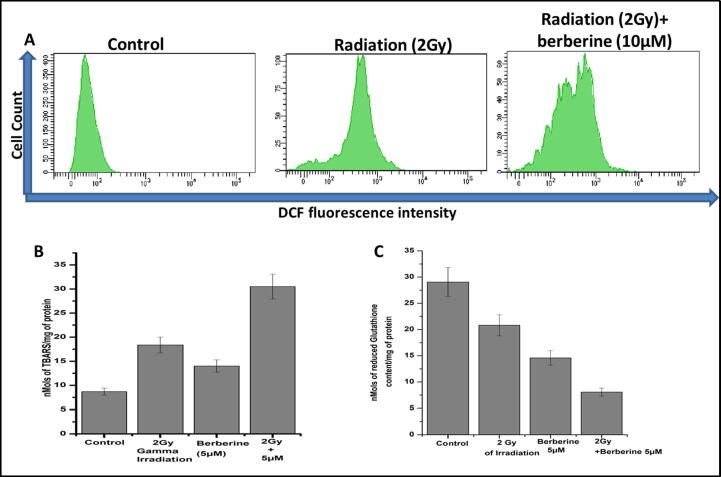
Apoptosis is caused by over production of ROS inside the cell, thereby damaging cellular constituent’s, and organelles (Redza-Dutordoir & Averill-Bates, 2016). Under the influence of ROS, H2DCFDA- a non-fluorescent molecule is oxidized to highly fluorescent molecule 2′,7′-dichlorofluorescein (DCF), which may found to be useful method to detect the elevated levels of ROS. The noticeably increase in the mean fluorescence intensity (MFI) of DCF was observed with berberine treatment after 2 Gy gamma irradiation while inspecting the ROS level (Fig. 3A). In case of 10 µM berberine pretreated 2 Gy gamma irradiated tube the MFI is significantly higher than only gamma irradiated tube, which were repeated multiple time to validate the ROS generation. The DCF + cell % was given in inset. Compared to gamma irradiation and berberine only, the level of reduced glutathione amount decreases (Fig. 3B). At the same time, higher rate of TBARS formation were displayed in combination groups (Fig. 3C), which established the incidence of oxidative surge in SKOV-3 cells.
Fig. 3.
(A) Flow cytometric presentation of ROS generation by DCFDA. (B) The bar diagram represented the TBARS level in control, irradiation, berberine, and irradiation + berberine treated groups. Nanomoles of TBARS/ mg of protein was plotted along the Y-axis and control irradiation, berberine, and irradiation + berberine were taken along the X-axis. (C) The bar diagram represented the reduced glutathione content in irradiation, berberine, and irradiation + berberine treated groups. Nanomoles of reduced glutathione/mg of protein was plotted along the Y-axis and irradiation, berberine, and irradiation + berberine were taken along the X-axis. Error bars were SEM for n = 3. p < 0.05 was considered significant.
3.3. Measurement of apoptosis
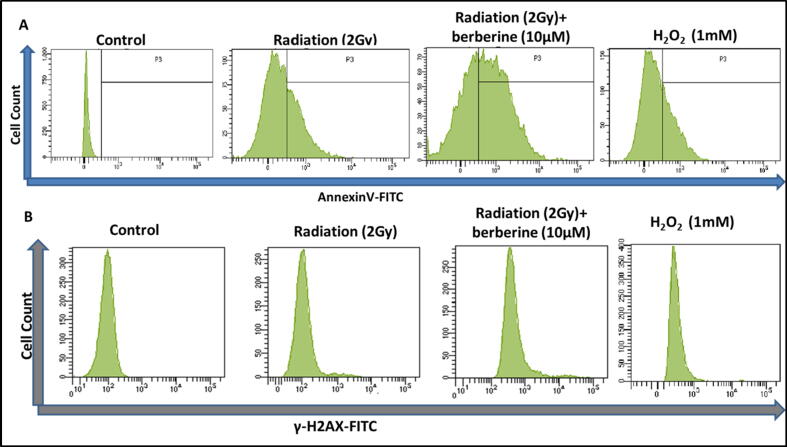
Flow cytometric assessment was carried out to investigate whether the berberine causes apoptosis/necrosis, by evaluating the level of phosphatidyl serine using Annexin-V-FITC/PI staining (Fig. 4A). The flowcytometric analysis suggested that berberine (10 µM) pretreated with 2 Gy gamma irradiated tube, the Annexin-V intensity has been significantly increased compare to only gamma irradiated and control tube. The PI intensity also increased with berberine treatment in presence of Gamma irradiation. This experiment was done for 24 h of incubation after irradiation·H2O2 of 1 mM strength has been taken as positive control. The ROS generation and the apoptosis confirmation suggested that the berberine induced radiosensitization is caused by ROS induced apoptosis.
