Abstract
Functional cellular structures with controllable mechanical and morphological properties are of great interest for applications including tissue engineering, energy storage, and aerospace. Additive manufacturing (AM), also referred to as 3D printing, has enabled the potential for fabrication of functional porous scaffolds (i.e., meta-biomaterials) with controlled geometrical, morphological, and mechanical properties. Understanding the biomechanical behavior of 3D printed porous scaffolds under physiologically relevant loading and environmental conditions is crucial in accurately predicting the in vivo performance. This study was aimed to investigate the environmental dependency of the mechanical responses of 3D printed porous scaffolds of poly(methyl methacrylate) (PMMA) Class IIa biomaterial that was based on triply periodic minimal surfaces – TPMS (i.e., Primitive and Schoen-IWP). The 3D printed scaffolds (n = 5/study group) were tested under compressive loading in both ambient and fluidic (distilled water with pH = 7.4) environments according to ASTM D1621 standards. Outcomes of this study showed that compressive properties of the developed scaffolds are significantly lower in the fluidic condition than the ambient environment for the same topological and morphological group (p ≤ 0.023). Additionally, compressive properties and flexural stiffness of the studied scaffolds were within the range of trabecular bone’s properties, for both topological classes. Relationships between predicted mechanical responses and morphological properties (i.e., porosity) were evaluated for each topological class. Quantitative correlation analysis indicated that mechanical behavior of the developed 3D printed scaffolds can be controlled based on both topology and morphology.
Keywords: Biomaterials, Additive manufacturing, Bone tissue regeneration, Mechanical responses, Body fluidic environment
1. Introduction
Inherently, the 3D porous scaffolds are aimed at better imitating the mechanical and biological characteristics of native tissue. The emergence of additive manufacturing (AM), also known as 3D printing, has unprecedentedly facilitated the control of mechanical and biological properties of porous scaffolds through multi-length-scale design and fabrication. 3D printing has also helped expedite the incorporation of topological optimization into scaffold design (Lynch et al., 2018; Tilton et al., 2021; Wang et al., 2016; Zadpoor, 2019). As an example, studies have utilized topological optimization for spatial tuning of mechanical (Lynch et al., 2018; Tilton et al., 2021; Wang et al., 2016; Zadpoor, 2019), biological (Karageorgiou and Ã, 2005; Li et al., 2020), mass transport (Bobbert et al., 2017), or combination of these properties of porous scaffolds for tissue regeneration. AM techniques have also facilitated the design of 3D functional structures tailored to tissue-specific properties for various biomedical applications (Zaszczyńska et al., 2021). Few of such examples include 3D printed retina and skin equivalent constructs (Solari et al., 2020) and 3D reconstruction of auricular cartilage structures and complex skull base structures post-surgery (Zaszczyńska et al., 2021). Particularly, 3D bioprinting, in which cells are encapsulated into the polymeric resin, allow for the fabrication of patient-specific constructs that mimic biological and anatomical structures for a range of biomedical applications including disease modeling, drug testing, and various therapeutic uses (Cidonio et al., 2019; Kim et al., 2020, 2012). The majority of the previous studies have used mechanical properties obtained in an ambient environment (i.e., dry-state and at room temperature) to understand only in vitro mechanical performance of the scaffolds. However, mechanical properties obtained under such boundary conditions are considerably different from those under the physiological condition, i.e., in vivo due to changes in temperature and medium. In the course of characterizing the mechanical properties of biomaterials for tissue engineering application, it is challenging to perform experiments using in-vivo animal models which could be extremely costly, particularly, at the early screening stages of the material (or scaffold) design. This also applies to assessing other biological and structural properties of biomaterials. Moreover, employing animal models in the research and development cycle is strictly controlled and is only allowed at the final development stages of the biomaterial (Fontana et al., 2021; Miramini et al., 2020; Mogil, 2009).
It is also important to recognize that mechanical responses are contingent on the type of biomaterial employed in developing the 3D porous scaffold and its performance in application-relevant environment. For instance, in the case of hydrophilic biomaterials, such as hydrogels, it has been shown that mechanical loading in aqueous environment results in substantial loss of compressive modulus (Hosaka et al., 2007). There has been considerable exploration of 3D porous scaffold of hydrophobic polymeric materials such as poly(methyl methacrylate) (PMMA), polydimethylsiloxane (PDMS), polycaprolactone (PCL), polyglycolic acid (PGA), and polylactic acid (PLA) (Bose et al., 2018). 3D printed PMMA hybrid composite resins have also been studied and optimized to harbor unique properties, such as antibacterial effects, with either comparable or enhanced mechanical properties (Chen et al., 2019; Marin et al., 2021). However, there has not been a clear understanding of the mechanical responses of such hydrophobic polymeric biomaterials under environment that is similar to their application domain, i.e., body fluidic aqueous environment. PMMA is one of the most widely used biocompatible hydrophobic biomaterials in orthopedics, orthodontics, and tissue engineering as both augmenting and injectable materials (Liu et al., 2019; Rezaei et al., 2020; Shimko and Nauman, 2006). Commercially available PMMA formula is primarily consisted of a liquid methyl methacrylate monomer and powdered MMA-styrene co-polymer, often including radiopacifiers such as BaSO4 or ZrO2. Polymerization of the liquid monomer around the co-polymer powder particles (which are pre-polymerized) and additives lead to producing a hardened and non-degradable PMMA for hard tissue augmentation purposes. Due to its inherent biocompatible and biomechanical properties, PMMA is the only FDA-approved injectable material used for percutaneous vertebroplasty (PVP) and kyphoplasty (PKP) (Fang et al., 2014; He et al., 2015; Münker et al., 2018; Shimko and Nauman, 2006), which are classified as minimally invasive surgical approaches for osteoporotic vertebral compression fractures. Overall, PMMA is lightweight, radiolucent, biocompatible, cost effective, and easy to handle and manufacture.
