Abstract
Stroke is a devastating cause of global morbidity and mortality. Ischemic brain injury triggers a profound local and systemic immune response that participates in stroke pathophysiology. In turn, this immune response has emerged as a potential therapeutic target. In order to maximize its therapeutic potential, it is critical to understand how the immune response to ischemic brain injury is affected by age - the strongest non-modifiable risk factor for stroke. The development of multi-omics and single cell technologies has provided a more comprehensive characterization of transcriptional and cellular changes that occur during aging. In this review, we summarize recent advances in our understanding of how age-related immune alterations shape differential stroke outcomes in older versus younger organisms, highlighting studies in both experimental mouse models and patient cohorts. Wherever possible, we emphasize outstanding questions that present important avenues for future investigation with therapeutic value for the aging population.
INTRODUCTION
Stroke is currently the second leading cause of death and third leading cause of disability worldwide [1]. The incidence of ischemic stroke, and its associated mortality and morbidity, increase markedly with age [2–5]. This trend persists despite similar rates in recanalization and hemorrhagic transformation among young and old patients that undergo intravenous thrombolysis and mechanical thrombectomy [6, 7]. Recent insights from experimental mouse models of ischemic stroke have shown that the immune response to brain ischemia actively participates in stroke pathophysiology and impacts outcomes [8–10]. How aging alters the immune response to ischemic brain injury and how this response impacts functional outcomes remains incompletely understood. Furthermore, the extent to which age-related changes in the immune response after ischemic stroke are cell-intrinsic, versus patterned by extrinsic signals derived from the brain and the peripheral microenvironment, remains unclear. Elucidating how neuroimmune interactions evolve with age is critical to effectively harness the immune system as a therapeutic tool in order to reduce injury propagation, enhance tissue repair, and maximize longitudinal functional recovery after ischemic brain injury. Here, we provide a brief overview of recent developments in our understanding of the age-related immunological response to stroke and highlight some open questions for future investigation.
Peripheral factors and resident CNS cells contribute to the proinflammatory signature of the aged brain
Aging is associated with a series of changes in the innate and adaptive immune system that manifest as a decline in immune function - termed “immunosenescence” - and an accumulation of inflammatory factors - termed “inflammaging” [11, 12]. Although our understanding of how immunosenescence and inflammaging impact brain physiology is in its infancy, aging is characterized by a progressive increase in neuroinflammation [13] (Figure 1). Single-cell transcriptomic studies of the central nervous system (CNS) suggest that this signature is largely driven by an expansion of pro-inflammatory subpopulations of both activated microglia and reactive astrocytes (Figure 1C, 1D) [13–17]. However, this signature is not cell-intrinsic. Metabolic restoration of peripheral myeloid cells in aged mice is sufficient to reduce age-related CNS inflammation and memory deficits [18]. Moreover, infusion of young blood and cerebrospinal fluid into aged animals reduces the pro-inflammatory signature of the aging brain and can restore synaptic function [19–21]. Together, these studies demonstrate that peripheral extrinsic factors instruct CNS resident cells to drive maladaptive age-related brain inflammation. Since endothelial cells of the cerebral vasculature and epithelial cells of the choroid plexus form the blood-brain barrier (BBB) and the blood-cerebrospinal fluid barrier (BCSFB) respectively, they have emerged as critical components that can either impede or propagate peripheral inflammatory cues [22–24]. In particular, age-related endothelial cell inflammation (Figure 1A) can provoke vascular dysfunction and contribute to the pathogenesis of cerebrovascular disease, such as ischemic stroke.
Figure 1. Age-related changes in neuro-immune crosstalk.
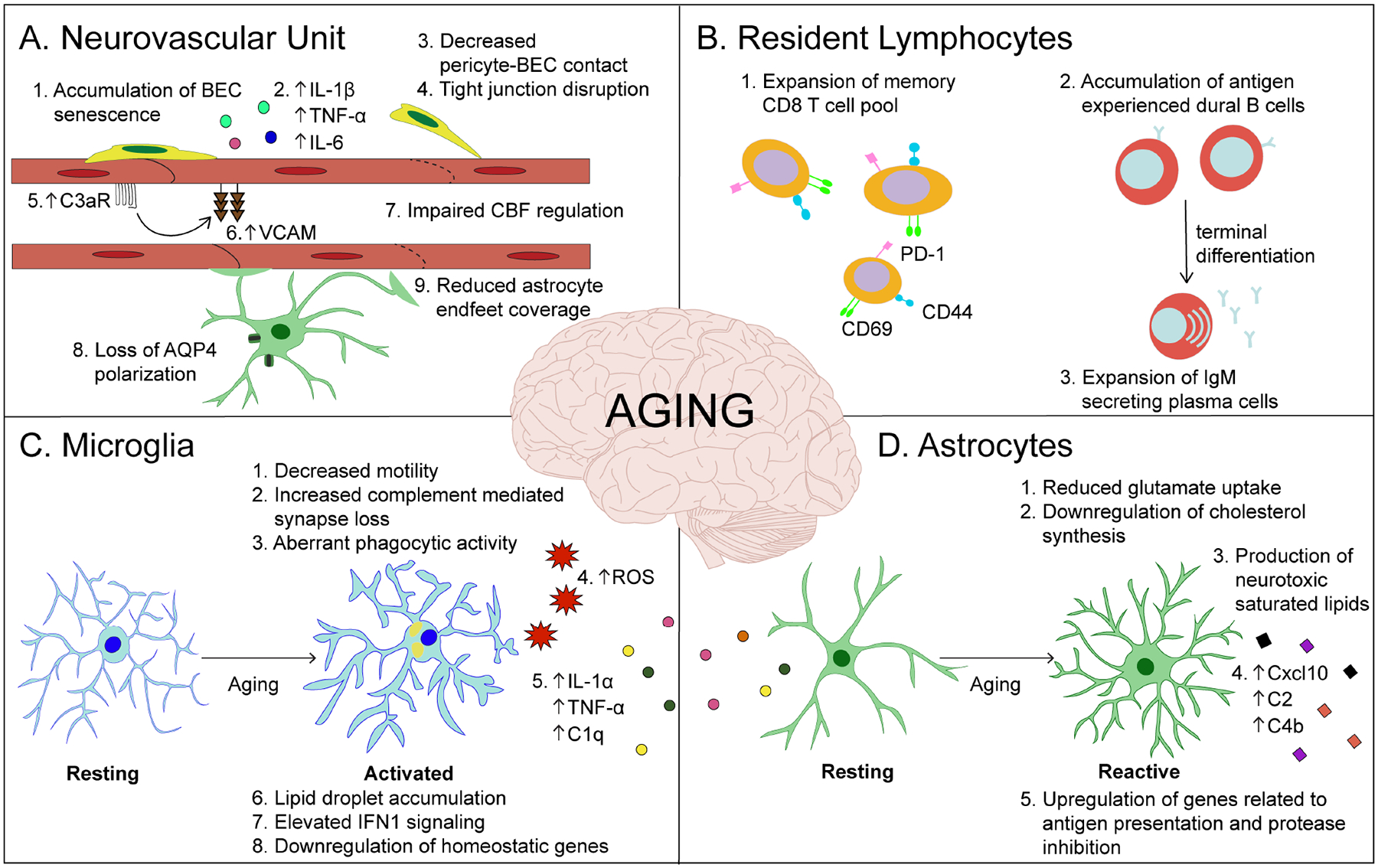
