Abstract
The present study was aimed to formulate and evaluate fast dissolving oral film of Rosuvastatin calcium to improve its bioavailability in comparison to typical solid oral dosage forms. The drug was formulated as solid dispersion with hydrophilic polymers and assessed for different constraints such as drug content, saturated solubility, and drug-polymer interaction. Best formula was selected and prepared in the form of orodispersible film. The films were developed by solvent casting method and examined for weight variations, drug content, folding endurance, pH, swelling profile, disintegration time, and in vitro dissolution. Further pharmacokinetic study was also performed on rabbit and compared with that of the marketed oral formulation. The drug and the polymers were found to be compatible with each other by FTIR study. Maximum solubility was found at drug polymer ratio of 1:4 and that was 54.53 ± 2.05 µg/mL. The disintegration time of the developed film was observed to be 10 ± 2.01 s, while release of the Rosuvastatin from the film was found to be 99.06 ± 0.40 in 10 min. Stability study shown that developed film was stable for three months. Further pharmacokinetic study revealed that developed orodispersible film had enhance oral bioavailability as compared to marketed product (Crestor® tablets). Conclusively, the study backs the development of a viable ODF of Rosuvastatin with better bioavailability.
Keywords: Orodispersible film, Rosuvastatin calcium, Solvent casting method, Pharmacokinetics
1. Introduction
Drug delivery by oral route is the most convenient, safe and economical (Samita et al., 2012). However, there is a growing need to enhance medication compliance for many patients, including children, the geriatric, and those who are mentally ill and have difficulty with swallowing of tablets. When compared to traditional oral medication, developing a rapid disintegrating or orally disintegrating dosage form that dissolves or disintegrates in the oral cavity without the need for water or chewing is a preferable option for such patients (Sutradhar et al., 2012, Basu et al., 2011). Because the oral mucosa is highly vascularized, it has a high permeability to many medications and serves as a good site for drug absorption. As per FDA definition, an oral disintegrating tablet (ODT) was defined as a solid-dosage form containing medicinal substances which disintegrates rapidly, usually within a matter of seconds, when placed on the tongue. Oro-dipersible film (ODF) also falls in the same category with extended definition as: a thin, flexible, non-friable polymeric film comprising active drug which is meant to be sited on the tongue for fast disintegration/dissolution in the saliva for delivery into the gastrointestinal tract (fda.gov). Fast dissolving oral film (FDOF) is a preferable technique since it dissolves quickly in the mouth and reaches the systemic circulation immediately. Several clinical studies have evident that fast disintegrating films can improve patient compliance, provide an immediate onset time of action with enhance bioavailability (Kumar and Sharma, 2012). Such delivery system is also very advantageous for the therapeutic molecules having first pass metabolism and lower oral bioavailability.
In the present work, Rosuvastatin was used as a model drug candidate for the development of an oral dissolving film. Rosuvastatin is a member of the drug class statins, commonly used for the treatment of dyslipidemia to lower high cholesterol level (Maria et al., 2019, Ahmed et al., 2020). The dose of Rosuvastatin usually starts with 5 mg once daily and could be increased if needed up to 20 mg/day, (max 40 mg/ day), following oral administration it reaches to maximum plasma concentration within 3–5 h and it has approximate elimination half-life of 19 h (Pal et al., 2016). Rosuvastatin is BCS class II drug with lower solubility profile and has poor absolute bioavailability 20% (Amr et al., 2011). The dissolution and permeability of Rosuvastatin oral conventional solid dosage forms could be regulated by the formulation excipients and method of manufacture (Pal et al., 2016).
In account of all these features, we develop oro-dispersible film (ODF) of rosuvastatin by enhancing the disintegration and dissolution of rosuvastatin to improve the bioavailability of the drug. ODF formulation properties are critically affected by the choice of its components. An appropriate drug selection is determined in part by its dosage, which would be usually confined to a maximum of 30% (w/w) of the film's weight. Rosuvastatin is a strong option for this sort of dosage form because its customary starting dose is 5 mg once day. Selection of appropriate polymer(s) for the formulation and its amount have a crucial role because it imparts as the key constituent in the formulation (as a minimum 45% of the dry weight), provides the obligatory mechanical strength to the oral film and persuading the drug release into the oral cavity. The purpose of this study was to choose the suitable polymer that can enhance the drug solubility. Two distinct hydrophilic polymers were chosen, and they were picked in terms of the ability to form an inclusion complex or a solid dispersion. To achieve greater bioavailability, PEG 4000 and poloxamer 407 (Pluronic® F127) were employed to formulate solid dispersions in drug to polymer ratios of 1:1, 1:2, and 1:4 (w/w), followed by the preparation of an oro-dispersible film.
2. Materials and methods
2.1. Materials
Rosuvastatin calcium was obtained as a gift from Aljazeera Pharma, KSA. Hydroxypropyl methyl cellulose (HPMC E15), Sodium carboxymethyl cellulose (Na CMC), glycerin, Polyethylene glycol (PEG 400 and PEG 4000) and Pluronic F-127 were purchased from Sigma-Aldrich, St. Louis, MI, USA. Crestor® tablets (Corden Pharma Gmbh, Germany) were purchased from local pharmaceutical store (Al Nahdi Pharmacy). All the used reagents and chemicals were of analytical reagent grade, unless otherwise stated.
2.2. Formulation of rosuvastatin calcium solid dispersions
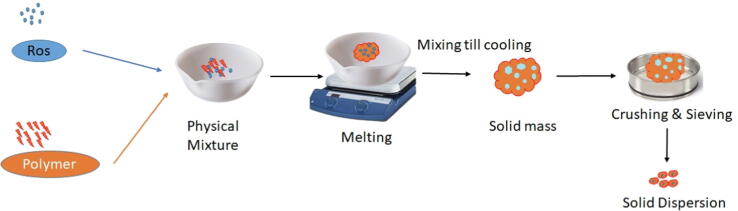
Rosuvastatin calcium solid dispersions (ROS-SDs) was developed by fusion method (Tran et al., 2019). Briefly, physical mixture of the hydrophilic carriers and drug (PEG 4000 or pluronic F-127 in ratio of 1:1, 1:2, 1:3 and 1:4, drug: polymer ratio) is heated until they melt. Then, this melt is cooled with continuous stirring. The resultant solid mass is crushed and sieved to get solid dispersion with homogenous particle size. Solid dispersion formulation illustration is represented in Fig. 1 and scomposition are shown in Table 1.
Fig. 1.
Schematic representation of solid dispersion preparation method.
Table 1.
