Abstract
Immunization with the whole-cell pertussis vaccine (Pw), while effective at preventing whooping cough in infants, has been associated with local, systemic, and neuronal reactions, including fevers and convulsions in children. In contrast, the new acellular pertussis vaccines (Pa) have a considerably improved safety profile. The lack of an appropriate animal model has restricted investigations into the mechanisms by which neurological reactions are induced by vaccination. Here we describe a novel murine model wherein seizure-like behavioral changes are induced following parenteral administration of Pw. The proinflammatory cytokine interleukin-β (IL-1β), production of which has been associated with many neurodegenerative conditions, was significantly increased in the hippocampus and hypothalamus of vaccinated animals. Accompanying this change was a decrease in release of the inhibitory neurotransmitters γ-aminobutyric acid and adenosine in the hippocampus. Seizure-like behavioral changes were significantly reduced following inhibition of IL-1β production by the administration of an inhibitor of IL-1β-converting enzyme and were almost completely abrogated in IL-1 receptor type I knockout mice. These results suggest a causal relationship between IL-1β induction and convulsive behavior following Pw vaccination. Significantly, Pa neither increased IL-1β nor induced behavioral changes in mice, but did induce the anti-inflammatory cytokine IL-10. In contrast, administration of active pertussis toxin and lipopolysaccharide, residual in Pw but absent from Pa, also induced convulsive activity. Our findings provide the first direct evidence of an immunological basis for pertussis vaccine reactogenicity and suggest that active bacterial toxins are responsible for the neurologic disturbances observed in children immunized with Pw.
Vaccines have probably made the single greatest contribution to human health in the last century and are still the most effective means of combating infectious diseases (1). However, certain vaccines, notably whole-cell pertussis vaccines (Pw), have been associated with mild to serious side effects. Although Pw are effective at preventing whooping cough in infants, they can induce local and systemic reactions in a high proportion of immunized infants (3, 5, 25). More significantly, convulsions and encephalopathy have been reported to be temporally associated with Pw administration (2, 3, 5, 20). Concerns about safety have adversely affected vaccine uptake and have motivated the development of acellular pertussis vaccines (Pa), prepared with highly purified antigens from Bordetella pertussis (8, 9, 25). These new vaccines, which have considerably reduced side effects, have been introduced into routine pediatric vaccination programs in most developed countries. However, their merits over the traditional vaccines in terms of potency are still being debated (5), and for reasons of cost and ease of production, Pw will continue to be used in most developing countries. Although it has been speculated that the reactogenicity of Pw may be related to residual active toxins (3), the mechanisms whereby the vaccine mediates neurological effects have not been defined.
Evidence suggests that the systemic effects of local exposure to live or killed bacteria may be mediated through proinflammatory cytokine induction within the central nervous system (CNS). Specifically, interleukin-1β (IL-1β) has been implicated in the neurologic manifestations of infectious diseases such as bacterial meningitis (17) and shigellosis (40) and in the fever response evoked following administration of lipopolysaccharide (LPS) (12). We have previously demonstrated that infection of mice with B. pertussis (14) or parenteral immunization with Pw (13) results in the induction of IL-1β production in the hippocampus and hypothalamus. In the present investigation, we sought to address the hypothesis that neurological responses, specifically convulsive activity, induced by Pw but not by Pa, were mediated by IL-1β induced in the brain in response to active bacterial toxins present in Pw.
The lack of an appropriate animal model has restricted investigations into the mechanisms by which neurological reactions are induced by Pw. Although it had been reported that repeated injection of Pw and bovine serum albumin (BSA) induced encephalopathy in mice (33), it was later demonstrated that the neurological responses observed resulted from the potentiating effect of pertussis toxin (PT) or LPS on anaphylaxis during sensitization to BSA (22, 28). In this study we describe a novel murine model where neurological changes are consistently induced following parenteral administration of Pw. We report that exposure of mice to high ambient temperature following subcutaneous (s.c.) injection of Pw induces seizure-like behavioral changes, which are associated with a significant augmentation of IL-1β induction in the hippocampus. The seizure activity was reduced by pretreatment with an inhibitor of IL-1β-converting enzyme (ICE) and was significantly attenuated in IL-1 receptor type 1-defective (IL-1RI−/−) mice. Increased IL-1β production and convulsive activity were also induced following parenteral injection of PT or LPS but was not observed following immunization with Pa. Our findings demonstrate that IL-1β, induced in the brain in response to active bacterial toxins residual in Pw, mediates certain neurological effects observed following immunization with Pw.
MATERIALS AND METHODS
Animal treatment.