The aging brain shows increased levels of inflammation. A) Aging results in vascular inflammation (red), which is characterized by increased expression of cell adhesion molecules (e.g. VCAM-1), cytokine production, blood-brain barrier permeability and dysregulation of vascular tone - all of which contribute to age-related atherosclerosis, hypertension, and stroke. B) There is a prominent increase in activated memory CD8+ T cells (orange) in the aged brain. Aged dura shows a distinct population of antigen experienced B cells (red) with an expansion of IgM plasma cells. C) Aged microglia (light blue) upregulate expression of proinflammatory cytokines and type one interferon (IFN1) signaling, downregulate homeostatic genes and accumulate lipid droplets. Functionally, aged microglia exhibit a reduced phagocytic capacity and motility. Microglia-derived C3 complement component mediates age-dependent synapse loss in neurons. Activated microglia further produce IL-1α, TNFα, and C1q, which are necessary and sufficient to induce a neuroinflammatory reactive astrocyte phenotype (green) exacerbated in the aged brain. D) Reactive astrocytes (green) in the aged brain significantly upregulate C3, C4b, Cxcl10, GFAP, Serpina3n and major histocompatibility complex (MHC) class I genes. The production of complement and saturated lipids by reactive astrocytes, as well as a downregulation of cholesterol synthesis, favor synapse elimination in the aged brain.
Transcriptomic characterization of age-related immune responses in ischemic stroke
Ischemic stroke triggers upregulation of selectins on brain endothelial cells [BECs; e.g. Vascular Cell Adhesion Molecule (VCAM)], which promote leukocyte adhesion (Figure 2; red cells) [8]. Ischemia-related BBB disruption allows danger associated molecular patterns (DAMPs), released from necrotic neurons, to enter the bloodstream and cerebrospinal fluid [25, 26]. DAMPs can further recruit immune cells directly through binding to pattern recognition receptors in the periphery, or indirectly through local activation and subsequent secretion of cytokines from CNS resident cells (Figure 2). Following this initial phase, there is T cell apoptosis and immunosuppression which predisposes patients to systemic infection [27, 28]. Thus, ischemic brain injury initiates profound changes in both local and systemic immune function.
Figure 2. The immune response to ischemic stroke in the aged brain.
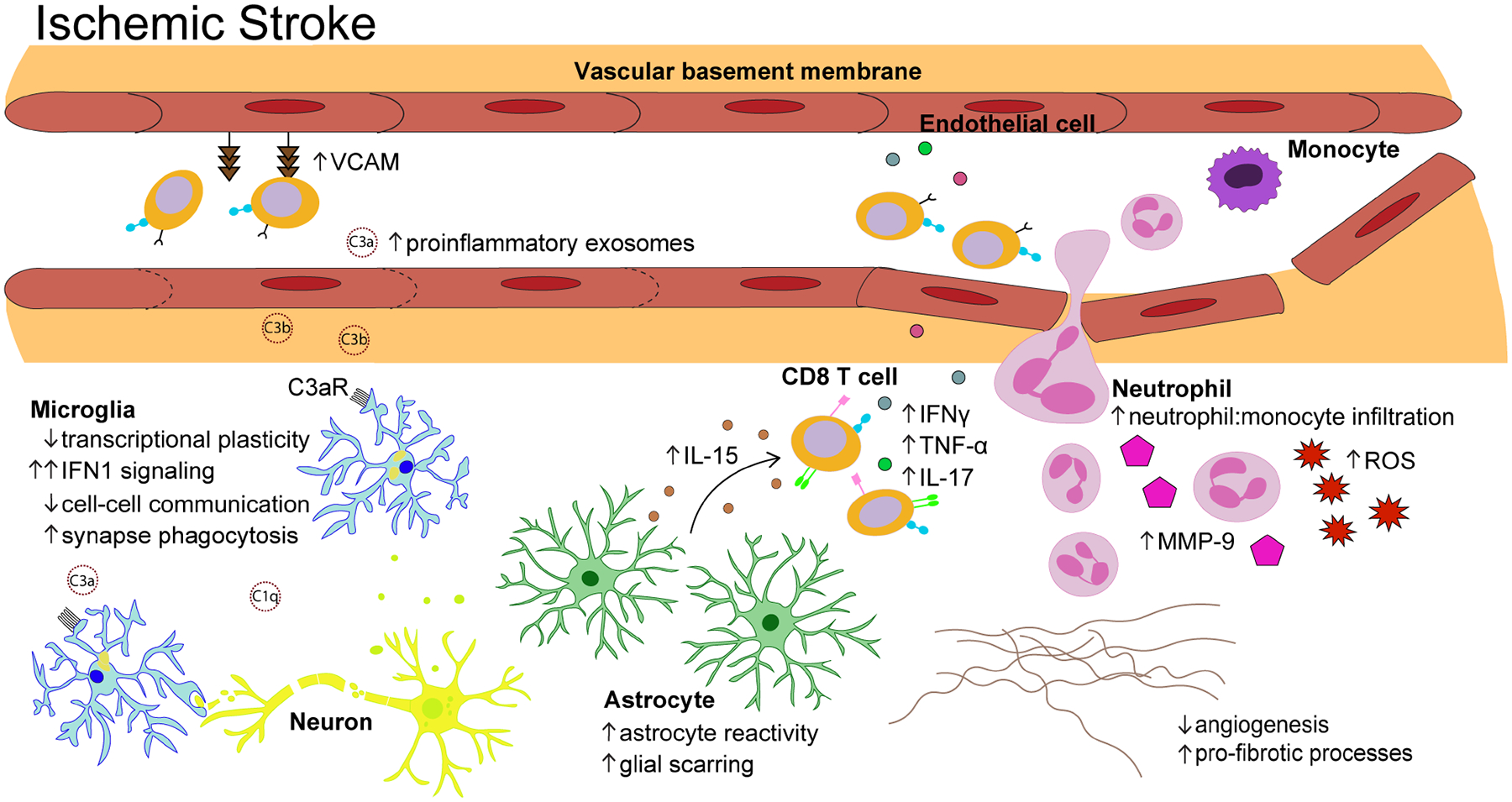
Following ischemic stroke, aged microglia (light blue) demonstrate a blunted transcriptional response dominated by genes associated with IFN1 signaling. The complement components that infiltrate the brain from serum exosomes bind to C3a receptors on aged microglia and trigger excessive synapse phagocytosis. Astrocyte reactivity (green) is exacerbated after ischemic stroke, leading to accelerated glial scar formation. Ischemic injury triggers aged astrocytes to significantly upregulate IL-15 which promotes further accumulation of CD8+ T cells (orange) that recruit other peripheral immune cells through secretion of proinflammatory cytokines. The ratio of neutrophils (pink) to monocytes (purple) is significantly increased in the aged brain after ischemic stroke and infiltrating neutrophils are highly metabolically active, secreting large amounts of matrix metalloproteinase (MMP)-9 and reactive oxygen species (ROS). Genes related to angiogenesis and extracellular matrix (ECM) remodeling (e.g. Col3a1, Col6a1, Pdgfrb, Lox, Angptl4, Ecm, Mmp12, Eln) are significantly downregulated in the aged brain relative to that observed in young animals, indicative of reduced vascular remodeling.
Recent transcriptomic studies in animal models have provided quantitative characterization of cell types and immune signaling pathways that are differentially affected by aging after ischemic stroke (Figure 2). A recent study using single cell RNA sequencing characterized the immune landscape in the aged mouse brain 72 hours after transient middle cerebral artery occlusion (t-MCAO), a mouse model for ischemic stroke [29]. Similar to findings reported in young animals after stroke, the frequency of aged microglia expressing homeostatic genes decreases after stroke and the aged microglia shift toward a highly proliferative state [29, 30]. However, aged microglia (Figure 2, light blue cells) are less responsive to ischemic injury, showing only 18 upregulated and 40 downregulated genes, as compared to young microglia which upregulated 250 and downregulated 21 genes after ischemic stroke [31, 32]. This lack of transcriptional plasticity may be a consequence of downregulation of purinergic receptors on aged microglia which normally respond to purines released after neuronal injury in ischemic stroke [33]. Yet, the functional implications of a dampened microglia response to injury are challenging to interpret given that resting aged microglia exhibit a heightened proinflammatory transcriptional