Abstract
Chronic complications are a significant concern for people living with HIV/AIDS (PLWHA) infection. HIV-associated neurocognitive disorders (HAND) are prevalent in PLWHA. Yet, the efficacy of medications that penetrate the central nervous system (CNS) at preventing or slowing the progression of HAND remains largely unknown. The objective of this study was to determine whether high CNS penetration effectiveness (CPE) regimens improve neurocognitive test scores in PLWHA on combined antiretroviral therapy (cART). Primary literature evaluating cognitive outcomes based on CPE score of cART regimens in PLWHA was assembled from Pubmed/Medline and EMBASE. Both randomized controlled trials and observational studies with at least 12 weeks of follow-up were included. A meta-analysis of four studies was conducted to calculate the standardized mean difference. Eight trials including a total of 3,303 patients with 13,103 person-years of follow-up were included in the systematic review. Four trials (n=366 patients) met our inclusion criteria and were included in the meta-analysis. In the meta-analysis, HIV regimens with a high CPE score did not affect NPZ-4 or GDS scores (standardized mean difference (SMD) = 0.10, 95% CI −0.19, 0.38; I2=26%). Future studies with larger sample sizes are warranted to prospectively evaluate the relationship between CPE and progression of HAND.
Keywords: HIV, AIDS dementia complex, HIV associated neurocognitive disease, central nervous system penetration, cognitive impairment
Introduction:
An estimated 37.7 million people worldwide live with human immunodeficiency virus (HIV) infection and almost 1.5 million individuals become infected each year (UNAIDS, 2020). The prognosis of HIV infection has drastically improved in recent years. With combination antiretroviral therapy (cART), HIV has transitioned from a life-threatening infection to a chronic disease (Deeks et al., 2013). Improvements in prevention, education, and treatment have led to decreases in incident cases while treatments have also increased life expectancy (Centers for Disease Control, 2021). However, complications associated with long-term HIV, such as renal and heart failure and HIV-associated neurocognitive disorders (HAND) have become increasingly burdensome (Deeks et al., 2013).
Nearly half of people living with HIV or AIDS (PLWHA) will develop some form of cognitive impairment during their lifetime (Clifford et al., 2013). HAND is defined in three stages (Antinori et al., 2007). Asymptomatic neurocognitive impairment (ANI) is defined by scoring 1 standard deviation (SD) below the age-adjusted normal mean score in at least two cognitive areas. Mild neurocognitive impairment (MND) is defined as ANI in addition to a patient having an impairment in activities of daily living. HIV-associated dementia (HAD) is defined as at least 2 SDs below the age-adjusted mean in at least two cognitive areas, significant difficulties in activities in daily living not caused by another comorbidity, and the absence of delirium or a different dementia.
The prevalence of ANI is higher than MND or HAD (Heaton et al., 2010). Antiretroviral therapy (ART) exposure has not been found to confer lifetime protection, as patients with cART exposure have similar rates of HAND than those who never receive cART (Heaton et al., 2011; Robertson et al., 2017). However, earlier ART initiation may delay the onset of HAND. Studies assessing measures of cognitive impairment suggest that initiation of cART within 3 months of HIV diagnosis appears to delay the diagnosis of HAND over a 48-month period compared to initiation of cART 24 months after diagnosis (Robertson et al., 2017). A recent meta-analysis of 19 studies conducted in 16 different countries and varying timespans (1984–2016) estimated the prevalence rate of HAND to be 43.9% (95% CI 36.7–51.4%) (Wei et al., 2020).
The efficacy of a cART regimen that penetrates the central nervous system to prevent or slow the progression or diagnosis of HAND remains largely unknown (Rumbaugh et al., 2015). The central nervous penetration effectiveness (CPE) score is a method of estimating an agent’s ability to penetrate the cerebrospinal fluid and can be calculated two ways. The original method, also known as the 2008 calculation, assigns each ARV medication a score of 0 (poor penetration), 0.5 (moderate penetration) or 1 (good penetration). For each medication in a cART regimen, CPE score is added together to calculate a total CPE score (Letendre et al., 2008). The updated method, also known as the 2010 calculation, assigns a score of 1 to 4 to each agent (Letendre et al., 2010). Each drug’s individual value is added for the total regimen CPE score. High CPE is defined as ≥2 in the 2008 calculation or ≥7 in the 2010 calculation (Letendre et al., 2008; Letendre et al., 2010).
