Abstract
Honey bee venom (BV) is a valuable product, and has a wide range of biological effects, and its use is rapidly increasing in apitherapy. Therefore, the current study, we reviewed the existing knowledge about BV composition and its numerous pharmacological properties for future research and use. Honey bee venom or apitoxin is produced in the venom gland in the honey bee abdomen. Adult bees use it as a primary colony defense mechanism. It is composed of many biologically active substances including peptides, enzymes, amines, amino acids, phospholipids, minerals, carbohydrates as well as some volatile components. Melittin and phospholipase A2 are the most important components of BV, having anti-cancer, antimicrobial, anti-inflammatory, anti-arthritis, anti-nociceptive and other curative potentials. Therefore, in medicine, BV has been used for centuries against different diseases like arthritis, rheumatism, back pain, and various inflammatory infections. Nowadays, BV or its components separately, are used for the treatment of various diseases in different countries as a natural medicine with limited side effects. Consequently, scientists as well as several pharmaceutical companies are trying to get a new understanding about BV, its substances and its activity for more effective use of this natural remedy in modern medicine.
Keywords: Bee venom, Chemical composition, Physical properties, Pharmacological properties, Therapeutic potential
Abbreviations
- Abbreviation
Definition
- BV
Bee venom
- PLA2
Phospholipase A2
- BVA
Bee venom acupuncture
- MCDP
Mast cell-degranulating peptide
- IgE
Immunoglobulin E
- NF-κB
Nuclear factor kappa B
- DRs’
Death receptor 3
- ROS
Reactive oxygen species
- AIF
Apoptosis-induced factors
- EndoG
Endonuclease G
- Akt
Protein kinase B
- Bcl-2
B-cell lymphoma 2
- DNA
Di oxy ribonucleic acid
- cDDP
cis-diamminedichloroplatinum
- HeLa
Henrietta Lacks
- CK
Cytokinin
- HPBLs
Human peripheral blood leukocytes
- MDA
Malondialdehyde
- GSH
Glutathione
- MIC
Minimum inhibitory concentrations
- TMV
Tobacco mosaic virus
- HIV
Human immunodeficiency virus
- VK2
Vaginal cell line
- HSV-1
Herpes simplex virus 1
- IFN
Interferon type I
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- H2O2
Hydrogen peroxide
- ALS
Amyotrophic lateral sclerosis
- HSCs
Hepatic stellate cells
- CCL4
Carbon tetrachloride
- LDH
Lactate dehydrogenase
- CK-MB
Creatine kinase myocardial band
- cTnI
Cardiac troponin 1
- MCF7
Human breast cancer cells line
- McARH7777
Rat hepatocellular carcinoma
- SKB R3
Human breast cancer cell line
- Cyt C
Cytochrome c
- TNBC
Triple-negative Breast cancer
- HER2
Human epidermal growth factor receptor 2
- PFC
Perfluorocarbon
- K14-HPV16
Human papillomavirus transgenic mice
- III sPLA2
Secreted Phospholipase A2
- FHC
Fetal human cells
- VEGF
Vascular endothelial growth factor
- VEGFR-2
Vascular endothelial growth factor receptor 2
- HCT116 cells
Human colorectal carcinoma cell line
- PGE2
Prostaglandin E2
- COX-2
Cyclooxygenase 2
- mRNA
Messenger RNA
- THP-1
Human leukemia monocytic cell line
- SBV
Sweet bee venom
- IL-1β
Interleukin 1 beta
- TNF-α
Tumor necrosis factor alpha
- TLR2
Toll-like receptors
- IGF-1
Insulin-like growth factor-1
- NO
Nitric oxide
- TNF
Tumor Necrosis Factor
- PG
Prostaglandin
- THP 1
Human leukemia monocytic cell line
- IKK
IκB kinase
- HaCaT
Human keratinocyte cell line
- MAPK
Mitogen-activated protein kinase
- MRTP
1-methyl-4- phenyl-1,2,3,6 tetrahydropyridine
- MS
Multiple sclerosis
- VGCs
voltage-gated channels
- AST
Aspartate aminotransferase
- LD50
lethal dose
- TZM-bl
A cell line (Uterus or cervix)
- SREBP
Sterol regulatory element-binding protein
- SMMC-7721
Human hepatocellular carcinoma
- N1S1 cells
Rat hepatocellular carcinoma
- MDA-MB-453
Human breast cancer cell line
1. Introduction
In apitherapy honey bee products including honey, beeswax, royal jelly, propolis and venom are used. These products are useful in treatment of various diseases and alterations of the human being (Ali, 2012, Silva et al., 2015, Abdela and Jilo, 2016, Basa et al., 2016, Azam et al., 2018, Šuran et al., 2021). Bee products were used in ancient times and their therapeutic efficacy is mentioned in the Holy Quran, Bible, and Vida (Münstedt and Bogdanov, 2009, Ali, 2012, Silva et al., 2015). Therefore, numerous studies are focused especially on the BV. Bee venom is produced in the venom gland placed in the honey bee’s abdominal cavity (Gajski and Garaj-Vrhovac, 2013, Samanci and Kekeçoğlu, 2019, Aufschnaiter et al., 2020, Kim et al., 2020a, Kim et al., 2020b, Nainu et al., 2021). However, bee holds an over 300 μg of venom in its venom sac (Bilo et al., 2005, Komi et al., 2018) and injects an average of about 50–140 μg of venom during a single sting (Ozdemir et al., 2011, Moreno and Giralt, 2015). Adult honey bees use it for hive defense (Frangieh et al., 2019, Ko et al., 2020). During the honey bee attack, the stinger (with barbs) is drawn out from the abdomen together with the venom pouch. Honey bees die after stinging once. The effect of bees’ stings on the host body can be local or systemic. The local reaction to the sting of all insects from the order Hymenoptera is similar, because of the presence of similar toxin components. The systemic reactions depend upon the allergens present in the BV (Fitzgerald and Flood, 2006, Komi et al., 2018)..
Honey bee venom consists of various bioactive compounds including several peptides, amines, enzymes, amino acids, lipids, and other water dissolvable substances (Chen and Lariviere, 2010, Sobral et al., 2016, Rady et al., 2017, Azam et al., 2018, Komi et al., 2018, El Adham et al., 2022). Due to these substances, BV has an anti-inflammatory, antibacterial, antiviral anti-cancer, anti-mutagenic, anti-nociceptive and radioprotective activity (Garaj-Vrhovac and Gajski, 2011, Samanci and Kekeçoğlu, 2019, Kim et al., 2020a, Kim et al., 2020b). Its medicinal properties have been recognized in the ancient ages. In antique medicine, BV was used for the treatments of dermal diseases, back pain, rheumatism, and arthritis (Kim et al., 2020a, Kim et al., 2020b, Abdela and Jilo, 2016, Uddin et al., 2016, Aliyazicioglu, 2019). Today, it is used for the cure of various human and animal diseases such as nervous system alterations, arthritis, blood circulatory system disease, tumors, skin diseases and a few immune-related defects (Bellik, 2015, Abdela and Jilo, 2016). Furthermore, the components of BV like phospholipase A2 and melittin can be used against numerous types of cancer cells such as prostate, liver, renal, cervical and mammary cancer cells (Abdela and Jilo 2016). Bee venom can be applied as cream, ointment, or liniment, through acupuncture or an injection. Also, BV can be applied via a honey bee sting (Ali, 2012, Silva et al., 2015). Therefore, on the market, BV is available in many forms, such as injections, ointments, creams, natural bee stings, tablets, balms, bee sting emergency kits, as well as pure liquid venom. Moreover, some specific laboratories can provide the components isolated from BV such as melittin, phospholipase A2 or other components for medicinal and scientific purposes (Ali 2012). The most used technique is bee venom acupuncture (BVA) in which a low concentration of BV can be applied to the patient's body (Silva et al., 2015, Abdela and Jilo, 2016, Ko et al., 2022). Bee venom acupuncture is a highly effective method for the treatment of osteoarthritis and rheumatoid arthritis (Hegazi, 2012, Silva et al., 2015, Abd El-Wahed et al., 2019, Chen et al., 2020). In a recent study, it was proved that the treatment with bee venom results in reproductive disorders in the mouse model, such as reduction in sperm count, motility of sperm, testosterone level as well as some irregular structural changes in sperm morphology, seminiferous tubules (Regeai et al., 2021). The aim of this study is to summarize the existing knowledge on BV with special emphasis on its various properties and therapeutic potential and suggest of new proposals for future research.
2. Physical properties of honey bee venom
Bee venom is an odorless, translucent fluid with pungent scent (Wehbe et al., 2019, Kim et al., 2020a, Kim et al., 2020b, Pattabhiramaiah et al., 2020, Choi et al., 2021). It has an unpleasant taste and pH from 4.5 to 5.5 (Ali, 2012, Abdela and Jilo, 2016, Pattabhiramaiah et al., 2020). It is dissolvable in water and insoluble in ammonium sulfate as well as alcohol (Hossen et al., 2017a, Hossen et al., 2017b). Due to the oxidation of BV protein, the dehydrated BV becomes light pale, while some variants available on the market are brown (Ali, 2012, Abdela and Jilo, 2016). Also, BV contains about 88 % of water (Hossen et al., 2017a, Hossen et al., 2017b, Wehbe et al., 2019, Nainu et al., 2021). Additionally, the venom contents like phospholipid, fructose and glucose are similar to the contents present in bee hemolymph. Due to its components, in direct contact with eyes or mucous membranes, BV causes mechanical damage (Ali 2012).
3. Chemical composition of honey bee venom
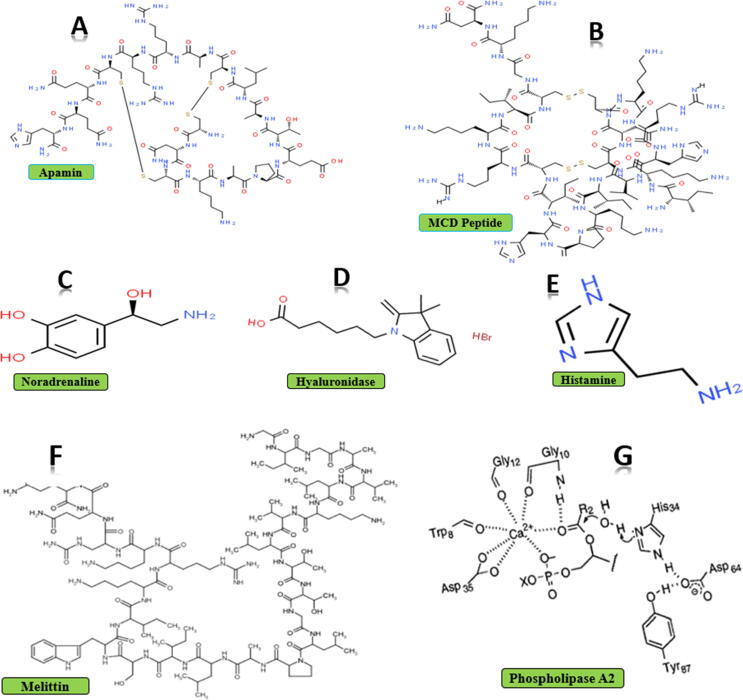
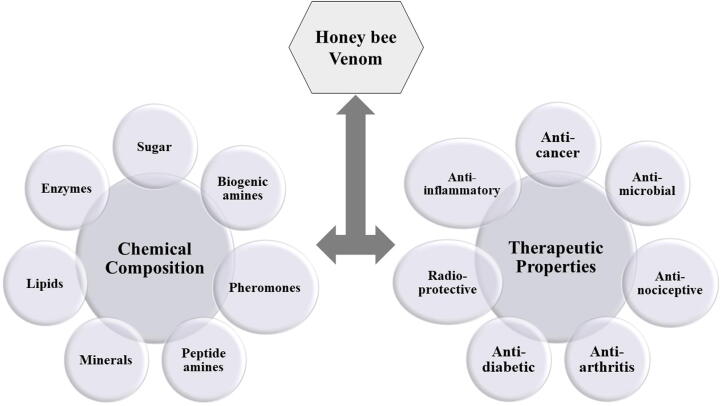
Bee venom contains 18 biologically active components including polypeptides, amines, enzymes, amino acids and lipids (Table 1; Fig. 1, Fig. 2) (Uddin et al., 2016, Hossen et al., 2017a, Hossen et al., 2017b, Abd El-Wahed et al., 2019, Frangieh et al., 2019, Wehbe et al., 2019, Ko et al., 2020, Kong et al., 2020, Shen et al., 2020). Also, there are various peptides in BV like mast cell-degranulating peptide (MCDP), melittin, adolapin and apamin (Zhang et al., 2018, Aufschnaiter et al., 2020, Kim et al., 2020a, Kim et al., 2020b, Lamas et al., 2020). But BV is mostly made of melittin which comprises 26 amino acids (Hossen et al., 2017a, Hossen et al., 2017b, Moga et al., 2018, Kong et al., 2020, Badawi, 2021). Melittin makes about 50 % part of the total BV dry weight (Gajski and Garaj-Vrhovac, 2010, Chen et al., 2016, Hossen et al., 2017a, Hossen et al., 2017b, El-Seedi et al., 2020). The molecular weight of melittin is about 2840 Daltons (Rady et al., 2017, Somwongin et al., 2018). Another important component of BV is called mast cell degranulating peptide (MCD peptide) or peptide 401. This peptide contains 22 amino acids and makes 2–3 % of BV dry weight (Baracchi et al., 2011, Wehbe et al., 2019). The name has been given because of its biotic property of histamine released from mast cells (Pucca et al., 2019). The enzymes in BV are hyaluronidase and phospholipase (Zhang et al., 2018, Kim et al., 2020a, Kim et al., 2020b, Pattabhiramaiah et al., 2020, Badawi, 2021).
