Abstract
In recent years, green corrosion inhibitors derived from natural plant resources have garnered much interest. In the present work, at first, we investigated the corrosion behavior of mild steel (st-37) in the presence, and absence of Dracocephalum extract based on bulk size as a corrosion inhibitor in two widely used acidic environments (0.5 M H2SO4, and 1.0 M HCl), at room temperature. Then, we used Dracocephalum extract based on nanometer size to reduce the optimal concentration of inhibitor, increase the corrosion resistant, and efficiency. Dracocephalum extract does not contain heavy metals or other toxic compounds, and also good characteristics such as low cost, eco-friendly, and widespread availability, make it suitable nature candidate as an environmentally safe green inhibitor. The anticorrosive behavior was assessed using electrochemical impedance spectroscopy (EIS), and potentiodynamic polarization (PP). In all of the studies, the inhibitory efficiency (IE%) increased as the extract dose was increased. But by using nano extract, in addition to maintaining high efficiency, the amount of inhibitor was reduced significantly. The highest IE% is 94% at the best dose of nano extract (75 ppm), but the highest IE% is 89% at the best dose of the bulk extract (200 ppm) in H2SO4 solution. Also, for the HCl solution, the highest IE% is 88% at the best dose of nano extract (100 ppm), but the highest IE% is 90% at the best dose of the bulk extract (400 ppm), by polarization method. The PP results suggest that this compound has an effect on both anodic, and cathodic processes, and that it adsorbs on mild steel surface according to the Langmuir adsorption isotherm. Optical microscopy, scanning electron microscopy (SEM) analysis, and a solid UV–Visible reflection spectrum were used to investigate the alloys' surface morphology.
Subject terms: Nanoscience and technology, Chemistry, Electrochemistry, Green chemistry, Physical chemistry
Introduction
Corrosion has claimed lives, and riches in practically every technical sector in the past1. Corrosion is defined as the deterioration of metals, and alloys as a result of chemical, and physical interactions with their surroundings. The anodic, and cathodic reactions are the chemical processes that create this behavior2. Not only that, but the expense of reviving corrosion-damaged manufacturing equipment contributed significantly to a country's gross domestic product. As a result, all hands must be on the desk to oppose this dangerous deed by doing a periodic study into its final resolution1.
Because of their great mechanical, and electrical qualities, metals are frequently utilized in human activities3. Mild steel is the most often used metal in major industrial businesses due to its cost-effectiveness, and excellent outstanding mechanical properties. However, because of its low corrosion resistance, particularly in acidic, and alkaline settings, its application has been restricted4. The utilization of acid solution in industrial applications has been chiefly used to study the occurrence of mild steel corrosion inhibition mechanisms in acidic environments. The refining process of crude oil, for example, results in various corrosive conditions. In most situations, refinery corrosion is caused by powerful acids attacking the equipment's surface5.
To avoid the corrosion of metals, many methods have been designed after analyzing the different forms of corrosion2. These methods include: inhibitors, electrical protection, surface coating, equipment design, and material selection6. Inhibitors are chemicals that, when applied in tiny amounts to corrosive conditions, inhibit electrochemical corrosion processes on metal surfaces1,7.
The use of corrosion inhibitors is a cost-effective way to reduce corrosion rate, shield metal surfaces against corrosion, and ultimately protect industrial equipment in harsh environments8. The inhibitors work at the interface between the corrosive aqueous solution, and the metal, influencing the electrochemical process procedures by adsorption on the metal's surface9. Polar functional groups10, which help to reduce the sensitivity of a metal surface to corrosion, are centers of reactivity that guarantee the stability of this adsorption process11,12.
Corrosion inhibitors have been extensively worked out in numerous industries to reduce the rate of dissolution of metal goods in contact with a damaging environment. The ability of corrosion inhibitors to adsorb on metal surfaces was linked to their high efficiency13.
Corrosion inhibitors' biodegradability, accumulation, and toxicity have all been questioned recently. Researchers' safety, environmental pollution, and economics are all significant concerns as researchers seek safe, non-polluting, and cost-effective inhibitors14.
Therefore, when selecting an inhibitor, several variables must be addressed, including cost, quantity, ease of availability, and, most importantly, safety to the ecosystem, and its species15.
In the recent decade, green chemistry has been attracting great interest in many contexts by commercial products, chemical technologies, and designing chemicals to reduce wastes, and avoiding toxins16. Green inhibitors are getting much attention in the corrosion field thanks to their renewability, ecologically acceptability, biodegradability, and safety17. These include, for example, polyphenols18, alkaloids15, amino acids19, and often extracts of plants20. As a result, scientists have been looking for green corrosion inhibitors that can retain high inhibitory efficiency while lowering toxicity in recent years21. Organic extracts having functional groups, including sulfur, nitrogen, and oxygen atoms in a conjugate system, are effective inhibitors22. Organic green corrosion inhibitors limit corrosion by eliminating water molecules from the surface of the metal/solution contact, resulting in the creation of a compact barrier layer23.
Nanostructured materials have been studied considerable because of their broad range of prominent applications because nanostructures exhibit novel size-dependent properties, such as magnetic and mechanical and chemical properties, that extensively differ from their bulk materials, that exhibit great potential in the novel fields24.
Several authors have reported using natural materials as corrosion inhibitors, such as extracted compounds from seeds or leaves. Gunasekaran et al.25 investigated the corrosion prevention of steel by the environmentally beneficial Zenthoxylum alatum plant extract in phosphoric acid. Corrosion inhibitors such as leaf extracts, and essential oils are commonly employed26. Corrosion inhibition of leaves extracts, and essential oils such as Acacia Arabica27, Annona squamosa28, Rosmarinous officinalis29, Aloysia citrodora30, and Lawsonia31, which were employed for steel in acid medium, was studied.
Dracocephalum is a genus of flowering plants in the Lamiaceae family with around 6032 to 70 species33 endemic to temperate parts of the Northern Hemisphere. These flowers, commonly known as dragonhead, are herbaceous perennials or subshrubs that grow to a height of 15 to 90 cm. This plant is widely utilized in contemporary medicine to treat a variety of viral disorders as well as to inhibit tumor progression over the world34. Dracocephalum has several biological, and pharmacological activities, including antibacterial35, antifungal36, and anti-inflammatory37.
Dracocephalum extract is a strong contender for use as an ecologically safe green inhibitor since it doesn't include heavy metals or other harmful substances. It also has favorable qualities including affordability, environmental friendliness, and wide availability. So, to overcome the disadvantages of widely used organic corrosion inhibitors, which are expensive and toxic to the environment, and in continuation of our previous works on the development of green corrosion inhibitors3, we report herein the inhibiting effect of Dracocephalum extract in bulk, and nanometer size on the corrosion of mild steel (st-37) in acidic media employing electrochemical impedance spectroscopy (EIS), and potentiodynamic polarization (PP) methods. In each study, the extract dosage was raised as the inhibitory effectiveness rose. However, employing nano extract significantly decreased the quantity of inhibitor while still retaining high efficiency. The experimental data obtained by optical microscopy, scanning electron microscopy, and UV–visible spectroscopy to confirm or reject the potential of this herbal extract as a novel green inhibitor. This article gives a vivid account of Dracocephalum extract as a natural product which is used as a corrosion inhibitor for mild steel alloy in aggressive media, with suitable efficiency, and the minimum concentration of inhibitor based on nanometer size.
Experimental details
Materials
Materials were commercially available and employed without further purification and prepared from Arshanzist Youtab Company. For the preparation of the electrolytes, and Dracocephalum extract, the following materials, and reagents were used: sulfuric acid (MW 98.08 g/mol, 96%), hydrochloric acid (MW 36.46 g/mol, 37%), ethyl alcohol (MW 46.07 g/mol, 99.5%), methanol (MW 32.04 g/mol, 99.8%), and distilled water (MW 18.02 g/mol).
Preparation of the st-37 electrodes for electrochemical test
The samples for the corrosion testing were made of mild steel. Table 1 shows the chemical composition of the alloy.
Table 1.
Chemical composition of mild steel (wt%).
| Element | C | Mn | P | Si | Cr | Al | Cu | Fe |
|---|---|---|---|---|---|---|---|---|
| Wt% | 0 0.076 | 0.192 | 0.012 | 0.026 | 0.050 | 0.023 | 0.123 | Balance |
Specimens with a surface area of 1 cm2 were used for all electrochemical experiments. The exposed side of the steel sheets was polished to a mirror shine with several grades of emery papers (100, 400, 1000, and 2500). Distilled water was used to clean the substrates, which were then degreased with ethyl alcohol and dried at room temperature.
Preparation of Dracocephalum extract
The healthy leaves of Dracocephalum were purchased from the local markets in Iran, which are completely designated for commercial usage. To eliminate dust, the gathered leaves were gently washed. The leaves were dried in the shade at room temperature. At ambient temperature and in the dark, 100 g of dried Dracocephalum leaves were soaked in methanol for 72 h. The surplus solvent was evaporated under reduced pressure in a rotary evaporator at 40 °C after filtering the solution. The recovered residue had a consistent weight of 2.0 g.