Fig. 4.
(A) Assessment of Annexin-V level in control, irradiation, berberine, and irradiation + berberine treated groups along with the positive control (H2O2 of 1 mM). (B) Expression of γ-H2AX in control, irradiation, berberine, and irradiation + berberine treated groups with the positive control (H2O2 of 1 mM).
3.4. Cellular pathway analysis via AKT mediated DNA damage axis
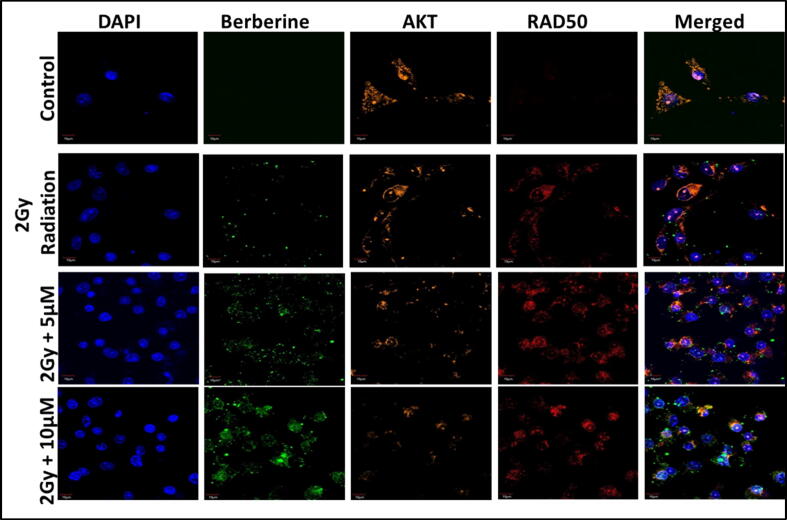
To justify whether the mechanism of apoptosis upon berberine pretreated gamma irradiated cells were DNA damage mediated or not, flow cytometric evaluation of γ-H2AX was conducted. It has been well-known that in case of berberine pretreated gamma irradiated tube the γ-H2AX expression was increased compared to only 2 Gy gamma-irradiated and control ones. The experiment was done after 24 h of incubation after irradiation. This data (Fig. 4B) further confirmed that the complete event is happening by ROS mediated DNA damage. For further affirmation, we have estimated RAD50 expression, a double-strand break repair protein by confocal microscopy (Fig. 5). In this experiment, we have estimated the expressions of AKT and RAD50 at 5 and 10 µM concentrations of berberine to visualize the green fluorescence. The experimental facts established that as the amount of berberine was higher, the expression of RAD50 (Red) was increased whereas the expression of AKT (Orange) was decreased. In this comment, DAPI was used as a nucleus stainer. Thus, berberine-induced radiosensitization was due to the oxidative burst in AKT-mediated DNA damage mode.
Fig. 5.
Expression of AKT and RAD50 in control, irradiation, berberine, and irradiation + berberine treated groups, in presence of the green fluorescence berberine.
3.5. Molecular docking
To give more insight into the interaction mode of berberine (PubChem ID: 2353), molecular docking studies were performed with receptor SKOV-3 (Pdb: 5IHJ) and PALB2/BRCA2 (Pdb: 3EU7) (Fig. 6). The basis of choosing these two receptors by keeping in mind to the repair of DNA damage and gram-negative coccobacillus that has recently appeared as a source of hospital-acquired infections. With the help of the Lamarckian Genetic Algorithm, the protein–ligand docked complex was undertaken to achieve the lowest free energy of binding (ΔG). Fig. 6 showed that berberine strongly recognizes the active site of these proteins and inhibit their enzymatic activity. We have selected the best conformation amongst the various conformations have been executed. The free energy of binding (ΔG) for 5IHJ obtained to be −7.5 kcal/mol, and inhibitory concentration (Ki) 322.453 nM. Similarly, in case of 3EU7 the free energy of binding (ΔG) was −8.8 kcal/mol, inhibitory concentration (Ki) 356.43 nM. Therefore, the outcomes revealed that berberine has a substantial character in the dealing of ovarian cancer treatment.