The primary objective of this study was to investigate the effects of physiologically relevant environment on biomechanical responses of porous scaffolds with inherent hydrophobic characteristics. In this study, PMMA porous scaffolds were fabricated based on two triply periodic minimal surface (TPMS) topologies (Primitive and Schoen-IWP) (Fig. 1A) using a relatively low-cost 3D printing technique called digital light processing (DLP) (Fig. 1B). As illustrated in Fig. 1B, the principle of DLP techniques is to selectively project light source to cure photopolymer resins in a layer-by-layer process in O2 controlled environment. It should be noted that the ability of DLP to simultaneously vary projection intensity within each layer enables parallel creation of part and support volume (if needed). Several studies have summarized the mechanisms, material options, characterization, and applications of DLP process (Layani et al., 2018; Quan et al., 2020; Zhao et al., 2020). In this current study, in order to evaluate the effects of physiologically relevant environment on the biomechanical responses of porous scaffolds, fabricated test specimens were tested under a quasi-static compressive loading condition in both ambient and fluidic (DI water with pH=7.4 and temperature maintained at 37 °C) environments (Fig. 1C).
Fig. 1: Additive manufacturing facilitates developing scalable tissue-inspired biomimetic scaffolds for a wide range of tissue regeneration applications.
(A) Schematic drawing depicting TPMS-based (i.e., Schoen-IWP and Primitive) scaffold designs and their potential in tissue regeneration. (B) Schematic diagram of the DLP process. (C) Experimental setup used for mechanical testing in the fluidic environment (DI water with pH=7.4); setup included a tank connected to a temperature control fluid circulating pump, a thermometer to maintain the temperature at 37 °C , and high-speed cameras to capture the failure progression during the compression testing and for DIC analysis.
Flexural behavior and fracture mechanisms of the scaffolds were analyzed through 3-point bending experiments in the ambient condition only. Additionally, the effects of morphological variations on the mechanical performance of the studied porous scaffolds were evaluated. In the present study, mechanical experiments were accompanied by a 3D digital image correlation (3D DIC) system (Fig. 1C) which provided additional information on surface strain and failure progression. In summary, findings from this study will evaluate the biomechanical response of hydrophobic 3D printed biomaterial (i.e., PMMA) under physiologically relevant environment which will further the understanding of mechanical behavior of 3D printed design topologies (i.e., TPMS) for biomedical applications.
2. Materials and Methods
2.1. Topological design and 3D fabrication process
In this study, biomimetic topological design addressing the main design objectives such as (1) 3D scaffolds with geometries aimed at mimicking the trabecular bone (Bobbert et al., 2017; Tilton et al., 2021; Yan et al., 2015), (2) with pore size greater than 300 μm (Murphy et al., 2010), and (3) high specific surface area (Han and Che, 2018; Sanjairaj et al., 2018)(i.e. ratio of surface area to volume), was implemented. Specifically, 3D scaffolds were designed based on two topological classes of TPMSs, Primitive and IWP which have been previously studied by several researchers (Abueidda et al., 2017; Bobbert et al., 2017; Tilton et al., 2021; Zhang et al., 2018). The 3D digital models of the sheet-based Primitive and IWP specimens were developed using mathematical equations (Eqn. 1-2) in MATLAB. The surface offsetting technique in MATLAB was employed to create the study groups (P0.25, P0.35, P0.45 and IWP0.25, IWP0.35, IWP0.45) with varying sheet-thicknesses (0.25, 0.35, 0.45 mm). Unit cell size of 2mm was consistent among all the study groups across the coordinate directions (i.e., x, y, and z). In order to ensure that the specimens reflect the scale-independent mechanical responses, compression specimens were designed to included 8-unit cells along each coordinate direction. Based on previous studies (Abueidda et al., 2017; Kadkhodapour et al., 2015; Smith et al., 2013; Yang et al., 2018; Zhang et al., 2018), a minimum of 5 unit cell tessellation in global coordinates is sufficient to characterize the mechanical properties of 3D printed porous scaffolds under compressive loading. However, flexural testing specimens included an arrangement of 20 × 4 × 2 unit cells along the x, y, and z directions. Two end plates (thickness = 3×sheet-thickness of the associated model (Shen et al., 2014)) were added to the porous scaffolds before fabrication.
| Eqn. 1 |
| Eqn. 2 |
Mathematically designed specimens were fabricated using NextDent 5100 3D printer (3D Systems, Inc., USA) from crown and bridge micro filled hybrid (C&B MFH) biomaterial (Kessler et al., 2019; Münker et al., 2018; Scotti et al., 2020). The C&B MFH biomaterial, which is a poly(methyl methacrylate) (PMMA) based material, has been qualified as a Class IIa CE-certified FDA clearance, and is currently used commercially for dental and other biomedical. In this study, the base composition of C&B MFH’s included methacrylate oligomer, methacrylate monomer, inorganic micro filler, and phosphine oxide. A roller mixer was used to mix the C&B MFH material overnight (12 hours) prior to dispensing it into the Figure 4 machine. In order to achieve the micro-scale geometrical features of the designed scaffolds, a layer thickness of 50 μm was implemented in the fabrication process. Other process parameters such as light intensity, wavelength, and layer exposure were assigned according to the manufacturer’s standard processing condition for this material. Post 3D printing process involved submerging the 3D printed scaffolds into the 99% isopropyl alcohol (IPA) in an ultrasonic cleaner for a duration of 20 minutes. IPA solution was replaced with a clean solution every 5 minutes. All samples were air dried at room temperature overnight (12 hours) prior to proceeding with the curing process using NextDent LC-3DPrint Box (3D Systems, Inc., USA). Lastly, all the samples were cured for one hour in a NextDent LC-3DPrint Box.
Fig. 4:
1st order power law relationship between the porosity variations of the 3D printed scaffolds and their mechanical responses including elastic gradient (A, C) and yield strength (B, D) in the ambient (A-B) and fluidic (C-D) environments at R2 ≥ 0.97. Similarly, data are reported as the mean values of 5 samples per study group per experiments ± the corresponding standard deviation.
2.2. Quasi-static compression testing: Ambient and body simulated fluidic environment
Quasi-static compressive experiments (n = 5 per test group) were conducted in two environments: ambient and body simulated fluidic environments. Both the compression, and flexural tests were performed on an electromechanical MTS load frame equipped with a 20 kN load cell. Compression testing in the body simulated environment included a temperature control 6-liter fluid bath (MTS Bionix EnviroBath) installed within the mechanical load frame as shown in Fig. 1C. The temperature of the circulated fluid, with a pH level of 7.4, was maintained at 37 °C to simulate human body temperature. During the compression testing in the fluid bath, samples were completely immersed inside the fluid, and the temperature of the environment was continuously monitored using a digital thermocouple.