It is noteworthy that, alcohol-based herbal preparations are those that use some form of alcohol as the solvent. Herbal tinctures, and herbal liniments are both considered alcohol-based preparations even though two different types of alcohol are used (ethyl alcohol and isopropyl alcohol, respectively). Alcohol preparations have a long shelf life as alcohol slows the decomposition of materials and bacterial growth, thus, increasing herbal preparation shelf life38.
Declaration for the usage of plant materials
We declare that in this research, we did not use or not going to use any plants (either cultivated or wild) irrespective of any location. Experimental research and field study in this study has complied with the IUCN Policy Statement on Research Involving Species at Risk of Extinction. The use of plants in the present study complies with international, national and/or institutional guidelines.
Preparation of Nanosized Dracocephalum extract
To obtain herbal nanostructures, the following method used. A specific value of pure Dracocephalum extract dissolved in 100 mL of ethanol in a beaker to have a solution. The solution stirred at room temperature via vigorous stirring for 30 min at 800 rpm, then the product filtered using filter papers (Whatman, 40 Ashless, Germany) to remove probable impurities. The filtered solution added at a 1:10 ratio to distilled water to isolate pure herbal particles. The suspensions placed in an ultrasonic bath for 20–30 min, and afterwards, to produce lower-sized nanostructures, ultra-prob sonication for 20 periods of 10 s (Hielscher, UP100H, Germany) used as well. Afterward, nanoparticles acquired in the colloid state. In this colloid, nanoparticles observed using dynamic light scattering (DLS) techniques.
Preparation of solutions
The corrosive media were 0.5 M H2SO4, and 1.0 M HCl, made by diluting analytical grade Merck H2SO4, and HCl with double distilled water, respectively. Before to each experiment, the test solutions were made fresh by mixing the extract with the corrosive solution. Experiments were conducted twice to verify repeatability. The extract concentrations were 50, 100, 150, 200, and 250 ppm for 0.5 M H2SO4, and 100, 200, 300, 400, and 500 ppm for 1.0 M HCl based on bulk size, and 25, 50, 75, and 100 ppm for 0.5 M H2SO4, and 50, 75, 100, and 125 ppm for 1.0 M HCl based on nanosized extract.
Noticeably, pentacyclic triterpenoids are one of the main functional components in Dracocephalum extract. Pentacyclic triterpenoids are practically insoluble in water and low concentration ethanol, but they are soluble in chloroform, HCl, and acidic media39.
Characterization
To investigate the size distribution or average sizes of the plant extract, dynamic light scattering (DLS) was employed. DLS data obtained using a Nano-ZS90 (Malvern) apparatus (Malvern Instruments, Malvern, UK). Electrochemical research such as electrochemical impedance spectroscopy, and potentiodynamic polarization were done using the AutoLab device (302 N potentiostat, Netherlands). Scanning electron microscopy (SEM FEI Quanta 200, accelerating voltage 20.0 kV), and optical microscopy (Leica zoom 2000 model) were used to investigate the surface morphology of mild steel submerged in sulfuric acid, and hydrochloric acid without and with the optimal concentration of Dracocephalum extract. The measurements of UV–Visible reflection spectra of surface species on the mild steel were performed by using UV–Vis spectrophotometer A SPECORD 210 (Analytik Jena, Germany) in the stainless tank (π × 12 × 1.5 cm) to avoid interference from ambient light. This spectrophotometer is controlled with the Spectra Manager software. For the last two tests, working electrodes were mechanically polished, and immersed in 0.5 M H2SO4, and 1.0 M HCl solutions in the absence, and presence of an inhibitor for about 24 h at room temperature and then removed and dried.
Statistical analysis
After exploring the normal distribution using the Kolmogorov-smearnov test, the data were subjected to One Way ANOVA, and Tukey Post Hoc tests (S = 0.05).
Stability study
The synthesized nanoparticles were stored at 4 °C, room temperature (24 °C), and physiologic temperature (37 °C) for 3 weeks in the glass vials. After a duration of storage, the distribution of nanoparticles size considered to detect the variations in the formulation with respect to time.
Procedures
Electrochemical measurements
EIS is a vital way of monitoring in situ electrochemical changes with critical knowledge of physical processes occurring at the metal/electrolyte interface40, so impedance diagrams may provide information on mechanistic, surface characteristics, and electrode kinetics41. In most applications, the basic lab setup comprises employing three electrodes in the electrochemical cell for the measurement: working, counter, and reference electrodes submerged in a specified volume and the concentration test solution. So in this work, a three-electrode cell containing Pt electrode, Ag/AgCl electrode, and st-37 specimen as a counter, a reference, and a working electrode, respectively, have been used. First, the open circuit potential (OCP) was recorded for 30 min, and then the EIS data were obtained. The experiment is carried out using a modest potential of 10 mV of AC voltage and frequencies ranging from 100 kHz to 100 mHz. The inhibition efficiency (IEI) of a corrosion inhibitor was estimated using the following equation utilizing electrochemical data collected from the workstation42:
| 1 |
where Rct, and R′ct are the polarization resistance of the sample in the presence, and absence of the corrosion inhibitor, respectively.
Potentiodynamic polarization is another electrochemical-based method for determining the corrosion mechanism protection, corrosion rate, and effectiveness of green corrosion inhibitors. The experiment is carried out in a three-electrode electrochemical cell, the same as EIS. The polarization scan rate was set at 1 mV/s to plot the Tafel polarization curves. The electrode potential was changed automatically from − 800 mV to − 100 mV vs. Ecorr at 25 ± 1 °C to create these graphs. After EIS, a potentiodynamic test was used to determine the polarization curve. The corrosion inhibitor's inhibition efficiency (IEP) is calculated using the following equation43:
| 2 |
where, i, and i′ are the current densities of the solution in the absence, and presence of the inhibitor, respectively.
Also, using NOVA 1–10 software, the suitable equivalent circuit, the corresponding EIS, and potentiodynamic polarization parameters can be prepared.
To check the reproducibility of the results, at least two experiments were performed at each concentration for EIS, and potentiodynamic polarization curve. The standard deviations (S.D.) were obtained and, S.D. values were small, suggesting that the electrochemical measurements had good reproducibility. In this work, S.D. is smaller than 0.5 for all electrochemical experiments, so these data were omitted in the following sections.
Results and discussion
Characterization of the nanosized plant extract
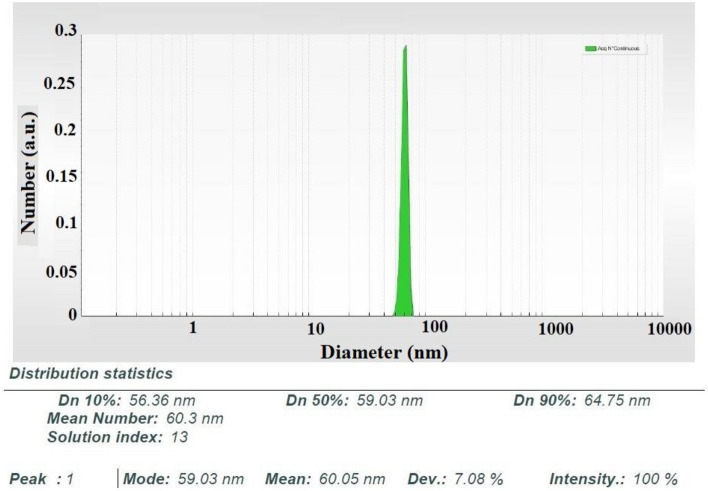
Dynamic light scattering is used for measuring average particle diameter, and particle diameter distribution of nanosized particles dispersed in the liquid. Extract Biomolecules like proteins, enzymes, terpenoids, and flavonoids cofactors play both capping, and reducing role. Furthermore, due to strong binding ability with amino acid residues (carbonyl group), agglomeration behavior was prevented, and stability of medium was provided. For a better understanding of the real size of nanoparticles, the nanosizer technique used for calculating particle size, and stated by SBL (Statistical Bin Limits) analysis. For this propose, reduction of agglomeration fault to state actual particle size was done by omitting hydrodynamic radius. Figure 1 reported the histogram of the SBL nanosizer of NPs, which showed the mean diameter of the size of the particle is ~ 64.75 nm for nanostructures. Reported results demonstrated narrow size distribution, and homogenous dispersity of NPs.
Figure 1.
The mean size of produced nanoparticles recorded by the nanosizer equipment (DLS technique).
Corrosion inhibition study
First, by immersing the working electrode in 0.5 M H2SO4, and 1.0 M HCl solution without, and with Dracocephalum extract based on bulk, and nanometer size for 1800s, open-circuit potential (OCP) was stabilized (Fig. 2), and then electrochemical tests were carried out. Figure 2 illustrated that the presence of extract in acidic solutions considerably changed the OCP curves.
Figure 2.
Variation of the OCP as a function of time, recorded for st-37 in 0.5 M H2SO4 (a) based on bulk, and (b) nano size of Dracocephalum, and in 1.0 M HCl (c) based on bulk, and (d) nano size of Dracocephalum, at 25 ± 1 °C.
The corrosion behavior of st-37 in 0.5 M H2SO4, and 1.0 M HCl solutions was determined by EIS, and PP methods under different concentrations of extract based on bulk, and nano size.
