Abstract
Passive aerosol exposure to Δ9-tetrahydrocannabinol (THC) in laboratory animals results in faster onset of action and less extensive liver metabolism compared to most other administration routes and might thus provide an ecologically relevant model of human cannabis inhalation. Previous studies have, however, overlooked the possibility that rodents, as obligate nose breathers, may accumulate aerosolized THC in the nasal cavity, from where the drug might directly diffuse to the brain. To test this, we administered THC (ten 5-s puffs of 100 mg/mL of THC) to adolescent (31-day-old) Sprague-Dawley rats of both sexes. We used liquid chromatography/tandem mass spectrometry to quantify the drug and its first-pass metabolites – 11-hydroxy-Δ9-THC (11-OH-THC) and 11-nor-9-carboxy-Δ9-THC (11-COOH-THC) – in nasal mucosa, lungs, plasma, and brain (olfactory bulb and cerebellum) at various time points after exposure. Apparent maximal THC concentration and area under the curve were ~5 times higher in nasal mucosa than in lungs and 50–80 times higher than in plasma. Concentrations of 11-OH-THC were also greater in nasal mucosa and lungs than other tissues, whereas 11-COOH-THC was consistently undetectable. Experiments with microsomal preparations confirmed local metabolism of THC into 11-OH-THC (not 11-COOH-THC) in nasal mucosa and lungs. Finally, whole-body exposure to THC deposited substantial amounts of THC (~150 mg/g) on fur but suppressed post-exposure grooming in rats of both sexes. The results indicate that THC absorption and metabolism in nasal mucosa and lungs, but probably not gastrointestinal tract, contribute to the pharmacological effects of aerosolized THC in male and female rats.
Keywords: cannabis, pharmacokinetics, metabolism, vaping, nasal mucosa, lungs
Graphical Abstract

1. Introduction
A substantial fraction of teenagers and young adults use cannabis on a regular basis. For example, in 2021 almost 6% of 12th graders in the USA reported using cannabis daily [1]. These data raise concern because human longitudinal studies have linked habitual cannabis use in adolescence to persistent deficits in executive functioning, impulse control, and cognition [2,3] which might be attributable to impaired prefrontal cortical development [4–6]. Animal experiments support these findings, showing that repeated adolescent administration of the intoxicating constituent of cannabis, Δ9-tetrahydrocannabinol (THC), produces dysregulations in synaptic plasticity, memory, and affect which persist until adulthood [7].
Smoking and ‘vaping’ (using either e-cigarettes or vaporizers) are the most frequent means of cannabis consumption in people of all ages, including adolescents [8]. By contrast, animal studies generally rely on the intraperitoneal route of administration, which is practical, reproducible, and elicits in rodents pharmacokinetic and pharmacodynamic responses that are in various ways comparable to those seen in cannabis smokers [9–12]. For example, intraperitoneal administration of 5 mg/kg THC produced, in adolescent and adult male mice, maximal circulating concentrations of the drug that were similar to those previously reported for adult human users [10, 11]. Nevertheless, to capture more closely the human experience, preclinical researchers are increasingly turning to aerosol (often referred to as ‘vapor’) administration as a potential alternative to intraperitoneal injection [13–19]. This effort is primarily motivated by the assumption that, like smoking or vaping, passive aerosol exposure would result in absorption of THC through the lungs, and thus in faster onset of drug action, less extensive first-pass liver metabolism, and distinct psychoactive effects, relative to intraperitoneal administration. Indeed, comparative studies identified significant differences in drug distribution and pharmacodynamics between the two routes. For example, in adult rats inhalation produced hypothermia of equal magnitude but shorter duration [17], as well as greater initial brain THC concentration than intraperitoneal injection [14]. Furthermore, it was shown that rats self-administer an aerosolized cannabis extract [20] or a THC/cannabidiol combination [21] in operant behavioral tests, suggesting that this mode of THC delivery may be useful to investigate the drug’s reinforcing properties.
However, there are noteworthy differences between cannabis vaping in humans and aerosol exposure in rodents. Even in operant behavioral settings, passive inhalation does not engage the same biomechanical and interoceptive processes involved in voluntary vaping or smoking. Moreover, unlike humans, mice and rats are obligate nasal breathers [22]. For this reason, a substantial fraction of THC-containing aerosol particles breathed in by the animals may remain trapped in the nasal mucosa, from where the compound might be rapidly absorbed into the brain [23]—a phenomenon that would not be expected to occur significantly during direct oral inhalation by humans.
To begin examining how the biomechanics of rodent breathing might influence the distribution of inhaled THC, in the present study we exposed male and female adolescent rats to a THC aerosol [13] and measured the concentrations of the drug and its main cytochrome P450 metabolites – 11-hydroxy-Δ9-THC (11-OH-THC) and 11-nor-9-carboxy-Δ9-THC (11-COOH-THC) – in nasal mucosa, lungs, and two brain structures (olfactory bulb and cerebellum). Because rodents routinely lick their fur during grooming [24, 25], we also quantified THC deposited on the animals’ fur, and grooming behavior. The results show that (i) the rat nasal mucosa effectively traps aerosolized THC and transforms it into its bioactive metabolite, 11-OH-THC (but not into inactive 11-COOH-THC); (ii) THC also accumulates in lungs, where is metabolized into 11-OH-THC; and (iii) aerosolized THC accumulates on fur, but this is accompanied by a marked suppression of body licking and washing and is thus unlikely to contribute to the drug’s acute pharmacological effects.
2. Materials and Methods
2.1. Chemicals and solvents
[2H3]-THC, [2H3]-11-OH-THC, and [2H3]-11-COOH-THC were purchased from Cerilliant (Round Rock, TX). THC was from Cayman Chemicals (Ann Arbor, MI), or the NIDA Drug Supply Program. All analytical solvents were of the highest grade and were obtained from Honeywell (Muskegon, MI) or Sigma-Aldrich (Saint Louis, MO). Formic acid was from Thermo Fisher (Houston, TX).
2.2. Animal subjects
Adolescent (post-natal day, PND, at arrival: 23, 30–70 g) male and female Wistar rats were purchased from Charles River (Wilmington, MA). They were housed in same-sex groups of 4 and were allowed to acclimate for 7 days before experiments. Housing rooms were maintained on a 12-h reverse light/dark cycle (lights off at 6:30 AM) under controlled conditions of temperature (20 ± 2°C) and relative humidity (55–60%). Food and water were available ad libitum. All procedures were approved by the Institutional Animal Care and Use Committee at the University of California, Irvine, and carried out in strict accordance with the National Institutes of Health guidelines for the care and use of experimental animals.
2.3. Aerosol equipment
The equipment was designed and manufactured by La Jolla Alcohol Research Inc. (LJARI; San Diego, CA) and were controlled by MedPC hardware and software. LJARI aerosol generators were fourth generation, model 0004–100 W, which rapidly heated the stainless-steel coil in the tanks at 61.1W, 0.4Ω, to 232.2°C during the 5 sec puff deliveries. Chambers consisted of a 52.7 × 58.4 × 48.8 cm clear, air-tight acrylic box, capable of holding four 22.9 × 21.0 × 43.8 cm clear plastic tub cages with wire tops. Two ports delivered aerosol into the chamber at the upper and lower levels and four outlet ports allowed a vacuum pump (1.42 psi air compressor) to pull air and aerosol out of the chamber at a steady rate of 1 L/min (achieved via a regulator and flow gauge), resulting in clearing of aerosol from the chamber approximately 3 min after completion of a puff. Exhaust was filtered through a Whatman HEPA-CAP filter and routed to a fume hood for safe clearance.
2.4. Aerosol THC administration
This was carried out as described previously [13]. THC in ethanol was obtained from the National Institute on Drug Abuse Drug Distribution Program. The solvent was evaporated under N2, and THC was dissolved in propylene glycol (100 mg/mL) with heat (37°C) and sonication (15 min). Vaporizer tanks (Smok TFV8 Baby) were filled with 3–4 mL of the THC solution immediately before testing. On PND31, 2–3 animals were placed in pairs with familiar cagemates into tub cages with bedding, on the lower shelf of the larger aerosol containment chambers. They received 30 min of THC vapor exposure consisting of ten 5-s puffs every 175 sec. Approximately 5 min after the 30 min session, PK animals were removed and returned to their home cages, and those for grooming evaluation were placed alone into behavioral testing chambers, and were video recorded for 2 h. PK rats were disorientated and decapitated without anesthetic to avoid any interference with isoflurane at various timepoints after exposure (5, 15, 60 min; n = 4 per timepoint), trunk blood was collected into 4 mL polypropylene plastic tubes containing spray-coated potassium-EDTA (K2-EDTA) and centrifuged at 500×g at 4°C for 10 min. Plasma was collected and centrifuged again at 2000×g at 4°C for 10 min to remove any trace of residual cells and transferred into polypropylene tubes. After decapitation, their brains were quickly removed, and olfactory bulb and cerebellum samples were dissected rapidly. Nasal mucosa were collected after removing dorsal bone anterior to the olfactory bulbs and lung samples were collected. The dorsum of each rat was shaved and approximately 300 mg of fur was collected. All tissue samples were immediately frozen on dry ice, and stored at −80°C until analyses.