Female BALB/c and C57BL/6 mice were purchased from Harlan (Bicester, Oxon, United Kingdom), and breeding pairs of IL-1RI−/− mice were purchased from The Jackson Laboratory, Bar Harbor, Maine. IL-1RI−/− mice were homozygous for the targeted mutation in the IL-1 type I receptor gene and had been generated from 129/Sv mice and backcrossed to C57BL/6 (H-2b) for five generations (6), prior to importation by The Jackson Laboratory. Mice were bred and maintained under the guidelines of the Irish Department of Health, and studies were approved by the NUI Maynooth Biology Department's Ethics Committee. Mice were 8 to 10 weeks old at the initiation of experiments. Mice were injected s.c. with 0.2 ml of either Pw (4 IU; 88/522; British reference preparation from the National Institute for Biological Standards and Control, Herts, United Kingdom, containing 4 × 109 heat-inactivated B. pertussis organisms per IU), Pa, prepared as described elsewhere (15, 18) with 25 μg of formaldehyde and glutaraldehyde-detoxified PT, 7.5 μg of pertactin and 7.5 μg of filamentous hemagglutinin (commercially prepared antigens supplied by a vaccine manufacturer), B. pertussis sonicate (prepared by sonicating a bacterial suspension in phosphate-buffered saline [PBS] and removal of debris by centrifugation), PT (0.1 μg), B. pertussis LPS (6 μg), or PBS. The Pa components were free of detectable active PT and LPS, and the Pw had 0.1 μg of active PT and 6 μg of LPS in 4 IU. In certain experiments, mice were injected intraperitoneally with 0.33 μM ICE inhibitor peptide Ac-YVAD-CMK (Calbiochem, Beeston, Nottingham, United Kingdom) 30 min prior to administration of Pw.
Detection of fever.
BALB/c mice were acclimated to 30°C. Minimeters were implanted into the peritoneal cavity 1 week before s.c. injection of Pw (0.8 U) or PBS. Body temperatures were continuously recorded by a biotelemetry system.
Murine model for B. pertussis-induced convulsive behavior.
Mice were injected s.c. with Pw or B. pertussis sonicate and exposed to a temperature of 37°C 2 h after injection. In preliminary experiments, the behavior of mice was observed for 120 min after injection and for 60 min after placing at 37°C. Once the model was established, behavior of mice was observed for a period of 60 min after placing at 37°C. Convulsive behavior of mice was scored blind by observers, who sat in the constant-temperature rooms with mice individually marked and housed six per cage. Reactions ranged from unresponsive and head nodding to myoclonic jerks and clonic seizures. For statistical analysis of behavior, each phase was given a numeric score adapted from that described by Yuhas et al. (40): 0, unresponsive; 1, head nodding; 2, mild contractions; and 3, clonic seizures. The response of each mouse was scored on the basis of the most severe reaction recorded during the 60-min observation period, and a mean convulsive score was calculated for each test population.
Analysis of cytokine protein.
Mice were sacrificed by cervical dislocation, and the hippocampus and hypothalamus were dissected free. Brain tissue was homogenized in 0.25 M Tris-HC1 (pH 7.5), and concentrations of IL-1β protein were quantified by specific immunoassay (mouse IL-1β Duoset; Genzyme, Cambridge, Mass.). Values were calculated with reference to the standard curve, corrected for protein content and expressed as picograms per milligram of protein.
Expression of cytokine mRNA.
Hippocampal and hypothalamic tissue was homogenized in RNA isolation buffer (Genosys Biotechnologies Inc., The Woodlands, Tex.), and total RNA was extracted according to the manufacturer's directions. First-strand cDNA was synthesized from 2 μg of total RNA with avian myeloblastosis virus reverse transcriptase and oligo (DT) primer (Life Technologies Ltd., Paisley, United Kingdom). A 5-μl aliquot of cDNA was subjected to PCR with primers specific for IL-1β (upstream, 5′-AAGGAGAACCAAGCAACGAC-3′; downstream, 5′-GATTCCATGGTGAAGTCAAT-3′) and β-actin (Stratagene, La Jolla Calif.). A plasmid containing cDNA to IL-1β was used as a positive template for amplification of cytokine mRNA.
Analysis of neurotransmitter release.
Synaptosomes, prepared as previously described (24), were incubated for 15 min at 37°C in oxygenated Krebs solution containing 2 mM CaCl2, 3H-labeled γ-aminobutyric acid (GABA), and [14C]adenosine (Amersham Pharmacia Biotech UK Ltd., Little Chalfont, Bucks, United Kingdom), aliquoted onto Millipore filters (0.45-μm pore size), and rinsed under vacuum. Samples were then incubated at 37°C for 3 min in Krebs solution in the presence or absence of 40 mM KC1. The filtrate was collected into tubes containing scintillation fluid, and labeled neurotransmitter was quantified by scintillation counting. Values for each sample were calculated with reference to total radioactivity at the beginning of the incubation period and expressed as percent counts per minute.
Statistical analysis.
Results, expressed as mean ± standard error (SE), were compared by the Mann-Whitney U test. P values less than 0.05 were considered statistically significant.
RESULTS
Induction of fever and IL-1β production in the brain following parenteral immunization with Pw.
