Abstract
Brain tumors are devastating diseases of the central nervous system. Brain tumor pathogenesis depends on both tumor-intrinsic oncogenic programs and extrinsic microenvironmental factors, including neurons and glial cells. Glial cells (oligodendrocytes, astrocytes, and microglia) make up half of cells in the brain, and interact with neurons to modulate neurodevelopment and plasticity. Many brain tumor cells exhibit transcriptomic profiles similar to macroglial cells (oligodendrocytes and astrocytes) and their progenitors, making them likely to subvert existing neuron-glial interactions to support tumor pathogenesis. For example, oligodendrocyte precursor cells, a putative glioma cell of origin, can form bona fide synapses with neurons. Such synapses were recently identified in gliomas and drive glioma pathophysiology, underscoring how brain tumor cells can take advantage of neuron-glial interactions to support cancer progression. In this review, we briefly summarize how neurons and their activity normally interact with glial cells and glial progenitors, and discuss how brain tumor cells utilize neuron-glial interactions to support tumor initiation and progression. We also point out unresolved questions on these topics and potential avenues to therapeutically target neuron-glia-cancer interactions in the brain.
Keywords: neuron-glial interactions, brain tumor, glioma, neuron-glioma synapse, cancer neuroscience
Brain cancers are devastating diseases that recapitulate and subvert mechanisms of healthy brain development and plasticity. We will therefore consider glial malignancies in the context of the neuron-glial interactions that regulate and modulate normal brain function, discussing how these powerful intercellular interactions are subverted in the context of brain cancer.
INTERACTIONS BETWEEN NEURONS AND OLIGODENDROGLIA
Neuronal activity modulates oligodendroglial plasticity
Oligodendrocytes are glial cells that myelinate central nervous system (CNS) neuronal axons and facilitate fast saltatory electrical conductance of action potentials. Myelination is critical to neural circuit function, and even subtle changes in myelin profiles can alter axon conduction velocity, spike time arrival, and thus circuit dynamics [1]. In humans, learning certain tasks (e.g., piano practicing, learning to juggle, and working memory training) induces white matter (myelinated axon tract) microstructural changes in corresponding brain regions that are evident with advanced neuroimaging techniques, suggesting that certain experiences may modulate myelination in an activity-dependent manner [2–5]. Activity-regulated myelination was first observed in developing mammalian optic nerves, where premature eye-opening increases myelination [6]. In contrast, dark-rearing (to reduce light-induced optic nerve neuronal activity) or intraocular tetrodotoxin injection (to inhibit action potentials) delays optic nerve myelination [7, 8]. Moreover, both monocular deprivation and inhibiting glutamate release from retinal ganglion cells shorten internode length in mouse optic nerves, which is accompanied by reduced conduction velocity [9]. In larval zebrafish, which are transparent and there an excellent model to visualize myelination in vivo, researchers showed that axonal activity increases myelin sheath growth, stabilizes myelination of the active axons, and increases the number of myelin sheaths formed by individual oligodendrocytes [10–12]. Collectively, these findings demonstrate that neuronal activity modulates myelination during development.
Numerous studies in mice have now demonstrated experience-dependent changes in oligodendrogenesis and myelination during adolescence and adulthood. Neuronal activity can modulate myelination via remodeling existing oligodendrocyte processes [10, 11, 13, 14] or via regulating oligodendrogenesis, the formation of new oligodendrocytes by oligodendrocyte precursor cells (OPCs), and different mechanisms of experience-dependent myelination may operate in different brain regions and neuronal subtypes. For example, sensory enrichment by whisker stimulation increases myelination by inducing oligodendrogenesis, rather than stimulating existing oligodendrocytes to form new myelin sheaths in the adult somatosensory cortex [15]. Likewise, motor skill learning (complex wheel) and spatial learning (water maze) also increase oligodendrogenesis in corresponding brain regions, and genetically blocking OPC differentiation (OPC-specific Mrf knockout) decreases experience-dependent oligodendrogenesis and impairs learning in adult mice [16, 17]. Sensory deprivation such as social isolation decreases oligodendrogenesis and myelin sheath thickness in the prefrontal cortex of both juvenile and adult mice [18–20], a process modulated by neuregulin-1/ErbB3 signaling (Makinodan et al., 2012), while monocular visual experience deprivation results in neuronal cell type-specific myelin remodeling in the visual cortex (Yang et al., 2020). These findings together underscore the importance of experience-dependent OPC responses in normal CNS function.
Experience elicits a complex interplay among various types of neurons. In order to demonstrate the direct relationship between neuronal activity and OPC dynamics, tools of modern neuroscience such as optogenetics and chemogenetics have been leveraged. Optogenetic stimulation of neuronal activity in premotor cortical projection neurons [21] causes increased proliferation of OPCs and less differentiated pre-OPCs, oligodendrogenesis, and myelination within the cortico-callosal projections of the stimulated circuit [21]. These circuit-specific changes were also neuronal subtype-specific, restricted to corticocallosal projection neuron axons but not corticofugal projection neuron axons [21]. the increased myelination of the premotor cortex and projections into the corpus callosum was associated with an improved motor function (increased swing speed of the corresponding forelimb), and pharmacologically inhibiting OPC differentiation blocked these activity-induced myelin and motor function changes [21]. Similar to findings with optogenetic models, chemogenetic elevation of neuronal activity in somatosensory cortical neurons elicits a circuit-specific increase in OPC proliferation, oligodendrogenesis, and myelination [22]. One required component of the mechanism mediating neuronal activity-regulated OPC proliferation and oligodendrogenesis in cortical projection neurons is activity-regulated BDNF signaling to the neurotrophin receptor tyrosine kinase (TrkB) receptor on OPCs [23]. Inhibiting adaptive myelination through OPC-specific deletion of Ntrk2 (encoding the BDNF receptor TrkB) during adulthood impairs attention and short-term memory function [23].
Taken together, these studies of normal neuron-glial interactions demonstrate that neuronal activity directly modulates oligodendrogenesis and myelination in certain brain regions, and adaptive myelination can lead to functional changes in the CNS.
Neuronal activity drives the proliferation and growth of gliomas
Numerous additional studies suggest an OPC or earlier pre-OPC cell of origin for multiple forms of gliomas [24–34]. Concordantly, neuronal activity drives the proliferation of malignant glioma cells just as it does their normal glial precursor counterparts. Optogenetic stimulation of cortical projection neurons drives the proliferation and growth of patient-derived high-grade glioma xenografts in a circuit-specific manner, a study that provided the first evidence that brain activity can influence brain cancer progression (Venkatesh et al., 2015). Similarly, optogenetic stimulation of the optic nerve promotes low-grade optic pathway glioma growth (Pan et al., 2021). In a mouse model of olfactory bulb high-grade glioma, olfactory experience modulates tumor progression [35].
OPCs form synapses with neurons, a mechanism hijacked by glioma cells
One prominent feature of OPCs is that they form bona fide synapses with both glutamatergic and GABAergic neurons [36–39]. Given the gliomagenic potential of OPCs, it is plausible that glioma cells form synapses with neurons like OPCs. Indeed, recent studies revealed that both high-grade (pediatric and adult) and low-grade glioma (adult IDH-mutant) cells form bona fide synapses with neurons [40, 41] (Fig. 1). Examination of primary glioma tissue samples and patient-derived xenografts by electron microscopy clearly demonstrates synaptic structures between presynaptic neurons and postsynaptic glioma cells [40, 41].
Figure 1. Mechanisms of neuronal activity-mediated glioma pathogenesis.
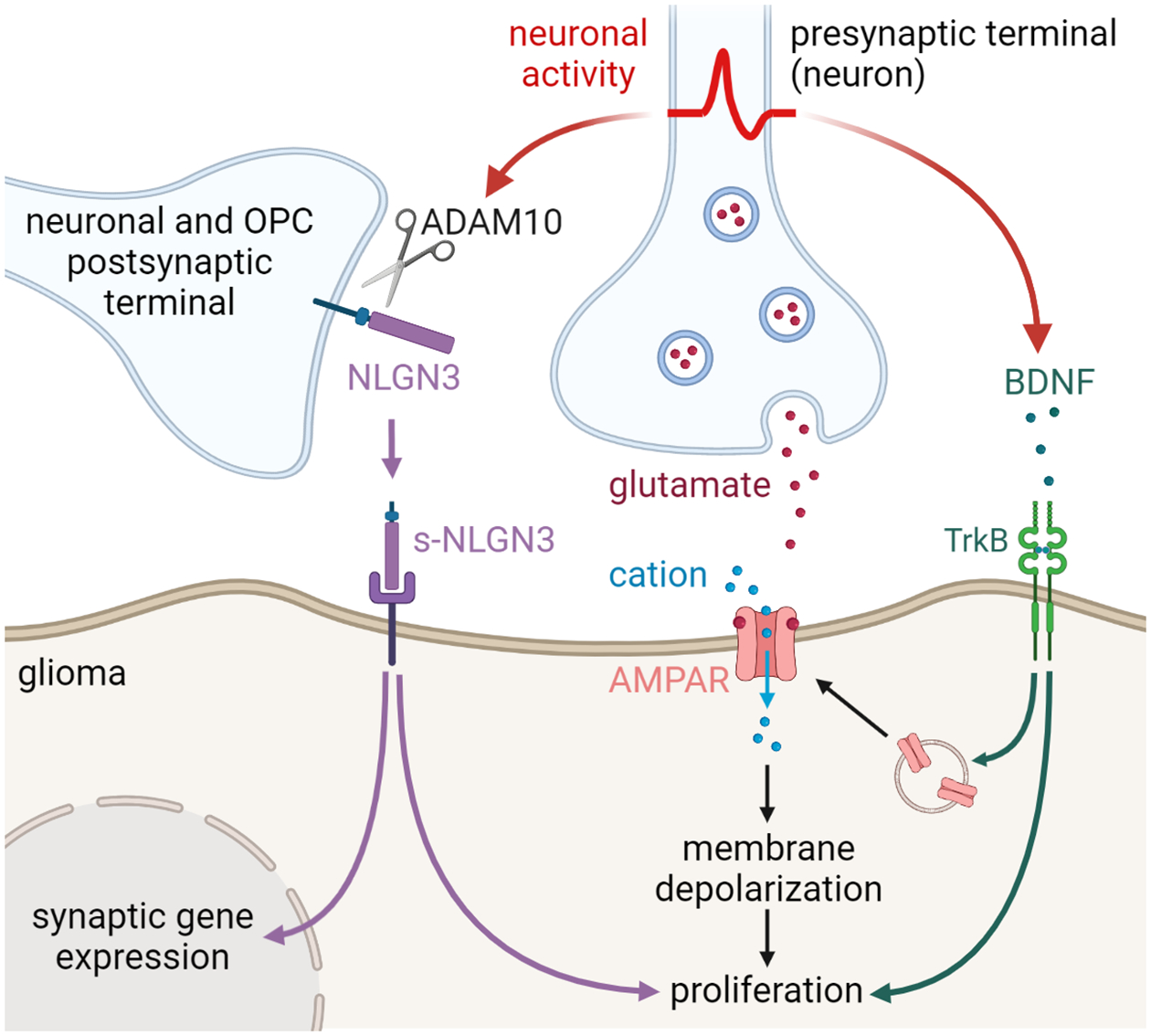
Neuronal activity drives glioma growth through both paracrine (ADAM10/NLGN3 and BDNF) signaling and bona fide synapses mediated by AMPA receptors (AMPAR).
Whole cell patch clamp electrophysiology of glioma cells illustrates fast inward currents in the glioma cells consistent with excitatory postsynaptic currents (EPSCs). These fast currents are inhibited by tetrodotoxin, indicating that the neuronal activity induces inward currents in glioma cells, and exhibit multiple electrophysiological characteristics of synapses, including paired-pulse facilitation [41] and mini-EPSCs representative of single vesicle events [40, 41]. These activity-induced glioma EPSCs are depolarizing [40, 41], and directly depolarizing glioma cells using optogenetics leads to increased glioma proliferation in vivo [41] (Fig. 1).
The neuron-to-glioma synapses described to date are glutamatergic, mediated by calcium-permeable AMPA receptors [40, 41]. Blocking neuron-glioma synaptic communication using AMPA receptor inhibitor, perampanel, or genetically expressing a dominant-negative form of GluA2, inhibited glioma growth in vivo [40, 41]. Importantly, perampanel is an anti-epileptic medication, and these findings underscore the potential of repurposing neuromodulatory compounds in treating gliomas.