2.5. Intranasal THC administration
THC was prepared shortly before use as described above (see section 2.4). Adolescent rats of both sexes were lightly anesthetized with isoflurane and held by the scruff with the nose positioned to facilitate dosing. A single dose of THC (5 mg/kg; half dose per nostril; n = 4 per sex) was administered intranasally with a Hamilton syringe (Microliter #705). The animals were euthanized by decapitation 35 min later. Trunk blood was collected into spray-coated potassium-EDTA (K2-EDTA) tubes, and plasma was harvested by centrifugation at 1450 × g at 4°C for 15 min. Brains were removed, and olfactory bulb, cerebellum, nasal mucosa, and lung samples were collected, frozen and stored as described above.
2.5. Intraperitoneal THC administration
THC was prepared shortly before experiments as described in section 2.4. Adolescent rats of both sexes were given a single intraperitoneal injection of 5 mg/kg THC (n = 4 per sex). They were euthanized by decapitation 35 min after injection. Trunk blood, nasal mucosa, lungs, and brain were processed as described above. All samples were frozen on dry ice and stored at 80°C until analyses.
2.6. Microsome preparation
Microsomes were prepared as previously described [26], with minor modifications, from naïve adolescent male and female rats (PND 31). Nasal mucosa and lung tissue were weighed and homogenized in extraction buffer (20%, w/v; 10 mM Tris pH 7.5, 250 mM sucrose, 1 mM phenylmethylsulfonyl fluoride, protease inhibitor cocktail, Nacalai Tesque, Kyoto, Japan cat no.: 04080–11). The homogenates were centrifuged at 3000×g at 4°C for 20 min. Supernatants were collected and centrifuged twice for 20 min at 10,000×g at 4°C. The supernatants from the second centrifugation were centrifuged again for 90 min at 100,000×g at 4°C. The microsome pellets were resuspended in 0.5 mL buffer (50 mM Tris pH 7.5, 20% glycerol, 1 mM dithiothreitol, 1 mM EDTA). Protein concentrations were measured using the bicinchoninic acid assay.
2.7. THC metabolism in nasal mucosa and lung microsomes
Microsomes (1 μg protein) were combined in a solution of potassium phosphate (0.1 M, pH 7.4) containing rat cytochrome P450 reductase (0.2 μM). After preincubation with THC (40 μM, 5 min, 37°C), reactions were initiated by adding 10 mM NADPH (0.1 mL,1 mM final) and allowed to proceed at 37°C for 30 min, at which point they were quenched with an equal volume of ethyl acetate. Extractions were performed as previously reported [27]. Briefly, the quenched reactions were stirred thoroughly, centrifuged for 5 min at 1800×g at 4°C, and the organic layers were transferred into clean tubes. Fresh ethyl acetate was added, and the cycle was repeated twice for a total of 3 extractions. After drying down the organic layer in a rotary evaporator, extracts were resuspended in 95% ethanol (0.1 mL) and shipped overnight on dry ice for LC/MS-MS analysis.
2.8. Liquid chromatography/tandem mass spectrometry (LC/MS-MS) analyses
2.8.1. Sample Preparation
Plasma (0.1 mL) was transferred into 8 mL glass vials (Thermo Fisher, catalog no.: B7999–3) and proteins were precipitated by addition of 0.5 mL of ice-cold acetonitrile containing 1% formic acid and the following internal standards (ISTD): [2H3]-THC, [2H3]-11-OH-THC, and [2H3]-11-COOH-THC, 50 pmol each. Lung, olfactory bulb, cerebellum, nasal mucosa and fur (20–25 mg) were homogenized using the Precellys CK-14 soft tissue homogenizing kit (Bertin Corp., Rockville, MD) in a Precellys Evolution apparatus (Bertin) at 4°C on pre-set setting #4 (6500 RPM × 20s × 2) in 0.5 mL of ice-cold acetonitrile containing 1% formic acid and 50 pmol ISTD. Plasma and tissue samples were stirred vigorously for 30 s and centrifuged at 2800×g at 4°C for 15 min. After centrifugation, the supernatants were loaded onto Captiva-Enhanced Matrix Removal (EMR)-Lipid cartridges (Agilent Technologies, Santa Clara, CA) and eluted under positive pressure (3–5 mmHg, 1 drop/5 sec; Agilent Technologies). For tissue fractionation, EMR cartridges were pre-washed with water/acetonitrile (1:4, v/v). No pretreatment was necessary for plasma fractionation. Tissue pellets were rinsed with water/acetonitrile (1:4, v/v; 0.2 mL), stirred for 30 s, and centrifuged at 2800×g at 4°C for 15 min. The supernatants were collected, transferred onto EMR cartridges, eluted, and pooled with the first eluate. The cartridges were washed again with water/acetonitrile (1:4, v/v; 0.2 mL), and pressure was increased gradually to 10 mmHg (1 drop/sec) to ensure maximal analyte recovery. Eluates were dried under N2 and reconstituted in 0.1 mL of methanol containing 0.1% formic acid. Samples were transferred to deactivated glass inserts (0.2 mL) placed inside amber glass vials (2 mL; Agilent Technologies).
2.8.2. LC/MS-MS Conditions
LC separations were carried out using a 1200 series LC system (Agilent Technologies), consisting of a binary pump, degasser, thermostated autosampler and column compartment coupled to a 6410B triple quadrupole mass spectrometric detector (MSD; Agilent Technologies). Analytes were separated on an Eclipse XDB C18 column (1.8 μm, 3.0 ×50.0 mm; Agilent Technologies). The mobile phase consisted of water containing 0.1% formic acid as solvent A and methanol containing 0.1% formic acid as solvent B. The flow rate was 1.0 mL/min. The gradient conditions were as follows: starting 75% B to 89% B in 3.0 min, changed to 95% B at 3.01 min, and maintained till 4.5 min to remove any strongly retained materials from the column. Equilibration time was 2.5 min. The column temperature was maintained at 40°C and the autosampler at 9°C. The total analysis time, including re-equilibrium, was 7 min. The injection volume was 5 μL. To prevent carry over, the needle was washed in the autosampler port for 30 s before each injection using a wash solution consisting of 10% acetone in water/methanol/isopropanol/acetonitrile (1:1:1:1, v/v). The MS was operated in the positive electrospray ionization (ESI) mode, and analytes were quantified by multiple reaction monitoring (MRM) of the following transitions: THC 315.2 > 193.0 m/z, [2H3]-THC 318.2 > 196.1 m/z, 11-OH-THC 331.2 > 313.1 m/z, [2H3]-11-OH-THC 334.2 > 316.1 m/z, 11-COOH-THC 345.2 > 299.2 m/z, [2H3]-11-COOH-THC 348.2 > 302.2 m/z. In select experiments, the identity of THC was further verified by monitoring the transition 315.2 > 135.0 m/z. The capillary voltage was set at 3500 V. The source temperature was 300°C and gas flow was set at 12.0 L/min. Nebulizer pressure was set at 40 psi. Collision energy and fragmentation voltage were set for each analyte as reported [28]. The MassHunter software (Agilent Technologies) was used for instrument control, data acquisition, and data analysis.
2.9. Grooming behavior
After being removed from aerosol exposure chambers following 30 min exposure to vacuum only (naïve), vehicle vapor, or THC vapor, rats were placed into 43 × 43 × 30.5 cm locomotor testing boxes without bedding, in a dimly lit room, for 2 h. Grooming behavior was recorded via infrared cameras from above and quantified manually by a blinded observer using Noldus Observer software. Total time spent grooming the head and face was quantified, as was duration of body/flank licking that typically occurs thereafter during normal grooming
3.0. Statistical analyses
Differences in apparent maximal concentration (apparent Cmax) and area under the curve (AUC) for each tissue compared to nasal mucosa were analyzed using one-way analysis of variance (ANOVA) with Dunnett’s post hoc test. All other data were analyzed using two-way ANOVA with Bonferroni or Tukey post hoc tests where appropriate. Differences between groups were considered statistically significant at values of P < 0.05.