Electrochemical impedance Spectroscopy, Bode, and Bode phase analysis in H2SO4 and HCl media
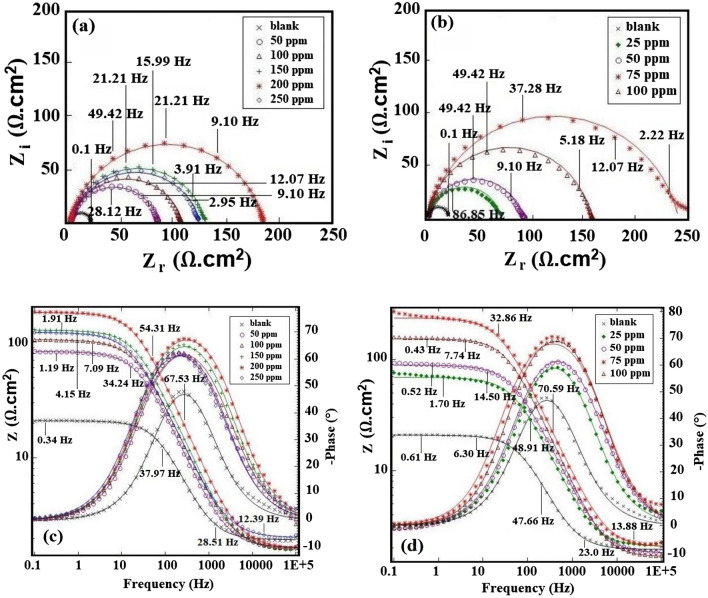
The EIS is a non-destructive, and very effective method for evaluating corrosion processes at the metal-corrosive electrolyte interface. The goal of EIS is to see how different concentrations of green inhibitors affect the impedance behavior of mild steel in 0.5 M H2SO4, and 1.0 M HCl. Figures 3, and 4 demonstrate Nyquist plots, Bode plots, and changes in phase angle for st-37 in 0.5 M H2SO4, and 1.0 M HCl solutions, respectively, with varying quantities of plant extract depending on bulk, and nanosize. Only one capacitive semicircle is seen in the Nyquist plots for the examined plant extract. The existence of charge transfer resistance (Rct) combined with the impact of ionic double-layer capacitance (Cdl) might explain this phenomenon44. The general semi-circle shape of the curves is fairly constant across the whole inhibitor concentration range, indicating that no change in corrosion mechanism has occurred as a result of the addition of plant extract45.
Figure 3.
The (a,b) Nyquist plots, (c,d) bode plots, and phase angle plots for st-37 with different concentrations of Dracocephalum based on bulk, and nano size, in 0.5 M H2SO4.
Figure 4.
The (a,b) Nyquist plots, (c,d) bode plots, and phase angle plots for st-37 with different concentrations of Dracocephalum based on bulk, and nano size, in 1.0 M HCl.
High-frequency capacitance circuits are generally generated by charge transfer resistance, as demonstrated in Figs. 3a, and 4a. It can be seen that adding inhibitor causes an increase in the radius of the capacitive ring, and inhibits electrochemical processes to some extent. It appears that adding the extract to mild steel reduces the rate of corrosion. Tables 2, and 3 show the EIS characteristics for mild steel with various concentrations of Dracocephalum extract (bulk, and nanosize) in acidic media, including the goodness of fit (chi-square), solution resistance (RS), double layer capacitance (Cdl), charge transfer resistance (Rct), and the degree of surface coverage (θ = IEI/100).
Table 2.
Corrosion parameters derived from Nyquist curves for st-37 in H2SO4 solution in the absence, and presence of different concentrations of inhibitor based on bulk, and nano size, at 25 ± 1 °C.
| C/ppm | Chi-square | RS/Ω cm2 | Rct/Ω cm2 | Cdl/μF cm−2 | θ | IE% |
|---|---|---|---|---|---|---|
| (a) Dracocephalum/H2SO4 0.5 M | ||||||
| Blank | 0.032 | 1.96 | 19 | 128 | – | – |
| 50 | 0.031 | 1.67 | 81 | 69 | 0.76 | 76 |
| 100 | 0.057 | 1.64 | 105 | 71 | 0.82 | 82 |
| 150 | 0.021 | 1.60 | 126 | 60 | 0.85 | 85 |
| 200 | 0.011 | 1.66 | 182 | 41 | 0.90 | 90 |
| 250 | 0.062 | 2.04 | 121 | 62 | 0.84 | 84 |
| (b) Dracocephalum (nano)/H2SO4 0.5 M | ||||||
| 25 | 0.065 | 2.08 | 68 | 47 | 0.72 | 72 |
| 50 | 0.024 | 1.80 | 88 | 37 | 0.78 | 78 |
| 75 | 0.16 | 2.06 | 237 | 32 | 0.92 | 92 |
| 100 | 0.019 | 1.69 | 153 | 28 | 0.88 | 88 |
Table 3.
Corrosion parameters derived from Nyquist curves for st-37 in HCl solution in the absence, and presence of different concentrations of inhibitor based on bulk, and nano size, at 25 ± 1 °C.
| C/ppm | Chi-square | RS/Ω cm2 | Rct/Ω cm2 | Cdl/μF cm-2 | θ | IE% |
|---|---|---|---|---|---|---|
| (a) Dracocephalum/HCl 1.0 M | ||||||
| Blank | 0.031 | 1.17 | 18 | 135 | – | – |
| 100 | 0.042 | 2.68 | 39 | 83 | 0.54 | 54 |
| 200 | 0.029 | 1.21 | 68 | 62 | 0.74 | 74 |
| 300 | 0.014 | 1.17 | 99 | 57 | 0.82 | 82 |
| 400 | 0.0082 | 1.21 | 203 | 37 | 0.91 | 91 |
| 500 | 0.012 | 1.13 | 145 | 40 | 0.88 | 88 |
| (b) Dracocephalum (nano)/HCl 1.0 M | ||||||
| 50 | 0.029 | 1.44 | 65 | 50 | 0.72 | 72 |
| 75 | 0.014 | 1.24 | 89 | 36 | 0.80 | 80 |
| 100 | 0.036 | 1.08 | 154 | 65 | 0.88 | 88 |
| 125 | 0.24 | 1.75 | 123 | 35 | 0.85 | 85 |
These semi-circles also show that IEI% increases with an increase in inhibitor concentrations. It is noted that the extract with nanosize possess better IEI% than the bulk extract, in the same amount, in both solutions.
Also, Rct increases when the concentration of Dracocephalum increases, owing to enhanced extract coverage on the steel surface, and higher inhibitor shielding efficiency against ion penetration of the corrosive medium46. When the inhibitor concentration is up to 200 ppm, and 75 ppm for 0.5 M H2SO4, and up to 400 ppm, and 100 ppm for 1.0 M HCl containing bulk, and nanosize of the extract, respectively, the Rct, and IEI% reaches the highest value (90, 92, 91, and 88%). This rise shows that the inhibitor builds an adsorption layer on the mild steel alloy's surface, preventing corrosion. Rct begins to decrease as the concentration of Dracocephalum extract increases, as the inhibitor is desorbed from the metallic surface. As the extract concentration grew, the electric double-layer capacitor, Cdl, dropped, which may be ascribed to a decrease in the local electric double layer constant47. In this case, inhibitor molecules adhered to the steel surface, and replaced the original water molecules that were present in the steel surface's interface layer. The Cdl decreased as the inhibitor concentration grew because the inhibitor molecules had a lower dielectric constant than water molecules, causing the inhibitor molecules to be loosely organized in the interface layer48. It was discovered that the extract might produce an inhibitor coating on the steel surface to prevent corrosion, indicating that Dracocephalum extract has high inhibition efficiency for mild steel.
The increase in phase angle with increasing extract content, as seen in the Bode plots in Figs. 3c,d, and 4c,d, further supports the prevention of corrosion13. The roughness of the electrode surface is linked to the value of the phase angle in these figures. The higher the value of θ, lower is the surface roughness. As the inhibitor concentration increases, the surface roughness decreases, implying that corrosion decreases.
The equivalent Randle's circuit model (Fig. 5) was used to examine all of the impedance curves illustrated in Figs. 3, and 4. This is made up of a series solution resistance (RS), a parallel resistance (Rct), and capacitor combination (Cdl).
Figure 5.