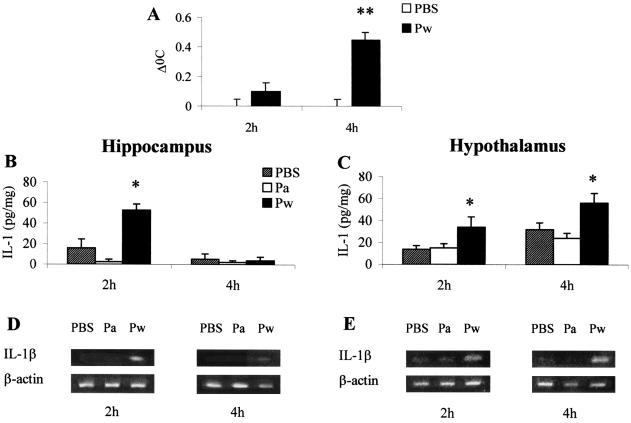
Initially we established an association between immunization with Pw and the development of a febrile response, indicating a central reaction to vaccination. Following s.c. injection of Pw, mean body temperatures of mice were significantly raised at 2 h postvaccination and elevated further at 4 h (Fig. 1A). The induction of fever with Pw was associated with elevated IL-1β production in the brain. We focused specifically on cytokine production within the hypothalamus and hippocampus, regions of the brain associated with control of fever and seizure activity, respectively. In both areas, concentrations of IL-1β protein were significantly elevated at 2 h following Pw administration (Fig. 1B and C). IL-1β returned to basal concentrations at 4 h in the hippocampus but increased further in the hypothalamus, corresponding to the raised body temperature. Accompanying this increase in IL-1β protein in both regions was an induction of IL-1β mRNA expression, indicating central synthesis of this cytokine by intrinsic brain cells in response to vaccination with Pw (Fig. 1 D and E). The marked increase in IL-1β observed 2 h before the onset of the peak febrile response suggests a role in the centrally controlled responses induced by systemic administration of Pw. In contrast to the induction of IL-1β by Pw, proinflammatory cytokine production was not significantly induced following immunization with Pa (Fig. 1B to E). Conversely, the anti-inflammatory cytokine IL-10 was significantly elevated in the hippocampus 4 h after parenteral injection of Pa (260 ± 9.6 pg/ml for Pa versus 100 ± 3.9 pg/ml for PBS- or Pw-injected mice).
FIG. 1.
Parenteral injection of mice with Pw induces IL-1β production in the brain and increases core body temperature. (A) BALB/c mice, housed at 30°C, were injected with Pw (0.8 U) or PBS, and temperatures were recorded using a biotelemetry system. BALB/c mice were injected with Pw, Pa, or PBS; 2 h and 4 h later, the hippocampus (B and D) and hypothalamus (C and E) were dissected free, and IL-1β protein was quantified by immunoassay (B, C) and IL-1β mRNA was analyzed by reverse transcription-PCR (D and E). Results are mean (SE) values for six mice in each experimental group. ∗, P < 0.05; ∗∗, P < 0.01 versus PBS controls.
A new murine model for neurological responses to pertussis vaccines.
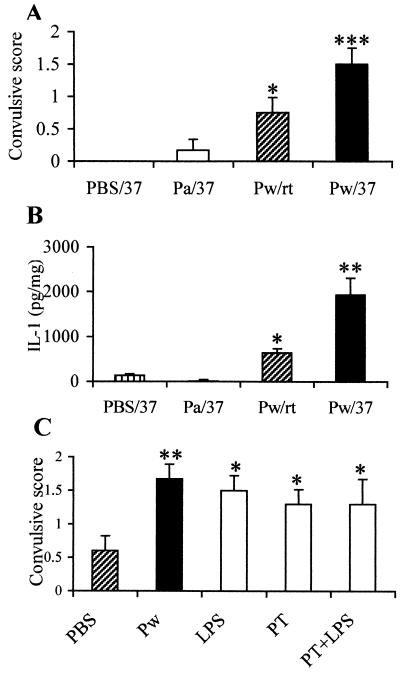
Having established that Pw induced IL-1β production in the brain (13), together with the knowledge that IL-1β is associated with seizure activity (4, 36), we set out to establish a model of neurological responses to pertussis vaccination by manipulating the early central response to Pw. In a series of preliminary experiments we observed that exposure of mice to a high ambient temperature enhanced the fever response to vaccination. We then examined the behavior of mice housed at 30 to 37°C either before or after injection of B. pertussis sonicate. When mice were injected with B. pertussis sonicate (from 1010 bacteria with an LPS content of 22 μg) and placed at 37°C, full clonic seizures were observed in 100% of animals (data not shown). Parenteral immunization with Pw and exposure to a temperature of 37°C 2 h after vaccination, the time at which IL-1β production was at a peak, induced behavioral changes ranging from head nodding to myoclonic jerks. While administration of Pw at room temperature itself induced mild behavioral changes, exposure to 37°C significantly enhanced the effect (Fig. 2A; P < 0.05). Commercially available alum-adsorbed Pw also induced behavioral changes similar to those observed with the reference vaccine (data not shown).
FIG. 2.
Parenteral injection with Pw, but not with Pa, induces convulsive behavior in mice. BALB/c mice were injected with Pw, Pa, or PBS and 2 h later were placed at 37°C (Pw/37, Pa/37, or PBS/37). Alternatively, mice were left at room temperature after injection of Pw (Pw/rt). (A) Behavioral activity was recorded for a further hour. (B) Mice were sacrificed, and hippocampal IL-1β protein concentrations were determined by immunoassay. (C) Behavioral changes were also recorded after injection of B. pertussis LPS (6 μg), PT (0.1 μg), LPS and PT, Pw, or PBS and exposure to 37°C. Results are mean (SE) values for six mice in each test group. ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001 Pw versus PBS controls.
Pw but not Pa induces seizure activity, which is mimicked by bacterial toxins and is associated with elevated IL-1β production in the hippocampus.