In accordance with ASTM D1621 (ASTM, 1991), displacement control at the displacement rate of 1.6 mm/min was applied during quasi-static compression testing regardless of the environmental condition. All the compression tests were terminated when a maximum densification of approximately 40% was reached. These experiments were accompanied by two 5.0 Megapixel digital cameras capturing the mechanical response in a stereo setting at 1 Hz. Due to the high deformation rate of the porous scaffolds under compressive loading, surface strain analysis was not performed for this loading condition.
The stress-strain curves were computed from the recorded load-displacement data from the load frames. Subsequently, the elastic gradient (E), yield stress (σy), ultimate compressive strength, and plateau stress (σpl- when > 20% strain was observed) were calculated from the stress-strain curves for every test specimen. The elastic gradient was measured from the slope of the linear elastic region. Yield stress was determined using the 0.2% offset of the elastic gradient line. Ultimate compressive strength was considered to be the maximum stress before a decrease in the stress level as the cellular layers start deforming. Plateau stress was determined by the arithmetic mean of the stresses between 20-30% of compressive strain (Bobbert et al., 2017; Tilton et al., 2021).
2.3. Three-point flexural experiment
The flexural tests were performed on five samples per study group in the same MTS load frame equipped with a 20 kN load cell in an ambient environment (Fig. 5A). In accordance to the ASTM C393, displacement control at the rate of 0.5 mm/min was applied during three-point bending tests (ASTM International, 2009). The tests were terminated when specimens fractured, or a sudden drop in load-displacement data was observed. Flexural stiffness was determined from the linear region of the load-deflection curves. Flexural strength (i.e., flexural yield strength) was recorded from the maximum stress in the specimen just prior to yielding. The linear region until the yield point of the load-deflection curves were used to compute flexural stress (Eqn. 3) and strain (Eqn. 4), where F, L, b, h, and l correspond to the applied force, span length between two supports, width, thickness, and maximum deflection of the center of the specimen, respectively.
Fig. 5:
(A) Flexural testing and surface strain analysis of the 3D printed TPMS scaffolds in an ambient environment. Force-deflection curves illustrating the flexural behavior of the 3D printed primitive (B) and IWP (C) scaffolds. All the flexural experiments were performed in the ambient environment. Full-field surface strain maps were associated with the exx field for each studied morphological group. Images are color and number coded according to the respective force-deflection curve (or different morphological group for each topological class). The shaded regions in the force-deflection curves correspond to standard deviation. (D-E) 1st order power law relationship between the porosity variations of the 3D printed scaffolds and their flexural properties including stiffness (D) and strength (E) at R2 ≥ 0.99. Similarly, data are reported as the mean values of 5 samples per study group per experiments ± the corresponding standard deviation.
| Eqn. 3 |
| Eqn. 4 |
All of the flexural experiments were accompanied by 3D digital image correlation (3D DIC) in order to evaluate the full-field surface strain of the porous specimens at different stages of deformation. DIC images were collected using the two 5.0 Megapixel digital cameras in a stereo setting at 1 Hz. Speckle patterns were created on the specimens by initially spray painting the specimens in white. After an interval of 10 minutes, random black speckles were sprayed onto the imaging surface of the specimens using pressure-controlled airbrush.
2.4. Post-processing and Analysis
Post-processing of the recorded images was performed in Vic-3D software (v8, Correlated Solutions, Inc., USA). Statistical analysis was performed in IBM SPSS Statistics 24.0 Software (IBM, USA). One-way ANOVA was used to test for statistical significance, followed by Tukey’s test for pairwise comparisons between relevant groups in order to further evaluate the effects of variation in testing environment and pore size (within a same topological group). This pairwise comparison method was employed to evaluate statistical significance in mechanical responses within the topological groups (i.e., Primitive and IWP) across different morphological conditions.
3. Results and Discussion
Environmental conditions play a critical role in the mechanical properties of biomaterials. Specifically, variations in biomechanical properties of scaffolds are influenced by loading, environmental and boundary conditions. In addition morphological variations are a co-variable in biomechanical responses between ambient and fluidic environments. This study examined mechanical responses of the 3D printed (i.e., DLP) PMMA-microfilled scaffolds at two different environments, ambient and body simulated fluid. The 3D printed scaffolds were designed based on two TPMS topologies, primitive and IWP, across a range of design morphologies (Table 1). The design of scaffolds for each topological class was controlled by varying the wall-thickness, which directly controls the pore size, porosity, and specific surface area. Specifically, increasing the wall-thickness, increases the other mentioned morphological parameters. The vat-polymerization technique employed in this study facilitated rapid high-fidelity printing of biomaterial specimens without any visible defects. This study correlated the mechanical responses of biomaterials across a range of morphological design in two varying environmental conditions. This is both novel and critical because multiple studies in the field of tissue engineering interpret the mechanical properties obtained in-vitro in an ambient environment to assume the mechanical performance in-vivo. In this study, an in-vitro experimental was set-up to assess the quasi-static compressive properties of the 3D printed PMMA-microfilled scaffolds in both ambient and fluidic conditions. Additionally, the flexural and failure properties of these scaffolds in the ambient condition that were not previously reported in the literature were analyzed in this current study.