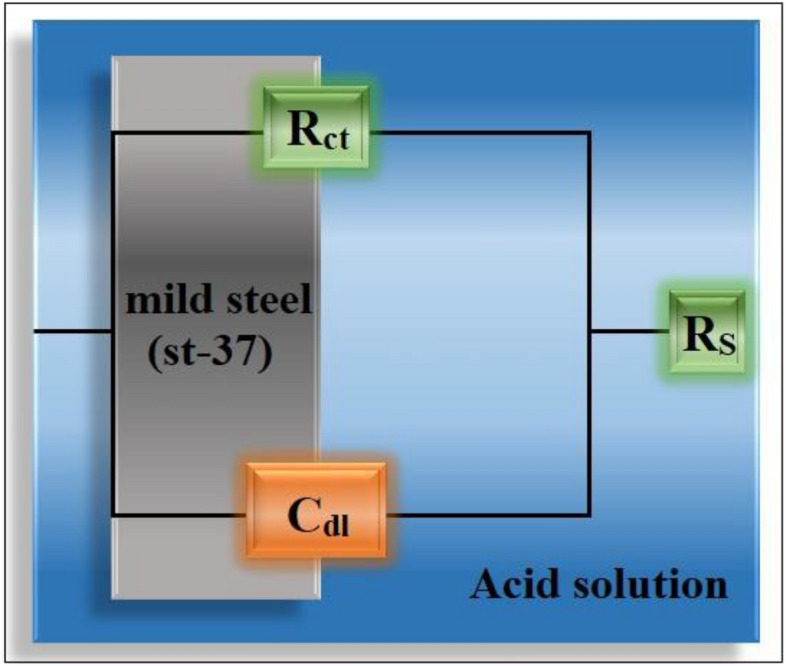
The electrical equivalent Randle's circuit model.
Potentiodynamic polarization in H2SO4 and HCl media
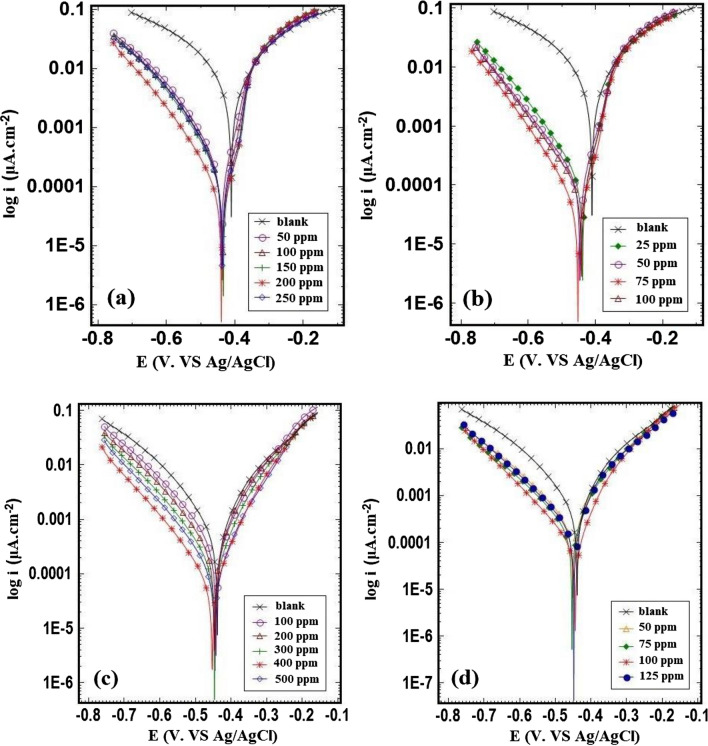
Figure 6 depicts the cathodic, and anodic polarization curves of mild steel following immersion in 0.5 M H2SO4, and 1.0 M HCl solutions in the absence, and presence of various amounts of extract. The experimental results including the corrosion current density (icorr), the cathodic, and anodic Tafel slopes (βc, and βa), the corrosion potential (Ecorr), the inhibition efficiency (IEp%), and the degree of surface coverage (θ) for different solutions are reported in Table 4. The corrosion current density was calculated using the intercept of extrapolated cathodic, and anodic Tafel lines at the corrosion potential. Also, the IEp% was calculated using Eq. (2).
Figure 6.
Polarization curves for st-37 in 0.5 M H2SO4 (a) based on bulk, and (b) nano size of Dracocephalum, and in 1.0 M HCl (c) based on bulk, and (d) nano size of Dracocephalum, at 25 ± 1 °C.
Table 4.
Corrosion parameters derived from polarization curves for st-37 in sulfuric acid solution with (a) inhibitor, (b) nano inhibitor, and in hydrochloric acid solution with (c) inhibitor, (d) nano inhibitor in uninhibited, and inhibited solution at 25 ± 1 °C.
| C/ppm | icorr/μA.cm−2 | − Ecorr/mV | βc/mV.dacade−1 | βa/mV.dacade-1 | θ | IE% |
|---|---|---|---|---|---|---|
| (a) Dracocephalum/H2SO4 0.5 M | ||||||
| Blank | 1427 | 413 | 67 | 59 | – | – |
| 50 | 327 | 440 | 61 | 130 | 0.77 | 77 |
| 100 | 249 | 438 | 55 | 126 | 0.82 | 82 |
| 150 | 222 | 435 | 53 | 129 | 0.84 | 84 |
| 200 | 151 | 440 | 51 | 137 | 0.89 | 89 |
| 250 | 199 | 438 | 53 | 115 | 0.86 | 86 |
| (b) Dracocephalum (nano)/H2SO4 0.5 M | ||||||
| 25 | 197 | 439 | 55 | 143 | 0.86 | 86 |
| 50 | 126 | 447 | 53 | 136 | 0.91 | 91 |
| 75 | 91 | 454 | 56 | 135 | 0.94 | 94 |
| 100 | 105 | 443 | 50 | 135 | 0.93 | 93 |
| (c) Dracocephalum/HCl 1.0 M | ||||||
| Blank | 752 | 440 | 106 | 138 | – | – |
| 100 | 363 | 445 | 92 | 127 | 0.52 | 52 |
| 200 | 209 | 446 | 67 | 111 | 0.72 | 72 |
| 300 | 156 | 447 | 77 | 117 | 0.79 | 79 |
| 400 | 73 | 455 | 75 | 117 | 0.90 | 90 |
| 500 | 111 | 445 | 78 | 119 | 0.85 | 85 |
| (d) Dracocephalum (nano)/HCl 1.0 M | ||||||
| 50 | 217 | 451 | 71 | 126 | 0.71 | 71 |
| 75 | 168 | 454 | 71 | 125 | 0.78 | 78 |
| 100 | 87 | 445 | 62 | 120 | 0.88 | 88 |
| 125 | 182 | 449 | 68 | 128 | 0.76 | 76 |
From the experimental values, it can be observed that the corrosion current density decreases significantly with an increase in inhibitor concentration up to 200 ppm, and 75 ppm for 0.5 M H2SO4, and up to 400 ppm, and 100 ppm for 1.0 M HCl containing bulk, and nano size of the extract, respectively, supports the retardation of the corrosion process49. The reduced current density in the presence of inhibitor in all four solutions suggests that the metal surface is passivated due to the creation of the inhibitor layer50. The results reveal that icorr of mild steel decreased from 1427 μA/cm to 151 μA/cm, and 1427 μA/cm to 91 μA/cm, and the IE% increased to 89%, and 94%, and also, 752 μA/cm to 73 μA/cm, and 752 μA/cm to 87 μA/cm, and the IE% increased to 90%, and 88%, for H2SO4, and HCl solutions with bulk, and nano size of the extract, respectively.
The findings of the investigation, suggest that the nano extract of the plant has greater inhibitory properties than the regular extract.
Furthermore, differences in the values of βc, and βa compared to blank solutions show that these inhibitors safeguard the corrosion process by adsorbing inhibitor molecules on both anodic, and cathodic sites.
With the addition of inhibitors, there is a distinct change in the cathodic, and anodic parts of curves in Tafel plots of H2SO4 solution. As a result, it's referred to as a mixed-type inhibitor. From Fig. 6c,d, and Table 4, in hydrochloric acid solution, the shape of the anodic, and cathodic curves, and the Tafel parameter (βc, and βa) did not change significantly after using the extract as an inhibitor, but in sulfuric acid solution βa changed (Fig. 6a,b), and this means that the inhibitor acts as a both anodic, and cathodic inhibitor (mixed one), with predominant anodic effect in H2SO4 medium. On the other hand, for H2SO4, and HCl solutions, the maximum shift in Ecorr value is positive/negative side 41, and 15 mV, respectively, and a literature survey revealed that if a shift in corrosion potential is less than ± 85 mV with respect to the blank solution, the inhibitor acts as a mixed-type inhibitor; thus, this inhibitor is a mixed-type inhibitor51.
Based on the above analysis, the values of IEI%, and IEP% rise as the concentration of inhibitors rises, with bulk, and nano size of extract in acidic media. The mean difference between the maximum values of %IEI, and %IEP using the best concentration of extract with bulk and nano size is 1.0, and 2.0%, in H2SO4, and 1.0, and 0.0%, in HCl solutions, respectively.
Figure 7 shows the influence of inhibitor concentration (ppm) on inhibition efficiency (IEp, and IEI, %) for st-37 steel in 0.5 M H2SO4, and 1.0 M HCl at 25 ± 1 °C, as measured by impedance, and polarization. It has been discovered that when the concentration of extract increases, the effectiveness of inhibition increases. Inhibition efficiency significantly increases as the extract concentration increases from 0 up to 200, 75, 400, and 100 ppm in acidic media. When the concentration of inhibitor exceeds from above values, the inhibition efficiency decreases slightly. The slight change in inhibition efficiency is due to the saturation adsorption of inhibitor molecules on the alloy surface. The higher inhibition efficiency indicates that the Dracocephalum extract is a suitable corrosion inhibitor for both acidic media.
Figure 7.