3. Results
3.1. Aerosolized THC accumulates in nasal mucosa
Adolescent (PND 31) male and female rats were either exposed to a THC aerosol (100 mg/mL) for 30 min or were given a single intraperitoneal injection of THC (5 mg/kg). The concentrations of THC, 11-OH-THC and 11-COOH-THC in nasal mucosa, lungs, plasma, olfactory bulb, and cerebellum were measured 5, 15 or 60 min after THC administration using a previously reported LC/MS-MS method [limit of quantification (LOQ) for THC and its metabolites: 1.0 pmol/mL] [9, 12, 28]. Figure 1 shows the maximal concentrations attained by THC (apparent Cmax) in nasal mucosa, lungs, plasma, olfactory bulb and cerebellum 5 min after removing the animals from the aerosol chamber or 35 min after intraperitoneal administration. This time point was selected to match the start of behavioral testing which, in this protocol, is initiated 5 min after THC exposure. Aerosol exposure yielded higher THC concentrations in nasal mucosa, lungs, olfactory bulb and cerebellum than did intraperitoneal administration (Fig. 1 and Table 1). For example, the apparent Cmax of THC in nasal mucosa of male animals after aerosol administration was approximately ~1000 times higher after aerosol than intraperitoneal administration (29,998 ± 12,660 pmol/g, mean ± SEM vs 21 ± 6 pmol/g; P < 0.001; Table 1). 11-OH-THC concentrations followed a similar pattern in both male and female animals (Table 1). By contrast, THC concentrations in plasma were similar with the two routes (Fig. 1 and Table 1). The concentration-time profiles of THC in surveyed tissues after aerosol administration are illustrated in Figure 2, which includes the 5-min aerosol timepoints reported in Figure 1. Table 2 lists apparent Cmax values for THC as well as the time at which apparent Cmax was reached (apparent Tmax), apparent half-life time of elimination (apparent t1/2), and AUC for the 60-min interval examined in the study. These parameters offer critical insights into the maximal level of systemic exposure to THC (apparent Cmax and AUC) and the temporal trajectory of such exposure (apparent Tmax and t1/2). In rats of both sexes, the apparent Cmax for THC was ~80 times higher in nasal mucosa than plasma, and ~5 times higher in nasal mucosa than lungs. In females, for example, the apparent Cmax was 19,175 ± 293 pmol/g in nasal mucosa compared to 349 ± 38 pmol/mL in plasma (P < 0.01) and 4,168 ± 502 pmol/g in lungs (P < 0.05) (Table 2). Only one statistically detectable sexual dimorphism was observed: male rats had higher THC concentration at 5 min in lungs compared to females (6,881 ± 1,026 vs 4,168 ± 502 pmol/g, P < 0.01, Fig. 2B, Table 2); the AUC was nearly double but not statistically different between the two groups (91,689 ± 18,654 vs 51,805 ± 7,543 pmol/min/mg, P > 0.05, Table 1). Thus, irrespective of sex, aerosol delivery resulted in substantially higher THC concentrations in nasal mucosa and lungs than in any other tissue included in the survey.
Figure 1.

THC concentrations in nasal mucosa, lungs, and plasma of adolescent male rats (A) and female rats (B) after passive exposure to a THC aerosol (100 mg/mL, ■•) or intraperitoneal administration (5 mg/kg, ■•). Tissues were collected 5 min after a 30-min aerosol exposure or 35 min after intraperitoneal injection. Bars represent mean ± SEM, n = 3–4 animals. ***P < 0.001, ****P < 0.0001, significant difference compared to aerosol administration, two-way ANOVA.
Table 1.
Apparent maximal concentration (Cmax) for THC and 11-OH-THC in nasal mucosa, lung, plasma, olfactory bulb and cerebellum of adolescent (PND 31) male and female rats after 30-min aerosol exposure to a THC aerosol (100 mg/mL) or 35 min after intraperitoneal (IP) administration (5 mg/kg).
| Tissue | Sex | Aerosol | IP | ||
|---|---|---|---|---|---|
| THC (pmol/mL or g) | 11-OH-THC (pmol/mL or g) | THC (pmol/mL or g) | 11-OH-THC (pmol/mL or g) | ||
| Nasal mucosa | Male | 29,998 ± 12,660 | 1,184 ± 300 | 21 ± 6*** | 4 ± 1**** |
| Female | 19,175 ± 293 | 986 ± 168 | 33 ± 14**** | 15 ± 9**** | |
| Lung | Male | 6,881 ± 1,026 | 174 ± 22 | 200 ± 29 | 22 ± 7 |
| Female | 4,168 ± 502## | 199 ± 17 | 459 ± 203**** | 121 ± 62# | |
| Plasma | Male | 279 ± 17 | 17 ± 2 | 287 ± 74 | 5 ± 2 |
| Female | 349 ± 38 | 32 ± 7## | 347±116 | 30 ± 12 | |
| Olfactory Bulb | Male | 464 ± 86 | 63 ± 4 | 47 ± 17 | 9 ± 3 |
| Female | 303 ± 8 | 111 ± 22# | 67 ± 26 | 36 ± 20 | |
| Cerebellum | Male | 331 ± 36 | 72 ± 7 | 86 ± 33 | 16 ± 5 |
| Female | 221 ± 26 | 142 ± 9## | 149 ± 65 | 85 ± 46 | |
Data are presented as means ± standard error of the mean, n = 3–4.
P < 0.001,
P < 0.0001 (significant effect compared to aerosol administration),
P < 0.05,
P < 0.001 (significant sex effect), two-way ANOVA.
Figure 2.
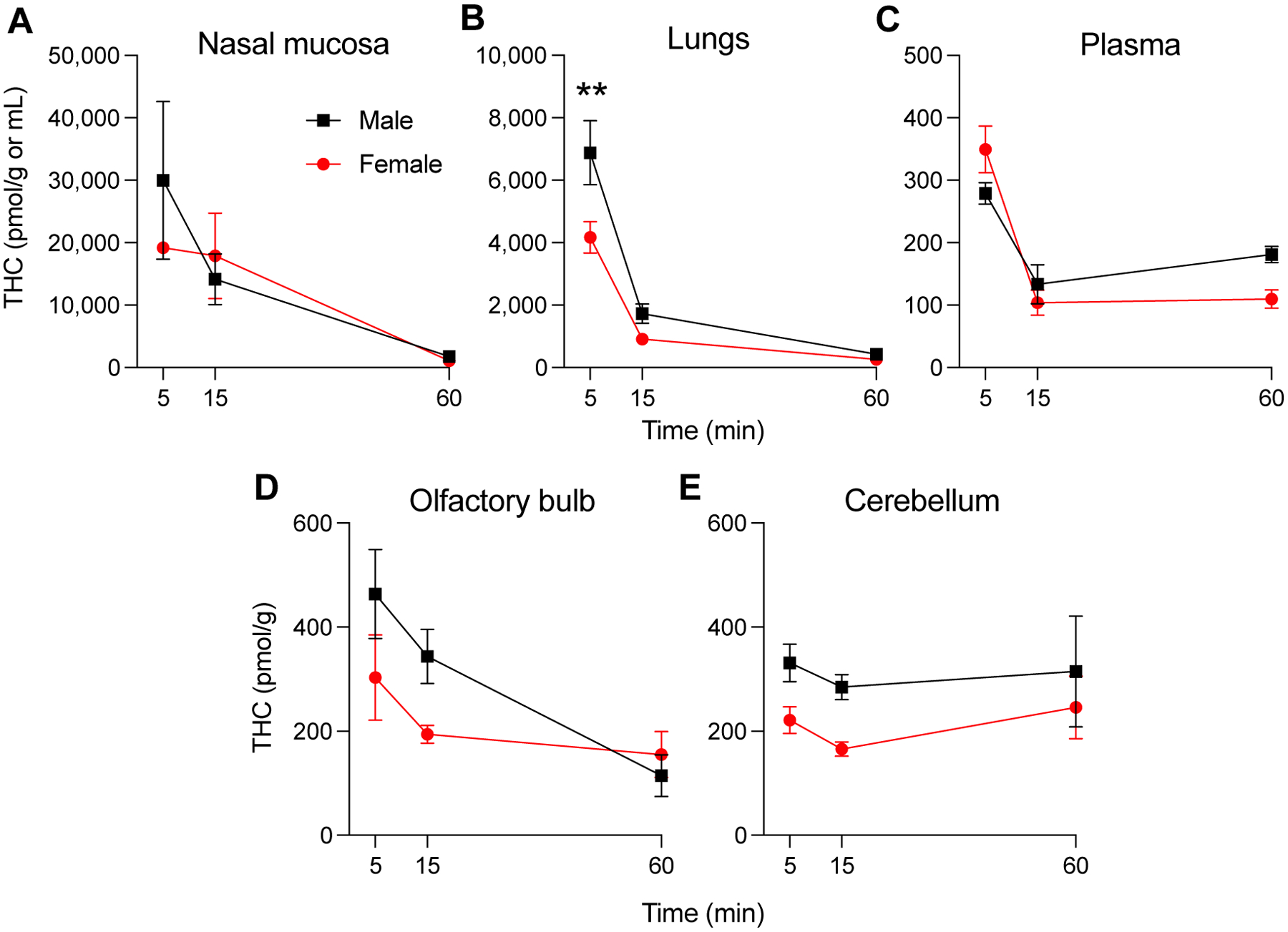
Concentrations of THC in nasal mucosa (A), lungs (B), plasma (C), olfactory bulb (D) and cerebellum (E) of adolescent male (■) and female (•) rats after passive exposure to a THC aerosol (100 mg/mL). The exposure lasted 30 min and tissues were collected 5, 15 and 60 min later. Symbols represent mean ± SEM, n = 3–4 animals. **P < 0.01, significant sex-difference, two-way ANOVA.