Composition of solid dispersion formulations with their responses.
| Formula code | Polymer | Drug: polymer ratio | % Drug content ± SD | Saturated solubility in phosphate buffer (µg/mL) ± SD |
|---|---|---|---|---|
| SD1 | Pluronic F-127 | 1:1 | 96.35 ± 1.75 | 30.17 ± 1.63 |
| SD2 | 1:2 | 97.14 ± 0.95 | 38.73 ± 0.98 | |
| SD3 | 1:3 | 95.87 ± 0.85 | 44.26 ± 1.64 | |
| SD4 | 1:4 | 98.53 ± 1.05 | 54.53 ± 2.05 | |
| SD5 | PEG 4000 | 1:1 | 96.82 ± 2.03 | 22.86 ± 1.96 |
| SD6 | 1:2 | 98.16 ± 1.63 | 29.56 ± 1.73 | |
| SD7 | 1:3 | 99.04 ± 1.87 | 37.27 ± 1.83 | |
| SD8 | 1:4 | 96.15 ± 2.06 | 42.58 ± 0.93 | |
| Pure drug | 14.54 ± 1.58 | |||
2.3. Evaluation of rosuvastatin calcium solid dispersions
2.3.1. Drug content
Drug content of SDs was assessed by dissolving an amount of SDs equivalent to 10 mg of rosuvastatin calcium in 100 mL of phosphate buffer (pH 6.8). The solutions were further filtered, diluted and the absorbances were measured spectrophotometrically at the predetermined λ max 246 nm (UV visible spectrophotometer UV‐1601 Shimadzu Corporation, Japan). All measurements were done in triplicates and the values were represented as mean ± SD.
2.3.2. Saturated solubility study
Saturated solubility of pure rosuvastatin calcium and its SDs were conducted by adding excess amount of pure rosuvastatin calcium and its SDs to glass tubes comprising of 10 mL phosphate buffer followed by sonication for 30 min. Furthermore, orbital shaker was employed for 24 h to attain the equilibrium solubility. Further, these solutions were filtered, diluted, and evaluated for drug content by spectrophotometer at λmax 246 nm. All measurements were done in triplicates and the values were represented as mean ± SD (Baka et al., 2008, Ali et al., 2022).
2.3.3. Fourier transform infra-red analysis (FT-IR)
The FT-IR spectroscopy (Thermo nicolet 6700, USA) was utilized as a tool to confirm any possible drug-polymer interaction. Drug and physical mixture were mixed with KBr in order to form a pellet, which was analyzed in the IR-range of 400–4000 cm−1. Based on the outcomes of these studies, the best SD was further evaluated for DSC, XRD and SEM studies.
2.3.4. X-ray diffraction XRD for the selected SD
X-ray diffractometer (Siemens, Munich, Germany) was employed to check the crystallinity of pure drug and selected formula SD4. All the specimens were imperiled to radiation operating at 40 KV and 40 mA (λkα = 1.5418 Å). The diffraction pattern was attained by employing continuous scan mode with 2θ˚ varying from 4° to 60°) (Ibrahim et al., 2014).
2.3.5. DSC evaluation
The Differential scanning calorimeter (Shimadzu DSC-60, Tokyo, Japan) were used for DSC scan of pure drug and selected SD4 formulation. The study was held out at a heating rate of 10 °C/min from 10 to 200 °C in the nitrogen atmosphere.
2.3.6. Scanning electron microscopy
Scanning Electron microscopy (SEM, Quanta 400; FEI, Cambridge, UK) was used for the study of surface morphology of the selected formula SD4.
2.4. Formulation of fast-dissolving films
Fast-dissolving films of Rosuvastatin calcium were developed by solvent-casting method (Shen et al., 2014, Chavan et al., 2020). The film-forming materials (HPMC E15 or Na CMC) were dissolved in distilled water in different weight ratios. The plasticizers (PEG 400 or glycerin) were mixed to the polymeric solution with continuous stirring. The drug solution was prepared by dissolving an amount of the best solid dispersion formula corresponding to 10 mg in phosphate buffer. Both polymeric and drug solution were mixed and stirred continuously at suitable RPM. The mixture was poured onto a glass Petri dish and allowed to dry at room temperature. Surface perfection, folding or breaking, and ease of detachment from the Petri dish were all tested on the prepared films. The composition of ODFs is shown in Table 2.
Table 2.
Different variables included into the prepared films.
| Formula No. | Rosuvastatin (mg) | Polymer type | Polymer concentration (% w/v) | Plasticizer type | Plasticizer concentration (% w/v) |
|---|---|---|---|---|---|
| ODF 1 | 10 | HPMC E15 | 2.5 | Glycerin | 7.5 |
| ODF 2 | 10 | HPMC E15 | 2.5 | PEG 400 | 7.5 |
| ODF 3 | 10 | HPMC E15 | 2.5 | Glycerin | 15 |
| ODF 4 | 10 | HPMC E15 | 2.5 | PEG 400 | 15 |
| ODF 5 | 10 | HPMC E15 | 5 | Glycerin | 7.5 |
| ODF 6 | 10 | HPMC E15 | 5 | PEG 400 | 7.5 |
| ODF 7 | 10 | HPMC E15 | 5 | Glycerin | 15 |
| ODF 8 | 10 | HPMC E15 | 5 | PEG 400 | 15 |
| ODF 9 | 10 | Na CMC | 2.5 | Glycerin | 7.5 |
| ODF 10 | 10 | Na CMC | 2.5 | PEG 400 | 7.5 |
| ODF 11 | 10 | Na CMC | 2.5 | Glycerin | 15 |
| ODF 12 | 10 | Na CMC | 2.5 | PEG 400 | 15 |
| ODF 13 | 10 | Na CMC | 5 | Glycerin | 7.5 |
| ODF 14 | 10 | Na CMC | 5 | PEG 400 | 7.5 |
| ODF 15 | 10 | Na CMC | 5 | Glycerin | 15 |
| ODF 16 | 10 | Na CMC | 5 | PEG 400 | 15 |
2.5. Evaluation of the prepared Rosuvastatin calcium fast dissolving oral films.
2.5.1. Physical appearance, weight variations and thickness
Physical appearance of developed films was observed visually. Five samples of each formulation were weighed individually, and average weight was computed. The film thickness of the ODFs was assessed with a micrometer screw gauge at various sites and an average was calculated.
2.5.2. pH evaluation
Because an acidic or alkaline pH may induce irritation to the buccal mucosa, the surface pH of the films was evaluated in order to explore the probable impacts of a change in pH in vivo. The film to be examined was moistened with 0.5 mL of distilled water and stored in a Petri dish for 1 h, after which the pH was measured with a pH meter (Jenway 3510, Swedesboro, USA). All measurements were done in triplicate.
2.5.3. Percentage moisture loss
The initial weight of every film was determined, and then the films were stored in a desiccator comprising anhydrous calcium carbonate for three days at room temperature to determine moisture loss %. Following that, these films were removed and reweighed (Satyanarayana et al. 2012). Moisture loss percent was calculated as per the following equation:
Moisture loss % = ((Initial weight − Final weight)\Initial weight) × 100.