signature compared to their young counterparts, thus resembling more “activated” cells (Figure 1C) [14, 31]. Do age-related inflammatory changes “prime” microglia prior to ischemic insult, or do aged microglia exhibit a senescence-associated phenotype that disrupts restoration of tissue homeostasis after ischemic injury? Single cell RNA sequencing studies have revealed that genes related not only to inflammatory processes, but also cell-cell communication, angiogenesis, and extracellular matrix (ECM) regulation are significantly upregulated in young compared to aged microglia after ischemic stroke [31]. Changes in microglia-mediated ECM remodeling may provide mechanistic insight into why ischemic injury preferentially pushes the aged brain towards fibrotic rather than regenerative processes (Figure 2) [34]. Studies in young mice have further demonstrated that upregulation of genes related to microglial phagocytosis and lipid metabolism modulate the clearance of lipid-rich tissue debris after ischemic stroke which is critical for functional recovery [35–37]. A recent study identified the presence of lipid droplet-accumulating microglia (LDAM) in the resting aged brain, which are defective in phagocytosis and secrete higher levels of proinflammatory cytokines and reactive oxygen species (ROS; Figure 1C) [16]. Future pharmacological and genetic ablation experiments will be useful to determine how LDAMs contribute to the blunted microglial response observed in aged animals after ischemic brain injury.
A striking hallmark of the transcriptional response to ischemic stroke in aged animals is the upregulation of type I interferon signaling (IFN1) in microglia (Figure 2, light blue cells) and oligodendrocytes [38]. This IFN1 response differs from that of young animals in both its magnitude and temporal profile, with persistent elevation in aged animals 14 days after stroke. Although the functional implications of this response remain to be determined, these findings align with previous data which identified chronically elevated IFN1 signaling in the aged brain (Figure 1C) [22, 39]. Importantly, blocking IFN1 signaling in aged mice (either pharmacologically or through ablation of the IFN1 receptor on microglia) attenuates maladaptive neuroinflammation and cognitive decline associated with normal aging [22, 39]. Moreover, the persistent elevation of IFNI signaling in the aged brain after ischemic insult suggests that the therapeutic window for immunomodulation after ischemic stroke may be age-dependent. Elongation of the therapeutic window in older patients has massive clinical implications; yet the majority of rodent studies focus on the first 72 hours after stroke. It will be crucial for future studies to precisely map how acute age-related alterations in immune function evolve after ischemic stroke and how this trajectory impacts longitudinal restoration of brain homeostasis. Longitudinal studies in aged animals are of particular relevance given that in the first year after ischemic stroke elderly patients show an initial improvement in cognition that is then followed by a significant decline in cognitive function [40]. Critically, older age and recurrent stroke (rather than stroke severity or other vascular risk factors) are associated with an increased risk and accelerated presentation of cognitive decline [40].
Age-related immune alterations contribute to differential stroke outcomes
It is challenging to bridge the mechanistic gap between RNA sequencing data and ischemic stroke outcomes. Furthermore, cells (particularly of the innate immune system) are exceedingly sensitive to in vitro artifacts induced during tissue dissociation and cell isolation [41]. This ex vivo gene expression signature can distort the interpretation of functional responses and obscure age-related changes. Therefore, following identification of age-related transcriptional alterations after ischemic stroke, it is necessary to validate them in vivo and obtain mechanistic insight to establish their role in disease pathogenesis. To what degree does the immune alteration reflect a cell-intrinsic change due to aging versus a consequence of extrinsic factors in the aging microenvironment? Does the immune alteration give rise to differential functional outcomes after ischemic stroke? Elucidating these questions is critical for identifying points of therapeutic intervention. Recent studies using experimental animal models have begun to examine these questions and demonstrate that age-related immune alterations after ischemic stroke 1) represent changes in both cell-intrinsic and extrinsic properties and 2) have a causal role in functional outcomes.