Table 1.
General composition of honey bee venom.
Fig. 1.
Chemical structures of important BV components [Data of A, B, C, D, E obtained from SpiderChem (Royal Society of Chemistry)] (F); (Jampilek and Kralova 2021), (G); (Annand et al., 1996).
Fig. 2.
Composition and pharmacological activities of bee venom.
Those enzymes are responsible for activating immunity and inducing IgE reactions in sensitive people (Moreno and Giralt, 2015, Abdela and Jilo, 2016). Furthermore, phospholipase A2 (PLA2) is the main allergen agent of BV and makes up about 10 to12% of the total weight of BV (Hossen et al., 2017a, Hossen et al., 2017b, Wehbe et al., 2019, Baek et al., 2020). In addition, PLA2 (a calcium-dependent enzyme), accelerates hydrolyze processes of the sn-2 ester of glycerophospholipids and liberates lysophospholipids and fatty acids (Oršolić, 2012, Frangieh et al., 2019, Baek et al., 2020). Hyaluronidase is also called the “spreading factor” and it is the second most important allergen of BV, disrupting the cell membrane which hydrolyzes the sticky polymer hyaluronic acid into non-sticky parts (Moreno and Giralt, 2015, Abdela and Jilo, 2016). Additionally, it facilitates the activity of other toxins among the cells, after the dissolution of extracellular substances(Bellik, 2015, Komi et al., 2018). Apamin, which is also called the smallest neurotoxin of BV, contains 18 amino acids (Abd El-Wahed et al., 2019, Wehbe et al., 2019), and has two disulfide bridges. Also, apamin is known as a well selective terminator of calcium ion (Ca2+) stimulated potassium (K+) channels (Chen and Lariviere, 2010, Bellik, 2015, Moga et al., 2018). Additionally, an important polypeptide of BV is adolapin. It has 103 amino acids and makes 1 % of BV dry weight (Abd El-Wahed et al., 2019, Wehbe et al., 2019). Other low molecular weight compounds like amino acids, minerals, catecholamines and sugars are also present in BV (Moreno and Giralt, 2015, Pucca et al., 2019). Also, BV contains amines such as dopamine, norepinephrine and histamine (Komi et al., 2018). The main component of amine is histamine, and it contributes to the inflammatory reaction by enhancing the penetrability into the blood vessels. Similarly, other components like catecholamines, norepinephrine and dopamine facilitate the dispersal and circulation of BV by enhancing the heart beat (Moreno and Giralt, 2015, Abdela and Jilo, 2016).
4. Pharmacological properties of honey bee venom
Bee venom and its components have great biotic and pharmaceutical potentials, including anti-cancer, anti-bacterial, anti-inflammatory, anti-viral, radioprotective, anti-nociceptive, anti-arthritis, antifungal as well as hepatoprotective properties (Fig. 2; Table 2).
Table 2.
Basic components of bee venom and its medicinal properties.
| Component | Biological properties of Bee venom | References |
|---|---|---|
| Melittin | anticancer, anti-tumor, anti-angiogenesis, anti-fungal, anti-parasitic, anti-microbial, anti-arthritic, anti-inflammatory, anti-psychotic, anti-atherosclerosis, anti-bacterial, anti-viral, | (Bellik, 2015, Liu Cui-Cui et al., 2016, Rady et al., 2017, Aliyazicioglu, 2019) |
| Apamin | anti-fungal, anti-viral, anti-inflammatory, analgesic | (Wehbe et al., 2019, Memariani et al., 2020) |
| Adolapin | anti-arthritic, analgesic, anti-inflammatory, antipyretic, anti-nociceptive | (Ali, 2012, Wehbe et al., 2019) |
| MCD peptide | anti-inflammatory | (Komi et al., 2018) |
| Phospholipase A2 | anticancer, antiprotozoal, anti-inflammatory, immunomodulatory, anti-bacterial, anti-viral | (Oršolić, 2012, Lee and Bae, 2016a, Lee and Bae, 2016b, Hossen et al., 2017a, Hossen et al., 2017b) |
| Hyaluronidase | hydrolysis of hyaluronic acid | (Moreno and Giralt 2015) |
| Histamine | perviousness of blood vessels | (Moreno and Giralt 2015) |
| Lasioglossins | anti-microbial, cytotoxicity | (Azam et al., 2018) |
4.1. Anti-cancer activity of BV
Studies have shown that BV has anti-cancer efficacy against prostate cancer cells, hepatocellular cancer cells, lung cancer cells, mammary cancer cells, bladder cancer cells, ovarian cancer cells, leukemia, and melanocyte cancer cells (Liu Cui-Cui et al., 2016, Rady et al., 2017, Abd El-Wahed et al., 2019, Wehbe et al., 2019, Varol et al., 2022). In lung cancer cells, BV causes apoptosis by activating the DRs’ (death receptor 3) (Rady et al., 2017). Similarly, it was shown that BV stops the apoptosis in melanocyte (melanoma cells) cancer by a calcium-dependent pathway, followed by reactive oxygen species (ROS) production, the liberation of apoptosis-induced factors (AIF), endonuclease G (EndoG), and calcium variation (Oršolić 2012). A component of BV, melittin, stops the spread of cancer cells trough the initiation of apoptosis (Moga et al., 2018, Aliyazicioglu, 2019). Also, melittin causes apoptosis in leukemia cells (U937 cells) via the suppression of Akt (protein kinase B) signal processes (Gajski and Garaj-Vrhovac, 2008, Bellik, 2015, Rady et al., 2017, Moga et al., 2018). Bee venom treatment also downregulated Bcl-2 (B-cell lymphoma 2) which limits the initiation of caspase-3 and cells reinstated their viability in leukemic cells (Varol et al., 2022). Furthermore, melittin inhibits calmodulin (calcium-binding protein) which restrains the progress of leukemia cells in the human body (Son et al., 2007, Liu Cui-Cui et al., 2016). Also, melittin triggers apoptosis in gastrointestinal cancer cells (SGC-7901 cells) (Rady et al., 2017).
Previous studies indicate that melittin disrupts and targets the whole-cell membrane structure, compared to other chemotherapeutic medicines which can’t distinguish typical from and cancer cells (Liu Cui-Cui et al., 2016). Furthermore, melittin can destroy the internal cytoplasm content and penetrate inside the cancer cell through endocytosis and destroys the cell membrane (Kohno et al., 2014). The mechanism of the anti-cancer capability of melittin depends upon the type of cancer cell. For instance, melittin can disrupt metastasis, proliferation, angiogenesis, cell cycle and apoptosis of cancer cells and regulate or activate these reactions via various genes, molecules and other signal pathways (Oršolić 2012). Gajski et al. (2014) investigated the combined effect of BV and cisplatin. Authors concluded those two substances have synergistic killing activity towards all tested cell lines (HeLa CK and CK2 cells etc). Because of the pretreatment with BV, these cell lines become more susceptible and the chances of resistance against cisplatin is reduced. Also, melittin causes distraction and the formation of pores in the cell membrane increasing the entry and deposition of cisplatin, which triggers the cytotoxicity of cancer cells (Gajski et al., 2014). Additionally, melittin and cisplatin have a synergistic effect on A1235 cells. That combination boosts the killing activity of BV and decreases the resistance of cDDP (cis-diamminedichloroplatinum) (Gajski et al., 2016a, Gajski et al., 2016b).
It was documented that the membrane disrupting action of melittin can enhance cell membrane permeability and open calcium ion channels which change and elevate free Ca2+ concentration inside the cell that causes necrosis or apoptosis in mammal cells (Saris and Carafoli 2005). Furthermore, previously published articles showed the destructive and cytotoxic effect of melittin in leukemia patients (L1210 cells) (Son et al., 2007, Liu Cui-Cui et al., 2016). Similarly, the BV significantly increases MDA (malondialdehyde) level while decreases the level of GSH (glutathione) and enhances the damage of DNA in human peripheral blood leukocytes (HPBLs). Because both MDA and GSH are oxidative stress factors that could be the cytotoxicity mechanism of BV. While BV also enhanced the formation of lipid peroxide in renal proximal tubule cells of rabbit which is involved for the expressions of oxidative pressure. But some studies showed the cytotoxic effect of BV or melittin on normal cells, indicating the need for further researches (Gajski et al., 2012). Additionally, melittin selectively targeted tumor cells because these cells possess high membrane potential as compared to normal cells (Gajski et al., 2016a, Gajski et al., 2016b) and the concentration, which was used against tumor cells, does not affect the normal cells growth (Gajski and Garaj-Vrhovac 2013). Bee venom holds anti-tumor efficacy against different cancer cells like Breast cancer cell, Liver and Cervix cancer cells in a time and dose dependent way and have no side effect on non-target cells (Salama et al., 2021).
According to Kong et al. (2016) melittin enhances the protein expressions of human digestive tumor cell’s mitochondrial pro-apoptotic factors as AIF (Apoptosis inducing factor), EndoG (endonuclease G) as well as cytochrome c (Cyt C) while reduces the Smac/Diablo which causes apoptosis by means of the mitochondrial-dependent pathway (Kong et al., 2016). Wang et al. (2009) investigated the anti-tumor activity of melittin in human liver cancer cells. Authors concluded that melittin can enhance the discharge of cytochrome c through the stimulation of caspase-9, caspase-3 and the calcium channels, causing disturbance of mitochondrial membrane penetrability (Wang et al., 2009). Also, previous studies showed that BV causes alterations in cell structure, reduces cell survival rate and necrosis in human lymphocyte cells in vitro. Because the main component of BV, melittin has a cell demolition property (Garaj-Vrhovac and Gajski, 2009, Gajski and Garaj-Vrhovac, 2011, Gajski et al., 2016a, Gajski et al., 2016b). It has been reported that BV restrains lung cancer cell development via the suppression of DNA binding potential of NF-κB. In addition, BV possesses anti-cancer capability which inactivates NF-κB and overexpresses DR3 in lung cancer cells therapy (Choi et al., 2014). Studies revealed that melittin is the potent agent toward TNBC (Triple-negative Breast cancer) (SUM149 and SUM159) and HER2 (Human epidermal growth factor receptor 2) enriched breast cancer (SKB R3 and MDA-MB-453) while having protective impact on normal cells (Duffy et al., 2020).
In another study, scientists observed the anti-angiogenesis activity of melittin on liver tumor cells of humans (Zhang et al., 2016). Wu et al. (2015) suggest that melittin can prevent the progression of cancer cells by affecting their cell cycle (Wu et al., 2015). Also, melittin can act as an antitumor and anti-vascular agent through a PFC (Perfluorocarbon) nanoparticles delivery system which has therapeutic targeted potential (Pan et al., 2011). Melittin nanoparticles revealed the anticancer capability via the immunomodulation of liver sinusoidal endothelial cells which suppress the liver malignant growth (Duffy et al., 2020). Additionally, melittin-laden nanoparticles significantly provided melittin to the mice model (having squamous dysplasia and carcinoma induced by human papillomavirus transgenic elements such as E6 and E7) intravenously which successfully targeted and killed precancerous lacerations in K14-HPV16 (Human papillomavirus transgenic mice) (Kasozi et al., 2020). Bee venom acupuncture has a favorable effect in the restriction of peripheral nervous system disease that is caused by the chemical treatment of cancer. The peptide of BV called lasioglossin II shows cytotoxicity against the different tumor cells ex vivo (Abdela and Jilo, 2016, Azam et al., 2018). It was demonstrated that BV component group III sPLA2 (secreted PLA2) as well as phosphatidylinositol-(3,4)-bisphosphate mutually disintegrate kidney tumor cell membrane and subsequently cause tumor cell death (Lee and Bae, 2016a, Lee and Bae, 2016b). Researchers examined the anti-tumor activity of BV in vitro and stated that BV restrains K1735M2 cancer cells in the mice model because it inhibits the cell cycle of these tumor cells at the G1 stage (Komi et al., 2018).
Also, the administration of BV intravenously decreases the spread of lungs tumors in the mice models (Oršolić 2012). Furthermore, BV stops the multiplication of B16 malignancy in C57BL/6 in vivo and K1735M2 tumor cells ex vivo in mice models (Liu et al., 2002, Rady et al., 2017). Both in vivo and ex vivo studies suggest that BV is useful in the fight against prostate cancer by the stimulation of caspase as well as through the deactivation of the NF-κB process. Bee venom is also preventing the progression of colon tumor cells via stimulation of apoptosis having no negative impact on normal FHC (fetal human cells) of colon epithelium (Zheng et al., 2015, Rady et al., 2017). It has been stated that the activation of phospholipase A2 by melittin can enhance the activity of calpain as well as necrosis of hepatic cancer cells including McARH7777 and N1S1 cells (Oršolić 2012). Huh et al. (2010) state that BV can obstruct metastasis and angiogenesis through the suppression of VEGFR-2 (Vascular endothelial growth factor receptor 2) and VEGF (Vascular endothelial growth factor) in pulmonary cancer cells (Huh et al., 2010). Moreover, the treatment of cancer with the melittin gene (gene therapy) in vivo causes apoptosis in tumor cells (Ling et al., 2005, Oršolić, 2012). The BV polypeptides have a major inhibition role in the suppression of human hepatocellular carcinoma (SMMC-7721) (Hu et al., 2006, Oršolić, 2012, Azam et al., 2018). Putz et al. (2006) concluded that a combination of anti-cancer effect of phospholipase A2 and phosphatidylinositol-homologs causes the interruption of membrane integrity, abolition of signal transduction and the obstruction of renal melanoma cell proliferation (Putz et al., 2006). Also, BV possesses cytotoxicity against leukemia, mammary carcinoma, osteosarcoma, and hepatoma cells (Moon et al., 2006, Chu et al., 2007).
