Abstract
The complicated multiple sclerosis (MS) can exhibit subacute sight deterioration and can lead to total deprivation of vision. In the current work, we explored the therapeutic outcome of Cathepsin B inhibitor (CA-074) against retinopathy and optic neuritis (ON) caused by experimental autoimmune encephalomyelitis (EAE) induced by proteolipid protein peptide (PLP) in female SJL/J mice. A daily dose of 10 mg/kg CA-074 was administered to the EAE mice intraperitoneally for 14 days from day 14 post-immunization until day 28. The Western blot and immunofluorescence analyses show inflammation in the optic nerve through the elevation of iNOS and NFkB markers in EAE mice. Optic neuritis was reported which is a consequence of demyelination and axon injury, estimated with the reduction in myelin basic protein (MBP). The glial fibrillary acidic protein (GFAP) expression level was found to be elevated in the retina of EAE mice which confirmed the retinopathy. The administration of CA-074 ameliorated optic neuritis and retinopathy by reducing inflammation. The treatment with CA-074 also reduced the demyelination and axonal injuries in the EAE mice. The findings of this study have shown the protective effect of CA-074 in the case of retinopathy and ON inflicted by EAE in SJL/J mice.
Keywords: Multiple sclerosis, Neurodegeneration, Demyelination, Retinopathy, Optic Neuritis
1. Introduction
Multiple Sclerosis (MS) is an autoimmune, multifocal inflammatory demyelination disorder of the central nervous system (CNS). MS is a disease marked by oligodendrocyte loss and clinical indicators such as paralysis and ataxia, which can lead to major disability and even death (Jin et al., 2019, Shindler et al., 2012, Wilmes et al., 2018, Yang et al., 2017). The pathological development of MS is manifested through myelin-specific autoreactive lymphocytes generated by autoimmune reactions (Allan & Yates, 2015). In the CNS, broad activation of macrophages and microglia occurs first, followed by continuous immunological infiltration of lymphocytes and macrophages (Goverman, 2009, Nakahara et al., 2010, Yamasaki et al., 2014). This process is complicated by glial activation and lymphocytic infiltration (Compston & Coles, 2008).
MS can cause subacute vision loss to full vision loss, impaired color sight, reduced contrast responsiveness, and retrobulbar discomfort at the time of eye movement, among other symptoms (Compston and Coles, 2008, Lampert et al., 2015). The reactive oxygen species along with the nitric oxide(NO) are released by activated immune cells during the disease, causing oxidative stress and further, the damaging processes of demyelination, neural destruction, and inflammation in the case of MS (Wang et al., 2014, Witherick et al., 2010) The patients' studies have revealed severe axon and retinal ganglion cells (RGC) damage as well as diminishing nerve fibers in the retina and vision deprivation (Shindler et al., 2006, Shindler et al., 2008). After the onset of the clinical symptoms of optic neuritis (ON), a reduction in macular volume occurs, which is found linked to the degree of remaining visual impairment. (Steel & Waldock, 1998). Studies have also shown that vision loss after the occurrence of ON is associated with RGCs death (Trip et al., 2005), However, sometimes patients have RGC damage in absence of an episode of optic nerve damage, implicating that losing RGC can be a mediator of visual loss in this condition (Fisher et al., 2006).
Monoclonal antibodies and immunosuppressive and immunomodulating drugs are among the various therapeutic options available for the treatment of MS. The multidimensional therapies and additional explanation related to neuroprotective pathways involved in the autoimmune process will help us find a greater comprehension of the disease's complexity as well as enhance treatment, particularly in the case of long-term disability and cognitive loss. (Filippini et al., 2013). The treatment with a high dose of corticosteroids is usually applied in clinical procedures to relieve MS and ON, while prolonged corticosteroid administration is avoided because of its harmful effects. As a result, it is critical to create innovative medicines that focus on the clinical conditions along with ON.
The experimental autoimmune encephalomyelitis (EAE) animal model is the most commonly used for studying the immunological mechanisms relevant to MS (Jin et al., 2019, Shindler et al., 2008). The EAE mimics the common markers of human disease, including CNS inflammation, demyelination, oxidative stress, and neurodegeneration. (Li et al., 2013). The EAE model of SJL/J mice exhibits remission and relapse similar to the relapsing, remitting (RR), and progressive MS in humans (Ransohoff, 2012). The EAE is arbitrated by T-cells with numerous similarities to MS. (Larabee et al., 2016). Elevated levels of iNOS and NFkB were found in activated microglia and optic nerve tissue, which have been reported to be associated with the activation of neurotoxic astrocytes (Yang et al., 2017).
In MS lesions and cerebrospinal fluid, acidic and neutral proteases have been found to be elevated. (Bever & Whitaker, 1985). The proteolytic breakdown of myelin proteins is the first step in the injury processes that occurs during the onset and progression of MS. The cysteine cathepsins found in lysosomes are a group of proteases that mainly control protein hydrolysis these proteases also activate the other proteases found in the endosomes and lysosomes (Lecaille et al., 2002, Rawlings et al., 2010). The cathepsin B (CTHB) are well-known lysosomal cysteine protease found in all mammalian cells including neuronal tissues (Rawlings et al., 2010). The main role of cathepsin B and L is the degradation of intracellular proteins, proenzyme activation, hormone maturation, epidermal homeostasis, and antigen presentation (Turk, Turk, & Turk, 2000). The CTHB in particular involved in several disorders, including cancer progression (Yan & Sloane, 2003), and arthritis (Baici, Lang, Zwicky, & Muntener, 2005).