The peripheral environment modulates age-related immune response to ischemic stroke
Transplantation of young bone marrow into aged mice reduces the number of brain-infiltrating neutrophils, occurrence of hemorrhagic transformation and severity of behavioral deficits after t-MCAO [32]. Conversely, transplantation of old bone marrow into young mice increases the number of brain-infiltrating neutrophils, reduces microglia phagocytosis and exacerbates behavioral deficits after t-MCAO. A similar rejuvenating effect can be recapitulated by transfusion of young blood and splenectomy [42, 43]. Replacement of aged blood 7 hours after t- MCAO with whole blood obtained from young mice results in a significant reduction in neurological deficits, peripheral neutrophilia, brain infiltrating neutrophils, and a decrease in matrix metalloproteinase-9 (MMP-9) levels [43]. The rescue effect of young blood is diminished upon the addition of MMP-9 which is highly expressed by brain-infiltrating neutrophils in aged mice (Figure 2) and has been associated with an increased risk of morbidity and mortality in ischemic stroke patients [29, 32, 43, 44]. Transfusion of young blood further: a) reduces circulating DAMPs released by the ischemic brain; b) removes activated aged leukocytes and other deleterious signals such as cytokines; and c) introduces young soluble factors that bathe barrier compartments – all of which may contribute to its overall neuroprotective effect.
Gut microbiome abnormalities and autonomic nervous system (ANS) dysfunction contribute to age-related systemic inflammation and stroke outcomes [45–48]. Importantly, the transfer of young doner microbiota to aged mice decreases systemic inflammation, morbidity, and mortality after t-MCAO [49]. Peripheral exosomes have also emerged as novel regulators of the age-related immune response to ischemic stroke. A recent study found that levels of serum exosomal complement components (C1q, C3a, and C3b) are increased with age and can cross the BBB, accumulate in the penumbra, and drive worse functional outcomes after ischemic stroke [50]. Complement present in aged exosomes exacerbates microglial activation and triggers excessive synapse phagocytosis (Figure 2). Strikingly, the delivery of young exosomes into aged rats after ischemic stroke significantly reduces infarct volume size, cognitive deficits, and sensorimotor deficits. Interestingly, C3a has further been implicated as a mediator of age-related vascular inflammation, lymphocyte infiltration, and BBB permeability through C3a receptor signaling on brain endothelial cells (Figure 1A) [51].
Together, these studies demonstrate that rejuvenation of the systemic environment can rescue maladaptive features of both chronic age-related neuroinflammation (discussed under “Peripheral factors and resident CNS cells contribute to the proinflammatory signature of the aged brain”) and the acute age-related immune response to ischemic brain injury. These findings have important therapeutic implications. Although the Plasma for Alzheimer Symptom Amelioration (PLASMA) Study has demonstrated the tolerability and safety of repetitive plasma transfusions, a clinical trial examining the therapeutic benefit of young plasma transfusion after ischemic stroke has not been performed [52]. Furthermore, it remains to be determined how age-related changes in other border compartments such as the meninges and cerebrospinal fluid prime the inflammatory response to ischemic injury in the aged brain [37].
The number and function of brain infiltrating immune cells impacts age-related stroke outcomes
The cellular immune response to ischemic stroke changes significantly with aging. Whereas monocytes make up a significantly larger proportion of immune cells migrating into the young brain after ischemic stroke, neutrophils (Figure 2, pink cells), known to produce high levels of ROS and MMPs, dominate the cellular response to ischemic stroke in the aged brain [29, 32]. Neutrophil depletion through anti-Ly6G treatment significantly improves long-term functional outcomes after ischemic stroke in aged, but not young, mice [53]. These findings suggest a unique age-dependent pathogenicity of the neutrophil response to ischemic stroke which may be caused by increased levels of the neutrophil activating cytokine, IL-6, or decreased levels of the bone marrow retention chemokine, CXCL12 [53]. Neutrophil accumulation may be amplified by age-associated microglia dysfunction, as microglial phagocytic activity in young mice controls the expansion of neutrophils beyond the infarct core [54]. Moreover, increased production of MMP-9 (Figure 2, pink pentagons) by aged neutrophils exacerbates BBB damage and may be responsible for worse age-related functional outcomes [32, 55]. However, brain infiltrating pro-inflammatory monocytes also play a crucial role in post-ischemic angiogenesis and tissue remodeling [56, 57]. Therefore, the skewed neutrophil to monocyte ratio in aged animals may contribute to failed functional recovery through promotion of pro-fibrotic processes (Figure 2). Understanding the mechanisms regulating fibrotic scar tissue formation after CNS ischemic injury and its impact on functional recovery will be an important point of future investigation in the aged brain [58–60].
Consistent with findings in young mice, microglia depletion prior to t-MCAO results in an increased number of brain-infiltrating immune cells and larger infarction size in aged mice [61]. Therefore, despite their unique age-dependent transcriptional profile, microglia beneficially contribute to ischemic stroke outcomes in both young and aged animals. A recent study using conditional phagocytic receptor knockout mice reveals that while microglia phagocytosis mediates the beneficial removal of debris after ischemic stroke, it also results in the detrimental removal of synapses [62]. These findings emphasize the need for future studies to use genetic strategies to assess the contribution of specific signaling pathways in aged microglia after ischemic brain injury.
