Abstract
BACKGROUND.
Here we report the residual efficacy of the neonicotinoid insecticide Clothianidin against pyrethroid-resistant Aedes aegypti. We first conducted a range-finding evaluation of Clothianidin on three different substrates (wall, wood, cloth) using three doses (100, 300 and 600 mg a.i./m2) and conducting WHO cone bioassays to assess acute (24h) and delayed (up to 7 days) mortality. In experimental houses located in Merida (Mexico) and using free-flying pyrethroid-resistant Ae. aegypti females, we then quantified the acute and delayed mortality after a 24-hour exposure to the targeted indoor residual spraying (TIRS) of two Clothianidin doses (100 and 300 mg a.i./m2).
RESULTS.
Range-finding studies with WHO cones showed low (<50%) acute mortality for all surfaces, doses and times post spraying. Delayed mortality was higher, with average values above or close the 60% mark (and 95% CI estimates crossing 80% for the 600 mg a.i./m2 dose). In experimental houses, a similar low acute mortality was quantified (range of mortality across 12 months, 2–44% for 100 mg a.i./m2 and 8–61% for 300 mg a.i/m2). However, delayed mortality showed a strong effect of Clothianidin on free-flying Ae. aegypti, with values above 80% up to 7 months post-TIRS.
CONCLUSION.
Novel residual insecticide molecules have a promising outlook for Ae. aegypti control and can contribute to the expansion and adoption of TIRS in urban areas. Clothianidin can contribute to the control of resistant Ae. aegypti and provide residual control for up to 7 months after application.
Keywords: neonicotinoid, Aedes, targeted indoor residual spraying, Mexico
Graphical Abstract

Insecticide resistance management in Aedes aegypti depends on the availability of active ingredients to which mosquitoes are susceptible. Here, we report the finding of up to seven months of residual efficacy of the neonicotinoid insecticide Clothianidin (formulated as SumiShield 50WDG) when applied as targeted indoor residual spraying in experimental houses of Merida, Mexico.
1. INTRODUCTION
Routine urban vector control strategies (e.g. truck-mounted ultra-low volume outdoor spraying of adulticides, larviciding) have proven only partially effective to contain dengue epidemics and failed to prevent the expansion of the other emerging Aedes-borne viruses (ABVs; e.g., chikungunya, Zika).(1) Although extensively used, the paradigm of reactive vector control with peridomiciliary ephemeral insecticide-based methods in response to cases/outbreaks(2) and the lack of preventive long-lasting strategies has failed to prevent ABV transmission. The classic deployment of interventions in response to clinical cases fails to account for the important contribution of out-of-home human mobility and asymptomatic infections.(3, 4) Such epidemiological and operational challenges, combined with the occurrence resistance to pyrethroids and other insecticide classes,(5, 6) highlight the need for novel vector control approaches and chemistries for successful ABV management.
Targeted indoor residual spraying (TIRS, preventively applying residual insecticides on Aedes aegypti resting sites such as exposed low walls [<1.5 m], under furniture and on dark surfaces) is a rational vector control approach that exploits Ae. aegypti resting behavior to focalize insecticide applications with no loss in residual efficacy (thus, reducing unnecessary exposure to pesticides in applicators and residents, and the time it takes to spray a premise).(7, 8) Recently published systematic reviews have identified TIRS as a very promising approach for ABV prevention.(9, 10) Mathematical models also predicted strong and sustained reductions in Ae. aegypti and ABV transmission(11, 12) and showed that greater epidemiological efficacy can be achieved as the insecticide residual efficacy increases.(13) TIRS expansion, adoption, and sustainability to the emergence of insecticide resistance will depend on the availability of insecticide molecules and formulations with high residual capacity and to which mosquitoes are susceptible.
Clothianidin ([C(E)]-N-[(2-chloro-5-thiazolyl)methyl]-N′-methyl-N″-nitroguanidine) is a nitroguanidine insecticide within the neonicotinoid class, with a basic mode of action targeting the nicotinic acetylcholine receptor (nAChR) in the insect central nervous system.(14) Clothianidin, applied for indoor residual spraying alone or in combination with other insecticides, is currently used to control malaria vectors as a new approach for managing insecticide resistance as they interact with a biochemical target not previously used in public health.(15, 16) Pre-qualified IRS formulations of clothianidin by the World Health Organization (WHO) have only been recently evaluated for malaria vector control,(17) one developed by Sumitomo (SumiShield 50WG) and another from Bayer has also incorporated clothianidin in a mixture IRS product with deltamethrin (Fludora Fusion WP-SB). Few studies are available on the efficacy of Clothianidin formulations against Aedes mosquitoes,(18) which is important to describe because its potential use to control vectors of ABV, but also because it has a mode of action different from molecules currently employed (v.gr. pyrethroids, organophosphates and carbamates), and because it causes a delayed effect on mortality of mosquitoes which is also different from other insecticides.(19)
The objective of this study was to quantify the residual bio-efficacy of Clothianidin on a pyrethroid-resistant strain of Ae. aegypti under semi-field conditions. Given Clothianidin has lower insecticidal activity on sessile than free-flying insects (19), we experimentally quantified residual efficacy under two conditions: a) by placing WHO cone bioassays on three treated surfaces (wood, cement, and cloth); and b) on free-flying mosquitoes released in experimental houses treated with targeted indoor residual spraying (TIRS) in cement walls, wooden doors and fabric curtains.
2. MATERIALS AND METHODS
2.1. Ethics statement.
This was an experimental study, and because mosquitoes were released into uninhabited houses rented on long-term contracts, we did not require an Institutional Review Board evaluation.