2.5.4. Drug content
To ensure the drug payload in the film, drug content analysis was done. A premeasured region of the film was dissolved in phosphate buffer (50 mL) by stirring followed by filtration through Whatman filter paper. Amount of drug in solution was analyzed using an UV–visible spectrophotometer. All measurements were performed in triplicate.
2.5.5. Folding endurance
Folding endurance was evaluated by folding the film several times at the same location until it broke, then calculating the number of times the film could be folded without breaking (Karen et al., 2013). The folding endurance is directly proportional with the film flexibility (Shivhare et al., 2010).
2.5.6. Tensile strength
Tensile strength can be defined as the highest force needed to break the films. This was done to evaluate the mechanical strength of the prepared films. Tensile strength was measured by tensile tester (Qualitest, model EMS301, USA) which consists of two clamps, one of them is fixed while the other one is movable in the opposite direction at a rate of 35 mm/min. The film was placed between these two clamps with recording the point at which the film was broken (Takeuchi et al., 2020). Tensile strength was stated as force/unit area and computed from the following equation (Ali et al., 2016, Rédai et al., 2021):
Tensile strength (N/cm2) = the force at which the film broke (Newton)/(Film thickness (cm) × film width (cm)).
2.5.7. In vitro disintegration and dissolution time
The film was placed on Petri dish comprising 10 mL of simulated salivary fluid with churning each 10 sec. both disintegration and dissolution time were determined for each formula in triplicate and presented as mean ± SD (Bharti et al., 2019).
2.5.8. In vitro dissolution
The in vitro release of Rosuvastatin loaded ODF was carried out by USP Apparatus I (Pharm Test, Hainburg, Germany). Simulated salivary fluid (SSF, 300 mL, pH 6.8) was taken as the dissolution medium and was kept at 37 ± 5 °C. A premeasured film was placed into the basket and set at 75 rpm. At definite time, 5 mL of the sample were taken and swapped with same amount of fresh buffer. The withdrawn samples were filtered and evaluated for the drug by using a UV spectrophotometer at 246 nm.
2.6. Stability study
Stability of any formulation is a prominent factor for its success. In order to evaluate the stability of selected ODF, it was first wrapped in a butter paper followed by aluminum foil and stored for three months at the specified relative humidity storage conditions (40 °C/75%). Samples were taken out at every month and analyzed for different parameters like physical appearance, pH, content of drug, moisture content, disintegration and dissolution time, tensile strength, and drug release profile (Hamza M. 2017).
2.7. In vivo pharmacokinetic study
In vivo study was granted by the Research Ethics Committee, Department of Pharmacology and Toxicology, Faculty of Pharmacy, Prince Sattam Bin Abdulaziz University, Al-Kharj, Saudi Arabia (Approval No: BERC-001-03-21). All the studies were done on male rabbits (2.6–3.1 kg). During the study, all the rabbits were healthy. The rabbits were fasted overnight prior to receiving the formulations. The investigation was divided into two phases with a two-sequence cross-over design. The rabbits were separated into two groups at random. The marketed formulation was given to one group, while the ODF (SD4) was given to the other. With the use of a body restraint apparatus in which the rabbit's head was exposed and the gums were pulled apart with a wooden tongue depressor, film was placed carefully on the rabbit's tongue. By wetting the mouth with a small bit of water, the film was inserted in the mouth. To ensure complete disintegration of the film, the innovator also added a slight strain to restrain the mouth. Blood samples were taken from each rabbit's peripheral vein at a specific interval of 0, 1, 2, 3, 4, 5, 7, 9, 12, 24 h for the pharmacokinetic analysis. The acquired samples were then centrifuged at 6000 rpm for 20 min to extract the plasma and reserved for future analysis.
2.8. Sample preparation for analysis
Protein precipitation method was employed to treat plasma samples. The internal standard (prednisolone) was prepared in concentration of 100 μg/mL. Briefly, mixture of 50 μL of internal standard solution and 0.75 mL methanol were added to about 0.2 mL of plasma sample followed by vortexing for one minute followed by centrifugation (15,000 rpm, 10 min). The supernatant was separated and placed into a vial. 5 μL of this supernatant was injected into the LC/MS/MS apparatus for quantitative determination of rosuvastatin. Chromatographic conditions: a validated UPLC-MS/MS (Waters Acquity, Milford, MA, USA) with C18 column (50 mm × 2.1 mm, 1.7 μm) was used to assess the Rosuvastatin concentration in rabbit plasma. The mobile phase consisted of acetonitrile and 0.1% formic acid mixture (35:65 v/v) and allowed to flow at a rate of 0.25 mL/min (Alshora et al., 2018).
2.9. Pharmacokinetic analysis
Different pharmacokinetics parameters such as maximum plasma concentration (Cmax), peak time (Tmax), area under the curve (AUC), mean residence time (MRT), and half-life (t1/2) were calculated using WinNonlin software (version 1.5, Scientific Consulting, Inc., Rockville, MD, USA). Results were represented as mean values ± standard deviations. Furthermore, Single way ANOVA was used to visualize the substantial variation between the pharmacokinetic parameters.
3. Results and discussion
3.1. Characterization of the solid dispersions
3.1.1. Drug content
Every developed SD was tested for its drug content and summarized in Table 1. Rosuvastatin content in solid dispersions formulations was found to be uniform and ranged from 96.15 ± 2.06 to 99.04 ± 1.87.
3.1.2. Saturated solubility
The solubility of rosuvastatin in phosphate buffer was observed to be 14.54 ± 1.58 (µg/mL ± SD) as shown in Table 1. To expedite the ODF integration of rosuvastatin in a solubilized form, two distinct polymers (PEG 4000 and poloxamer 407) were explored. Poloxamer has bland taste, therefore it was chosen as stabilizer. The outcomes of saturated solubility studies of rosuvastatin with the poloxamer is demonstrated in Table 1. It can be observed that by adding different polymer ratios of 1:1, 1:2,1:3, and 1:4 (w/w) of either P407 or PEG 4000 considerably boosted solubility of rosuvastatin in phosphate buffer, 1:4 polymer ratios of P407 (SD4) significantly increased the solubility of rosuvastatin in phosphate buffer compared to PEG 4000. Based on saturated solubility data the formula SD4 was selected as a best formula and characterization of the SD4 was performed using differential scanning calorimetry (DSC), X-ray diffractometry (XRD), and scanning electron microscopy (SEM).