Resident lymphocytes have emerged as another immune population that modulates functional outcomes after ischemic stroke [63, 64]. Aging is associated with an increased number of CNS resident memory CD8+ T cells (CD44+) and dural antigen experienced B cells (Figure 1B) [63, 65, 66]. This expanded population of CD8+ T cells (Figure 2, orange cells) amplifies ischemic brain injury through IL-15 mediated production of pro-inflammatory cytokines (IFNγ, TNFα, and IL-17) which increase peripheral immune cell recruitment [63, 65]. Both depletion of CD8+ T cells and ablation of IL-15 in astrocytes (Figure 2, green cells) attenuates ischemic brain damage in aged mice and improves functional outcomes [67]. However, it is unclear how an accumulation of CD8+ T cells and B cells in the aged brain may longitudinally promote or suppress auto-reactive responses to self-antigens following ischemic tissue injury. Moreover, it remains to be determined whether the persistent accumulation of CD8+ T cells in the aged brain after ischemic injury represents an increase in recruitment, or local proliferation of an already expanded resident population. In contrast, recruitment of regulatory T cells to sites of tissue damage is severely dampened with age [68, 69]. This age-related decrease in recruitment is of particular interest given that regulatory T cells in young mice promote functional recovery after ischemic stroke by enhancing microglia-mediated white matter repair and suppressing neurotoxic astrogliosis [70, 71].
Overall, these studies demonstrate that age-related immune alterations contribute to differential experimental stroke outcomes that can be modulated through targeting of distinct immune components. It will be important for future studies to further investigate the potential immunomodulatory functions of non-classical immune cells. For example, emerging evidence supports the capacity of endothelial cells to facilitate immune homeostasis beyond regulation of immune cell recruitment [72]. Age-associated changes in brain endothelial cell inflammatory signaling suggest a potential age-specific immunomodulatory role of the neurovasculature that may be of therapeutic importance for ischemic stroke risk and functional outcomes [23, 24].
Identifying functionally-relevant age-dependent therapeutic targets in stroke patients
Animal models have been indispensable for elucidating how the immune response to brain ischemia has a causal role in ischemic stroke outcomes. However, many immunomodulatory drugs found to be effective in experimental stroke have failed in clinical trials [8]. The predominance of young animals in preclinical studies has likely contributed to the lack of clinical translation for a disease that occurs largely in an aged population. Furthermore, there are significant drawbacks of studying neuroimmune interactions in mice that have an underdeveloped immune system due to clean pathogen-free living conditions in the laboratory [73, 74]. Lastly, poor functional outcomes in older patients with ischemic stroke can be partially explained by age-related comorbidities such as hyperlipidemia, diabetes mellitus, and hypertension [75–77]. These comorbidities have not been widely incorporated in preclinical ischemic stroke models; thus, it is critical that experimental findings are validated in patient cohorts [78, 79]
Recent cohort studies have characterized the acute and longitudinal peripheral immune response to ischemic stroke. The magnitude of the innate immune response in the acute period is associated with poorer cognitive outcomes, while an increase in peripheral regulatory T cells is associated with improved functional outcomes and smaller infarction size [80, 81]. Older age is associated with an increase in peripheral IL-6, soluble tumor necrosis factor receptor I, and neutrophil-lymphocyte ratio in patients with both ST-segment-elevation myocardial infarction and acute ischemic stroke [82]. This data suggests a potentially unified systemic immune response to ischemic tissue injury that is age-dependent. Transcriptomic data further supports age-related changes in lymphocyte function after ischemic stroke and suggests that B cells may play a unique role in age-related stroke outcomes [83, 84].
It is necessary to distinguish between chronological (time passed since birth) and biological (level of physiological functioning) aging [85]. While the chronological age of women at stroke onset is older than men, DNA methylation profiles at time of stroke onset do not differ [86]. Understanding how sex-specific epigenetic aging impacts downstream physiology, such as the immune response to ischemic brain injury, will be of significant therapeutic value. It will also be important to investigate how epigenetic changes mediated by psychosocial factors modify the process of aging itself and the risk for age-related disease [87]. Recent studies highlight the complexity of “aging” which differs across individuals and within different cells of a single individual [88]. They emphasize the need for future studies to incorporate multidimensional and longitudinal immune profiling after ischemic stroke to validate age-dependent signatures. Incorporating this depth and temporal dimension with stroke outcomes in individuals of different ages with different genotypes, comorbidities, and environmental exposures will best inform risk prediction and drug discovery.
CONCLUDING REMARKS
Considerable progress has been made in identifying how aging alters the immune response to ischemic stroke. An improved understanding of how age-related immune alterations causally influence stroke risk and functional outcomes will have a substantial clinical impact. Recent multi-omics profiling studies demonstrate the complexity by which the immune system remodels during aging. Leveraging the data derived from patient cohorts as testable hypotheses in experimental animal models will elucidate to what degree age-related immune alterations represent correlative or causative regulators of stroke pathophysiology. This future work is needed to establish a robust framework for the development of effective immunotherapeutic strategies in stroke.
HIGHLIGHTS.
Omics approaches have expanded our understanding of immune aging and the immune response to ischemic stroke
Age-related intrinsic and extrinsic changes shape the immune response to ischemic stroke
The immune response to ischemic stroke contributes to differential outcomes in young and old hosts
Targeting age-dependent immune alterations after ischemic stroke has therapeutic potential
FUNDING
Mary Claire Tuohy and Dritan Agalliu are supported by grants from the NIH/NIMH (R01 MH112849), NIH/NINDS (R21 NS118891, F31 NS130983-01), NIH/NHLBI (R61HL159949), NIH/NEI (R01EY033994), and the National Multiple Sclerosis (MS) Society (RG-1901-33218). Elizabeth Hillman is supported by grants from the NIH/NIMH (RF1 MH114276) and NIH/NINDS (RO1 NS063226). Randolph Marshall is supported by grants from the NIH/NINDS (R01NS097876, U01NS095869, U24NS107237).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
COMPETING INTERESTS
The authors declare that the research was conducted without any commercial or financial relationships that can be construed as potential conflicts of interest.