Table 1:
Morphological parameters of the mathematically designed 3D scaffolds –for compression testing
| Group | Wall-Thickness (μm) | Porosity (%) | Pore Size (μm) | Specific Surface Area (mm−1) |
|---|---|---|---|---|
| P0.25 | 250 | 66.7 | 1630 | 6.78 |
| P0.35 | 350 | 55.5 | 1530 | 4.81 |
| P0.45 | 450 | 45.6 | 1440 | 3.73 |
| IWP0.25 | 250 | 53.7 | 1000 | 6.90 |
| IWP0.35 | 350 | 39.3 | 920 | 4.82 |
| IWP0.45 | 450 | 26.9 | 850 | 3.58 |
3.1. Effects of fluidic environment on the compressive mechanical responses
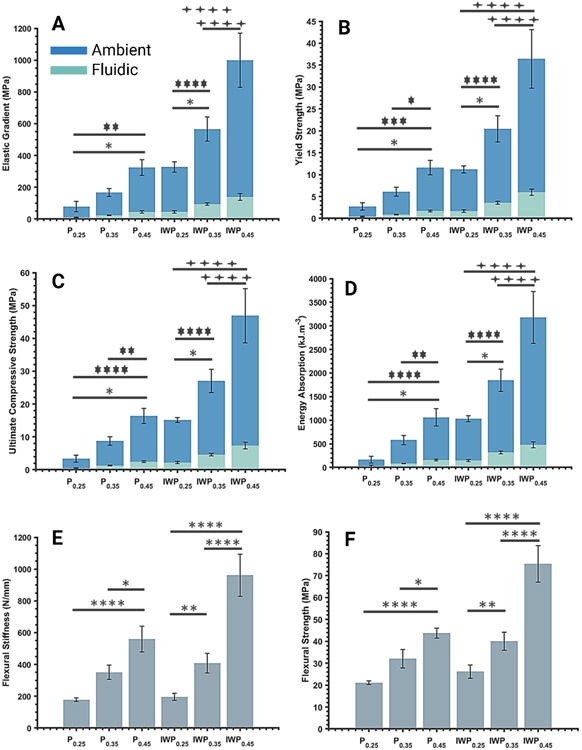
Quasi-static compressive loading was performed in both ambient and fluidic environments. Examining the stress-strain curves indicated that, independent of topology or porosity, all the study groups showed three distinct stages of deformation (Fig. 2A-D): (1) linear elastic region, (2) long plateau (plastic deformation) region, and (3) failure and densification of the scaffolds. The fluidic environment accelerated the failure of the scaffolds, which was evident from the smaller strain condition when the specimens failed and could no longer carry load (Fig. 2C-D). All the quasi-static compressive properties showed statistically significantly lower performance in the fluidic condition than the ambient environment for the same topological and morphological group (p ≤ 0.023) (Fig. 3A-D). Specifically, the compressive properties, including elastic modulus, yield strength, ultimate compressive strength, and energy absorption, showed more than 82% average reduction when testing an identical scaffold in the fluidic environment. These findings indicated a significant dependency of the mechanical properties on the environmental conditions. Previous studies (Guan and Davies, 2004; Wu et al., 2006) on the characteristics of poly(D,L-lactic-co-glycolic acid) (PLGA) porous scaffolds have shown that mechanical properties are significantly decreased when temperature was raised (25 to 37 °C) and/or the testing environment was changed from a dry-state to a wet-state. Our results on PMMA-microfilled scaffolds were consistent with these studies. Additionally, it was observed that biomechanical responses of the tested specimens in the fluidic environment were more consistent within each study group (Fig. 2C-D). This was also indicated by lower standard deviations, across all the groups, when comparing outcomes between ambient and fluidic environments. In order to verify any potential effects of polymer degradation, random samples were weighted in the dry-state (i.e., initial mass), then immersed into the prepared fluidic environment for 1 hour; specimens were vacuum dried and weighed again. Observation of lack of any differences in recorded mass confirmed that mechanical responses excluded possible polymer degradation. The 3D printed PMMA-microfilled scaffolds showed failure patterns that included either diagonal shear-band formation or longitudinal layer-by-layer collapse of unit cells (Fig. 2) which were observed in both topological classes, Primitive (Fig. 3A, C) and IWP (Fig. 3B, D) which was consistent with observations in previous similar studies (Abueidda et al., 2017; Karimipour-Fard et al., 2020; Ma et al., 2020). In the ambient environment, failure pattern, in both topological groups, was changed from longitudinal layer-by-layer collapse (from bottom layer) to diagonal shear band failure as the porosity was reduced (or wall-thickness was increased) (Fig. 2A-B). The localized shear band collapse occurred at 45° to the loading vector with the failure starting from the outer most unit cell on the first layer under the top face-sheet (Fig. 2A-B). These observations could be attributed to the weaker sites, at the higher porosity, distributed across the layer of unit cells in contact with the bottom solid face-sheet. However, increasing the wall-thickness shifted the weaker regions to the corner unit cells causing them to experience greater applied load such that they were unable to transfer the load to the entire structure uniformly. In the fluidic environment, IWP groups, across design morphologies, showed localized shear band formation with minimal deformation of the off-diagonal unit cells (Fig. 2D). On the other hand, Primitive groups showed layer-by-layer collapse at a higher porosity (i.e., Primitive 0.25) and diagonal 45° shear band failure at lower porosities (i.e., Primitive 0.35 and Primitive 0.45). Such concentrated and non-uniform failure of structures could be partly explained by presence of micro- or nano-scale defects introduced during fabrication process (Zhang et al., 2021).
Fig. 2: Effects of physiologically relevant testing environment on the mechanical responses.
Stress-strain of compressive behavior of the 3D printed primitive (A, C) and IWP (B, D) scaffolds in the ambient (A-B) and fluidic (C-D) environments. Representative images of the final failure stage associated with each studied pore size or porosity (i.e., wall-thickness variations) are aligned next to graphs. Images are color coded according to the respective stress-strain curve. The shaded regions in the stress-strain curves correspond to standard deviation.
Fig. 3: Compressive mechanical properties of the 3D printed TPMS scaffolds are significantly lower in the fluidic condition than the ambient environment.
Summary of the quasi-static compressive (A-D) and flexural (E-F) properties of the studied 3D printed scaffolds under ambient and fluidic environments. Data are reported as the mean values of 5 samples per study group per experiment ± the corresponding standard deviation. The statistical analysis was done according to the methods explained in “Post-processing and analysis” section. Asterisks show the results that are statically significant with p-values < 0.05 (*), 0.01 (**), 0.001 (***), or 0.0001 (****). Noting that asterisks {*}, { }, and {
}, and { } correspond to statistical significance in ambient, fluidic, or similar outcome in both tested environments, respectively. The “P” in the axis labeling was used to refer to “Primitive” study groups.
} correspond to statistical significance in ambient, fluidic, or similar outcome in both tested environments, respectively. The “P” in the axis labeling was used to refer to “Primitive” study groups.