3.2. Fourier transform infra-red analysis
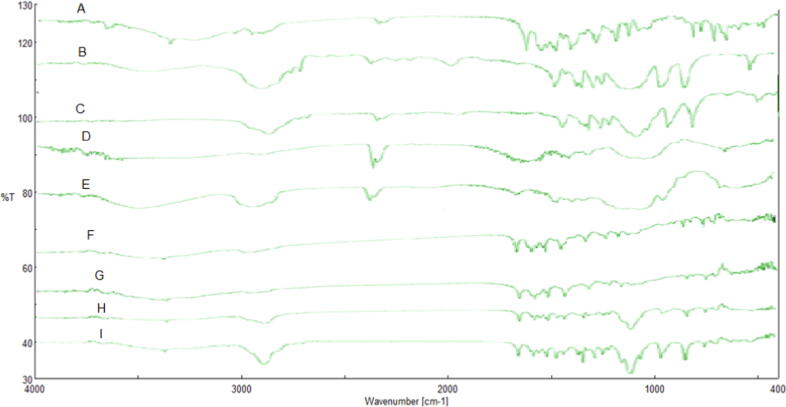
The FTIR spectra of Rosuvastatin, PEG 4000, poloxamer 407, Na CMC, HPMC E15, Drug-HPMC physical mixture, Drug- Na CMC physical mixture, Drug- poloxamer 407 physical mixture and Drug-PEG 4000 are shown in Fig. 2. Rosuvastatin exhibited distinctive absorption bands at 3337.90 cm−1, 2968.23 cm−1 and 1435.48 cm−1 comparable to cyclic amines, CH stretching, C O stretching, O—H bending. These agreed with the previously reported monograph for rosuvastatin (Schachter et al. 2005). These characteristics peaks of the drug were also found in the different physical mixture of drug-polymer, which suggest the compatibility of drug-polymer.
Fig. 2.
FTIR spectra of A. Rosuvastatin, B. PEG 4000, C. Pluronic F 127, D. Na CMC, E. HPMC E15, F. Drug-HPMC physical mixture, G. Drug- Na CMC physical mixture, H. Drug- Pluronic F127 physical mixture, and I. Drug-PEG 4000.
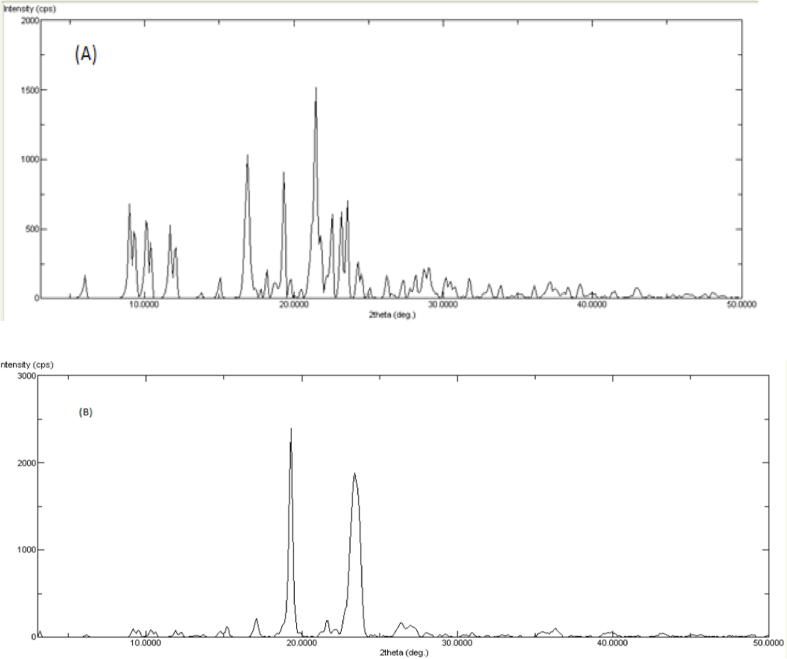
3.3. X-ray diffraction study
The pure drug's diffraction spectrum (Fig. 3) revealed a highly crystalline component, as evidenced by the distinctive intense peaks at 2θ values of 16.04, 22.45, and 34, and these were similar to those discussed in the literature (Sarfraz et al., 2015). Rosuvastatin's XRD pattern also revealed several strong distinctive diffraction lines, showing that it is a fully crystalline substance. When compared to the parent elements, the drug–SD4 XRD spectra displayed a less intense spectrum. XRD studies showed decrease in the peak intensity or absence of peaks which indicated the amorphous nature of the drug in solid dispersions (SD4).
Fig. 3.
XRD pattern of (A) pure drug and (B) Solid dispersion formula SD4).
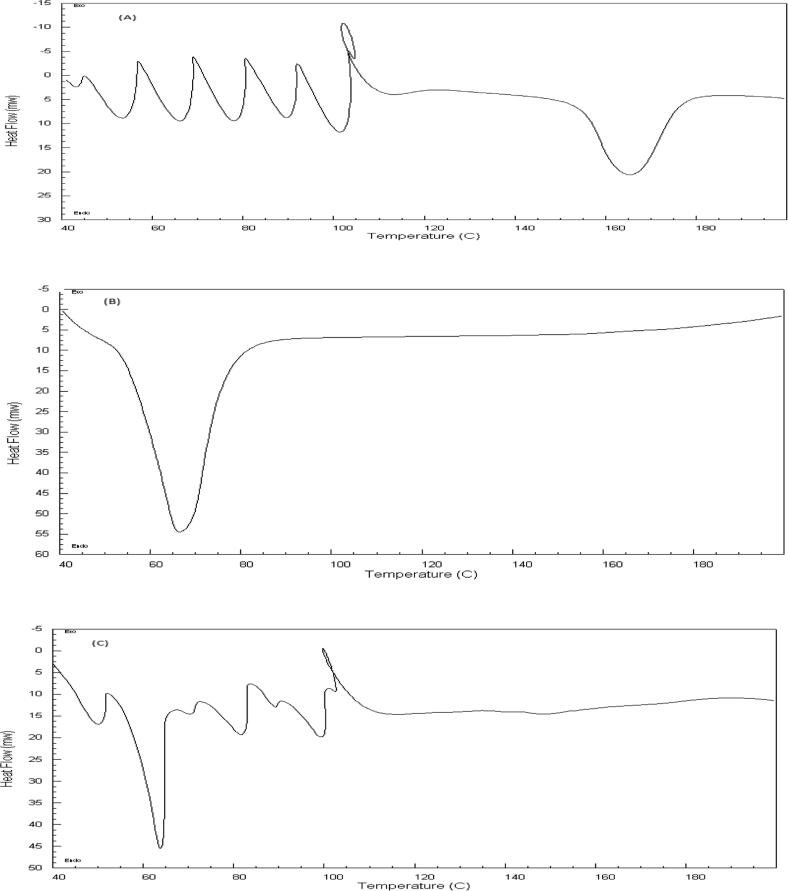
3.4. DSC evaluation for the formula SD4
Fig. 4 illustrates the DSC thermograms of Rosuvastatin, poloxamer 407 and solid dispersion formula (SD4) which was designated as a optimized formulation. Rosuvastatin's thermogram included an endothermic peak at about 165 °C, which corresponds to the drug's melting point (Kapure et al., 2013). Also showed endothermic peaks at 50 °C up to 100 °C, correspond to the loss of water from Rosuvastatin (Angelo et al., 2019). Similarly, the DSC of both poloxamer 407 and SD4 revealed endothermic peaks at 65 °C correspond to the melting point of poloxamer 407. It can be observed that the peaks of Rosuvastatin is totally disappeared in the thermogram of SD4 indicating that the drug was in amorphous state and completely entrapped by the polymer. In the solid dispersion formula (SD4), this also ensures the development of an uniform distribution with complete molecular miscibility of the various polymer components.