2.2. Experimental Design.
We used a commercial formulation of Clothianidin 50% (SumiShield 50 WDG). Residual efficacy was quantified in two phases. We first conducted a range-finding evaluation of Clothianidin on three different substrates (wall, wood, cloth) using three doses (100, 300 and 600 mg a.i./m2) and WHO cone bioassays to assess acute (24h) and delayed (up to 7 days) mortality. In experimental houses located in Merida (Mexico) and using free-flying pyrethroid-resistant Ae. aegypti females, we then quantified the acute and delayed mortality after a 24-hour exposure to the targeted indoor residual spraying (TIRS) of two Clothianidin doses (100 and 300 mg a.i./m2), as in.(20)
2.2.1. Mosquito strain.
Mosquitoes originated from a pyrethroid-resistant population from Merida (Juan Pablo strain, from F4 generation), used for resistance evolution and operational studies(21) kept at the insectary of Unidad Colaborativa para Bioensayos Entomologicos of the Universidad Autonoma de Yucatan (UCBE-UADY). The strain undergoes periodic mixing with field populations every 6 months and undergoes routine CDC cone bioassays to quantify levels of phenotypic resistance to the four major groups of insecticides (pyrethroid, carbamate, organophosphate, organochlorine).
2.2.2. WHO cone tests.
Given the poorly understood effect of Clothianidin on Ae. aegypti, we conducted a range-finding study using WHO cone bioassays to identify the effect of insecticide dose on acute and delayed mortality. Three substrates measuring 2.50 cm × 1.22 cm were tested (cement wall, black cloth, and wood) using three insecticide doses of 100 mg a.i./m2, 300 mg a.i./m2 and 600 mg a.i./m2 (Fig. 1). The substrates were impregnated following the PAHO/WHO guidelines for indoor residual spraying (described below) using a manual compression sprayer IK-Vector Control Super® (Fig. 1).
Figure 1.

Surfaces used for the range-finding study quantifying the residual efficacy of Clothianidin 50% using WHO cones.
After 24 hours post-impregnation, four WHO cones were fixed and distributed vertically on the center of each substrate/treatment (dose) at a height of 0.5, 1.0, 1.5 and 2.0 meters (Fig. 1). Groups of 10 female Ae. aegypti (3–4-day old non-bloodfed) were exposed per cone. One control (substrate impregnated with water) was also included. After 30 min of exposure on the substrates, mosquitoes were placed in recovery cups and transferred to UCBE-UADY and kept under insectary conditions (fed only with a 10% sucrose solution). Knock-down (KD) at 30 min, acute mortality (24 h post-exposure) and delayed mortality (up to 10 days post-exposure) were recorded. Bioassays and evaluations were repeated at 7- and 30-days post impregnation with a new groups of mosquitoes.
2.2.3. Experimental houses.
Methods were similar as previously described by Che-Mendoza et al. (21). Briefly, three experimental houses (typical middle- to low-income housing) were equally furnished and double-screened with mosquito mesh on outdoor and interior doors and windows to impede mosquito entry/exit (see (20, 21) for more details). Two experimental houses were randomly assigned to one of the following Clothianidin treatments: a) 100 mg a.i./m2 or b) 300 mg a.i./m2. A third house was sprayed with tap water without insecticide and used as a control. TIRS was implemented using an IK-Vector Control Super® (Goizper Group, Antzuola, Spain) manual pump fitted with a mouthpiece 8002EVP and Goizper Low Pressure Control Flow Valve (output pressure 1.5 bar to provide a flow rate of 580 milliliters per minute ± 5%). The following specifications were followed to generate the desired doses: for 100 mg a.i./m2, 1/3 (50 g) sachet was diluted in 7.5 L of water; for 300 mg a.i./m2, 1 sachet was diluted in 7.5 L of water. TIRS was applied to walls below 1.5 m and under furniture or to the undersides of furniture, according to standard TIRS protocols (7, 21).
After 24-h of TIRS application, groups of 3–7-day old female mosquitoes (n=50) were released into each house (bedrooms) and left in the environment for 24 h. After this exposure period, all live mosquitoes were collected using a Prokopack aspirator(22) whereas dead mosquitoes were searched and collected by hand individually.(20, 21) Mosquitoes found alive were placed in recovery cups and transferred to UCBE-UADY insectary (and fed only with a sugar solution). Acute mortality was measured based on the proportion of live and dead mosquitoes in the houses, whereas delayed mortality quantified by monitoring mosquitoes from the collection cups for up to 7 days after collection. We followed this procedure at different times post-TIRS: 15 days and after 1, 2, 5, 7 8 9, 10, 11 and 12 months. Replication on each evaluation period occurred by conducting three consecutive release events on different days within the same week, as described in Che-Mendoza et al.(21)
2.3. Analysis.
For cone tests, mortality from treatments was calculated as the number of dead mosquitoes out of the total mosquitoes tested for each of the four cones. Delayed mortality was quantified as the daily cumulative percentage (from the total of live mosquitoes) by day and up to 10 days post-exposure. Mortality was corrected using Abbott’s formula(23) when mortality in the control was ≥ 5%. Mean and standard error of the mean (SEM) were calculated for each period and compared to the threshold mortality of 80% required for a residual insecticide to be considered efficacious.