Variation of IE% with the concentration of inhibitor for both (a) impedance, and (b) polarization experiments.
Adsorption isotherm
Adsorption isotherms serve a critical function in providing extensive information about the current interaction behavior between metal surfaces, and Dracocephalum extract molecules52.
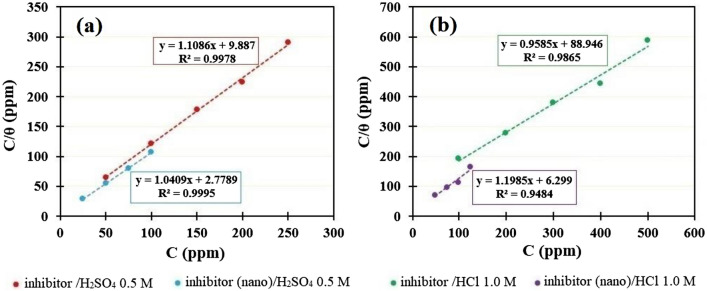
Different adsorption isotherm models were utilized in this work to suit the experimental results. The Langmuir isotherm is in good agreement with the experimental findings. The general form of the Langmuir isotherm model is shown in below equation53–57:
| 3 |
where, , Kads, and C are the metal surface coverage, the equilibrium constant for the adsorption–desorption process, and the inhibitor concentration, respectively. As can be seen, when a graph is drawn between (C/θ), and C, a straight line (R2 > 0.9) is formed for all samples, as shown in Fig. 8, with a gradient (slope) near to the unit and an intercept equal to Kads. The fact that all linear correlation coefficients (R) are almost equal to one shows that plant extract adsorption on mild steel surfaces follows the Langmuir adsorption isotherm. The Langmuir isotherm implies inhibitor molecule monolayer adsorption, or the inhibitor molecule occupies one active site on a metal surface58. Furthermore, the Langmuir adsorption isotherm revealed that organic components in plant extracts with polar atoms or groups adsorbed on the metal surface may interact via mutual attraction or repulsion59.
Figure 8.
Langmuir adsorption isotherm of the inhibitor determined by Tafel polarization data for st-37 in (a) 0.5 M H2SO4, and (b) 1.0 M HCl solutions at 25 ± 1 °C.
The calculated adsorption coefficient, Kads, was larger in H2SO4 than in HCl, indicating that the adsorption of inhibitor molecules on active sites of steel surfaces was easier in H2SO4 than in HCl solution60. The strength, and stability of the adsorbed layer formed by nano extract in both solutions could also be evaluated from the higher Kads value compared to the other situation.
The standard adsorption free energy () is also calculated using the Kads values. In the context of corrosion inhibition, physisorption, and chemisorption are two adsorption mechanisms that are frequently studied61. For the physical adsorption, values of the standard adsorption free energy are until − 20 kJ/mol, while those lower than − 40 kJ/mol are correlated with the chemical adsorption3,53.
of the adsorption process linked with Kads, and determined using below equation62:
| 4 |
where, R, and T are the universal gas constant, and thermodynamic temperature, respectively, and 106 points to the ppm concentration (mg/L) of water.
For H2SO4 solution with bulk, and nano size of extract the calculated value of is 28.54, and − 31.71 kJ/mol, respectively. For HCl solution with bulk, and nano size of extract, the calculated value of is 22.83, and − 29.70 kJ/mol, respectively. As a result of the obtained value for , it can be concluded that Dracocephalum adsorption is not solely chemisorption or physisorption, but also includes comprehensive adsorption (both chemical and physical), and that the negative sign of indicates that inhibitor molecule adsorption on the metal surface is spontaneous3. Table 5 lists the results, including Kads, and .
Table 5.
Thermodynamic characteristics of adsorption obtained by PP measurements for st-37 in acidic media in the presence of varying concentration of extract.
| Sample | Kads (L/mg) | (kJ/mol) |
|---|---|---|
| Dracocephalum/H2SO4 | 0.10 | − 28.54 |
| Dracocephalum (nano)/H2SO4 | 0.36 | − 31.71 |
| Dracocephalum /HCl | 0.01 | − 22.83 |
| Dracocephalum (nano)/HCl | 0.16 | − 29.70 |
A comparison of the present research with similar studies that have used plant extract as a corrosion inhibitor in acidic media presented in Table 6. It can be concluded that Dracocephalum extract in bulk, and especially in nanometer size is a suitable candidate for boosting the corrosion resistance of mild steel alloy in 0.5 M H2SO4, and 1.0 M HCl.
Table 6.
Summary of similar studies for plant extract as a corrosion inhibitor and in comparison to this study.
| Refs | inhibitor | Concentration of inhibitor | alloy | Corrosive medium | PCE (%) |
|---|---|---|---|---|---|
| 63 | Origanum Compactum extract | 400 ppm | mild steel | 1.0 M HCl | 90 |
| 64 | Ammi visnaga extract | 700 ppm | Carbon steel | 1.0 M HCl | 84 |
| 65 | Quince seed extract | 800 ppm | Mild steel | 1.0 M HCl | 95 |
| 66 | Inula viscosa leaves extract | 600 ppm | Carbon steel | 1.0 M HCl | 92 |
| 67 | Mish Gush leaves extract | 1200 ppm | Mild steel | 1.0 M HCl | 96 |
| 68 | Sugarcane purple rind extract | 800 ppm | Carbon steel | 1.0 M HCl | 96.2 |
| 69 | Artabotrys odoratissimus extract | 1250 ppm | Mild steel | 0.5 M H2SO4 | 93 |
| 70 | Cinnamoum tamala extract | 100 ppm | Carbon steel | 0.5 M H2SO4 | 96.76 |
| This work | Dracocephalum extract | 200 ppm | Mild steel | 0.5 M H2SO4 | 89 |
| This work | Dracocephalum extract (nano) | 75 ppm | Mild steel | 0.5 M H2SO4 | 94 |
| This work | Dracocephalum extract | 400 ppm | Mild steel | 1.0 M HCl | 90 |
| This work | Dracocephalum extract (nano) | 100 ppm | Mild steel | 1.0 M HCl | 88 |
Mechanism of corrosion inhibition
The examined compounds' ability to prevent carbon steel corrosion is mostly owing to their physical or chemical adsorption on the metal surface, where they replace H2O molecules on the steel surface, and form a compact barrier coating71. Electrostatic contact occurs between charged inhibitor molecules, and charged metal surfaces in the event of physical adsorption (Fig. 9a). During chemical adsorption, the pair electron on the π-electron of multiple bonds, and heteroatoms interact with the iron's unoccupied d-orbitals (Fig. 9b)13. In this work, the values of are − 22.83, and 29.70 kJ mol−1, in HCl solution, indicating that the examined compound molecules are adsorbed by a mix of chemical, and physical adsorption. It is known experimentally that the steel surface is positively charged in acidic solutions, Cl− ions may be adsorbed on the positively charged steel surface, and subsequently, the protonated inhibitor molecules are adsorbed via electrostatic attraction (physical adsorption). But at the same time, d-orbitals of iron atoms get a lone pair of electrons on π-electron, and heteroatoms in the extract structure (Chemical adsorption). In the H2SO4 solution, the values of are -28.54, and 31.71 kJ mol−1, but due to the low electron charge density on the surface of ions, the examined compound molecules are more adsorbed by chemical adsorption.
Figure 9.
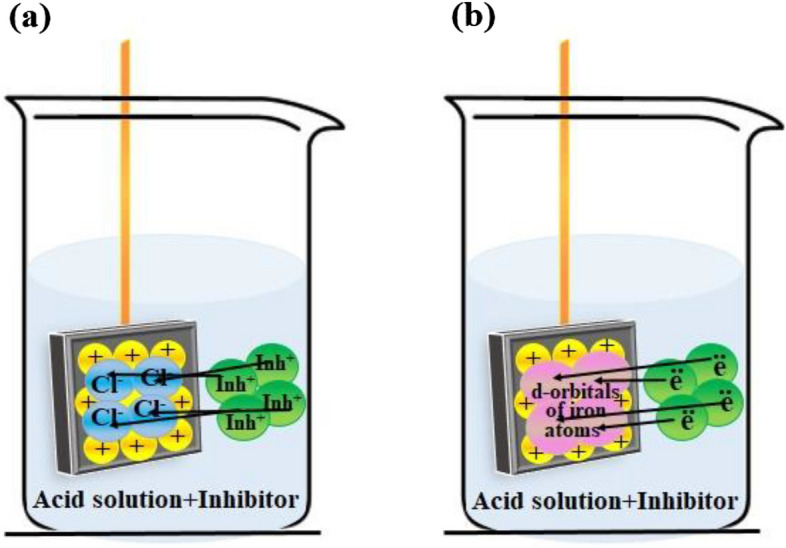
Mechanism of corrosion inhibition adsorption on the metal surface: (a) physical adsorption, and (b) chemical adsorption.
UV–visible reflection measurements
The present surface analysis gives the reflectance of metal specimens before, and after immersion in 0.5 M H2SO4, and 1.0 M HCl in the absence, and the presence of the best inhibitor concentration. The results shown in Fig. 10 indicate that the reflectance of the st-37 specimen has decreased after immersion in acidic media in the absence of extract. Whereas, adding the corrosion inhibitor to the test solution increases the reflectance value until it is close to the specimen reflectance before immersion in the acidic solution.