Fig. 4.
DSC thermograms of (A) Rosuvastatin, (B) poloxamer 407 and (C) solid dispersion formula SD4).
3.5. Scanning electron microscopy
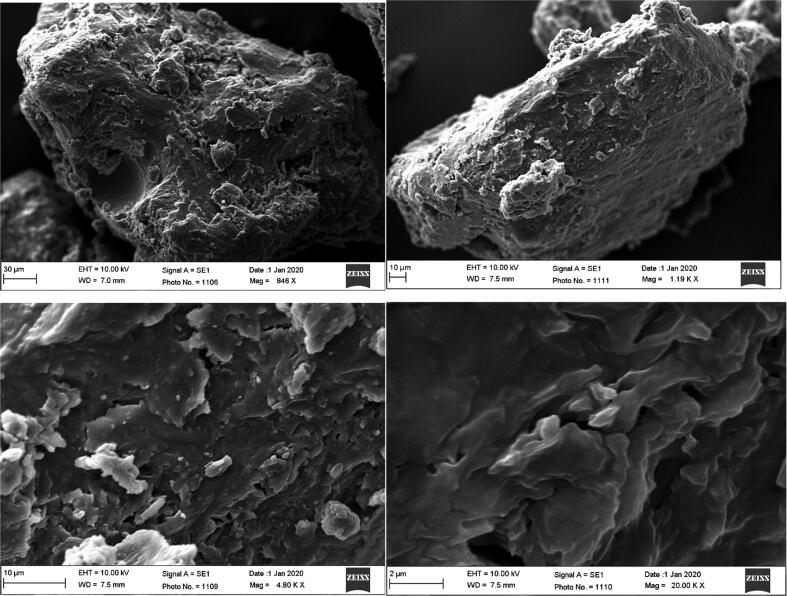
The findings of SEM images revealed that SD4 formulation showed distinctive amorphous aggregates and lack of the distinct nature of crystals (Fig. 5).
Fig. 5.
SEM photographs of the SD4 at the magnification of (A) 846 X, (B) 1.19 KX, (C) 4.8 KX and (D) 20.0 KX.
3.6. Experimental design
Orodispersible films were prepared by solvent casting method using the best solid dispersion formula (SD4). The effect of HPMC E15 concentration of (2.5 or 5, %w/v) and Na CMC concentration of 2.5 (% w/v), plasticizer type (PEG 400 and glycerin), and plasticizer concentration (7.5% and 15%, w/v) was evaluated. The composition of different coded formulations was summarized in Table 2.
3.7. Physicochemical characterization of Rosuvastatin calcium fast dissolving oral films
Developed ODFs were transparent, flexible, non-sticky, smooth, and homogenous. The pH, weight and thickness, % drug content, % moisture loss and folding endurance of developed films are shown in Fig. 6. Film’s surface pH varied from 5.97 to 7.87, which is near to the neutral pH of the buccal cavity indicating no irritation for the buccal cavity. The weight of ODFs varied from 157.36 to 193.33 mg. Determination of thickness and area weight are the general requirement for ODF (El-Feky et al., 2020). A typical film thickness ranges from 0.05 and 1 mm (El-Feky, et al., 2020). The average thickness of the ODFs spanned from 0.157 ± 0.023 to 0.181 ± 0.013 mm, as shown in Fig. 6B, validating acceptable reliability. The findings of rosuvastatin loading content in ODFs preparations were also reported in Fig. 6A and found to be consistent, ranging from 96.3 to 102.1.
Fig. 6.
Evaluation parameters of Rosuvastatin calcium fast dissolving oral films.
3.7.1. Determination of Moisture loss
The moisture sorption properties of the ODF formulations are so important to visualize the stability of films (Chavan et al., 2020). It was expected to be affected by the polymers used in the formulation. The percent moisture loss of different ODF formulations are shown Fig. 6B. The percentage moisture loss ranged from 1.01 ± 0.017% to 1.72 ± 0.024%, with a general downward trend in moisture loss when both the plasticizer level and the polymer concentration increased. This range of moisture loss was acceptable, indicating low moisture loss and good stability for the prepared films (Bharti et al., 2019).
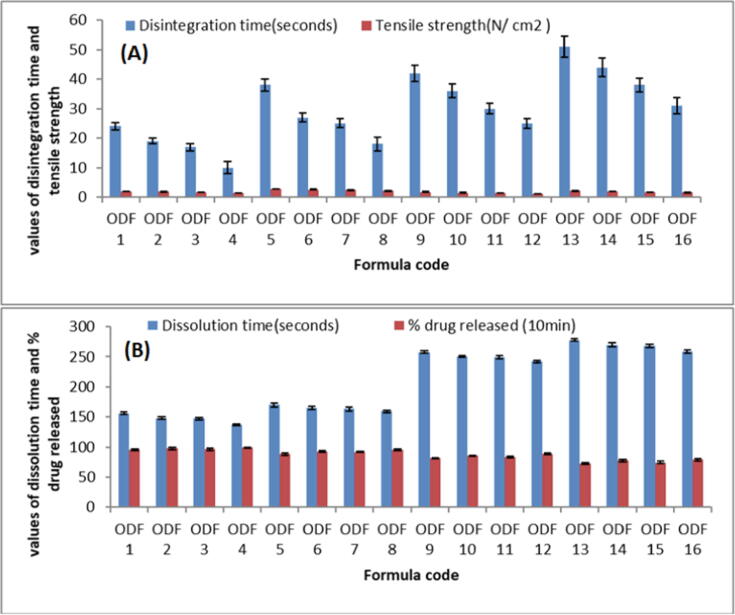
3.7.2. Folding endurance
For measuring the folding endurance, films were repeatedly folded at the same position until they break (Khurana et al., 2000). Highest folding endurance was shown by film ODF 4 and the lowest was found to be for film ODF 13. Regardless the type of the polymer in the prepared films, folding endurance was found to be affected by the type of plasticizer. Films prepared with PEG 400 have slightly increased folding endurance compared to films contained glycerol, for example, ODF 4 (PEG 400) has folding endurance of 38, while ODF 3 (Glycerol) has folding endurance of 30 Fig. 6A. Our finding was in agreement with Bariya and Koradiya (2016) who reported that orodispersable films prepared with PEG400 as a plasticizer showed better folding endurance than those prepared with glycerol.