It is not yet clear whether other primary brain tumors form synapses with neurons. While brain tumor cells of origin remain an area of intensive research and discussion, many forms of brain tumors exhibit gene expression profiles like stem/progenitor cells. For example, in medulloblastomas, the SHH-subtype is thought to arise from granule neuron precursor cells in the cerebellum [42, 43], WNT-subtype may develop from precursor cells in the embryonic dorsal brainstem [44], and group 4 likely originate from glutamatergic neuron cellular origins [45]. How these tumor cells interact with neurons may be informed by the known neuronal regulation of their putative cellular origin, but much remains to be demonstrated for non-glial primary brain malignancies.
Neuroligin-3 and BDNF are activity-dependent factors involved in neuron-glioma interactions
In addition to synaptic signaling, neuronal activity promotes glioma proliferation and growth through activity-regulated paracrine signaling [46]. In order to identify paracrine signaling molecules responsible for neuronal activity-driven glioma pathogenesis, the secretome of cortical or retinal+optic nerve explants was evaluated at varying levels of neuronal activity (tetrodotoxin-silences, optogenetically stimulated, or spontaneously active). This paradigm discovered that activity-regulated paracrine factors promote the proliferation of a range of pediatric and adult low-grade and high-grade glioma types [46, 47] (Fig. 1). Proteomic analyses followed by candidate factor necessity and sufficiency testing reveal two key glioma mitogens. Unsurprisingly, BDNF promotes the proliferation of glioma cells [46], just as do healthy OPCs [23]. Activity-regulated paracrine factors driving glioma growth may be tumor type or region/circuit-specific to some extent, as IGF-1 was found to be a critical paracrine factor driving olfactory bulb high-grade gliomas in mice (Chen et al., 2022). While activity-regulated neurotrophic and growth factor signaling parallels known mechanisms of normal neurodevelopment and plasticity, quite unexpected was the discovery that a shed form of neuroligin-3 (Nlgn3), a postsynaptic membrane protein, functions to promote glioma proliferation [46, 47]. Neuronal activity regulates Nlgn3 shedding [46, 48], and Nlgn3 increases the growth of several types of glioma (H3K27M-altered diffuse midline glioma, H3WT pediatric hemispheric high-grade glioma, IDH WT adult high-grade glioma, IDH-mutant oligodendroglioma, and Nf1 optic glioma) in vitro.
Besides functioning as a paracrine mitogen for glioma, functioning to stimulate multiple oncogenic signaling pathways (FAK, PI3K-mTOR, RAS, and SRC), shed Nlgn3 also serves as a synaptogenic factor for gliomas, in part by upregulating the expression of synapse-associated genes in glioma cells [41, 46, 48]. Consistent with the synaptogenic and mitogenic effect of Nlgn3 in gliomas, glioma growth and progression are markedly reduced in the brains of Nlgn3−/− mice [46].
Neuronal activity and consequent neuroligin-3 shedding are also required for the initiation of certain gliomas. In the neurofibromatosis type 1 (NF1) cancer predisposition syndrome, patients often develop gliomas in the optic nerve (optic glioma). The optic nerve contains axons of retinal ganglion cells, whose neuronal activity is modulated by light. Blocking light-induced optic nerve neuronal activity (dark-rearing) prevents optic glioma initiation in a genetically engineered mouse model of Nf1 optic glioma [47]. In this NF1 optic glioma mouse model (Nf1flox/mut;Gfap-Cre), all somatic cells are Nf1 heterozygous, thus recapitulating the germline NF1 mutations in NF1 patients. Interestingly, light exposure increases neuroligin-3 shedding only in the Nf1+/−, but not wild-type, optic nerves, indicating that cancer predisposition mutations can alter the tumor microenvironment and suggesting that an aspect of the predisposition to optic nerve gliomas is this aberrantly increased Nlgn3 shedding in the optic nerve. Indeed, it was later discovered that this aberrantly increased Nlgn3 shedding is due to hyperexcitability of the Nf1-mutant optic nerve [49]. Nlgn3 knockout in the optic glioma mouse model results in fewer and smaller optic gliomas formed compared to Nlgn3 wild-type littermate control mice. Together, these findings demonstrate that the cancer predisposition Nf1 mutation synergizes with neuronal activity to increase the shedding of Nlgn3, which drives optic glioma initiation.
Like the role that optic nerve activity plays in optic pathway low-grade glioma initiation, olfactory neuronal activity regulates the incidence of high-grade gliomas of the olfactory bulb in a genetically engineered mouse model of IDH WT glioblastoma. In this case, IGF-1 is the key activity-regulated paracrine factor [35].
Glioma cells recruit mechanisms of adaptive plasticity, and in this regard, activity-regulated BDNF functions not only as a paracrine growth factor but also to elaborate and reinforce neuron-to-glioma synapses, modulating the strength of glutamatergic currents in glioma cells and regulating the number of neuron-to-glioma synapses [50] (Fig. 1). BDNF increases the amplitude of glioma currents elicited by glutamate, increases glutamate-induced glioma Ca2+ influx, and enhances AMPA receptor surface trafficking in glioma cells. Consistently, Ntrk2 (gene encoding TrkB) knockdown in human glioma cells reduces glioma cell response to glutamate, decreases neuron-glioma synaptic connectivity, and abrogates neuronal activity-induced glioma proliferation. Importantly, blocking activity-dependent BDNF expression is sufficient to increase the survival of glioma-bearing mice [50].
These discoveries suggest that NLGN3 and BDNF are potential therapeutic targets for gliomas. Neuroligin-3 (NLGN3) shedding is mediated by the sheddase ADAM10. ADAM10 inhibitors reduce high-grade glioma growth in mouse xenograft models [48] and inhibit the initiation and maintenance of Nf1 optic glioma in a genetic mouse model [47]. INCB7839, an ADAM10 inhibitor, is currently being tested in a phase I clinical trial for recurrent/progressive pediatric high-grade gliomas (NCT04295759, Table 1). By targeting the BDNF-TrKB signaling axis, the pan-Trk inhibitor entrectinib has shown efficacy in reducing glioma growth in mouse xenograft models of high-grade glioma; working by targeting neuron-glioma interactions, these effects were seen in glioma models that lack NTRK-fusions [50]. Entrectinib has been approved by the FDA for the treatment of cancers with NTRK fusions, and its effect on CNS tumors with NTRK fusions is currently being tested in clinical trials (NCT02650401, NCT02568267, Table 1). The preclinical results described above suggest that Trk inhibitors may provide benefit more broadly in gliomas, and this hypothesis should be tested in clinical studies.
Table 1.
Clinical trials targeting interactions in the brain tumor neural microenvironment
| Clinical trial # | Name of the study | Molecular target | Target interaction |
|---|---|---|---|
| NCT04295759 | INCB7839 in Treating Children With Recurrent/Progressive High-Grade Gliomas | ADAM10-mediated neuroligin-3 shedding | Neuron-Glioma |
| NCT02568267 | Basket Study of Entrectinib (RXDX-101) for the Treatment of Patients With Solid Tumors Harboring NTRK 1/2/3 (Trk A/B/C), ROS1, or ALK Gene Rearrangements (Fusions) (STARTRK-2) | TrkA,B,C | Neuron-Glioma |
| NCT02650401 | Study of Entrectinib (Rxdx-101) in Children and Adolescents With Locally Advanced or Metastatic Solid or Primary CNS Tumors and/or Who Have No Satisfactory Treatment Options (STARTRK-NG) | TrkA,B,C | Neuron-Glioma |
| NCT02363933 | Perampanel in Seizure Patients With Primary Glial Brain Tumors | AMPA receptor | Glioma-Neuron |
| NCT03636958 | Efficacy and Safety of Perampanel in Combination in Glioma-refractory Epilepsy | AMPA receptor | Glioma-Neuron |
| NCT04497142 | Effect of Perampanel on Peritumoral Hyperexcitability in HGG | AMPA receptor | Glioma-Neuron |
| NCT05169944 | Magrolimab in Children and Adults With Recurrent or Progressive Malignant Brain Tumors (PNOC025) | CD47 | microglia/macrophage-tumor cell |
| NCT02429570 | Meclofenamate in Subjects With Recurrent or Progressive Brain Metastasis From Solid Tumor Primary | Gap junctions | Astrocyte-tumor cell |
INTERACTIONS BETWEEN NEURONS AND ASTROCYTES
Astrocytes modulate neuronal circuit assembly and plasticity
Astrocytes are one of the most abundant glial cells in the CNS. It has been long appreciated that astrocytes play supportive roles such as providing substrates to neurons, maintaining extracellular neurotransmitter and ion homeostasis, and maintaining the blood-brain barrier. It was later found that astrocytes also actively communicate with neurons and modulate circuit assembly and plasticity [51, 52]. Astrocytes produce synaptogenic factors (e.g., thrombospondin, hevin, glypicans) that promote synapse formation between neurons and thereby play a key role in neurodevelopment [53–55]. Astrocytes also assist synaptic pruning either directly via phagocytic receptors (MERTK and MEGF10) or indirectly by modulating microglia-driven synaptic elimination by IL-33 [56, 57]. Moreover, astrocytes are involved in synaptic maturation, during which postsynaptic calcium-permeable AMPA receptor subunits are gradually switched to calcium-impermeable ones to promote synaptic stability. Astrocytes secrete chordin-like 1 (Chrdl1) that facilitates the switch to GluA2-containing calcium-impermeable AMPA receptors at postsynaptic terminals, and thus limit synaptic plasticity [58].
Astrocytes also support synaptic function. At the ultrastructural level, perisynaptic astrocyte processes form a tripartite structure (tripartite synapse) with an astrocytic process cupping a presynaptic terminal and a postsynaptic terminal of neurons. The close association of astrocytes to neuron-neuron synapses facilitates the clearance of neurotransmitters and stabilizes synaptic connections (reviewed in [59]). The morphogenesis of astrocyte processes are regulated by neuroligins. Knocking down neuroligin-1, 2, or 3 reduces astrocyte arborization in vitro (co-culture with neurons) and in vivo [60]. In addition, genetically ablating neuroligin-2 in astrocytes inhibits the formation and function of cortical excitatory synapses, and enhances the function of inhibitory synapses [60]. These findings demonstrate the critical role of neuroligins in modulating astrocyte morphogenesis and astrocyte-mediated synaptogenesis. While most studies have focused on excitatory neurons/synapses, recent studies demonstrated that astrocytes also support the formation and function of GABAergic synapses via the neuronal cell adhesion molecule (NRCAM) expressed by both astrocytes and the inhibitory postsynaptic terminals [61].
In addition to forming direct contacts with synapses, astrocytes secrete factors (e.g., glutamate, ATP, D-serine) that participate in neuromodulation [62, 63]. For example, D-serine is released by astrocytes to modulate long-term potentiation in a Ca2+-dependent manner [64]. Furthermore, lactate transportation from astrocytes to neurons is critical for maintaining long-term potentiation and long-term memory formation [65].
Together, these findings underscore the critical role of astrocytes in modulating neurodevelopment and neuronal function.
Astrocytes respond to neuronal activity
The interactions between astrocytes and neurons are bi-directional. Astrocytes modulate neuronal function and neuronal activity also guides astrocyte behaviors. Changes in intracellular Ca2+ concentrations in astrocytes are often observed upon neuronal activation [66–69]. In the mouse hippocampal slice culture system, both intracellular Ca2+ concentrations and the motility of perisynaptic astrocyte processes are increased by neuronal activity [70]. Similarly in the mouse somatosensory cortex, whisker stimulation increases perisynaptic astrocyte process motility [70].
Astrocytes express a variety of neurotransmitter receptors that can respond to neuronal activity by sensing extracellular neurotransmitters [71]. Adult human astrocytes respond to glutamate and display increased intracellular Ca2+ levels, which can be inhibited by the mGluR5-specific antagonist 2-methyl-6-phenylethynyl-pyridine (MPEP) [72]. Decreasing neuronal glutamate release inhibits astrocyte maturation in vivo [73]. The inhibitory neurotransmitter GABA also induces astrocytic calcium signaling during neurodevelopment in a GABA receptor-dependent manner [74]. These findings indicate that astrocytes express the molecular machinery that senses neuronal activity and activity-dependent factors.
