Abstract
Although numerous studies have reported the association between sarcopenia and the prognosis of hepatocellular carcinoma (HCC) patients, there is lack of a newer and more comprehensive meta-analysis. Herein, a comprehensive literature search was performed on PubMed, Web of Science, the Cochrane Library, and Embase databases to identify relevant studies published up to February 2022. The outcomes were overall survival (OS), recurrence, progression‐free survival, tumor response, severe postoperative complications, and toxicity of drugs. A total of 57 studies involving 9790 HCC patients were included in the meta-analysis. The pooled prevalence of sarcopenia in HCC patients was 41.7% (95% CI 36.2–47.2%). Results demonstrated that sarcopenia was significantly associated with impaired OS (HR: 1.93, 95% CI 1.73–2.17, P < 0.001), higher risk of tumor recurrence (HR: 1.75, 95% CI 1.56–1.96, P < 0.001), lower objective response rate (OR: 0.37 95% CI 0.17–0.81, P = 0.012), and more drug-related adverse events (OR: 2.23, 95% CI 1.17–4.28, P = 0.015) in HCC patients. The subgroup analyses revealed that the OS of patients at the early stage of tumor was more severely affected by sarcopenia than for patients at other stages. Moreover, the presence of cirrhosis and Child Pugh class B increased the hazard of mortality from sarcopenia. This study has shown that sarcopenia is highly associated with poor prognosis in HCC patients. In addition, cirrhosis and poor liver functional reserve increase the danger of sarcopenia. OS was more impaired in HCC patients with sarcopenia at early stage of tumor than at other tumor stages.
Subject terms: Cancer, Computational biology and bioinformatics
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common cancer and the fourth leading cause of cancer-related death1. Studies have reported that HCC is closely associated with chronic liver disease and cirrhotic livers2, and patients with these comorbidities commonly suffer poor appetite, malabsorption, and anorexia, which ultimately leads to the abnormality of nutritional status and skeletal muscle condition3. Although there are some methods that can predict the prognosis of patients according to tumor stage, such as Barcelona Clinic Liver Cancer (BCLC) staging classification and tumor-node-metastasis (TNM) classification4,5, these methods do not evaluate the nutritional status and skeletal muscle condition. In addition, despite the Child–Pugh score including the indicators of albumin and ascites which reflect the nutritional status to some degree, it is still limited by its inherent subjectivity in the assessment of Child–Pugh6. Moreover, although an objective laboratory parameter is used in the Albumin-bilirubin (ALBI) score7, it is hard to comprehensively assess the nutritional status using albumin alone. Therefore, this calls for studies to develop a new method that comprehensively reflects the nutritional status and skeletal muscle status of patients.
Sarcopenia is a skeletal muscle disorder characterized by progressive loss of skeletal muscle mass and strength8–10. Studies have proved that reduced muscle mass is associated with decreased immunity, reduced quality of life, and higher prevalence of fractures or falls, which eventually results in poor clinical outcomes11–13. In recent years, an increasing number of studies have reported that the loss of skeletal muscle mass is associated with poor prognoses in cancer patients, including HCC14–16. However, to date, there is no large prospective study which has explored the relationship between sarcopenia and HCC. A recent meta-analysis indicated that sarcopenia impairs clinical outcomes in patients with cirrhosis6. However, although the study included a certain number of HCC patients, it still proved inadequate in determining the association between sarcopenia and HCC. In addition, the meta-analyses on sarcopenia and HCC published before 2019 included very few patients and studies, which limited their statistical power for subgroup analyses or clinical outcomes17,18.
Therefore, there is need for a newer and comprehensive meta-analysis to evaluate the influence of sarcopenia on prognosis of HCC patients, with more detailed subgroup analyses, larger sample sizes, and more clinical outcomes, such as overall survival (OS), recurrence, tumor response, and adverse events. This study investigated a large number of patients and conducted subgroup analyses of different clinical outcomes, with the overarching goal of exploring the association between sarcopenia and HCC.
Methods
Search strategy
This meta-analysis was performed in accordance with the PRISMA guidelines19 and the protocol for this meta‐analysis was available in PROSPERO (CRD42022310433). A comprehensive search was performed on PubMed, Web of Science, the Cochrane Library, and Embase databases to identify relevant studies published up to February 2022. The following key words were used: “sarcopenia”, “sarcopenic”, “skeletal muscle”, “muscle atrophy”, “muscle wasting”, “muscular depletion “, “HCC”, “liver cancer”, “liver neoplasm”, and “hepatocellular carcinoma”. In addition, the references of included studies were manually scanned to retrieve potentially missing studies.
Inclusion and exclusion criteria
Two independent authors (Yusheng Guo and Yanqiao Ren) conducted the preliminary review of literature identified in the databases by reading titles and abstracts. Studies were considered eligible if they met the following inclusion criteria: (1) were limited to English articles; (2) evaluated the impact of sarcopenia in HCC patients; (3) reported OS, disease-free survival (DFS), recurrence-free survival (RFS), objective response rate (ORR), disease control rate (DCR), toxicity of drugs, or postoperative complications were reported. In instances where multiple publications reported overlapping data, the study with the largest sample size was considered. Exclusion Criteria: (1) Comments, editorials, letters, case reports, reviews, and meta-analyses were not considered. (2) Duplicate documents were deleted.
Data extraction
Two authors independently extracted the following data from the included studies: year of publication, name of first author, region, treatment mean, diagnostic method, cut‐off value, HCC stage, outcomes, number of enrolled patients, number of patients with sarcopenia, and sex ratio. Each study was independently assessed by the two authors using the Newcastle–Ottawa scale (NOS)20, and studies with NOS score ≥ 6 were considered high‐quality studies. Any disagreements were resolved by discussion or consensus with a third author (Lian Yang or Chuansheng Zheng).
Statistical analyses
All statistical analyses were performed using R software (version 4.1.0). Before conducting the meta-analysis, a heterogeneity test was performed using χ2 tests (α = 0.10) and the I2 metric. P < 0.05 indicated the existence of heterogeneity, and studies with I2 > 35% were considered as having high heterogeneity. Notably, a random effects model (high heterogeneity) or a fixed effects model (low heterogeneity) was used to pool data for meta-analysis. Next, a forest map was drawn, and the HR or OR and its 95% confidence interval (CI) were described and discussed. Possible sources of heterogeneity were determined using Baujat plots and sensitivity analyses were then conducted through sequential omission of studies. Subgroup analyses of OS, recurrence, and tumor response were performed based on patients’ characteristics. Finally, Egger’s tests and funnel plot were performed to evaluate publication biases. Two-sided P < 0.05 were considered statistically significant for all statistical procedures.