In this study, mathematically designed porous scaffolds resulted in achieving a range of mechanical properties within the range of human trabecular bones. The PMMA-microfilled (i.e., C&B MFH) exhibits a bulk elastic modulus of 2400-2600 MPa (Revilla-León et al., 2019); integration of bioinspired TPMS micro-architectural design enabled achieving elastic modulus ranging 9-150 MPa in fluidic and 77-1000 MPa in an ambient environment. Elastic modulus of human bone ranges between 16 to 4500 MPa on average (Goulet et al., 1994; Morgan et al., 2003); studied 3D printed scaffolds exhibit compressive moduli at the lower range of the trabecular bone, making them an ideal candidate for bone reconstruction in clinical needs for osteoporosis. Similarly, the yield strength of the specimens (ambient: 2-40 MPa and fluidic: 0.40-6.5 MPa) are also within the previously reported range of human trabecular bone’s yield strength (0.56-64 MPa) (Morgan et al., 2003). Additionally, the 3D printed porous scaffolds resulted in ultimate compressive strength (ambient: 3-50 MPa fluidic: 0.40-8 MPa) are also within the range of reported compressive strength of trabecular bone (0.15-13.5 MPa) (Misch et al., 1999; Schoenfeld et al., 1974).
The compression properties showed increasing trends as the wall-thickness of the 3D printed TPMS-based scaffolds was increased, regardless of the topological class. For a similar porosity level between primitive and IWP groups (e.g., Primitive 0.35 and IWP 0.25), IWP scaffolds exhibited compressive mechanical properties approximately twofold in magnitude as compared with the primitive scaffolds (Fig. 3A-D). This finding is consistent with results from previous studies comparing the mechanical performance of these two topological classes of 3D printed TPMS scaffolds (Abueidda et al., 2017; Bobbert et al., 2017; Tilton et al., 2021). Additionally, in both topological classes, as the wall-thickness was increased, the standard deviation across the compressive mechanical properties was increased (Fig. 3A-D), especially in an ambient environment. Regression analysis indicated that variations in morphological parameters (e.g., porosity, pore size, specific surface area, etc.) has substantial influence (R2 ≥ 0.97) on the quasi-static mechanical performance of the 3D printed porous scaffolds (Fig. 4). Fig. 4 illustrates an example of these correlations between elastic gradient, yield strength, and porosity. These correlations followed a 1st order power law; the reported empirical relationship could be utilized to predict the biomechanical responses of the desired PMMA-microfilled TPMS scaffolds for a given porosity and boundary condition.
3.2. Flexural behavior and failure mechanism
Based on the load-deflection curves shown in Fig. 5, all the Primitive groups showed a flexural response of yielding of the specimens after the linear region followed by plastic deformation then failure of the specimen. However, in the IWP topological class, while the IWP0.25 specimens performed similar to the Primitive groups, the IWP0.35 and IWP0.45 specimen’s flexural response after the linear region consisted of yield of the specimens followed by rapid brittle failure (Fig. 5B-C). Based on these observations, the flexural response of the 3D printed PMMA-microfilled scaffolds was dependent on both topology and morphology. Specifically, highly porous Primitive groups exhibited larger deflection before the failure of the specimens when compared with other study groups, particularly the less porous IWP specimens (i.e., IWP 0.45). The IWP groups showed substantially higher flexural stiffness (range: 10-71%) and strength (range: 24-72%) when compared with the Primitive specimens of similar as-designed wall-thickness (Fig. 3E-F). In this current study, 3D printed TPMS PMMA-microfilled scaffolds showed flexural stiffness values (Primitive: 160-660 N/mm and IWP: 170-1200 N/mm) within the lower limit of those previously reported for human bone (300-2500 N/mm) (Lotz et al., 1991; Yeh et al., 2019). Fracture mechanisms of the studied 3D printed scaffolds were observed to involve core yielding and buckling, top face sheet compression failure, and bottom face sheet tension failure (Fig. 5). Yielding and buckling of the core structure initiated from the unit cells underneath the top solid face-sheet along the loading vector which indicates stress concentration at those sites.
Regression analysis of flexural responses (i.e., flexural stiffness and strength) and porosity revealed that morphological parameters used as design inputs could be highly effective to predict flexural properties with high accuracy (R2 ≥ 0.99). As observed in Fig. 5D-E, these correlations follow 1st order power law equations, which associate an individual flexural response to the porosity for each topological class. In addition, the outcomes of this study indicated that compressive and flexural responses of the 3D printed TPMS PMMA-microfilled scaffolds were highly sensitive to morphological changes. For instance, minor variations in porosity (or pore size) (≤ 17%) led to statistically significant variations in compressive and flexural responses in the ambient environment across each topological class (Fig. 3).
3.3. Limitations and future research directions
Successful clinical translation of the engineered biomaterials relies on the adequacy of comprehensive characterizations that have been performed using in vitro and in vivo assays. When investigating the mechanical characteristics of the tissue engineering scaffolds, many published reports have used the properties obtained in an ambient environment to describe the expected performance of biomaterials in-vivo. Therefore, there is a critical need to develop standardized mechanical characterization protocols for tissue engineering scaffolds, particularly for load-bearing orthopedic applications. This study demonstrated the significant dependency of the mechanical performance of 3D printed PMMA-microfilled scaffolds to the testing environment condition (i.e., boundary conditions). It is worth noting that environmental-dependent variations in mechanical responses are contingent on the type of biomaterial employed in the development of the 3D porous scaffold. While this study was focused specifically on the PMMA-microfilled (C&B MFH) biomaterial, there are also few published studies (Guan and Davies, 2004; Wu et al., 2006) on the characteristics of PLGA porous scaffolds in different environments. However, similar to many previous works, this study was limited in loading conditions used for mechanical characterization. A comprehensive evaluation of these biomaterials for load-bearing orthopedic applications requires the implementation of additional physiologically relevant loading and boundary conditions; compression-only loading does not replicate the full range of physiological loading conditions that scaffolds experience in vivo. Additionally, future efforts are needed to investigate the effects of environmental conditions on the fatigue properties of such 3D printed scaffolds.