Fig. 6.
(a) The surface view of SKOV-3 (Pdb: 5IHJ) and PALB2/BRCA2 (Pdb: 3EU7); (b) Docked pose of Berberine with SKOV-3 (Pdb: 5IHJ) and PALB2/BRCA2 (Pdb: 3EU7) showing 2D interaction plot originating from several non-covalent weak interactions.
4. Discussion
Paying emphasis to the overview of natural-derived radiosensitizer in order to increase the radiation capability towards ovarian cancer. Isoquinoline based flavonoids owns various applications with unique mode of action (Dey et al., 2020, Liskova et al., 2021). Particularly, berberine exhibited cytotoxicity through direct effect on progression of cancer cell (Y. Liu et al., 2008, Zhang et al., 2020). The major cellular enzymes that lead to the anticancer potential of berberine is Akt, mitogen activated protein kinases (MAPKs), cell cycle checkpoint kinases, etc (Habtemariam, 2020; Y.-B. Yan et al., 2020). However, the direct influence of berberine on cancer cells is comparatively weak but its effect enhances many folds once combined with radiotherapy (Pauwels et al., 2005). Despite the impressive experimental evidence, the radio-sensitizing potential of berberine on ovarian cancer cells using gamma radiation is scarcely reported. Owing to its weak lipid solubility, it is tough for berberine to enter the cytomembrane and grasp through the gastrointestinal route (Lu et al., 2006, Tiwari and Mishra, 2020, Zou et al., 2017). Keep in mind, the multifunctional role of berberine, we hypothesize that it might increase the sensitivity of tumor radiotherapy. Marverti et al showed that the development of cisplatin-resistant ovarian cancer cells has been inhibited through berberine via preventing the role of enzymes that are essential for DNA synthesis (MARVERTI et al., 2013). Hou et al has also ascertained that berberine enhances the sensitivity of ovarian cancer cells to PARP inhibitors as a result of apoptosis through DNA damage (Hou et al., 2017). In this article, we observed the application of berberine with gamma radiation boosts the radiosensitivity of ovarian cancer cells. The analysis showed that the ovarian cancer cells treated with berberine increased levels of oxidative stress and hence, DNA damage. As anticipated, displaying a synergistic radiosensitizing efficacy once composed with gamma irradiation; induces apoptosis and inhibited tumor expansion.
Thus, radiosensitization potential of berberine in SKOV-3 has been evaluated. First, we have checked the cytotoxicity of berberine and gamma irradiation individually. From the initial MTT data 2 Gy and 10 µM concentration of gamma irradiation and berberine concentration was selected respectively because these concentrations are significantly less toxic to healthy cell, HEK 293 cell line. To find out the fundamental mechanism of the radiosensitization potential of berberine on SKOV-3 cells, flow cytometric analysis was implemented using Annexin V-FITC staining assay. The flow cytometric results reflected that the radiosensitization by berberine (10 µM) is due to apoptosis. Previous reports said that ROS play a major role in apoptosis (Kamogashira et al., 2015, Slika et al., 2022). So, we have calculated the ROS generation by H2DCFH-DA and the analysis confirmed that the apoptotic cell death was due to ROS generation. Furthermore, higher rate of TBARS formation and decreased level of (GSH) content supported the elevated ROS generation data.