Results
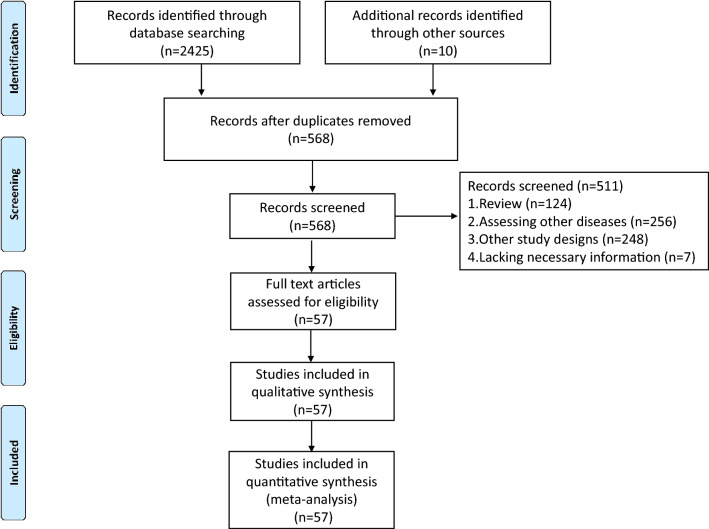
A total of 2435 studies were identified after screening the databases, from which 1867 studies were excluded, followed by reviewing the abstracts of 568 studies in accordance with the inclusion criteria. Finally, 57 studies3,21–76 were included in this meta-analysis after detailed full-text examination (Fig. 1). Notably, sarcopenia was defined based on computed tomography (CT) or magnetic resonance imaging (MRI) in all enrolled studies. Given that two studies by Saeki et al.46,56 had duplicated the patients, the study with more patients was included in OS and subgroup analyses56. Saeki et al.46 was only used to explore the prevalence of sarcopenia. All enrolled studies were retrospective in design.
Figure 1.
Flow diagram of study selection for inclusion in this meta-analysis.
Characteristics of included studies
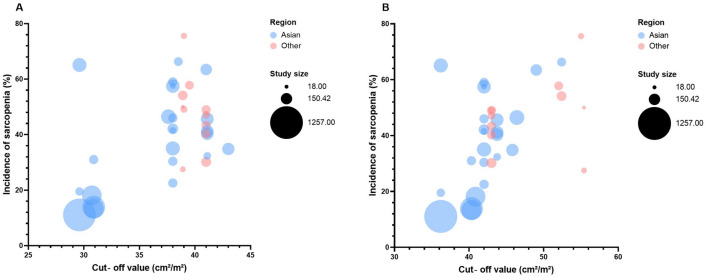
A total of 57 studies involving 9790 HCC patients were included in the meta-analysis. The studies were conducted in different regions, with 41 studies3,24,26–28,32–37,39–41,43–51,54–60,62,63,66–69,71,73–76 (Japan provided the largest volume of studies, followed by the South Korea) from Asia, and 16 studies21–23,25,29–31,38,42,52,53,61,64,65,70,72 conducted in non-Asia regions. The enrolled patients covered all stages of HCC (from BCLC stage 0/A to BCLC stage D) and the corresponding treatments (RFA, hepatectomy, LDLT, TACE, TARE, radiotherapy, sorafenib, lenvatinib, and ICIs). Sarcopenia was diagnosed through CT or (MRI) in all studies. Two studies3,71 included only males and three studies identified sarcopenia using the change of muscle mass after or during treatment. Among all included studies, different diagnostic methods and cut‐off values were utilized to identify sarcopenia, with the skeletal mass index (SMI) being the most commonly used method. Figure 2 indicates that the cut-off value of SMI in non-Asia regions tended to be higher than in Asian regions in both sexes. Notably, as the cut-off value increased, the prevalence of sarcopenia increased. The prevalence of sarcopenia ranged from 11.1% to 78.3% in 52 studies available21,22,24–34,36–52,54–70,72–76 for the prevalence data, and the pooled prevalence was 41.7% (95% CI 36.2–47.2%) (Fig. S1).
Figure 2.
A bubble plot showing the cut-off values of SMI.
Overall survival
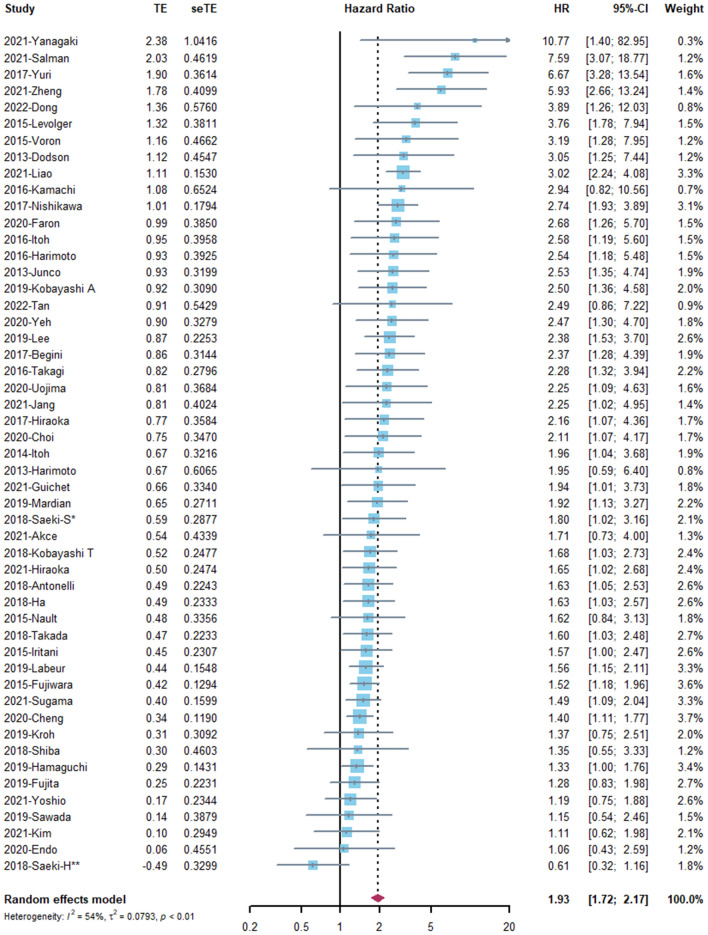
The association between OS and sarcopenia was reported in 51 studies3,23–34,36,38–43,45–71,73–76 involving 8768 patients. Considering the high heterogeneity (I2 = 54%), a random effects model was used for analysis (Fig. 3). The pooled HR was 1.93 (95% CI 1.73–2.17, P < 0.001), which suggested that the presence of sarcopenia was significantly associated with improved mortality. A Baujat plot showed that the study by Liao et al.69 significantly contributed to the overall result on both heterogeneity and influence (Fig. S2). Sensitivity analysis was conducted through sequential omission of studies, from which a similar result was obtained (Fig. S3).
Figure 3.
The forest plot of overall survival.
Recurrence
A total of 17 studies3,24,26,28,31–35,37,44,50,51,67,69,73,74 involving 3615 patients provided DFS or RFS data. The fixed effects model was used, with the pooled HR (HR: 1.75, 95% CI 1.56–1.96, P < 0.001) indicating a higher risk of recurrence in patients with sarcopenia (Fig. S4). Notably, the sensitivity analysis obtained a similar result (Fig. S5).
Progression‐free survival
Six studies40,47,57,64,68,76 involving 581 patients reported the association between PFS and sarcopenia. There was no heterogeneity (I2 = 0) in these studies and the fixed effects model showed impaired PFS in patients with sarcopenia, but the trend was not significant (HR: 1.20, 95% CI 0.98–1.48, P = 0.082) (Fig. S6).
Tumor response
Tumor response was evaluated using the modified Response Evaluation Criteria in Solid Tumors (mRECIST) in three studies40,49,72, and based on Response Evaluation Criteria in Solid Tumors (RECIST) in another three studies22,68,76.
The ORR ranged from 0 to 45.5% in the sarcopenia group and 12.7% to 66.7% in the non-sarcopenia group. The OR was 0.37 (95% CI 0.17–0.81, P = 0.012), which indicated that sarcopenia was significantly associated with worse tumor response (Fig. S7). Similar results were obtained after conducting sensitivity analysis (Fig. S8).