The venom of A. mellifera syriaca was used against human colon cancer cells, which showed the combined cytotoxicity activity of melittin and phospholipase A2 towards HCT116 cells (Human colorectal carcinoma cell line) (Yaacoub et al., 2021). In addition, BV has an anti-cancer capability toward NCI-H1299 cells (human lung cancer) via the initiation of apoptosis and the synthesis of PGE2 (Prostaglandin E2) because of the obstruction of COX-2 (Cyclooxygenase 2) mRNA expression (Bellik 2015). It was discovered that the compounds of BV have anti-cancer activity against MCF7 cells (human breast cancer cells), which cause apoptosis via stimulating caspase-3 and −9 or by the discharge of AIF and EndoG from mitochondria (Moga et al., 2018). A recent study has shown that the sweet bee venom revealed cell death of THP-1 (human leukemia monocytic cell line) cells at a concentration of 20 µg/mL (Ryu et al., 2022). Similarly, due to the increased expression of protein of p21 and p53, bee venom also showed anti-tumor efficacy towards pancreatic cancer cell lines (Zhao et al., 2022). The current literature shows that bee venom or its individual component melittin possess anti-cancer activity against various cell lines, but some obstacles remain for successful therapy, like non-specificity in cytotoxicity, in vivo lysis activity and ineffective system delivery. However, modern strategies such as nanotechnology, gene therapy and immunoconjugation will overcome these restrictions and bee venom or its components will be used as a therapeutic agent in clinical applications in the near future.
4.2. Antimicrobial action of bee venom
4.2.1. Antifungal activity of BV
It has been documented that melittin possesses anti-fungal activity (Rady et al., 2017, Pattabhiramaiah et al., 2020) against Candida albicans by destroying membrane and causing the cell apoptosis in a caspase/mitochondrial-dependent way (Liu Cui-Cui et al., 2016). Also, BV is a strong agent against Trichophyton rubrum, Trichophyton mentagrophytes (El-Seedi et al., 2020), Malassezia furfur and Candida albicans (Kim et al., 2019, Kurek-Górecka et al., 2020). It is proved that the effect of BV toward Trichophyton rubrum and Trichophyton mentagrophytes is stronger than of commercially available antifungal medicine, fluconazole (Park et al., 2018, El-Seedi et al., 2020). Furthermore, sweet bee venom (SBV) (BV without enzymes and histamines) possesses stronger antifungal efficacy compared to BV (Lee 2016). Both SBV and BV show antifungal capability on 10 experimental C. albicans strains isolated from vagina and blood through broth micro-dilution assay, disk diffusion assay and killing-curve assay (Park et al., 2018). Surendra et al. (2011) presented that BV of Apis cerana has strong inhibitory activity towards C. albicans as compared to Apis dorsata and Apis florea, respectively (Surendra et al., 2011). The two constituents of BV, apamin and melittin, show inhibitory effects against Aspergillus pillows and Alternaria alternate which cause inflammatory disease in the nasal cavity (El-Seedi et al., 2020). Based on the literature studies, a larger and long-term follow-up trial is needed to determine the safety and efficacy of BV because its safety is still a strong limiting consideration during clinical treatment.
4.2.2. Anti-protozoal activity of BV
Studies revealed that BV group III sPLA2 has anti-trypanosomiasis properties (Lee and Bae, 2016a, Lee and Bae, 2016b, Pucca et al., 2019). The expression of BV group III sPLA2 in a genetically modified mosquito’s midgut has inhibitory action toward Plasmodium ookinetes (Bellik, 2015, Lee and Bae, 2016a, Lee and Bae, 2016b). Additionally, cecropin (a hybrid of melittin) has antileishmanial efficacy for Leishmania donavani promastigote via disrupting its plasma membrane (Bellik 2015). Melittin disrupts the membrane’s integrity of both prokaryotic and eukaryotic organisms which causes lysis of membrane and permeability. This kind of reaction makes melittin an anti-microbial, anti-fungal, and antileishmanial agent (Bellik 2015). According to previous research, PLA2 shows anti-protozoal action towards Trypanosoma brucei brucei in controlled conditions. Bee venom peptide called Lasioglossins, possesses greater antimicrobial action because of its membrane interaction, as well as DNA binding (Bandyopadhyay et al., 2013, Ali, 2014). It has been reported that, the peptide melittin possesses antiprotozoal activity against Toxoplasma gondii, Trypanosoma cruzi, Plasmodium and Leishmania. Furthermore, melittin has been utilized in vaccine preparation to enhance immunity to leishmaniasis. Also, melittin shows killing activity towards Trypanosoma cruzi (Memariani and Memariani 2021). As we reviewed, BV possesses antiprotozoal activities but the exact effect of its compounds along with mode of action and its commercialization as a therapeutic medicine is still unknown.
4.2.3. Anti-bacterial activity of BV
Researchers documented that BV group III sPLA2 has anti-bacterial efficacy against Gram-negative bacteria (Boutrin et al., 2008, Lee and Bae, 2016a, Lee and Bae, 2016b). However, many studies revealed that melittin shows anti-bacterial activity toward both types of bacteria, Gram-negative and Gram-positive bacteria (Lee et al., 2015, Komi et al., 2018). It was demonstrated that BV possesses anti-bacterial activity towards various inflammatory dermal diseases (Lee et al., 2015). Purified BV has anti-bacterial capability toward Propionibacterium acnes, clindamycin-resistant Propionibacterium acnes, Streptococcus pyogenes, Staphylococcus aureus, Staphylococcus epidermidis as well as Staphylococcus pyrogenes (Han et al., 2012, Kim et al., 2019, Kurek-Górecka et al., 2020). Some studies have exposed that the treatment of melittin recovered the skin lesions induced by MRSA (methicillin-resistant S. aureus) in mice models (Kim et al., 2019). Furthermore, the application of BV against Acne vulgaris significantly restricts the propagation of S. aureus which causes inflamed lesions (Lee 2016). It was observed in Korea that BV stopped the development of two Gram-negative bacteria while seventeen strains of gram-positive bacteria separated from bovine mastitis (Hegazi et al., 2015). Experiments have shown that BV has bactericidal action against Salmonella spp., Enterobacter cloacae, Citrobacter freundii, E. coli, S. aureus and Coagulase-negative Staphylococcus spp. (Hegazi et al., 2014).
Han et al. (2013) documented that BV displayed antibacterial efficacy towards Vibrio ichthyoenteri, Streptococcus iniae and Edwardsiella tarda (Han et al., 2013). Also, melittin has antibacterial activity against Vibrio parahaemolyticus (Memariani et al., 2019). It was indicated that the use of BV significantly lowered IL-1β (Interleukin 1 beta) along with TNF-α (Tumor necrosis factor alpha) level and decreased the inflammatory cells' population in mice skin which was caused by Cutibacterium acnes injection into ears. In the same way, BV also retarded the expression of CD14 (protein coding gene) and TLR2 (Toll-like receptors) in tissues that were induced by C. acnes injection (An et al., 2014, Kurek-Górecka et al., 2020). Additionally, due to its anti-lipogenesis and anti-acne activity BV and its component melittin obstructed the high expression of both pro-inflammatory factor and lipogenic in an IGF-1 (Insulin-like growth factor-1) stimulated lipogenic disorder and Cutibacterium acnes model via the inhibition of Akt/mTOR/SREBP signaling pathways (Gu et al., 2022). Due to its antibiotic properties, BV obstructs the progression of Listeria monocytogenes strains (foodborne pathogens) at low concentrations (Lamas et al., 2020). Bee venom along with melittin possesses anti-bacterial properties against penicillin resisting S. aureus strains (Memariani et al., 2019). Furthermore, melittin is a potent anti-bacterial agent for Borrelia burgdorferi which causes Lyme malady (Socarras et al., 2017). The expression of melittin in a plasmid can significantly inhibit genitourinary microbes such as Chlamydia trachomatis and Mycoplasma hominis intracellularly (Lazarev et al., 2002, Memariani et al., 2019). Likewise, melittin shows antibacterial efficacy against various plants' pathogenic bacteria, e.g., Xanthomonas oryzae pathovar oryzae (Shi et al., 2016). Correspondingly, melittin has inhibitory activity against 41 experimental bacterial types including 15 methicillin-sensitive S. aureus, 11 methicillin resistive S. aureus and 15 E. faecalis strains (Dosler and Gerceker, 2012, Memariani et al., 2019).
In another study, it was documented that melittin exhibited antibacterial activity toward 32 types of antibiotics opposing bacteria, which include P. aeruginosa, E. coli, Salmonella typhimurium, S. aureus at about 16 μM concentration (Gopal et al., 2013, Memariani et al., 2019). The combined effect of melittin along with erythromycin showed inhibitory action on K. pneumoniae (Liu et al., 2002, Moerman et al., 2002). In the same way, melittin also had antibiotic efficacy against A. baumannii isolates once united with imipenem as well as colistin (Bardbari et al., 2018). Lazarev et al. (2004) acknowledged that a plasmid containing the melittin gene had the potential to restrain Mycoplasma gallisepticum septicity in poultry (Lazarev et al., 2004). A new study revealed that melittin invades biofilm strata of P. aeruginosa progressively and attacks the biofilm existing bacteria via destroying their membranes (Khozani et al., 2019, Memariani et al., 2019). Bee venom and its components, melittin and PLA2, were used against various oral microbes which were responsible for dental decay. Consequently, BV minimum inhibitory concentrations (MIC) were about 20 to40 µg/mL when applied against Streptococcus mutans, S. salivarius, S. mitis, S. sobrinus, S. sanguinis, Enterococcus faecalis and Lactobacillus casei (Leandro et al., 2015, El-Seedi et al., 2020). Bee venom is effective against 14 out of 16 Salmonella strains of poultry due to its antibiofilm and antibacterial capability with MIC varying between 256 and 1024 µg/mL (Arteaga et al., 2019). Additionally, BV enhanced the production of antibodies against S. gallinarum (formalin-killed) in broiler chickens (Jung et al., 2013, El-Seedi et al., 2020). It has been shown that the bee venom of two sub-species of Apis mellifera (Apis mellifera carnica and Apis mellifera yemenitica) exhibited parallel anti-bacterial activity against Pseudomonas aeruginosa, Escherichia coli and Salmonella typhimurium with MIC (minimum inhibitory concentration) of 10 mg/ml (Alajmi et al., 2022). The above summarized antibacterial, antibiofilm and antifungal properties of bee venom revealed its therapeutic potential against microbial pathogens. Future clinical studies including detailed experimental investigation may eventually yield treatment of various disorders. So, we believe that bee venom can be used for therapeutic purposes if careful provisions are taken to avoid adverse effects.
4.2.4. Anti-viral activity of BV
Melittin possesses anti-viral activity against herpes simplex virus, TMV (Tobacco mosaic virus) and murine retrovirus (Uddin et al., 2016, Kurek-Górecka et al., 2020, Lamas et al., 2020). Additionally, nanoparticles holding melittin can demolish HIV (human immunodeficiency virus), having no harmful effect on adjacent cells (Eze et al., 2016, Azam et al., 2018, Wehbe et al., 2019). Likewise, it also showed viricidal capability toward HIV-1 in the epithelial vaginal cell line, VK2 (Vaginal cell line) and blocked the infection of HIV in TZM-bl (derived from HeLa cell) reporter cells (Ratcliffe et al., 2014, Da Mata et al., 2017). The analogous melittin called Hecate remarkably diminished the synthesis of virus-specific proteins (glycoproteins B, C, D, H) of herpes simplex virus type 1 (Da Mata et al., 2017). Melittin can disrupt the production of the viral proteins HSV-1 (Herpes simplex virus 1) and it also diminishes the expression of the HIV-1 viral genes and inhibits its replication (Bellik 2015). PLA2 also acts as an anti-viral agent for HIV (Bellik, 2015, Wehbe et al., 2019). It was demonstrated that BV and melittin had viricidal activity ex vivo against various enveloped and unenveloped viruses including herpes simplex virus, influenza A virus, vesicular stomatitis virus besides coxsackievirus, enterovirus-71 respectively along with Respiratory Syncytial Influenza A (El-Seedi et al., 2020).