An increased risk of incident HIV-associated dementia has been reported in a retrospective cohort of patients on high CPE score antiretroviral therapy (Caniglia et al., 2014). Patients started on low, medium, or high CPE score regimens were analyzed with a median of 37 months of follow-up for incidence of HAND. The researchers used the 2010 calculation high CPE threshold of at least 10, which is higher than the cut-off of 7 used more commonly (Antinori et al., 2007). This higher cutoff score was chosen based on the distribution of CPE scores in their cohort and may be a result of confounding by indication; that is, patients with more severe disease receiving additional agents would be more likely to have a higher CPE score for the regimen (Caniglia et al., 2014).
Because cognitive impairment and dementia are gradually worsening conditions, encouraging early initiation of combination antiretroviral therapy and discovery of an antiretroviral regimen that slows the progression of cognitive decline has potential to improve quality of life and health outcomes. To our knowledge, there have been no systematic reviews and/or meta-analysis that has been conducted on this topic. While there has been interest in the area of central nervous system penetration and HIV associated neurocognitive disorders, the available evidence has yet to be quantitively synthesized in a systematic review and meta-analysis. To address this question, we assembled and systematically assessed available literature that evaluated whether high CPE cART impacts neurocognitive scores and performed a meta-analysis to assess this association by combining evidence from the relevant studies.
Methods:
A systematic literature review was conducted of PubMed/Medline as well as EMBASE databases from inception through October 2020 which followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [Appendix Table 1; Appendix Table 2] (Moher et al., 2009). The gray literature was identified by reviewing the abstracts from the EMBASE database. Randomized controlled trials and prospective and retrospective cohort studies were included in the analysis and required a study duration of 12 weeks or longer. A review of citations was also conducted of all full-text studies reviewed to identify additional studies that may not have been identified through our search. Articles went through three stages of review: title, abstract, and full text (Moher et al., 2009). To be included, studies had to compare cART regimens based on CPE score. If CPE scores were not reported, regimens needed to be delineated so CPE scores could be calculated (Table 1) (Letendre et al., 2008; Letendre et al., 2010; Robertson et al., 2017). We included studies in which patients were adults (≥ 18 years) and did not have pre-existing cognitive impairment unrelated to HIV. For each included study, patients could be ART-exposed at the baseline. Neuropsychiatric Z-score (NPZ) or global deficit score (GDS) were required to be evaluated as study outcomes. Studies that did not measure a difference in neurocognitive scores were not included in this analysis.
Table 1:
2010 method for calculating CPE, regimen CPE is calculated through addition of individual scores
| CPE Score | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Integrase inhibitors | Raltegravir Elvitegravir |
Dolutegravir | ||
| Fusion inhibitors | Enfuvirtide | Maraviroc | ||
| Protease inhibitors | Nelfinavir Ritonavir Saquinavir Tipranavir |
Atazanavir Atazanavir/r Fosamprenavir |
Lopinavir Darunavir/r Fosamprenavir/r Indinavir |
Indinavir/r |
| Non-Nucleoside Reverse Transcriptase Inhibitors (NNRTIs) | Etravirine | Delavirdine Efavirenz |
Nevirapine | |
| Nucleoside Reverse Transcriptase Inhibitors (NRTIs) | Tenofovir Zalcitabine |
Didanosine Lamivudine Stavudine |
Abacavir Emtricitabine |
Zidovudine |
Adapted from Letendre 2008
All stages of review were conducted by two team members (AW, EB). Data was abstracted into a Microsoft Excel spreadsheet by a single reviewer with data abstraction verification being conducted by a separate reviewer. Elements of the abstraction consisted of study design, interventions, patient population and demographics, outcomes assessed, effectiveness results, and safety results. Study quality was assessed by the Cochrane Risk of Bias tool for randomized controlled trials (Higgins et al., 2011) [Appendix Table 3] and the STROBE Checklist for nonrandomized observational studies (von Elm et al., 2007) [Appendix Table 4].