Here, it was shown that mechanical responses of the developed 3D printed TPMS PMMA-microfilled scaffolds were similar to those of human trabecular bone; however, additional experiments are required to further confirm the potential correlations Some deviations were observed in the mechanical properties within the same study group, which could be attributed to minor manufacturing irregularities and/or micro/nano structural defects. This study was limited to the morphological and mechanical characterization of the studied 3D scaffolds; understanding the biological properties of these scaffolds would be an essential step in future works. Specifically, understanding the effects of computational topological and morphological variations in 3D printed TPMS scaffolds on their biological behavior at different length-scales should be extensively investigated to improve the mathematical models linking structural designs to mechanobiological responses. Lastly, this study considered the static state of bone tissue when developing the scaffolds while human tissue experiences dynamic adaption and a non-equilibrium state in vivo. This limitation could be addressed by developing multi-physics computational models which facilitate adaptive design and tuning of the scaffolds.
4. Conclusion
In this study, the environmental-dependency of the 3D printed TPMS PMMA-microfilled scaffold’s mechanical responses was investigated. The goal of clinical translation of biomimetic scaffolds is to develop safe and medically relevant materials and devices that reliably mimic tissue-specific properties, while improving the patient’s quality of life, without compromising the biomaterial’s integrity to regenerate or repair tissue. Adequately estimating the mechanical properties of biomaterials in physiologically relevant conditions will further improve our understanding of engineered implant (or scaffold) properties when used in vivo and for clinical translation. Therefore, the results of our study highlight the need to test biomaterials in physiologically relevant environmental conditions that are also clinically relevant. All the measured compressive properties were significantly lower in magnitude in the fluidic condition when compared with the ambient environment. It was observed that failure mechanism of the studied 3D printed PMMA-microfilled scaffolds was independent of the environmental conditions and was mainly dictated by the topology and morphology of the tested scaffolds. Additionally, correlation between the morphological variations and the mechanical responses was performed, which indicated that compressive and flexural properties of the studied scaffolds could be estimated knowing the design morphological parameters. It was found that at a similar porosity, IWP scaffolds exhibited mechanical properties substantially higher than those of Primitive specimens. Overall, the developed TPMS PMMA-microfilled scaffolds were able to mimic the compressive properties and flexural stiffness of native human trabecular bone. In this study, it was shown that physiological, environmental conditions play a critical role in estimating the mechanical properties of biomaterials.
Acknowledgement
MT is supported by National Institute of Health grant (T32AR56950) for Musculoskeletal Research Training. Authors acknowledge the support of Ryan Overdorff from 3D Systems Inc. in 3D printing processing.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Abueidda DW, Bakir M, Al-rub RKA, Bergström JS, Sobh NA, Jasiuk I, 2017. Mechanical properties of 3D printed polymeric cellular materials with triply periodic minimal surface architectures. Mater. Des 122, 255–267. 10.1016/j.matdes.2017.03.018 [DOI] [Google Scholar]
- ASTM, 1991. Standard test method for compressive properties of rigid cellular plastics. ASTM Stand. D1621-16, 1621–1673. 10.1520/D1621-16.2 [DOI] [Google Scholar]
- International ASTM, 2009. ASTM C393/C 393 M - 06 Standard Test Method for Core Shear Properties of Sandwich Constructions by Beam. Annu. B. ASTM Stand, i, 1–8. 10.1520/C0393 [DOI] [Google Scholar]
- Bobbert FSL, Lietaert K, Eftekhari AA, Pouran B, Ahmadi SM, Weinans H, Zadpoor AA, 2017. Additively manufactured metallic porous biomaterials based on minimal surfaces: A unique combination of topological, mechanical, and mass transport properties. Acta Biomater. 53, 572–584. 10.1016/j.actbio.2017.02.024 [DOI] [PubMed] [Google Scholar]
- Bose S, Ke D, Sahasrabudhe H, Bandyopadhyay A, 2018. Additive manufacturing of biomaterials. Prog. Mater. Sci 93, 45–111. 10.1016/J.PMATSCI.2017.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SG, Yang J, Jia YG, Lu B, Ren L, 2019. TiO2 and PEEK Reinforced 3D Printing PMMA Composite Resin for Dental Denture Base Applications. Nanomater. (Basel, Switzerland) 9. 10.3390/NANO9071049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cidonio G, Glinka M, Dawson JI, Oreffo ROC, 2019. The cell in the ink: Improving biofabrication by printing stem cells for skeletal regenerative medicine. Biomaterials. 10.1016/j.biomaterials.2019.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Z, Giambini H, Zeng H, Camp JJ, Dadsetan M, Robb RA, An KN, Yaszemski MJ, Lu L, 2014. Biomechanical evaluation of an injectable and biodegradable copolymer P(PF-co-CL) in a cadaveric vertebral body defect model. Tissue Eng. - Part A 20, 1096–1102. 10.1089/TEN.TEA.2013.0275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana F, Figueiredo P, Martins JP, Santos HA, 2021. Requirements for Animal Experiments: Problems and Challenges. Small 17, 2004182. 10.1002/SMLL.202004182 [DOI] [PubMed] [Google Scholar]