For experimental houses, mortality per house was calculated by dividing the number of dead individuals by the number of individuals released for each release period. Missing individuals for all treatments (and the control) were assumed to be dead. Mosquito recovery averaged 98.4 ± 0.78% (Mean ± SEM; n = 99 releases). Acute (24h) mortality in the control group ranged between 2–23% (considering each replicate separately), leading to the calculation of corrected mortalities when appropriate. On each evaluation date, corrected acute mortalities were compared to the 80% threshold. Further, delayed mortalities were compared between treatment and control using binomial generalized linear mixed models (GLMM) in R 4.0.5 statistical software (https://www.r-project.org/) using package lme4. For each date, treatment was classified as fixed effect and time was classified as a random effect. All data generated in this study is available from [MendeleyData URL:DOI].
3. RESULTS
3.1. Range-finding tests for different substrates using WHO cone bioassays.
Acute mortality (24h) and daily mortality during the next 10 days (249h) after 1 day, 7 days and 1 month post-application of Clothianidin 50% on different substrates is shown in Fig. 2. As shown for Clothianidin in malaria vectors, both KD30 and 24-hour mortality were very low regardless of the treated surface and the insecticide dose used, with average 24h mortalities being lower than 50% for all surfaces, doses, and times post spraying (Fig. 2). Delayed mortality was important and led to increases in efficacy over time, with 95% CI estimates crossing the 80% mark for all tested times (1day and up to 30 days) for cement at a dose of 600 mg a.i./m2. Most other doses, surfaces, and times after spraying led to delayed mortalities being above or close to 60% (Fig. 2). A common issue identified with Clothianidin and Chlorfenapyr (21, 24) is that mosquitoes that are not actively flying tend to show much lower mortalities than mosquitoes that are moving in large enclosures. As such, we considered these results preliminary and proceeded to test two doses (100 and 300 mg a.i./m2) in experimental houses to compare their impact on free flying mosquitoes.
Figure 2.
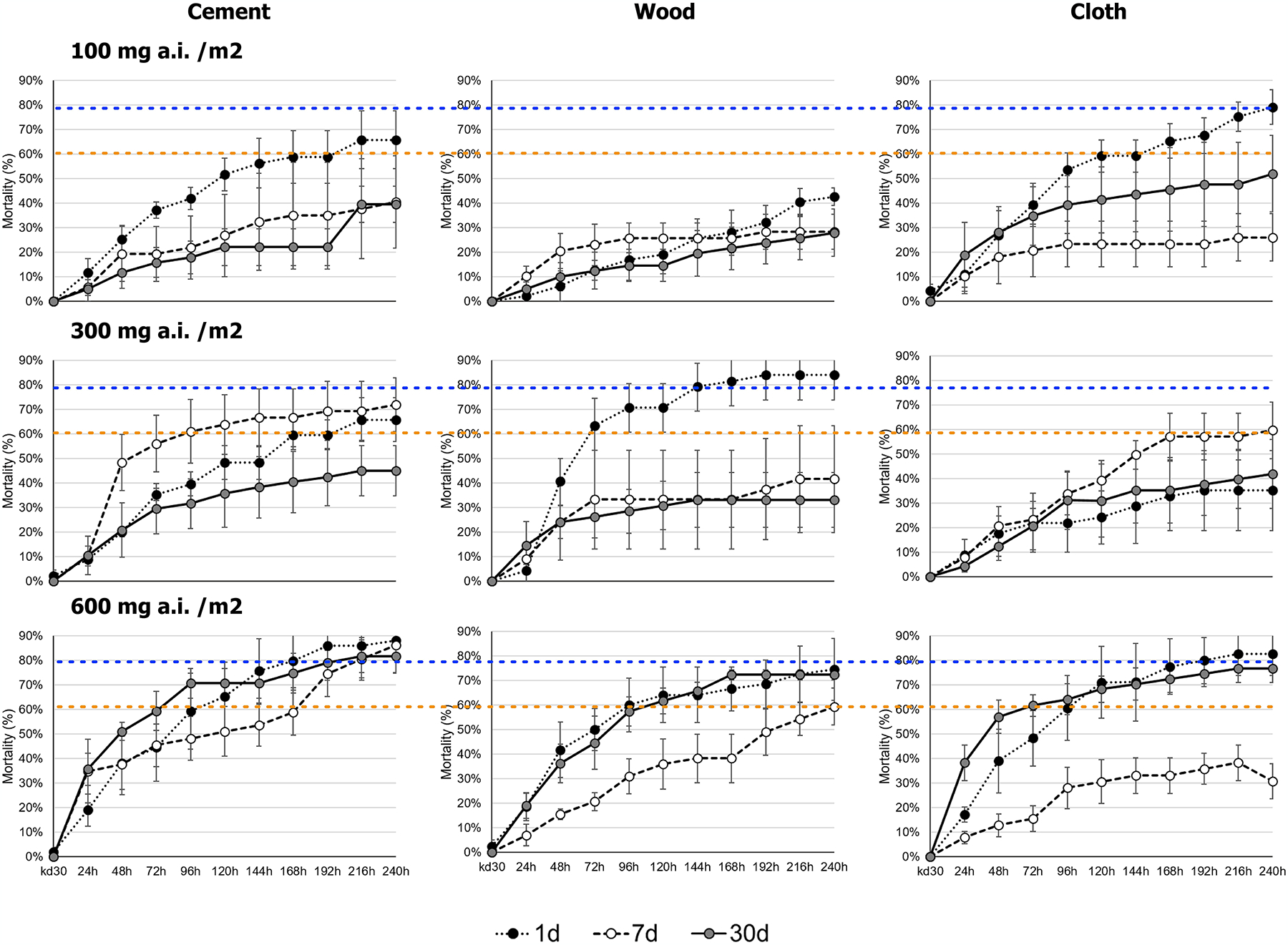
Knock-down, 24-h, and delayed mortality after Ae. aegypti exposure to Clothianidin 50% on different surface materials/substrates using WHO cone tests and different insecticide doses (100, 300 and 600 mg a.i./m2) after 1-, 7- and 30-days post insecticide application.