REFERENCES
- 1.Collaborators, G.B.D.S., Global, regional, and national burden of stroke and its risk factors, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol, 2021. 20(10): p. 795–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bamford J, et al. , A prospective study of acute cerebrovascular disease in the community: the Oxfordshire Community Stroke Project--1981–86. 2. Incidence, case fatality rates and overall outcome at one year of cerebral infarction, primary intracerebral and subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry, 1990. 53(1): p. 16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonita R, Solomon N, and Broad JB, Prevalence of stroke and stroke-related disability. Estimates from the Auckland stroke studies. Stroke, 1997. 28(10): p. 1898–902. [DOI] [PubMed] [Google Scholar]
- 4.Kammersgaard LP, et al. , Short- and long-term prognosis for very old stroke patients. The Copenhagen Stroke Study. Age Ageing, 2004. 33(2): p. 149–54. [DOI] [PubMed] [Google Scholar]
- 5.Rothwell PM, et al. , Population-based study of event-rate, incidence, case fatality, and mortality for all acute vascular events in all arterial territories (Oxford Vascular Study). Lancet, 2005. 366(9499): p. 1773–83. [DOI] [PubMed] [Google Scholar]
- 6.Kim D, et al. , Intra-arterial thrombolysis for acute stroke in patients 80 and older: a comparison of results in patients younger than 80 years. AJNR Am J Neuroradiol, 2007. 28(1): p. 159–63. [PMC free article] [PubMed] [Google Scholar]
- 7.Furlan NE, et al. , The Impact of Age on Mortality and Disability in Patients With Ischemic Stroke Who Underwent Cerebral Reperfusion Therapy: A Brazilian Cohort Study. Front Aging Neurosci, 2021. 13: p. 649902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iadecola C, Buckwalter MS, and Anrather J, Immune responses to stroke: mechanisms, modulation, and therapeutic potential. J Clin Invest, 2020. 130(6): p. 2777–2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doyle KP and Buckwalter MS, Immunological mechanisms in poststroke dementia. Curr Opin Neurol, 2020. 33(1): p. 30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Endres M, et al. , Immune Pathways in Etiology, Acute Phase, and Chronic Sequelae of Ischemic Stroke. Circ Res, 2022. 130(8): p. 1167–1186. [DOI] [PubMed] [Google Scholar]
- 11.Panda A, et al. , Human innate immunosenescence: causes and consequences for immunity in old age. Trends Immunol, 2009. 30(7): p. 325–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Franceschi C, et al. , Inflammaging: a new immune-metabolic viewpoint for age-related diseases. Nat Rev Endocrinol, 2018. 14(10): p. 576–590. [DOI] [PubMed] [Google Scholar]
- 13.Schaum N, et al. , Ageing hallmarks exhibit organ-specific temporal signatures. Nature, 2020. 583(7817): p. 596–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ximerakis M, et al. , Single-cell transcriptomic profiling of the aging mouse brain. Nat Neurosci, 2019. 22(10): p. 1696–1708. [DOI] [PubMed] [Google Scholar]; * This study performs a single-cell transcriptomic analysis of young and old mouse brains to develop a dataset of age-related changes in gene expression and ligand-receptor interactions. They find that while age-related genes are differentially regulated across different cell types, a common hallmark of aging across cell populations is inflammation, mitochondrial dysfunction, and loss of proteostasis.
- 15.Tabula Muris C, A single-cell transcriptomic atlas characterizes ageing tissues in the mouse. Nature, 2020. 583(7817): p. 590–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marschallinger J, et al. , Lipid-droplet-accumulating microglia represent a dysfunctional and proinflammatory state in the aging brain. Nat Neurosci, 2020. 23(2): p. 194–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clarke LE, et al. , Normal aging induces A1-like astrocyte reactivity. Proc Natl Acad Sci U S A, 2018. 115(8): p. E1896–E1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Minhas PS, et al. , Restoring metabolism of myeloid cells reverses cognitive decline in ageing. Nature, 2021. 590(7844): p. 122–128. [DOI] [PMC free article] [PubMed] [Google Scholar]; * This study finds that increased prostaglandin E2-EP2 receptor signaling in aged myeloid cells reduces glucose flux and mitochondrial respiration, resulting in maladaptive systemic and brain inflammation. They show that administration of a brain-penetrant EP2 antagonist or brain-impermeant EP2 antagonist are both sufficient to rescue age-related inflammation and cognitive deficits in aged mice.
- 19.Katsimpardi L, et al. , Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors. Science, 2014. 344(6184): p. 630–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Villeda SA, et al. , Young blood reverses age-related impairments in cognitive function and synaptic plasticity in mice. Nat Med, 2014. 20(6): p. 659–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iram T, et al. , Young CSF restores oligodendrogenesis and memory in aged mice via Fgf17. Nature, 2022. 605(7910): p. 509–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baruch K, et al. , Aging. Aging-induced type I interferon response at the choroid plexus negatively affects brain function. Science, 2014. 346(6205): p. 89–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yousef H, et al. , Aged blood impairs hippocampal neural precursor activity and activates microglia via brain endothelial cell VCAM1. Nat Med, 2019. 25(6): p. 988–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]; * This study finds that aged plasma upregulates vascular cell adhesion molecule 1 (VCAM1) on BECs, which impairs neural precursor cell activity and increases microglia reactivity. Genetic ablation or pharmacological inhibition of VCAM1 prevents detrimental effects of aged plasma in young mice and rescues cogntive impairments in aged mice. These findings provide mechanistic insight into earlier observations that systemic factors promoting rejuvenation or aging can modulate brain function and highlight the critical position of BECs at the interface of systemic and brain aging.
- 24.Chen MB, et al. , Brain Endothelial Cells Are Exquisite Sensors of Age-Related Circulatory Cues. Cell Rep, 2020. 30(13): p. 4418–4432 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liesz A, et al. , DAMP signaling is a key pathway inducing immune modulation after brain injury. J Neurosci, 2015. 35(2): p. 583–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Esposito E, et al. , Brain-to-cervical lymph node signaling after stroke. Nat Commun, 2019. 10(1): p. 5306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prass K, et al. , Stroke-induced immunodeficiency promotes spontaneous bacterial infections and is mediated by sympathetic activation reversal by poststroke T helper cell type 1-like immunostimulation. J Exp Med, 2003. 198(5): p. 725–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roth S, et al. , Post-injury immunosuppression and secondary infections are caused by an AIM2 inflammasome-driven signaling cascade. Immunity, 2021. 54(4): p. 648–659 e8. [DOI] [PubMed] [Google Scholar]
- 29.Li X, et al. , Single-cell transcriptomic analysis of the immune cell landscape in the aged mouse brain after ischemic stroke. J Neuroinflammation, 2022. 19(1): p. 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zheng K, et al. , Single-cell RNA-seq reveals the transcriptional landscape in ischemic stroke. J Cereb Blood Flow Metab, 2022. 42(1): p. 56–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shi L, et al. , Genome-wide transcriptomic analysis of microglia reveals impaired responses in aged mice after cerebral ischemia. J Cereb Blood Flow Metab, 2020. 40(1_suppl): p. S49–S66. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** This study explores aging-associated transcriptional alterations in microglia five days after ischemic stroke in young and aged mice. The authors find that the transcriptional reponse to brain ischemia in aged microglia is severely blunted compared to young microglia and implicates maladaptive age-related changes in post stroke angiogenesis, extracellular matrix reorganization, and cell-cell communication.