We have also evaluated that DNA damage, whether it is endogenous or exogenous, forms of double stranded breaks (DSBs), it is continuously followed by a series of nuclear protein such as phosphorylation of the histone, H2AX. H2AX is a variant of the H2A protein. So, the expression of γ-H2AX was monitored before and after irradiation of berberine (10 µM) treated SKOV-3 cells. The flow cytometric data established that the γ-H2AX expression was elevated in berberine treated cells that confirmed the DNA double strand breaks. This is already reported that RAD50 plays a key role in double-strand break (DSB) of DNA. Hence, the RAD50 expression on gamma irradiated berberine treated SKOV-3 cells result further established that the radiosensitization is happening due to double stranded breaks of DNA. The DNA damage response (DDR) signaling pathway orchestrated by the ATM ATR kinases and RAD50 is the central regulator of this network in response to DNA damage. Both ATM and ATR are activated by DNA damage and DNA replication stress, but their DNA-damage specificities are distinct and their functions are not redundant. We have checked the expression of γ-H2AX to check the ROS induced DNA damage. After confirmation the DNA damage, DNA damage regulatory proteins (including ATM, ATR, RAD50) are involved in AKT activation, so the expression of AKT was measured in only gamma irradiated cells and berberine pre-treated gamma irradiated cells. The data validated that the expression of AKT was increased owing to radiosensitization by berberine. Finally, we established that berberine encouraged radiosensitization is triggered by oxidative burst and activate γ-H2AX in the DNA damage response pathways via AKT axis.
5. Conclusion
Natural products may ascertain to be a great asset for the development of therapeutic candidate mainly due to low toxicity. Due to unique structure and biochemical pathways of active molecules that can be used as ovarian cancer inhibitor against SKOV-3 and PALB2/BRCA2 is of prime importance. In summary, we present strong in vitro evidence that berberine functions as a radiosensitizer in SKOV-3. Further, we demonstrated that berberine specifically induce oxidative burst and activate γ-H2AX in the DNA damage response pathways. Additionally, molecular modelling studies have been shown that berberine recognizes the active residues of SKOV-3 (Pdb: 5IHJ) and PALB2/BRCA2 (Pdb: 3EU7) in a highly specific binding pattern. In future work we will check the in vivo radiosensitization efficacy of berberine along with dosimetry analysis.
6. Availability of data
Online data is available without request.
7. Authors' contributions
Mohammed Aleissa, and Lina M Alneghery were investigated the oxidative stress levels and Quantified apoptosis. Mohammed AL-Zharani were estimated iROS. Mohammed Aleissa, Lina M Alneghery and Abdulmalik were involved in the conception and design of the study.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at Imam Mohammad Ibn Saud Islamic University for funding this work through Research Group no. RG-21-09-88.
Footnotes
Peer review under responsibility of King Saud University.
References
- Aga S., Jaha R., Khan R., Junaydi D., Hakami A., Khan M., Alsaab H. Detailed demographics and the prevalence of comorbidities in ovarian cancer patients in Western Region of Saudi Arabia. J. Nat. Sci. Med. 2022;5(3):254. doi: 10.4103/jnsm.jnsm_158_21. [DOI] [Google Scholar]
- Ajjarapu S.M., Tiwari A., Taj G., Singh D.B., Singh S., Kumar S. Simulation studies, 3D QSAR and molecular docking on a point mutation of protein kinase B with flavonoids targeting ovarian Cancer. BMC Pharmacol. Toxicol. 2021;22(1):68. doi: 10.1186/s40360-021-00512-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman H.M. The protein data bank. Nucleic Acids Res. 2000;28(1):235–242. doi: 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhanja P., Mishra S., Manna K., Mallick A., Das Saha K., Bhaumik A. Covalent organic framework material bearing phloroglucinol building units as a potent anticancer agent. ACS Appl. Mater. Interfaces. 2017;9(37):31411–31423. doi: 10.1021/acsami.7b07343. [DOI] [PubMed] [Google Scholar]