Bee venom and its constituents can accelerate IFN (interferon type I), hence restrain viral multiplication in the host cell (Wehbe et al., 2019). Masuda et al. (2005) justified that phospholipase A2 acts as an anti-viral agent and has the proficiency to destroy the phospholipids of cell membranes (Masuda et al., 2005). Immediate application of phospholipase A2 against 293A cells reduced the number and the size of plaque formation of adenovirus (Mitsuishi et al., 2006, Mansour et al., 2016). BV (non-cytotoxic amount) notably repressed the multiplication of HSV along with the stimulation of IFN-1 that induces the inhibition of virus replication after the initiation of host antiviral communication (Kim et al., 2019). Moreover, BV efficiently obstructs the proliferation of cervical tumor cells via the suppression of HPV viruses E6/E7 proteins (El-Seedi et al., 2020). Melittin exhibits anti-viral activity toward HSV-1 as well as HSV-2 (Herpes simplex virus 2) and Arenavirus Junin through the prevention of virus proliferation, adsorption in addition to diffusion and also impede K + along with Na + pumps in host tissue cells (Matanic and Castilla, 2004, El-Seedi et al., 2020). The combination of BV constituents, melittin and apamin, show anti-viral activity for the bovine viral diarrhea virus (Picoli et al., 2018). Studies revealed that influenza A virus- infected chick embryos demonstrate a survival rate of 80 % after melittin inoculation as compared to non-melittin inoculated chicks with a 40 % viability rate (Memariani et al., 2020). Bee venom or melittin and PLA2 individually possesses strong antiviral capabilities against different viruses. In addition, antiviral property of BV could stand with other remedies as the possible candidate in future to investigate its activity against SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) (Al Naggar et al., 2020, Kasozi et al., 2020, Lima et al., 2021). Therefore, like other remarkable activities of bee BV against various pathogens, it also boosts immunity and adopts a defensive response towards different viral infections and could be effective as a natural remedy against SARS-Covid-19.
4.3. Anti-arthritis activity of BV
Studies revealed that BV was used against arthritis disease through the prevention of rheumatoid joint cell proliferation and also hindering the assemblage of the pro stimulating ingredients like PGE-2, cytokinin, NO (Nitric oxide), Enzyme COX-2, and Tumor Necrosis Factor (TNF-2) (Eze et al., 2016, Aliyazicioglu, 2019). Additionally, melittin has anti-arthritic efficacy due to the reduced expression of phospholipase A2 and cyclooxygenase-2 while it decreases the levels of interleukin-1, TNF-α (tumor necrosis factor-alpha), IL-6 (interleukin 6), oxygen reactive species and nitric oxide (Bellik, 2015, Moga et al., 2018). The anti-arthritis capability of adolapin is due to the inhibition of the prostaglandin (PG) production system. Additionally, it was also discovered that BV inhibits arthritis disease caused by Mycobacterium butyricum in Lewis mice. Previous reports have shown that BV reduces the level of TNF-α and NO synthesis which are responsible for the abrasion of joint cartilage, invasion of inflammatory cells as well as bone damage in inflammatory arthritis (Son et al., 2007). Due to its anti-arthritis property, BV blocks the DNA transcriptional and binding action of NF-κB in synoviocytes, THP 1 (human leukemia monocytic cell line) and Raw 264.7 cells (which are the monocyte cell line) in a dosage-related manner (Park et al., 2004).
Furthermore, BV stops the propagation of rheumatoid synovial cells and causes apoptosis through the stimulation of caspase-3 (Son et al., 2007). Additionally, the injection of apamin and melittin to patients having arthritis disease, significantly cures edema. Also, administration of adolapin and protease inhibitor also treats 15 to 40 % of edema disease (Han et al., 2012). It has been proven that the injection of BV via Zusanli acupoint in mice having arthritis which was induced by Freund's adjuvant, revealed both anti-nociceptive and anti-inflammatory results (Kim et al., 2003, Azam et al., 2018). Bee venom apitherapy exhibited anti-arthritis activity both in vivo and ex vivo for osteoarthritis and rheumatoid arthritis. It also protects the body from oxidative stress caused by rheumatoid arthritis both in human and animal experimental models (Bellik 2015). It was stated that BV acupuncture treatment inhibits the immune responses induced by type-II collagen afterward blocking the progression of arthritis disease (Mansour et al., 2016). Studies revealed that BV acupuncture significantly protected tissue impairment through the downregulation of lysosomal, cytoplasmic as well as matrix protease while decreasing the level of reactive oxygen species (ROS) in type-II arthritis induced by collagen in the mice model (Zhang et al., 2018). In conclusion, numerous evidence regarding the broad-spectrum of anti-arthritis properties of BV have encouraged physicians to use it against osteoarthritis and rheumatoid arthritis carefully to avoid side effects.
4.4. Anti-inflammatory activity of BV
It was documented that the use of melittin reduces the phosphorylation of IκB, IKK (IκB kinase), NF-κB along with p38 via heat-killed Propionibacterium acnes in HaCaT (human keratinocyte cell line) cells. This evidence demonstrated that melittin therapy abolishes the inflammatory cytokine formation by P. acnes via preventing p38 MAPK (Mitogen-activated protein kinase) and NF-κB signals in HaCaT cells (Lee et al., 2015, Lee and Bae, 2016a, Lee and Bae, 2016b). Moreover, melittin shows anti-inflammatory activity in a live model animal having inflammatory dermal disease induced by P. acnes which exhibited distinctly lower granulomatous and swelling reactions as compared to P. acnes injected alone (Memariani et al., 2019). Melittin has the potential to cure neurodegenerative abnormalities along with the activation of microglial cells because it suppresses the pro-inflammatory reactions of BV2 glial cells (Hegazi, 2012, Lee and Bae, 2016a, Lee and Bae, 2016b). It was detected that the use of melittin restrained the reduction of anti-apoptotic factor Bcl-2 (B-cell lymphoma 2) expression induced by H2O2 (Hydrogen peroxide), enhancing the pro-apoptotic factor Bax (Bcl2-associated X protein) expressions in order to reduce apoptotic DNA division and increasing the viability of cell (Han et al., 2014). Additionally, the administration of melittin against animal models having amyotrophic lateral sclerosis (ALS) of lung and spleen shows accelerated signaling while diminishing inflammation for the survival of cells (Lee et al., 2014, Lee and Bae, 2016a, Lee and Bae, 2016b). Studies have exposed that melittin possesses anti-psychotic activity which can be used for the treatment of psychosis instead of the use of other therapeutic drugs having side effects (Dantas et al., 2014). In the same way, melittin was used against High-Fat/LPS mice in vivo which displayed anti-atherosclerosis activity through the suppression of atherosclerosis disease in tested mice (Moreno and Giralt 2015).
Melittin reduces hepatic fibrosis, inflammation, and hepatic injury through the expression of IL-6, as well as IL-1β, and also prevents the secretion of TNF-α in the TNF-α-tested HSCs (hepatic stellate cells). Similarly, the treatment of the bile duct using melittin diminished the inflammation and fibrosis (Lee and Bae, 2016a, Lee and Bae, 2016b). In a recent study, it was reported that BV diminishes the lipid polysaccharides (LPS) induced kidney malfunction and physical defects through the downregulation of oxidative stress, tubular cell apoptosis and inflammation of an acute kidney wound in a mice (Kim et al., 2020a, Kim et al., 2020b). The group III sPLA2 of BV has anti-inflammatory activity against mice having asthma disease through Treg cells (Park et al., 2015). Likewise, the comparable activity of BV group III sPLA2 was also shown in hepatic and renal wound induced by acetaminophen and cisplatin respectively (Kim et al., 2015a, Kim et al., 2015b, Lee and Bae, 2016a, Lee and Bae, 2016b). Palm et al. (2013) proved that the component of BV group III sPLA2 enhanced the immunity of the mice after injection of a high dose of group III sPLA2 showing positive immune responses of IgE toward group III sPLA2 and protected mice from future alterations (Palm et al., 2013). Investigators reported that BV group III sPLA2 can stop the inflammation and death of nerve cells caused by prion protein fragment106-126 (Hossen et al., 2017a, Hossen et al., 2017b). New research show that the treatment with phospholipase A2 significantly cured cholestatic liver injury in mice via the obstruction of inflammation and liver cell apoptosis (Kim et al., 2021). The BV constituent called MCD peptide is composed of 22 amino acids having 2 disulfide bridges and possesses anti-inflammatory potential (Bellik, 2015, Komi et al., 2018). Moreover, the administration of melittin can induce the body to produce cortisol and acts as a potent anti-inflammatory representative of BV (Eze et al., 2016). It was investigated that BV component adolapin possesses anti-inflammatory action towards the adjuvant polyarthritis and mice posterior foot oedema caused by PG (prostaglandin), carrageenan and adjuvant (Son et al., 2007).
Similarly, the apamin acts as an anti-inflammatory agent which stops inflammation of foot edema in animal models induced by dextran and serotonin hypodermically (Chen and Lariviere, 2010, Bellik, 2015). Due to its anti-inflammatory activity, apamin also restrains phospholipase A2 and COX −2 (Varol et al., 2022). Studies have shown that BV contains anti-inflammatory activity against various diseases. For instance, Herpes zoster, arthritis, osteoarthritis, bursitis, keloids, rheumatoid arthritis, tendinitis, multiple sclerosis as well as Lyme disease (Basa et al., 2016). BV can be used against various neuroinflammatory diseases such as Parkinson's disease (PD), amyotrophic lateral sclerosis and multiple sclerosis (Zhang et al., 2018, Wehbe et al., 2019). In addition, BV exhibited anti-neuroinflammatory activity toward PD through the control of apoptotic and neuroinflammatory signs of PD induced in mice (Silva et al., 2015). Similarly, BV also decreases the inflammation of nerve cells in MRTP (1-methyl-4- phenyl-1,2,3,6 tetrahydropyridine) treated mouse model with PD (Azam et al., 2018). It was also reported that phospholipase A2 causes the downregulation of neuroinflammatory reactions in mice models having Parkinson's disease (Baek et al., 2020). Also, apamin can be used as a healing agent for the cure of Alzheimer's disease and Multiple sclerosis (MS) (Azam et al., 2018). In addition, BV along with other antiepileptic drugs can have curative potential for epileptic disease. As BV enhances alterations in the expression of VGCs (voltage-gated channels) and reverses balance in neurotransmitters and blood electrolytes, because of the prevention of proinflammatory cytokine stimulation (Abd El-Hameed et al., 2021).
Furthermore, PLA2 has immuno-protective capability against various diseases like PD, asthma and Alzheimer’s disease (Park et al., 2015, Wehbe et al., 2019). Shin et al. (2018) proved that PLA2 possesses anti-inflammatory capability against dermal infections and reduces atopic dermal inflammation via collaboration with CD206 (Shin et al., 2018). The application of BV efficiently blocked DNA impairment while repressed caspase-3, apoptotic Bax, in addition to Bcl-2 gene expression in mice having PD (Khalil et al., 2015, Wehbe et al., 2019). Also, BV decreases the synthesis of pro-inflammatory cytokines, PGE2, NO and COX-2 in murine glial cells (macrophage in the brain) cultures which were accelerated by lipopolysaccharides (Silva et al., 2015). It was documented that BV diminishes hepatic fibrosis caused by CCL4 (Carbon tetrachloride) via the downregulation of fibrogenic cytokines in animal models (Lee et al., 2015, Zhang et al., 2018). PLA2 medication treated the liver malfunction and provoked the synthesis of anti-inflammatory cytokines in acetaminophen-treated mice (Hossen et al., 2017a, Hossen et al., 2017b, Zhang et al., 2018). The wound healing potential of BV has been shown in many studies. Bee venom along with chitosan and polyvinyl alcohol synergistically enhances the wound recovery process via accelerating hydroxyproline and glutathione while suppressing the IL-6 level in injured tissues (Kurek-Górecka et al., 2021). The anti-inflammatory activities of bee venom and its compounds provide scientific evidence supporting the use of bee venom as an alternative therapeutic medicine.
4.5. Anti-nociceptive property of BV
Bee venom has anti-nociceptive capability toward inflammatory pain (Seo et al., 2018). Therefore, BV is used traditionally for the cure of visceral, inflammatory, thermal, and many others discomfort responses (Son et al., 2007). Hypodermic apipuncture treatment with BV reduces thermal and mechanical hyperplasia (Kwon et al., 2001a, Kwon et al., 2001b, Kwon et al., 2001c, Kwon et al., 2001d), arthritic pain induced by collagen (Komi et al., 2018) and pain induced by formalin, and knee osteoarthritis associated pain (Kwon et al., 2001a, Kwon et al., 2001b, Kwon et al., 2001c, Kwon et al., 2001d, Son et al., 2007). Also, one component of BV, adolapin, shows analgesic efficacy, on its own (Son et al., 2007). Moreover, the anti-nociceptive impact of BV is established when BV is directly inoculated into acupoint ST36 (which is recognized as Zusanli) to an animal model having persistent arthritis as compared with a non-acupoint inoculation (Azam et al., 2018). Pretreatment with BV shows anti-nociceptive power against spinal cord Fos expression associated with formalin-caused distress behavior in rats (Lee et al., 2001, Son et al., 2007). Apamin can act as a pain killer and is significantly effective in reduction of arthritis, gout, rheumatoid arthritis, neuralgia and muscular pain (Han et al., 2012). Also, apamin has analgesic activity via prohibiting lipoxygenase from human blood platelets (Wehbe et al., 2019). The phospholipase A2 improves the negative consequences of mechanical allodynia and coldness induced by oxaliplatin (Hossen et al., 2017a, Hossen et al., 2017b).