- 32.Ritzel RM, et al. , Aging alters the immunological response to ischemic stroke. Acta Neuropathol, 2018. 136(1): p. 89–110. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** This study finds that neutrophil brain infiltration after ischemic stroke is increased in aged mice relative to young mice. Through flow cytometry and bone marrow chimera experiments, the authors identify that aged neutrophils produce greater levels of ROS and MMP-9, leading to exacerbation of hemorrhagic transformation in aged mice which can be rescued through reconstitution of young bone marrow.
- 33.Hickman SE, et al. , The microglial sensome revealed by direct RNA sequencing. Nat Neurosci, 2013. 16(12): p. 1896–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buga AM, et al. , Transcriptomics of post-stroke angiogenesis in the aged brain. Front Aging Neurosci, 2014. 6: p. 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kawabori M, et al. , Triggering receptor expressed on myeloid cells 2 (TREM2) deficiency attenuates phagocytic activities of microglia and exacerbates ischemic damage in experimental stroke. J Neurosci, 2015. 35(8): p. 3384–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kurisu K, et al. , Triggering receptor expressed on myeloid cells-2 expression in the brain is required for maximal phagocytic activity and improved neurological outcomes following experimental stroke. J Cereb Blood Flow Metab, 2019. 39(10): p. 1906–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beuker C, et al. , Stroke induces disease-specific myeloid cells in the brain parenchyma and pia. Nat Commun, 2022. 13(1): p. 945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Androvic P, et al. , Decoding the Transcriptional Response to Ischemic Stroke in Young and Aged Mouse Brain. Cell Rep, 2020. 31(11): p. 107777. [DOI] [PubMed] [Google Scholar]; ** This is one of the first studies to longitudinally assess differential gene expression in young and aged mice after ischemic stroke. The authors find that IFN1 signaling is highly upregulated in aged animals and suggest that this neuroinflammatory signature may exacerbate neuronal injury after ischemic stroke in an age-dependent manner.
- 39.Deczkowska A, et al. , Mef2C restrains microglial inflammatory response and is lost in brain ageing in an IFN-I-dependent manner. Nat Commun, 2017. 8(1): p. 717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lo JW, et al. , Long-Term Cognitive Decline After Stroke: An Individual Participant Data Meta-Analysis. Stroke, 2022. 53(4): p. 1318–1327. [DOI] [PubMed] [Google Scholar]
- 41.Marsh SE, et al. , Dissection of artifactual and confounding glial signatures by single-cell sequencing of mouse and human brain. Nat Neurosci, 2022. 25(3): p. 306–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chauhan A, et al. , Splenectomy protects aged mice from injury after experimental stroke. Neurobiol Aging, 2018. 61: p. 102–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ren X, et al. , Blood substitution therapy rescues the brain of mice from ischemic damage. Nat Commun, 2020. 11(1): p. 4078. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Using a murine t-MCAO stroke model, this study demonstrates that transfusion of relatively young mouse blood in the acute period after ischemic injury reduces infarct volume and improves neurological deficits. A reduction in brain infiltrating neutrophils and MMP-9 is identified as a potential neuroprotective mechanism of post stroke blood replacement therapy.
- 44.Zhong C, et al. , Serum matrix metalloproteinase-9 levels and prognosis of acute ischemic stroke. Neurology, 2017. 89(8): p. 805–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kenney MJ and Ganta CK, Autonomic nervous system and immune system interactions. Compr Physiol, 2014. 4(3): p. 1177–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bosco N and Noti M, The aging gut microbiome and its impact on host immunity. Genes Immun, 2021. 22(5–6): p. 289–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Honarpisheh P, Bryan RM, and McCullough LD, Aging Microbiota-Gut-Brain Axis in Stroke Risk and Outcome. Circ Res, 2022. 130(8): p. 1112–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhu L, et al. , Interactions between the Autonomic Nervous System and the Immune System after Stroke. Compr Physiol, 2022. 12(3): p. 3665–3704. [DOI] [PubMed] [Google Scholar]
- 49.Spychala MS, et al. , Age-related changes in the gut microbiota influence systemic inflammation and stroke outcome. Ann Neurol, 2018. 84(1): p. 23–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang H, et al. , Circulating Pro-Inflammatory Exosomes Worsen Stroke Outcomes in Aging. Circ Res, 2021. 129(7): p. e121–e140. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** This study demonstrates that aging is associated with an increase in serum exosomal complement components which exacerbate microglia-mediated synapse phagocytosis after ischemic stroke. The authors find that substituting exosomes in aged rats with exosomes from young rats improves post stroke functional recovery.