The CTHB has been found linked to the degradation of myelin basic protein (MBP) (Whitaker, Heinemann, & Uzman, 1982). If produced by macrophages or other cells, it could therefore play a role in inflammatory demyelination alongside other enzymes. The CA-074 is a powerful cathepsin B inhibitor that inhibits cysteine proteases irreversibly (Murata et al., 1991). Recently, we have reported the targeted pharmacological inhibition of CTHB with CA-074 in the relapsing and remitting form of EAE and have shown that the treatment with CA-074 has ameliorated the clinical symptoms of EAE and improved the neuro-axonal damage (Ansari et al., 2022).
The SJL/J mouse model of the RR form of MS expresses relevant disease conditions to investigate the effectiveness of a medicinal compound in some preclinical settings. The present study explored the therapeutic outcome of CTHB inhibitor CA-074 administration to evaluate whether it would reduce the inflammatory responses in retinal tissue, optic nerve, and clinical signs of retinopathy in EAE. We found that in EAE mice the inflammatory markers of retinopathy and ON were elevated in the retina and optic nerve respectively, which is linked with elevated inflammation in the RR model of EAE. The CA-074 treatment protected the severity of the neuronal damage caused by EAE which in turn reduced the potential of ON and the retinopathy inflicted by EAE in the SJL/J mice.
2. Materials and methods
2.1. Animals
The 9–10-week old adult female SJL/J mice were procured from Jackson Laboratories (Bar Harbor, ME, USA). The two weeks of acclimatization was ensured before the immunization of the mice. The room temperature of 22 °C ± 2 °C, 12:12-h light/dark cycle, and 40 to 60 % humidity were maintained for the mice They were accommodated in pathogen-free conditions. The mice were provided with the standard rodent diet, and water ad libitum. All experimental procedures were carried out in accordance with the guidelines of the Institutional Research Ethics Committee (REC), King Saud University as well as in compliance with the ARRIVE guidelines.
2.2. EAE induction in SJL/J mice
The isoflurane was used to anesthetize the mice (Abbott Laboratories). The 200 μg proteolipid protein peptide (PLP-amino acids 139–151), 50 μg/site emulsified with complete Freund’s adjuvant (CFA) (Hooke Laboratories, USA), was administered subcutaneously at four sites In addition, immunization with the intraperitoneal injection of 200 ng lyophilized pertussis toxin (PTX) (Hooke Laboratories, USA) was also performed on the same day of immunization. the PTX injection was repeated in the mice on the third-day post-encephalitogenic injection. The Clinical scoring (0–5 scale) was carried out daily as reported previously (Horstmann et al., 2013). The scoring system was as follows: 0 = normal; 1 = flaccid tail; 2 = partial hind limb paralysis; 3 = both hind limb paralysis; 4 = both hind limb paralysis and forelimb frailty; 5 = moribund, requiring sacrifice or inadvertent death.
2.3. Experimental groups and CA-074 administration
To investigate the effect of CA-074 on retinopathy and optic ON the mice were administered CA-074 (Tocris, Bristol, UK),10 mg/kg i.p, in a vehicle (0.9 % DMSO in PBS) or only vehicle daily from day 14 to day 28 after immunization. The CA-074 dose was chosen as has been previously reported (Tang et al., 2018). The mice were randomized into the following groups (N = 6) after the onset of the disease symptoms: Group A, control: were administered with vehicle only. Group B, Drug-Control: treated with CA-074, as written above. Group C, EAE: injected with PLP for immunization, Group D, EAE + CA-074: administered with PLP treated with CA-074. The mice were anesthetized and sacrificed at the completion of the experiment. The retinas and optic nerves were harvested for western blot and immunofluorescence analyses.
2.4. Mouse retina and optic nerve processing
The sodium pentobarbital i.p. (100 mg/kg body weight) was used to anesthetize the mice. The eyes were dissected and submerged for 3 h into 4 % paraformaldehyde (PFA) for fixation then placed into 30 % sucrose for cryoprotection. The eyes and optic nerves were prepared for cross-sectioning by placing them into a mold and covered with Optimal Cutting Temperature compound (O.C.T.) (VWR, Radnor, PA, USA) followed by snap freezing with liquid nitrogen. The 16 μm vertical sections of eyes were prepared and then mounted on the slides (Thermo Scientific, Rockford, IL, USA). The optic nerves were separated out from the eyes and placed upright into a mold pre-filled with O.C.T. and similarly sectioned as explained above for eye sectioning. The 10 μm thick optic nerve sections were prepared and mounted onto slides followed by staining for immunofluorescence analyses (Jin et al., 2019).