Given the high heterogeneity (I2 = 57%), a random effects model for analysis was used to pool HR of DCR before the sensitivity analysis. Results showed that there was no significant association between DCR and sarcopenia (OR: 0.56, 95% CI 0.31–1.01, P = 0.055) (Fig. S9). In addition, the Baujat plot indicated that the study by Fujita et al.49 contributed significantly to heterogeneity (Fig. S10). After it was excluded, the heterogeneity decreased substantially (I2 = 14%), with the obtained result (OR: 0.46, 95% CI 0.30–0.69, P < 0.001) indicating that sarcopenia was significantly associated with worse disease control (Fig. S11).
Severe postoperative complications and toxicity of drugs
A total of four studies24,29,31,52 reported the rate of severe postoperative complications in two groups (three studies addressed hepatectomy and one study addressed RFA). All postoperative complications were evaluated by Clavien-Dindo classification. The OR was 1.15 (95% CI 0.46–2.88, P = 0.772) and the sensitivity analyses yielded similar findings (Fig. S12, S13). After omitting the study by Levolger et al.29, the obtained result (OR: 0.78, 95% CI 0.36–1.67, P = 0.519) revealed that sarcopenia was not associated with the occurrences of severe postoperative complications on hepatectomy (Fig. S14).
Drug toxicity data was available in six studies21,39,40,57,62,76, of which four studies21,39,40,57 addressed sorafenib and two studies62,76 addressed lenvatinib. Results indicated that sarcopenia was significantly associated with higher occurrences of severe drug-related adverse events (OR: 2.23, 95% CI 1.17–4.28, P = 0.015) (Fig. S15). Moreover, the Baujat plot showed that the study by Mir et al.21 contributed significantly to heterogeneity (Fig. S16), and the sensitivity analysis provided similar results (Fig. S17).
Subgroup analyses
Subgroup analyses of OS were conducted according to the treatments that patients underwent, BCLC stages, diagnostic methods, regions, gender, and the time points of diagnosis. With regard to the seven different treatment methods, results showed that the efficacy of most treatments (six out of the seven) could be influenced by sarcopenia (Table 1). Sarcopenia increased the risk of mortality in most patients who underwent RFA (HR: 4.46, 95% CI 2.64–7.54, P < 0.001), but it did not increase the risk of mortality in patients treated with ICIs (HR: 1.27, 95% CI 0.79–2.05, P = 0.323). It is worth noting that the earlier the BCLC stage, the higher the risk of sarcopenia. The pooled HR from three studies56,58,68 involving 620 advanced HCC patients indicated that sarcopenia may be not associated with the OS of patients at the BCLC C stage (HR: 1.20, 95% CI 0.83–1.75, P = 0.331). In addition, subgroup analyses based on the different diagnostic methods, regions, gender, and the time points of diagnosis provided similar results.
Table 1.
Subgroup analyses of overall survival.
| Subgroup | No. of studies | No. of patients | Estimates (HR) | Lower limit to Upper limit | P‐value |
|---|---|---|---|---|---|
| Treatment | |||||
| RFA | 4 | 505 | 4.46 | 2.64–7.54 | < 0.001 |
| Hepatectomy | 12 | 3172 | 2.03 | 1.56–2.64 | < 0.001 |
| TACE/TARE | 6 | 975 | 2.23 | 1.47–3.39 | < 0.001 |
| Radiotherapy | 2 | 224 | 2.13 | 1.43–3.17 | < 0.001 |
| Sorafenib | 8 | 1458 | 1.69 | 1.41–2.06 | < 0.001 |
| Lenvatinib | 4 | 354 | 1.80 | 1.27–2.55 | 0.001 |
| ICIs | 2 | 159 | 1.27 | 0.79–2.05 | 0.323 |
| BCLC stage | |||||
| 0/A | 2 | 233 | 4.13 | 1.38–12.36 | 0.011 |
| 0/A and B | 5 | 660 | 3.93 | 2.38–6.50 | < 0.001 |
| B and C | 13 | 1715 | 1.51 | 1.33–1.72 | < 0.001 |
| C | 3 | 620 | 1.20 | 0.83–1.75 | 0.331 |
| PMI or SMI | |||||
| SMI | 33 | 6727 | 1.87 | 1.63–2.13 | < 0.001 |
| PMI | 8 | 1115 | 2.26 | 1.56–3.28 | < 0.001 |
| Regions | |||||
| Asia | 37 | 7469 | 1.87 | 1.64–2.13 | < 0.001 |
| Non-Asia | 13 | 1299 | 2.16 | 1.73–2.72 | < 0.001 |
| Gender | |||||
| Only males | 2 | 111 | 1.55 | 1.15–2.10 | 0.004 |
| Both | 48 | 8657 | 1.95 | 1.73–2.20 | < 0.001 |
| Delta or baseline* | |||||
| Delta | 3 | 356 | 2.41 | 1.34–4.33 | 0.003 |
| Baseline | 47 | 8412 | 1.93 | 1.72–2.16 | < 0.001 |
ICIs immune checkpoint inhibitors, RFA radiofrequency ablation, TACE transarterial chemoembolization, TARE transarterial radioembolization, BCLC Barcelona Clinic Liver Cancer, SMI skeletal muscle index, PMI psoas muscle index.
*Delta: sarcopenia was defined with the change of skeleton muscle during or after the treatment.
Furthermore, we evaluated the association between the proportion of patients with different liver diseases and liver functional reserve in every cohort and OS. The results were consistent across all subgroups (Table 2) (Tables S2–S16). Specifically, it was found that the higher the proportion of patients with cirrhosis in a cohort, the more increased the risk of mortality due to sarcopenia (Tables S2–S4). Meanwhile, the lower the proportion of patients with Child–Pugh class A and the higher the proportion of patients with Child–Pugh class B, the more increased the risk of mortality (Tables S13–S16).
Table 2.
Different liver diseases and liver functional reserve.
| Subgroup | Proportion | No. of studies | No. of patients | Estimates (HR) | Lower limit to Upper limit | P‐value |
|---|---|---|---|---|---|---|
| Cirrhosis | ||||||
| All cirrhosis | 100% | 5 | 641 | 2.61 | 1.76–3.86 | < 0.001 |
| Proportion > median | 62.67–100% | 8 | 1130 | 2.47 | 1.77–3.43 | < 0.001 |
| Proportion < median | 21.58–61.43% | 9 | 2120 | 1.72 | 1.46–2.03 | < 0.001 |
| ALD | ||||||
| Proportion > median | 16.67–47.5% | 9 | 1132 | 1.53 | 1.23–1.91 | < 0.001 |
| Proportion < median | 3.26–15.52% | 9 | 966 | 1.84 | 1.50–2.26 | < 0.001 |
| NASH | ||||||
| Proportion > median | 11.46–100% | 5 | 541 | 1.67 | 1.30–2.15 | < 0.001 |
| Proportion < median | 6.7–10.1% | 5 | 687 | 1.79 | 1.44–2.22 | < 0.001 |
| HBV | ||||||
| proportion > median | 25.1–85.18% | 15 | 2515 | 1.64 | 1.36–1.98 | < 0.001 |
| proportion < median | 6.59–20.18% | 18 | 3662 | 2.01 | 1.69–2.37 | < 0.001 |
| HCV | ||||||
| Proportion > median | 39.19–100% | 18 | 3641 | 2.07 | 1.71–2.50 | < 0.001 |
| Proportion < median | 6.86–38.18% | 16 | 2174 | 1.78 | 1.45–2.18 | < 0.001 |
| Child–Pugh class A | ||||||
| Proportion > median | 80.7–100% | 17 | 3157 | 1.61 | 1.44–1.81 | < 0.001 |
| Proportion < median | 50–78.66% | 18 | 3790 | 2.27 | 1.83–2.81 | < 0.001 |
| Child–Pugh class B | ||||||
| Proportion > median | 22.75–62.67% | 16 | 3550 | 2.17 | 1.75–2.69 | < 0.001 |
| Proportion < median | 2.15–16.67% | 15 | 3056 | 1.64 | 1.45–1.84 | < 0.001 |
AH alcohol-related liver disease, NASH nonalcoholic steatohepatitis, HBV hepatitis B Virus, HCV hepatitis C Virus.