Figure 10.

UV-Vis spectrum for st-37 specimens in 0.5 M H2SO4, and 1.0 M HCl solutions in the absence, and presence of an optimum concentration of Dracocephalum extract as a corrosion inhibitor.
Scanning electron and optical microscopic observations
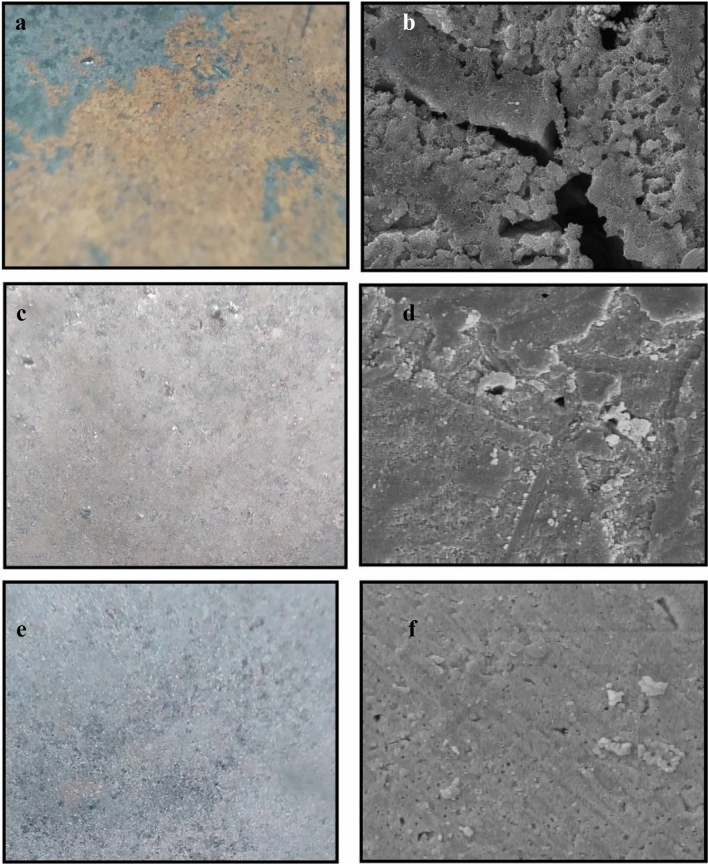
In Figs. 11, 12, optical microscopy, and scanning electron micrographs, were used to understand the surface morphology of mild steel after 24 h of immersion in 0.5 M H2SO4, and 1.0 M HCl, without, and with the best concentration of extract. In the case of blank solutions, Figs. 11(a,b) and 12(a,b) reveal a highly rough the specimen surface with serious damage, obvious pits, and cracks. However, after adding a corrosion inhibitor to the acidic media, the corrosion was visibly reduced, and the surface of the samples became reasonably smooth (Figs. 11(c–f) and 12(c–f), the corrosion inhibition efficacy showed up, and the protective inhibitor coating was produced. Figures 11(e,f) and 12(e,f) illustrate that in the presence of the optimal inhibitor concentration depending on nano size, surface corrosion of the alloy decreased substantially. It confirms that the Dracocephalum extract (nano) molecules cover the metal surface better than the bulk size. When comparing the images related to both solutions, it can be observed that the extract as a corrosion inhibitor in H2SO4 has a better impact than the HCl solution.
Figure 11.
The images of the st-37 surface after 24 h immersion in 0.5 M H2SO4 solution in the (a,b) absence, (c,d) presence of 200 ppm of Dracocephalum extract in bulk size (e,f), and presence of 75 ppm of Dracocephalum extract in nano size, using optical, and Scanning electron microscopy, respectively.
Figure 12.
The images of the st-37 surface after 24 h immersion in 1.0 M HCl solution in the (a,b) absence, (c,d) presence of 400 ppm of Dracocephalum extract in bulk size (e,f), and presence of 100 ppm of Dracocephalum extract in nano size, using optical, and Scanning electron microscopy, respectively.
Conclusions
The effect of Dracocephalum extract based on bulk, and nanometer size as a corrosion inhibitor for mild steel in 0.5 M H2SO4, and 1.0 M HCl solutions was investigated:
The data derived from EIS, and PP curves indicate that the inhibition efficiency augmented with the increase in extract concentration up to a special dose.
By polarization method, in HCl solution, the highest IE% is 88% at the best dose of nano extract (100 ppm), but the highest IE% is 90% at best dose of the bulk extract (400 ppm). In the H2SO4 solution, the highest IE% is 89% at the best dose of the bulk extract (200 ppm), but the corrosion inhibitor had the best inhibition efficiency (94%), at the minimum concentration (75 ppm) of nano extract. It was worth noting that the value of IE% calculated by PP shows the same trend as that obtained from the EIS curves method.
In both acidic environments, PP measurements reveal that this examined chemical reduced corrosion by mixed-type inhibition, impacting both hydrogen evolution, and metal dissolution, with a predominant anodic action in the H2SO4 medium.
According to EIS, this compound reduced corrosion through adsorption on the metal/solution contact.
suggested that Dracocephalum adsorption is not only chemisorption or physisorption but also includes comprehensive adsorption. That means, the investigated compound adsorbed both chemical, and physical adsorption on the st-37 surface while following the Langmuir isotherm. Furthermore, the negative value of shows that inhibitor molecules adsorb spontaneously on the metal surface.
Optical, and SEM microscopy were used to confirm the corrosion testing. Thus, a uniform and less damaged surface was found with the optimal concentration of Dracocephalum extract in both acid solutions. The corrosion inhibition effectiveness showed up, and the protective inhibitor film was formed.
Finally, compared to the results of other researchers, it can be concluded that the Dracocephalum extract has the lowest optimal concentration, and proper efficiency. Therefore, by using Dracocephalum extract based on nanometer size, we could reduce the optimal concentration of inhibitor significantly, and increase the corrosion resistant, as well as efficiency. That is a cheap, eco-friendly, and efficient method to reduce the corrosion of mild steel in acidic media. So, Dracocephalum extract can be a suitable candidate for boosting the corrosion resistance of mild steel alloy in 0.5 M H2SO4, and 1.0 M HCl.
Acknowledgements
The authors would like to express their sincere appreciation to the founders of Shahid Bahonar University of Kerman, Mr. Alireza Afzalipour and his wife, Mrs. Fakhereh Saba, for their foresight and generosity in training future generations. Additionally, the authors are grateful to the Iran High-Tech Laboratory Network [Grant Number: 29473] for their support of this work.
Author contributions
All authors conceived and designed the experiments. Z.G. wrote the main manuscript text, performed the experiment, fabricated the devices, and analyzed the data and results with support from Dr. M. A., and Dr. S.M.A. H. The bulk extract prepared by F. A., and S. J. F., and also, the extract in nanometer size prepared by Dr. M. A.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mahnaz Amiri, Email: ma.amiri@kmu.ac.ir.
Seyed Mohammad Ali Hosseini, Email: s.m.a.hosseini@uk.ac.ir.