It has previously been suggested that the neurological side effects of Pw may be related to residual active bacterial toxins, especially PT and LPS (3), and we have previously reported that both of these toxins induce IL-1β production in the hippocampus (13). Here we demonstrate that PT, LPS, or both toxins, given s.c. 2 h prior to exposure to 37°C, induced convulsive behavior with a mean score similar to that observed with Pw and significantly greater than that induced by PBS (P < 0.05) (Fig. 2C). In contrast, exposure to 37°C alone or after injection of Pa did not induce behavioral changes (Fig. 2A).
While vaccination alone induced IL-1β production in the hippocampus, exposure to 37°C 2 h after administration of Pw resulted in a significantly greater (P < 0.01) elevation of IL-1β concentration (Fig. 2B). This correlates tightly with the intensity of the seizure activity observed in the same mice. In contrast, Pa- and PBS-treated mice, both of which demonstrated minimal behavioral changes (Fig. 2A), produced negligible increases in IL-1β concentration in the hippocampus (Fig. 2B).
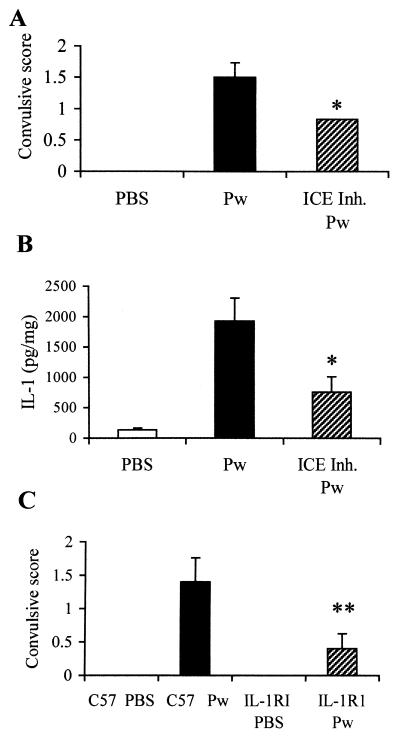
IL-1β mediates the convulsive activity of Pw.
To provide definitive evidence of a role for IL-1β in the neurological responses to Pw, we examined the effect of an ICE inhibitor in vivo and the responses to Pw in IL-1RI−/− mice. Pretreatment of BALB/c mice with the ICE inhibitor 30 min before s.c. vaccination with Pw significantly decreased the concentration of IL-1β in the hippocampus, and this correlated with a reduced convulsive response (Fig. 3). Treatment with an ICE inhibitor alone, without vaccination, had no effect (data not shown). Although ICE was originally identified as an IL-1-specific protease, it is also essential for the conversion of IL-18 to its active form. Therefore, we confirmed the role of IL-1β using IL-1RI−/− mice, in which all IL-1-mediated signaling events are abrogated. The seizure response to Pw was abrogated or significantly reduced in IL-1RI−/− mice (Fig. 3C).
FIG. 3.
Pw-induced convulsive behavior is attenuated by pretreatment with an ICE inhibitor and abrogated in IL-1RI−/− mice. Mice were injected intraperitoneally with 0.33 μM ICE inhibitor peptide (ICE Inh.) Ac-YVAD-CMK before s.c. administration of Pw. Two hours later, mice were placed at 37°C, and behavioral activity recorded for a further hour (A). Mice were sacrificed, and hippocampal IL-1β protein concentrations were determined by immunoassay (B). IL-1RI−/− (IL-1R1) and wild-type C57BL/6 (C57) mice were injected s.c. with Pw and placed at 37°C 2 h later, and behavioral activity recorded for a further hour (C). Results are mean (SE) values for six mice in each group. ∗ P < 0.05; ∗∗, P < 0.01, Pw versus ICE inhibitor plus Pw or IL-1RI−/− versus C57BL/6 wild-type mice.
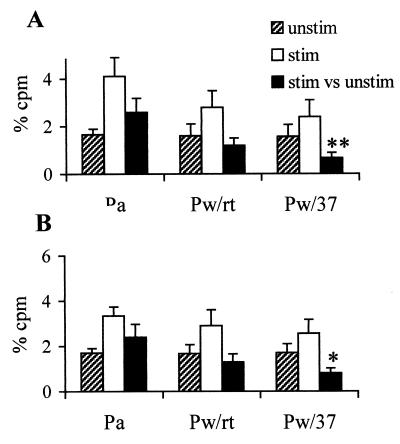
Pw immunization inhibits neurotransmitter release.
To investigate the possibility that IL-1β, synthesized in the CNS in response to Pw, might alter neuronal excitability and therefore induce convulsive behavior, we analyzed release of the neurotransmitters GABA and adenosine in hippocampal synaptosomes prepared from Pw-treated mice. Analysis of neurotransmitter release revealed similar levels of unstimulated release in synaptosomes prepared from all groups of mice (Fig. 4). However, following administration of Pw, KC1-evoked release of GABA and adenosine was reduced compared with Pa-vaccinated mice. More importantly, in mice with the highest concentrations of hippocampal IL-1β (Pw/37), release of both adenosine and GABA was significantly inhibited.
FIG. 4.