Subgroup analyses of recurrence were performed based on patients treated with hepatectomy or LDLT. The pooled HR from three studies3,33,44 involving 295 HCC patients who underwent LDLT were much higher than the pooled HR from the 12 studies24,26,31,32,35,37,50,51,67,69,73,74 on hepatectomy (Table 3).
Table 3.
Subgroup analyses of recurrence and tumor response.
| Subgroup | No. of studies | No. of patients | Estimates (HR/OR) | Lower limit to upper limit | P‐value |
|---|---|---|---|---|---|
| DFS/RFS | |||||
| Hepatectomy | 12 | 3155 | 1.70 | 1.51–1.92 | < 0.001 |
| LDLT | 3 | 295 | 4.13 | 2.14–7.97 | < 0.001 |
| ORR | |||||
| Systemic therapy | 4 | 396 | 0.21 | 0.09–0.46 | < 0.001 |
| TACE | 2 | 265 | 0.70 | 0.28–1.72 | 0.434 |
| DCR | |||||
| Systemic therapy | 4 | 396 | 0.52 | 0.34–0.82 | 0.004 |
| TACE | 2 | 265 | 0.55 | 0.10–3.06 | 0.500 |
LDLT Living-Donor Liver Transplantation, TACE transarterial chemoembolization, DFS disease-free survival, RFS recurrence-free survival, ORR objective response rate, DCR disease control rates.
Subgroup analyses of ORR and DCR were conducted on patients who received systemic therapy or TACE. The detailed data of ORR and DCR were available in four studies22,40,68,76 on systemic therapy (two studies on lenvatinib, one study on sorafenib, and one study on gemcitabine and oxaliplatin) and two studies49,72 on TACE. Results indicated that sarcopenia was associated with lower ORR and DCR in patients that received systemic therapy instead of TACE (Table 3).
Publication bias
Funnel plots of the OS, recurrence, and PFS provided little indication of asymmetry suggestive of publication bias (Fig. S18A–F). The P values from the Egger's test on OS, recurrence, PFS, ORR, DCR, severe postoperative complications, and severe toxicity of drugs were 0.004, 0.024, 0.882, 0.056, 0.277, 0.219, and 0.272, respectively. The Trim and Fill method led to addition of 17 potential unpublished studies (Fig. S19), and the pooled HR of OS was 1.59 (95% CI 1.40–1.81, P < 0.001). Similarly, the pooled HR of recurrence was 1.62 (95% CI 1.36–1.93, P < 0.001) after five potential unpublished studies were added (Fig.S20).
Discussion
To date, this study involving 9790 patients is the largest study that has explored the impact of sarcopenia in HCC. Although two previous systematic reviews and meta-analyses17,18 described the negative influence of sarcopenia in HCC, they only included 13 studies and 3111 patients, which resulted in the absence of detailed subgroup analyses. The two studies also included patients with other cancers (such as intrahepatic cholangiocarcinoma), which may limit interpretation of their conclusions77. In this study, a more comprehensive literature search was conducted, which resulted in more HCC patients being included thereby providing more data for effective subgroup analyses. To determine and decrease the potential heterogeneity, Baujat plots and sensitivity analyses, respectively, were performed to identify the sources of heterogeneity and ensure the stability of obtained results. In addition, to avoid the selection bias and possible calculation error, data was not extracted from the reported Kaplan–Meier curves.
Results demonstrated that sarcopenia was significantly associated with impaired OS, higher risk of tumor recurrence, worse tumor response, and more drug-related adverse events in HCC patients. The calculation results showed that HCC patients with sarcopenia had a 1.93 times higher risk of death, 1.75 times higher risk of recurrence, 0.37 times lower odds of tumor response, and 2.23 times higher odds of adverse drug reactions than HCC patients without sarcopenia. Despite the existence of heterogeneity in the OS data analysis, the sensitivity analyses and the consistency of results derived from different subgroups further validated our results.
In the subgroup analyses, we did not divide the treatment means into curative therapy or palliative treatment like previous meta-analysis17 because many studies using curative therapy included patients at the BCLC B or C stage who were beyond the indications of curative therapy4,24,29,31. Therefore, the subgroups were clearly divided according to specific therapies and BCLC stages. It was found that patients with sarcopenia at early stage were more vulnerable, and thus we speculated that other risk factors such as tumor metastasis or tumor thrombus were the more important factors leading to death in the patients at more advanced stages. However, the skeletal muscle mass representing systemic nutritional states was associated with the tolerance of operation on the liver like RFA, hepatectomy or LDLT. Meanwhile, HCC patients with sarcopenia suffered higher rates of liver failure, major complications, and intra-abdominal abscess formation78,79. Ultimately, OS was significantly impaired in patients with sarcopenia at early stage. In addition, frailty has a very close overlap with sarcopenia80. Frailty was associated with an increased risk for mortality and morbidity related to cancer and worse response to treatment81, therefore, this could result in decreased numbers of frail patients who received TACE, hepatectomy, or systemic treatment. This selection bias could explain why we found more impact of sarcopenia in the BCLC-0/BCLC-A groups in comparison with those treated with TACE or systemic treatment. Moreover, it could also explain why sarcopenia was not associated with complications after hepatectomy (Fig. S14).
A previous study reported that chronic underlying liver diseases contributed to the process of hepatocarcinogenesis82. Moreover, a recent meta-analysis revealed that sarcopenia was highly associated with higher risk of mortality in patients with cirrhosis6. Similarly, this study found that HCC patients with more proportion of cirrhosis were at a higher risk of mortality, suggesting the synergistic effect of cirrhosis and HCC. Therefore, more emphasis should be given for the influence of sarcopenia in HCC patients with cirrhosis. In addition, we found that the proportion of patients with different Child–Pugh classes may affect the association between sarcopenia and OS.
Given the impact of sarcopenia on HCC, more attention should be paid on the prevention of sarcopenia and rehabilitation treatment in HCC patients with sarcopenia. Nutritional support and physical exercise are two promising strategies that can improve the skeletal muscle state and long-term prognosis78. A previous retrospective study reported that L-carnitine improved sarcopenia progression in HCC patients treated with lenvatinib, and patients with L-carnitine supplementation tended to have a longer median time to treatment failure compared to patients without L-carnitine supplementation83. A study conducted in Japan found that in-hospital exercise may prevent sarcopenia in HCC patients who underwent TACE84, which suggested the necessity and feasibility of sarcopenia prevention.