Reactive astrocytes in brain tumors
Reactive astrocytes are astrocytes that display morphological, molecular, and/or functional changes in response to various conditions [75]. Multiple states of reactive astrocytes have been identified and contribute to a range of CNS disorders [72, 75–78]. Using markers such as GFAP (glial fibrillary acidic protein) together with morphological changes of hypertrophy, reactive astrocytes are observed in the microenvironment of brain tumors, including glioma, medulloblastoma, and brain metastases [79, 80], but a more granular understanding of the precise cell state and role in pathogenesis – as has been accomplished in neurodegenerative disease research using single-cell transcriptomics - is largely lacking at present in the brain tumor literature. Early studies using astrocyte conditioned media demonstrate that secreted factors from astrocytes can promote the proliferation, stemness, invasion and/or therapy resistance of brain tumor cells (primary and metastatic) in vitro [81]. Since GFAP does not distinguish astrocytes from some brain tumor cells in primary samples, two groups recently used HepaCAM (Hepatic and Glial Cell Adhesion Molecule) antibodies to isolate glioblastoma (GBM)-associated astrocytes, and found that these astrocytes exhibit expression signatures resembling human fetal astrocytes [72, 82]. The GBM-associated astrocytes exhibit JAK/STAT hyperactivation and an anti-inflammatory state. JAK inhibition increases inflammation in the GBM microenvironment ex vivo [82]. These findings suggest that astrocytes can shape the brain tumor immune microenvironment, but much work remains to be done.
Some brain tumor cells are astrocyte-like
In glioma, some tumor cells display astrocyte-like gene expression signatures [32, 83, 84]. Similarly, in SHH-medulloblastoma, a population of tumor cells assumes an astrocyte-like state [85]. Many cancer cells metastatic to the brain also exhibit astrocytic features. Zeng et al. showed that breast-to-brain metastasis cells form pseudo-tripartite synapses with glutamatergic neurons and leverage NMDAR-mediated signaling to promote metastatic colonization and growth of breast cancer brain metastases [86].
A central role for astrocytes in brain metastases is well-established. Brain metastatic cancer cells form gap junctions with astrocytes, a phenomenon observed both in vitro and in vivo [79, 80, 87]. The brain metastatic cells (breast and lung cancer) transfer secondary messenger cGAMP to astrocytes, leading to astrocytic secretion of inflammatory cytokines (e.g., IFNa and TNFa) that increase growth and chemoresistance of the cancer cells. Gap junction inhibitors (meclofenamate and tonabersat) inhibit the outgrowth of the brain metastases in vivo [87]. Meclofenamate is currently undergoing being tested in a clinical trial for brain metastases (NCT02429570, Table 1). Furthermore, multiple models of brain metastasis (lung cancer, breast cancer, and melanoma) exhibit the presence of a subpopulation of reactive astrocytes with active STAT3. These astrocytes alter the immune microenvironment to support metastasis [88]. Genetically ablating Stat3 in astrocytes or pharmacologically inhibiting STAT3 activity reduces the growth of brain metastases in vivo [88]. Collectively, these findings suggest that reactive astrocytes could serve as a therapeutic target for preventing or treating brain metastases.
Glioma cells produce factors that increase neuronal excitability and remodel local neuronal networks
Given their phenotypic similarity to astrocytes, subpopulations of glioma cells may produce synaptogenic factors to shape the neuron-neuron and neuron-glioma connections in the tumor microenvironment. One study in the adult mouse brain identified several subtypes of normal astrocytes, with one subtype enriched in genes regulating synapse formation and function, as well as enhanced synaptogenic ability in astrocyte-neuron co-culture [84]. These prospective astrocyte signatures are found in mouse and human high-grade non-GSC (non-glioma stem cell) glioma cells. Moreover, the expression of certain astrocytic signatures increases together with the progression of glioma-associated seizures [84], implicating astrocyte-like glioma cells in increasing neuronal hyperexcitability.
Astrocyte-derived glypicans (glypican 4 and 6) are known to drive the formation of excitatory synapses in the healthy brain [55]. In high-grade gliomas, Yu et al. discovered that glypican-3 is responsible for glioma-driven synaptogenesis and network hyperexcitability, and that this can occur in a mutation-specific way such that glioma cells with certain point mutations in PIK3CA drive neuronal hyperexcitability, while gliomas with other point mutations in the same gene do not. [89]. Similarly, thrombospondin-1, which is made by astrocytes to enhance synaptogenesis, can be produced by glioma cells to increase tumor invasion and growth [90]. In IDH-WT glioblastoma (GBM), tumor regions that are more functionally connected to neuronal network produce higher levels of thrombospondin-1 than regions that are less integrated into the network [91]. Single-cell RNA sequencing analysis further demonstrated that in the high functionally connected regions, both glioma and astrocytes produce thrombospondin-1, whereas only non-malignant astrocytes produce thrombospondin-1 in the low functionally connected regions. High functional connectivity of the tumor with the rest of the brain is strongly correlated with shorter survival. These findings suggest that some tumor-derived astrocytic factors can remodel synaptic connections in the brain tumor microenvironment, and that functional connectivity of the tumor negatively influences patient survival [91].
In addition to synaptogenic factors, glioblastoma cells also secrete glutamate to induce neuronal hyperexcitability and epileptic activity, which is a common comorbidity associated with brain tumors. Glioblastoma cells secrete glutamate in a system xc− cystine-glutamate transporter-dependent manner and pharmacological inhibition of system xc− using sulfasalazine reduced the glioma-associated epileptic event frequency (Buckingham et al., 2011; Campbell et al., 2012). Moreover, glioma-induced loss of inhibitory interneurons also contributes to glioma-induced neuronal hyperexcitability [92–94]. Campbell et al. also revealed that neurons in the brain tumor microenvironment exhibit decreased KCC2 (potassium-chloride cotransporter) and increased intracellular Cl− concentration, rendering GABA responses depolarizing and excitatory rather than inhibitory. Hatcher et al. performed a study monitoring the evolution of hyperexcitability in glioma peritumoral microenvironment in immunocompetent mice, and found that early inhibition of glial glutamate antiporter xCT and genetically suppressing neuronal excitability reduce glioma-induced seizure activity [95]. Together, these findings demonstrate that glioma cells can produce neurotransmitters and synaptogenic factors that induce neuronal hyperexcitability, thereby contributing to glioma-associated seizures and augmenting the neuron-glioma signaling mechanisms that drive glioma progression. The hyperexcitability of the glioma-infiltrated cortex has been confirmed in humans using intraoperative electrocorticography (Venkatesh et al., 2019), and magnetic encephalography (MEG) studies in adult patients with glioblastoma revealed that elevated neuronal activity, measured as broadband power, predicted a worse prognosis in both the pre-operative [96] and post-operative [97] settings.
Glioma cells exhibit activity-dependent potassium-evoked currents reminiscent of astrocytes
In astrocytes, neuronal activity-mediated rise in extracellular potassium induces inward, potassium-evoked currents [98–100]. Similarly, in a subgroup of glioma cells, neuronal activity induces non-synaptic potassium-evoked currents [40, 41]. The amplitude of these currents increases with increasing neuronal activity, and the current is largely blocked by inhibiting the inward rectifying potassium channels (Venkatesh et al., 2019).
Both adult [40, 101] and pediatric [26, 41] glioma cells form tumor-to-tumor gap junction-coupled networks, much like normal astrocytes. This gap junction coupling in glioma is responsible for the synchrony of calcium transients in glioma cells upon neuronal activation. Gap junction blockers decrease the amplitude of prolonged currents, reduce calcium transient synchrony, increase input resistance in glioma cells, and inhibit glioma growth in vivo [40, 41]. Collectively, these findings show that glioma cells, and perhaps other brain tumor cells, can resemble astrocytes to modulate synaptic connectivity and glioma inter-connectivity to support tumor growth.
INTERACTIONS BETWEEN NEURONS AND MICROGLIA
Microglia sculpt neuronal connections and modulate neuronal activity
Microglia are brain-resident immune cells derived primarily from yolk sac myeloid precursor cells and comprise approximately 5–15% of cells in the CNS. During neurodevelopment, microglia sculpt neuronal connections such that precise synaptic connections are achieved. In the developing lateral geniculate nucleus (LGN), retinal ganglion cell axon terminals from both eyes initially overlap with each other but are later pruned to eye-specific territories (left vs right eye territories). During this eye segregation process, C1q and C3, the “eat me” signals of the complement immune pathway (Elward and Gasque, 2003; Lauber et al., 2004), are expressed in the synapses. These complement components activate microglia, which express the receptor of C3, CR3 (complement receptor 3), to engulf the excess synapses. Genetically ablating C3 or CR3 in mice impairs synaptic pruning and decreases synaptic connectivity in the developing LGN [102, 103]. In addition, microglia can engulf axonal/presynaptic materials through trogocytosis and induce dendritic spine head filopodia in the hippocampus [104].
Throughout life, microglia regulate neuronal function [105]. Extracellular vesicles secreted by microglia carry lipids that are capable of enhancing excitatory synaptic transmission (sphingolipid) and suppressing inhibitory synaptic transmission (N-arachidonoylethanolamine) [106, 107]. Moreover, microglial P2Y12 is required for experience-dependent synaptic plasticity of the visual cortex in vivo [108]. Microglia also mediate the activity-dependent plasticity of glycinergic synapses via prostaglandin E2 and neuronal EP2 receptors [109]. Several studies in mice have demonstrated that disrupting microglial function leads to social behavioral deficits and repetitive behaviors in adulthood [110–112].
Badimon et al. recently identified an important function of microglia similar to inhibitory neurons [113], such that microglial depletion results in hyperexcitable neurons and increased neurostimulant-induced seizures. The authors showed that microglial depletion increases striatal neuron synchrony and lowers the threshold for activating these neurons. This process is dependent on regional ATP production from neurons and astrocytes. Microglia convert the ATP into adenosine, which suppresses neuronal activity by binding to neuronal adenosine receptor A1R [113], demonstrating that neuronal activation in the striatum triggers a negative feedback signaling in microglia to suppress neuronal activity. Concordantly, microglia were shown to protect mice from epilepsy [112, 114]. These findings together highlight the importance of microglia-neuron communications in normal CNS development and function.
Neuronal activity guides microglial dynamics
Microglia processes constantly survey the CNS parenchyma. Neuronal activation increases targeted microglial branch extension and microglia-neuron proximity [113, 115–118], and alters microglial transcriptional profiles [113], indicating that neuronal activity conveys information to microglia. At ultrastructural levels, microglial processes contact neuronal cell body and form junctions that display unique features such as closely apposed mitochondria. The formation of these microglia-neuron junctions is enhanced by neuronal activity in a P2Y12-dependent manner [119].
Studies in the visual system demonstrated that microglia-mediated synaptic pruning is guided by neuronal activity in vivo. Pharmacologically inhibiting retinal ganglion cell (RGC) firing increases the likelihood of microglial engulfing of RGC axon terminals, whereas increasing RGC neuronal activity decreases microglial synaptic pruning [103]. Consistently, blocking visual experience in mice using dark-rearing increases the number of microglia at synapses that eventually decrease in size. This process can be rescued by reintroducing mice to light [120]. Together, these studies demonstrate that neuronal activity modulates neuron-microglia interactions.
Tumor-associated microglia in brain tumors
In the homeostatic state, microglia usually display a ramified morphology. In response to various stimuli, microglia can adopt an amoeboid morphology (round cell body with few processes) referred to as “reactive microglia”. Microglial reactivity is a generalized term and was used to describe various reactive states/phenotypes, including brain tumor-associated microglia [121, 122]; new insights from single-cell genomics define multiple states of both healthy and reactive microglia [123].
Both microglia and macrophages are commonly observed in brain tumors, often accounting for a large fraction of the cells within the tumor [124–129]. For example, in low-grade gliomas such as pilocytic astrocytomas, TAMs account for 35–50% of the cells in the tumor [130, 131]. The expression of conventional markers that distinguish microglia and peripherally infiltrated macrophages in a healthy state (e.g., CD45, CX3CR1, and CCR2) changes when microglia/macrophages encounter the brain tumor microenvironment [132–134]. While intriguing and underscoring tumor-mediated molecular changes, this phenomenon imposes challenges to distinguish microglia from macrophages in brain tumors. As such, new molecular markers and tools are being proposed to separate brain tumor-associated microglia and macrophages [89, 134–136]. The number and molecular phenotype of tumor-associated microglia and macrophages (TAMs) in brain tumors depends on the type/subtype of the brain tumors [128], and can also be influenced by age, brain location, and sex. The role of TAMs in brain tumor pathogenesis also varies in different brain tumor types [121, 122].