- Bi Y., Verginadis I.I., Dey S., Lin L., Guo L., Zheng Y., Koumenis C. Radiosensitization by the PARP inhibitor olaparib in BRCA1-proficient and deficient high-grade serous ovarian carcinomas. Gynecol. Oncol. 2018;150(3):534–544. doi: 10.1016/j.ygyno.2018.07.002. [DOI] [PubMed] [Google Scholar]
- Bonvino N.P., Liang J., McCord E.D., Zafiris E., Benetti N., Ray N.B., Hung A., Boskou D., Karagiannis T.C. OliveNetTM: a comprehensive library of compounds from Olea europaea. Database. 2018;2018 doi: 10.1093/database/bay016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buege, J.A., Aust, S.D., 1978. [30] Microsomal lipid peroxidation, pp. 302–310. https://doi.org/10.1016/S0076-6879(78)52032-6. [DOI] [PubMed]
- Chan K.K.L., Siu M.K.Y., Jiang Y., Wang J., Leung T.H.Y., Ngan H.Y.S. Estrogen receptor modulators genistein, daidzein and ERB-041 inhibit cell migration, invasion, proliferation and sphere formation via modulation of FAK and PI3K/AKT signaling in ovarian cancer. Cancer Cell Int. 2018;18(1):65. doi: 10.1186/s12935-018-0559-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q., Qin R., Fang Y., Li H. Berberine sensitizes human ovarian cancer cells to cisplatin through miR-93/PTEN/Akt signaling pathway. Cell. Physiol. Biochem. 2015;36(3):956–965. doi: 10.1159/000430270. [DOI] [PubMed] [Google Scholar]
- Dey P., Kundu A., Kumar A., Gupta M., Lee B.M., Bhakta T., Dash S., Kim H.S. Recent Advances in Natural Products Analysis. Elsevier; 2020. Analysis of alkaloids (indole alkaloids, isoquinoline alkaloids, tropane alkaloids) pp. 505–567. [DOI] [Google Scholar]
- Forli S., Huey R., Pique M.E., Sanner M.F., Goodsell D.S., Olson A.J. Computational protein–ligand docking and virtual drug screening with the AutoDock suite. Nat. Protoc. 2016;11(5):905–919. doi: 10.1038/nprot.2016.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparri M., Bardhi E., Ruscito I., Papadia A., Farooqi A., Marchetti C., Bogani G., Ceccacci I., Mueller M., Benedetti Panici P. PI3K/AKT/mTOR Pathway in Ovarian Cancer Treatment: Are We on the Right Track? Geburtshilfe Frauenheilkd. 2017;77(10):1095–1103. doi: 10.1055/s-0043-118907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel A., Aggarwal B.B. Curcumin, the Golden Spice From Indian Saffron, Is a Chemosensitizer and Radiosensitizer for Tumors and Chemoprotector and Radioprotector for Normal Organs. Nutr. Cancer. 2010;62(7):919–930. doi: 10.1080/01635581.2010.509835. [DOI] [PubMed] [Google Scholar]
- Gong L., Zhang Y., Liu C., Zhang M., Han S. Application of Radiosensitizers in Cancer Radiotherapy. Int. J. Nanomed. 2021;16:1083–1102. doi: 10.2147/IJN.S290438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habtemariam S. Recent advances in berberine inspired anticancer approaches: from drug combination to novel formulation technology and derivatization. Molecules. 2020;25(6):1426. doi: 10.3390/molecules25061426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B., Aruoma O.I. DNA damage by oxygen-derived species Its mechanism and measurement in mammalian systems. FEBS Lett. 1991;281(1–2):9–19. doi: 10.1016/0014-5793(91)80347-6. [DOI] [PubMed] [Google Scholar]
- Hogle W.P. The state of the art in radiation therapy. Semin. Oncol. Nurs. 2006;22(4):212–220. doi: 10.1016/j.soncn.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Hou D., Xu G., Zhang C., Li B., Qin J., Hao X., Liu Q., Zhang X., Liu J., Wei J., Gong Y., Liu Z., Shao C. Berberine induces oxidative DNA damage and impairs homologous recombination repair in ovarian cancer cells to confer increased sensitivity to PARP inhibition. Cell Death Dis. 2017;8(10):e3070–e. doi: 10.1038/cddis.2017.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagetia G.C., Venkatesha V.A.K. Enhancement of radiation effect by Aphanamixis polystachya in mice transplanted with ehrlich ascites carcinoma. Biol. Pharm. Bull. 2005;28(1):69–77. doi: 10.1248/bpb.28.69. [DOI] [PubMed] [Google Scholar]