References
- 1.Popoola LT. Progress on pharmaceutical drugs, plant extracts and ionic liquids as corrosion inhibitors. Heliyon. 2019;5:e01143. doi: 10.1016/j.heliyon.2019.e01143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nduma RC, et al. Review of metal protection techniques and application of drugs as corrosion inhibitors on metals. IOP Conf. Ser. Mater. Sci. Eng. 2021;1107:012023. doi: 10.1088/1757-899X/1107/1/012023. [DOI] [Google Scholar]
- 3.Golshani Z, et al. Effect of Thyme extract as an ecofriendly inhibitor for corrosion of mild steel in acidic media. Mater. Corros. 2022;73:460–469. doi: 10.1002/maco.202112769. [DOI] [Google Scholar]
- 4.Oyekunle DT, Agboola O, Ayeni AO. Corrosion inhibitors as building evidence for mild steel: A review. J. Phys. Conf. Ser. 2019;1378:032046. doi: 10.1088/1742-6596/1378/3/032046. [DOI] [Google Scholar]
- 5.Scattergood GL. Corrosion inhibitors for crude oil refineries. ASM Handb. 1987;13:485–486. [Google Scholar]
- 6.Abdel-Gaber AM, Abdel-Rahman HH, Ahmed AM, Fathalla MH. Corrosion behaviour of zinc in alcohol-water solvents. Anti-Corros. Methods Mater. 2006 doi: 10.1108/00035590610678910. [DOI] [Google Scholar]
- 7.Arthur DE, Jonathan A, Ameh PO, Anya C. A review on the assessment of polymeric materials used as corrosion inhibitor of metals and alloys. Int. J. Ind. Chem. 2013;4:1–9. doi: 10.1186/2228-5547-4-2. [DOI] [Google Scholar]
- 8.Saini N, et al. Minified dose of urispas drug as better corrosion constraint for soft steel in sulphuric acid solution. J. Mol. Liq. 2018;269:371–380. doi: 10.1016/j.molliq.2018.08.070. [DOI] [Google Scholar]
- 9.Chidiebere MA, Oguzie EE, Liu L, Li Y, Wang F. Ascorbic acid as corrosion inhibitor for Q235 mild steel in acidic environments. J. Ind. Eng. Chem. 2015;26:182–192. doi: 10.1016/j.jiec.2014.11.029. [DOI] [Google Scholar]
- 10.Cowan RL, Tedmon CS. Advances in Corrosion Science and Technology. Springer; 1973. Intergranular corrosion of iron-nickel-chromium alloys; pp. 293–400. [Google Scholar]
- 11.Umoren SA, Li Y, Wang FH. Influence of aluminium microstructure on corrosion and Corrosion inhibitor performance in acidic medium. J. Mater. Environ. Sci. 2010;1:189–196. [Google Scholar]
- 12.Umoren SA, Ebenso EE, Okafor PC, Ogbobe O. Water-soluble polymers as corrosion inhibitors. Pigment Resin. Technol. 2006 doi: 10.1108/03699420610711353. [DOI] [Google Scholar]
- 13.Fouda AS, El-Desoky HS, Abdel-Galeil MA, Mansour D. Niclosamide and dichlorphenamide: New and effective corrosion inhibitors for carbon steel in 1M HCl solution. SN Appl. Sci. 2021;3:1–20. doi: 10.1007/s42452-021-04155-w. [DOI] [Google Scholar]
- 14.Hosseini SMA, Amiri M, Momeni A. inhibitive effect of L-OH on the corrosion of austenitic chromium-nickel steel in H2SO4 solution. Surf. Rev. Lett. 2008;15:435–442. doi: 10.1142/S0218625X08011561. [DOI] [Google Scholar]
- 15.Rani BE, Basu BBJ. Green inhibitors for corrosion protection of metals and alloys: An overview. Int. J. Corros. 2012;2012:1–15. doi: 10.1155/2012/380217. [DOI] [Google Scholar]
- 16.Amiri M, Mahmoudi-Moghaddam H. Green synthesis of ZnO/ZnCo2O4 and its application for electrochemical determination of bisphenol A. Microchem. J. 2021;160:105663–105670. doi: 10.1016/j.microc.2020.105663. [DOI] [Google Scholar]
- 17.Darling, D. & Rakshpal, R. Green chemistry applied to corrosion and scale inhibitors. In Corrosion 98 (OnePetro, 1998).
- 18.Radojčić I, Berković K, Kovač S, Vorkapić-Furač J. Natural honey and black radish juice as tin corrosion inhibitors. Corros. Sci. 2008;50:1498–1504. doi: 10.1016/j.corsci.2008.01.013. [DOI] [Google Scholar]
- 19.Bereket G, Yurt A. The inhibition effect of amino acids and hydroxy carboxylic acids on pitting corrosion of aluminum alloy 7075. Corros. Sci. 2001;43:1179–1195. doi: 10.1016/S0010-938X(00)00135-9. [DOI] [Google Scholar]
- 20.Sanyal B. Organic compounds as corrosion inhibitors in different environments: A review. Prog. Org. Coatings. 1981;9:165–236. doi: 10.1016/0033-0655(81)80009-X. [DOI] [Google Scholar]
- 21.Pal A, Dey S, Sukul D. Effect of temperature on adsorption and corrosion inhibition characteristics of gelatin on mild steel in hydrochloric acid medium. Res. Chem. Intermed. 2016;42:4531–4549. doi: 10.1007/s11164-015-2295-8. [DOI] [Google Scholar]
- 22.Alaneme KK, Olusegun SJ. Corrosion inhibition performance of lignin extract of sun flower (Tithonia diversifolia) on medium carbon low alloy steel immersed in H2SO4 solution. Leonardo J. Sci. 2012;20:59–70. [Google Scholar]
- 23.Finšgar M, Jackson J. Application of corrosion inhibitors for steels in acidic media for the oil and gas industry: A review. Corros. Sci. 2014;86:17–41. doi: 10.1016/j.corsci.2014.04.044. [DOI] [Google Scholar]
- 24.Amiri M, Salavati-Niasari M, Akbari A, Razavi R. Sol–gel auto-combustion synthesize and characterization of a novel anticorrosive cobalt ferrite nanoparticles dispersed in silica matrix. J. Mater. Sci. Mater. Electron. 2017;28:10495–10508. doi: 10.1007/s10854-017-6823-8. [DOI] [Google Scholar]
- 25.Gunasekaran G, Chauhan LR. Eco friendly inhibitor for corrosion inhibition of mild steel in phosphoric acid medium. Electrochim. Acta. 2004;49:4387–4395. doi: 10.1016/j.electacta.2004.04.030. [DOI] [Google Scholar]
- 26.Khedr MGA, Lashien AMS. The role of metal cations in the corrosion and corrosion inhibition of aluminium in aqueous solutions. Corros. Sci. 1992;33:137–151. doi: 10.1016/0010-938X(92)90023-V. [DOI] [Google Scholar]
- 27.Verma SA, Mehta GN. Effect of acid extracts of Acacia arabica on acid corrosion of mild steel. Bull. Electrochem. 1999;15:67–70. [Google Scholar]
- 28.Lebrini M, Robert F, Roos C. Inhibition effect of alkaloids extract from Annona squamosa plant on the corrosion of C38 steel in normal hydrochloric acid medium. Int. J. Electrochem. Sci. 2010;5:1698–1712. [Google Scholar]
- 29.Kliškić M, Radošević J, Gudić S, Katalinić V. Aqueous extract of Rosmarinus officinalis L. as inhibitor of Al–Mg alloy corrosion in chloride solution. J. Appl. Electrochem. 2000;30:823–830. doi: 10.1023/A:1004041530105. [DOI] [Google Scholar]
- 30.Dehghani A, Bahlakeh G, Ramezanzadeh B, Ramezanzadeh M. Aloysia citrodora leaves extract corrosion retardation effect on mild-steel in acidic solution: Molecular/atomic scales and electrochemical explorations. J. Mol. Liq. 2020;310:113221. doi: 10.1016/j.molliq.2020.113221. [DOI] [Google Scholar]
- 31.El-Etre AY, Abdallah M, El-Tantawy ZE. Corrosion inhibition of some metals using lawsonia extract. Corros. Sci. 2005;47:385–395. doi: 10.1016/j.corsci.2004.06.006. [DOI] [Google Scholar]
- 32.Sonboli A. Molecular characterization of Iranian Dracocephalum (Lamiaceae) species based on RAPD data. Acta Biol. Szeged. 2011;55:227–230. [Google Scholar]
- 33.Lazarević P, Lazarević M, Krivošej Z, Stevanović V. On the distribution of Dracocephalum ruyschiana (Lamiaceae) in the Balkan Peninsula. Phytol. Balc. 2009;15:175–179. [Google Scholar]
- 34.Talari M, et al. Dracocephalum: Novel anticancer plant acting on liver cancer cell mitochondria. Biomed. Res. Int. 2014;2014:1–10. doi: 10.1155/2014/892170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kamali M, Khosroyar S, Mohammadi A. Antibacterial activity of various extracts from Dracocephalum kotschyi against food pathogenic microorganisms. Int. J. PharmTech Res. 2015;8:158–163. [Google Scholar]
- 36.Mashak B, Hoseinzadeh M, Ehsanpour A, Ghanbaran AR, Vakili M. Evaluation of treatment response and side effects of spinal-Z in patients with metastatic gastroesophageal adenocarcinoma: A double-blind randomized controlled trial. Jundishapur J. Chronic Dis. Care. 2017;6:57870. doi: 10.5812/jjcdc.57870. [DOI] [Google Scholar]
- 37.Aćimović M, et al. Chemical composition, antioxidant, and antimicrobial activity of Dracocephalum moldavica L. essential oil and hydrolate. Plants. 2022;11:941. doi: 10.3390/plants11070941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Green J. The Herbal Medicine-Maker’s Handbook: A Home Manual. Crossing Press; 2011. [Google Scholar]
- 39.Numonov S, et al. The ursolic acid-rich extract of Dracocephalum heterophyllum Benth. with potent antidiabetic and cytotoxic activities. Appl. Sci. 2020;10:6505. doi: 10.3390/app10186505. [DOI] [Google Scholar]