Neuron-immune crosstalk in brain tumor pathogenesis
TAMs are often tumor-promoting in gliomas. In high-grade gliomas, TAMs produce cytokines and growth factors that increase tumor growth and invasion [127, 137], either directly or through immune-suppressive effects on tumor-infiltrating lymphocytes that would otherwise fight the tumor. Depletion of TAMs through CSF1R inhibition in preclinical models of adult high-grade glioma – a high-grade glioma type in which tumor-infiltrating lymphocytes occur – slows growth and improves outcomes [127]. In a genetically engineered mouse model of NF1-associated low-grade glioma (NF1 optic glioma), genetically (Cx3cr1 knockout) or pharmacologically (minocycline) inhibiting microglia delayed glioma formation [138–140].
It was further demonstrated in this NF1-associated low-grade glioma model that T cell secretion of Ccl4 in the tumor microenvironment induces microglial Ccl5 production that is required for Nf1 optic glioma growth [141, 142]. Importantly, Ccl4 production from T cells is mediated by neurons that harbor Nf1 mutations. Nf1-mutant neurons upregulate secretion of midkine that activates CD8 T cells to produce Ccl4 [141].
In the brain, neurons express various signaling molecules that can influence the function of T cells (e.g., TGFβ and FasL) [143, 144] and microglia (e.g., CX3CL1, CD22, and ICAM5) [145–147], suggesting that the neuron-immune crosstalk can modulate the tumor immune microenvironment and may be a potential driver of brain tumor pathogenesis. Such interactions may also influence the efficacy of immuno-therapies, and more work is required to understand the neuronal influence on immune-suppressive aspects of the tumor immune microenvironment.
Microglia in cancer therapy-related cognitive impairments
Besides driving brain tumor cell growth, dysregulated microglia/macrophages can lead to radiation/chemotherapy-induced neurocognitive impairments, which are common neurological sequelae for brain tumor survivors. Part of the mechanism of radiation-induced and chemotherapy-induced cognitive impairment is through acute, direct damage to neural progenitor cells and thus neurogenesis and oligodendrogenesis [148–150]. These direct effects are largely transient, and yet cognitive impairment is typically persistent and progressive. Contributing importantly to these persistent deficits is cancer therapy-induced microglial/macrophage reactivity and consequent dysregulation of neural precursor cells. In the hippocampus, cranial radiation results in microglial reactivity that persistently inhibits adult neurogenesis via secreted cytokines such as IL-6 [148]. In addition, microglial C1q expression is elevated following irradiation, causing aberrantly increased synaptic pruning, and knocking out C1q specifically in microglia rescues the irradiation-induced microglia activation and synaptic loss [151].
In a mouse model of chemotherapy-related cognitive impairment (CRCI), persistent white matter microglial reactivity is observed after methotrexate (a chemotherapy agent used in treating brain tumors, leukemia, and many other cancers) exposure. Methotrexate directly induces microglial reactivity, which in turn induces neurotoxic astrocyte reactivity, oligodendroglial lineage dysregulation, and loss of myelin plasticity. This multi-lineage glial dysregulation leads to cognitive impairments such as deficits in attention and memory [23, 152]. These glial and cognitive deficits can be rescued by depleting microglia using a CSF1R inhibitor following the methotrexate exposure [23, 152]. Together, these studies highlight microglia/macrophage dysregulation as potential therapeutic targets for cancer therapy-related cognitive impairment.
Brain tumors subvert microglia-neuron interactions
Microglial interact with neurons via various molecular mechanisms. Microglia express neurotransmitter receptors (e.g., glutamate, ATP, GABA) [153], as well as surface (e.g., CD200R, CX3CR1, CSF1R, and TREM2) and secreted (e.g., IL-1β, TNFα, PGE2, BDNF) factors that interact with neurons [154]. CD47 is one critical molecule involved in neuronal activity-dependent microglial synaptic pruning. CD47 is a widely expressed cell surface protein that provides a “don’t eat me” signal to prevent the innate immune cells from attacking host cells [155, 156]. In the CNS, CD47 expression is found at synapses [157–159], and the expression of CD47 binding partner SIRPα (signal regulatory protein alpha) is enriched in microglia during the peak of developmental synaptic pruning [159]. Moreover, CD47 preferentially localizes to more active synapses during the eye segregation [159]. Mice lacking CD47 or SIRPα exhibit excess microglial synaptic engulfment and decreased synaptic density [159, 160]. These findings indicate that the CD47/SIRPα axis protects active synapses from microglial engulfment.
Brain tumors take advantage of this elegant CD47/SIRPα “don’t eat me” signaling axis. Several brain tumor cells upregulate CD47 to escape phagocytosis [161]. In patients with gliomas, higher expression of CD47 is correlated with higher tumor grade and poorer overall survival [162]. Importantly, anti-CD47 antibodies can disrupt CD47/SIRPα signaling and increase phagocytosis of high-grade glioma cells by both macrophages and microglia [162–164]. In addition, anti-CD47 treatment decreases the growth of several types of pediatric brain tumors (group 3 medulloblastoma, atypical teratoid rhabdoid tumor, primitive neuroectodermal tumor, pediatric glioblastoma, and diffuse midline glioma) in vivo, and improves mouse survival in both patient-derived xenograft models and an immunocompetent glioma model [164]. The efficacy of anti-CD47 treatment on glioblastoma in preclinical models is further increased when combined with irradiation or temozolomide [165], indicating that CD47 is a potential therapeutic target for certain types of brain tumors. Anti-CD47 treatment is currently in a clinical trial for patients with malignant brain tumors (NCT05169944, Table 1). Given the role of CD47 in inhibiting microglial engulfment of synapses, glioma CD47 could also engage in the maintenance or refinement of neuron-glioma synapses, which is an important question to address in the future.
SUMMARY
The interactions among neurons, glia, and brain tumor cells are intertwined and complex. Neuron-glia and neuron-glioma interactions are bi-directional, and can influence the behavior of other cell types. For example, glioma induces hyperexcitability of neurons via secretion of neurotransmitters and synaptogenic factors, driving the growth-promoting effects of neurons on tumor growth and further influencing other glial cell types in the tumor microenvironment in a malignant cycle of cell-cell interactions (Fig. 2). Furthermore, glial cells interact with each other [166–168]. For instance, astrocyte-derived IL33 stimulates microglial synaptic engulfment during neurodevelopment [57], and such glia-glia interactions should also be considered when examining the pathogenesis of brain tumors. The complex interaction between neurons, stromal cells, and tumor cells exists in both the central and peripheral nervous systems. A number of studies have demonstrated the role of neurons in the pathogenesis of other cancers (e.g., prostate cancer, pancreatic cancer, etc.) [169, 170]. In pancreatic cancer, both tumor-promoting and tumor-inhibiting effects of different types of peripheral nerves have been observed. Whereas sensory and sympathetic nerves are required for tumor initiation and progression [171, 172], parasympathetic nerves suppress tumorigenesis and cancer stemness [173].
Figure 2. The crosstalk between neuron, glia, and brain tumor cells.

The interaction between neuronal activity, microglia, oligodendroglial cells, astrocytes, and brain tumor cells. OPC, oligodendrocyte precursor cells; OL, Oligodendrocyte; TME, tumor microenvironment.
To date, most studies in neuron-brain tumor interactions have focused on gliomas. Other primary brain tumors such as medulloblastoma and ependymoma have different tumor-initiating cells and oncogenic programs. It will be important for future investigations to determine how neurons interact with each type of brain tumor, and more specifically, with the molecular subtypes of each tumor type that differ in pathophysiology, treatment responses, and clinical outcome. Elucidating complex neuron-glia-immune-tumor crosstalk and identifying the molecular mechanisms are critical for understanding brain tumor pathophysiology, identifying potential therapeutic targets, and improving outcomes for these debilitating and lethal cancers.
ACKNOWLEDGMENTS
The authors gratefully acknowledge Alex’s Lemonade Stand Foundation (M.M. and 19-16681 to Y.P), the National Cancer Institute (P50CA165962, R01CA258384, U19CA264504 to MM), National Institute of Neurological Disorders and Stroke (R01NS092597 to M.M.), NIH Director’s Pioneer Award (DP1NS111132 to M.M.), Robert J. Kleberg, Jr. and Helen C. Kleberg Foundation (to M.M.), Cancer Research UK (to M.M.), Cancer Prevention and Research Institute of Texas (RR210085 to Y.P.).
REFERENCES
- 1.Pajevic S, Basser PJ, Fields RD, Role of myelin plasticity in oscillations and synchrony of neuronal activity. Neuroscience 2014;276,135–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bengtsson SL, Nagy Z, Skare S, Forsman L, Forssberg H, Ullen F, Extensive piano practicing has regionally specific effects on white matter development. Nat Neurosci 2005;8(9),1148–50. [DOI] [PubMed] [Google Scholar]
- 3.Scholz J, Klein MC, Behrens TE, Johansen-Berg H, Training induces changes in white-matter architecture. Nat Neurosci 2009;12(11),1370–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lakhani B, Borich MR, Jackson JN, Wadden KP, Peters S, Villamayor A, MacKay AL, Vavasour IM, Rauscher A, Boyd LA, Motor Skill Acquisition Promotes Human Brain Myelin Plasticity. Neural Plast 2016;2016,7526135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takeuchi H, Taki Y, Sassa Y, Hashizume H, Sekiguchi A, Fukushima A, Kawashima R, White matter structures associated with creativity: evidence from diffusion tensor imaging. Neuroimage 2010;51(1),11–8. [DOI] [PubMed] [Google Scholar]
- 6.Tauber H, Waehneldt TV, Neuhoff V, Myelination in rabbit optic nerves is accelerated by artificial eye opening. Neurosci Lett 1980;16(3),235–8. [DOI] [PubMed] [Google Scholar]
- 7.Gyllensten L, Malmfors T, Myelinization of the optic nerve and its dependence on visual function--a quantitative investigation in mice. J Embryol Exp Morphol 1963;11,255–66. [PubMed] [Google Scholar]