However, this meta-analysis had several limitations. First, all the included studies are retrospective studies, which leads to inevitable selection bias and confounding bias. Second, it was hard to compare differences between the hazard of sarcopenia occurring in the course of treatment and the hazard of baseline sarcopenia because only three studies on the change of skeleton muscle mass during or after the treatment were included in the subgroup analyses. Similarly, only two studies explored the association between sarcopenia and prognosis in patients who underwent immunotherapy, thus, it was hard to draw conclusions on the effect of sarcopenia on the efficacy of ICIs. Third, most studies involved patients with different etiologies of chronic liver diseases and Child Pugh classes, thus, it was hard to directly evaluate the impact of chronic liver diseases and liver functional reserve on sarcopenia. This is despite the fact that we divided studies according to the proportion of specific liver disease or Child Pugh class. Fourth, the funnel plots and Egger's test of OS and recurrence implied the presence of publication bias, even though similar results were achieved from the Trim and Fill method (OS: 1.59 vs. 1.93; recurrence: 1.62 vs. 1.75). Fifth, the cut‐off values varied from different diagnostic methods to different research teams. Therefore, meta‐regression analyses should be used to investigate the effect of cut‐off values in future studies. Sixth, to unify the inclusion criteria and facilitate subgroup analyses, we only included studies defining sarcopenia with radiological evaluation. Finally, it should be noted that the level of evidence from this study was rated as low according to GEADE because of the inclusion of retrospective studies which led to inevitable selection bias and confounding bias.
Conclusion
This meta-analysis demonstrated that sarcopenia was associated with significantly impaired OS, higher risk of tumor recurrence, worse tumor response, and more drug-related adverse events in HCC patients. The presence of cirrhosis and Child Pugh class B increased the hazard of mortality from sarcopenia. HCC patients at early stage of tumor had more impaired OS resulting from sarcopenia than other tumor stages.
Supplementary Information
Acknowledgements
We thank Tianxiang Li (Peking Union Medical College Hospital) and Zheng Zhong (Jinan University) for their support in statistics. We also thank the reviewers’ helpful comments and suggestions.
Author contributions
Conception and design: Y.G., L.Y., C.Z.; Collection of data from literatures: Y.G., Y.R.; Data analysis and interpretation: Y.G. and L.Z.; Manuscript writing: All authors; Final approval of manuscript: All authors; Accountable for all aspects of the work: All authors.
Funding
This work was supported by grant from National Nature Science Foundation of China (No. 81873919).
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Yusheng Guo, Yanqiao Ren and Licheng Zhu.
Contributor Information
Lian Yang, Email: yanglian@hust.edu.cn.
Chuansheng Zheng, Email: hqzcsxh@sina.com.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-27238-z.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Mancone C, Steindler C, Santangelo L, Simonte G, Vlassi C, Longo MA, et al. Hepatitis C virus production requires apolipoprotein A-I and affects its association with nascent low-density lipoproteins. Gut. 2011;60(3):378–386. doi: 10.1136/gut.2010.211292. [DOI] [PubMed] [Google Scholar]
- 3.Tan Y, Duan T, Li B, Zhang B, Zhu Y, Yan K, et al. Sarcopenia defined by psoas muscle index independently predicts long-term survival after living donor liver transplantation in male recipients. Quant. Imaging Med. Surg. 2022;12(1):215–228. doi: 10.21037/qims-21-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J. Hepatol. 2022;76(3):681–693. doi: 10.1016/j.jhep.2021.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu H, Yan Y, Chen R, Zhu M, Lin J, He C, et al. Integrated nomogram based on five stage-related genes and TNM stage to predict 1-year recurrence in hepatocellular carcinoma. Cancer Cell Int. 2020;20:140. doi: 10.1186/s12935-020-01216-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tantai X, Liu Y, Yeo YH, Praktiknjo M, Mauro E, Hamaguchi Y, et al. Effect of sarcopenia on survival in patients with cirrhosis: A meta-analysis. J. Hepatol. 2022;76(3):588–599. doi: 10.1016/j.jhep.2021.11.006. [DOI] [PubMed] [Google Scholar]
- 7.Hiraoka A, Kumada T, Michitaka K, Kudo M. Newly proposed ALBI grade and ALBI-T score as tools for assessment of hepatic function and prognosis in hepatocellular carcinoma patients. Liver Cancer. 2019;8(5):312–325. doi: 10.1159/000494844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cruz-Jentoft AJ, Sayer AA. Sarcopenia. Lancet. 2019;393(10191):2636–2646. doi: 10.1016/S0140-6736(19)31138-9. [DOI] [PubMed] [Google Scholar]
- 9.Choi Y, Cho J, No MH, Heo JW, Cho EJ, Chang E, et al. Re-setting the circadian clock using exercise against sarcopenia. Int. J. Mol. Sci. 2020;21(9):3106. doi: 10.3390/ijms21093106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nishikawa H, Shiraki M, Hiramatsu A, Moriya K, Hino K, Nishiguchi S. Japan Society of Hepatology guidelines for sarcopenia in liver disease (1st edition): Recommendation from the working group for creation of sarcopenia assessment criteria. Hepatol. Res. Off. J. Jpn. Soc. Hepatol. 2016;46(10):951–963. doi: 10.1111/hepr.12774. [DOI] [PubMed] [Google Scholar]
- 11.Martin L, Birdsell L, Macdonald N, Reiman T, Clandinin MT, McCargar LJ, et al. Cancer cachexia in the age of obesity: Skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2013;31(12):1539–1547. doi: 10.1200/JCO.2012.45.2722. [DOI] [PubMed] [Google Scholar]
- 12.Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16–31. doi: 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lai JC, Tandon P, Bernal W, Tapper EB, Ekong U, Dasarathy S, et al. Malnutrition, frailty, and sarcopenia in patients with cirrhosis: 2021 Practice guidance by the American association for the study of liver diseases. Hepatology. 2021;74(3):1611–1644. doi: 10.1002/hep.32049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takenaka Y, Oya R, Takemoto N, Inohara H. Predictive impact of sarcopenia in solid cancers treated with immune checkpoint inhibitors: a meta-analysis. J. Cachexia. Sarcopenia Muscle. 2021;12(5):1122–1135. doi: 10.1002/jcsm.12755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feliciano EMC, Kroenke CH, Meyerhardt JA, Prado CM, Bradshaw PT, Kwan ML, et al. Association of systemic inflammation and sarcopenia with survival in nonmetastatic colorectal cancer: Results from the C scans study. JAMA Oncol. 2017;3(12):e172319. doi: 10.1001/jamaoncol.2017.2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caan BJ, Cespedes Feliciano EM, Prado CM, Alexeeff S, Kroenke CH, Bradshaw P, et al. Association of muscle and adiposity measured by computed tomography with survival in patients with nonmetastatic breast cancer. JAMA Oncol. 2018;4(6):798–804. doi: 10.1001/jamaoncol.2018.0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang KV, Chen JD, Wu WT, Huang KC, Hsu CT, Han DS. Association between Loss of skeletal muscle mass and mortality and tumor recurrence in hepatocellular carcinoma: A systematic review and meta-analysis. Liver Cancer. 2018;7(1):90–103. doi: 10.1159/000484950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang G, Meng S, Li R, Ye J, Zhao L. Clinical significance of sarcopenia in the treatment of patients with primary hepatic malignancies, a systematic review and meta-analysis. Oncotarget. 2017;8(60):102474–102485. doi: 10.18632/oncotarget.19687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxfordasp.