Table 2.
Apparent maximal concentration (Cmax), time at which apparent maximal concentration was reached (Tmax), area under the curve (AUC) and apparent half-life of elimination (t1/2) for THC in nasal mucosa, lung, plasma, olfactory bulb and cerebellum of adolescent (PND 31) male and female rats after 30-min aerosol exposure to a THC aerosol (100 mg/mL).
| Tissue | Male | Female | ||||||
|---|---|---|---|---|---|---|---|---|
| Cmax (pmol/mL or g) | AUC (pmol•min/mL or g) | Tmax (min) | t1/2 (min) | Cmax (pmol/mL or g) | AUC (pmol•min/mL or g) | Tmax (min) | t1/2 (min) | |
| Nasal mucosa | 29,998 ± 12,660 | 578,266 ± 230,738 | 5 | 3.9 ± 0.2 | 19,175 ± 293 | 613,129 ± 317,513 | 5 | 3.9 ± 0.0 |
| Lung | 6,881 ± 1,026* | 91,689 ± 18,654* | 5 | 4.4 ± 0.1 | 4,168 ± 502***,## | 51,805 ± 7,543* | 5 | 4.6 ± 0.1 |
| Plasma | 279 ± 17** | 9,148 ± 1,537** | 5 | 8.5 ± 0.5 | 349 ± 38*** | 7,080 ± 1,151* | 5 | 6.9 ± 0.2# |
| Olfactory Bulb | 464 ± 86** | 14,356 ± 3,099** | 5 | 6.3 ± 0.3 | 303 ± 82*** | 10,348 ± 2,261* | 5 | 7.2 ± 0.3 |
| Cerebellum | 331 ± 36** | 16,557 ± 4,935** | 5 | NA | 246 ± 60*** | 11,195 ± 2,781* | 60 | NA |
Data are represented as means ± standard error of the mean, n = 3–4.
P < 0.05,
P < 0.01,
P < 0.001 (significant tissue effect compared to nasal mucosa), one-way ANOVA;
P < 0.05,
P < 0.01 (significant sex effect), two-way ANOVA.
3.2. Aerosolized THC is metabolized to 11-OH-THC in nasal mucosa
Figure 3 depicts the concentration-time profiles of the psychoactive THC metabolite, 11-OH-THC. As shown in a previous study with aerosolized THC [13], the concentrations of 11-COOH-THC were below the LOQ of our LC/MS-MS assay. As seen with THC, the apparent Cmax for 11-OH-THC was, for both sexes, substantially higher in nasal mucosa than other sampled tissues. For example, in male rats, apparent Cmax and AUC were 5–6 times higher in nasal mucosa than in lungs (1,239 ± 231 vs 247 ± 42 pmol/g, P < 0.01; AUC: 58,227 ± 19,133 vs 8,825 ± 1,731 pmol/min/g, P < 0.05, Table 3). There were no detectable sex differences in 11-OH-THC concentrations in nasal mucosa or lungs (Fig. 3A and B, Table 3). However, compared to males, female animals exhibited significantly higher apparent Cmax for 11-OH-THC in plasma, olfactory bulb, and cerebellum (38 ± 5 vs 17 ± 2 pmol/mL, plasma, P < 0.01; 188 ± 43 vs 87 ± 41 pmol/g, olfactory bulb, P < 0.05; 186 ± 30 vs 91 ± 27 pmol/g, cerebellum, P < 0.01).
Figure 3.
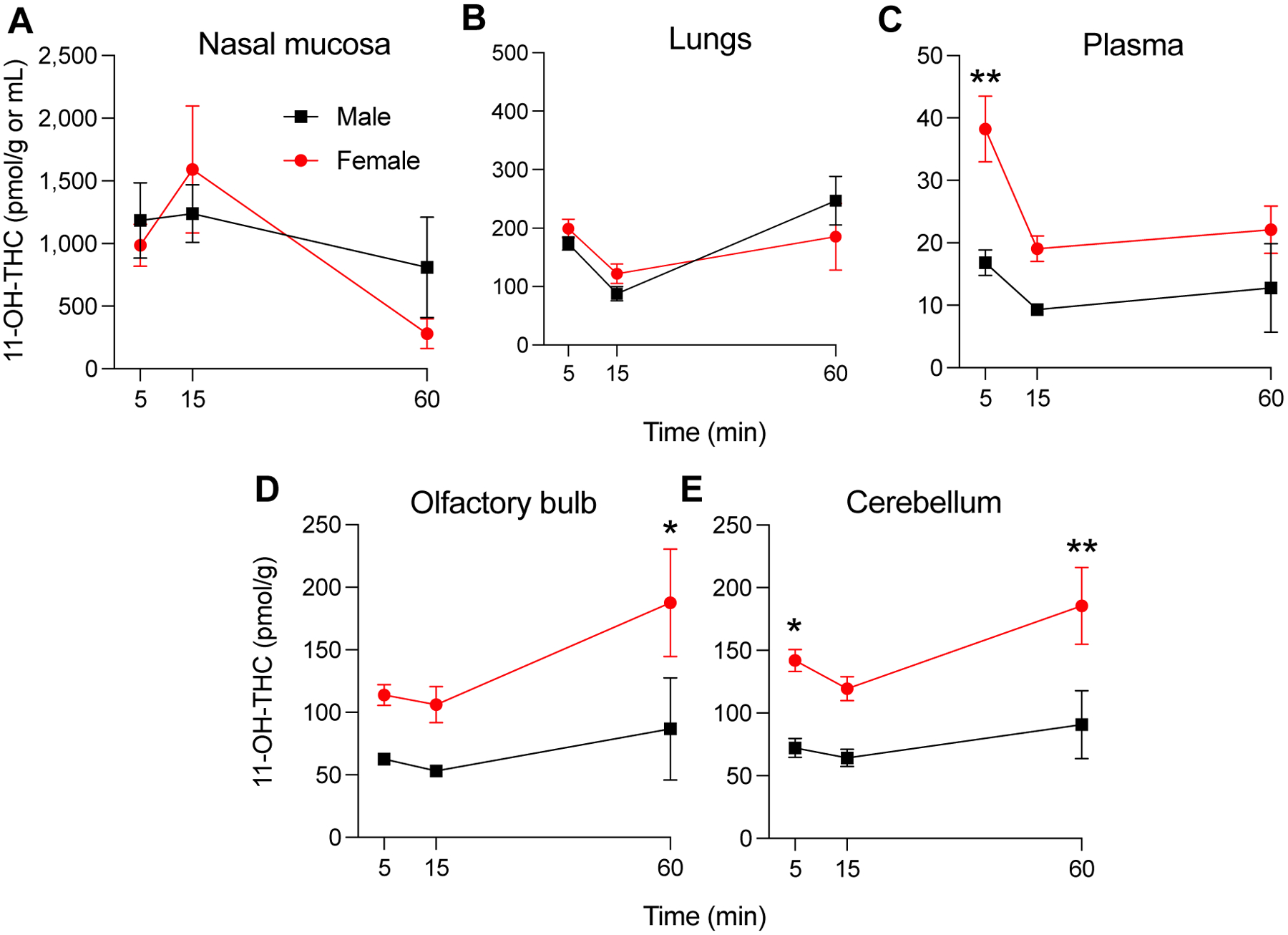
Concentrations of 11-OH-THC in nasal mucosa (A), lungs (B), plasma (C), olfactory bulb (D) and cerebellum (E) of adolescent male (■) and female (•) rats after passive exposure to a THC aerosol (100 mg/mL). The exposure lasted 30 min and tissues were collected 5, 15 and 60 min later. Symbols represent mean ± SEM, n = 3–4. *P < 0.05, **P < 0.01, significant sex-difference, two-way ANOVA.
Table 3.
Apparent maximal concentration (Cmax), time at which apparent maximal concentration was reached (Tmax) and area under the curve (AUC) for 11-OH-THC in nasal mucosa, lung, plasma, olfactory bulb and cerebellum of adolescent (PND 31) male and female rats after 30-min aerosol exposure to a THC aerosol (100 mg/mL).
| Tissue | Male | Female | ||||
|---|---|---|---|---|---|---|
| Cmax (pmol/mL or g) | AUC (pmol•min/mL or g) | Tmax (min) | Cmax (pmol/mL or g) | AUC (pmol•min/mL or g) | (min) | |
| Nasal mucosa | 1,239 ± 231 | 58,227 ± 19,133 | 15 | 1,591 ± 506 | 54,987 ± 23,995 | 15 |
| Lung | 247 ± 42*** | 8,825 ± 1,731** | 60 | 199 ± 16** | 8,520 ± 2,686* | 5 |
| Plasma | 17 ± 2*** | 627 ± 280*** | 5 | 38 ± 5##, ** | 1,213 ± 195* | 5 |
| Olfactory Bulb | 87 ± 41*** | 3,728 ± 1,610** | 60 | 188 ± 43#, * | 7,712 ± 1,770* | 60 |
| Cerebellum | 91 ± 27*** | 4,167 ± 1,105** | 60 | 186 ± 30##, * | 8,174 ± 1,257* | 60 |
Data are represented as means ± standard error of the mean, n = 3–4.