NPZ scores and GDS scores were analyzed together because both were based on similar neurocognitive assessments. The sign of the GDS was changed to match the sign of the NPZ score (higher scores indicate better cognitive function). A sub-analysis was completed of studies utilizing GDS. Sub-analysis of studies utilizing NPZ was not completed because only one trial utilized NPZ alone (Valcour et al., 2015).
Quantitative analysis was represented using a forest plot. Mean NPZ and GDS scores, number of patients in each trial, and SD of high and low CPE regimens was compared. High CPE was defined as ≥7 or ≥2.0, using the 2010 or 2008 method, respectively (Letendre et al., 2008; Letendre et al., 2010). A standardized mean difference was calculated to obtain a combined estimate using a random effects model. Pooled SD was calculated for each group and was used to calculate the standardized mean difference. Hedges correction was applied, and 95% confidence intervals were calculated. An I2 statistic was used to measure the degree of heterogeneity across studies. While bias assessment is an important part of meta-analysis, given the small number of studies included in the meta-analysis, a funnel plot could not be constructed. Statistical analysis was completed in R version 3.3.2 using the meta package (Schwarzer et al., 2007). This study was deemed exempt by the Investigational Review Board at the University of Rhode Island.
Results:
There were 184 articles initially identified from the systematic literature review with 168 unique articles remaining after removal of duplicates. Of those, 106 titles were unrelated to the study question and were excluded. After reviewing the abstracts, 14 articles were fully reviewed, upon which six were excluded. No additional articles reviewed from the review of citations or the grey literature search met our inclusion criteria, so no additional studies were added. Reasons for exclusion include outcomes not assessing NPZ or GDS, not controlling for CPE changes, and study follow-up of less than 12 weeks. Eight studies (two RCTs, six observational studies) were included for qualitative review only (Figure 1) (Cross et al., 2013; Cysique et al., 2009; Ellis et al., 2014; Marra et al., 2009; Perez-Valero et al., 2014; Smurzynski et al., 2011; Tozzi et al., 2009; Valcour et al., 2015).