2.5. Western blotting
In present study, we analyzed expression levels of retinopathy and optic neuritis markers in the tissue lysates of the retina and optic nerves respectively. Briefly, the tissues of all groups of mice (n = 6 mice per group) were homogenized in RIPA buffer containing phosphatase and protease inhibitors. The protein concentrations of samples homogenate of tissues from all the groups were measured using BCA protein estimation kit from Bio-Rad, then mixed with 2X lamellae buffer, denatured at 95 °C for 10 min, 10 % SDS-PAGE gel was used to separate the protein. The Proteins from the gel were transferred to the nitrocellulose membrane. Before probing with the primary antibodies, the membranes were treated with a blocking buffer (3 % BSA in TBS-T) for 30 min. The membranes were then incubated with the following primary antibodies overnight at 4 °C: Rabbit anti-MBP (1:500, Novus); Mouse anti-GFAP (1:500, Novus); Guinea Pig anti-IBA1 (1:500, Novus); Mouse anti-iNOS (1:500, Santa Cruz); mouse anti-NFkB p65 (1:500 Santa Cruz), and after striping, the same blots were probed with mouse anti-β-actin (1:3000 Santa Cruz). The membranes were then incubated with HRP-conjugated secondary antibodies for 1 h and the blots were then developed with ECL reagents. The chemiluminescence signals were detected with the image analyzer (Bio-Rad). The quantifications and analyses of protein expression were performed with the help of ImageJ software (NIH, USA), by measuring the intensities of the bands corresponding to the proteins of interest (Alshammari et al., 2019).
2.6. Immunofluorescence staining
In this study immunofluorescence analyses have been used to score the semi-quantitative expression of retinopathy markers in the retinas and optic nerve slices of control, EAE, and EAE + CA-074 mice. We expected that the molecular architecture of the retina and optic nerves would be changed in EAE mice. These alterations might be rescued in CA-treated mice. The frozen mouse eyes and optic nerves were sectioned vertically (16 µm and 10 µm thickness respectively) using a cryostat (Leica CM3050s). The eyes and optic nerve sections were washed with PBS (1x) and permeabilized with a permeabilizing agent (1 % Triton, 0.5 % tween in PBS). The slices were then washed thoroughly with 1x PBS, blocked with 10 % normal goat serum (Sigma-Aldrich)for 1 hr. The slides were then incubated with the primary antibodies in 3 % BSA dissolved in 1x PBS overnight at 4 °C. The primary antibodies used were; mouse anti-GFAP (1: 300, Novus Biologicals USA) and rabbit anti-MBP (1: 250, Novus Biologicals USA). On the next day, the slides were washed with 1x PBS, and treated with specific secondary antibodies: Alexa 568-conjugated goat-anti-mouse for mouse GFAP (1:250, Invitrogen) Alexa 488-conjugated goat-anti-rabbit for MBP (1:250, Invitrogen) for 1 hr. at room temperature. The sections were washed with 1x PBS buffer, followed by counterstaining with DAPI (1:3000 in PBS). In the final step the sections were washed with 1x PBS and rinsed with distilled water, dried, overlaid with anti-fade mountant (Life Technologies), and cover-slipped with Premium Cover Glass coverslip (Fisher Scientific) (Jin et al., 2019).
2.7. Immunofluorescence imaging
To capture the images the Zeiss Axio Observer microscope equipped with the specific excitation and emission filters and Zen Pro software (Carl Zeiss, Oberkochen, Germany) was used. The Comparisons of control, EAE, and EAE + CA-074 groups were performed on sections arranged in a row, and images were captured on single exposure times. The image acquisitions were executed at 488 nm excitation for Alexa 488 and 568 nm for Alexa 568. The binary images of the staining and their intensities were quantified with the help of ImageJ software (NIH). The regions of interest (ROI) from the images were analyzed as per the previously published report (Jin et al., 2019).
2.8. Statistical analysis
The Data were analyzed by either one-way ANOVA using Bonferroni post hoc, or two-way repeated measures. All the analyses were performed by GraphPad Prism 5 software (San Diego, USA). The data are reported as the mean ± standard error of the mean (SEM), and the differences were considered significant at P < 0.05.
3. Results
3.1. The CA-074 treatment alleviates the optic neuritis in EAE mice induced by PLP
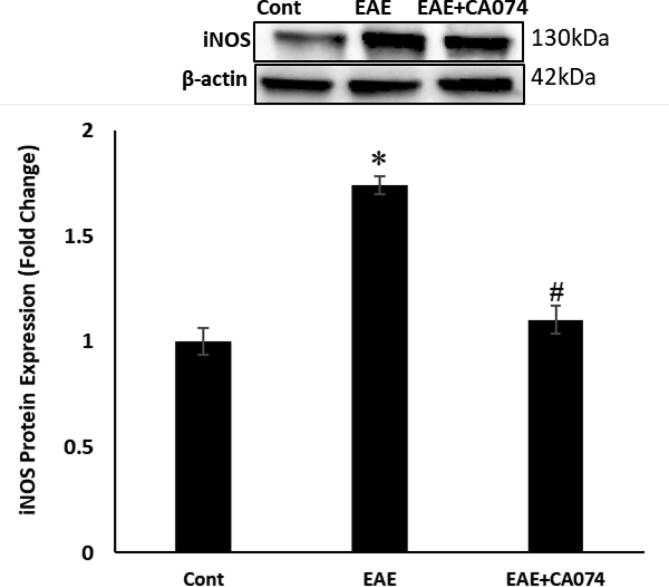
In the current study, the outcomes of CA-074 treatment on retinopathy and optic neuritis caused by EAE were evaluated on SJL/J mice immunized with PLP. The dose of CA-074 was injected immediately after the onset of the disease. The expression of inflammatory marker iNOS, which produces nitric oxide as well as the alteration of NFkB expression was investigated. The transcription factor NFkB actively participates in the inflammatory process. The activated NF-kB guides the expression of several inflammatory cytokines. The NF-kB has been an important therapeutic target for many inflammatory disorders. In this study, we explored the alteration in the expression levels of iNOS and nuclear NF-kB in the optic nerves. The Western blot study has shown that the expression of iNOS (Fig. 1) and nuclear NF-kB (Fig. 2) are up-regulated in the optic nerves of the EAE mice compared to the control group. The treatment with the CA-074 restored these changes in comparison to the untreated EAE mice.