3.2. Residual efficacy on free-flying Aedes in experimental houses.
At 24 hours post-TIRS, Abbot-corrected acute mortality varied markedly by dose, being 12.9% and 45.8% for 100 and 300 mg a.i./m2, respectively (Fig. 3). For all periods post-TIRS, there was a clear dose-response of Ae. aegypti evident in the lower acute mortality at 100 mg a.i./m2 compared to 300 mg a.i./m2 (Fig. 3). Acute mortality varied widely at 300 mg a.i./m2 throughout the 12-month evaluation, showing a bimodal behavior with peaks at 14 days and 5 months post-TIRS (Fig. 3). Mortality in the control house was always below 20% (Fig. 3). When delayed mortality was accounted for (accumulating acute mortality through seven days after the capture of live mosquitoes from houses), total delayed mortality (100%) was observed after 5 days post-exposure for both insecticide doses at one month after TIRS (Fig. 4). Residual efficacy in producing high (above 80%) delayed mortality was observed up to 5 months for 100 mg a.i./m2 and up to 7 months for 300 mg a.i./m2 (Fig. 4). For most doses and periods post-TIRS, the biggest increase in delayed mortality was observed one day after the recovery of live mosquitoes from houses (Fig. 4). Delayed mortality (measured at 7 days post-recovery of live mosquitoes) was significantly higher in the treated houses compared to the control throughout the duration of the study for both doses (binomial GLMM, t-values 7.5 and 8.5 for 100 mg a.i./m2 and 300 mg a.i. m2, respectively, Table 1). Using the GLMM we predicted the overall (throughout the 12-month period) delayed mortality by treatment, finding that while the natural mosquito mortality in houses averaged 5%, the application of Clothianidin led to an increase of 12-month mortality of 53% mortality for 100 mg a.i./m2 and of 61% for 300 mg a.i./m2 (Fig. 5A). Only for 300 mg a.i./m2 the 95% CI of the predicted 12-month mortality reached 80% (Fig. 5A). The model also confirmed the significant residual efficacy up to 7 months post-TIRS, evidenced by the significantly high random effects (95% CI values being positive and not overlapping with 0) for up to 210 days (Fig. 5B). This interpretation of the random effects statistically confirms the findings from Figure 4, where delayed mortality reached 80% up to 7 months after insecticide application.
Figure 3.
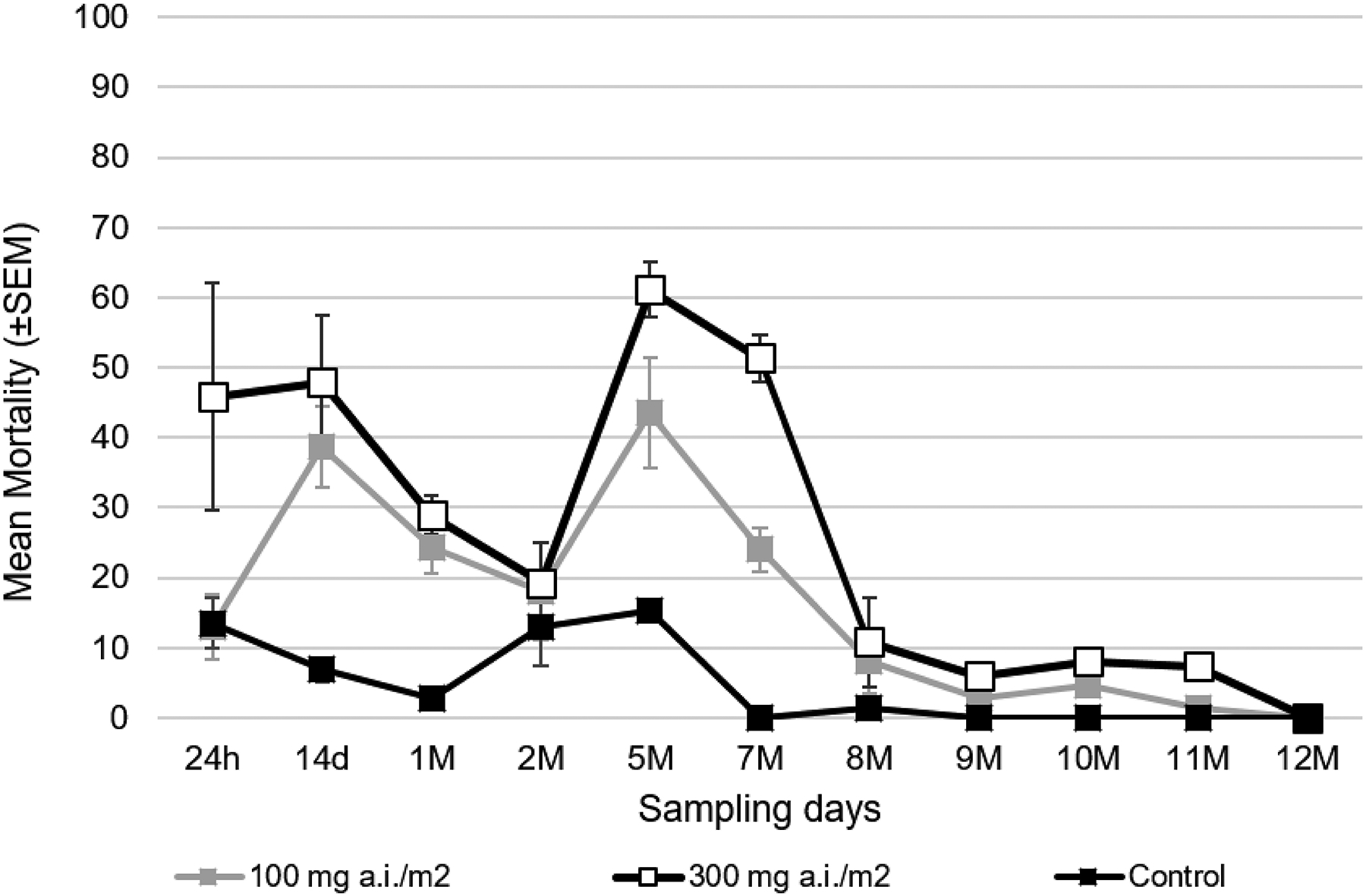
Acute mortality of pyrethroid-resistant Ae. aegypti after TIRS application of Clothianidin 50% at two doses (100 & 300 mg a.i./m2, gray and white squares respectively) compared to a control treated with water (black squares).