- 40.Mourya P, Banerjee S, Singh MM. Corrosion inhibition of mild steel in acidic solution by Tagetes erecta (Marigold flower) extract as a green inhibitor. Corros. Sci. 2014;85:352–363. doi: 10.1016/j.corsci.2014.04.036. [DOI] [Google Scholar]
- 41.Lorenz WJ, Mansfeld F. Determination of corrosion rates by electrochemical DC and AC methods. Corros. Sci. 1981;21:647–672. doi: 10.1016/0010-938X(81)90015-9. [DOI] [Google Scholar]
- 42.Cotting F, Aoki IV. Octylsilanol and Ce (III) ions–Alternative corrosion inhibitors for carbon steel in chloride neutral solutions. J. Mater. Res. Technol. 2020;9:8723–8734. doi: 10.1016/j.jmrt.2020.06.011. [DOI] [Google Scholar]
- 43.Du P, Deng S, Li X. Mikania micrantha extract as a novel inhibitor for the corrosion of cold rolled steel in Cl2HCCOOH solution. J. Mater. Res. Technol. 2022 doi: 10.1016/j.jmrt.2022.06.026. [DOI] [Google Scholar]
- 44.Omran MA, Fawzy M, Mahmoud AED, Abdullatef OA. Optimization of mild steel corrosion inhibition by water hyacinth and common reed extracts in acid media using factorial experimental design. Green Chem. Lett. Rev. 2022;15:216–232. doi: 10.1080/17518253.2022.2032844. [DOI] [Google Scholar]
- 45.Labjar N, et al. Corrosion inhibition of carbon steel and antibacterial properties of aminotris-(methylenephosphonic) acid. Mater. Chem. Phys. 2010;119:330–336. doi: 10.1016/j.matchemphys.2009.09.006. [DOI] [Google Scholar]
- 46.Obot IB, Madhankumar A. Synergistic effect of iodide ion addition on the inhibition of mild steel corrosion in 1 M HCl by 3-amino-2-methylbenzylalcohol. Mater. Chem. Phys. 2016;177:266–275. doi: 10.1016/j.matchemphys.2016.04.027. [DOI] [Google Scholar]
- 47.Aljourani J, Raeissi K, Golozar MA. Benzimidazole and its derivatives as corrosion inhibitors for mild steel in 1M HCl solution. Corros. Sci. 2009;51:1836–1843. doi: 10.1016/j.corsci.2009.05.011. [DOI] [Google Scholar]
- 48.Yan R, He W, Zhai T, Ma H. Anticorrosion organic–inorganic hybrid films constructed on iron substrates using self-assembled polyacrylic acid as a functional bottom layer. Electrochim. Acta. 2019;295:942–955. doi: 10.1016/j.electacta.2018.11.117. [DOI] [Google Scholar]
- 49.Iroha NB, Nnanna LA. Electrochemical and Adsorption Study of the anticorrosion behavior of Cefepime on Pipeline steel surface in acidic Solution. J. Mater. Environ. Sci. 2019;10:898–908. [Google Scholar]
- 50.Vishwanatham S, Haldar N. Corrosion inhibition of N80 steel in hydrochloric acid by phenol derivatives. Corros. Sci. 2007;50:2999–30004. doi: 10.1016/j.corsci.2008.08.005. [DOI] [Google Scholar]
- 51.Raviprabha K, Bhat RS. 5-(3-Pryridyl)-4H-1, 2, 4-triazole-3-thiol as potential corrosion inhibitor for AA6061 aluminium alloy in 01 M hydrochloric acid solution. Surf. Eng. Appl. Electrochem. 2019;55:723–733. doi: 10.3103/S1068375519060103. [DOI] [Google Scholar]
- 52.Herrag L, et al. Adsorption properties and inhibition of mild steel corrosion in hydrochloric solution by some newly synthesized diamine derivatives: Experimental and theoretical investigations. Corros. Sci. 2010;52:3042–3051. doi: 10.1016/j.corsci.2010.05.024. [DOI] [Google Scholar]
- 53.Golshani Z, Hosseini SMA, Shahidizandi M, Bahrami MJ. Increase corrosion resistance of mild steel in sulfuric acid and hydrochloric acid solutions by metoclopramide tablet. Mater. Corros. 2019;70:1862–1871. doi: 10.1002/maco.201910896. [DOI] [Google Scholar]
- 54.Aslam R, Mobin M, Shoeb M, Aslam J. Novel ZrO2-glycine nanocomposite as eco-friendly high temperature corrosion inhibitor for mild steel in hydrochloric acid solution. Sci. Rep. 2022;12:1–19. doi: 10.1038/s41598-022-13359-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Deyab MA, Mohsen Q, Guo L. Theoretical, chemical, and electrochemical studies of Equisetum arvense extract as an impactful inhibitor of steel corrosion in 2 M HCl electrolyte. Sci. Rep. 2022;12:1–14. doi: 10.1038/s41598-022-06215-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Al-Moubaraki AH, Al-Malwi SD. Experimental and theoretical evaluation of aqueous black mustard seeds extract as sustainable-green inhibitor for mild steel corrosion in H2SO4 acid solutions. J. Adhes. Sci. Technol. 2022;36:2612–2643. doi: 10.1080/01694243.2022.2062955. [DOI] [Google Scholar]
- 57.Munawaroh HSH, et al. Protoporphyrin extracted from biomass waste as sustainable corrosion inhibitors of T22 carbon steel in acidic environments. Sustainability. 2022;14:3622. doi: 10.3390/su14063622. [DOI] [Google Scholar]
- 58.Chaudhary S, Tak RK. Tribulus terrestris Extracts: An eco-friendly corrosion inhibitor for mild steel in H2SO4 medium. Asian J. Chem. 2017;29:1859–1865. doi: 10.14233/ajchem.2017.20702. [DOI] [Google Scholar]
- 59.Chaudhary S, Tak RK. Natural corrosion inhibition and adsorption characteristics of tribulus terrestris plant extract on aluminium in hydrochloric acid environment. Biointerface Res. Appl. Chem. 2022;12:2603–2617. [Google Scholar]
- 60.Srivastava M, Tiwari P, Srivastava SK, Prakash R, Ji G. Electrochemical investigation of Irbesartan drug molecules as an inhibitor of mild steel corrosion in 1 M HCl and 05 M H2SO4 solutions. J. Mol. Liq. 2017;236:184–197. doi: 10.1016/j.molliq.2017.04.017. [DOI] [Google Scholar]
- 61.Lai C, et al. Adsorption and corrosion inhibition of mild steel in hydrochloric acid solution by S-allyl-O, O′-dialkyldithiophosphates. Results Phys. 2017;7:3434–3443. doi: 10.1016/j.rinp.2017.09.012. [DOI] [Google Scholar]
- 62.Mohammadinejad F, Hosseini SMA, Zandi MS, Bahrami MJ, Golshani Z. Metoprolol: New and efficient corrosion inhibitor for mild steel in hydrochloric and sulfuric acid solutions. Acta Chim. Slov. 2020;67:710–719. doi: 10.17344/acsi.2019.5301. [DOI] [PubMed] [Google Scholar]
- 63.Berrissoul A, et al. Assessment of corrosion inhibition performance of origanum compactum extract for mild steel in 1 M HCl: Weight loss, electrochemical, SEM/EDX, XPS, DFT and molecular dynamic simulation. Ind. Crops Prod. 2022;187:115310. doi: 10.1016/j.indcrop.2022.115310. [DOI] [Google Scholar]
- 64.Zaher A, et al. A combined computational & electrochemical exploration of the Ammi visnaga L. extract as a green corrosion inhibitor for carbon steel in HCl solution. Arab. J. Chem. 2022;15:103573. doi: 10.1016/j.arabjc.2021.103573. [DOI] [Google Scholar]
- 65.Shahmoradi AR, et al. Molecular-MD/atomic-DFT theoretical and experimental studies on the quince seed extract corrosion inhibition performance on the acidic-solution attack of mild-steel. J. Mol. Liq. 2022;346:117921. doi: 10.1016/j.molliq.2021.117921. [DOI] [Google Scholar]
- 66.Kouache A, et al. Experimental and theoretical studies of Inula viscosa extract as a novel eco-friendly corrosion inhibitor for carbon steel in 1 M HCl. J. Adhes. Sci. Technol. 2022;36:988–1016. doi: 10.1080/01694243.2021.1956215. [DOI] [Google Scholar]
- 67.Shahini MH, Ramezanzadeh M, Bahlakeh G, Ramezanzadeh B. Superior inhibition action of the Mish Gush (MG) leaves extract toward mild steel corrosion in HCl solution: Theoretical and electrochemical studies. J. Mol. Liq. 2021;332:115876. doi: 10.1016/j.molliq.2021.115876. [DOI] [Google Scholar]
- 68.Meng S, et al. Efficient corrosion inhibition by sugarcane purple rind extract for carbon steel in HCl solution: Mechanism analyses by experimental and in silico insights. RSC Adv. 2021;11:31693–31711. doi: 10.1039/D1RA04976C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rathod MR, Minagalavar RL, Rajappa SK. Effect of Artabotrys odoratissimus extract as an environmentally sustainable inhibitor for mild steel corrosion in 0.5 M H2SO4 media. J. Indian Chem. Soc. 2022;99:100445. doi: 10.1016/j.jics.2022.100445. [DOI] [Google Scholar]
- 70.Prasad D, Dagdag O, Safi Z, Wazzan N, Guo L. Cinnamoum tamala leaves extract highly efficient corrosion bio-inhibitor for low carbon steel: Applying computational and experimental studies. J. Mol. Liq. 2022;347:118218. doi: 10.1016/j.molliq.2021.118218. [DOI] [Google Scholar]
- 71.Fouda AS, Abd El-Maksoud SA, El-Hossiany A, Ibrahim A. Corrosion protection of stainless steel 201 in acidic media using novel hydrazine derivatives as corrosion inhibitors. Int. J. Electrochem. Sci. 2019;14:2187–2207. doi: 10.20964/2019.03.15. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.