Figure 1:
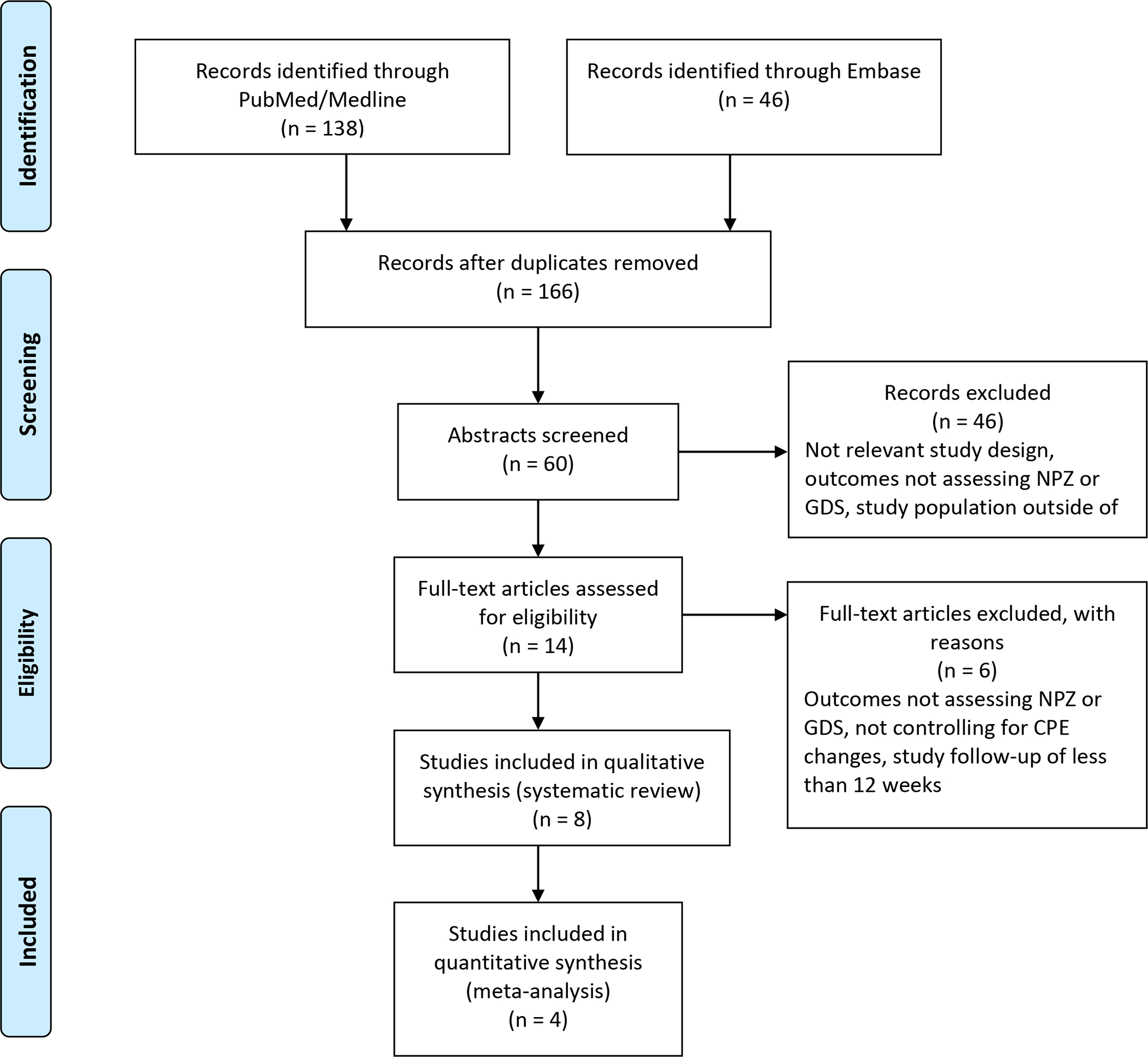
PRISMA flowchart of the systematic review of research articles completed through October 2020
Table 2 describes characteristics of the included studies and Table 3 describes design and outcomes of each study. Six prospective cohort studies (Cross et al., 2013; Cysique et al., 2009; Marra et al., 2009; Perez-Valero et al., 2014; Smurzynski et al., 2011; Tozzi et al., 2009) and two randomized controlled trials (Ellis et al., 2014; Valcour et al., 2015) were included in the systematic review. Samples size for prospective cohort studies ranged from 37 to 2,636 patients (Cross et al., 2013; Cysique et al., 2009; Marra et al., 2009; Perez-Valero et al., 2014; Smurzynski et al., 2011; Tozzi et al., 2009). Two studies were completed in Europe (Perez-Valero et al., 2014; Tozzi et al., 2009), four in the United States (Cysique et al., 2009; Ellis et al., 2014; Marra et al., 2009; Smurzynski et al., 2011), one in Africa (Cross et al., 2013), and one in Asia (Valcour et al., 2015). Mean age of the patients ranged from 27 to 45 years (Cross et al., 2013; Cysique et al., 2009; Ellis et al., 2014; Marra et al., 2009; Perez-Valero et al., 2014; Smurzynski et al., 2011; Tozzi et al., 2009; Valcour et al., 2015). Inclusion criteria was similar among studies, with two requiring patients to have symptoms of HAND at baseline (Ellis et al., 2014; Tozzi et al., 2009) and the others not specifying. Exclusion criteria was similar among studies and most excluded patients with severe psychiatric illness, serious brain injury, or other forms of neurocognitive impairment. Length of follow up ranged from 16 weeks to 4.7 years (Cross et al., 2013; Cysique et al., 2009; Ellis et al., 2014; Marra et al., 2009; Perez-Valero et al., 2014; Smurzynski et al., 2011; Tozzi et al., 2009; Valcour et al., 2015).