Figure 4.
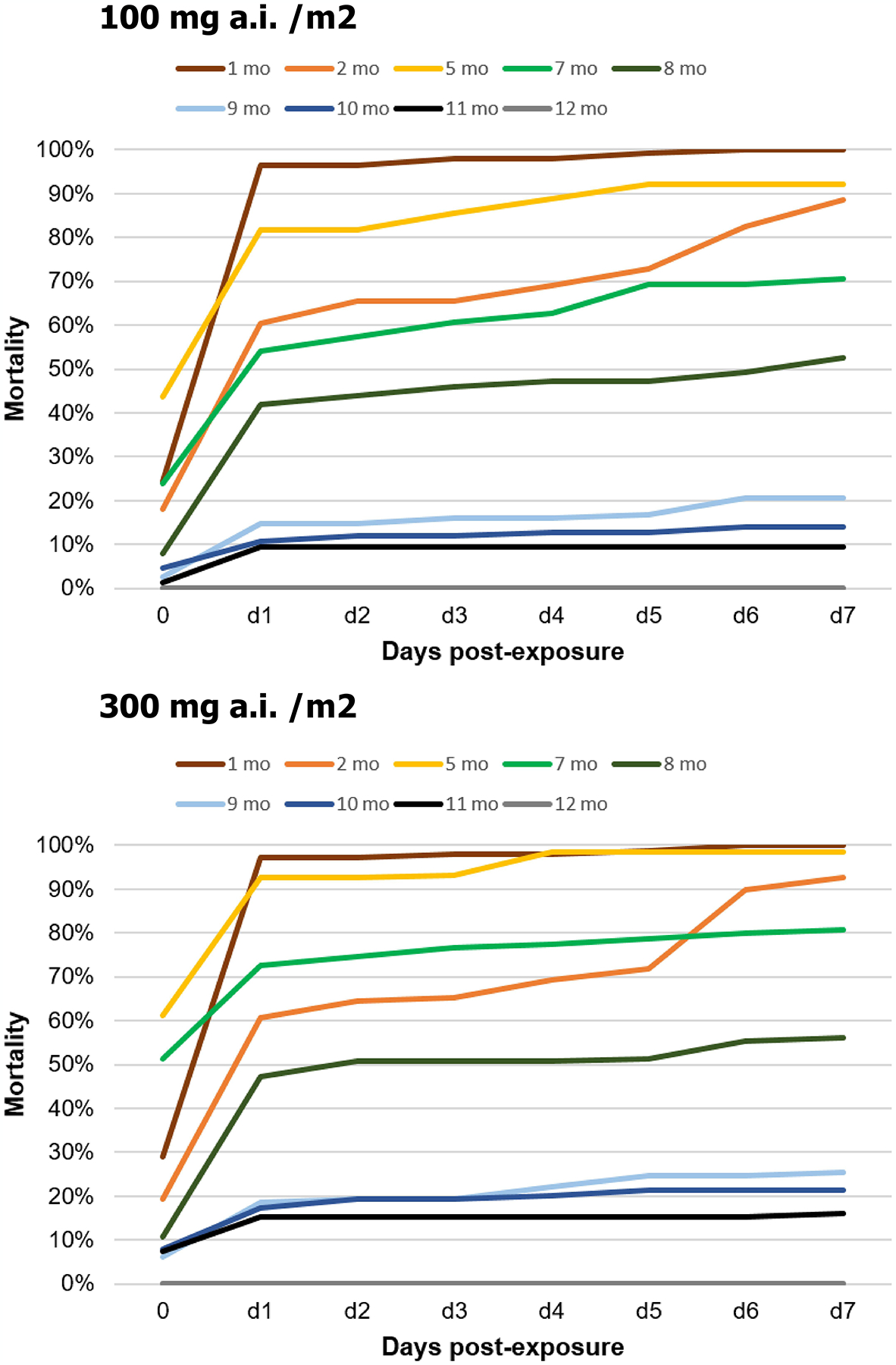
Delayed mortality of Clothianidin 50% applied as TIRS at two doses (100 and 300 mg a.i./m2) in experimental houses from Merida, Mexico. Mortality is expressed as the cumulative percentage of live mosquitoes collected in houses after being exposed for 24h to the insecticide and monitored daily under laboratory conditions for 7 days (d1 to d7). The acute mortality after the 24h exposure in houses is represented as “0”. For the evaluation at 12 months post-TIRS, mortality was 0 throughout all days.
Table 1.