Parenteral injection with Pw, but not with Pa, inhibited neurotransmitter release. BALB/c mice were injected s.c. with Pw, Pa, or PBS and 2 h later were placed at 37°C. Alternatively, mice were left at room temperature after injection of Pw (Pw/rt). Synaptosomes prepared from hippocampal tissue were assessed for KC1-induced release of endogenous adenosine (A) and GABA (B). Results are mean (SE) values for unstimulated (unstim), KC1-stimulated (stim), and stimulated versus unstimulated (stim vs unstim) for six mice in each test group. ∗, P < 0.05; ∗∗, P < 0.01, Pw versus Pa.
DISCUSSION
Respiratory infection of children with B. pertussis has been associated with mild to severe neurological responses, including, fever, convulsions, and in a small but significant number of cases, encephalitis, encephalopathy, permanent brain damage, and death. Centrally controlled responses, including fevers and seizures, are also detected at a relatively high frequency following parenteral immunization with Pw, and although controversial, there is also evidence of the more severe CNS complications in a small proportion of vaccinated children (2, 3, 5, 20). It has been suggested that active toxins, produced by the bacteria during infection or present in the Pw, may be responsible for the neurological responses (3, 32); however, the mechanisms underlying these centrally controlled clinical manifestations of the infection and reactogenicity of the vaccine are poorly understood. The present report details a murine model in which neuronal responses were consistently induced by pertussis vaccination. The most significant and novel finding of our study is that certain neurological effects of pertussis whole-cell vaccines are mediated through the induction of IL-1β production in the brain in response to active bacterial toxins present in the vaccine.
Previous animal models designed to study the neurological responses to pertussis vaccine focused on an encephalopathic syndrome in mice generated by the administration of BSA and Pw (33). However, data from a number of groups indicate that the protocol induced an acute anaphylactic reaction and did not cause an encephalopathy (22, 28). The model was therefore deemed unsuitable for testing the encephalopathic potential of pertussis vaccines. In this study we sought to develop an alternative murine model, in which it was possible to consistently induce neurological responses to Pw. The majority of children displaying a seizure response to Pw have documented high temperatures. Indeed, a typical presentation of pertussis vaccine-induced encephalopathy is that of a generalized seizure frequently associated with a high fever within 48 h of pertussis vaccination (2, 3). Furthermore, prophylactic acetaminophen administration reduces the frequency and severity of adverse reactions following primary vaccination with DTPw (11). Therefore, our initial investigations focused on the fever response to vaccination in mice. We observed that core body temperatures were significantly elevated 2 to 4 h after injection of Pw. This was accompanied by an increase in the production of IL-1β in the hippocampus and hypothalamus, detectable at the protein and mRNA levels. This is consistent with the established role for IL-1β in the fever response to LPS and in gram-negative and other infections (12, 30, 40).
Having demonstrated a temporal relationship between IL-1β and induction of fever, we hypothesized that there may also be a correlation between IL-1β and convulsive behavior induced by Pw vaccination, a hypothesis supported by an association between elevated IL-1β in the CNS and the pathogenesis of febrile convulsions in children (10) and chemically induced seizures in mice (4, 36, 40). Our rationale in establishing a model to study neurological responses to vaccination was to manipulate the early central response to Pw, by increasing core temperature and therefore exacerbating IL-1β production to a level that might induce seizure activity. We observed that placing the mice at high ambient temperature after injection of B. pertussis sonicate or Pw induced behavioral changes ranging from head nodding to full clonic seizures.
Although there may be a number of possible mechanisms underlying the enhanced seizure-like activity following exposure of Pw-immunized mice to a high ambient temperature, our data indicate that it may reflect a synergistic effect of the heat stress response on IL-1β production. The convulsive activity was associated with IL-1β concentrations in the hippocampus which was significantly elevated compared with that in the mice injected with Pw and left at room temperature. Neurologic disorders, including seizures, have been described during cancer therapy with IL-1β (29). Furthermore, elevated IL-1β has been linked with the seizure responses to glycerol (4), pentylenetetrazole, and Shigella dysenteriae (40), and intrahippocampal administration of IL-1 receptor antagonist was found to inhibit electroencephalographic seizures induced by bicuculline methiodide in mice (36). In the present study, definitive evidence of a role for IL-1β in the convulsive activity of Pw was provided by the demonstration that behavioral changes were significantly reduced by in vivo administration of an ICE inhibitor or in IL-1RI−/− mice.
Although the stimulus for IL-1β induction in the brain following parenteral injection with Pw has not been definitively identified, our findings suggest a role for bacterial toxins. It has been reported that LPS and PT are present in Pw (32). Indeed, we were able to detect these active toxins in the reference vaccine used for this study. Removal of active PT and LPS through deletion or mutations in the genes coding for these toxins (16, 26) may provide safer Pw. However, we do not rule out a role for other active bacterial toxins or virulence factors in IL-1 induction and neurological effects of Pw. In contrast, Pa are prepared with highly purified B. pertussis proteins, and although they include PT as a protective antigen in a chemically or genetically detoxified form, they are free of active bacterial toxins. Compared with Pw, these new vaccines are associated with significantly fewer adverse reactions, such as high fevers and convulsions (3, 5, 8, 9). We observed that Pa did not induce IL-1β but did induce IL-10 production in the hippocampus of immunized mice. This is consistent with our observations that Pw selectively induce systemic Th1 cells and pro-inflammatory responses, whereas Pa or their purified components induce Th2 cells and anti-inflammatory cytokine production (15, 18, 19, 21, 31). Significantly, these new generation Pa do not induce behavioral changes in mice. Direct evidence of the primary role of active bacterial toxins in inducing the effects of Pw was provided by our demonstration that PT or LPS given s.c. prior to exposure to 37°C induced convulsive behavior in mice. We have already reported that PT and LPS, like Pw, induce IL-1β production and c-Jun-N-terminal kinase (JNK) activation in the hippocampus (13).