- 21.Mir O, Coriat R, Blanchet B, Durand JP, Boudou-Rouquette P, Michels J, et al. Sarcopenia predicts early dose-limiting toxicities and pharmacokinetics of sorafenib in patients with hepatocellular carcinoma. PLoS ONE. 2012;7(5):e37563. doi: 10.1371/journal.pone.0037563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mir O, Coriat R, Boudou-Rouquette P, Ropert S, Durand JP, Cessot A, et al. Gemcitabine and oxaliplatin as second-line treatment in patients with hepatocellular carcinoma pre-treated with sorafenib. Med. Oncol. 2012;29(4):2793–2799. doi: 10.1007/s12032-012-0208-x. [DOI] [PubMed] [Google Scholar]
- 23.Dodson RM, Firoozmand A, Hyder O, Tacher V, Cosgrove DP, Bhagat N, et al. Impact of sarcopenia on outcomes following intra-arterial therapy of hepatic malignancies. J. Gastrointest. Surg. Off. J. Soc. Surg. Alimentary Tract. 2013;17(12):2123–2132. doi: 10.1007/s11605-013-2348-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harimoto N, Shirabe K, Yamashita YI, Ikegami T, Yoshizumi T, Soejima Y, et al. Sarcopenia as a predictor of prognosis in patients following hepatectomy for hepatocellular carcinoma. Br. J. Surg. 2013;100(11):1523–1530. doi: 10.1002/bjs.9258. [DOI] [PubMed] [Google Scholar]
- 25.Meza-Junco J, Montano-Loza AJ, Baracos VE, Prado CM, Bain VG, Beaumont C, et al. Sarcopenia as a prognostic index of nutritional status in concurrent cirrhosis and hepatocellular carcinoma. J. Clin. Gastroenterol. 2013;47(10):861–870. doi: 10.1097/MCG.0b013e318293a825. [DOI] [PubMed] [Google Scholar]
- 26.Itoh S, Shirabe K, Matsumoto Y, Yoshiya S, Muto J, Harimoto N, et al. Effect of body composition on outcomes after hepatic resection for hepatocellular carcinoma. Ann. Surg. Oncol. 2014;21(9):3063–3068. doi: 10.1245/s10434-014-3686-6. [DOI] [PubMed] [Google Scholar]
- 27.Fujiwara N, Nakagawa H, Kudo Y, Tateishi R, Taguri M, Watadani T, et al. Sarcopenia, intramuscular fat deposition, and visceral adiposity independently predict the outcomes of hepatocellular carcinoma. J. Hepatol. 2015;63(1):131–140. doi: 10.1016/j.jhep.2015.02.031. [DOI] [PubMed] [Google Scholar]
- 28.Iritani S, Imai K, Takai K, Hanai T, Ideta T, Miyazaki T, et al. Skeletal muscle depletion is an independent prognostic factor for hepatocellular carcinoma. J. Gastroenterol. 2015;50(3):323–332. doi: 10.1007/s00535-014-0964-9. [DOI] [PubMed] [Google Scholar]
- 29.Levolger S, van Vledder MG, Muslem R, Koek M, Niessen WJ, de Man RA, et al. Sarcopenia impairs survival in patients with potentially curable hepatocellular carcinoma. J. Surg. Oncol. 2015;112(2):208–213. doi: 10.1002/jso.23976. [DOI] [PubMed] [Google Scholar]
- 30.Nault JC, Pigneur F, Nelson AC, Costentin C, Tselikas L, Katsahian S, et al. Visceral fat area predicts survival in patients with advanced hepatocellular carcinoma treated with tyrosine kinase inhibitors. Digest. Liver Dis. Off. J. Italian Soc. Gastroenterol. Italian Assoc. Study Liver. 2015;47(10):869–876. doi: 10.1016/j.dld.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 31.Voron T, Tselikas L, Pietrasz D, Pigneur F, Laurent A, Compagnon P, et al. Sarcopenia impacts on short- and long-term results of hepatectomy for hepatocellular carcinoma. Ann. Surg. 2015;261(6):1173–1183. doi: 10.1097/SLA.0000000000000743. [DOI] [PubMed] [Google Scholar]
- 32.Harimoto N, Yoshizumi T, Shimokawa M, Sakata K, Kimura K, Itoh S, et al. Sarcopenia is a poor prognostic factor following hepatic resection in patients aged 70 years and older with hepatocellular carcinoma. Hepatol. Res. Off. J. Jpn. Soc. Hepatol. 2016;46(12):1247–1255. doi: 10.1111/hepr.12674. [DOI] [PubMed] [Google Scholar]
- 33.Itoh S, Yoshizumi T, Kimura K, Okabe H, Harimoto N, Ikegami T, et al. Effect of sarcopenic obesity on outcomes of living-donor liver transplantation for hepatocellular carcinoma. Anticancer Res. 2016;36(6):3029–3034. [PubMed] [Google Scholar]
- 34.Kamachi S, Mizuta T, Otsuka T, Nakashita S, Ide Y, Miyoshi A, et al. Sarcopenia is a risk factor for the recurrence of hepatocellular carcinoma after curative treatment. Hepatol. Res. Off. J. Jpn. Soc. Hepatol. 2016;46(2):201–208. doi: 10.1111/hepr.12562. [DOI] [PubMed] [Google Scholar]
- 35.Kobayashi A, Kaido T, Hamaguchi Y, Okumura S, Taura K, Hatano E, et al. Impact of postoperative changes in sarcopenic factors on outcomes after hepatectomy for hepatocellular carcinoma. J. Hepatobiliary Pancreat. Sci. 2016;23(1):57–64. doi: 10.1002/jhbp.302. [DOI] [PubMed] [Google Scholar]
- 36.Takagi K, Yagi T, Yoshida R, Shinoura S, Umeda Y, Nobuoka D, et al. Sarcopenia and American society of anesthesiologists physical status in the assessment of outcomes of hepatocellular carcinoma patients undergoing hepatectomy. Acta Med. Okayama. 2016;70(5):363–370. doi: 10.18926/AMO/54594. [DOI] [PubMed] [Google Scholar]
- 37.Yabusaki N, Fujii T, Yamada S, Suzuki K, Sugimoto H, Kanda M, et al. Adverse impact of low skeletal muscle index on the prognosis of hepatocellular carcinoma after hepatic resection. Int. J. Surg. 2016;30:136–142. doi: 10.1016/j.ijsu.2016.04.049. [DOI] [PubMed] [Google Scholar]
- 38.Begini P, Gigante E, Antonelli G, Carbonetti F, Iannicelli E, Anania G, et al. Sarcopenia predicts reduced survival in patients with hepatocellular carcinoma at first diagnosis. Ann. Hepatol. 2017;16(1):107–114. doi: 10.5604/16652681.1226821. [DOI] [PubMed] [Google Scholar]
- 39.Hiraoka A, Hirooka M, Koizumi Y, Izumoto H, Ueki H, Kaneto M, et al. Muscle volume loss as a prognostic marker in hepatocellular carcinoma patients treated with sorafenib. Hepatol. Res. Off. J. Jpn. Soc. Hepatol. 2017;47(6):558–565. doi: 10.1111/hepr.12780. [DOI] [PubMed] [Google Scholar]