- 8.Demerens C, Stankoff B, Logak M, Anglade P, Allinquant B, Couraud F, Zalc B, Lubetzki C, Induction of myelination in the central nervous system by electrical activity. Proc Natl Acad Sci U S A 1996;93(18),9887–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Etxeberria A, Hokanson KC, Dao DQ, Mayoral SR, Mei F, Redmond SA, Ullian EM, Chan JR, Dynamic Modulation of Myelination in Response to Visual Stimuli Alters Optic Nerve Conduction Velocity. J Neurosci 2016;36(26),6937–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hines JH, Ravanelli AM, Schwindt R, Scott EK, Appel B, Neuronal activity biases axon selection for myelination in vivo. Nat Neurosci 2015;18(5),683–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mensch S, Baraban M, Almeida R, Czopka T, Ausborn J, El Manira A, Lyons DA, Synaptic vesicle release regulates myelin sheath number of individual oligodendrocytes in vivo. Nat Neurosci 2015;18(5),628–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Almeida RG, Williamson JM, Madden ME, Early JJ, Voas MG, Talbot WS, Bianco IH, Lyons DA, Myelination induces axonal hotspots of synaptic vesicle fusion that promote sheath growth. Curr Biol 2021;31(17),3743–54 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swire M, Assinck P, McNaughton PA, Lyons DA, Ffrench-Constant C, Livesey MR, Oligodendrocyte HCN2 Channels Regulate Myelin Sheath Length. J Neurosci 2021;41(38),7954–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang SM, Michel K, Jokhi V, Nedivi E, Arlotta P, Neuron class-specific responses govern adaptive myelin remodeling in the neocortex. Science 2020;370(6523). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hughes EG, Orthmann-Murphy JL, Langseth AJ, Bergles DE, Myelin remodeling through experience-dependent oligodendrogenesis in the adult somatosensory cortex. Nat Neurosci 2018;21(5),696–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McKenzie IA, Ohayon D, Li H, de Faria JP, Emery B, Tohyama K, Richardson WD, Motor skill learning requires active central myelination. Science 2014;346(6207),318–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steadman PE, Xia F, Ahmed M, Mocle AJ, Penning ARA, Geraghty AC, Steenland HW, Monje M, Josselyn SA, Frankland PW, Disruption of Oligodendrogenesis Impairs Memory Consolidation in Adult Mice. Neuron 2020;105(1),150–64 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu J, Dietz K, DeLoyht JM, Pedre X, Kelkar D, Kaur J, Vialou V, Lobo MK, Dietz DM, Nestler EJ, Dupree J, Casaccia P, Impaired adult myelination in the prefrontal cortex of socially isolated mice. Nat Neurosci 2012;15(12),1621–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Makinodan M, Rosen KM, Ito S, Corfas G, A critical period for social experience-dependent oligodendrocyte maturation and myelination. Science 2012;337(6100),1357–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu J, Dupree JL, Gacias M, Frawley R, Sikder T, Naik P, Casaccia P, Clemastine Enhances Myelination in the Prefrontal Cortex and Rescues Behavioral Changes in Socially Isolated Mice. J Neurosci 2016;36(3),957–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gibson EM, Purger D, Mount CW, Goldstein AK, Lin GL, Wood LS, Inema I, Miller SE, Bieri G, Zuchero JB, Barres BA, Woo PJ, Vogel H, Monje M, Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science 2014;344(6183),1252304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mitew S, Gobius I, Fenlon LR, McDougall SJ, Hawkes D, Xing YL, Bujalka H, Gundlach AL, Richards LJ, Kilpatrick TJ, Merson TD, Emery B, Pharmacogenetic stimulation of neuronal activity increases myelination in an axon-specific manner. Nat Commun 2018;9(1),306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geraghty AC, Gibson EM, Ghanem RA, Greene JJ, Ocampo A, Goldstein AK, Ni L, Yang T, Marton RM, Pasca SP, Greenberg ME, Longo FM, Monje M, Loss of Adaptive Myelination Contributes to Methotrexate Chemotherapy-Related Cognitive Impairment. Neuron 2019;103(2),250–65 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Monje M, Mitra SS, Freret ME, Raveh TB, Kim J, Masek M, Attema JL, Li G, Haddix T, Edwards MS, Fisher PG, Weissman IL, Rowitch DH, Vogel H, Wong AJ, Beachy PA, Hedgehog-responsive candidate cell of origin for diffuse intrinsic pontine glioma. Proc Natl Acad Sci U S A 2011;108(11),4453–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagaraja S, Vitanza NA, Woo PJ, Taylor KR, Liu F, Zhang L, Li M, Meng W, Ponnuswami A, Sun W, Ma J, Hulleman E, Swigut T, Wysocka J, Tang Y, Monje M, Transcriptional Dependencies in Diffuse Intrinsic Pontine Glioma. Cancer Cell 2017;31(5),635–52 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagaraja S, Quezada MA, Gillespie SM, Arzt M, Lennon JJ, Woo PJ, Hovestadt V, Kambhampati M, Filbin MG, Suva ML, Nazarian J, Monje M, Histone Variant and Cell Context Determine H3K27M Reprogramming of the Enhancer Landscape and Oncogenic State. Mol Cell 2019;76(6),965–80 e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alcantara Llaguno SR, Wang Z, Sun D, Chen J, Xu J, Kim E, Hatanpaa KJ, Raisanen JM, Burns DK, Johnson JE, Parada LF, Adult Lineage-Restricted CNS Progenitors Specify Distinct Glioblastoma Subtypes. Cancer Cell 2015;28(4),429–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alcantara Llaguno S, Sun D, Pedraza AM, Vera E, Wang Z, Burns DK, Parada LF, Cell-of-origin susceptibility to glioblastoma formation declines with neural lineage restriction. Nat Neurosci 2019;22(4),545–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haag D, Mack N, Benites Goncalves da Silva P, Statz B, Clark J, Tanabe K, Sharma T, Jager N, Jones DTW, Kawauchi D, Wernig M, Pfister SM, H3.3-K27M drives neural stem cell-specific gliomagenesis in a human iPSC-derived model. Cancer Cell 2021;39(3),407–22 e13. [DOI] [PubMed] [Google Scholar]
- 30.Solga AC, Gianino SM, Gutmann DH, NG2-cells are not the cell of origin for murine neurofibromatosis-1 (Nf1) optic glioma. Oncogene 2014;33(3),289–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Solga AC, Toonen JA, Pan Y, Cimino PJ, Ma Y, Castillon GA, Gianino SM, Ellisman MH, Lee DY, Gutmann DH, The cell of origin dictates the temporal course of neurofibromatosis-1 (Nf1) low-grade glioma formation. Oncotarget 2017;8(29),47206–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Filbin MG, Tirosh I, Hovestadt V, Shaw ML, Escalante LE, Mathewson ND, Neftel C, Frank N, Pelton K, Hebert CM, Haberler C, Yizhak K, Gojo J, Egervari K, Mount C, van Galen P, Bonal DM, Nguyen QD, Beck A, Sinai C, Czech T, Dorfer C, Goumnerova L, Lavarino C, Carcaboso AM, Mora J, Mylvaganam R, Luo CC, Peyrl A, Popovic M, Azizi A, Batchelor TT, Frosch MP, Martinez-Lage M, Kieran MW, Bandopadhayay P, Beroukhim R, Fritsch G, Getz G, Rozenblatt-Rosen O, Wucherpfennig KW, Louis DN, Monje M, Slavc I, Ligon KL, Golub TR, Regev A, Bernstein BE, Suva ML, Developmental and oncogenic programs in H3K27M gliomas dissected by single-cell RNA-seq. Science 2018;360(6386),331–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neftel C, Laffy J, Filbin MG, Hara T, Shore ME, Rahme GJ, Richman AR, Silverbush D, Shaw ML, Hebert CM, Dewitt J, Gritsch S, Perez EM, Gonzalez Castro LN, Lan X, Druck N, Rodman C, Dionne D, Kaplan A, Bertalan MS, Small J, Pelton K, Becker S, Bonal D, Nguyen QD, Servis RL, Fung JM, Mylvaganam R, Mayr L, Gojo J, Haberler C, Geyeregger R, Czech T, Slavc I, Nahed BV, Curry WT, Carter BS, Wakimoto H, Brastianos PK, Batchelor TT, Stemmer-Rachamimov A, Martinez-Lage M, Frosch MP, Stamenkovic I, Riggi N, Rheinbay E, Monje M, Rozenblatt-Rosen O, Cahill DP, Patel AP, Hunter T, Verma IM, Ligon KL, Louis DN, Regev A, Bernstein BE, Tirosh I, Suva ML, An Integrative Model of Cellular States, Plasticity, and Genetics for Glioblastoma. Cell 2019;178(4),835–49 e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu C, Sage JC, Miller MR, Verhaak RG, Hippenmeyer S, Vogel H, Foreman O, Bronson RT, Nishiyama A, Luo L, Zong H, Mosaic analysis with double markers reveals tumor cell of origin in glioma. Cell 2011;146(2),209–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen P, Wang W, Liu R, Lyu J, Zhang L, Li B, Qiu B, Tian A, Jiang W, Ying H, Jing R, Wang Q, Zhu K, Bai R, Zeng L, Duan S, Liu C, Olfactory sensory experience regulates gliomagenesis via neuronal IGF1. Nature 2022;606(7914),550–6. [DOI] [PubMed] [Google Scholar]
- 36.Bergles DE, Roberts JD, Somogyi P, Jahr CE, Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus. Nature 2000;405(6783),187–91. [DOI] [PubMed] [Google Scholar]
- 37.Lin SC, Bergles DE, Synaptic signaling between GABAergic interneurons and oligodendrocyte precursor cells in the hippocampus. Nat Neurosci 2004;7(1),24–32. [DOI] [PubMed] [Google Scholar]
- 38.Mount CW, Yalcin B, Cunliffe-Koehler K, Sundaresh S, Monje M, Monosynaptic tracing maps brain-wide afferent oligodendrocyte precursor cell connectivity. Elife 2019;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karadottir R, Cavelier P, Bergersen LH, Attwell D, NMDA receptors are expressed in oligodendrocytes and activated in ischaemia. Nature 2005;438(7071),1162–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Venkataramani V, Tanev DI, Strahle C, Studier-Fischer A, Fankhauser L, Kessler T, Korber C, Kardorff M, Ratliff M, Xie R, Horstmann H, Messer M, Paik SP, Knabbe J, Sahm F, Kurz FT, Acikgoz AA, Herrmannsdorfer F, Agarwal A, Bergles DE, Chalmers A, Miletic H, Turcan S, Mawrin C, Hanggi D, Liu HK, Wick W, Winkler F, Kuner T, Glutamatergic synaptic input to glioma cells drives brain tumour progression. Nature 2019;573(7775),532–8. [DOI] [PubMed] [Google Scholar]
- 41.Venkatesh HS, Morishita W, Geraghty AC, Silverbush D, Gillespie SM, Arzt M, Tam LT, Espenel C, Ponnuswami A, Ni L, Woo PJ, Taylor KR, Agarwal A, Regev A, Brang D, Vogel H, Hervey-Jumper S, Bergles DE, Suva ML, Malenka RC, Monje M, Electrical and synaptic integration of glioma into neural circuits. Nature 2019;573(7775),539–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang ZJ, Ellis T, Markant SL, Read TA, Kessler JD, Bourboulas M, Schuller U, Machold R, Fishell G, Rowitch DH, Wainwright BJ, Wechsler-Reya RJ, Medulloblastoma can be initiated by deletion of Patched in lineage-restricted progenitors or stem cells. Cancer Cell 2008;14(2),135–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oliver TG, Read TA, Kessler JD, Mehmeti A, Wells JF, Huynh TT, Lin SM, Wechsler-Reya RJ, Loss of patched and disruption of granule cell development in a pre-neoplastic stage of medulloblastoma. Development 2005;132(10),2425–39. [DOI] [PubMed] [Google Scholar]
- 44.Gibson P, Tong Y, Robinson G, Thompson MC, Currle DS, Eden C, Kranenburg TA, Hogg T, Poppleton H, Martin J, Finkelstein D, Pounds S, Weiss A, Patay Z, Scoggins M, Ogg R, Pei Y, Yang ZJ, Brun S, Lee Y, Zindy F, Lindsey JC, Taketo MM, Boop FA, Sanford RA, Gajjar A, Clifford SC, Roussel MF, McKinnon PJ, Gutmann DH, Ellison DW, Wechsler-Reya R, Gilbertson RJ, Subtypes of medulloblastoma have distinct developmental origins. Nature 2010;468(7327),1095–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lin CY, Erkek S, Tong Y, Yin L, Federation AJ, Zapatka M, Haldipur P, Kawauchi D, Risch T, Warnatz HJ, Worst BC, Ju B, Orr BA, Zeid R, Polaski DR, Segura-Wang M, Waszak SM, Jones DT, Kool M, Hovestadt V, Buchhalter I, Sieber L, Johann P, Chavez L, Groschel S, Ryzhova M, Korshunov A, Chen W, Chizhikov VV, Millen KJ, Amstislavskiy V, Lehrach H, Yaspo ML, Eils R, Lichter P, Korbel JO, Pfister SM, Bradner JE, Northcott PA, Active medulloblastoma enhancers reveal subgroup-specific cellular origins. Nature 2016;530(7588),57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Venkatesh HS, Johung TB, Caretti V, Noll A, Tang Y, Nagaraja S, Gibson EM, Mount CW, Polepalli J, Mitra SS, Woo PJ, Malenka RC, Vogel H, Bredel M, Mallick P, Monje M, Neuronal Activity Promotes Glioma Growth through Neuroligin-3 Secretion. Cell 2015;161(4),803–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pan Y, Hysinger JD, Barron T, Schindler NF, Cobb O, Guo X, Yalcin B, Anastasaki C, Mulinyawe SB, Ponnuswami A, Scheaffer S, Ma Y, Chang KC, Xia X, Toonen JA, Lennon JJ, Gibson EM, Huguenard JR, Liau LM, Goldberg JL, Monje M, Gutmann DH, NF1 mutation drives neuronal activity-dependent initiation of optic glioma. Nature 2021;594(7862),277–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Venkatesh HS, Tam LT, Woo PJ, Lennon J, Nagaraja S, Gillespie SM, Ni J, Duveau DY, Morris PJ, Zhao JJ, Thomas CJ, Monje M, Targeting neuronal activity-regulated neuroligin-3 dependency in high-grade glioma. Nature 2017;549(7673),533–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anastasaki C, Mo J, Chen JK, Chatterjee J, Pan Y, Scheaffer SM, Cobb O, Monje M, Le LQ, Gutmann DH, Neuronal hyperexcitability drives central and peripheral nervous system tumor progression in models of neurofibromatosis-1. Nat Commun 2022;13(1),2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Taylor KR, Barron T, Zhang H, Hui A, Hartmann G, Ni L, Venkatesh HS, Du P, Mancusi R, Yalçin B, Chau I, Ponnuswami A, Aziz-Bose R, Monje M, Glioma synapses recruit mechanisms of adaptive plasticity. bioRxiv 2021,2021.11.04.467325. [Google Scholar]