It has been reported that subcutaneous injection of BV causes anti-nociceptive activity in many rodent models for both visceral and somatic nuisance, and prohibits the nociceptive reactions in mice models induced by acetic acid (Costa et al., 2014). Likewise, BV administration considerably reduces the inflammation-associated cytokines expressions like phospholipase A2, NO, IL-1, ROS, IL-6, COX-2 and TNF-α through NF-kB in rheumatoid arthritis (Costa et al., 2014). It has been documented that that treatment with BV in the acupoint substantially decreases nociceptive reactions and arthritis-related oedema (Kwon et al., 2001a, Kwon et al., 2001b, Kwon et al., 2001c, Kwon et al., 2001d, Kim et al., 2003). Additionally, the administration of BV through an acupoint had more significant anti-nociceptive efficacy on mice having writhing reflex (induced by acetic acid) as compared to non-acupoint injection (Kwon et al., 2001a, Kwon et al., 2001b, Kwon et al., 2001c, Kwon et al., 2001d, Son et al., 2007). Comparatively, the treatment of arthritic pain induced by an adjuvant through BV is better because of its anti-nociceptive efficiency (Kwon et al., 2001a, Kwon et al., 2001b, Kwon et al., 2001c, Kwon et al., 2001d). It was detected that BV acupuncture contains pain-relieving properties against neuritis pain induced by paclitaxel in the mice model (Shen et al., 2020). The injection of bee venom has some deleterious effects on allergic people, like anaphylactic or systemic reactions. Also, the cellular and molecular mechanism of bee venom as an anti-nociceptive agent is still unclear and needs further extensive investigation to minimize its undesirable effects.
4.6. Radioprotective potential of BV
Studies revealed that BV show radioprotective effects on the harmful consequences of ionizing radiation. Bee venom protects from the gamma and X-ray radiation in numerous assessment systems (Gajski and Garaj-Vrhovac 2009). Also, BV has antioxidant efficacy and can neutralize free radicals and protect the body from toxic radiation (Shaaban and Hamza 2019). BV defends the bone marrow cells from chromosomal abnormalities (aberrations) in vivo in the Wistar mice model (Varanda and Tavares, 1998, Bellik, 2015). It also stimulates hematopoiesis as well as MCD-induced histamine release, phospholipase A2-induced decrease of blood oxygen pressure (Gajski and Garaj-Vrhovac, 2009, Garaj-Vrhovac and Gajski, 2011). One of the BV component, PLA2, possesses a Foxp3 + CD4 + CD25 + Treg cell which has a defensive effect towards severe lung inflammation caused by radiotherapy (Hossen et al., 2017a, Hossen et al., 2017b). Moreover, BV treatment considerably reduces the level of both, IL-6 and TNF-α, after exposure to gamma radiation (Park et al., 2004, Shaaban and Hamza, 2019). Also, BV through the decline of higher hepatic NF-kB expression, efficiently reduces the serum AST (Aspartate aminotransferase), LDH (Lactate dehydrogenase), CK-MB (Creatine kinase myocardial band), cTnI (cardiac troponin 1) and ALT levels in Wistar mice increased after the gamma radiation. (Darwish et al., 2013, Shaaban and Hamza, 2019).
Research declared that BV has the radioprotective potential for oxidative and basal DNA destruction (Gajski and Garaj-Vrhovac, 2009, Garaj-Vrhovac and Gajski, 2011, El Adham et al., 2022). The administration of melittin 24 h before exposure to the X-rays (8.5 Gy), significantly increases the survival rate in the mice model (Varanda and Tavares 1998). Also, BV protects peripheral blood lymphocytes of the human from harmful gamma radiation (Varanda and Takahashi, 1993, Varanda and Tavares, 1998). Similarly, the components of BV reduce chromosomal damage to bone marrow cells induced by radiation in Wistar mice, reducing the number of cells having chromosomal abnormalities along with aberration frequency as well as fragments in an animal model 24 h before irradiation (3–4 Gy) than radiation subjected group individually (Varanda et al., 1992, Gajski and Garaj-Vrhovac, 2009). A similar experiment revealed the radioprotective mechanism of BV when applied to an animal model (blood lymphocytes) 24 h before exposure to radiation (3–4 Gy) (Varanda and Takahashi 1993). The use of modern technology and non-ionizing radiation in all spheres of human life, has increased in recent years, increasing the number of harmful effects on the human body. As BV shows radioprotective capability against X-rays and gamma radiations in various experimental trials, it is proposed that BV can be used as a nontoxic and effective radioprotector agent in the future.
4.7. Anti-diabetic activity of BV
Bee venom contain many beneficial therapeutic activities against various diseases of human being including Diabetes mellitus. Diabetes mellitus is a common human disease characterized by hyperglycemia and hyperlipidemia and other defects. The use of both metformin (an oral diabetic medicine) and BV show anti-diabetic activity in diabetic mice (Sattar 2022). Previous studies have shown that melittin improves the synthesis of insulin by downregulating the inflammatory process of pancreatic Islets (Pollak, 2014, Sattar, 2022). Additionally, melittin also depolarizes pancreatic Islets cell membrane which opens Ca2+ channels and permitting Calcium ions enhanced entry and activating β-cells to synthesize insulin (Mousavi et al., 2012, Zahran et al., 2021a, Zahran et al., 2021b, Sattar, 2022). The administration of BV renovated the usual physiology and anatomy of the pancreas because of its anti-inflammatory and antioxidant activities (Kim et al., 1999, Sattar, 2022). In another study, two types (different concentrations) of BV were used for 35 days against alloxan-induced diabetes mellitus in a mice model. Authors conclude that BV improved insulin production and glucose control as well as diminished infertility alterations (Al-Shaeli et al., 2022a, AL-Shaeli et al., 2022b).
Furthermore, the administration of BV reduces cholesterol and triglyceride (TG) levels, and concentration of glucose while improving the level of HDL (high-density lipoprotein) and insulin production in diabetic mice (Al-Shaeli et al., 2022a, AL-Shaeli et al., 2022b). Also, Melittin and phospholipase A2 can improve the level of insulin and glucose and cure the inflammation in islets of Langerhans (Elkotby et al., 2018, Zidan et al., 2018, Zahran et al., 2021a, Zahran et al., 2021b). Additionally, BV also restrains the synthesis of free radicals and proinflammatory cytokines which may trigger the death of β-cells (Badr et al., 2016, Zahran et al., 2021a, Zahran et al., 2021b). BV is alternative medicine for various diseases, including diabetes mellitus and obesity. Currently, the clinical use of BV as an anti-diabetic agent is limited but the ongoing research findings will clarify incomplete and contradictory findings and make BV a better remedy for the treatment of diabetic patients in the near future.
5. Bee venom safety
As we have already mentioned, the current findings on the safety of BV are incomplete and contradictory. Nowadays, BV is considered a preferable therapeutic remedy to synthetic medicine against many diseases. But the application of BV sometimes causes allergic and anaphylactic reactions, depending on the individual immune system and the dose given. The components of BV such as phospholipase A2, melittin and hyaluronidase are the main allergens of BV. In sensitive people, the administration of 100 µg/mL BV to the human body, can cause severe implications such as limb paralysis, pain, dyspnea, nausea, unconsciousness, and lymphocyte instability etc. Moreover, the doses from 2.8 mg to 3.5 mg of BV/kg of human body can be lethal (LD50) for an allergic individual. The severity of BV application mainly depends on the individual body weight, age, number of stings, immunity and previously sensitivity (Pucca et al., 2019). The effect of a bee’s sting on the host body can be local or systemic. The local reaction include redness of the sting site, swelling and oedema (Annila 2000). The systemic reactions depend upon effects of the allergens present in the venom which can develop angioedema, urticaria, vomiting, pruritus, and diarrhea in allergic patients (Fitzgerald and Flood 2006). Furthermore, some occasional clinical trials also showed Fisher’s syndrome, peripheral neuritis, optic neuropathy, bilateral empyema, septicemia and acute inflammatory polyradiculoneuropathy after bee sting (Pucca et al., 2019). Therefore, the knowledge of allergic reactions and side effects of BV should be the present and future research focus. New knowledge of BV safety is of great importance for clinical practitioners to avoid the negative aspects and hazardous consequences of this fundamental bee product.
6. Conclusive remarks and future studies
Bee venom has been traditionally used as a natural therapeutic medicine for centuries. Today, crude BV or its components are used for the treatment of various diseases such as cancer, arthritis, neurodegenerative ailments, inflammatory disorders, liver problems as well as skin infections in many countries of the world. Moreover, BV possesses anti-cancer and antimicrobial activity. Previous studies improved our knowledge about BV composition and its biomedical application. However, the clinical application of BV is limited. Also, extraction technologies still need further standardization which would be sustainable. Therefore, future studies should have greater focus on BV and its specific components, as well as its physiochemical activities and medical performance. In that way, BV will have a greater application in advanced medicine.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors from Department of Zoology, KUST Kohat, acknowledge the financial support provided by the Higher Education Commission of Pakistan under the project No.10615 entitled ‘‘Exploring Pathogen Web Affecting Honey Bee Health and its Effective Treatment in Pakistan”. Fahad Aldakheel is grateful to the Deanship of Scientific Research, King Saud University, Riyadh, Kingdom of Saudi Arabia for funding through the Vice Deanship of Scientific Research Chairs.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Fahad Mohammed Aldakheel, Email: faldakheel@ksu.edu.sa.
Syed Ishtiaq Anjum, Email: ishtiaq@kust.edu.pk.
Ghulam Raza, Email: Ghulam.raza@uobs.edu.pk.
Saeed Ahmad Khan, Email: saeedkhanphd@gmail.com.
Ivana Tlak Gajger, Email: ivana.tlak@vef.hr.