Results from binomial generalized linear mixed model (GLMM) testing the residual efficacy of two doses of Clothianidin 50% applied as TIRS in experimental houses on Ae. aegypti delayed mortality (measured at 7 days post-recovery of live mosquitoes) and analyzed throughout the 12-month period. The control group was set as the reference for the comparison of the two doses.
| Fixed Effects | Estimate | Std. Error | t-value |
|---|---|---|---|
| Intercept | 0.17162 | 0.11184 | 1.534 |
| 100mg a.i./m2 | 0.32626 | 0.04342 | 7.513*** |
| 300mg a.i./m2 | 0.37329 | 0.04342 | 8.597*** |
| *** P<0.001 | |||
| Random Effects | Variance | Std.Dev. | |
| Time | 0.10409 | 0.3226 | |
| Residual | 0.02546 | 0.1595 |
Figure 5.
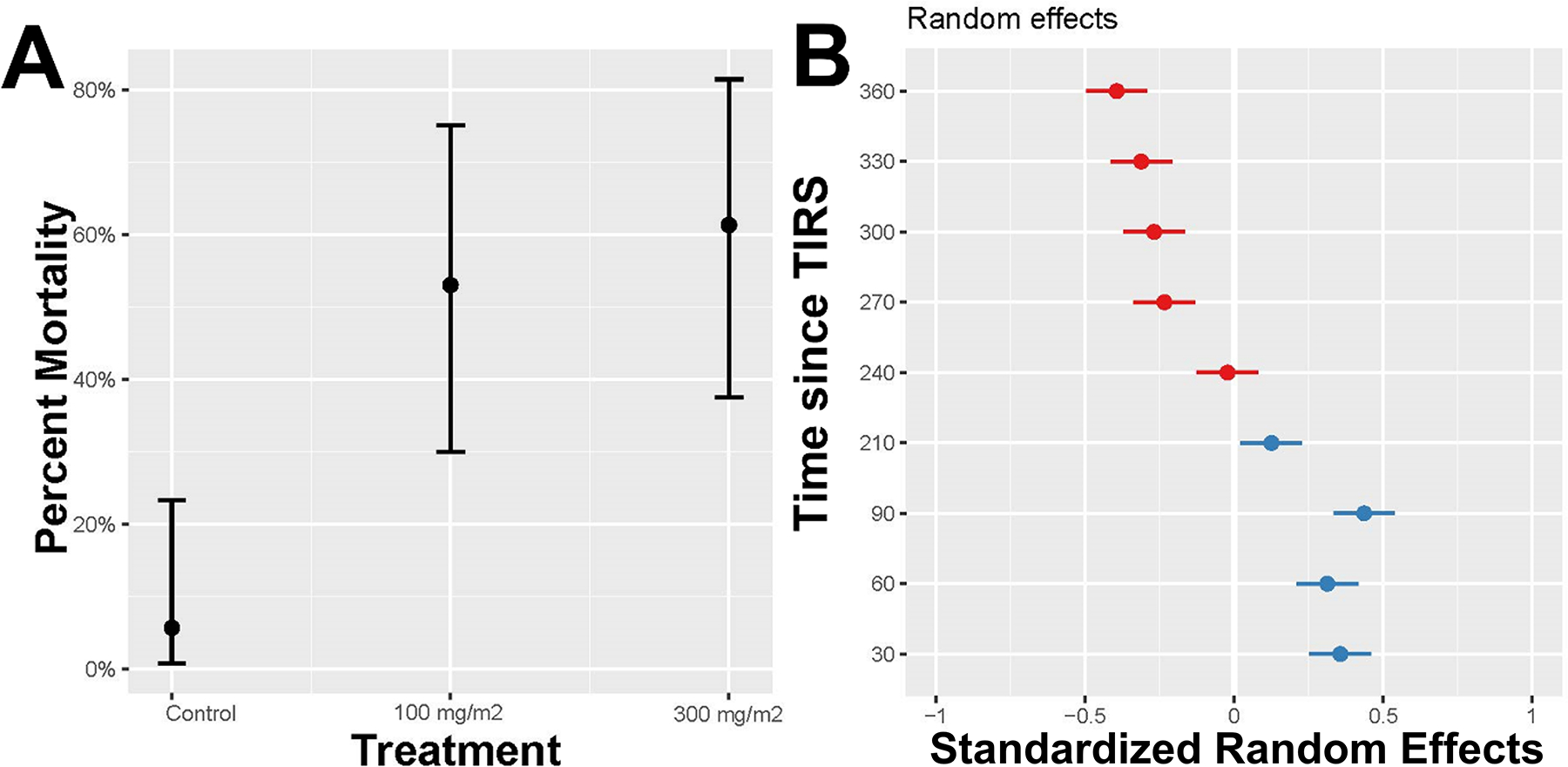
Predicted mortality (from a GLMM) for each treatment, averaged over the 12-month period (A) and plot of the random effects by time since TIRS application, with blue indicating positive effects (higher mortality than the control) and red negative effects (lower than the control). An effect is significant when the confidence bar does not overlap with zero.
4. DISCUSSION
Our study, conducted with pyrethroid-resistant Ae. aegypti, show that Clothianidin 50% is a viable candidate for TIRS in urban areas, after accounting for the delayed mortality effect of this type of insecticide. While acute mortality is low, leading to the false conclusion that this product is not efficacious against Ae. aegypti, the strong 7-day delayed mortality observed up to 7 months post-application in experimental houses provide a different perspective of the efficacy of Clothianidin 50%. The recorded 7-month duration of residual efficacy is much longer than previously recorded in the same experimental houses using bendiocarb (4 months)(20) and similar to the reported residual efficacy of Chlorfenapyr.(21) The results also highlight the utility of delayed mortality assessments for novel insecticide formulations that lie beyond the classic modes of action that lead to strong acute mortality.