- 51.Farhy-Tselnicker I, Allen NJ, Astrocytes, neurons, synapses: a tripartite view on cortical circuit development. Neural Dev 2018;13(1),7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Allen NJ, Eroglu C, Cell Biology of Astrocyte-Synapse Interactions. Neuron 2017;96(3),697–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Christopherson KS, Ullian EM, Stokes CC, Mullowney CE, Hell JW, Agah A, Lawler J, Mosher DF, Bornstein P, Barres BA, Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell 2005;120(3),421–33. [DOI] [PubMed] [Google Scholar]
- 54.Kucukdereli H, Allen NJ, Lee AT, Feng A, Ozlu MI, Conatser LM, Chakraborty C, Workman G, Weaver M, Sage EH, Barres BA, Eroglu C, Control of excitatory CNS synaptogenesis by astrocyte-secreted proteins Hevin and SPARC. Proc Natl Acad Sci U S A 2011;108(32),E440–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Allen NJ, Bennett ML, Foo LC, Wang GX, Chakraborty C, Smith SJ, Barres BA, Astrocyte glypicans 4 and 6 promote formation of excitatory synapses via GluA1 AMPA receptors. Nature 2012;486(7403),410–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chung WS, Clarke LE, Wang GX, Stafford BK, Sher A, Chakraborty C, Joung J, Foo LC, Thompson A, Chen C, Smith SJ, Barres BA, Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature 2013;504(7480),394–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vainchtein ID, Chin G, Cho FS, Kelley KW, Miller JG, Chien EC, Liddelow SA, Nguyen PT, Nakao-Inoue H, Dorman LC, Akil O, Joshita S, Barres BA, Paz JT, Molofsky AB, Molofsky AV, Astrocyte-derived interleukin-33 promotes microglial synapse engulfment and neural circuit development. Science 2018;359(6381),1269–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Blanco-Suarez E, Liu TF, Kopelevich A, Allen NJ, Astrocyte-Secreted Chordin-like 1 Drives Synapse Maturation and Limits Plasticity by Increasing Synaptic GluA2 AMPA Receptors. Neuron 2018;100(5),1116–32 e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Araque A, Parpura V, Sanzgiri RP, Haydon PG, Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci 1999;22(5),208–15. [DOI] [PubMed] [Google Scholar]
- 60.Stogsdill JA, Ramirez J, Liu D, Kim YH, Baldwin KT, Enustun E, Ejikeme T, Ji RR, Eroglu C, Astrocytic neuroligins control astrocyte morphogenesis and synaptogenesis. Nature 2017;551(7679),192–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Takano T, Wallace JT, Baldwin KT, Purkey AM, Uezu A, Courtland JL, Soderblom EJ, Shimogori T, Maness PF, Eroglu C, Soderling SH, Chemico-genetic discovery of astrocytic control of inhibition in vivo. Nature 2020;588(7837),296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Harada K, Kamiya T, Tsuboi T, Gliotransmitter Release from Astrocytes: Functional, Developmental, and Pathological Implications in the Brain. Front Neurosci 2015;9,499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Volterra A, Meldolesi J, Astrocytes, from brain glue to communication elements: the revolution continues. Nat Rev Neurosci 2005;6(8),626–40. [DOI] [PubMed] [Google Scholar]
- 64.Henneberger C, Papouin T, Oliet SH, Rusakov DA, Long-term potentiation depends on release of D-serine from astrocytes. Nature 2010;463(7278),232–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Suzuki A, Stern SA, Bozdagi O, Huntley GW, Walker RH, Magistretti PJ, Alberini CM, Astrocyte-neuron lactate transport is required for long-term memory formation. Cell 2011;144(5),810–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Porter JT, McCarthy KD, Hippocampal astrocytes in situ respond to glutamate released from synaptic terminals. J Neurosci 1996;16(16),5073–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pasti L, Volterra A, Pozzan T, Carmignoto G, Intracellular calcium oscillations in astrocytes: a highly plastic, bidirectional form of communication between neurons and astrocytes in situ. J Neurosci 1997;17(20),7817–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hirase H, Qian L, Bartho P, Buzsaki G, Calcium dynamics of cortical astrocytic networks in vivo. PLoS Biol 2004;2(4),E96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang X, Lou N, Xu Q, Tian GF, Peng WG, Han X, Kang J, Takano T, Nedergaard M, Astrocytic Ca2+ signaling evoked by sensory stimulation in vivo. Nat Neurosci 2006;9(6),816–23. [DOI] [PubMed] [Google Scholar]
- 70.Bernardinelli Y, Randall J, Janett E, Nikonenko I, Konig S, Jones EV, Flores CE, Murai KK, Bochet CG, Holtmaat A, Muller D, Activity-dependent structural plasticity of perisynaptic astrocytic domains promotes excitatory synapse stability. Curr Biol 2014;24(15),1679–88. [DOI] [PubMed] [Google Scholar]
- 71.Porter JT, McCarthy KD, Astrocytic neurotransmitter receptors in situ and in vivo. Prog Neurobiol 1997;51(4),439–55. [DOI] [PubMed] [Google Scholar]
- 72.Zhang Y, Sloan SA, Clarke LE, Caneda C, Plaza CA, Blumenthal PD, Vogel H, Steinberg GK, Edwards MS, Li G, Duncan JA 3rd, Cheshier SH, Shuer LM, Chang EF, Grant GA, Gephart MG, Barres BA, Purification and Characterization of Progenitor and Mature Human Astrocytes Reveals Transcriptional and Functional Differences with Mouse. Neuron 2016;89(1),37–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Morel L, Higashimori H, Tolman M, Yang Y, VGluT1+ neuronal glutamatergic signaling regulates postnatal developmental maturation of cortical protoplasmic astroglia. J Neurosci 2014;34(33),10950–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Meier SD, Kafitz KW, Rose CR, Developmental profile and mechanisms of GABA-induced calcium signaling in hippocampal astrocytes. Glia 2008;56(10),1127–37. [DOI] [PubMed] [Google Scholar]
- 75.Liddelow SA, Barres BA, Reactive Astrocytes: Production, Function, and Therapeutic Potential. Immunity 2017;46(6),957–67. [DOI] [PubMed] [Google Scholar]
- 76.Zamanian JL, Xu L, Foo LC, Nouri N, Zhou L, Giffard RG, Barres BA, Genomic analysis of reactive astrogliosis. J Neurosci 2012;32(18),6391–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hasel P, Rose IVL, Sadick JS, Kim RD, Liddelow SA, Neuroinflammatory astrocyte subtypes in the mouse brain. Nat Neurosci 2021;24(10),1475–87. [DOI] [PubMed] [Google Scholar]
- 78.Guttenplan KA, Weigel MK, Prakash P, Wijewardhane PR, Hasel P, Rufen-Blanchette U, Munch AE, Blum JA, Fine J, Neal MC, Bruce KD, Gitler AD, Chopra G, Liddelow SA, Barres BA, Neurotoxic reactive astrocytes induce cell death via saturated lipids. Nature 2021;599(7883),102–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lin Q, Balasubramanian K, Fan D, Kim SJ, Guo L, Wang H, Bar-Eli M, Aldape KD, Fidler IJ, Reactive astrocytes protect melanoma cells from chemotherapy by sequestering intracellular calcium through gap junction communication channels. Neoplasia 2010;12(9),748–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kim SJ, Kim JS, Park ES, Lee JS, Lin Q, Langley RR, Maya M, He J, Kim SW, Weihua Z, Balasubramanian K, Fan D, Mills GB, Hung MC, Fidler IJ, Astrocytes upregulate survival genes in tumor cells and induce protection from chemotherapy. Neoplasia 2011;13(3),286–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Placone AL, Quinones-Hinojosa A, Searson PC, The role of astrocytes in the progression of brain cancer: complicating the picture of the tumor microenvironment. Tumour Biol 2016;37(1),61–9. [DOI] [PubMed] [Google Scholar]
- 82.Henrik Heiland D, Ravi VM, Behringer SP, Frenking JH, Wurm J, Joseph K, Garrelfs NWC, Strahle J, Heynckes S, Grauvogel J, Franco P, Mader I, Schneider M, Potthoff AL, Delev D, Hofmann UG, Fung C, Beck J, Sankowski R, Prinz M, Schnell O, Tumor-associated reactive astrocytes aid the evolution of immunosuppressive environment in glioblastoma. Nat Commun 2019;10(1),2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Venteicher AS, Tirosh I, Hebert C, Yizhak K, Neftel C, Filbin MG, Hovestadt V, Escalante LE, Shaw ML, Rodman C, Gillespie SM, Dionne D, Luo CC, Ravichandran H, Mylvaganam R, Mount C, Onozato ML, Nahed BV, Wakimoto H, Curry WT, Iafrate AJ, Rivera MN, Frosch MP, Golub TR, Brastianos PK, Getz G, Patel AP, Monje M, Cahill DP, Rozenblatt-Rosen O, Louis DN, Bernstein BE, Regev A, Suva ML, Decoupling genetics, lineages, and microenvironment in IDH-mutant gliomas by single-cell RNA-seq. Science 2017;355(6332). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.John Lin CC, Yu K, Hatcher A, Huang TW, Lee HK, Carlson J, Weston MC, Chen F, Zhang Y, Zhu W, Mohila CA, Ahmed N, Patel AJ, Arenkiel BR, Noebels JL, Creighton CJ, Deneen B, Identification of diverse astrocyte populations and their malignant analogs. Nat Neurosci 2017;20(3),396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yao M, Ventura PB, Jiang Y, Rodriguez FJ, Wang L, Perry JSA, Yang Y, Wahl K, Crittenden RB, Bennett ML, Qi L, Gong CC, Li XN, Barres BA, Bender TP, Ravichandran KS, Janes KA, Eberhart CG, Zong H, Astrocytic trans-Differentiation Completes a Multicellular Paracrine Feedback Loop Required for Medulloblastoma Tumor Growth. Cell 2020;180(3),502–20 e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zeng Q, Michael IP, Zhang P, Saghafinia S, Knott G, Jiao W, McCabe BD, Galvan JA, Robinson HPC, Zlobec I, Ciriello G, Hanahan D, Synaptic proximity enables NMDAR signalling to promote brain metastasis. Nature 2019;573(7775),526–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen Q, Boire A, Jin X, Valiente M, Er EE, Lopez-Soto A, Jacob L, Patwa R, Shah H, Xu K, Cross JR, Massague J, Carcinoma-astrocyte gap junctions promote brain metastasis by cGAMP transfer. Nature 2016;533(7604),493–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Priego N, Zhu L, Monteiro C, Mulders M, Wasilewski D, Bindeman W, Doglio L, Martinez L, Martinez-Saez E, Ramon YCS, Megias D, Hernandez-Encinas E, Blanco-Aparicio C, Martinez L, Zarzuela E, Munoz J, Fustero-Torre C, Pineiro-Yanez E, Hernandez-Lain A, Bertero L, Poli V, Sanchez-Martinez M, Menendez JA, Soffietti R, Bosch-Barrera J, Valiente M, STAT3 labels a subpopulation of reactive astrocytes required for brain metastasis. Nat Med 2018;24(7),1024–35. [DOI] [PubMed] [Google Scholar]
- 89.Yu K, Lin CJ, Hatcher A, Lozzi B, Kong K, Huang-Hobbs E, Cheng YT, Beechar VB, Zhu W, Zhang Y, Chen F, Mills GB, Mohila CA, Creighton CJ, Noebels JL, Scott KL, Deneen B, PIK3CA variants selectively initiate brain hyperactivity during gliomagenesis. Nature 2020;578(7793),166–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Daubon T, Leon C, Clarke K, Andrique L, Salabert L, Darbo E, Pineau R, Guerit S, Maitre M, Dedieu S, Jeanne A, Bailly S, Feige JJ, Miletic H, Rossi M, Bello L, Falciani F, Bjerkvig R, Bikfalvi A, Deciphering the complex role of thrombospondin-1 in glioblastoma development. Nat Commun 2019;10(1),1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Krishna S, Choudhury A, Seo K, Ni L, Kakaizada S, Lee A, Aabedi A, Cao C, Sudharshan R, Egladyous A, Almeida N, Venkatesh HS, Findlay A, Nagarajan S, Raleigh D, Brang D, Monje M, Hervey-Jumper SL, Glioblastoma remodeling of neural circuits in the human brain decreases survival. bioRxiv 2021,2021.02.18.431915. [Google Scholar]