References
- Abd El-Hameed A.M., Abuelsaad A.S., Khalil A. Bee venom acupuncture therapy ameliorates neuroinflammatory alterations in a pilocarpine-induced epilepticus model. Metab. Brain Dis. 2021;1–12 doi: 10.1007/s11011-021-00766-9. [DOI] [PubMed] [Google Scholar]
- Abd El-Wahed A.A., Khalifa S.A., Sheikh B.Y., et al. Bee venom composition: From chemistry to biological activity. Stud. Nat. Products Chem. 2019;60:459–484. [Google Scholar]
- Abdela N., Jilo K. Bee venom and its therapeutic values: a review. Adv Life Sci Technol. 2016;44:18–22. [Google Scholar]
- Al Naggar Y., Giesy J.P., Abdel-Daim M.M., et al. Fighting against the second wave of COVID-19: Can honeybee products help protect against the pandemic? Saudi J. Biol. Sci. 2020 doi: 10.1016/j.sjbs.2020.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alajmi R.A., Barakat I.A., Alfozan L., et al. Microbiological investigation study for Apis mellifera yemenitica and Apis mellifera carnica bee venoms on selected bacterial strains. Braz. J. Microbiol. 2022:1–6. doi: 10.1007/s42770-021-00656-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali M. Studies on bee venom and its medical uses. Int. J. Adv. Res. Technol. 2012;1:69–83. [Google Scholar]
- Ali E.M. Contributions of some biological activities of honey bee venom. J. Apic. Res. 2014;53:441–451. doi: 10.3896/IBRA.1.53.4.13. [DOI] [Google Scholar]
- Aliyazicioglu R. Therapeutic effects of bee venom. Chem. Sci. Int. J. 2019:1–5. [Google Scholar]
- Al-Shaeli S., Ethaeb A., Al-Zaidi E. Serological and histological evaluation of the effect of honeybee venom on pancreas and liver in Diabetic Mice. Arch. Razi Institute. 2022;77:1125–1131. doi: 10.22092/ARI.2022.357385.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AL-Shaeli S.J., Hussen T.J., Ethaeb A.M. Effect of honey bee venom on the histological changes of testes and hormonal disturbance in diabetic mice. Veterinary World. 2022;15 doi: 10.14202/vetworld.2022.2357-2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An H.-J., Lee W.-R., Kim K.-H., et al. Inhibitory effects of bee venom on Propionibacterium acnes-induced inflammatory skin disease in an animal model. Int. J. Mol. Med. 2014;34:1341–1348. doi: 10.3892/ijmm.2014.1933. [DOI] [PubMed] [Google Scholar]
- Annand R.R., Kontoyianni M., Penzotti J.E., et al. Active site of bee venom phospholipase A2: the role of histidine-34, aspartate-64 and tyrosine-87. Biochemistry. 1996;35:4591–4601. doi: 10.1021/bi9528412. [DOI] [PubMed] [Google Scholar]
- Annila I. Bee venom allergy. Clin. Exp. Allergy. 2000;30:1682–1687. doi: 10.1046/j.1365-2222.2000.00885.x. [DOI] [PubMed] [Google Scholar]
- Arteaga V., Lamas A., Regal P., et al. Antimicrobial activity of apitoxin from Apis mellifera in Salmonella enterica strains isolated from poultry and its effects on motility, biofilm formation and gene expression. Microb. Pathog. 2019;137 doi: 10.1016/j.micpath.2019.103771. [DOI] [PubMed] [Google Scholar]
- Aufschnaiter A., Kohler V., Khalifa S., et al. Apitoxin and its components against cancer, neurodegeneration and rheumatoid arthritis: Limitations and possibilities. Toxins. 2020;12:66. doi: 10.3390/toxins12020066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azam M.N.K., Ahmed M.N., Biswas S., et al. A review on bioactivities of honey bee venom. Annu Res Rev Biol. 2018:1–13. [Google Scholar]
- Badawi J.K. Bee Venom Components as Therapeutic Tools against Prostate Cancer. Toxins. 2021;13:337. doi: 10.3390/toxins13050337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badr G., Hozzein W.N., Badr B.M., et al. Bee venom accelerates wound healing in diabetic mice by suppressing activating transcription factor-3 (ATF-3) and inducible nitric oxide synthase (iNOS)-mediated oxidative stress and recruiting bone marrow-derived endothelial progenitor cells. J. Cellular Physiol. 2016;231:2159–2171. doi: 10.1002/jcp.25328. [DOI] [PubMed] [Google Scholar]
- Baek H., Park S.-Y., Ku S.J., et al. Bee Venom Phospholipase A2 Induces Regulatory T Cell Populations by Suppressing Apoptotic Signaling Pathway. Toxins. 2020;12:198. doi: 10.3390/toxins12030198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandyopadhyay S., Lee M., Sivaraman J., et al. Model membrane interaction and DNA-binding of antimicrobial peptide Lasioglossin II derived from bee venom. Biochem Bioph Res Co. 2013;430:1–6. doi: 10.1016/j.bbrc.2012.11.015. [DOI] [PubMed] [Google Scholar]
- Baracchi D., Francese S., Turillazzi S. Beyond the antipredatory defence: honey bee venom function as a component of social immunity. Toxicon: Official J. Int. Soc. Toxinol. 2011;58:550–557. doi: 10.1016/j.toxicon.2011.08.017. [DOI] [PubMed] [Google Scholar]
- Bardbari A.M., Arabestani M.R., Karami M., et al. Highly synergistic activity of melittin with imipenem and colistin in biofilm inhibition against multidrug-resistant strong biofilm producer strains of Acinetobacter baumannii. Eur. J. Clin. Microbiol. Infectious Dis.: Official Publication Eur. Soc. Clin. Microbiol. 2018;37:443–454. doi: 10.1007/s10096-018-3189-7. [DOI] [PubMed] [Google Scholar]
- Basa B., Belay W., Tilahun A., et al. Review on medicinal value of honeybee products: apitherapy. Adv. Biol. R. 2016;10:236–247. [Google Scholar]
- Bellik Y. Bee venom: its potential use in alternative medicine. Antiinfect Agents. 2015;13:3–16. [Google Scholar]
- Bilo B., Rueff F., Mosbech H., et al. Diagnosis of Hymenoptera venom allergy. Allergy. 2005;60:1339–1349. doi: 10.1111/j.1398-9995.2005.00963.x. [DOI] [PubMed] [Google Scholar]
- Boutrin M.-C., Foster H., Pentreath V. The effects of bee (Apis mellifera) venom phospholipase A2 on Trypanosoma brucei brucei and enterobacteria. Exp. Parasitol. 2008;119:246–251. doi: 10.1016/j.exppara.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Chen X., Fan H., Chen J., et al. Bee venom acupuncture for adhesive capsulitis: a protocol for systematic review and meta-analysis. Medicine. 2020;99:e19975. doi: 10.1097/MD.0000000000019975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Guan S.M., Sun W., et al. Melittin, the Major Pain-Producing Substance of Bee Venom. Neurosci. Bull. 2016;32:265–272. doi: 10.1007/s12264-016-0024-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Lariviere W.R. The nociceptive and anti-nociceptive effects of bee venom injection and therapy: a double-edged sword. Prog. Neurobiol. 2010;92:151–183. doi: 10.1016/j.pneurobio.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi K.E., Hwang C.J., Gu S.M., et al. Cancer cell growth inhibitory effect of bee venom via increase of death receptor 3 expression and inactivation of NF-kappa B in NSCLC cells. Toxins. 2014;6:2210–2228. doi: 10.3390/toxins6082210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi G.-M., Lee B., Hong R., et al. Bee venom phospholipase A2 alleviates collagen-induced polyarthritis by inducing Foxp3+ regulatory T cell polarization in mice. Sci. Rep. 2021;11:3511. doi: 10.1038/s41598-021-82298-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu S.-T., Cheng H.-H., Huang C.-J., et al. Phospholipase A2-independent Ca2+ entry and subsequent apoptosis induced by melittin in human MG63 osteosarcoma cells. Life Sci. 2007;80:364–369. doi: 10.1016/j.lfs.2006.09.024. [DOI] [PubMed] [Google Scholar]
- Costa M.F., Campos A.R., Abdon A.P., et al. Study of visceral antinociceptive potential of bee Apis mellifera venom. Afr. J. Pharm. Pharmacol. 2014;8:781–785. [Google Scholar]
- Da Mata É.C.G., Mourão C.B.F., Rangel M., et al. Antiviral activity of animal venom peptides and related compounds. J. Venom. Anim. Tox. incl. Trop. Dis. 2017;23:3. doi: 10.1186/s40409-016-0089-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantas C.G., Nunes T.L., Nunes T.L., et al. Pharmacological evaluation of bee venom and melittin. Rev Bras Farmacogn. 2014;24:67–72. [Google Scholar]
- Darwish S.F., El-Bakly W.M., Arafa H.M., et al. Targeting TNF-α and NF-κB activation by bee venom: role in suppressing adjuvant induced arthritis and methotrexate hepatotoxicity in rats. PLoS One. 2013;8:e79284. doi: 10.1371/journal.pone.0079284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosler S., Gerceker A.A. In vitro activities of antimicrobial cationic peptides; melittin and nisin, alone or in combination with antibiotics against Gram-positive bacteria. J. Chemother. 2012;24:137–143. doi: 10.1179/1973947812Y.0000000007. [DOI] [PubMed] [Google Scholar]
- Duffy C., Sorolla A., Wang E., et al. Honeybee venom and melittin suppress growth factor receptor activation in HER2-enriched and triple-negative breast cancer. NPJ Precis Oncol. 2020;4:1–16. doi: 10.1038/s41698-020-00129-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Adham E.K., Hassan A.I., Dawoud M.A. Evaluating the role of propolis and bee venom on the oxidative stress induced by gamma rays in rats. Sci. Rep. 2022;12:1–22. doi: 10.1038/s41598-022-05979-1. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Elkotby D., Hassan A.K., Emad R., et al. Histological changes in islets of Langerhans of pancreas in alloxan-induced diabetic rats following Egyptian honey bee venom treatments. Int. J. Pure Appl. Zool. 2018;6:1–6. [Google Scholar]
- El-Seedi H., El-Wahed A., Yosri N., et al. Antimicrobial Properties of Apis mellifera’s Bee Venom. Toxins. 2020;12:451. doi: 10.3390/toxins12070451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eze O., Nwodo O., Ogugua V.N. Therapeutic effect of honey bee venom. Proteins (enzymes) 2016;1 [Google Scholar]
- Fitzgerald K.T., Flood A.A. Hymenoptera stings. Clin Tech Small Anim Pract. 2006;21:194–204. doi: 10.1053/j.ctsap.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Frangieh J., Salma Y., Haddad K., et al. First Characterization of The Venom from Apis mellifera syriaca, A honey bee from the Middle East Region. Toxins. 2019;11 doi: 10.3390/toxins11040191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajski G., Garaj-Vrhovac V. Genotoxic potential of bee venom (Apis mellifera) on human peripheral blood lymphocytes in vitro using single cell gel electrophoresis assay. J. Environ. Sci. Health A. 2008;43:1279–1287. doi: 10.1080/10934520802177862. [DOI] [PubMed] [Google Scholar]
- Gajski G., Garaj-Vrhovac V. Radioprotective effects of honeybee venom (Apis mellifera) against 915-mhz microwave radiation–induced DNA damage in wistar rat lymphocytes: In vitro study. Int. J. Toxicol. 2009;28:88–98. doi: 10.1177/1091581809335051. [DOI] [PubMed] [Google Scholar]
- Gajski G., Garaj-Vrhovac V. Increased frequency of sister chromatid exchanges and decrease in cell viability and proliferation kinetics in human peripheral blood lymphocytes after in vitro exposure to whole bee venom. J. Environ. Sci. Health A. 2010;45:1654–1659. doi: 10.1080/10934529.2010.506144. [DOI] [PubMed] [Google Scholar]
- Gajski G., Garaj-Vrhovac V. Bee venom induced cytogenetic damage and decreased cell viability in human white blood cells after treatment in vitro: a multi-biomarker approach. Environ. Toxicol. Pharmacol. 2011;32:201–211. doi: 10.1016/j.etap.2011.05.004. [DOI] [PubMed] [Google Scholar]
- Gajski G., Čimbora-Zovko T., Rak S., et al. Combined antitumor effects of bee venom and cisplatin on human cervical and laryngeal carcinoma cells and their drug resistant sublines. J. Appl. Toxicol. 2014;34:1332–1341. doi: 10.1002/jat.2959. [DOI] [PubMed] [Google Scholar]
- Gajski G., Čimbora-Zovko T., Rak S., et al. Antitumour action on human glioblastoma A1235 cells through cooperation of bee venom and cisplatin. Cytotechnology. 2016;68:1197–1205. doi: 10.1007/s10616-015-9879-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajski G., Domijan A.-M., Žegura B., et al. Melittin induced cytogenetic damage, oxidative stress and changes in gene expression in human peripheral blood lymphocytes. Toxicon: Official J. Int. Soc. Toxinol. 2016;110:56–67. doi: 10.1016/j.toxicon.2015.12.005. [DOI] [PubMed] [Google Scholar]
- Gajski G., Garaj-Vrhovac V. Melittin: a lytic peptide with anticancer properties. Environ. Toxicol. Pharmacol. 2013;36:697–705. doi: 10.1016/j.etap.2013.06.009. [DOI] [PubMed] [Google Scholar]
- Gajski G., Domijan A.M., Garaj-Vrhovac V. Alterations of GSH and MDA levels and their association with bee venom-induced DNA damage in human peripheral blood leukocytes. Environ. Mol. Mutag. 2012;53:469–477. doi: 10.1002/em.21708. [DOI] [PubMed] [Google Scholar]
- Garaj-Vrhovac V., Gajski G. Evaluation of the cytogenetic status of human lymphocytes after exposure to a high concentration of bee venom in vitro. Arhiv za higijenu rada i toksikologiju. 2009;60:27–34. doi: 10.2478/10004-1254-60-2009-1896. [DOI] [PubMed] [Google Scholar]
- Garaj-Vrhovac V., Gajski G. Institute for Medical Research and Occupational Health; Zagreb, Croatia: 2011. Radioprotection of Wistar Rat Lymphocytes Against Microwave Radiation Mediated by Bee Venom. [Google Scholar]
- Gopal R., Lee J.H., Kim Y.G., et al. Anti-microbial, anti-biofilm activities and cell selectivity of the NRC-16 peptide derived from witch flounder, Glyptocephalus cynoglossus. Mar. Drugs. 2013;11:1836–1852. doi: 10.3390/md11061836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H., An H.-J., Gwon M.-G., et al. Bee venom and its major component melittin attenuated Cutibacterium acnes-and IGF-1-induced acne vulgaris via inactivation of Akt/mTOR/SREBP signaling pathway. Int. J. Mol. Sci. 2022;23:3152. doi: 10.3390/ijms23063152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, S. M., K. G. Lee, J. H. Yeo, et al., 2012. Composition containing bee venom as an active ingredient for preventing and treating acne, Google Patents.