3.8. Tensile strength
As shown in Fig. 7A, both polymer type and concentration directly affect the tensile strength of film. As earlier stated, with the plasticizer PEG 400, the film's tensile strength was at its lowest. It was also observed that increasing the concentration of either of the plasticizers resulted in a significant reduction in film strength. An ideal ODF should have adequate tensile strength to withstand mechanical stress, but extremely high tensile strength is undesirable because it may slow down the release of the medication from the polymer matrix (Nair et al., 2013). The developed ODFs had tensile strength from 1.18 ± 0.009 to 2.81 ± 0.063 N/cm2, as shown in Fig. 7A. By changing the polymer types, tensile strength changed significantly. ODFs prepared with HPMC polymer had much greater tensile strength as compared with CMC polymer. This might be attributed to the type and molecular weight variations between these polymers. Maher et al., also stated the similar outcomes (Maher et al., 2016). Furthermore, it was observed that upping the concentration of polymer enhanced the tensile strength of the formed ODFs substantially. That might be because at higher concentrations, the utilized polymer chains create a tightly packed network, result in the formation of a stronger matrix. Similar findings were reported by Bharti et al., who discovered that raising the film's former concentration enhanced the tensile strength of the constructed films (Wong et al., 1999).
Fig. 7.
Different Evaluation parameters of developed films (A) Disintegration time & Tensile strength; (B) Dissolution time & % drug release.
3.9. In vitro disintegration and dissolution time of films
The disintegration time of ODF formulations ranged from 10 ± 2.01 to 51 ± 3.48 s seconds, as shown in Fig. 7A. All the developed ODFs had disintegration time <1 min, which was in agreement with the previous study done by Liew et al. (Liew et al., 2012). It was seen that PEG 400 resulted in significantly shorter disintegration time than glycerin. When compared to Na CMC, the disintegration period of polymer HPMC E15 films was much shorter. This may be owing to the increased E15 content in HPMC E15 film, which, due to its high-water solubility, allows water to penetrate into the film. This result in accordance with Maher et al. who studied the effect of polymer type on the disintegration time of olanzapine loaded orodispersable film and reported that films prepared with HPMC showed lower disintegration time than those prepared with NaCMC.
In vitro dissolution time of different films are shown in Fig. 7B. The impacts of polymer nature and concentration on film dissolution time revealed that polymer 1 (HPMC E15) dissolving slower than polymer 2. (NaCMC). Fig. 7B shows that the duration was also extended at higher polymer concentrations (5 mg). When compared to glycerin, the plasticizer PEG 400 had the shortest film dissolving time, and the time reduced linearly when the plasticizer concentration was raised.
3.10. In vitro drug release
Fig. 7B shows the release of drug from ODF formulations. The ODF constructed with HPMC E15 had a higher release profile than the ODFs made with Na CMC as the polymer matrix. However, increasing the concentration of HPMC E15 decreased the % release. This might be due to formation of a relatively strong matrix layer with greater deformability and poor water permeability for drug diffusion affected by more physical intimacy between HPMC particles at high concentrations (Sapkal et al., 2011). Furthermore, increased polymer concentrations created a viscous environment in the system, which slowed water transport into the matrix and drug diffusion into the milieu (Dunn & English, 2002). While, NaCMC has a greater molecular weight than HPMC E5, allowing for the development of a stronger matrix and slower release. This adds to the evidence that HPMC E15 has an essential role in improving Rosuvastatin dissolution from prepared films.
3.11. Assessment of optimized ODF
Based on in vitro evaluations of ODFs, it can be concluded that the optimum formulation was ODF4. So, ODF4 was picked for further evaluation of stability and in vivo study.
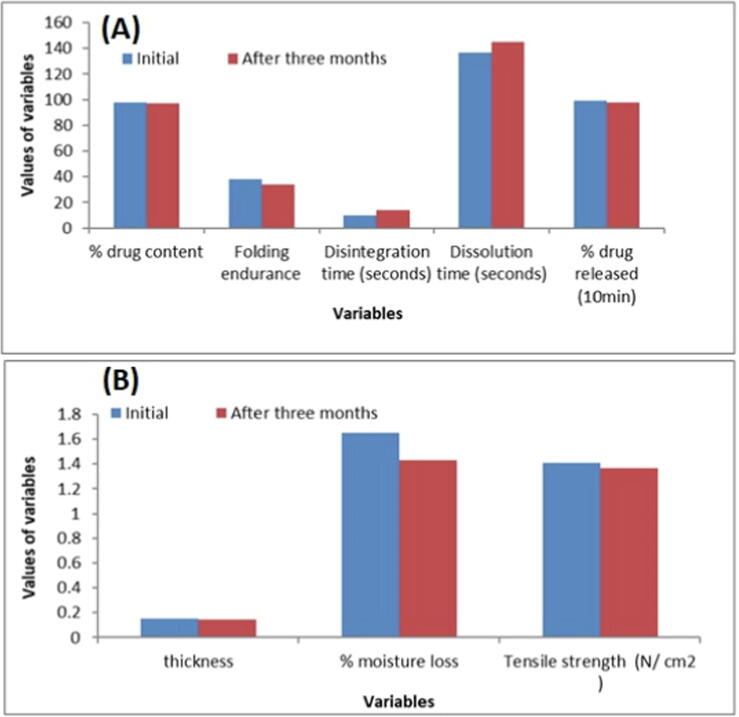
3.12. Stability study of optimized film
The formulated orodispersible film ODF4 was observed for visual appearance, thickness, % drug content, % moisture loss, folding endurance, Disintegration time (seconds), Tensile strength, % drug released and Dissolution time (seconds) after a period of three month and the results was summarized in Fig. 8A and B. The ODF4 profile indicated that the physical appearance remains transparent over the indicated period. The thickness properties of ODF4 not largely affected by time, the initial thickness was 0.151 mm, while after three month it was observed 0.147 mm. The percentage of moisture loss, tensile strength, and folding endurance slightly decreased over a period of three month. The drug content in the formulation was varied from 97.4% to 96.8%. This indicates that there was no significant loss of drug from the film during storage. Initially, 99.06% of drug release was observed from the optimized film within the first 10 min and after a period of three-month, release of drug was found to 97.8%. So, from the stability data of developed film, it can be concluded that the changes in the physical and in vitro characteristics of developed film were insignificant after the storage period of three months.
Fig. 8.
Stability study of the selected formula ODF4), initial and after 3 months.