Table 2:
Baseline study characteristics of studies identified in the systematic review
| Author, year | Country | Study Type | Number of patients | Mean age (years) | Inclusion Criteria | Exclusion criteria | Mean follow-up |
|---|---|---|---|---|---|---|---|
| Cross HM, et al. 2013 * | South Africa | Prospective cohort | 111 | 29.6 | HIV infected, age 18 to 35, completed at least 7 years of school | Uncontrolled medical conditions, psychiatric or neurologic condition, substance or alcohol abuse disorder, or previous moderate to severe head injury | 52 weeks |
| Cysique LA, et al. 2009 | United States | Prospective cohort | 37 | 39.7 | HIV infected, ART naïve or ART treatment failure (defined as HIV RNA >5,000 copies/mL), HAND at baseline | History of neurologic disease unrelated to HIV, psychotic psychiatric disease, drug abuse disorder within last 6 months, history of traumatic brain injury with loss of consciousness for greater than 1 hour | 48 weeks |
| Ellis RJ, et al. 2014 * | United States | Randomized controlled trial | 59 | 44.9 | HIV infected, current symptoms of HAND, on stable ARV for at least 8 weeks, and under consideration to change ARV regimen | Active opportunistic infection, neuropsychiatric (NP) impairment due to a disorder other than HIV, substance use disorder as diagnosed by DSM-IV within past 12 months, active severe psychiatric disorder, positive urine drug screening | 16 weeks |
| Marra CM, et al. 2009 | United States | Prospective cohort | 79 | 39 | HIV infected, CD4+ count <200/μL with HIV RNA >2000 copies/mL or HIV RNA >50,000 regardless of CD4+ count, either initiating cART or changing therapy | Not on a cART regimen with ≥3 agents | 52 weeks |
| Perez-Valero I, et al. 2014 * | Spain | Prospective cohort | 134 | 44.6 | HIV infected, undetectable viral load for ≥1 year, treated with either darunavir/r or lopinavir/r as monotherapy or with emtricitabine-tenofovir as triple therapy | Non-Spanish speaking | 52 weeks |
| Smurzynski M, et al. 2011 | United States | Prospective cohort | 2,636 | 40 | HIV infected, either treatment naïve or experienced, included in AIDS Clinical Trials Group Longitudinal Linked Randomized TRIAL (ALLRT) cohort | Factors that would compromise the ability to comply with scheduled visits | 4.7 years |
| Tozzi V, et al. 2009 | Italy | Prospective cohort | 185 | 39.3 | HIV infected, previous symptoms of HAND, symptomatic HIV, CD4+ count <200/μL | Current or past opportunistic infection, tumor of the CNS, non-HIV related neuropsychiatric illness, current illicit drug use, or use of sedative hypnotics | 39 months |
| Valcour VG, et al. 2015 * | Thailand | Randomized controlled trial | 62 | 27 | HIV infected | CNS opportunistic infection | 24 weeks |
Included in quantitative meta-analysis
Table 3:
Outcomes of studies identified for the systematic review
| Author | Number in each study group | Description of intervention | Measured outcome | Outcome |
|---|---|---|---|---|
| Cross HM, et al., 2013* | 69 patients were included in CPE analysis CPE ≤7 (31), CPE >7 (38) |
cART regimen was chosen by the provider. The 2010 CPE method was used, ≤7 was defined as low CPE and >7 was defined as high CPE | Patients underwent NP testing at baseline. Tests consisted of the following domains: attention, motor, learning, processing, executive function, and verbal skills. Results were converted to z scores and then translated to GDS sore. NP testing was completed 1 year later for all patients and data on what cART regimen for each patient was collected. | No significant difference between GDS scores of low and high CPE groups. Median difference was 0.13 (p=0.32). |
| Cysique LA, et al., 2009 | High CPE, ≥2 (10), medium CPE, 1.5 (11), low CPE <1.5 (16) | cART was chosen by providers. CPE scores were assigned to each regimen using the 2008 method. High CPE was defined as ≥2, medium CPE was 1.5, and low CPE was <1.5. |