- Han S.M., Lee K.G., Park K.K., et al. Antimicrobial Activity of Honey Bee Venom against Select Infectious Fish Pathogens. N. Am. J. Aquacult. 2013;75:445–448. doi: 10.1080/15222055.2013.802264. [DOI] [Google Scholar]
- Han S.M., Kim J.M., Park K.K., et al. Neuroprotective effects of melittin on hydrogen peroxide-induced apoptotic cell death in neuroblastoma SH-SY5Y cells. BMC Complem Altern M. 2014;14:286. doi: 10.1186/1472-6882-14-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegazi A.G. Medical importance of bee products. U Arı D. 2012;12:136–146. [Google Scholar]
- Hegazi A., Abdou A.M., El-Moez S., et al. Evaluation of the antibacterial activity of bee venom from different sources. World Appl Sci J. 2014;30:266–270. [Google Scholar]
- Hegazi A.G., El-Feel M., Abdel-Rahman E., et al. Antibacterial activity of bee venom collected from Apis mellifera carniolan pure and hybrid races by two collection methods. Int. J. Curr. Microbiol. App. Sci. 2015;4:141–149. [Google Scholar]
- Hossen M., Gan S.H., Khalil M. Melittin, a potential natural toxin of crude bee venom: probable future arsenal in the treatment of diabetes mellitus. J. Chem. 2017 [Google Scholar]
- Hossen M., Shapla U.M., Gan S.H., et al. Impact of bee venom enzymes on diseases and immune responses. Molecules. 2017;22:25. doi: 10.3390/molecules22010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H., Chen D., Li Y., et al. Effect of polypeptides in bee venom on growth inhibition and apoptosis induction of the human hepatoma cell line SMMC-7721 in-vitro and Balb/c nude mice in-vivo. J. Pharm. Pharmacol. 2006;58:83–89. doi: 10.1211/jpp.58.1.0010. [DOI] [PubMed] [Google Scholar]
- Huh J.-E., Baek Y.-H., Lee M.-H., et al. Bee venom inhibits tumor angiogenesis and metastasis by inhibiting tyrosine phosphorylation of VEGFR-2 in LLC-tumor-bearing mice. Cancer Lett. 2010;292:98–110. doi: 10.1016/j.canlet.2009.11.013. [DOI] [PubMed] [Google Scholar]
- Jampilek J., Kralova K. Advances in drug delivery nanosystems using graphene-based materials and carbon nanotubes. Materials. 2021;14:1059. doi: 10.3390/ma14051059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung B.-G., Lee J.-A., Park S.-B., et al. Immunoprophylactic effects of administering honey bee (Apis melifera) venom spray against Salmonella gallinarum in broiler chicks. J Vet Sci. 2013:13–0045. doi: 10.1292/jvms.13-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasozi K.I., Niedbała G., Alqarni M., et al. Bee Venom—A Potential Complementary Medicine Candidate for SARS-CoV-2 Infections. Front public health. 2020;8:755. doi: 10.3389/fpubh.2020.594458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil W.K., Assaf N., ElShebiney S.A., et al. Neuroprotective effects of bee venom acupuncture therapy against rotenone-induced oxidative stress and apoptosis. Neurochem. Int. 2015;80:79–86. doi: 10.1016/j.neuint.2014.11.008. [DOI] [PubMed] [Google Scholar]
- Khozani R.S., Shahbazzadeh D., Harzandi N., et al. Kinetics study of antimicrobial peptide, melittin, in simultaneous biofilm degradation and eradication of potent biofilm producing MDR Pseudomonas aeruginosa isolates. Int J Pept Res Ther. 2019;25:329–338. [Google Scholar]
- Kim Y.-W., Chaturvedi P.K., Chun S.N., et al. Honeybee venom possesses anticancer and antiviral effects by differential inhibition of HPV E6 and E7 expression on cervical cancer cell line. Oncol. Rep. 2015;33:1675–1682. doi: 10.3892/or.2015.3760. [DOI] [PubMed] [Google Scholar]
- Kim J.-Y., Cho S.-H., Kim Y.-W., et al. Effects of BCG, lymphotoxin and bee venom on insulitis and development of IDDM in non-obese diabetic mice. J. Korean Med. Sci. 1999;14:648–652. doi: 10.3346/jkms.1999.14.6.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.-Y., Jang H.-J., Leem J., et al. Protective Effects of Bee Venom-Derived Phospholipase A2 against Cholestatic Liver Disease in Mice. Biomedicines. 2021;9:992. doi: 10.3390/biomedicines9080992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.-W., Kwon Y.-B., Ham T.-W., et al. Acupoint stimulation using bee venom attenuates formalin-induced pain behavior and spinal cord fos expression in rats. J. Vet. Sci. 2003;65:349–355. doi: 10.1292/jvms.65.349. [DOI] [PubMed] [Google Scholar]
- Kim H., Lee H., Lee G., et al. Phospholipase A2 inhibits cisplatin-induced acute kidney injury by modulating regulatory T cells by the CD206 mannose receptor. Kidney Int. 2015;88:550–559. doi: 10.1038/ki.2015.147. [DOI] [PubMed] [Google Scholar]
- Kim A., Lee S.Y., Kim B.Y., et al. Elimination of Teratogenic Human Induced Pluripotent Stem Cells by Bee Venom via Calcium-Calpain Pathway. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21093265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.-Y., Lee S.-J., Maeng Y.-I., et al. Protective effects of bee venom against endotoxemia-related acute kidney injury in mice. Biology. 2020;9:154. doi: 10.3390/biology9070154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H., Park S.-Y., Lee G. Potential therapeutic applications of bee venom on skin disease and its mechanisms: a literature review. Toxins. 2019;11:374. doi: 10.3390/toxins11070374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko S.-H., Oh H.-M., Kwon D.-Y., et al. Incidence Rate of Bee Venom Acupuncture Related Anaphylaxis: A Systematic Review. Toxins. 2022;14:238. doi: 10.3390/toxins14040238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko S.J., Park E., Asandei A., et al. Bee venom-derived antimicrobial peptide melectin has broad-spectrum potency, cell selectivity, and salt-resistant properties. Sci. Rep. 2020;10:1–12. doi: 10.1038/s41598-020-66995-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno M., Horibe T., Ohara K., et al. The membrane-lytic peptides K8L9 and melittin enter cancer cells via receptor endocytosis following subcytotoxic exposure. Chem. Biol. 2014;21:1522–1532. doi: 10.1016/j.chembiol.2014.09.008. [DOI] [PubMed] [Google Scholar]
- Komi D.E.A., Shafaghat F., Zwiener R.D. Immunology of bee venom. Clin. Rev. Allergy Immunol. 2018;54:386–396. doi: 10.1007/s12016-017-8597-4. [DOI] [PubMed] [Google Scholar]
- Kong R., Lee Y.-S., Kang D.-H., et al. The antibacterial activity and toxin production control of bee venom in mouse MRSA pneumonia model. BMC Complem Altern M. 2020;20:1–12. doi: 10.1186/s12906-020-02991-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong G.-M., Tao W.-H., Diao Y.-L., et al. Melittin induces human gastric cancer cell apoptosis via activation of mitochondrial pathway. World J. Gastroenterol. 2016;22:3186. doi: 10.3748/wjg.v22.i11.3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurek-Górecka A., Górecki M., Rzepecka-Stojko A., et al. Bee Products in Dermatology and Skin Care. Molecules. 2020;25:556. doi: 10.3390/molecules25030556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurek-Górecka A., Komosinska-Vassev K., Rzepecka-Stojko A., et al. Bee Venom in Wound Healing. Molecules. 2021;26:148. doi: 10.3390/molecules26010148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon Y.-B., Kang M.-S., Han H.-J., et al. Visceral antinociception produced by bee venom stimulation of the Zhongwan acupuncture point in mice: role of α2 adrenoceptors. Neurosci. Lett. 2001;308:133–137. doi: 10.1016/s0304-3940(01)01989-9. [DOI] [PubMed] [Google Scholar]
- Kwon Y.-B., Kang M.-S., Kim H.-W., et al. Antinociceptive effects of bee venom acupuncture (apipuncture) in rodent animal models: a comparative study of acupoint versus non-acupoint stimulation. Acupunct Electrother Res. 2001;26:59–68. doi: 10.3727/036012901816356054. [DOI] [PubMed] [Google Scholar]
- Kwon Y.-B., Kim J.-H., Yoon J.-H., et al. The analgesic efficacy of bee venom acupuncture for knee osteoarthritis: a comparative study with needle acupuncture. Am. J. Chin. Med. 2001;29:187–199. doi: 10.1142/S0192415X01000228. [DOI] [PubMed] [Google Scholar]
- Kwon Y.-B., Lee J.-D., Lee H.-J., et al. Bee venom injection into an acupuncture point reduces arthritis associated edema and nociceptive responses. Pain. 2001;90:271–280. doi: 10.1016/S0304-3959(00)00412-7. [DOI] [PubMed] [Google Scholar]
- Lamas A., Arteaga V., Regal P., et al. Antimicrobial Activity of Five Apitoxins from Apis mellifera on Two Common Foodborne Pathogens. Antibiotics. 2020;9:367. doi: 10.3390/antibiotics9070367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarev V., Parfenova T., Gularyan S., et al. Induced expression of melittin, an antimicrobial peptide, inhibits infection by Chlamydia trachomatis and Mycoplasma hominis in a HeLa cell line. Int. J. Antimicrob. Agents. 2002;19:133–137. doi: 10.1016/s0924-8579(01)00479-4. [DOI] [PubMed] [Google Scholar]
- Lazarev V.N., Stipkovits L., Biro J., et al. Induced expression of the antimicrobial peptide melittin inhibits experimental infection by Mycoplasma gallisepticum in chickens. Microb. Infect. 2004;6:536–541. doi: 10.1016/j.micinf.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Leandro L.F., Mendes C.A., Casemiro L.A., et al. Antimicrobial activity of apitoxin, melittin and phospholipase A2 of honey bee (Apis mellifera) venom against oral pathogens. An Acad Bras Cienc. 2015;87:147–155. doi: 10.1590/0001-3765201520130511. [DOI] [PubMed] [Google Scholar]
- Lee S.-B. Antifungal activity of bee venom and sweet bee venom against clinically isolated Candida albicans. J Pharmacopunct. 2016;19:45. doi: 10.3831/KPI.2016.19.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G., Bae H. Anti-inflammatory applications of melittin, a major component of bee venom: Detailed mechanism of action and adverse effects. Molecules. 2016;21:616. doi: 10.3390/molecules21050616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G., Bae H. Bee venom phospholipase A2: Yesterday’s enemy becomes today’s friend. Toxins. 2016;8:48. doi: 10.3390/toxins8020048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.-H., Choi S.-M., Yang E.J. Melittin ameliorates the inflammation of organs in an amyotrophic lateral sclerosis animal model. Exp Neurobiol. 2014;23:86. doi: 10.5607/en.2014.23.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.-H., Kwon Y.-B., Han H.-J., et al. Bee venom pretreatment has both an antinociceptive and anti-inflammatory effect on carrageenan-induced inflammation. J. Veterinary Med. Sci. 2001;63:251–259. doi: 10.1292/jvms.63.251. [DOI] [PubMed] [Google Scholar]
- Lee W.-R., Pak S.C., Park K.-K. The protective effect of bee venom on fibrosis causing inflammatory diseases. Toxins. 2015;7:4758–4772. doi: 10.3390/toxins7114758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima W.G., Brito J.C., da Cruz Nizer W.S. Bee products as a source of promising therapeutic and chemoprophylaxis strategies against COVID-19 (SARS-CoV-2) Phytother. Res. 2021;35:743–750. doi: 10.1002/ptr.6872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling C.-Q., Li B., Zhang C., et al. Inhibitory effect of recombinant adenovirus carrying melittin gene on hepatocellular carcinoma. Ann. Oncol. 2005;16:109–115. doi: 10.1093/annonc/mdi019. [DOI] [PubMed] [Google Scholar]
- Liu X., Chen D., Xie L., et al. Effect of honey bee venom on proliferation of K1735M2 mouse melanoma cells in-vitro and growth of murine B16 melanomas in-vivo. J. Pharm. Pharmacol. 2002;54:1083–1089. doi: 10.1211/002235702320266235. [DOI] [PubMed] [Google Scholar]
- Liu Cui-Cui D.-J., Hao Q.Z., et al. Application of bee venom and its main constituent melittin for cancer treatment. Cancer Chemother. Pharmacol. 2016;78:1113–1130. doi: 10.1007/s00280-016-3160-1. [DOI] [PubMed] [Google Scholar]
- Mansour A.M., Elfiky A.A., Fahmy A., et al. Therapeutic effect of bee venom formulation in the treatment of FMD viral infection: Preclinical and clinical evaluation. IJSR. 2016;6:711–729. [Google Scholar]
- Masuda S., Murakami M., Takanezawa Y., et al. Neuronal expression and neuritogenic action of group X secreted phospholipase A2. J. Biol. Chem. 2005;280:23203–23214. doi: 10.1074/jbc.M500985200. [DOI] [PubMed] [Google Scholar]
- Matanic V.C.A., Castilla V. Antiviral activity of antimicrobial cationic peptides against Junin virus and herpes simplex virus. Int. J. Antimicrob. Agents. 2004;23:382–389. doi: 10.1016/j.ijantimicag.2003.07.022. [DOI] [PubMed] [Google Scholar]
- Memariani H., Memariani M. Melittin as a promising anti-protozoan peptide: current knowledge and future prospects. AMB Express. 2021;11:1–16. doi: 10.1186/s13568-021-01229-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memariani H., Memariani M., Shahidi-Dadras M., et al. Melittin: from honeybees to superbugs. Appl. Microbiol. Biotechnol. 2019;103:3265–3276. doi: 10.1007/s00253-019-09698-y. [DOI] [PubMed] [Google Scholar]
- Memariani H., Memariani M., Moravvej H., et al. Melittin: a venom-derived peptide with promising anti-viral properties. Eur. J. Clin. Microbiol. Infectious Dis.: Official Publication Eur. Soc. Clin. Microbiol. 2020;39:5–17. doi: 10.1007/s10096-019-03674-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuishi M., Masuda S., Kudo I., et al. Group V and X secretory phospholipase A2 prevents adenoviral infection in mammalian cells. Biochem. J. 2006;393:97–106. doi: 10.1042/BJ20050781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moerman L., Bosteels S., Noppe W., et al. Antibacterial and antifungal properties of α-helical, cationic peptides in the venom of scorpions from southern Africa. Eur. J. Biochem. 2002;269:4799–4810. doi: 10.1046/j.1432-1033.2002.03177.x. [DOI] [PubMed] [Google Scholar]