Fig. 1.
Western blot analysis of inducible nitric oxide synthase (iNOS) expression has shown that the treatment with CA-074 attenuated the expression of iNOS in the optic nerve of the EAE Mice. (A)Western blot analysis of iNOS expression. (B) NF-kB expression. The Data are shown as mean ± S.E.M, n = 6. p < 0.05 is accepted as the level of significance; ∗p < 0.05. *p < 0.05 compared to the SJL/J control group; **p < 0.05 compared to EAE group.
Fig. 2.
Western blot analysis of NF-kB expression has shown that the treatment with CA-074 downregulated the activation of NFkB in the optic nerve of the EAE Mice. The Data are shown as mean ± S.E.M, n = 6. p < 0.05 is accepted as the level of significance; ∗p < 0.05. *p < 0.05 compared to the SJL/J control group; **p < 0.05 compared to EAE group.
3.2. CA-074 reduces the activation of the retinal-glial cells of EAE mice
In the retinas of EAE mice, the activated glial cells expressed GFAP. The Western blot results have shown that GFAP-positive glial cells had stronger GFAP expression in the EAE mice in contrast to the control group, indicating activation of glia (Fig. 3A). We also estimated the expression of ionized Calcium-binding adaptor molecule (IBA1) to find the presence of microglia. The IBA1 expression was also found to be elevated in EAE mice indicating the activation of phagocytes (Fig. 3B). The immunofluorescence analyses also showed elevated expression intensity of retinal GFAP of EAE mice in contrast to the control group (Fig. 4). The treatment with CA-074 has reduced the expression of both GFAP and IBA1, suggestive of a reduction in activated Müller glia and phagocytes which in turn indicates reduced inflammation.
Fig. 3.
The CA-074 reduces the activation of glial cells in the inner retina in EAE mice. Western blot analysis. The treatment of CA-074 downregulated the expression of GFAP (A) and IBA1 (B). The Data were shown as mean ± S.E.M, n = 6. p < 0.05 is accepted as the level of significance; ∗p < 0.05. *p < 0.05 compared to the SJL/J control group; **p < 0.05 compared to EAE group.
Fig. 4.
The activation of Glial cells occurred in the retinas of EAE mice, the treatment with CA-074 decreases the activation of glial cells in EAE mice. The analyses of GFAP immunofluorescence in the retina (n = 6), of control SJL/J (A), The increased GFAP + glia cells in EAE mouse (B) The reduced staining intensity of GFAP in EAE + CA-074 treated mice (C). The Data were shown as mean ± S.E.M, n = 6. p < 0.05 is accepted as the level of significance; ∗p < 0.05. *p < 0.05 compared to the SJL/J control group; **p < 0.05 compared to EAE group. Scale bars represent 20 μm.
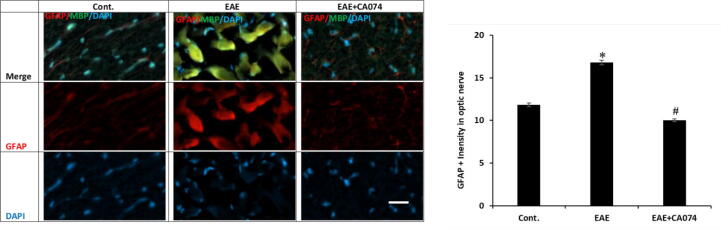
3.3. The treatment of CA-074 reduced the activation of astrocytes in EAE mice
On day 28, in the optic nerves of the control mice the GFAP expression was lesser as compared to the EAE mice and the GFAP positive cells have shown denser processes in the EAE group compared to the controls showing that the astrocytes are altered in EAE. Immunofluorescence analyses supported the elevated GFAP expression in the glial cells of the optic nerves at the advanced stage of EAE mice (Fig. 5). These findings have shown the alteration of the astrocytes in the EAE. In the group that received CA-074, a significantly reduced area of GFAP positive signal was detected than in the EAE group.
Fig. 5.
The activation of glial cells occurred in the optic nerves of the EAE mice, the treatment with the CA-074 reduces the activation of glial cells in the optic nerve of EAE mice. The estimation of GFAP immunofluorescence in the optic nerve (n = 6), of control, the increased GFAP positive glia cells in EAE mouse and reduced staining intensity of GFAP in EAE + CA-074 treated mice. The Data were shown as mean ± S.E.M, n = 6. p < 0.05 is accepted as the level of significance; ∗p < 0.05. *p < 0.05 compared to the SJL/J control group; **p < 0.05 compared to EAE group. Scale bars represent 20 μm.
3.4. The CA-074 treatment reduced the demyelination in EAE mice
The demyelination is the hallmark of EAE pathogenesis. There was significant demyelination as shown by the reduced level of MBP in the immunofluorescence analyses of the optic nerve tissues of the EAE mice as compared to the control group. The CA-074 intervention markedly reduced the demyelination and axonal injury to the optic nerve (Fig. 6).
Fig. 6.