- Goulet RW, Goldstein SA, Ciarelli MJ, Kuhn JL, Brown MB, Feldkamp LA, 1994. The relationship between the structural and orthogonal compressive properties of trabecular bone. J. Biomech 27, 375–389. 10.1016/0021-9290(94)90014-0 [DOI] [PubMed] [Google Scholar]
- Guan L, Davies JE, 2004. Preparation and characterization of a highly macroporous biodegradable composite tissue engineering scaffold. J Biomed Mater Res 71, 480–487. 10.1002/jbm.a.30173 [DOI] [PubMed] [Google Scholar]
- Han L, Che S, 2018. An Overview of Materials with Triply Periodic Minimal Surfaces and Related Geometry: From Biological Structures to Self-Assembled Systems. Adv. Mater 30, 1–22. 10.1002/adma.201705708 [DOI] [PubMed] [Google Scholar]
- He Z, Zhai Q, Hu M, Cao C, Wang J, Yang H, Li B, 2015. Bone cements for percutaneous vertebroplasty and balloon kyphoplasty: Current status and future developments. J. Orthop. Transl 3, 1. 10.1016/J.JOT.2014.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosaka K, Tagami J, Nishitani Y, Yoshiyama M, Carrilho M, Tay FR, Agee KA, Pashley DH, 2007. Effect of wet vs. dry testing on the mechanical properties of hydrophilic self-etching primer polymers. Eur. J. Oral Sci 115, 239–245. 10.1111/J.1600-0722.2007.00452.X [DOI] [PubMed] [Google Scholar]
- Kadkhodapour J, Montazerian H, Darabi AC, Anaraki AP, Ahmadi SM, Zadpoor AA, Schmauder S, 2015. Failure mechanisms of additively manufactured porous biomaterials: Effects of porosity and type of unit cell. J. Mech. Behav. Biomed. Mater 50, 180–191. 10.1016/j.jmbbm.2015.06.012 [DOI] [PubMed] [Google Scholar]
- Karageorgiou V, Ã DK, 2005. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 26, 5474–5491. 10.1016/j.biomaterials.2005.02.002 [DOI] [PubMed] [Google Scholar]
- Karimipour-Fard P, Behravesh AH, Jones-Taggart H, Pop-Iliev R, Rizvi G, 2020. Effects of design, porosity and biodegradation on mechanical and morphological properties of additive-manufactured triply periodic minimal surface scaffolds. J. Mech. Behav. Biomed. Mater 112, 104064. 10.1016/J.JMBBM.2020.104064 [DOI] [PubMed] [Google Scholar]
- Kessler A, Reymus M, Hickel R, Kunzelmann KH, 2019. Three-body wear of 3D printed temporary materials. Dent. Mater 35, 1805–1812. 10.1016/J.DENTAL.2019.10.005 [DOI] [PubMed] [Google Scholar]
- Kim J, Kong JS, Han W, Kim BS, Cho DW, 2020. 3D Cell Printing of Tissue/Organ-Mimicking Constructs for Therapeutic and Drug Testing Applications. Int. J. Mol. Sci 21, 1–27. 10.3390/IJMS21207757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TG, Shin H, Lim DW, 2012. Biomimetic scaffolds for tissue engineering. Adv. Funct. Mater 22, 2446–2468. 10.1002/adfm.201103083 [DOI] [Google Scholar]
- Layani M, Wang X, Magdassi S, 2018. Novel Materials for 3D Printing by Photopolymerization. Adv. Mater 30, 1706344. 10.1002/ADMA.201706344 [DOI] [PubMed] [Google Scholar]
- Li J, Cui X, Hooper GJ, Lim KS, Woodfield TBF, 2020. Rational design, bio-functionalization and biological performance of hybrid additive manufactured titanium implants for orthopaedic applications: A review. J. Mech. Behav. Biomed. Mater 10.1016/j.jmbbm.2020.103671 [DOI] [PubMed] [Google Scholar]
- Liu X, Miller AL, Xu H, Waletzki BE, Lu L, 2019. Injectable Catalyst-Free Poly(Propylene Fumarate) System Cross-Linked by Strain Promoted Alkyne-Azide Cycloaddition Click Chemistry for Spine Defect Filling. Biomacromolecules 20, 3352–3365. 10.1021/acs.biomac.9b00133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotz JC, Gerhart TN, Hayes WC, 1991. Mechanical properties of metaphyseal bone in the proximal femur. J. Biomech 24, 317–329. 10.1016/0021-9290(91)90350-V [DOI] [PubMed] [Google Scholar]
- Lynch ME, Mordasky M, Cheng L, To A, 2018. Design , testing , and mechanical behavior of additively manufactured casing with optimized lattice structure. Addit. Manuf 22, 462–471. 10.1016/j.addma.2018.05.021 [DOI] [Google Scholar]
- Ma S, Song K, Lan J, Ma L, 2020. Biological and mechanical property analysis for designed heterogeneous porous scaffolds based on the refined TPMS. J. Mech. Behav. Biomed. Mater 107, 103727. 10.1016/J.JMBBM.2020.103727 [DOI] [PubMed] [Google Scholar]
- Marin E, Mukai M, Boschetto F, Sunthar TPM, Adachi T, Zhu W, Rondinella A, Lanzutti A, Kanamura N, Yamamoto T, Fedrizzi L, Pezzotti G, 2021. Antibacterial 3D-printed PMMA/ceramic composites. bioRxiv 2021.10.11.463892 10.1101/2021.10.11.463892 [DOI] [Google Scholar]
- Miramini S, Fegan KL, Green NC, Espino DM, Zhang L, Thomas-Seale LEJ, 2020. The status and challenges of replicating the mechanical properties of connective tissues using additive manufacturing. J. Mech. Behav. Biomed. Mater 103. 10.1016/J.JMBBM.2019.103544 [DOI] [PubMed] [Google Scholar]
- Misch CE, Qu Z, Bidez MW, 1999. Mechanical properties of trabecular bone in the human mandible: Implications for dental implant treatment planning and surgical placement. J. Oral Maxillofac. Surg 57, 700–706. 10.1016/S0278-2391(99)90437-8 [DOI] [PubMed] [Google Scholar]
- Mogil JS, 2009. Animal models of pain: progress and challenges. Nat. Rev. Neurosci 10, 283–294. 10.1038/NRN2606 [DOI] [PubMed] [Google Scholar]
- Morgan EF, Bayraktar HH, Keaveny TM, 2003. Trabecular bone modulus-density relationships depend on anatomic site. J. Biomech 36, 897–904. 10.1016/S0021-9290(03)00071-X [DOI] [PubMed] [Google Scholar]
- Münker TJAG, van de Vijfeijken SECM, Mulder CS, Vespasiano V, Becking AG, Kleverlaan CJ, Becking AG, Dubois L, Karssemakers LHE, Milstein DMJ, van de Vijfeijken SECM, Depauw PRAM, Hoefnagels FWA, Vandertop WP, Kleverlaan CJ, Münker TJAG, Maal TJJ, Nout E, Riool M, Zaat SAJ, 2018. Effects of sterilization on the mechanical properties of poly(methyl methacrylate) based personalized medical devices. J. Mech. Behav. Biomed. Mater 81, 168–172. 10.1016/j.jmbbm.2018.01.033 [DOI] [PubMed] [Google Scholar]