P < 0.05,
P < 0.01,
P < 0.001 (significant tissue effect compared to nasal mucosa), one-way ANOVA;
P < 0.05,
P < 0.01 (significant sex effect), two-way ANOVA.
3.3. Nasal mucosa and lung microsomes metabolize THC in vitro
We also assessed whether microsomes prepared from nasal mucosa and lung tissue of naïve adolescent male and female rats metabolize THC. Both preparations converted THC into 11-OH-THC, whereas 11-COOH-THC was undetectable. The rate of 11-OH-THC production was 3 times higher in lung than nasal mucosa microsomes (0.10 ± 0.01 vs 0.03 ± 0.006 pmol/min/mg, males; 0.12 ± 0.02 vs 0.03 ± 0.009 pmol/min/mg, females; P < 0.01, Fig. 4). Confirming in vivo data, nasal mucosal microsomes from male and female animals did not differ in their ability to transform THC into 11-OH-THC (Fig. 4).
Figure 4.
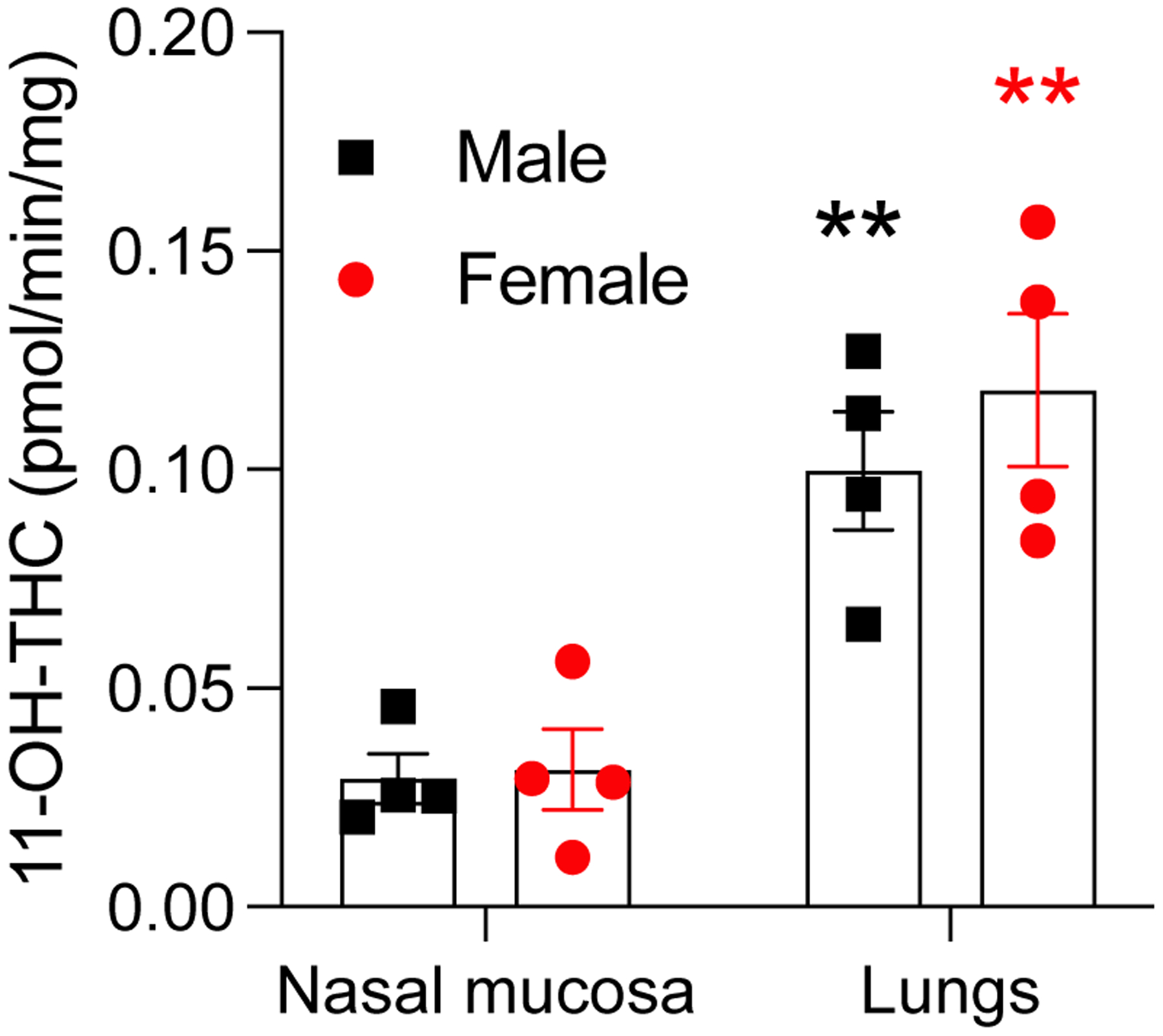
Rate of production of 11-OH-THC from THC in nasal mucosa and lung microsomes from naïve adolescent male (■) and female (•) rats. 11-COOH-THC was below the limit of detection in both tissues. Data are shown as pmol/min/mg of protein. Bars represent mean ± SEM, n = 4. **P < 0.01, significant difference compared to nasal mucosa, two-way ANOVA.
3.4. Distribution and metabolism of intranasally administered THC
Next, to determine whether THC in nasal mucosa might reach the brain and other organs, we infused the drug intranasally (5 mg/kg) and quantified its concentrations in nasal mucosa, lungs, plasma, olfactory bulb, and cerebellum 35 min later (to match the protocol of previous experiments). As expected, we found that nasal mucosa contained substantial amounts of THC and, to a lesser extent, 11-OH-THC (Fig. 5A, C). Additionally, significant concentrations of the drug and its bioactive metabolite were detected in all other tissues included in the survey (Fig. 5B, D).
Figure 5.

Concentrations of THC (top) and 11-OH-THC (bottom) in nasal mucosa (A, C) or lungs, plasma, olfactory bulb, and cerebellum (B, D) of adolescent male (■) and female (•) rats after intranasal infusion of THC (5 mg/kg). To match the aerosol exposure protocol, tissues were collected 35 min after administration. Symbols represent mean ± SEM, n = 4.
3.5. Aerosolized THC deposits on fur
Finally, we asked whether aerosolized THC might deposit on the rats’ fur, where it could be ingested during grooming. We found that submicromolar amounts of the drug accumulate on dorsal fur of male and female animals (513,298 ± 123,289 pmol/g, males; 437,231 ± 53,312 pmol/g, females) (Fig. 6). To assess whether THC deposited on fur might be ingested by licking, and thus absorbed through the buccal or oral route, we placed male and female rats in aerosol containment chambers and exposed them for 30 min to vehicle, THC, or no aerosol (vacuum only, naïve). At the end of the exposure period, we recorded duration of self-grooming for 2 h (Fig. 7). Both sex and aerosol treatment condition impacted total grooming duration (main effects of sex: F3,24 = 21, P < 0.0001; and treatment: F2,24 = 21, P < 0.0001), though males and females did not differ in head grooming after any treatment. However, vehicle vapor induced large increases in body (flank) grooming in females that were contrasted by a decrease of body grooming in males (P < 0.0001, females; P < 0.05 males). Aerosolized THC markedly suppressed grooming in both sexes relative to vehicle and naïve animals, with a significant suppression of body licking (P < 0.001), and a trend toward suppression of head grooming. Relative to vehicle-exposed rats, THC significantly suppressed body grooming in females (P < 0.001), and only trended toward suppressing all grooming for both sexes.
Figure 6.