The Demyelination and axonal injury occurred in the optic nerves of the EAE mice, the treatment with CA-074-protected axonal demyelination is shown by estimation of MBP immunofluorescence in the optic nerve (n = 6), of control, the demyelination in EAE mice and reduced demyelination in EAE + CA-074 treated mice. The Data were shown as mean ± S.E.M, n = 6. p < 0.05 is accepted as the level of significance; ∗p < 0.05. *p < 0.05 compared to the SJL/J control group; **p < 0.05 compared to EAE group. Scale bars represent 20 μm.
4. Discussion
Multiple sclerosis (MS) is an autoimmune disease characterized by inflammation, demyelination, and axonal damage in the brain and spinal cord. There is no effective treatment available until now. At the early stage of MS, optic nerve damage occurs, resulting in retinopathy and optic neuritis (Hobom et al., 2004). A cysteine protease, Cathepsin B (CTHB), present in neutral lysosomes found to be been involved in the demeaning of MBP (Rawlings et al., 2010, Whitaker et al., 1982). If produced by macrophages or other cells, it could thus play a role in inflammatory demyelination alongside other enzymes. The CA-074 is a Cathepsin B inhibitor (CA-074) that irreversibly inhibits cysteine proteases. (Murata et al., 1991).The mechanistic inhibition of CTHB to investigate its role in retinopathy and optic neuritis in the EAE model of SJL/J mice has not been reported previously. hence, the present study was designed to investigate the possible protective role of CA-074 on the retinopathy and optic neuritis developed in EAE Mice.
The EAE SJL/J mice in this work mimicked the symptoms of MS. The distinct feature of SJL/J mice inoculated with PLP139-151 is the relapsing and remitting of clinical symptoms, and it is strikingly analogous to the medical events seen in human RRMS. This model is optimal for studying the effect of targeted therapy on RRMS, as opposed to the EAE model produced through MOG35-55-immunization in C57BL/6 mice, which develops single-phase MS conditions without relapses (Jin et al., 2019, Wilmes et al., 2018). In the present study female SJL/J mice were used because they develop severe EAE episodes as compared to the male SJL/J mice. The human counterparts, have a larger frequency of MS opposite to men, have shown similar clinical findings (Ransohoff, 2012). The nuclear factor NF-kB modulates the transcription of the diverse inflammatory as well as immune-regulatory components. The inhibition of the calpain by its inhibitor calpeptin caused an indirect reduction in NF-kB expression and is effective in improving ON in EAE animal models (Das et al., 2013). In the optic nerve tissues of EAE mice, we investigated the inhibitory effect of CA-074 on NFkB as well as the expression of the NF-kB-linked inflammatory protein iNOS. The overproduction of NO is caused by iNOS overexpression, which can lead to cell injury and myelin loss. (Danilov et al., 2003, Iwahashi et al., 1999). One of the previous studies has reported that the EAE starts with the intrusion of T-cells,iNOS generating microglia, and proliferation of astrocytes occurs at the severe stage of the disease in the optic nerves of the MOG35-55 induced EAE model (Jin et al., 2019). We reported that CA-074 successfully inhibited NF-kB Expression, and downregulated iNOS production which could minimize the demyelination and inflammation caused by EAE in the optic nerves and retinas. The present outcome could be due to the anti-inflammatory effect of the CA-074. The administration of CA-074 may have protected RGCs in our study probably by reducing inflammatory conditions.
In addition, this could be because CA-074 therapy might have reduced the retinal microglia’s activation, and infiltration of macrophages by reducing the proapoptotic conditions and also could have inhibited the activation of astrocytic NF-kB which might have downgraded the apoptosis in the retina. In line with the previous finding, the suppression of NF-kB is linked to a slowdown in RGC death in EAE mice. (Brambilla et al., 2012). These results stipulate that the CA-074 intervention attenuates the pathologic intensity of EAE by reducing demyelination, apoptosis as well as inflammation in the Optic nerve and retinas of EAE mice.
We focused on astrocytes among macroglia. Astrocytes can become reactive and produce a glial scar when they are injured in the brain. (Correale and Farez, 2015, Pekny et al., 1998, Sun and Jakobs, 2012). The infiltration of immune cells, the formation of cytotoxic substances, the weakening of remyelination, and reducing regeneration of axons all are the effects of astrocytes in MS (Correale & Farez, 2015).
In one of the frequently used models of EAE generated by the immunization with MOG 35–55 peptide to the C57/B6 mice, it was previously documented that neurotoxic astrocytes are prominent in optic neuritis and retinopathy, are responsible for eventual RGC loss (Jin et al., 2019). Similarly, we also reported the GFAP-positive astrocytes in the retina and optic nerves of PLP-induced EAE in SJL/J mice and we report that the administration of CA-074 has reduced GFAP-positive microglia in the same tissues. These findings are consistent with studies in EAE mice's spinal cords, where laquinimod reduced the population and activation of the microglia (Mishra et al., 2014, Wilmes et al., 2018). The inhibition of CTHB with CA-074 might have reduced the RGC loss. These findings expand on a recent study that used investigated the gene expression profiles in the astrocytes derived from optic nerves of EAE mice and found that multiple A1 phenotype astrocyte genes were highly expressed (Itoh et al., 2018). As we reported recently, that CA-074 treatment attenuated the signaling pathways mediated by Th1/Th17/Th22 involved in the progression of EAE in SJL/J mice. The protective effect of CA-074 against retinopathy and ON in EAE might have accrued through the immunomodulatory action.