- 51.Propson NE, et al. , Endothelial C3a receptor mediates vascular inflammation and blood-brain barrier permeability during aging. J Clin Invest, 2021. 131(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sha SJ, et al. , Safety, Tolerability, and Feasibility of Young Plasma Infusion in the Plasma for Alzheimer Symptom Amelioration Study: A Randomized Clinical Trial. JAMA Neurol, 2019. 76(1): p. 35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roy-O’Reilly MA, et al. , Aging exacerbates neutrophil pathogenicity in ischemic stroke. Aging (Albany NY), 2020. 12(1): p. 436–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Otxoa-de-Amezaga A, et al. , Microglial cell loss after ischemic stroke favors brain neutrophil accumulation. Acta Neuropathol, 2019. 137(2): p. 321–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rosell A, et al. , MMP-9-positive neutrophil infiltration is associated to blood-brain barrier breakdown and basal lamina type IV collagen degradation during hemorrhagic transformation after human ischemic stroke. Stroke, 2008. 39(4): p. 1121–6. [DOI] [PubMed] [Google Scholar]
- 56.Mastorakos P, et al. , Temporally distinct myeloid cell responses mediate damage and repair after cerebrovascular injury. Nat Neurosci, 2021. 24(2): p. 245–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pedragosa J, et al. , CCR2 deficiency in monocytes impairs angiogenesis and functional recovery after ischemic stroke in mice. J Cereb Blood Flow Metab, 2020. 40(1_suppl): p. S98–S116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dorrier CE, et al. , CNS fibroblasts form a fibrotic scar in response to immune cell infiltration. Nat Neurosci, 2021. 24(2): p. 234–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dias DO and Goritz C, Fibrotic scarring following lesions to the central nervous system. Matrix Biol, 2018. 68–69: p. 561–570. [DOI] [PubMed] [Google Scholar]
- 60.Dias DO, et al. , Pericyte-derived fibrotic scarring is conserved across diverse central nervous system lesions. Nat Commun, 2021. 12(1): p. 5501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Marino Lee S, et al. , Microglia depletion increase brain injury after acute ischemic stroke in aged mice. Exp Neurol, 2021. 336: p. 113530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shi X, et al. , Stroke subtype-dependent synapse elimination by reactive gliosis in mice. Nat Commun, 2021. 12(1): p. 6943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ritzel RM, et al. , Age-Associated Resident Memory CD8 T Cells in the Central Nervous System Are Primed To Potentiate Inflammation after Ischemic Brain Injury. J Immunol, 2016. 196(8): p. 3318–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liesz A, et al. , Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nat Med, 2009. 15(2): p. 192–9. [DOI] [PubMed] [Google Scholar]
- 65.Selvaraj UM, et al. , Delayed diapedesis of CD8 T cells contributes to long-term pathology after ischemic stroke in male mice. Brain Behav Immun, 2021. 95: p. 502–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brioschi S, et al. , Heterogeneity of meningeal B cells reveals a lymphopoietic niche at the CNS borders. Science, 2021. 373(6553). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li M, et al. , Astrocyte-derived interleukin-15 exacerbates ischemic brain injury via propagation of cellular immunity. Proc Natl Acad Sci U S A, 2017. 114(3): p. E396–E405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Panduro M, Benoist C, and Mathis D, Tissue Tregs. Annu Rev Immunol, 2016. 34: p. 609–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Guo Z, et al. , DCAF1 regulates Treg senescence via the ROS axis during immunological aging. J Clin Invest, 2020. 130(11): p. 5893–5908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shi L, et al. , Treg cell-derived osteopontin promotes microglia-mediated white matter repair after ischemic stroke. Immunity, 2021. 54(7): p. 1527–1542 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ito M, et al. , Brain regulatory T cells suppress astrogliosis and potentiate neurological recovery. Nature, 2019. 565(7738): p. 246–250. [DOI] [PubMed] [Google Scholar]
- 72.Amersfoort J, Eelen G, and Carmeliet P, Immunomodulation by endothelial cells - partnering up with the immune system? Nat Rev Immunol, 2022. 22(9): p. 576–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Beura LK, et al. , Normalizing the environment recapitulates adult human immune traits in laboratory mice. Nature, 2016. 532(7600): p. 512–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tao L and Reese TA, Making Mouse Models That Reflect Human Immune Responses. Trends Immunol, 2017. 38(3): p. 181–193. [DOI] [PubMed] [Google Scholar]
- 75.Di Carlo A, et al. , Stroke in the very old : clinical presentation and determinants of 3-month functional outcome: A European perspective. European BIOMED Study of Stroke Care Group. Stroke, 1999. 30(11): p. 2313–9. [DOI] [PubMed] [Google Scholar]
- 76.Sharma JC, Fletcher S, and Vassallo M, Strokes in the elderly - higher acute and 3-month mortality - an explanation. Cerebrovasc Dis, 1999. 9(1): p. 2–9. [DOI] [PubMed] [Google Scholar]
- 77.Knoflach M, et al. , Functional recovery after ischemic stroke--a matter of age: data from the Austrian Stroke Unit Registry. Neurology, 2012. 78(4): p. 279–85. [DOI] [PubMed] [Google Scholar]
- 78.Reeson P, et al. , Delayed inhibition of VEGF signaling after stroke attenuates blood-brain barrier breakdown and improves functional recovery in a comorbidity-dependent manner. J Neurosci, 2015. 35(13): p. 5128–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mehina EMF, et al. , Invasion of phagocytic Galectin 3 expressing macrophages in the diabetic brain disrupts vascular repair. Sci Adv, 2021. 7(34). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Santamaria-Cadavid M, et al. , Regulatory T cells participate in the recovery of ischemic stroke patients. BMC Neurol, 2020. 20(1): p. 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tsai AS, et al. , A year-long immune profile of the systemic response in acute stroke survivors. Brain, 2019. 142(4): p. 978–991. [DOI] [PMC free article] [PubMed] [Google Scholar]; * This study uses single-cell mass cytometry to characterize the systemic immune response in patients over the course of one year after ischemic stroke and identify immune correlates of post-stroke cognitive outcomes.
- 82.Bochaton T, et al. , Impact of Age on Systemic Inflammatory Profile of Patients With ST-Segment-Elevation Myocardial Infarction and Acute Ischemic Stroke. Stroke, 2022. 53(7): p. 2249–2259. [DOI] [PubMed] [Google Scholar]
- 83.Sykes GP, et al. , Aging Immune System in Acute Ischemic Stroke: A Transcriptomic Analysis. Stroke, 2021. 52(4): p. 1355–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** This study assesses the transcriptional profile of peripheral leukocytes in relationship to age in patients with ischemic stroke.
- 84.Korf JM, et al. , CD11b(high) B Cells Increase after Stroke and Regulate Microglia. J Immunol, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rutledge J, Oh H, and Wyss-Coray T, Measuring biological age using omics data. Nat Rev Genet, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gallego-Fabrega C, et al. , Biological Age Acceleration Is Lower in Women With Ischemic Stroke Compared to Men. Stroke, 2022. 53(7): p. 2320–2330. [DOI] [PubMed] [Google Scholar]; * This study uses DNA methylation to evaluate biological age of men and women at time of ischemic stroke. Authors find that while women’s chronological age at time of stroke is older than men, there is no difference in biological age - demonstrating sex-dependent age acceleration in stroke patients.
- 87.McEwen BS, Allostasis and the Epigenetics of Brain and Body Health Over the Life Course: The Brain on Stress. JAMA Psychiatry, 2017. 74(6): p. 551–552. [DOI] [PubMed] [Google Scholar]
- 88.Sayed N, et al. , An inflammatory aging clock (iAge) based on deep learning tracks multimorbidity, immunosenescence, frailty and cardiovascular aging. Nat Aging, 2021. 1: p. 598–615. [DOI] [PMC free article] [PubMed] [Google Scholar]