- 40.Nishikawa H, Nishijima N, Enomoto H, Sakamoto A, Nasu A, Komekado H, et al. Prognostic significance of sarcopenia in patients with hepatocellular carcinoma undergoing sorafenib therapy. Oncol. Lett. 2017;14(2):1637–1647. doi: 10.3892/ol.2017.6287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yuri Y, Nishikawa H, Enomoto H, Ishii A, Iwata Y, Miyamoto Y, et al. Implication of psoas muscle index on survival for hepatocellular carcinoma undergoing radiofrequency ablation therapy. J. Cancer. 2017;8(9):1507–1516. doi: 10.7150/jca.19175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Antonelli G, Gigante E, Iavarone M, Begini P, Sangiovanni A, Iannicelli E, et al. Sarcopenia is associated with reduced survival in patients with advanced hepatocellular carcinoma undergoing sorafenib treatment. United Eur. Gastroenterol. J. 2018;6(7):1039–1048. doi: 10.1177/2050640618781188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ha Y, Kim D, Han S, Chon YE, Lee YB, Kim MN, et al. Sarcopenia predicts prognosis in patients with newly diagnosed hepatocellular carcinoma, independent of tumor stage and liver function. Cancer Res. Treat. 2018;50(3):843–851. doi: 10.4143/crt.2017.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim YR, Park S, Han S, Ahn JH, Kim S, Sinn DH, et al. Sarcopenia as a predictor of post-transplant tumor recurrence after living donor liver transplantation for hepatocellular carcinoma beyond the Milan criteria. Sci. Rep. 2018;8(1):7157. doi: 10.1038/s41598-018-25628-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kobayashi T, Kawai H, Nakano O, Abe S, Kamimura H, Sakamaki A, et al. Rapidly declining skeletal muscle mass predicts poor prognosis of hepatocellular carcinoma treated with transcatheter intra-arterial therapies. BMC Cancer. 2018;18(1):756. doi: 10.1186/s12885-018-4673-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saeki I, Yamasaki T, Maeda M, Kawano R, Hisanaga T, Iwamoto T, et al. No muscle depletion with high visceral fat as a novel beneficial biomarker of sorafenib for hepatocellular carcinoma. Liver Cancer. 2018;7(4):359–371. doi: 10.1159/000487858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shiba S, Shibuya K, Katoh H, Koyama Y, Okamoto M, Abe T, et al. No deterioration in clinical outcomes of carbon ion radiotherapy for sarcopenia patients with hepatocellular carcinoma. Anticancer Res. 2018;38(6):3579–3586. doi: 10.21873/anticanres.12631. [DOI] [PubMed] [Google Scholar]
- 48.Takada H, Kurosaki M, Nakanishi H, Takahashi Y, Itakura J, Tsuchiya K, et al. Impact of pre-sarcopenia in sorafenib treatment for advanced hepatocellular carcinoma. PLoS ONE. 2018;13(6):e0198812. doi: 10.1371/journal.pone.0198812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fujita M, Takahashi A, Hayashi M, Okai K, Abe K, Ohira H. Skeletal muscle volume loss during transarterial chemoembolization predicts poor prognosis in patients with hepatocellular carcinoma. Hepatol. Res. Off. J. Jpn. Soc. Hepatol. 2019;49(7):778–786. doi: 10.1111/hepr.13331. [DOI] [PubMed] [Google Scholar]
- 50.Hamaguchi Y, Kaido T, Okumura S, Kobayashi A, Shirai H, Yao S, et al. Preoperative visceral adiposity and muscularity predict poor outcomes after hepatectomy for hepatocellular carcinoma. Liver Cancer. 2019;8(2):92–109. doi: 10.1159/000488779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kobayashi A, Kaido T, Hamaguchi Y, Okumura S, Shirai H, Yao S, et al. Impact of sarcopenic obesity on outcomes in patients undergoing hepatectomy for hepatocellular carcinoma. Ann. Surg. 2019;269(5):924–931. doi: 10.1097/SLA.0000000000002555. [DOI] [PubMed] [Google Scholar]
- 52.Kroh A, Uschner D, Lodewick T, Eickhoff RM, Schöning W, Ulmer FT, et al. Impact of body composition on survival and morbidity after liver resection in hepatocellular carcinoma patients. Hepatobiliary Pancreatic Dis. Int. HBPD INT. 2019;18(1):28–37. doi: 10.1016/j.hbpd.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 53.Labeur TA, van Vugt JL, Ten Cate DW, Takkenberg RB, Ijzermans JN, Koerkamp BG, et al. Body composition is an independent predictor of outcome in patients with hepatocellular carcinoma treated with sorafenib. Liver Cancer. 2019;8(4):255–270. doi: 10.1159/000493586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee J, Cho Y, Park S, Kim JW, Lee IJ. Skeletal muscle depletion predicts the prognosis of patients with hepatocellular carcinoma treated with radiotherapy. Front. Oncol. 2019;9:1075. doi: 10.3389/fonc.2019.01075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mardian Y, Yano Y, Ratnasari N, Choridah L, Wasityastuti W, Setyawan NH, et al. Sarcopenia and intramuscular fat deposition are associated with poor survival in Indonesian patients with hepatocellular carcinoma: A retrospective study. BMC Gastroenterol. 2019;19(1):229. doi: 10.1186/s12876-019-1152-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Saeki I, Yamasaki T, Maeda M, Hisanaga T, Iwamoto T, Matsumoto T, et al. Effect of body composition on survival benefit of hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma: A comparison with sorafenib therapy. PLoS ONE. 2019;14(6):e0218136. doi: 10.1371/journal.pone.0218136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sawada K, Saitho Y, Hayashi H, Hasebe T, Nakajima S, Ikuta K, et al. Skeletal muscle mass is associated with toxicity, treatment tolerability, and additional or subsequent therapies in patients with hepatocellular carcinoma receiving sorafenib treatment. JGH Open Open Access J. Gastroenterol. Hepatol. 2019;3(4):329–337. doi: 10.1002/jgh3.12167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cheng TY, Lee PC, Chen YT, Chao Y, Hou MC, Huang YH. Pre-sarcopenia determines post-progression outcomes in advanced hepatocellular carcinoma after sorafenib failure. Sci. Rep. 2020;10(1):18375. doi: 10.1038/s41598-020-75198-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Choi K, Jang HY, Ahn JM, Hwang SH, Chung JW, Choi YS, et al. The association of the serum levels of myostatin, follistatin, and interleukin-6 with sarcopenia, and their impacts on survival in patients with hepatocellular carcinoma. Clin. Mol. Hepatol. 2020;26(4):492–505. doi: 10.3350/cmh.2020.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Endo K, Kuroda H, Kanazawa J, Sato T, Fujiwara Y, Abe T, et al. Impact of grip strength in patients with unresectable hepatocellular carcinoma treated with lenvatinib. Cancers. 2020;12(8):2146. doi: 10.3390/cancers12082146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Faron A, Sprinkart AM, Pieper CC, Kuetting DLR, Fimmers R, Block W, et al. Yttrium-90 radioembolization for hepatocellular carcinoma: Outcome prediction with MRI derived fat-free muscle area. Eur. J. Radiol. 2020;125:108889. doi: 10.1016/j.ejrad.2020.108889. [DOI] [PubMed] [Google Scholar]
- 62.Uojima H, Chuma M, Tanaka Y, Hidaka H, Nakazawa T, Iwabuchi S, et al. Skeletal muscle mass influences tolerability and prognosis in hepatocellular carcinoma patients treated with lenvatinib. Liver cancer. 2020;9(2):193–206. doi: 10.1159/000504604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yeh WS, Chiang PL, Kee KM, Chang CD, Lu SN, Chen CH, et al. Pre-sarcopenia is the prognostic factor of overall survival in early-stage hepatoma patients undergoing radiofrequency ablation. Medicine. 2020;99(23):e20455. doi: 10.1097/MD.0000000000020455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Akce M, Liu Y, Zakka K, Martini DJ, Draper A, Alese OB, et al. Impact of Sarcopenia, BMI, and inflammatory biomarkers on survival in advanced hepatocellular carcinoma treated with anti-PD-1 antibody. Am. J. Clin. Oncol. 2021;44(2):74–81. doi: 10.1097/COC.0000000000000787. [DOI] [PubMed] [Google Scholar]