3.13. In vivo pharmacokinetic study
The bioavailability of rosuvastatin orodispersible film was tested and compared to that of a commercially available product (Crestor® tablets). The mean plasma concentration time curve is illustrated in Fig. 9. The mean Cmax estimated from Crestor® tablets and the selected formula ODF4 were 410 ng/m and 491 ng/mL respectively as shown in Fig. 10B. The variations in Cmax between the two treatments were statistically significant (p < 0.05) (Table 3). When comparing the fast-dissolving film ODF4 to the Crestor® pill, the mean AUC0-∞, which represents the total amount of drug absorbed over a 24-hour period, was considerably higher (p < 0.05). Additionally, tmax and t1/2 were calculated Fig. 10A. Tmax and t1/2 of ODF4 were found to be significantly different from crestor® tablet with p value 0.001 and 0.10, respectively as shown in Table 3. The higher Cmax, faster tmax with the enhanced bioavailability noted for ODF4 than the marketed Crestor® tablets may be credited to prompt disintegration and dissolution of the drug in the saliva followed by higher permeability through rich vascularized oral mucosa.
Fig. 9.
Plasma concentration of Rosuvastatin following the oral administration of Crestor® tablets compared to the selected formula ODF4.
Fig. 10.
Pharmacokinetic parameters of Rosuvastatin after oral administration of marketed product Crestor® tablets) compared to the selected ODF4.
Table 3.
Single way ANOVA for pharmacokinetic parameters.
| Pharmacokinetic Parameters | SS | Df | MS | F | P-value |
|---|---|---|---|---|---|
| Cmax | 9760.667 | 1 | 9760.667 | 1195.184 | 4.18E−06 |
| Tmax | 6 | 1 | 6 | 72 | 0.001058 |
| AUC 0-t | 1,636,470 | 1 | 1,636,470 | 405.7284 | 3.59E−05 |
| Auc0-∞ | 3,152,180 | 1 | 3,152,180 | 35.77696 | 0.003927 |
| MRT0-∞ | 6.437106 | 1 | 6.437106 | 6.344264 | 0.065442 |
| t1/2 | 10.3494 | 1 | 10.3494 | 21.01835 | 0.010148 |
4. Conclusion
The results show that rosuvastatin solubility was enhanced by the production of solid dispersion, particularly with Poloxamer 407, which was successfully incorporated into orodispersible films using a variety of film forming polymers. The optimized film (ODF 4) constitutes of 2.5% w/v, HPMC E15 and plasticized with 15% w/v, PEG 400. When compared to CMC-based films, the optimized ODF4 had a much shorter disintegration time (10 ± 2.01 s), better folding endurance, and a higher percentage of drug release. Shorter disintegration time is prerequisite for oral disintegration tablet or orodispersable film as per the FDA definition. Stability study revealed that the developed formulation was stable for a period of three months. Furthermore, in vivo bioavailability study in rabbits showed that there was significant increase in rosuvastatin bioavailability compared to marketed tablet. Finally, it can be concluded that the optimized ODF can be a promising, easy, and cost-effective approach to improve the rosuvastatin bioavailability.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Randa Mohammed Zaki, Email: r.abdelrahman@psau.edu.sa.
Munerah Alfadhel, Email: m.alfadhel@psau.edu.sa.
Vidya DevanathaDesikan Seshadri, Email: v.adri@psau.edu.sa.
Faisal Albagami, Email: f.albaqami@psau.edu.sa.
Majed Alrobaian, Email: majed.alrobaian@tu.edu.sa.
Salha M Tawati, Email: salha.tawati@glasgow.ac.uk.
Musarrat Husain Warsi, Email: mvarsi@tu.edu.sa.
Alanood S. Almurshedi, Email: marshady@ksu.edu.sa.
References
- Ahmed M.Z., Khan U.A., Haye A., Agarwal N.B., Alhakamy N.A., Alhadrami H.A., Warsi M.H., Jain G.K. Liquid crystalline nanoparticles for nasal delivery of Rosuvastatin: implications on therapeutic efficacy in management of Epilepsy. Pharmaceuticals. 2020;13(11):356. doi: 10.3390/ph13110356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali M., Vijendar C., Kumar S., Krishnaveni J. Formulation and evaluation of fast dissolving oral films of diazepam. Aust. J. Pharm. 2016;4:1–5. [Google Scholar]
- Ali A.M.A., Warsi M.H., Abourehab M.A., Ali A.A. Preparation and transformation of solid glass solutions of Clotrimazole to Nanosuspensions with improved physicochemical and antifungal properties. J. Pharm. Innov. 2022:1–14. doi: 10.1007/s12247-021-09595-w. [DOI] [Google Scholar]
- Alshora D.H., Ibrahim M.A., Elzayat E., Almeanazel O.T., Alanazi F., Mukherjee A. Rosuvastatin calcium nanoparticles: improving bioavailability by formulation and stabilization codesign. PLoS One. 2018;13(7):e0200218. doi: 10.1371/journal.pone.0200218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amr M., Badawy N.M.M., Abd El-Aziz B., El-Aleem A., Nesrine T. Stability indicating spectrophotometric methods for determination of rosuvastatin in the presence of its acid degradation products by derivative spectrophotometric techniques. J. Adv. Pharm. Res. 2011;2:44–55. [Google Scholar]
- Ângelo M.L., Ruela A.L., Ferreira A.C., Ramos M.V., Montanari C.M., Silva L.M., Araújo M.B. Evaluating the discriminatory power of a dissolution assay for rosuvastatin calcium capsules: Solid-state properties and dissolution media. Braz. J. Pharm. Sci. 2019;23:55. [Google Scholar]
- Baka E., Comer J.E.A., Takács-Novák K. Study of equilibrium solubility measurement by saturation shake-flask method using hydrochlorothiazide as model compound. J. Pharm. Biomed. Anal. 2008;46(2):335–341. doi: 10.1016/j.jpba.2007.10.030. [DOI] [PubMed] [Google Scholar]
- Bariya J.G., Koradiya H.D. Formulation and in-vitro evaluation of Orodispersible film of Torsemide. J. Pharm. Sci. Bioscient. Res. 2016;6(5):666–676. [Google Scholar]
- Basu B., Bagadiya A., Makwana S., Kapadiya M. Design and evaluation of sublimed orodispersible tablets of cetirizine HCl masked valdecoxib tablets. J. Chem. Pharm. Res. 2011;3:882–892. [Google Scholar]
- Bharti K., Mittal P., Mishra B. Formulation and characterization of fast dissolving oral films containing buspirone hydrochloride nanoparticles using design of experiment. J. Drug Deliv. Sci. Technol. 2019;49:420–432. [Google Scholar]
- Chavan D.U., Marques S.M., Bhide P.J., Kumar L., Shirodkar R.K. Rapidly dissolving Felodipine nanoparticle strips— formulation using design of experiment and characterisation. J. Drug Deliv. Sci. Technol. 2020;60:102053. [Google Scholar]
- Dunn, R.L., English, J.P., 2002. Biodegradable polymer composition, US Patent 20,020,090,398.