IL-1β and tumor necrosis factor alpha induction has been detected at the protein and mRNA levels in the hypothalamus and hippocampus of mice during infection with B. pertussis (14). Since the expression is more persistent in the brain than in the lungs or circulation, it was concluded that the production is induced locally in the brain, by either activated macrophages, bacterial toxins, or other mediators that have crossed the blood-brain barrier. Significantly, it has been suggested that PT may enhance histamine-induced vascular permeability (39). The demonstration of antibodies to PT and filamentous hemagglutinin in the cerebrospinal fluid of a child with pertussis has been used as evidence to support the suggestion that pertussis antigens may gain entry to the CNS during infection (7). It has also been reported that another AB toxin, cholera toxin, can be transported to the brain and can enhance trafficking of third party antigens to neural tissue following nasal delivery in mice (35).
There are several possible pathways by which IL-1β can affect brain function. Some reports suggest that behavioral changes are mediated by cytokines produced in the periphery, which stimulate the CNS through afferent nerves (38). However, there is increasing evidence that cytokines, including IL-1β, are synthesized by glial and neuronal cells during inflammation, suggesting a pathophysiological role for IL-1β in the brain (30). In support of this, several studies have demonstrated that IL-1β can affect neuroendocrine functions and modulate release of neurotransmitters (34, 37). To investigate the possibility that IL-1β, synthesized in the CNS in response to Pw, might alter neuronal excitability and therefore induce convulsive behavior, we demonstrated an inhibition of release of the neurotransmitters GABA and adenosine by hippocampal synaptosomes from Pw-treated mice. Modulation of these neurotransmitters has been reported during epilepsy and febrile convulsions in children (27). A significant correlation was reported between seizure excitability and low concentrations of GABA in cerebrospinal fluid. It is possible that this decrease in inhibitory transmitter release occurs as a direct result of increased IL-1β in response to Pw vaccination. Indeed, it has been reported that IL-1β has the potential to exert an inhibitory effect on neurotransmitter release and synaptic plasticity (23, 37).
Although we do not rule out a role for other inflammatory mediators, including tumor necrosis factor alpha or IL-6, our results demonstrate that the neurological response to Pw is dependent on the central production of IL-1β, which may exert its effect via the modulation of inhibitory neurotransmission. To our knowledge, this is the first time a causal relationship between Pw vaccination and neuronal responses has been demonstrated in an animal model. Our findings provide the first direct evidence of a mechanism of pertussis vaccine reactogenicity and suggest that active bacterial toxins are responsible for the neurologic reactions observed in children immunized with Pw.
ACKNOWLEDGMENTS
This work was supported by a basic research grant from an Enterprise Ireland. Christine Loscher is supported by a fellowship from the Irish Health Research Board.
We thank Patricia Byrne, Patricia Johnson, and Edel McNeela for assistance in assessing convulsive scores.
REFERENCES
- 1.Bloom B B, Widdus R. Vaccine visions and their global impact. Nat Med. 1998;4:480–484. doi: 10.1038/nm0598supp-480. [DOI] [PubMed] [Google Scholar]
- 2.Blumberg D A, Lewis K, Mink C M, Christenson P D, Chatfield P, Cherry J D. Severe reactions associated with diphtheria-tetanus-pertussis vaccine: detailed study of children with seizures, hypotonic-hyporesponsive episodes, high fevers and persistent crying. Pediatrics. 1993;91:1158–1165. [PubMed] [Google Scholar]
- 3.Cherry J D, Brunell P A, Golden G S, Karzon D T. Report of the task force on pertussis and pertussis immunization. Pediatrics. 1988;81:939–984. [Google Scholar]
- 4.Donnelly S, Loscher C, Mills K H G, Lynch M A. Glycerol-induced seizures: involvement of IL-1β and glutamate. NeuroReport. 1999;10:1821–1825. doi: 10.1097/00001756-199906230-00004. [DOI] [PubMed] [Google Scholar]
- 5.Edwards K M, Decker M D, Mortimer E A. Pertussis vaccine. In: Plotkin S A, Orenstein W A, editors. Vaccines. 3rd ed. Philadelphia, Pa: W. B. Saunders; 1999. pp. 293–344. [Google Scholar]
- 6.Glaccum M B, Stocking K L, Charrier K, Smith J L, Willis C R, Maliszewski C, Livingston D J, Peschon J J, Morrissey P J. Phenotypic and functional characterization of mice that lack the type I receptor for IL-1. J Immunol. 1997;159:3364–3371. [PubMed] [Google Scholar]