- 65.Guichet PL, Taslakian B, Zhan C, Aaltonen E, Farquharson S, Hickey R, et al. MRI-derived sarcopenia associated with increased mortality following yttrium-90 radioembolization of hepatocellular carcinoma. Cardiovasc. Intervent. Radiol. 2021;44(10):1561–1569. doi: 10.1007/s00270-021-02874-6. [DOI] [PubMed] [Google Scholar]
- 66.Hiraoka A, Kumada T, Kariyama K, Tada T, Tani J, Fukunishi S, et al. Clinical importance of muscle volume in lenvatinib treatment for hepatocellular carcinoma: Analysis adjusted with inverse probability weighting. J. Gastroenterol. Hepatol. 2021;36(7):1812–1819. doi: 10.1111/jgh.15336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jang HY, Choi GH, Hwang SH, Jang ES, Kim JW, Ahn JM, et al. Sarcopenia and visceral adiposity predict poor overall survival in hepatocellular carcinoma patients after curative hepatic resection. Transl Cancer Res. 2021;10(2):854–866. doi: 10.21037/tcr-20-2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim N, Yu JI, Park HC, Yoo GS, Choi C, Hong JY, et al. Incorporating sarcopenia and inflammation with radiation therapy in patients with hepatocellular carcinoma treated with nivolumab. Cancer Immunol. Immunother. CII. 2021;70(6):1593–1603. doi: 10.1007/s00262-020-02794-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liao C, Li G, Bai Y, Zhou S, Huang L, Yan M, et al. Prognostic value and association of sarcopenic obesity and systemic inflammatory indexes in patients with hepatocellular carcinoma following hepatectomy and the establishment of novel predictive nomograms. J. Gastrointest. Oncol. 2021;12(2):669–693. doi: 10.21037/jgo-20-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Salman A, Salman M, Moustafa A, Shaaban HE, El-Mikkawy A, Labib S, et al. Impact of sarcopenia on two-year mortality in patients with HCV-associated hepatocellular carcinoma after radiofrequency ablation. J. Hepatocell. Carcinoma. 2021;8:313–320. doi: 10.2147/JHC.S300680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sugama Y, Miyanishi K, Osuga T, Tanaka S, Hamaguchi K, Ito R, et al. Combination of psoas muscle mass index and neutrophil/lymphocyte ratio as a prognostic predictor for patients undergoing nonsurgical hepatocellular carcinoma therapy. JGH Open Open Access J. Gastroenterol. Hepatol. 2021;5(12):1335–1343. doi: 10.1002/jgh3.12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vallati GE, Trobiani C, Teodoli L, Lai Q, Cappelli F, Ungania S, et al. Sarcopenia worsening one month after transarterial radioembolization predicts progressive disease in patients with advanced hepatocellular carcinoma. Biology. 2021;10(8):728. doi: 10.3390/biology10080728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yanagaki M, Haruki K, Yasuda J, Furukawa K, Onda S, Tsunematsu M, et al. The significance of the rapid turnover protein score as a predictor of the long-term outcomes in hepatocellular carcinoma after hepatic resection. Ann. Surg. Oncol. 2021;28(13):8130–8139. doi: 10.1245/s10434-021-10704-9. [DOI] [PubMed] [Google Scholar]
- 74.Yoshio S, Shimagaki T, Hashida R, Kawaguchi T, Tsutsui Y, Sakamoto Y, et al. Myostatin as a fibroblast-activating factor impacts on postoperative outcome in patients with hepatocellular carcinoma. Hepatol. Res. Off. J. Jpn. Soc. Hepatol. 2021;51(7):803–812. doi: 10.1111/hepr.13667. [DOI] [PubMed] [Google Scholar]
- 75.Zheng X, Cao F, Qian L, Dong J. Body composition changes in hepatocellular carcinoma: Prediction of survival to transcatheter arterial chemoembolization in combination with clinical prognostic factors. Cancer Control J. Moffitt Cancer Center. 2021;28:10732748211038445. doi: 10.1177/10732748211038445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dong D, Shi JY, Shang X, Liu B, Xu WL, Cui GZ, et al. Prognostic significance of sarcopenia in patients with hepatocellular carcinoma treated with lenvatinib: A retrospective analysis. Medicine. 2022;101(5):e28680. doi: 10.1097/MD.0000000000028680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Valero V, 3rd, Amini N, Spolverato G, Weiss MJ, Hirose K, Dagher NN, et al. Sarcopenia adversely impacts postoperative complications following resection or transplantation in patients with primary liver tumors. J. Gastrointest. Surg. Off. J. Soc. Surg. Aliment. Tract. 2015;19(2):272–281. doi: 10.1007/s11605-014-2680-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Perisetti A, Goyal H, Yendala R, Chandan S, Tharian B, Thandassery RB. Sarcopenia in hepatocellular carcinoma: Current knowledge and future directions. World J. Gastroenterol. 2022;28(4):432–448. doi: 10.3748/wjg.v28.i4.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Marasco G, Serenari M, Renzulli M, Alemanni LV, Rossini B, Pettinari I, et al. Clinical impact of sarcopenia assessment in patients with hepatocellular carcinoma undergoing treatments. J. Gastroenterol. 2020;55(10):927–943. doi: 10.1007/s00535-020-01711-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Minaglia C, Giannotti C, Boccardi V, Mecocci P, Serafini G, Odetti P, et al. Cachexia and advanced dementia. J. Cachexia. Sarcopenia Muscle. 2019;10(2):263–277. doi: 10.1002/jcsm.12380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dudzisz-Śledź M, Bylina E, Teterycz P, Rutkowski P. Treatment of metastatic gastrointestinal stromal tumors (GIST): A focus on older patients. Drugs Aging. 2021;38(5):375–396. doi: 10.1007/s40266-021-00841-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Poon D, Anderson BO, Chen LT, Tanaka K, Lau WY, Van Cutsem E, et al. Management of hepatocellular carcinoma in Asia: Consensus statement from the Asian Oncology Summit 2009. Lancet Oncol. 2009;10(11):1111–1118. doi: 10.1016/S1470-2045(09)70241-4. [DOI] [PubMed] [Google Scholar]
- 83.Okubo H, Ando H, Nakadera E, Ikejima K, Shiina S, Nagahara A. Levocarnitine supplementation suppresses Lenvatinib-related sarcopenia in hepatocellular carcinoma patients: Results of a propensity score analysis. Nutrients. 2021;13(12):4428. doi: 10.3390/nu13124428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Koya S, Kawaguchi T, Hashida R, Hirota K, Bekki M, Goto E, et al. Effects of in-hospital exercise on sarcopenia in hepatoma patients who underwent transcatheter arterial chemoembolization. J. Gastroenterol. Hepatol. 2019;34(3):580–588. doi: 10.1111/jgh.14538. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.