Patients underwent NP testing (grooved pegboard, paced auditory serial addition test, trail making test A & B, and letter fluency) at baseline and at follow up. Tests were translated into a GDS score and a mean score regression-based change score (MS-Reg-CS). CPE was compared to MS-Reg-CS in a univariate and multivariate model. | CPE score ≥2 was associated with a significant overall improvement in MS-Reg-CS of 2.46 points over 12 weeks (95% CI: 1.02, 3.91). There was not a significant time interaction effect (p=0.57). In multivariable analysis, higher CPE score was significantly positively correlated with MS-Reg-CS improvement (R2 = 0.59, p=0.002). |
| Ellis RJ, et al., 2014* | CNS-Targeted (29), Non-CNS-T (30) | cART regimen was chosen by the provider. CPE score (2008 method) was ≥2 in the CNS-T group and <2 in the non-CNS-T group. | Patients underwent NP testing at baseline (wide range achievement test, trail making test A, color-word test, brief visuospatial memory test) and at 16 weeks (Wisconsin card sorting test, trail making test B, color-word test, controlled oral word association test, category fluency, WMS-III, PASAT, grooved pegboard test) and scores were converted into a GDS score. Testing evaluated the following domains: intellectual function, speed of information processing, learning and delayed recall, executive function, verbal fluency, working memory, and motor function. Difference in GDS score between groups were compared at 16 weeks. | Median CPE in the CNS-T group was 2.5 and 1 in the non-CNS-T group. In the 49 patients included in the intent-to-treat analysis, there was a 7% difference between GDS scores for each group (95% CI: −31%, 62%). GDS decreased by 0.27 in the CNS-T group (SD = 0.52) and by 0.17 in the non-CNS-T group (SD=0.41). |
| Marra CM, et al., 2009 | Treatment naïve (44), treatment experienced (35) | cART regimen was chosen by provider but needed to have ≥3 agents. CPE was calculated using the 2008 method with high CPE being defined as ≥2. | Patients underwent NP testing (timed gait, grooved pegboard, digit symbol, finger tapping, trail making A and B, Rey auditory verbal learning test trial, basic choice reaction time) before beginning or changing cART regimen. Tests were repeated at 24 and 52 weeks. Results were translated into NPZ-4 scores or NPZ-8. A univariate model was made comparing CPE score and change in NPZ-4 or NPZ-8. A secondary analysis of changes among patients whose baseline NPZ-4 score was ≤−0.5 was also completed. | In the univariate model, CPE ≥2 was associated with an NPZ-4 decrease of 1.08 (95% CI: 0.50, 1.66). A significant effect did not exist for NPZ-8 score, with CPE rank ≥2 associated with an NPZ-8 decrease of 0.29 (95% CI: −0.41, 1.00). Among patients with baseline NPZ-4 ≤2, CPE score <2 was associated with an improvement in NPZ-4 of 0.28 (95% CI: 0.19, 0.87) while CPE score ≥2 led to a increase of 0.01 (95% CI: −0.64, 0.59). |
| Perez-Valero I, et al., 2014* | Monotherapy (67) or triple therapy (67) | Darunavir/r or lopinavir/r (2010 CPE score 3) versus darunavir/r or lopinavir/r plus tenofovir/emtricitabine (2010 CPE score 7) for 1 year | Patients underwent NP testing in Spanish (Wechsler adult intelligence scale digit span, digit symbol, vocabulary, and digit search subtests, Stroop color and word test, trailmaking test A and B, controlled oral word association test, Buschke selecting reminding test, brief visuospatial test, grooved pegboard test) at baseline and at follow-up. NP scores were translated to GDS scores and clinical rating (CR) score. | Both monotherapy (low CPE) and triple therapy (high CPE) decreased GDS scores by significant amounts. Monotherapy decreased GDS by 0.08 (95% CI: 0.02, 0.14) and triple therapy decreased GDS by 0.09 (95% CI: 0.01, 0.16). Difference between groups was not significant (p=0.82). |
| Smurzynski M, et al., 2011 | HIV-infected, either treatment naïve (1,819) or experienced (817) | ARV regimen was chosen by the provider and changed as needed. CPE score was calculated for each regimen at each visit by the 2008 method. | Patients underwent NP testing (trail making test A and B, Wechsler adult intelligence scale digit symbol) at initiation of ARV and at each follow-up. Scores were translated into adjusted z scores (adjusted for sex, age, education, and ethnicity) and reported as NPZ-3. Changes in NPZ-3 were correlated with CPE scores of each patient regimen. | Median CPE for patients taking ≤3 ARVs was 1.5. Median CPE for patients taking >3 ARVs was 2.5. Higher CPE score was found to positively correlated with an increase in NPZ-3 score only in regimens with >3 ARVs (0.07 SD change per 1-point increase in CPE, p = 0.005) but not significantly correlated with regimens with ≤3 ARVs (0.01 SD change per 1-point increase in CPE, p = 0.5) in multivariable analysis. |