- Moga M.A., Dimienescu O.G., Arvătescu C.A., et al. Anticancer activity of toxins from bee and snake venom—an overview on ovarian cancer. Molecules. 2018;23:692. doi: 10.3390/molecules23030692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon D.-O., Park S.-Y., Heo M.-S., et al. Key regulators in bee venom-induced apoptosis are Bcl-2 and caspase-3 in human leukemic U937 cells through downregulation of ERK and Akt. Int. Immunopharmacol. 2006;6:1796–1807. doi: 10.1016/j.intimp.2006.07.027. [DOI] [PubMed] [Google Scholar]
- Moreno M., Giralt E. Three valuable peptides from bee and wasp venoms for therapeutic and biotechnological use: melittin, apamin and mastoparan. Toxins. 2015;7:1126–1150. doi: 10.3390/toxins7041126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousavi S.M., Imani S., Haghighi S., et al. Effect of Iranian honey bee (Apis mellifera) venom on blood glucose and insulin in diabetic rats. J. Arthropod-borne Dis. 2012;6:136. [PMC free article] [PubMed] [Google Scholar]
- Münstedt K., Bogdanov S. Bee products and their potential use in modern medicine. JAAS. 2009;1:57–63. [Google Scholar]
- Nainu F., Masyita A., Bahar M.A., et al. Pharmaceutical Prospects of Bee Products: Special Focus on Anticancer, Antibacterial, Antiviral, and Antiparasitic Properties. Antibiotics. 2021;10:822. doi: 10.3390/antibiotics10070822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oršolić N. Bee venom in cancer therapy. Cancer Metastasis Rev. 2012;31:173–194. doi: 10.1007/s10555-011-9339-3. [DOI] [PubMed] [Google Scholar]
- Ozdemir C., Kucuksezer U., Akdis M., et al. Mechanisms of immunotherapy to wasp and bee venom. Clin. Exp. Allergy. 2011;41:1226–1234. doi: 10.1111/j.1365-2222.2011.03812.x. [DOI] [PubMed] [Google Scholar]
- Palm N.W., Rosenstein R.K., Yu S., et al. Bee venom phospholipase A2 induces a primary type 2 response that is dependent on the receptor ST2 and confers protective immunity. Immunity. 2013;39:976–985. doi: 10.1016/j.immuni.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan H., Soman N.R., Schlesinger P.H., et al. Cytolytic peptide nanoparticles (‘NanoBees’) for cancer therapy. Wiley Interdiscip Rev: Nanomed Nanobiotechnol. 2011;3:318–327. doi: 10.1002/wnan.126. [DOI] [PubMed] [Google Scholar]
- Park S., Baek H., Jung K.H., et al. Bee venom phospholipase A2 suppresses allergic airway inflammation in an ovalbumin-induced asthma model through the induction of regulatory T cells. Immun Inflamm Dis. 2015;3:386–397. doi: 10.1002/iid3.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J., Kwon O., An H.-J., et al. Antifungal effects of bee venom components on trichophyton rubrum: A novel approach of bee venom study for possible emerging antifungal agent. Ann Dermatol. 2018;30:202–210. doi: 10.5021/ad.2018.30.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H.J., Lee S.H., Son D.J., et al. Antiarthritic effect of bee venom: Inhibition of inflammation mediator generation by suppression of NF-κB through interaction with the p50 subunit. Arthritis Rheum. 2004;50:3504–3515. doi: 10.1002/art.20626. [DOI] [PubMed] [Google Scholar]
- Pattabhiramaiah M., Ramesh K., Kv V., et al. Computational analysis of PhospholipaseA2 in the honey bee venom. J. Apic. Res. 2020:1–16. [Google Scholar]
- Picoli T., Peter C.M., Vargas G.D., et al. Antiviral and virucidal potential of melittin and apamin against bovine herpesvirus type 1 and bovine viral diarrhea virus. Pesqui Vet Bras. 2018;38:595–604. [Google Scholar]
- Pollak M. Overcoming drug development bottlenecks with repurposing: repurposing biguanides to target energy metabolism for cancer treatment. Nat. Med. 2014;20:591–593. doi: 10.1038/nm.3596. [DOI] [PubMed] [Google Scholar]
- Pucca M.B., Cerni F.A., Oliveira I.S., et al. Bee Updated: Current Knowledge on Bee Venom and Bee Envenoming Therapy. Frontiers in immunology. 2019;10:2090. doi: 10.3389/fimmu.2019.02090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putz T., Ramoner R., Gander H., et al. Antitumor action and immune activation through cooperation of bee venom secretory phospholipase A2 and phosphatidylinositol-(3, 4)-bisphosphate. Cancer Immunol Immunother. 2006;55:1374–1383. doi: 10.1007/s00262-006-0143-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rady I., Siddiqui I.A., Rady M., et al. Melittin, a major peptide component of bee venom, and its conjugates in cancer therapy. Cancer Lett. 2017;402:16–31. doi: 10.1016/j.canlet.2017.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliffe N., Azambuja P., Mello C.B. Recent advances in developing insect natural products as potential modern day medicines. Evid Based Complement Alternat Med. 2014 doi: 10.1155/2014/904958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regeai S.O., Abusrer S.A., Shibani N.S. Low semen quality and adverse histological changes in testes of adult male mice treated with bee venom (Apis mellifera) Open Vet J. 2021;11:70–79. doi: 10.4314/ovj.v11i1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu J.-M., Na H.-H., Park Y.-J., et al. Sweet Bee Venom Triggers Multiple Cell Death Pathways or Spurs Acute Cell Rupture According to Its Concentration in THP-1 Monocytic Leukemia Cells. Genes. 2022;13:223. doi: 10.3390/genes13020223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salama M.A., Younis M.A., Talaat R.M. Cytokine and inflammatory mediators are associated with cytotoxic, anti-inflammatory and apoptotic activity of honeybee venom. J. Altern. Complement Med. 2021;18:75–86. doi: 10.1515/jcim-2019-0182. [DOI] [PubMed] [Google Scholar]
- Samanci T., Kekeçoğlu M. Comparison of commercial and anatolian bee venom in terms of chemical composition. U Bee J. 2019;19 [Google Scholar]
- Saris N.-E., Carafoli E. A historical review of cellular calcium handling, with emphasis on mitochondria. Biochemistry (Moscow). 2005;70:187–194. doi: 10.1007/s10541-005-0100-9. [DOI] [PubMed] [Google Scholar]
- Sattar A.-S. Metformin and Bee Venom Enhanced Histological Changes of the Pancreas in Diabetic Mice. Wasit J. Pure Sci. 2022;1:192–201. [Google Scholar]
- Seo Y.J., Jeong Y.S., Park H.S., et al. Late-Onset Post-radiation Lymphedema Provoked by Bee Venom Therapy: A Case Report. Ann. Rehabilitation Med. 2018;42:626–629. doi: 10.5535/arm.2018.42.4.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaaban A.M.M., Hamza R.G. Studying the Ameliorative Effect of Bee Venom Against Damage and Inflammation Induced in Gamma-Irradiated Rats. AJNSA. 2019;52:178–184. [Google Scholar]
- Shen L., Lee J.H., Joo J.C., et al. Bee Venom Acupuncture for Shoulder Pain: A Systematic Review and Meta-analysis of Randomized Controlled Trials. J. Pharmacopuncture. 2020;23:44. doi: 10.3831/KPI.2020.23.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi W., Li C., Li M., et al. Antimicrobial peptide melittin against Xanthomonas oryzae pv. oryzae, the bacterial leaf blight pathogen in rice. Appl. Microbiol. Biotechnol. 2016;100:5059–5067. doi: 10.1007/s00253-016-7400-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin D., Choi W., Bae H. Bee venom phospholipase A2 alleviate house dust mite-induced atopic dermatitis-like skin lesions by the CD206 mannose receptor. Toxins. 2018;10:146. doi: 10.3390/toxins10040146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva J., Monge-Fuentes V., Gomes F., et al. Pharmacological alternatives for the treatment of neurodegenerative disorders: Wasp and bee venoms and their components as new neuroactive tools. Toxins. 2015;7:3179–3209. doi: 10.3390/toxins7083179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobral F., Sampaio A., Falcão S., et al. Chemical characterization, antioxidant, anti-inflammatory and cytotoxic properties of bee venom collected in Northeast Portugal. Food Chem. Toxicol. 2016;94:172–177. doi: 10.1016/j.fct.2016.06.008. [DOI] [PubMed] [Google Scholar]
- Socarras K.M., Theophilus P.A., Torres J.P., et al. Antimicrobial activity of bee venom and melittin against Borrelia burgdorferi. Antibiotics. 2017;6:31. doi: 10.3390/antibiotics6040031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somwongin S., Chantawannakul P., Chaiyana W. Antioxidant activity and irritation property of venoms from Apis species. Toxicon: Official J. Int. Soc. Toxinol. 2018;145:32–39. doi: 10.1016/j.toxicon.2018.02.049. [DOI] [PubMed] [Google Scholar]
- Son D.J., Lee J.W., Lee Y.H., et al. Therapeutic application of anti-arthritis, pain-releasing, and anti-cancer effects of bee venom and its constituent compounds. Pharmacol. Ther. 2007;115:246–270. doi: 10.1016/j.pharmthera.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Šuran J., Cepanec I., Mašek T., et al. Nonaqueous polyethylene glycol as a safer alternative to ethanolic propolis extracts with comparable antioxidant and antimicrobial activity. Antioxidants. 2021;10:978. doi: 10.3390/antiox10060978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surendra N., Jayaram G., Reddy M. Antimicrobial activity of crude venom extracts in honeybees (Apis cerana, Apis dorsata, Apis florea) tested against selected pathogens. Afr. J. Microbiol. Res. 2011;5:2765–2772. [Google Scholar]
- Uddin M.B., Lee B.H., Nikapitiya C., et al. Inhibitory effects of bee venom and its components against viruses in vitro and in vivo. J. Microbiol. (Seoul, Korea). 2016;54:853–866. doi: 10.1007/s12275-016-6376-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varanda E.A., Takahashi C., Soares A., et al. Effect of Apis mellifera bee venom and gamma radiation on bone marrow cells of wistar rats treated in vivo. Rev Bras Genet. 1992;15:807–819. [Google Scholar]
- Varanda E.A., Takahashi C.S. Effect of pretreatment with venom of Apis mellifera bees on the yield of gamma-ray induced chromosome aberrations in human blood lymphocytes. Rev Bras Genet. 1993;16:551–559. [Google Scholar]
- Varanda E., Tavares D. Radioprotection: mechanisms and radioprotective agents including honeybee venom. J. Venom. Anim. Toxins. 1998;4:5–21. [Google Scholar]
- Varol A., Sezen S., Evcimen D., et al. Cellular targets and molecular activity mechanisms of bee venom in cancer: recent trends and developments. Toxin Rev. 2022:1–14. [Google Scholar]
- Wang C., Chen T., Zhang N., et al. Melittin, a major component of bee venom, sensitizes human hepatocellular carcinoma cells to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis by activating CaMKII-TAK1-JNK/p38 and inhibiting IκBα kinase-NFκB. J. Biol. Chem. 2009;284:3804–3813. doi: 10.1074/jbc.M807191200. [DOI] [PubMed] [Google Scholar]
- Wehbe R., Frangieh J., Rima M., et al. Bee venom: Overview of main compounds and bioactivities for therapeutic interests. Molecules. 2019;24:2997. doi: 10.3390/molecules24162997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Zhao B., Cheng Y., et al. Melittin induces PTCH1 expression by down-regulating MeCP2 in human hepatocellular carcinoma SMMC-7721 cells. Toxicol. Appl. Pharmacol. 2015;288:74–83. doi: 10.1016/j.taap.2015.07.010. [DOI] [PubMed] [Google Scholar]
- Yaacoub C., Rifi M., El-Obeid D., et al. The Cytotoxic Effect of Apis mellifera Venom with a Synergistic Potential of Its Two Main Components—Melittin and PLA2—On Colon Cancer HCT116 Cell Lines. Molecules. 2021;26:2264. doi: 10.3390/molecules26082264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahran F., Mohamad A., Zein N. Bee venom ameliorates cardiac dysfunction in diabetic hyperlipidemic rats. Exp. Biol. Med. 2021;246:2630–2644. doi: 10.1177/15353702211045924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahran F., Mohamed A., Zein N. Bee venom attenuates degenerative effects of diabetes associated with hyperlipidemia in rats. Biochemistry Letters. 2021;17:77–107. [Google Scholar]
- Zhang S., Liu Y., Ye Y., et al. Bee venom therapy: Potential mechanisms and therapeutic applications. Toxicon: Official J. Int. Soc. Toxinol. 2018;148:64–73. doi: 10.1016/j.toxicon.2018.04.012. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Zhang H., Peng T., et al. Melittin suppresses cathepsin S-induced invasion and angiogenesis via blocking of the VEGF-A/VEGFR-2/MEK1/ERK1/2 pathway in human hepatocellular carcinoma. Oncol Lett. 2016;11:610–618. doi: 10.3892/ol.2015.3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Hu W., Zhang Z., et al. Bee venom protects against pancreatic cancer via inducing cell cycle arrest and apoptosis with suppression of cell migration. J. Gastrointestinal Oncol. 2022;13:847. doi: 10.21037/jgo-22-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J., Lee H.L., Ham Y.W., et al. Anti-cancer effect of bee venom on colon cancer cell growth by activation of death receptors and inhibition of nuclear factor kappa B. Oncotarget. 2015;6:44437. doi: 10.18632/oncotarget.6295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zidan H.-A.-E.-G., Mostafa Z.K., Ibrahim M.A., et al. Venom Composition of Egyptian and Carniolan Honeybee, Apis mellifera L. affected by collection methods. Egyptian Academic J. Biol. Sci. A, Entomol. 2018;11:59–71. [Google Scholar]