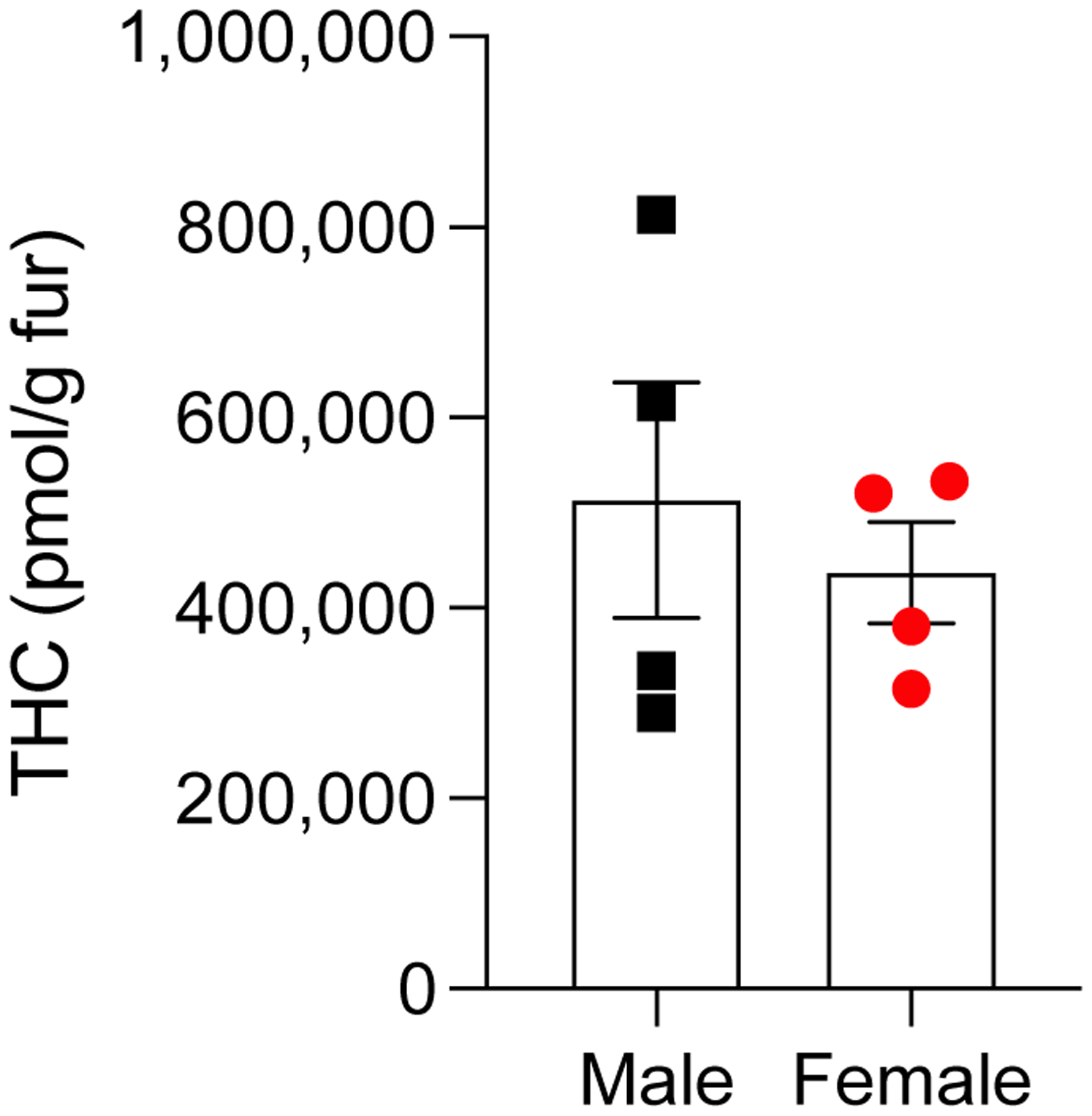
THC deposits on the fur of adolescent male (■) and female (•) rats after passive exposure to THC aerosol. Fur was collected 60 min after removal of the animals from the aerosol chambers. Data are shown as pmol/g of fur. Bars represent mean ± SEM, n = 4.
Figure 7:

Impacts of vehicle or THC vapor on grooming behavior of the head (unfilled symbols) or body (filled symbols) of adolescent male (■) and female (•) rats after exposure to the chamber/vacuum only (naïve), vehicle vapor (VEH), or THC vapor (100mg/ml). Bars represent mean ± SEM, n = 3 animals. *P < 0.05, ***P < 0.001, ****P < 0.0001, two-way ANOVA, not all statistics are shown for clarity.
4. Discussion
This study examined potential routes through which THC might be absorbed in adolescent male and female rats following aerosol (“vapor”) delivery. Addressing this issue is important to interpret experimental data obtained with this increasingly popular route of administration, because accumulation in the nasal cavity would enable a lipophilic drug such as THC to enter the brain through the cribriform plate, in addition to being absorbed via the lungs [29]. Nasal absorption is expected to result in faster onset of action and greater initial brain concentration than lung absorption alone, the primary route through which THC reaches the circulation in cannabis smokers.
The first finding of our study is that, irrespective of sex, the rat nasal mucosa captures substantial amounts of aerosolized THC. After a 30-min exposure, average concentrations of the drug were approximately 5 times higher in mucosal tissue than in lungs and 80 times higher than in plasma. A direct comparison with intraperitoneal administration highlighted the ability of aerosol exposure to deliver THC to the nasal mucosa, as well as to the lungs. The large amount of THC found in these two organs suggests that absorption may take place via both routes. Confirming this possibility, we found that intranasal infusion delivered THC to the brain, though less effectively than inhalation. The findings are consistent with prior reports, which have shown that aerosol THC exposure results in shorter onset of action and greater initial concentrations in brain, compared to the intraperitoneal and subcutaneous routes [14, 30]. The fast kinetics of nasal and pulmonary absorption might also contribute to why rats self-administer aerosols of THC-rich cannabis extracts in a response-contingent setting [20, 21].
Another noteworthy finding is that nasal mucosa tissue converts inhaled THC into the highly psychoactive metabolite 11-OH-THC. This finding is novel, but not completely unexpected. Previous research showed that rat nasal mucosa and lungs express various cytochrome P450 isoforms [31, 32] which catalyze this reaction [33–35]. THC biotransformation in nasal mucosa is likely to be functionally relevant because the maximal concentrations reached by 11-OH-THC in the nasal cavity were sizeable (double-digit micromolar) and approximately 80 times higher than those found in plasma. Of note, the ratios of 11-OH-THC to THC (AUC11-OH-THC/AUCTHC) found in plasma and brain are comparable to those previously reported for intraperitoneal injection. For example, in males, plasma 11-OH-THC/THC ratios are: aerosol (100 mg/mL), 0.07 ± 0.005; intraperitoneal (0.5 mg/kg), 0.07 ± 0.01; and intraperitoneal (5 mg/kg) 0.10 ± 0.01 [11]. The findings thus suggest that aerosolized THC undergoes significant metabolism in both nose and lungs. Interestingly, however, in these two tissues THC biotransformation seemingly stops with 11-OH-THC and does not proceed to the inactive product 11-COOH-THC. Nasal mucosa and lungs thus appear to differ from liver, which readily converts THC into both 11-OH-THC and 11-COOH-THC [36]. This discrepancy might be attributed to possible, but as-yet unidentified, tissue-selective differences in alcohol dehydrogenase and aldehyde dehydrogenase activities [37, 38], which oxidize 11-OH-THC into 11-COOH-THC. Supporting the possibility that aerosolized THC bypasses liver metabolism, 11-COOH-THC was also undetectable in plasma and brain.
In rodents and humans, the conversion of THC into 11-OH-THC is sexually dimorphic, with adult females showing higher rates of conversion compared to adult males [9, 39]. However, in nasal mucosa or lungs of adolescent rats, we found no sex difference in the apparent Cmax and AUC of 11-OH-THC, or in the rate at which this compound is produced from THC. By contrast, as anticipated from previous studies [9,11,13], significantly higher apparent Cmax and AUC for 11-OH-THC were observed in female plasma and brain, compared to males. It appears therefore that nasal mucosa and lungs – which, as the present results indicate, may be two major sites of THC absorption and biotransformation following aerosol administration – do not metabolize THC in a sexually dimorphic manner.
We expected that THC would deposit on the fur of rats undergoing whole-body aerosol exposure but were surprised by the quantities we found. In animals of both sexes, the fur was covered with sub-milligram amounts of the drug (approximately 150 mg/g of fur). As this layer is accessible to licking during self-grooming in the rat [24, 25], we asked whether THC might be absorbed through buccal or oral absorption after self-grooming. A study designed to explore this possibility showed, however, that rats exhibit little or no grooming behavior in the two hours following aerosol THC exposure, which might be due either to aversion to the organoleptic properties of THC or to a generally sedative effect of the drug [13]. It is thus unlikely that buccal or oral absorption contribute to the pharmacological effects of aerosolized THC, at least following acute administration.
The study has several limitations. First, it was conducted on adolescent animals only. The fastest growing segment of cannabis users are persons 55 years and older [42], so adult animals of different age groups should be evaluated to determine potential age-related differences in aerosolized THC accumulation in lungs, nose, and fur. Second, concentrations of THC and its metabolites were only assessed in the 60 min interval following the aerosol session. This protocol is standard in the field [13, 14, 17, 19, 43, 44], but its selection prevented us from obtaining a complete pharmacokinetic profile of inhaled THC. Third, the preponderant quantities of THC found in nasal mucosa and lungs, compared to plasma, suggests that the drug reaches the brain through both nasal and pulmonary absorption after inhalation by rats. However, the exact contributions of these two routes were not fully investigated, and we note that although THC concentrations were higher in nasal mucosa than lungs, the substantially larger surface area of the latter likely supports greater and faster drug absorption. One last caveat is that variables such as the drug vehicle employed, and the duration/parameters of exposure may affect THC accumulation in nose, lungs, and fur.