A recent article applying Clothianidin 50% on different surfaces and exposing pyrethroid resistant and susceptible Ae. aegypti to different surfaces (cement, mud or wood) using WHO cone assays reported 72-hour mortalities below 50% regardless of the surface(18) Interestingly, the mortalities reported at 1 month for such study (~25%) were slightly lower than the ones we registered for the same surface types (range, 25–40%, Fig. 2). Further, the significant contrast in mortality observed between those studies conducted using WHO cones and our bioassay in experimental houses with free-flying mosquitoes emphasize the need to rethink the approaches to evaluate new insecticide chemistries that deviate from the pyrethroid-centric neurotoxic mode of action. This observation is also backed by prior research conducted in experimental houses in Merida using the pyrrole Chlorfenapyr, which also showed residual efficacy of up to 7 months when delayed mortality was measured.(21)
Clothianidin 50% showed variable efficacy when sprayed in different surfaces, with cement showing the highest mortality for the standard dose, followed by cloth and wood. Cement (plastered or not) is by far the most common housing material in urban areas of Merida and Latin America, so the higher efficacy on those surfaces is encouraging. Furthermore, since we conducted TIRS and that experimental houses are built with painted cement walls and have wooden doors and cloth curtains, the higher mortality observed in them is indicative of Ae. aegypti resting in those surfaces below 1.5 m of height. Whether our measured efficacy would apply to locations in which wood is a primary building material remains to be evaluated.
In experimental houses, we found a bimodal behavior of mosquito mortality over the 12-month evaluation period. We cannot rule out an effect of humidity on the performance of the insecticide as a cause of such unusual behavior. Some studies have reported the effect of atmospheric humidity on the biological activity of the residual insecticides sprayed on the surfaces.(25) An increase to high humidity can result in sufficient migration of insecticide to the surface and increase bioavailability to the mosquito and then increased mortality. Precisely, in our study, the months of evaluation at 2 months (April) and between 5 and 7 months (August and October), corresponded to the dry and rainy seasons, respectively. The increase of humidity during these months might have contributed to the increase of mortality observed in the experimental houses in that period.
Indoor residual spraying, including TIRS, depend on the ability of the insecticide to remain active on treated surfaces for months.(7, 26) What epidemiological gains one may accrue when using insecticides that have longer residuality (for instance, 7 versus 4 months, as our comparison between bendiocarb and Clothianidine) remains a key knowledge gap for residual insecticide applications. Mathematical models evaluating the epidemiological impact of TIRS on dengue found that increasing the residuality of an insecticide from 3 to 5 months would lead to an increase in the effectiveness in preventing transmission but also would allow interventions to begin 1 month earlier.(13) Increasing residuality to 7 months, as it is the case of Clothianidin and Chlorfenapyr,(21) could provide full-season TIRS protection in areas where dengue and other Aedes-borne viruses are seasonal. While estimates of the epidemiological impact of using Clothianidin 50% may remain speculative at this point, the findings from this study provide solid evidence for advancing to a pre-clinical evaluation phase, where entomological field trials quantify the effect of Clothianidin 50% on indoor Ae. aegypti density and ABV infection.
ACKNOWLEDGMENTS
The authors would like to thank Yolanda Carolina Carmona Carballo, Suemy Analí Gutiérrez Martín, Eduardo José Geded Moreno, and Ana Laura Marrufo Tamayo for their dedication and efforts. Thanks are extended to John Lucas, Sumitomo Chemical, for consultation on unique properties of clothianidin prior to study initiation. This project received support from Innovative Vector Control Consortium (Award ID:48835), Emory Global Health Institute and Marcus Foundation (00052002), and partly by the National Institutes of Health, National Institute of Allergy and Infectious Disease (U01AI148069; Vazquez-Prokopec, PI). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
REFERENCES
- 1.Achee NL, Gould F, Perkins TA, Reiner RC Jr., Morrison AC, Ritchie SA, et al. A critical assessment of vector control for dengue prevention. PLoS neglected tropical diseases. 2015;9(5):e0003655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Esu E, Lenhart A, Smith L, Horstick O. Effectiveness of peridomestic space spraying with insecticide on dengue transmission; systematic review. Trop Med Int Health. 2010;15(5):619–31. [DOI] [PubMed] [Google Scholar]
- 3.Ten Bosch QA, Clapham HE, Lambrechts L, Duong V, Buchy P, Althouse BM, et al. Contributions from the silent majority dominate dengue virus transmission. PLoS Pathog. 2018;14(5):e1006965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stoddard ST, Forshey BM, Morrison AC, Paz-Soldan VA, Vazquez-Prokopec GM, Astete H, et al. House-to-house human movement drives dengue virus transmission. Proc Natl Acad Sci U S A. 2013;110(3):994–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guedes RNC, Beins K, Navarro Costa D, Coelho GE, Bezerra H. Patterns of insecticide resistance in Aedes aegypti: meta-analyses of surveys in Latin America and the Caribbean. Pest Manag Sci. 2020;76(6):2144–57. [DOI] [PubMed] [Google Scholar]