The improvement of the pathogenic condition in EAE mice administered with CA-074 could be attributed to a variety of mechanisms. In the EAE model of SJL/J mice, our findings imply that the CTHB activation of the mouse eye is connected to the formation and the release of inflammatory mediators. The inhibition of CTHB activity with CA-074 resulted in a decrease in inflammatory mediators in the retina and optic nerve, which improved illness symptoms. Our findings suggest that targeting CTHB with a CA-074 inhibitor could help alleviate neuronal/peripheral auto-inflammation in RRMS patients. Overall, the current research supports CA-074′s protective effects against retinopathy and optic neuritis caused by EAE in this animal model owing to its neuroprotective potential. These findings add to the increasing pieces of evidence supporting the intervention of the CA-074 for the management of retinopathy and optic neuritis inflicted by MS. To better recognize the role of CA-074 in MS-related retinopathy and ON, more research is needed.
5. Conclusion
The present study showed that in the course of the EAE model of SJL/J mice, the increased level of Cathepsin B causes damage to the optic and retinal neurons, resulting in retinopathy and optic neuritis. The inhibition of Cathepsin B by its inhibitor CA-074 resulted in a decrease in inflammatory mediators in the optic nerve and retina, which improved the severity of retinopathy and optic neuritis. Overall, the current research supports CA-074′s protective effects against retinopathy and optic neuritis caused by EAE in this animal model owing to its neuroprotective potential. Therefore, these findings suggest that targeting Cathepsin B with CA-074 could help to alleviate vision impairment in MS patients. The improvement of the pathogenic condition in EAE mice administered with CA-074 could be attributed to a variety of mechanisms. To better recognize the role of CA-074 in MS-related retinopathy and ON, more research is needed.
Funding
This research project was supported by the research initiative from King Saud University, Riyadh, Kingdom of Saudi Arabia. The Research Supporting Project (RSP-2021/352).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
The authors extend their appreciation to the Research Supporting Project (RSP) by King Saud University, Riyadh, Kingdom of Saudi Arabia.
Author contributions
M.R.K. and K.A designed the experiments. MRK. Performed and analyzed the data. K.A and M.R.K interpreted the results. M.R.K. prepared figures and drafted the manuscript. K.A edited and revised the manuscript. F.I., M.A. and M.S.K. helped in the data curation. A.N., S.F.A. M.R.K., M.S.K., and K.A performed proofreading and editing.
Footnotes
Peer review under responsibility of King Saud University.
References
- Allan E.R.O., Yates R.M., Lees J.R. Redundancy between Cysteine Cathepsins in Murine experimental autoimmune Encephalomyelitis. PLoS One. 2015;10(6):e0128945. doi: 10.1371/journal.pone.0128945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alshammari M.A., Khan M.R., Alasmari F., Alshehri A.O., Ali R., Boudjelal M., Alhosaini K.A., Niazy A.A., Alshammari T.K. Changes in the fluorescence tracking of NaV1.6 protein expression in a BTBR T+Itpr3tf/J autistic mouse model. Neural Plast. 2019;2019:1–12. doi: 10.1155/2019/4893103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari M.A., Nadeem A., Alshammari M.A., Attia S.M., Bakheet S.A., Khan M.R., Albekairi T.H., Alasmari A.F., Alhosaini K., Alqahtani F., Al-Mazroua H.A., Ahmad S.F. Cathepsin B inhibitor alleviates Th1, Th17, and Th22 transcription factor signaling dysregulation in experimental autoimmune encephalomyelitis. Exp. Neurol. 2022;351:113997. doi: 10.1016/j.expneurol.2022.113997. [DOI] [PubMed] [Google Scholar]
- Baici A., Lang A., Zwicky R., Muntener K. Cathepsin B in osteoarthritis: uncontrolled proteolysis in the wrong place. Semin. Arthritis Rheum. 2005;34(6 Suppl 2):24–28. doi: 10.1016/j.semarthrit.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Bever C.T., Whitaker J.N. Proteinases in inflammatory demyelinating disease. Springer Semin. Immunopathol. 1985;8(3):235–250. doi: 10.1007/BF00197298. [DOI] [PubMed] [Google Scholar]
- Brambilla R., Dvoriantchikova G., Barakat D., Ivanov D., Bethea J.R., Shestopalov V.I. Transgenic inhibition of astroglial NF-κB protects from optic nerve damage and retinal ganglion cell loss in experimental optic neuritis. J. Neuroinflamm. 2012;9:213. doi: 10.1186/1742-2094-9-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compston A., Coles A. Multiple sclerosis. Lancet. 2008;372(9648):1502–1517. doi: 10.1016/S0140-6736(08)61620-7. [DOI] [PubMed] [Google Scholar]
- Correale J., Farez M.F. The role of astrocytes in multiple sclerosis progression. Front. Neurol. 2015;6:180. doi: 10.3389/fneur.2015.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danilov A.I., Andersson M., Bavand N., Wiklund N.P., Olsson T., Brundin L. Nitric oxide metabolite determinations reveal continuous inflammation in multiple sclerosis. J. Neuroimmunol. 2003;136(1–2):112–118. doi: 10.1016/s0165-5728(02)00464-2. [DOI] [PubMed] [Google Scholar]