- Kamogashira T., Fujimoto C., Yamasoba T. Reactive oxygen species, apoptosis, and mitochondrial dysfunction in hearing loss. Biomed Res. Int. 2015;2015:1–7. doi: 10.1155/2015/617207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Chen J., Cheng T., Gindulyte A., He J., He S., Li Q., Shoemaker B.A., Thiessen P.A., Yu B., Zaslavsky L., Zhang J., Bolton E.E. PubChem 2019 update: improved access to chemical data. Nucleic Acids Res. 2019;47(D1):D1102–D1109. doi: 10.1093/nar/gky1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurman R.J., Shih I.-M. The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. Am. J. Surg. Pathol. 2010;34(3):433–443. doi: 10.1097/PAS.0b013e3181cf3d79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Li D., Kuang H., Feng X., Ai W., Wang Y., Shi S., Chen J., Fan R. Berberine increases glucose uptake and intracellular ROS levels by promoting Sirtuin 3 ubiquitination. Biomed. Pharmacother. 2020;121 doi: 10.1016/j.biopha.2019.109563. [DOI] [PubMed] [Google Scholar]
- Lim W., Jeong W., Song G. Delphinidin suppresses proliferation and migration of human ovarian clear cell carcinoma cells through blocking AKT and ERK1/2 MAPK signaling pathways. Mol. Cell. Endocrinol. 2016;422:172–181. doi: 10.1016/j.mce.2015.12.013. [DOI] [PubMed] [Google Scholar]
- Liskova A., Samec M., Koklesova L., Brockmueller A., Zhai K., Abdellatif B., Siddiqui M., Biringer K., Kudela E., Pec M., Gadanec L.K., Šudomová M., Hassan S.T.S., Zulli A., Shakibaei M., Giordano F.A., Büsselberg D., Golubnitschaja O., Kubatka P. Flavonoids as an effective sensitizer for anti-cancer therapy: insights into multi-faceted mechanisms and applicability towards individualized patient profiles. EPMA Journal. 2021;12(2):155–176. doi: 10.1007/s13167-021-00242-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Liu Q., Xu B., Wu J., Guo C., Zhu F., Yang Q., Gao G., Gong Y., Shao C. Berberine induces p53-dependent cell cycle arrest and apoptosis of human osteosarcoma cells by inflicting DNA damage. Mutation Research/Fundamental Mol. Mech. Mutagenesis. 2009;662(1–2):75–83. doi: 10.1016/j.mrfmmm.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Liu Y., Yu H., Zhang C., Cheng Y., Hu L., Meng X., Zhao Y. Protective effects of berberine on radiation-induced lung injury via intercellular adhesion molecular-1 and transforming growth factor-beta-1 in patients with lung cancer. Eur. J. Cancer. 2008;44(16):2425–2432. doi: 10.1016/j.ejca.2008.07.040. [DOI] [PubMed] [Google Scholar]
- Lu Y.-C., Lin Q., Luo G.-S., Dai Y.-Y. Solubility of berberine chloride in various solvents. J. Chem. Eng. Data. 2006;51(2):642–644. doi: 10.1021/je0504360. [DOI] [Google Scholar]
- Mallick I., Waldron J.N. Radiation Therapy for Head and Neck Cancers. Semin. Oncol. Nurs. 2009;25(3):193–202. doi: 10.1016/j.soncn.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Manna K., Mishra S., Saha M., Mahapatra S., Saha C., Yenge G., Gaikwad N., Pal R., Oulkar D., Banerjee K., Das Saha K. Amelioration of diabetic nephropathy using pomegranate peel extract-stabilized gold nanoparticles: assessment of NF-κB and Nrf2 signaling system. Int. J. Nanomed. 2019;14:1753–1777. doi: 10.2147/IJN.S176013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maria Neag, Andrei Mocan, Javier Echeverría, Raluca Pop, Corina Bocsan, Gianina Crişan, Anca Buzoianu, et al. Berberine: Botanical Occurrence, Traditional Uses, Extraction Methods, and Relevance in Cardiovascular, Metabolic, Hepatic, and Renal Disorders. Front Pharmacol. 2018;9:557. doi: 10.3389/fphar.2018.00557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marverti G., Ligabue A., Lombardi P., Ferrari S., Monti M.G., Frassineti C., Costi M.P. Modulation of the expression of folate cycle enzymes and polyamine metabolism by berberine in cisplatin-sensitive and -resistant human ovarian cancer cells. Int. J. Oncol. 2013;43(4):1269–1280. doi: 10.3892/ijo.2013.2045. [DOI] [PubMed] [Google Scholar]