- 6.Kuri-Morales PA, Correa-Morales F, Gonzalez-Acosta C, Moreno-Garcia M, Santos-Luna R, Roman-Perez S, et al. Insecticide susceptibility status in Mexican populations of Stegomyia aegypti (= Aedes aegypti): a nationwide assessment. Med Vet Entomol. 2018;32(2):162–74. [DOI] [PubMed] [Google Scholar]
- 7.Pan American Health Organization. Manual for Indoor Residual Spraying in Urban Areas for Aedes aegypti Control. PAHO, editor: Pan American Health Organization; 2019. [Google Scholar]
- 8.Manrique-Saide P, Dean NE, Halloran ME, Longini IM, Collins MH, Waller LA, et al. The TIRS trial: protocol for a cluster randomized controlled trial assessing the efficacy of preventive targeted indoor residual spraying to reduce Aedes-borne viral illnesses in Merida, Mexico. Trials. 2020;21(1):839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ritchie SA, Devine GJ, Vazquez-Prokopec G, Lenhart A, Manrique-Saide P, Scott TW. Insecticide-based approaches for dengue vector control. Innovative strategies for vector control. 6. Wageningen, ND: Wageningen Academic Publishers; 2021. p. 59–89. [Google Scholar]
- 10.Roiz D, Wilson AL, Scott TW, Fonseca DM, Jourdain F, Muller P, et al. Integrated Aedes management for the control of Aedes-borne diseases. PLoS neglected tropical diseases. 2018;12(12):e0006845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cavany SM, Espana G, Lloyd AL, Waller LA, Kitron U, Astete H, et al. Optimizing the deployment of ultra-low volume and indoor residual spraying for dengue outbreak response. PLoS Comput Biol. 2020;16(4):e1007743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hladish TJ, Pearson CAB, Toh KB, Rojas DP, Manrique-Saide P, Vazquez-Prokopec GM, et al. Designing effective control of dengue with combined interventions. Proc Natl Acad Sci U S A. 2020;117(6):3319–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hladish TJ, Pearson CAB, Patricia Rojas D, Gomez-Dantes H, Halloran ME, Vazquez-Prokopec GM, et al. Forecasting the effectiveness of indoor residual spraying for reducing dengue burden. PLoS neglected tropical diseases. 2018;12(6):e0006570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tomizawa M, Casida JE. Neonicotinoid insecticide toxicology: mechanisms of selective action. Annu Rev Pharmacol Toxicol. 2005;45:247–68. [DOI] [PubMed] [Google Scholar]
- 15.Darriet F, Chandre F. Efficacy of six neonicotinoid insecticides alone and in combination with deltamethrin and piperonyl butoxide against pyrethroid-resistant Aedes aegypti and Anopheles gambiae (Diptera: Culicidae). Pest Manag Sci. 2013;69(8):905–10. [DOI] [PubMed] [Google Scholar]
- 16.Zoh MG, Bonneville JM, Tutagata J, Laporte F, Fodjo BK, Mouhamadou CS, et al. Experimental evolution supports the potential of neonicotinoid-pyrethroid combination for managing insecticide resistance in malaria vectors. Sci Rep. 2021;11(1):19501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dagg K, Irish S, Wiegand RE, Shililu J, Yewhalaw D, Messenger LA. Evaluation of toxicity of clothianidin (neonicotinoid) and chlorfenapyr (pyrrole) insecticides and cross-resistance to other public health insecticides in Anopheles arabiensis from Ethiopia. Malar J. 2019;18(1):49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lees RS, Praulins G, Lissenden N, South A, Carson J, Brown F, et al. The Residual Efficacy of SumiShield 50WG and K-Othrine((R)) WG250 IRS Formulations Applied to Different Building Materials against Anopheles and Aedes Mosquitoes. Insects. 2022;13(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ngufor C, Fongnikin A, Rowland M, N’Guessan R. Indoor residual spraying with a mixture of clothianidin (a neonicotinoid insecticide) and deltamethrin provides improved control and long residual activity against pyrethroid resistant Anopheles gambiae sl in Southern Benin. PLoS One. 2017;12(12):e0189575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dunbar MW, Correa-Morales F, Dzul-Manzanilla F, Medina-Barreiro A, Bibiano-Marín W, Morales-Ríos E, et al. Efficacy of Novel Indoor Residual Spraying Methods Targeting Pyrethroid-Resistant Aedes aegypti. PLoS neglected tropical diseases. 2019;13(2):e0007203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Che-Mendoza A, Gonzalez-Olvera G, Medina-Barreiro A, Arisqueta-Chable C, Bibiano-Marin W, Correa-Morales F, et al. Efficacy of targeted indoor residual spraying with the pyrrole insecticide chlorfenapyr against pyrethroid-resistant Aedes aegypti. PLoS neglected tropical diseases. 2021;15(10):e0009822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vazquez-Prokopec GM, Galvin WA, Kelly R, Kitron U. A new, cost-effective, battery-powered aspirator for adult mosquito collections. Journal of medical entomology. 2009;46(6):1256–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abbott WS. A method of computing the effectiveness of an insecticide. 1925. J Am Mosq Control Assoc. 1987;3(2):302–3. [PubMed] [Google Scholar]
- 24.Oxborough RM, N’Guessan R, Jones R, Kitau J, Ngufor C, Malone D, et al. The activity of the pyrrole insecticide chlorfenapyr in mosquito bioassay: towards a more rational testing and screening of non-neurotoxic insecticides for malaria vector control. Malar J. 2015;14:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hadaway AB, Barlow F. The Residual Action of Two Organophosphorus Compounds and a Carbamate on Dried Muds. Bull World Health Organ. 1963;28(1):69–76. [PMC free article] [PubMed] [Google Scholar]
- 26.World Health Organization. Indoor residual spraying. An operational manual for indoor residual spraying (IRS) for malaria transmission control and elimination. WHO, editor. Geneva, Switzerland: 2015. [Google Scholar]