- 7.Grant C C, McKay E J, Simpson A, Buckley D. Pertussis encephalopathy with high cerebrospinal fluid antibody titers to pertussis toxin and filamentous hemagglutinin. Pediatrics. 1998;102:986–990. doi: 10.1542/peds.102.4.986. [DOI] [PubMed] [Google Scholar]
- 8.Greco D, Salmaso S, Mastrantonio P, Giuliano M, Tozzi A E, Anemona A, Ciofi degli Atti M L, Giammanco A, Panei P, Blackwelder W C, Klein D L, Wassilak S G. A controlled trial of two acellular vaccines and one whole cell vaccine against pertussis. N Engl J Med. 1996;334:341–348. doi: 10.1056/NEJM199602083340601. [DOI] [PubMed] [Google Scholar]
- 9.Gustafsson L, Hallander H O, Olin P, Reizenstein O, Storsaeter J A. A controlled trial of a two-component acellular, a five component acellular, and a whole cell pertussis vaccine. N Engl J Med. 1996;334:349–355. doi: 10.1056/NEJM199602083340602. [DOI] [PubMed] [Google Scholar]
- 10.Helminen M, Vesikare T. Increased interleukin-1 (IL-1) production from LPS-stimulated peripheral blood monocytes in children with febrile convulsions. Acta Paedtiar Scand. 1990;79:810–816. doi: 10.1111/j.1651-2227.1990.tb11559.x. [DOI] [PubMed] [Google Scholar]
- 11.Ipp M M, Gold R, Greenberg S, Goldbach M, Kupfert B B, Lloyd D D, Maresky D C, Saunders N, Wise S A. Acetaminophen prophylaxis of adverse reactions following vaccination of infants with diphtheria-pertussis-tetanus toxoids-polio vaccine. Pediatr Infect Dis J. 1987;6:721–725. doi: 10.1097/00006454-198708000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Kluger M J, Kozak W, Leon L R, Soszynski D, Conn C A. Cytokines and fever. Neuroimmunomodulation. 1995;2:216–223. doi: 10.1159/000097199. [DOI] [PubMed] [Google Scholar]
- 13.Loscher C E, Donnelly S, Mills K H G, Lynch M. Interleukin-1β-dependent changes in hippocampal function following parenteral immunization with a whole cell pertussis vaccine. J Neuroimmunol. 2000;111:68–76. doi: 10.1016/s0165-5728(00)00366-0. [DOI] [PubMed] [Google Scholar]
- 14.Loscher C E, Donnelly S, Lynch M, Mills K H G. Pro-inflammatory cytokine induction in the brain following respiratory infection with Bordetella pertussis. J Neuroimmunol. 2000;102:172–181. doi: 10.1016/s0165-5728(99)00177-0. [DOI] [PubMed] [Google Scholar]
- 15.Mahon B P, Sheahan B J, Griffin F, Murphy G, Mills K H G. Atypical disease after Bordetella pertussis respiratory infection of mice with targeted disruptions of interferon-γ receptor or immunoglobulin μ chain genes. J Exp Med. 1997;186:1843–1851. doi: 10.1084/jem.186.11.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marsili I, Pizza M, Giovannoni F, Volpini G, Bartalini M, Olivieri R, Rappuoli R, Nencioni L. Cellular pertussis vaccine containing a Bordetella pertussis strain that produces a nontoxic pertussis toxin molecule. Infect Immun. 1992;60:1150–1155. doi: 10.1128/iai.60.3.1150-1155.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCracken G H, Mustafa M M, Ramilo O, Olsen K D, Risser R C. Cerebrospinal fluid interleukin Iβ and tumour necrosis factor concentrations and outcome from neonatal gram negative enteric bacillary meningitis. Pediatr Infect Dis. 1989;8:155–159. [PubMed] [Google Scholar]
- 18.McGuirk P, Mills K H G. A regulatory role for IL-4 in differential inflammatory responses in the lung following infection of mice primed with Th1- or Th2-inducing pertussis vaccines. Infect Immun. 2000;68:1383–1391. doi: 10.1128/iai.68.3.1383-1390.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McGuirk P, Mills K H G. Direct anti-inflammatory effect of a bacterial virulence factor: IL-10-dependent suppression of IL-12 production by filamentous haemagglutinin from Bordetella pertussis. Eur J Immunol. 2000;30:415–422. doi: 10.1002/1521-4141(200002)30:2<415::AID-IMMU415>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 20.Miller D, Madge N, Diamond J, Wadsworth J, Ross E. Pertussis immunization and serious acute neurological illnesses in children. Br Med J. 1993;307:1171–1176. doi: 10.1136/bmj.307.6913.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mills K H G, Ryan M, Ryan E, Mahon B P. A murine model in which protection correlates with pertussis vaccine efficacy in children demonstrates complementary roles for humoral and cell-mediated immunity in protection against Bordetella pertussis. Infect Immun. 1998;66:594–602. doi: 10.1128/iai.66.2.594-602.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Munoz J J, Peacock M G, Hadlow W J. Anaphylaxis or so-called encephalopathy in mice sensitized to an antigen with the aid of pertussigen (pertussis toxin) Infect Immun. 1987;55:1004–1008. doi: 10.1128/iai.55.4.1004-1008.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murray C A, Lynch M A. Evidence that increased hippocampal expression of the cytokine IL-1β is a common trigger for age- and stress-induced impairments in long term potentiation. J Neurosci. 1998;18:2974–2981. doi: 10.1523/JNEUROSCI.18-08-02974.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murray C A, McGahon B, McBennett S, Lynch M A. Interleukin-1 beta inhibits glutamate release in hippocampus of young, but not aged, rats. Neurobiol Aging. 1997;18:343–348. doi: 10.1016/s0197-4580(97)80317-x. [DOI] [PubMed] [Google Scholar]