- Medhat A.M., Azab K.S., Said M.M., El Fatih N.M., El Bakary N.M. Antitumor and radiosensitizing synergistic effects of apigenin and cryptotanshinone against solid Ehrlich carcinoma in female mice. Tumor Biol. 2017;39(10) doi: 10.1177/1010428317728480. 101042831772848. [DOI] [PubMed] [Google Scholar]
- Mishra S., Manna K., Kayal U., Saha M., Chatterjee S., Chandra D., Hara M., Datta S., Bhaumik A., Das Saha K. Folic acid-conjugated magnetic mesoporous silica nanoparticles loaded with quercetin: a theranostic approach for cancer management. RSC Adv. 2020;10(39):23148–23164. doi: 10.1039/D0RA00664E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee A., Mishra S., Kotla N.K., Manna K., Roy S., Kundu B., Bhattacharya D., Das Saha K., Talukdar A. Semisynthetic quercetin derivatives with potent antitumor activity in colon carcinoma. ACS Omega. 2019;4(4):7285–7298. doi: 10.1021/acsomega.9b00143. [DOI] [Google Scholar]
- Nandi R., Mishra S., Maji T.K., Manna K., Kar P., Banerjee S., Dutta S., Sharma S.K., Lemmens P., Saha K.D., Pal S.K. A novel nanohybrid for cancer theranostics: folate sensitized Fe2O3 nanoparticles for colorectal cancer diagnosis and photodynamic therapy. J. Mater. Chem. B. 2017;5(21):3927–3939. doi: 10.1039/C6TB03292C. [DOI] [PubMed] [Google Scholar]
- O’Boyle N.M., Banck M., James C.A., Morley C., Vandermeersch T., Hutchison G.R. Open Babel: An open chemical toolbox. J. Cheminf. 2011;3(1):33. doi: 10.1186/1758-2946-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ognjanović B.I., Djordjević N.Z., Matić M.M., Obradović J.M., Mladenović J.M., Štajn A.Š., Saičić Z.S. Lipid peroxidative damage on cisplatin exposure and alterations in antioxidant defense system in rat kidneys: a possible protective effect of selenium. Int. J. Mol. Sci. 2012;13(2):1790–1803. doi: 10.3390/ijms13021790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olszewska-Słonina D.M., Mątewski D., Czajkowski R., Olszewski K.J., Woźniak A., Odrowąż-Sypniewska G., Lis K., Musiałkiewicz D., Kowaliszyn B. The concentration of thiobarbituric acid reactive substances (TBARS) and paraoxonase activity in blood of patients with osteoarthrosis after endoprosthesis implantation. Medical Sci. Monitor. 2011;17(9):CR498–CR504. doi: 10.12659/MSM.881936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauwels B., Korst A.E.C., Lardon F., Vermorken J.B. Combined modality therapy of gemcitabine and radiation. Oncologist. 2005;10(1):34–51. doi: 10.1634/theoncologist.10-1-34. [DOI] [PubMed] [Google Scholar]
- Pettersen E.F., Goddard T.D., Huang C.C., Couch G.S., Greenblatt D.M., Meng E.C., Ferrin T.E. UCSF Chimera?A visualization system for exploratory research and analysis. J. Comput. Chem. 2004;25(13):1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- Ponte L.G.S., Pavan I.C.B., Mancini M.C.S., da Silva L.G.S., Morelli A.P., Severino M.B., Bezerra R.M.N., Simabuco F.M. The Hallmarks of Flavonoids in Cancer. Molecules. 2021;26(7):2029. doi: 10.3390/molecules26072029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redza-Dutordoir M., Averill-Bates D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochimica et Biophysica Acta (BBA) - Molecular. Cell Res. 2016;1863(12):2977–2992. doi: 10.1016/j.bbamcr.2016.09.012. [DOI] [PubMed] [Google Scholar]
- Sarkar C., Chaudhary P., Jamaddar S., Janmeda P., Mondal M., Mubarak M.S., Islam M.T. Redox activity of flavonoids: impact on human health, therapeutics, and chemical safety. Chem. Res. Toxicol. 2022;35(2):140–162. doi: 10.1021/acs.chemrestox.1c00348. [DOI] [PubMed] [Google Scholar]