- El-Feky Y.A., Mostafa D.A., Al-Sawahli M.M., El-Telbany R.F., Zakaria S., Fayez A.M., Ahmed K.A., Alolayan E.M., El-Telbany D.F. Reduction of intraocular pressure using timolol orally dissolving strips in the treatment of induced primary open-angle glaucoma in rabbits. J. Pharm. Pharmacol. 2020;72:682–698. doi: 10.1111/jphp.13239. [DOI] [PubMed] [Google Scholar]
- Hamza M. Development and evaluation of orodispersible films of lamotrigine: hydroxypropyl β cyclodextrin inclusion complex. Al-Azhar J. Pharm. Sci. 2017;56(2):31–46. [Google Scholar]
- Ibrahim M.A. Tenoxicam-Kollicoat IR® binary system: physicochemical and biological evaluation. Acta Polon. Pharm. Drug Res. 2014;71:647–659. [PubMed] [Google Scholar]
- Kapure V.J., Pande V.V., Deshmukh P.K. Dissolution enhancement of rosuvastatin calcium by liquisolid compact technique. J. Pharm. (Cairo) 2013;2013(2013):315902. doi: 10.1155/2013/315902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karen H.D., Patel D.M., Jasakiya A.R., Patel C.N. Development of oral strip for Loratadine and in vitro evaluation. Int. J. Pharm. Pharmacol. 2013;2:125–130. [Google Scholar]
- Khurana R., Ahuja A., Khar R.K. Development and evaluation of mucoadhesive film of miconazole nitrate. Indian J. Pharm. Sci. 2000;62:447–453. [Google Scholar]
- Kumar S., Gupta S., Sharma P. A review on recent trends in oral drug delivery- fast dissolving formulation. Adv. Bio Res. 2012;6:6–13. [Google Scholar]
- Liew K.B., Tan Y.T.F., Peh K.K. Characterization of oral disintegrating film containing donepezil for Alzheimer disease. AAPS Pharm. Sci. Tech. 2012;13(1):134–142. doi: 10.1208/s12249-011-9729-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher E.M., Mahmoud A., Ali A., Farouk H., Abdelrahman A.A., Magdy E., Mahmoud A., Ali A., Salem H.F., Maher E.M., et al. In vitro/in vivo evaluation of an optimized fast dissolving oral film containing olanzapine co-amorphous dispersion with selected carboxylic acids. Drug Deliv. 2016;23:3088–3100. doi: 10.3109/10717544.2016.1153746. [DOI] [PubMed] [Google Scholar]
- Maria S., Khan A., Zainuddin R., Haw F. Formulation and optimization of mouth dissolving film of rosuvastatin calcium using QbD approach. Int. Res. J. Pharm. 2019;10:11–14. [Google Scholar]
- Nair A.B., Kumria R., Harsha S., Attimarad M., Al-Dhubiab B.E., Alhaider I.A. In vitro techniques to evaluate buccal films. J. Control. Release. 2013;166:10–21. doi: 10.1016/j.jconrel.2012.11.019. [DOI] [PubMed] [Google Scholar]
- Pal T.P., Saha D., Maity S. Bioequivalence modulation with modified starch in orodispersible tablets in comparison to marketed conventional tablets of rosuvastatin calcium. Eur. J. Pharm. Med. Res. 2016;3:236–249. [Google Scholar]
- Rédai E.M., Antonoaea P., Todoran N., Vlad R.A., Bîrsan M., Tătaru A., Ciurba A. Development and evaluation of fluoxetine fast dissolving films: an alternative for noncompliance in pediatric patients. Processes. 2021;9:778. [Google Scholar]
- Samita G., Kumar G. Fast dissolving drug delivery and its technologies. Pharma Innovation. 2012;1:34–39. [Google Scholar]
- Sapkal N.P., Kilor V.A., Daud A.S., Bonde M.N. Development of fast dissolving oral thin films of ambroxol hydrochloride: effect of formulation variables. J. Adv. Pharm. Res. 2011;2:102–109. [Google Scholar]
- Sarfraz R.M., Ahmad M., Mahmood A., Minhas M.U., Yaqoob A. Fabrication and evaluation of rosuvastatin calcium fast-disintegrating tablets using β-cyclodextrin and superdisintegrants. Trop. J. Pharm. Res. 2015;14:1961–1968. [Google Scholar]
- Satyanarayana D.A., Keshavarao K.P. Fast disintegrating films containing anastrazole as a dosage form for dysphagia patients. Arch. Pharm. Res. 2012;35:2171–2182. doi: 10.1007/s12272-012-1215-3. [DOI] [PubMed] [Google Scholar]
- Schachter M. Chemical, pharmacokinetic and pharmacodynamic properties of statins: an update. Funda. Clin. Pharmacol. 2005;19:117–125. doi: 10.1111/j.1472-8206.2004.00299.x. [DOI] [PubMed] [Google Scholar]
- Shen C., Shen B., Xu H., Bai J., Dai L., Lv Q., Han J., Yuan H. Formulation and optimization of a novel oral fast dissolving film containing drug nanoparticles by Box-Behnken design response surface methodology. Drug Dev. Ind. Pharm. 2014;40:649–656. doi: 10.3109/03639045.2014.884116. [DOI] [PubMed] [Google Scholar]
- Shivhare U.D., Bodkhe P.D., Bhusari K.P., Mathur V.B. Formulation and evaluation of buccoadhesive films of losartan potassium. Pharm. Lett. 2010;2:251–260. [Google Scholar]
- Sutradhar K.B., Akhter D.T., Uddin R. Formulation and evaluation of taste masked oral dispersible tablets of domepridone using sublimation method. Int. J. Pharm. Sci. 2012;4:727–732. [Google Scholar]
- Takeuchi Y., Ikeda N., Tahara K., Takeuchi H. Mechanical characteristics of orally disintegrating films: Comparison of folding endurance and tensile properties. Int. J. Pharm. 2020;589 doi: 10.1016/j.ijpharm.2020.119876. [DOI] [PubMed] [Google Scholar]
- Tran P., Pyo Y.C., Kim D.H., Lee S.E., Kim J.K., Park J.S. Overview of the manufacturing methods of solid dispersion technology for improving the solubility of poorly water-soluble drugs and application to anticancer drugs. Pharmaceutics. 2019;11:132. doi: 10.3390/pharmaceutics11030132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong C.F., Yuen K.H., Peh K.K. An in vitro method for buccal adhesion studies: importance of instrument variables. Int. J. Pharm. 1999;180:47–57. doi: 10.1016/s0378-5173(98)00402-5. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2010/022524Orig1s000ChemR [DOI] [PubMed] [Google Scholar]