- Das A., Guyton M.K., Smith A., Wallace G., McDowell M.L., Matzelle D.D., Ray S.K., Banik N.L. Calpain inhibitor attenuated optic nerve damage in acute optic neuritis in rats. J. Neurochem. 2013;124(1):133–146. doi: 10.1111/jnc.12064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippini, G., Del Giovane, C., Vacchi, L., D'Amico, R., Di Pietrantonj, C., Beecher, D., & Salanti, G. (2013). Immunomodulators and immunosuppressants for multiple sclerosis: a network meta-analysis. Cochrane Database Syst Rev(6), CD008933. doi:10.1002/14651858.CD008933.pub2. [DOI] [PMC free article] [PubMed]
- Fisher J., Jacobs D., Markowitz C., Galetta S., Volpe N., Nanoschiavi M., Baier M., Frohman E., Winslow H., Frohman T. Relation of visual function to retinal nerve fiber layer thickness in multiple sclerosis. Ophthalmology. 2006;113(2):324–332. doi: 10.1016/j.ophtha.2005.10.040. [DOI] [PubMed] [Google Scholar]
- Goverman J. Autoimmune T cell responses in the central nervous system. Nat. Rev. Immunol. 2009;9(6):393–407. doi: 10.1038/nri2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobom M., Storch M.K., Weissert R., Maier K., Radhakrishnan A., Kramer B., Diem R. Mechanisms and time course of neuronal degeneration in experimental autoimmune encephalomyelitis. Brain Pathol. 2004;14(2):148–157. doi: 10.1111/j.1750-3639.2004.tb00047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horstmann L., Schmid H., Heinen A.P., Kurschus F.C., Dick H.B., Joachim S.C. Inflammatory demyelination induces glia alterations and ganglion cell loss in the retina of an experimental autoimmune encephalomyelitis model. J. Neuroinflamm. 2013;10:120. doi: 10.1186/1742-2094-10-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh N., Itoh Y., Tassoni A., Ren E., Kaito M., Ohno A.i., Ao Y., Farkhondeh V., Johnsonbaugh H., Burda J., Sofroniew M.V., Voskuhl R.R. Cell-specific and region-specific transcriptomics in the multiple sclerosis model: focus on astrocytes. Proc. Natl. Acad. Sci. U. S. A. 2018;115(2) doi: 10.1073/pnas.1716032115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwahashi T., Inoue A., Koh C.S., Shin T.K., Kim B.S. Expression and potential role of inducible nitric oxide synthase in the central nervous system of Theiler's murine encephalomyelitis virus-induced demyelinating disease. Cell Immunol. 1999;194(2):186–193. doi: 10.1006/cimm.1999.1482. [DOI] [PubMed] [Google Scholar]
- Jin J., Smith M.D., Kersbergen C.J., Kam T.-I., Viswanathan M., Martin K., Dawson T.M., Dawson V.L., Zack D.J., Whartenby K., Calabresi P.A. Glial pathology and retinal neurotoxicity in the anterior visual pathway in experimental autoimmune encephalomyelitis. Acta Neuropathol. Commun. 2019;7(1) doi: 10.1186/s40478-019-0767-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampert E.J., Andorra M., Torres-Torres R., Ortiz-Pérez S., Llufriu S., Sepúlveda M., Sola N., Saiz A., Sánchez-Dalmau B., Villoslada P., Martínez-Lapiscina E.H. Color vision impairment in multiple sclerosis points to retinal ganglion cell damage. J. Neurol. 2015;262(11):2491–2497. doi: 10.1007/s00415-015-7876-3. [DOI] [PubMed] [Google Scholar]
- Larabee C.M., Hu Y., Desai S., Georgescu C., Wren J.D., Axtell R.C., Plafker S.M. Myelin-specific Th17 cells induce severe relapsing optic neuritis with irreversible loss of retinal ganglion cells in C57BL/6 mice. Mol. Vis. 2016;22:332–341. [PMC free article] [PubMed] [Google Scholar]
- Lecaille F., Kaleta J., Bromme D. Human and parasitic papain-like cysteine proteases: their role in physiology and pathology and recent developments in inhibitor design. Chem. Rev. 2002;102(12):4459–4488. doi: 10.1021/cr0101656. [DOI] [PubMed] [Google Scholar]
- Li B., Cui W., Liu J., Li R.u., Liu Q., Xie X.-H., Ge X.-L., Zhang J., Song X.-J., Wang Y., Guo L.i. Sulforaphane ameliorates the development of experimental autoimmune encephalomyelitis by antagonizing oxidative stress and Th17-related inflammation in mice. Exp. Neurol. 2013;250:239–249. doi: 10.1016/j.expneurol.2013.10.002. [DOI] [PubMed] [Google Scholar]
- Mishra M.K., Wang J., Keough M.B., Fan Y., Silva C., Sloka S., Hayardeny L., Brück W., Yong V.W. Laquinimod reduces neuroaxonal injury through inhibiting microglial activation. Ann. Clin. Transl. Neurol. 2014;1(6):409–422. doi: 10.1002/acn3.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata M., Miyashita S., Yokoo C., Tamai M., Hanada K., Hatayama K., Towatari T., Nikawa T., Katunuma N. Novel epoxysuccinyl peptides. selective inhibitors of cathepsin B, in vitro. FEBS Lett. 1991;280(2):307–310. doi: 10.1016/0014-5793(91)80318-w. [DOI] [PubMed] [Google Scholar]