- Slika H., Mansour H., Wehbe N., Nasser S.A., Iratni R., Nasrallah G., Shaito A., Ghaddar T., Kobeissy F., Eid A.H. Therapeutic potential of flavonoids in cancer: ROS-mediated mechanisms. Biomed. Pharmacother. 2022;146 doi: 10.1016/j.biopha.2021.112442. [DOI] [PubMed] [Google Scholar]
- Tang L., Wei F., Wu Y., He Y., Shi L., Xiong F., Gong Z., Guo C., Li X., Deng H., Cao K., Zhou M., Xiang B., Li X., Li Y., Li G., Xiong W., Zeng Z. Role of metabolism in cancer cell radioresistance and radiosensitization methods. J. Exp. Clin. Cancer Res. 2018;37(1):87. doi: 10.1186/s13046-018-0758-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teckie S., Makker V., Tabar V., Alektiar K., Aghajanian C., Hensley M., Beal K. Radiation therapy for epithelial ovarian cancer brain metastases: clinical outcomes and predictors of survival. Radiat. Oncol. 2013;8(1):36. doi: 10.1186/1748-717X-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teekaraman D., Elayapillai S.P., Viswanathan M.P., Jagadeesan A. Quercetin inhibits human metastatic ovarian cancer cell growth and modulates components of the intrinsic apoptotic pathway in PA-1 cell line. Chem. Biol. Interact. 2019;300:91–100. doi: 10.1016/j.cbi.2019.01.008. [DOI] [PubMed] [Google Scholar]
- Tiwari P., Mishra K.P. Flavonoids sensitize tumor cells to radiation: molecular mechanisms and relevance to cancer radiotherapy. Int. J. Radiat Biol. 2020;96(3):360–369. doi: 10.1080/09553002.2020.1694193. [DOI] [PubMed] [Google Scholar]
- Vafadar A., Shabaninejad Z., Movahedpour A., Fallahi F., Taghavipour M., Ghasemi Y., Akbari M., Shafiee A., Hajighadimi S., Moradizarmehri S., Razi E., Savardashtaki A., Mirzaei H. Quercetin and cancer: new insights into its therapeutic effects on ovarian cancer cells. Cell Biosci. 2020;10(1):32. doi: 10.1186/s13578-020-00397-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter M.H., Hays J.L. Use of targeted therapeutics in epithelial ovarian cancer: a review of current literature and future directions. Clin. Ther. 2018;40(3):361–371. doi: 10.1016/j.clinthera.2018.01.012. [DOI] [PubMed] [Google Scholar]
- Wang J., Kang M., Wen Q., Qin Y.-T., Wei Z.-X., Xiao J.-J., Wang R.-S. Berberine sensitizes nasopharyngeal carcinoma cells to radiation through inhibition of Sp1 and EMT. Oncol. Rep. 2017;37(4):2425–2432. doi: 10.3892/or.2017.5499. [DOI] [PubMed] [Google Scholar]
- Wu Y.-H., Huang Y.-F., Chen C.-C., Huang C.-Y., Chou C.-Y. Comparing PI3K/Akt inhibitors used in ovarian cancer treatment. Front. Pharmacol. 2020;11 doi: 10.3389/fphar.2020.00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H., Pan L., Gao C., Xu H., Li Y., Zhang L., Ma L., Meng L., Sun X., Qin H. Quercetin inhibits the proliferation of glycolysis-addicted HCC cells by reducing Hexokinase 2 and Akt-mTOR pathway. Molecules. 2019;24(10):1993. doi: 10.3390/molecules24101993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J., Milosevic M., Fyles A., Manchul L., Kelly V., Levin W. A Hypofractionated radiotherapy Regimen (0–7-21) for advanced gynaecological cancer patients. Clin. Oncol. 2011;23(7):476–481. doi: 10.1016/j.clon.2011.01.001. [DOI] [PubMed] [Google Scholar]
- Yan Y.-B., Tian Q., Zhang J.-F., Xiang Y. Antitumor effects and molecular mechanisms of action of natural products in ovarian cancer (Review) Oncol. Lett. 2020;20(5):1. doi: 10.3892/ol.2020.12001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Sheng J., Li G., Zhao L., Wang Y., Yang W., Yao X., Sun L., Zhang Z., Cui R. Effects of berberine and its derivatives on cancer: a systems pharmacology review. Front. Pharmacol. 2020;10 doi: 10.3389/fphar.2019.01461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou K., Li Z., Zhang Y., Zhang H., Li B., Zhu W., Shi J., Jia Q., Li Y. Advances in the study of berberine and its derivatives: a focus on anti-inflammatory and anti-tumor effects in the digestive system. Acta Pharmacol. Sin. 2017;38(2):157–167. doi: 10.1038/aps.2016.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Online data is available without request.