- 25.Olin P, Rasmussen F, Gustafsson L, Hallander H O, Heijbel H. Randomised controlled trial of two-component, three component and five-component acellular vaccines compared with whole cell pertussis vaccine. Lancet. 1997;ii:1569–1577. doi: 10.1016/s0140-6736(97)06508-2. [DOI] [PubMed] [Google Scholar]
- 26.Preston A, Allen A G, Cadisch J, Thomas R, Stevens K, Churcher C M, Badcock K L, Parkhill J, Barrell B, Maskell D J. Genetic basis for lipopolysaccharide O-antigen biosynthesis in bordetellae. Infect Immun. 1999;67:3763–3767. doi: 10.1128/iai.67.8.3763-3767.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rating D, Siemes H, Loscher W. Low CSF GABA concentration in children with febrile convulsions, untreated epilepsy, and menigitis. J Neurol. 1983;230:217–225. doi: 10.1007/BF00313697. [DOI] [PubMed] [Google Scholar]
- 28.Redhead K, Robinson A, Ashworth L A E, Melville-Smith M. The activity of purified Bordetella pertussis components in murine encephalopathy. J Biol Stand. 1987;15:341–351. doi: 10.1016/s0092-1157(87)80007-0. [DOI] [PubMed] [Google Scholar]
- 29.Redmond B G, Abubakr Y, Chou T, Esper P, Flaherty L E. Phase II trial of recombinant interleukin-1 beta in patients with metastatic renal cell carcinoma. J Immunother Emphas Tumour Immunol. 1994;16:211–215. doi: 10.1097/00002371-199410000-00005. [DOI] [PubMed] [Google Scholar]
- 30.Rothwell N J. Cytokines—killers in the brain? J Physiol. 1999;514:3–17. doi: 10.1111/j.1469-7793.1999.003af.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ryan M, Murphy G, Nilsson L, Shackley F, Gothefors L, O/mar K, Miller E, Storsaeter J, Mills K H G. Distinct Th cell subtypes induced with whole cell and acellular pertussis vaccines in children. Immunology. 1998;93:1–10. doi: 10.1046/j.1365-2567.1998.00401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sidey F M, Furmean B L, Wardlaw A C. Effect of hyperactivity to endotoxin on the toxicity of pertussis vaccine and pertussis toxin in mice. Vaccine. 1989;7:237–241. doi: 10.1016/0264-410x(89)90236-3. [DOI] [PubMed] [Google Scholar]
- 33.Steinmann L, Sriram S, Adelman N E, Zamvil S, McDevitt H O, Urich H. Murine model for pertussis vaccine encephalopathy: linkage to H-2. Nature. 1982;299:738–740. doi: 10.1038/299738a0. [DOI] [PubMed] [Google Scholar]
- 34.Uehara A, Gottschall P E, Dahl R R, Arimura A. Interleukin-1 stimulates ACTH release by indirect action which requires endogenous corticotropin releasing factor. Endocrinology. 1987;4:1580–1582. doi: 10.1210/endo-121-4-1580. [DOI] [PubMed] [Google Scholar]
- 35.van Ginkel F W, Jackson R J, Yuki Y, McGhee J R. Cutting edge: the mucosal adjuvant cholera toxin redirects vaccine proteins into olfactory tissues. J Immunol. 2000;165:4778–4782. doi: 10.4049/jimmunol.165.9.4778. [DOI] [PubMed] [Google Scholar]
- 36.Vezzani A, Moneta D, Conti M, Richichi C, Ravizza T, De Luigi A, De Simoni M G, Sperk G, Andell-Jonsson S, Lundkvist J, Iverfeldt K, Bartfai T. Powerful anticonvulsant action of IL-1 receptor antagonist on intracerebral injection and astrocytic overexpression in mice. Proc Natl Acad Sci USA. 2000;97:11534–11539. doi: 10.1073/pnas.190206797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang S, Cheng Q, Malik S, Yang J. Interleukin-1beta inhibits gamma-aminobutyric acid type A (GABA(A)) receptor current in cultured hippocampal neurons. J Pharmacol Exp Ther. 2000;292:497–504. [PubMed] [Google Scholar]
- 38.Watkins L R, Maier S F, Goehler L E. Immune activation: the role of pro-inflammatory cytokines in inflammation, illness response and pathological pain states. Pain. 1995;6:289–302. doi: 10.1016/0304-3959(95)00186-7. [DOI] [PubMed] [Google Scholar]
- 39.Yong T, Meininger G A, Linthicum D S. Enhancement of histamine-induced vascular leakage by pertussis toxin in SJL/J mice but not BALB/c mice. J Neuroimmunol. 1993;45:47–52. doi: 10.1016/0165-5728(93)90162-r. [DOI] [PubMed] [Google Scholar]
- 40.Yuhas Y, Shulman L, Weizman A, Kaminsky E, Vanichkin A, Ashkenazi S. Involvement of tumour necrosis factor alpha and interleukin-1β in enhancement of pentylenetetrazole-induced seizures caused by Shigella dysenteriae. Infect Immun. 1999;67:1455–1460. doi: 10.1128/iai.67.3.1455-1460.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]