Despite these limitations, the present results demonstrate that the nasal mucosa of adolescent rats captures large amounts of THC, and effectively transforms it into the bioactive metabolite 11-OH-THC, resulting in nasal absorption of both psychoactive compounds. The findings also show that aerosolized THC accumulates on rat fur, though it is unlikely to be ingested at pharmacologically relevant levels due to a suppression of grooming behavior by the drug. These findings do not detract from the value of aerosol THC delivery as an experimental animal model, but they should be taken into consideration when interpreting the relevance such data to human vaping behavior. Further, we suggest that THC aerosol exposure may capture features of human second-hand cannabis exposure – especially in infants [45], who (like rats) are preferential nose breathers [46]. This hypothesis deserves further examination.
Funding
The study was funded by the National Institute on Drug Abuse (NIDA) [Center of Excellence Grant DA044118], the National Institutes of Health [R01 GM1155884; U01 DA053826] and the State of California Tobacco-Related Disease Research Program grants [T31IR1767, T29IR0618, T30FT0967].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CRediT Authorship Statement
Alexa Torrens: Conceptualization, Investigation, Formal Analysis, Writing – Original draft preparation, Writing – Review & Editing Christina M. Ruiz: Conceptualization, Investigation Maricela X. Martinez: Investigation, Formal Analysis Alex Mabou Tagne: Investigation Pritam Roy: Investigation Dakota Grimes: Investigation Faizy Ahmed: Methodology Valeria Lallai: Investigation Victoria Inshishian: Investigation Malia Bautista: Investigation Yen-Chu Chen: Investigation Marilyn A. Huestis: Writing – Original draft preparation Aditi Das: Conceptualization, Supervision Christie D. Fowler: Conceptualization, Investigation, Supervision Stephen V. Mahler: Conceptualization, Formal Analysis, Writing – Original draft preparation, Writing – Review & Editing, Supervision Daniele Piomelli: Conceptualization, Writing – Original draft preparation, Writing – Review & Editing, Supervision
Declaration of Interest
No competing interests exist.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Miech RA, Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE, & Patrick ME (2022). Monitoring the Future National Survey Results on Drug Use, 1975–2021: Volume 1, Secondary School Students. Institute for Social Research. [Google Scholar]
- 2.Morin JG, Afzali MH, Bourque J, Stewart SH, Séguin JR, O’Leary-Barrett M, & Conrod PJ (2019). A Population-Based Analysis of the Relationship Between Substance Use and Adolescent Cognitive Development. The American journal of psychiatry, 176(2), 98–106. 10.1176/appi.ajp.2018.18020202 [DOI] [PubMed] [Google Scholar]
- 3.Power E, Sabherwal S, Healy C, O’ Neill A, Cotter D, & Cannon M (2021). Intelligence quotient decline following frequent or dependent cannabis use in youth: a systematic review and meta-analysis of longitudinal studies. Psychological medicine, 51(2), 194–200. 10.1017/S0033291720005036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Albaugh MD, Ottino-Gonzalez J, Sidwell A, Lepage C, Juliano A, Owens MM, Chaarani B, Spechler P, Fontaine N, Rioux P, Lewis L, Jeon S, Evans A, D’Souza D, Radhakrishnan R, Banaschewski T, Bokde A, Quinlan EB, Conrod P, Desrivières S, … IMAGEN Consortium (2021). Association of Cannabis Use During Adolescence With Neurodevelopment. JAMA psychiatry, 78(9), 1–11. Advance online publication. 10.1001/jamapsychiatry.2021.1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Owens MM, Albaugh MD, Allgaier N, Yuan D, Robert G, Cupertino RB, Spechler PA, Juliano A, Hahn S, Banaschewski T, Bokde A, Desrivières S, Flor H, Grigis A, Gowland P, Heinz A, Brühl R, Martinot JL, Martinot MP, Artiges E, … IMAGEN Consortium (2022). Bayesian causal network modeling suggests adolescent cannabis use accelerates prefrontal cortical thinning. Translational psychiatry, 12(1), 188. 10.1038/s41398-022-01956-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Batalla A, Bhattacharyya S, Yücel M, Fusar-Poli P, Crippa JA, Nogué S, Torrens M, Pujol J, Farré M, & Martin-Santos R (2013). Structural and functional imaging studies in chronic cannabis users: a systematic review of adolescent and adult findings. PloS one, 8(2), e55821. 10.1371/journal.pone.0055821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rubino T, & Parolaro D (2016). The Impact of Exposure to Cannabinoids in Adolescence: Insights From Animal Models. Biological psychiatry, 79(7), 578–585. 10.1016/j.biopsych.2015.07.024 [DOI] [PubMed] [Google Scholar]
- 8.Knapp AA, Lee DC, Borodovsky JT, Auty SG, Gabrielli J, & Budney AJ (2019). Emerging Trends in Cannabis Administration Among Adolescent Cannabis Users. The Journal of adolescent health : official publication of the Society for Adolescent Medicine, 64(4), 487–493. 10.1016/j.jadohealth.2018.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Torrens A, Roy P, Lin L, Vu C, Grimes D, Inshishian VC, Montesinos JS, Ahmed F, Mahler SV, Huestis MA, Das A, & Piomelli D (2022). Comparative Pharmacokinetics of Δ9-Tetrahydrocannabinol in Adolescent and Adult Male and Female Rats. Cannabis and cannabinoid research, 10.1089/can.2021.0205. Advance online publication. 10.1089/can.2021.0205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee HL, Jung KM, Fotio Y, Squire E, Palese F, Lin L, Torrens A, Ahmed F, Mabou Tagne A, Ramirez J, Su S, Wong CR, Jung DH, Scarfone VM, Nguyen PU, Wood M, Green K, & Piomelli D (2022). Frequent Low-Dose Δ9-Tetrahydrocannabinol in Adolescence Disrupts Microglia Homeostasis and Disables Responses to Microbial Infection and Social Stress in Young Adulthood. Biological psychiatry, S0006–3223(22)01237–9. Advance online publication. 10.1016/j.biopsych.2022.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruiz CM, Torrens A, Castillo E, Perrone CR, Cevallos J, Inshishian VC, Harder EV, Justeson DN, Huestis MA, Swarup V, Piomelli D, & Mahler SV (2021a). Pharmacokinetic, behavioral, and brain activity effects of Δ9-tetrahydrocannabinol in adolescent male and female rats. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology, 46(5), 959–969. 10.1038/s41386-020-00839-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Torrens A, Vozella V, Huff H, McNeil B, Ahmed F, Ghidini A, Mahler SV, Huestis MA, Das A, & Piomelli D (2020). Comparative Pharmacokinetics of Δ9-Tetrahydrocannabinol in Adolescent and Adult Male Mice. The Journal of pharmacology and experimental therapeutics, 374(1), 151–160. 10.1124/jpet.120.265892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruiz CM, Torrens A, Lallai V, Castillo E, Manca L, Martinez MX, Justeson DN, Fowler CD, Piomelli D, & Mahler SV (2021b). Pharmacokinetic and pharmacodynamic properties of aerosolized (“vaped”) THC in adolescent male and female rats. Psychopharmacology, 238(12), 3595–3605. 10.1007/s00213-021-05976-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baglot SL, Hume C, Petrie GN, Aukema RJ, Lightfoot S, Grace LM, Zhou R, Parker L, Rho JM, Borgland SL, McLaughlin RJ, Brechenmacher L, & Hill MN (2021). Pharmacokinetics and central accumulation of delta-9-tetrahydrocannabinol (THC) and its bioactive metabolites are influenced by route of administration and sex in rats. Scientific reports, 11(1), 23990. 10.1038/s41598-021-03242-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Javadi-Paydar M, Nguyen JD, Kerr TM, Grant Y, Vandewater SA, Cole M, & Taffe MA (2018). Effects of Δ9-THC and cannabidiol vapor inhalation in male and female rats. Psychopharmacology, 235(9), 2541–2557. 10.1007/s00213-018-4946-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marshell R, Kearney-Ramos T, Brents LK, Hyatt WS, Tai S, Prather PL, & Fantegrossi WE (2014). In vivo effects of synthetic cannabinoids JWH-018 and JWH-073 and phytocannabinoid Δ9-THC in mice: inhalation versus intraperitoneal injection. Pharmacology, biochemistry, and behavior, 124, 40–47. 10.1016/j.pbb.2014.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nguyen JD, Aarde SM, Vandewater SA, Grant Y, Stouffer DG, Parsons LH, Cole M, & Taffe MA (2016). Inhaled delivery of Δ(9)-tetrahydrocannabinol (THC) to rats by e-cigarette vapor technology. Neuropharmacology, 109, 112–120. 10.1016/j.neuropharm.2016.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manwell LA, Ford B, Matthews BA, Heipel H, & Mallet PE (2014). A vapourized Δ(9)-tetrahydrocannabinol (Δ(9)-THC) delivery system part II: comparison of behavioural effects of pulmonary versus parenteral cannabinoid exposure in rodents. Journal of pharmacological and toxicological methods, 70(1), 112–119. 10.1016/j.vascn.2014.06.004 [DOI] [PubMed] [Google Scholar]