- Murphy CM, Haugh MG, O’Brien FJ, 2010. The effect of mean pore size on cell attachment, proliferation and migration in collagen-glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials 31, 461–466. 10.1016/j.biomaterials.2009.09.063 [DOI] [PubMed] [Google Scholar]
- Quan H, Zhang T, Xu H, Luo S, Nie J, Zhu X, 2020. Photo-curing 3D printing technique and its challenges. Bioact. Mater 5, 110–115. 10.1016/J.BIOACTMAT.2019.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revilla-León M, Meyers MJ, Zandinejad A, Özcan M, 2019. A review on chemical composition, mechanical properties, and manufacturing work flow of additively manufactured current polymers for interim dental restorations. J. Esthet. Restor. Dent 31, 51–57. 10.1111/jerd.12438 [DOI] [PubMed] [Google Scholar]
- Rezaei A, Giambini H, Miller AL, Liu X, Elder BD, Yaszemski MJ, Lu L, 2020. OPF/PMMA cage system as an alternative approach for the treatment of vertebral corpectomy. Appl. Sci 10, 6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanjairaj V, Zhang L, Fuh JYH, Lu W, 2018. Triply Periodic Minimal Surfaces Sheet Scaffolds for Tissue Engineering Applications : An Optimization Approach towards Biomimetic Scaffold Design Triply Periodic Minimal Surfaces Sheet Scaffolds for Tissue Engineering Applications : An Optimization Appro. 10.1021/acsabm.8b00052 [DOI] [PubMed] [Google Scholar]
- Schoenfeld CM, Lautenschlager EP, Meyer PR, 1974. Mechanical properties of human cancellous bone in the femoral head. Med. Biol. Eng 12, 313–317. 10.1007/BF02477797 [DOI] [PubMed] [Google Scholar]
- Scotti CK, Velo M.M. de A.C., Rizzante FAP, Nascimento T.R. de L., Mondelli RFL, Bombonatti JFS, 2020. Physical and surface properties of a 3D-printed composite resin for a digital workflow. J. Prosthet. Dent 124, 614.e1–614.e5. 10.1016/J.PROSDENT.2020.03.029 [DOI] [PubMed] [Google Scholar]
- Shen Y, Cantwell W, Li Y, 2014. Skin-core adhesion in high performance sandwich structures 15, 61–67. 10.1631/jzus.A1300283 [DOI] [Google Scholar]
- Shimko DA, Nauman EA, 2006. Development and Characterization of a Porous Poly(methyl methacrylate) Scaffold With Controllable Modulus and Permeability. 10.1002/jbm.b.30605 [DOI] [PubMed] [Google Scholar]
- Smith M, Guan Z, Cantwell WJ, 2013. Finite element modelling of the compressive response of lattice structures manufactured using the selective laser melting technique. Int. J. Mech. Sci 67, 28–41. 10.1016/j.ijmecsci.2012.12.004 [DOI] [Google Scholar]
- Solari D, Papallo I, Ugga L, Cavallo LM, Onofrio I, Cuocolo R, Improta G, Brunetti A, Martorelli M, Gloria A, Cappabianca P, Russo T, 2020. Novel concepts and strategies in skull base reconstruction after endoscopic endonasal surgery. Acta IMEKO 9, 67–73. 10.21014/ACTA_IMEKO.V9I4.745 [DOI] [Google Scholar]
- Tilton M, Borjali A, Isaacson A, Varadarajan KM, Manogharan GP, 2021. On structure and mechanics of biomimetic meta-biomaterials fabricated via metal additive manufacturing. Mater. Des 201, 109498. 10.1016/j.matdes.2021.109498 [DOI] [Google Scholar]
- Wang X, Xu S, Zhou S, Xu W, Leary M, Choong P, Qian M, Brandt M, Xie YM, 2016. Topological design and additive manufacturing of porous metals for bone scaffolds and orthopaedic implants: A review. Biomaterials 83, 127–141. 10.1016/j.biomaterials.2016.01.012 [DOI] [PubMed] [Google Scholar]
- Wu L, Zhang J, Jing D, Ding J, 2006. “Wet-state” mechanical properties of three-dimensional polyester porous scaffolds. J. Biomed. Mater. Res. - Part A 76, 264–271. 10.1002/JBM.A.30544 [DOI] [PubMed] [Google Scholar]
- Yan C, Hao L, Hussein A, Young P, 2015. Ti–6A1–4V triply periodic minimal surface structures for bone implants fabricated via selective laser melting. J. Mech. Behav. Biomed. Mater 51, 61–73. 10.1016/J.JMBBM.2015.06.024 [DOI] [PubMed] [Google Scholar]
- Yang L, Yan C, Han C, Chen P, Yang S, Shi Y, 2018. Mechanical response of a triply periodic minimal surface cellular structures manufactured by selective laser melting. Int. J. Mech. Sci 148, 149–157. 10.1016/j.ijmecsci.2018.08.039 [DOI] [Google Scholar]
- Yeh P-S, Lee Y-W, Chang W-H, Wang W, Wang J-L, Liu S-H, Chen R-M, 2019. Biomechanical and tomographic differences in the microarchitecture and strength of trabecular and cortical bone in the early stage of male osteoporosis. PLoS One 14, e0219718. 10.1371/journal.pone.0219718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zadpoor AA, 2019. Additively manufactured porous metallic biomaterials. J. Mater. Chem. B 7, 4088–4117. 10.1039/c9tb00420c [DOI] [PubMed] [Google Scholar]
- Zaszczyńska A, Moczulska-Heljak M, Gradys A, Sajkiewicz P, 2021. Advances in 3D Printing for Tissue Engineering. Materials (Basel). 14. 10.3390/MA14123149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Jiang Zhaoliang, Zhao L, Guo W, Jiang Zongxiang, Li X, Chen G, 2021. Mechanical characteristics and deformation mechanism of functionally graded triply periodic minimal surface structures fabricated using stereolithography. Int. J. Mech. Sci 208, 106679. 10.1016/j.ijmecsci.2021.106679 [DOI] [Google Scholar]
- Zhang L, Feih S, Daynes S, Chang S, Yu M, Wei J, Feng W, 2018. Energy absorption characteristics of metallic triply periodic minimal surface sheet structures under compressive loading. Addit. Manuf 23, 505–515. 10.1016/j.addma.2018.08.007 [DOI] [Google Scholar]
- Zhao Z, Tian X, Song X, 2020. Engineering materials with light: recent progress in digital light processing based 3D printing. J. Mater. Chem. C 8, 13896–13917. 10.1039/D0TC03548C [DOI] [Google Scholar]