- 92.Buckingham SC, Campbell SL, Haas BR, Montana V, Robel S, Ogunrinu T, Sontheimer H, Glutamate release by primary brain tumors induces epileptic activity. Nat Med 2011;17(10),1269–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Campbell SL, Buckingham SC, Sontheimer H, Human glioma cells induce hyperexcitability in cortical networks. Epilepsia 2012;53(8),1360–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Campbell SL, Robel S, Cuddapah VA, Robert S, Buckingham SC, Kahle KT, Sontheimer H, GABAergic disinhibition and impaired KCC2 cotransporter activity underlie tumor-associated epilepsy. Glia 2015;63(1),23–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hatcher A, Yu K, Meyer J, Aiba I, Deneen B, Noebels JL, Pathogenesis of peritumoral hyperexcitability in an immunocompetent CRISPR-based glioblastoma model. J Clin Invest 2020;130(5),2286–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Derks J, Dirkson AR, de Witt Hamer PC, van Geest Q, Hulst HE, Barkhof F, Pouwels PJ, Geurts JJ, Reijneveld JC, Douw L, Connectomic profile and clinical phenotype in newly diagnosed glioma patients. Neuroimage Clin 2017;14,87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Belgers V, Numan T, Kulik SD, Hillebrand A, de Witt Hamer PC, Geurts JJG, Reijneveld JC, Wesseling P, Klein M, Derks J, Douw L, Postoperative oscillatory brain activity as an add-on prognostic marker in diffuse glioma. J Neurooncol 2020;147(1),49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bergles DE, Jahr CE, Synaptic activation of glutamate transporters in hippocampal astrocytes. Neuron 1997;19(6),1297–308. [DOI] [PubMed] [Google Scholar]
- 99.Luscher C, Malenka RC, Nicoll RA, Monitoring glutamate release during LTP with glial transporter currents. Neuron 1998;21(2),435–41. [DOI] [PubMed] [Google Scholar]
- 100.Sibille J, Pannasch U, Rouach N, Astroglial potassium clearance contributes to short-term plasticity of synaptically evoked currents at the tripartite synapse. J Physiol 2014;592(1),87–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Osswald M, Jung E, Sahm F, Solecki G, Venkataramani V, Blaes J, Weil S, Horstmann H, Wiestler B, Syed M, Huang L, Ratliff M, Karimian Jazi K, Kurz FT, Schmenger T, Lemke D, Gommel M, Pauli M, Liao Y, Haring P, Pusch S, Herl V, Steinhauser C, Krunic D, Jarahian M, Miletic H, Berghoff AS, Griesbeck O, Kalamakis G, Garaschuk O, Preusser M, Weiss S, Liu H, Heiland S, Platten M, Huber PE, Kuner T, von Deimling A, Wick W, Winkler F, Brain tumour cells interconnect to a functional and resistant network. Nature 2015;528(7580),93–8. [DOI] [PubMed] [Google Scholar]
- 102.Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, Micheva KD, Mehalow AK, Huberman AD, Stafford B, Sher A, Litke AM, Lambris JD, Smith SJ, John SW, Barres BA, The classical complement cascade mediates CNS synapse elimination. Cell 2007;131(6),1164–78. [DOI] [PubMed] [Google Scholar]
- 103.Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, Ransohoff RM, Greenberg ME, Barres BA, Stevens B, Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 2012;74(4),691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Weinhard L, di Bartolomei G, Bolasco G, Machado P, Schieber NL, Neniskyte U, Exiga M, Vadisiute A, Raggioli A, Schertel A, Schwab Y, Gross CT, Microglia remodel synapses by presynaptic trogocytosis and spine head filopodia induction. Nat Commun 2018;9(1),1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, Giustetto M, Ferreira TA, Guiducci E, Dumas L, Ragozzino D, Gross CT, Synaptic pruning by microglia is necessary for normal brain development. Science 2011;333(6048),1456–8. [DOI] [PubMed] [Google Scholar]
- 106.Antonucci F, Turola E, Riganti L, Caleo M, Gabrielli M, Perrotta C, Novellino L, Clementi E, Giussani P, Viani P, Matteoli M, Verderio C, Microvesicles released from microglia stimulate synaptic activity via enhanced sphingolipid metabolism. EMBO J 2012;31(5),1231–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gabrielli M, Battista N, Riganti L, Prada I, Antonucci F, Cantone L, Matteoli M, Maccarrone M, Verderio C, Active endocannabinoids are secreted on extracellular membrane vesicles. EMBO Rep 2015;16(2),213–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sipe GO, Lowery RL, Tremblay ME, Kelly EA, Lamantia CE, Majewska AK, Microglial P2Y12 is necessary for synaptic plasticity in mouse visual cortex. Nat Commun 2016;7,10905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cantaut-Belarif Y, Antri M, Pizzarelli R, Colasse S, Vaccari I, Soares S, Renner M, Dallel R, Triller A, Bessis A, Microglia control the glycinergic but not the GABAergic synapses via prostaglandin E2 in the spinal cord. J Cell Biol 2017;216(9),2979–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhan Y, Paolicelli RC, Sforazzini F, Weinhard L, Bolasco G, Pagani F, Vyssotski AL, Bifone A, Gozzi A, Ragozzino D, Gross CT, Deficient neuron-microglia signaling results in impaired functional brain connectivity and social behavior. Nat Neurosci 2014;17(3),400–6. [DOI] [PubMed] [Google Scholar]
- 111.Kim HJ, Cho MH, Shim WH, Kim JK, Jeon EY, Kim DH, Yoon SY, Deficient autophagy in microglia impairs synaptic pruning and causes social behavioral defects. Mol Psychiatry 2017;22(11),1576–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sanchez JMS, DePaula-Silva AB, Doty DJ, Truong A, Libbey JE, Fujinami RS, Microglial cell depletion is fatal with low level picornavirus infection of the central nervous system. J Neurovirol 2019;25(3),415–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Badimon A, Strasburger HJ, Ayata P, Chen X, Nair A, Ikegami A, Hwang P, Chan AT, Graves SM, Uweru JO, Ledderose C, Kutlu MG, Wheeler MA, Kahan A, Ishikawa M, Wang YC, Loh YE, Jiang JX, Surmeier DJ, Robson SC, Junger WG, Sebra R, Calipari ES, Kenny PJ, Eyo UB, Colonna M, Quintana FJ, Wake H, Gradinaru V, Schaefer A, Negative feedback control of neuronal activity by microglia. Nature 2020;586(7829),417–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wu W, Li Y, Wei Y, Bosco DB, Xie M, Zhao MG, Richardson JR, Wu LJ, Microglial depletion aggravates the severity of acute and chronic seizures in mice. Brain Behav Immun 2020;89,245–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Eyo UB, Peng J, Swiatkowski P, Mukherjee A, Bispo A, Wu LJ, Neuronal hyperactivity recruits microglial processes via neuronal NMDA receptors and microglial P2Y12 receptors after status epilepticus. J Neurosci 2014;34(32),10528–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bernier LP, Bohlen CJ, York EM, Choi HB, Kamyabi A, Dissing-Olesen L, Hefendehl JK, Collins HY, Stevens B, Barres BA, MacVicar BA, Nanoscale Surveillance of the Brain by Microglia via cAMP-Regulated Filopodia. Cell Rep 2019;27(10),2895–908 e4. [DOI] [PubMed] [Google Scholar]
- 117.Madry C, Kyrargyri V, Arancibia-Carcamo IL, Jolivet R, Kohsaka S, Bryan RM, Attwell D, Microglial Ramification, Surveillance, and Interleukin-1beta Release Are Regulated by the Two-Pore Domain K(+) Channel THIK-1. Neuron 2018;97(2),299–312 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dissing-Olesen L, LeDue JM, Rungta RL, Hefendehl JK, Choi HB, MacVicar BA, Activation of neuronal NMDA receptors triggers transient ATP-mediated microglial process outgrowth. J Neurosci 2014;34(32),10511–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cserep C, Posfai B, Lenart N, Fekete R, Laszlo ZI, Lele Z, Orsolits B, Molnar G, Heindl S, Schwarcz AD, Ujvari K, Kornyei Z, Toth K, Szabadits E, Sperlagh B, Baranyi M, Csiba L, Hortobagyi T, Magloczky Z, Martinecz B, Szabo G, Erdelyi F, Szipocs R, Tamkun MM, Gesierich B, Duering M, Katona I, Liesz A, Tamas G, Denes A, Microglia monitor and protect neuronal function through specialized somatic purinergic junctions. Science 2020;367(6477),528–37. [DOI] [PubMed] [Google Scholar]
- 120.Tremblay ME, Lowery RL, Majewska AK, Microglial interactions with synapses are modulated by visual experience. PLoS Biol 2010;8(11),e1000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gutmann DH, Kettenmann H, Microglia/Brain Macrophages as Central Drivers of Brain Tumor Pathobiology. Neuron 2019;104(3),442–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Keane L, Cheray M, Blomgren K, Joseph B, Multifaceted microglia - key players in primary brain tumour heterogeneity. Nat Rev Neurol 2021;17(4),243–59. [DOI] [PubMed] [Google Scholar]
- 123.Hammond TR, Dufort C, Dissing-Olesen L, Giera S, Young A, Wysoker A, Walker AJ, Gergits F, Segel M, Nemesh J, Marsh SE, Saunders A, Macosko E, Ginhoux F, Chen J, Franklin RJM, Piao X, McCarroll SA, Stevens B, Single-Cell RNA Sequencing of Microglia throughout the Mouse Lifespan and in the Injured Brain Reveals Complex Cell-State Changes. Immunity 2019;50(1),253–71 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ochocka N, Segit P, Walentynowicz KA, Wojnicki K, Cyranowski S, Swatler J, Mieczkowski J, Kaminska B, Single-cell RNA sequencing reveals functional heterogeneity of glioma-associated brain macrophages. Nat Commun 2021;12(1),1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Szulzewsky F, Arora S, de Witte L, Ulas T, Markovic D, Schultze JL, Holland EC, Synowitz M, Wolf SA, Kettenmann H, Human glioblastoma-associated microglia/monocytes express a distinct RNA profile compared to human control and murine samples. Glia 2016;64(8),1416–36. [DOI] [PubMed] [Google Scholar]
- 126.Szulzewsky F, Pelz A, Feng X, Synowitz M, Markovic D, Langmann T, Holtman IR, Wang X, Eggen BJ, Boddeke HW, Hambardzumyan D, Wolf SA, Kettenmann H, Glioma-associated microglia/macrophages display an expression profile different from M1 and M2 polarization and highly express Gpnmb and Spp1. PLoS One 2015;10(2),e0116644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pyonteck SM, Gadea BB, Wang HW, Gocheva V, Hunter KE, Tang LH, Joyce JA, Deficiency of the macrophage growth factor CSF-1 disrupts pancreatic neuroendocrine tumor development. Oncogene 2012;31(11),1459–67. [DOI] [PubMed] [Google Scholar]
- 128.Lin GL, Nagaraja S, Filbin MG, Suva ML, Vogel H, Monje M, Non-inflammatory tumor microenvironment of diffuse intrinsic pontine glioma. Acta Neuropathol Commun 2018;6(1),51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wang Q, Hu B, Hu X, Kim H, Squatrito M, Scarpace L, deCarvalho AC, Lyu S, Li P, Li Y, Barthel F, Cho HJ, Lin YH, Satani N, Martinez-Ledesma E, Zheng S, Chang E, Sauve CG, Olar A, Lan ZD, Finocchiaro G, Phillips JJ, Berger MS, Gabrusiewicz KR, Wang G, Eskilsson E, Hu J, Mikkelsen T, DePinho RA, Muller F, Heimberger AB, Sulman EP, Nam DH, Verhaak RGW, Tumor Evolution of Glioma-Intrinsic Gene Expression Subtypes Associates with Immunological Changes in the Microenvironment. Cancer Cell 2017;32(1),42–56 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yang I, Han SJ, Sughrue ME, Tihan T, Parsa AT, Immune cell infiltrate differences in pilocytic astrocytoma and glioblastoma: evidence of distinct immunological microenvironments that reflect tumor biology. J Neurosurg 2011;115(3),505–11. [DOI] [PubMed] [Google Scholar]