- 19.Taffe MA, Creehan KM, Vandewater SA, Kerr TM, & Cole M (2021). Effects of Δ9-tetrahydrocannabinol (THC) vapor inhalation in Sprague-Dawley and Wistar rats. Experimental and clinical psychopharmacology, 29(1), 1–13. 10.1037/pha0000373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freels TG, Baxter-Potter LN, Lugo JM, Glodosky NC, Wright HR, Baglot SL, Petrie GN, Yu Z, Clowers BH, Cuttler C, Fuchs RA, Hill MN, & McLaughlin RJ (2020). Vaporized Cannabis Extracts Have Reinforcing Properties and Support Conditioned Drug-Seeking Behavior in Rats. The Journal of neuroscience : the official journal of the Society for Neuroscience, 40(9), 1897–1908. 10.1523/JNEUROSCI.2416-19.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spencer S, Neuhofer D, Chioma VC, Garcia-Keller C, Schwartz DJ, Allen N, Scofield MD, Ortiz-Ithier T, & Kalivas PW (2018). A Model of Δ9-Tetrahydrocannabinol Self-administration and Reinstatement That Alters Synaptic Plasticity in Nucleus Accumbens. Biological psychiatry, 84(8), 601–610. 10.1016/j.biopsych.2018.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Negus VE (1927). The Function of the Epiglottis. Journal of anatomy, 62(Pt 1), 1–8. [PMC free article] [PubMed] [Google Scholar]
- 23.Johnston M (2003). The importance of lymphatics in cerebrospinal fluid transport. Lymphatic research and biology, 1(1), 41–45. 10.1089/15396850360495682 [DOI] [PubMed] [Google Scholar]
- 24.Bolles RC (1960). Grooming behavior in the rat. Journal of comparative and physiological psychology, 53, 306–310. 10.1037/h0045421 [DOI] [PubMed] [Google Scholar]
- 25.Schweinfurth MK (2020). The social life of Norway rats (Rattus norvegicus). eLife, 9, e54020. 10.7554/eLife.54020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McDougle DR, Kambalyal A, Meling DD, & Das A (2014). Endocannabinoids anandamide and 2-arachidonoylglycerol are substrates for human CYP2J2 epoxygenase. The Journal of pharmacology and experimental therapeutics, 351(3), 616–627. 10.1124/jpet.114.216598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huff HC, Maroutsos D, & Das A (2019). Lipid composition and macromolecular crowding effects on CYP2J2-mediated drug metabolism in nanodiscs. Protein science : a publication of the Protein Society, 28(5), 928–940. 10.1002/pro.3603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vozella V, Zibardi C, Ahmed F, & Piomelli D (2019). Fast and Sensitive Quantification of Δ9-Tetrahydrocannabinol and Its Main Oxidative Metabolites by Liquid Chromatography/Tandem Mass Spectrometry. Cannabis and cannabinoid research, 4(2), 110–123. 10.1089/can.2018.0075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grassin-Delyle S, Buenestado A, Naline E, Faisy C, Blouquit-Laye S, Couderc LJ, Le Guen M, Fischler M, & Devillier P (2012). Intranasal drug delivery: an efficient and non-invasive route for systemic administration: focus on opioids. Pharmacology & therapeutics, 134(3), 366–379. 10.1016/j.pharmthera.2012.03.003 [DOI] [PubMed] [Google Scholar]
- 30.Hložek T, Uttl L, Kadeřábek L, Balíková M, Lhotková E, Horsley RR, Nováková P, Šíchová K, Štefková K, Tylš F, Kuchař M, & Páleníček T (2017). Pharmacokinetic and behavioural profile of THC, CBD, and THC+CBD combination after pulmonary, oral, and subcutaneous administration in rats and confirmation of conversion in vivo of CBD to THC. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology, 27(12), 1223–1237. 10.1016/j.euroneuro.2017.10.037 [DOI] [PubMed] [Google Scholar]
- 31.Lingappan K, Maity S, Jiang W, Wang L, Couroucli X, Veith A, Zhou G, Coarfa C, & Moorthy B (2017). Role of Cytochrome P450 (CYP)1A in Hyperoxic Lung Injury: Analysis of the Transcriptome and Proteome. Scientific reports, 7(1), 642. 10.1038/s41598-017-00516-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thornton-Manning JR, Nikula KJ, Hotchkiss JA, Avila KJ, Rohrbacher KD, Ding X, & Dahl AR (1997). Nasal cytochrome P450 2A: identification, regional localization, and metabolic activity toward hexamethylphosphoramide, a known nasal carcinogen. Toxicology and applied pharmacology, 142(1), 22–30. 10.1006/taap.1996.7975 [DOI] [PubMed] [Google Scholar]
- 33.Christensen HD, Freudenthal RI, Gidley JT, Rosenfeld R, Boegli G, Testino L, Brine DR, Pitt CG, & Wall ME (1971). Activity of delta8- and delta9-tetrahydrocannabinol and related compounds in the mouse. Science (New York, N.Y.), 172(3979), 165–167. 10.1126/science.172.3979.165 [DOI] [PubMed] [Google Scholar]
- 34.Agurell S, Halldin M, Lindgren JE, Ohlsson A, Widman M, Gillespie H, & Hollister L (1986). Pharmacokinetics and metabolism of delta 1-tetrahydrocannabinol and other cannabinoids with emphasis on man. Pharmacological reviews, 38(1), 21–43. [PubMed] [Google Scholar]
- 35.Watanabe K, Narimatsu S, Matsunaga T, Yamamoto I, & Yoshimura H (1993). A cytochrome P450 isozyme having aldehyde oxygenase activity plays a major role in metabolizing cannabinoids by mouse hepatic microsomes. Biochemical pharmacology, 46(3), 405–411. 10.1016/0006-2952(93)90516-y [DOI] [PubMed] [Google Scholar]
- 36.Halldin MM, Widman M, V Bahr C, Lindgren JE, & Martin BR (1982). Identification of in vitro metabolites of delta 1-tetrahydrocannabinol formed by human livers. Drug metabolism and disposition: the biological fate of chemicals, 10(4), 297–301. [PubMed] [Google Scholar]
- 37.Julià P, Farrés J, & Parés X (1987). Characterization of three isoenzymes of rat alcohol dehydrogenase. Tissue distribution and physical and enzymatic properties. European journal of biochemistry, 162(1), 179–189. 10.1111/j.1432-1033.1987.tb10559.x [DOI] [PubMed] [Google Scholar]
- 38.Morris JB (1997). Uptake of acetaldehyde vapor and aldehyde dehydrogenase levels in the upper respiratory tracts of the mouse, rat, hamster, and guinea pig. Fundamental and applied toxicology : official journal of the Society of Toxicology, 35(1), 91–100. 10.1006/faat.1996.2263 [DOI] [PubMed] [Google Scholar]
- 39.Sholler DJ, Strickland JC, Spindle TR, Weerts EM, & Vandrey R (2021). Sex differences in the acute effects of oral and vaporized cannabis among healthy adults. Addiction biology, 26(4), e12968. 10.1111/adb.12968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mu MD, Geng HY, Rong KL, Peng RC, Wang ST, Geng LT, Qian ZM, Yung WH, & Ke Y (2020). A limbic circuitry involved in emotional stress-induced grooming. Nature communications, 11(1), 2261. 10.1038/s41467-020-16203-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rojas-Carvajal M, & Brenes JC (2020). Acute stress differentially affects grooming subtypes and ultrasonic vocalisations in the open-field and home-cage test in rats. Behavioural processes, 176, 104140. 10.1016/j.beproc.2020.104140 [DOI] [PubMed] [Google Scholar]
- 42.Maxwell CJ, Jesdale BM, & Lapane KL (2021). Recent Trends in Cannabis Use in Older Americans. Annals of internal medicine, 174(1), 133–135. 10.7326/M20-0863 [DOI] [PubMed] [Google Scholar]
- 43.Wiley JL, Taylor SI, & Marusich JA (2021). Δ9-Tetrahydrocannabinol discrimination: Effects of route of administration in rats. Drug and alcohol dependence, 225, 108827. 10.1016/j.drugalcdep.2021.108827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nguyen JD, Grant Y, Kerr TM, Gutierrez A, Cole M, & Taffe MA (2018). Tolerance to hypothermic and antinoceptive effects of Δ9-tetrahydrocannabinol (THC) vapor inhalation in rats. Pharmacology, biochemistry, and behavior, 172, 33–38. 10.1016/j.pbb.2018.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sangmo L, Braune T, Liu B, Wang L, Zhang L, Sosnoff CS, Blount BC, & Wilson KM (2021). Secondhand marijuana exposure in a convenience sample of young children in New York City. Pediatric research, 89(4), 905–910. 10.1038/s41390-020-0958-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bergeson PS, & Shaw JC (2001). Are infants really obligatory nasal breathers?. Clinical pediatrics, 40(10), 567–569. 10.1177/000992280104001006 [DOI] [PubMed] [Google Scholar]