- Nakahara J., Aiso S., Suzuki N. Autoimmune versus oligodendrogliopathy: the pathogenesis of multiple sclerosis. Arch. Immunol. Ther. Exp. (Warsz) 2010;58(5):325–333. doi: 10.1007/s00005-010-0094-x. [DOI] [PubMed] [Google Scholar]
- Pekny M., Eliasson C., Chien C.L., Kindblom L.G., Liem R., Hamberger A., Betsholtz C. GFAP-deficient astrocytes are capable of stellation in vitro when cocultured with neurons and exhibit a reduced amount of intermediate filaments and an increased cell saturation density. Exp. Cell Res. 1998;239(2):332–343. doi: 10.1006/excr.1997.3922. [DOI] [PubMed] [Google Scholar]
- Ransohoff R.M. Animal models of multiple sclerosis: the good, the bad and the bottom line. Nat. Neurosci. 2012;15(8):1074–1077. doi: 10.1038/nn.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlings N.D., Barrett A.J., Bateman A. MEROPS: the peptidase database. Nucleic Acids Res. 2010;38(suppl_1):D227–D233. doi: 10.1093/nar/gkp971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shindler K.S., Guan Y., Ventura E., Bennett J., Rostami A. Retinal ganglion cell loss induced by acute optic neuritis in a relapsing model of multiple sclerosis. Mult. Scler. 2006;12(5):526–532. doi: 10.1177/1352458506070629. [DOI] [PubMed] [Google Scholar]
- Shindler K.S., Ventura E., Dutt M., Rostami A. Inflammatory demyelination induces axonal injury and retinal ganglion cell apoptosis in experimental optic neuritis. Exp. Eye Res. 2008;87(3):208–213. doi: 10.1016/j.exer.2008.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shindler K.S., Revere K., Dutt M., Ying G.S., Chung D.C. In vivo detection of experimental optic neuritis by pupillometry. Exp. Eye Res. 2012;100:1–6. doi: 10.1016/j.exer.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steel D.H., Waldock A. Measurement of the retinal nerve fibre layer with scanning laser polarimetry in patients with previous demyelinating optic neuritis. J. Neurol. Neurosurg. Psychiatry. 1998;64(4):505–509. doi: 10.1136/jnnp.64.4.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D., Jakobs T.C. Structural remodeling of astrocytes in the injured CNS. Neuroscientist. 2012;18(6):567–588. doi: 10.1177/1073858411423441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y., Cao G., Min X., Wang T., Sun S., Du X., Zhang W. Cathepsin B inhibition ameliorates the non-alcoholic steatohepatitis through suppressing caspase-1 activation. J. Physiol. Biochem. 2018;74(4):503–510. doi: 10.1007/s13105-018-0644-y. [DOI] [PubMed] [Google Scholar]
- Trip S.A., Schlottmann P.G., Jones S.J., Altmann D.R., Garway-Heath D.F., Thompson A.J., Plant G.T., Miller D.H. Retinal nerve fiber layer axonal loss and visual dysfunction in optic neuritis. Ann. Neurol. 2005;58(3):383–391. doi: 10.1002/ana.20575. [DOI] [PubMed] [Google Scholar]
- Turk B., Turk D., Turk V. Lysosomal cysteine proteases: more than scavengers. Biochim. Biophys. Acta. 2000;1477(1–2):98–111. doi: 10.1016/s0167-4838(99)00263-0. [DOI] [PubMed] [Google Scholar]
- Wang P., Xie K., Wang C., Bi J. Oxidative stress induced by lipid peroxidation is related with inflammation of demyelination and neurodegeneration in multiple sclerosis. Eur. Neurol. 2014;72(3–4):249–254. doi: 10.1159/000363515. [DOI] [PubMed] [Google Scholar]
- Whitaker J.N., Heinemann M.A., Uzman B.G. The renal degradation of myelin basic protein peptide 43–88 by two enzymes in different subcellular fractions. Biochem. J. 1982;201(3):543–553. doi: 10.1042/bj2010543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmes A.T., Reinehr S., Kühn S., Pedreiturria X., Petrikowski L., Faissner S., Ayzenberg I., Stute G., Gold R., Dick H.B., Kleiter I., Joachim S.C. Laquinimod protects the optic nerve and retina in an experimental autoimmune encephalomyelitis model. J. Neuroinflamm. 2018;15(1) doi: 10.1186/s12974-018-1208-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witherick J., Wilkins A., Scolding N., Kemp K. Mechanisms of oxidative damage in multiple sclerosis and a cell therapy approach to treatment. Autoimmune Dis. 2011;2011:1–11. doi: 10.4061/2011/164608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki R., Lu H., Butovsky O., Ohno N., Rietsch A.M., Cialic R., Wu P.M., Doykan C.E., Lin J., Cotleur A.C., Kidd G., Zorlu M.M., Sun N., Hu W., Liu L., Lee J.-C., Taylor S.E., Uehlein L., Dixon D., Gu J., Floruta C.M., Zhu M., Charo I.F., Weiner H.L., Ransohoff R.M. Differential roles of microglia and monocytes in the inflamed central nervous system. J. Exp. Med. 2014;211(8):1533–1549. doi: 10.1084/jem.20132477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan S., Sloane B.F. Molecular regulation of human cathepsin B: implication in pathologies. Biol. Chem. 2003;384(6):845–854. doi: 10.1515/BC.2003.095. [DOI] [PubMed] [Google Scholar]
- Yang H., Liu C., Jiang J., Wang Y., Zhang X. Celastrol attenuates multiple sclerosis and optic neuritis in an experimental autoimmune encephalomyelitis model. Front. Pharmacol. 2017;8:44. doi: 10.3389/fphar.2017.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]