- 131.Griesinger AM, Birks DK, Donson AM, Amani V, Hoffman LM, Waziri A, Wang M, Handler MH, Foreman NK, Characterization of distinct immunophenotypes across pediatric brain tumor types. J Immunol 2013;191(9),4880–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Muller A, Brandenburg S, Turkowski K, Muller S, Vajkoczy P, Resident microglia, and not peripheral macrophages, are the main source of brain tumor mononuclear cells. Int J Cancer 2015;137(2),278–88. [DOI] [PubMed] [Google Scholar]
- 133.Chen Z, Feng X, Herting CJ, Garcia VA, Nie K, Pong WW, Rasmussen R, Dwivedi B, Seby S, Wolf SA, Gutmann DH, Hambardzumyan D, Cellular and Molecular Identity of Tumor-Associated Macrophages in Glioblastoma. Cancer Res 2017;77(9),2266–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bowman RL, Klemm F, Akkari L, Pyonteck SM, Sevenich L, Quail DF, Dhara S, Simpson K, Gardner EE, Iacobuzio-Donahue CA, Brennan CW, Tabar V, Gutin PH, Joyce JA, Macrophage Ontogeny Underlies Differences in Tumor-Specific Education in Brain Malignancies. Cell Rep 2016;17(9),2445–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.McKinsey GL, Lizama CO, Keown-Lang AE, Niu A, Santander N, Larpthaveesarp A, Chee E, Gonzalez FF, Arnold TD, A new genetic strategy for targeting microglia in development and disease. Elife 2020;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Muller S, Kohanbash G, Liu SJ, Alvarado B, Carrera D, Bhaduri A, Watchmaker PB, Yagnik G, Di Lullo E, Malatesta M, Amankulor NM, Kriegstein AR, Lim DA, Aghi M, Okada H, Diaz A, Single-cell profiling of human gliomas reveals macrophage ontogeny as a basis for regional differences in macrophage activation in the tumor microenvironment. Genome Biol 2017;18(1),234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hambardzumyan D, Gutmann DH, Kettenmann H, The role of microglia and macrophages in glioma maintenance and progression. Nat Neurosci 2016;19(1),20–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pong WW, Higer SB, Gianino SM, Emnett RJ, Gutmann DH, Reduced microglial CX3CR1 expression delays neurofibromatosis-1 glioma formation. Ann Neurol 2013;73(2),303–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Daginakatte GC, Gutmann DH, Neurofibromatosis-1 (Nf1) heterozygous brain microglia elaborate paracrine factors that promote Nf1-deficient astrocyte and glioma growth. Hum Mol Genet 2007;16(9),1098–112. [DOI] [PubMed] [Google Scholar]
- 140.Pan Y, Bush EC, Toonen JA, Ma Y, Solga AC, Sims PA, Gutmann DH, Whole tumor RNA-sequencing and deconvolution reveal a clinically-prognostic PTEN/PI3K-regulated glioma transcriptional signature. Oncotarget 2017;8(32),52474–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Guo X, Pan Y, Xiong M, Sanapala S, Anastasaki C, Cobb O, Dahiya S, Gutmann DH, Midkine activation of CD8(+) T cells establishes a neuron-immune-cancer axis responsible for low-grade glioma growth. Nat Commun 2020;11(1),2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Pan Y, Xiong M, Chen R, Ma Y, Corman C, Maricos M, Kindler U, Semtner M, Chen YH, Dahiya S, Gutmann DH, Athymic mice reveal a requirement for T-cell-microglia interactions in establishing a microenvironment supportive of Nf1 low-grade glioma growth. Genes Dev 2018;32(7–8),491–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Liu Y, Teige I, Birnir B, Issazadeh-Navikas S, Neuron-mediated generation of regulatory T cells from encephalitogenic T cells suppresses EAE. Nat Med 2006;12(5),518–25. [DOI] [PubMed] [Google Scholar]
- 144.Flugel A, Schwaiger FW, Neumann H, Medana I, Willem M, Wekerle H, Kreutzberg GW, Graeber MB, Neuronal FasL induces cell death of encephalitogenic T lymphocytes. Brain Pathol 2000;10(3),353–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Harrison JK, Jiang Y, Chen S, Xia Y, Maciejewski D, McNamara RK, Streit WJ, Salafranca MN, Adhikari S, Thompson DA, Botti P, Bacon KB, Feng L, Role for neuronally derived fractalkine in mediating interactions between neurons and CX3CR1-expressing microglia. Proc Natl Acad Sci U S A 1998;95(18),10896–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mizuno T, Yoshihara Y, Kagamiyama H, Ohsawa K, Imai Y, Kohsaka S, Mori K, Neuronal adhesion molecule telencephalin induces rapid cell spreading of microglia. Brain Res 1999;849(1–2),58–66. [DOI] [PubMed] [Google Scholar]
- 147.Mott RT, Ait-Ghezala G, Town T, Mori T, Vendrame M, Zeng J, Ehrhart J, Mullan M, Tan J, Neuronal expression of CD22: novel mechanism for inhibiting microglial proinflammatory cytokine production. Glia 2004;46(4),369–79. [DOI] [PubMed] [Google Scholar]
- 148.Monje ML, Toda H, Palmer TD, Inflammatory blockade restores adult hippocampal neurogenesis. Science 2003;302(5651),1760–5. [DOI] [PubMed] [Google Scholar]
- 149.Dietrich J, Monje M, Wefel J, Meyers C, Clinical patterns and biological correlates of cognitive dysfunction associated with cancer therapy. Oncologist 2008;13(12),1285–95. [DOI] [PubMed] [Google Scholar]
- 150.Monje ML, Mizumatsu S, Fike JR, Palmer TD, Irradiation induces neural precursor-cell dysfunction. Nat Med 2002;8(9),955–62. [DOI] [PubMed] [Google Scholar]
- 151.Markarian M, Krattli RP Jr., Baddour JD, Alikhani L, Giedzinski E, Usmani MT, Agrawal A, Baulch JE, Tenner AJ, Acharya MM, Glia-Selective Deletion of Complement C1q Prevents Radiation-Induced Cognitive Deficits and Neuroinflammation. Cancer Res 2021;81(7),1732–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gibson EM, Nagaraja S, Ocampo A, Tam LT, Wood LS, Pallegar PN, Greene JJ, Geraghty AC, Goldstein AK, Ni L, Woo PJ, Barres BA, Liddelow S, Vogel H, Monje M, Methotrexate Chemotherapy Induces Persistent Tri-glial Dysregulation that Underlies Chemotherapy-Related Cognitive Impairment. Cell 2019;176(1–2),43–55 e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lee M, Neurotransmitters and microglial-mediated neuroinflammation. Curr Protein Pept Sci 2013;14(1),21–32. [DOI] [PubMed] [Google Scholar]
- 154.Szepesi Z, Manouchehrian O, Bachiller S, Deierborg T, Bidirectional Microglia-Neuron Communication in Health and Disease. Front Cell Neurosci 2018;12,323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Oldenborg PA, Zheleznyak A, Fang YF, Lagenaur CF, Gresham HD, Lindberg FP, Role of CD47 as a marker of self on red blood cells. Science 2000;288(5473),2051–4. [DOI] [PubMed] [Google Scholar]
- 156.Oldenborg PA, Gresham HD, Lindberg FP, CD47-signal regulatory protein alpha (SIRPalpha) regulates Fcgamma and complement receptor-mediated phagocytosis. J Exp Med 2001;193(7),855–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Jiang P, Lagenaur CF, Narayanan V, Integrin-associated protein is a ligand for the P84 neural adhesion molecule. J Biol Chem 1999;274(2),559–62. [DOI] [PubMed] [Google Scholar]
- 158.Mi ZP, Jiang P, Weng WL, Lindberg FP, Narayanan V, Lagenaur CF, Expression of a synapse-associated membrane protein, P84/SHPS-1, and its ligand, IAP/CD47, in mouse retina. J Comp Neurol 2000;416(3),335–44. [PubMed] [Google Scholar]
- 159.Lehrman EK, Wilton DK, Litvina EY, Welsh CA, Chang ST, Frouin A, Walker AJ, Heller MD, Umemori H, Chen C, Stevens B, CD47 Protects Synapses from Excess Microglia-Mediated Pruning during Development. Neuron 2018;100(1),120–34 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ding X, Wang J, Huang M, Chen Z, Liu J, Zhang Q, Zhang C, Xiang Y, Zen K, Li L, Loss of microglial SIRPalpha promotes synaptic pruning in preclinical models of neurodegeneration. Nat Commun 2021;12(1),2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Jaiswal S, Jamieson CH, Pang WW, Park CY, Chao MP, Majeti R, Traver D, van Rooijen N, Weissman IL, CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell 2009;138(2),271–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Li F, Lv B, Liu Y, Hua T, Han J, Sun C, Xu L, Zhang Z, Feng Z, Cai Y, Zou Y, Ke Y, Jiang X, Blocking the CD47-SIRPalpha axis by delivery of anti-CD47 antibody induces antitumor effects in glioma and glioma stem cells. Oncoimmunology 2018;7(2),e1391973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hutter G, Theruvath J, Graef CM, Zhang M, Schoen MK, Manz EM, Bennett ML, Olson A, Azad TD, Sinha R, Chan C, Assad Kahn S, Gholamin S, Wilson C, Grant G, He J, Weissman IL, Mitra SS, Cheshier SH, Microglia are effector cells of CD47-SIRPalpha antiphagocytic axis disruption against glioblastoma. Proc Natl Acad Sci U S A 2019;116(3),997–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Gholamin S, Mitra SS, Feroze AH, Liu J, Kahn SA, Zhang M, Esparza R, Richard C, Ramaswamy V, Remke M, Volkmer AK, Willingham S, Ponnuswami A, McCarty A, Lovelace P, Storm TA, Schubert S, Hutter G, Narayanan C, Chu P, Raabe EH, Harsh Gt, Taylor MD, Monje M, Cho YJ, Majeti R, Volkmer JP, Fisher PG, Grant G, Steinberg GK, Vogel H, Edwards M, Weissman IL, Cheshier SH, Disrupting the CD47-SIRPalpha anti-phagocytic axis by a humanized anti-CD47 antibody is an efficacious treatment for malignant pediatric brain tumors. Sci Transl Med 2017;9(381). [DOI] [PubMed] [Google Scholar]
- 165.Gholamin S, Youssef OA, Rafat M, Esparza R, Kahn S, Shahin M, Giaccia AJ, Graves EE, Weissman I, Mitra S, Cheshier SH, Irradiation or temozolomide chemotherapy enhances anti-CD47 treatment of glioblastoma. Innate Immun 2020;26(2),130–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Yalcin B, Monje M, Microenvironmental interactions of oligodendroglial cells. Dev Cell 2021;56(13),1821–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Matejuk A, Ransohoff RM, Crosstalk Between Astrocytes and Microglia: An Overview. Front Immunol 2020;11,1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Han RT, Kim RD, Molofsky AV, Liddelow SA, Astrocyte-immune cell interactions in physiology and pathology. Immunity 2021;54(2),211–24. [DOI] [PubMed] [Google Scholar]
- 169.Zahalka AH, Frenette PS, Nerves in cancer. Nat Rev Cancer 2020;20(3),143–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Monje M, Borniger JC, D’Silva NJ, Deneen B, Dirks PB, Fattahi F, Frenette PS, Garzia L, Gutmann DH, Hanahan D, Hervey-Jumper SL, Hondermarck H, Hurov JB, Kepecs A, Knox SM, Lloyd AC, Magnon C, Saloman JL, Segal RA, Sloan EK, Sun X, Taylor MD, Tracey KJ, Trotman LC, Tuveson DA, Wang TC, White RA, Winkler F, Roadmap for the Emerging Field of Cancer Neuroscience. Cell 2020;181(2),219–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Saloman JL, Albers KM, Li D, Hartman DJ, Crawford HC, Muha EA, Rhim AD, Davis BM, Ablation of sensory neurons in a genetic model of pancreatic ductal adenocarcinoma slows initiation and progression of cancer. Proc Natl Acad Sci U S A 2016;113(11),3078–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Renz BW, Takahashi R, Tanaka T, Macchini M, Hayakawa Y, Dantes Z, Maurer HC, Chen X, Jiang Z, Westphalen CB, Ilmer M, Valenti G, Mohanta SK, Habenicht AJR, Middelhoff M, Chu T, Nagar K, Tailor Y, Casadei R, Di Marco M, Kleespies A, Friedman RA, Remotti H, Reichert M, Worthley DL, Neumann J, Werner J, Iuga AC, Olive KP, Wang TC, beta2 Adrenergic-Neurotrophin Feedforward Loop Promotes Pancreatic Cancer. Cancer Cell 2018;33(1),75–90 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Renz BW, Tanaka T, Sunagawa M, Takahashi R, Jiang Z, Macchini M, Dantes Z, Valenti G, White RA, Middelhoff MA, Ilmer M, Oberstein PE, Angele MK, Deng H, Hayakawa Y, Westphalen CB, Werner J, Remotti H, Reichert M, Tailor YH, Nagar K, Friedman RA, Iuga AC, Olive KP, Wang TC, Cholinergic Signaling via Muscarinic Receptors Directly and Indirectly Suppresses Pancreatic Tumorigenesis and Cancer Stemness. Cancer Discov 2018;8(11),1458–73. [DOI] [PMC free article] [PubMed] [Google Scholar]