| Tozzi V, et al., 2009 | HIV infected, previous symptoms of HAND (n=93) or CD4+ <200 (n=92) | HAART regimen was chosen by provider. CPE score was calculated for each regimen by the 2008 method. | Patients underwent NP testing (tested for mental flexibility, concentration and speed of mental processing, memory, visuospatial and constructional abilities, and fine motor functioning) at baseline and follow-up. Results were translated into z scores and reported as NPZ-4 and NPZ-8. Changes in NPZ-4 and NPZ-8 were correlated with CPE scores of each patient regimen. | Mean CPE score at baseline was 1.65 (range 0–3) and at follow up was 1.55 (range 0–3). All patient’s NPZ-4 scores improved over time. Higher CPE scores were positively correlated with improvements in NPZ-4 (R2 = 0.028, p=0.0283) and NPZ-8 (R2 = 0.0486, p=0.0071) at the second assessment. In patients without prior symptoms of HAND, higher CPE scores were non-significantly correlated with improvements in NPZ-4 (R2=0.0242, p=0.095) and NPZ-8 (R2=0.0030, p=0.276). |
| Valcour VG, et al., 2015* | cART (30) and cART+ (32) | cART is defined as efavirenz, tenofovir, and either emtricitabine or lamivudine (CPE 6 or 7) and cART+ is defined as cART plus raltegravir and maraviroc (CPE 12 or 13) | Patients underwent NP testing (color trails I and II, grooved pegboard, trail making test A) at baseline and at follow up. Scores were translated into a z-score and are reported as NPZ-4. | NPZ-4 improved significantly for both groups. cART patients improved by 0.59 (p<0.001) and cART+ improved by 0.69 (p<0.001). The mean difference between groups was not significant (p=0.19). |
Four articles, two RCTs (Ellis et al., 2014; Valcour et al., 2015) and two prospective observational cohort studies (Cross et al., 2013; Perez-Valero et al., 2014), were included in the quantitative meta-analysis. Four studies from the systematic review were excluded for the meta-analysis because they did not contain the required continuous data for outcomes (Cysique et al., 2009; Marra et al., 2009; Tozzi et al., 2009,Smurzynski et al., 2011). The data from Cross et al. was reanalyzed to include patients with CPE score of 7 in the high CPE group (Cross et al., 2013).
Blinding occurred in only one of the studies (Ellis et al., 2014), while the rest of the studies were open-labeled (Cross et al., 2013; Cysique et al., 2009; Ellis et al., 2014; Marra et al., 2009; Perez-Valero et al., 2014; Smurzynski et al., 2011; Tozzi et al., 2009; Valcour et al., 2015). Most studies did not specify which cART regimens patients were taking and allowed prescriber autonomy in agent selection (Cross et al., 2013; Cysique et al., 2009; Ellis et al., 2014; Marra et al., 2009; Smurzynski et al., 2011; Tozzi et al., 2009). Two studies analyzed patients by whether they were ARV naïve or experienced (Marra et al., 2009; Smurzynski et al., 2011). The remainder made distinctions based on either CPE status or specific regimen where CPE could be calculated (Cross et al., 2013; Cysique et al., 2009; Ellis et al., 2014; Perez-Valero et al., 2014; Tozzi et al., 2009; Valcour et al., 2015). Four studies utilized NPZ scores as their outcome measure (Marra et al., 2009; Smurzynski et al., 2011; Tozzi et al., 2009; Valcour et al., 2015) and the remainder used GDS scores (Cross et al., 2013; Cysique et al., 2009; Ellis et al., 2014; Perez-Valero et al., 2014). No study appeared to have a differential loss to follow-up (Cross et al., 2013; Cysique et al., 2009; Ellis et al., 2014; Marra et al., 2009; Perez-Valero et al., 2014; Smurzynski et al., 2011; Tozzi et al., 2009; Valcour et al., 2015).
Adjustment for confounding was performed in different ways in each study. Ellis et al. adjusted GDS scores for age and race/ethnicity, while accounting for correlation due to repeated measures (Ellis et al., 2014). Perez-Valero et al. adjusted for 20 variables that could contribute to confounding, including age, gender, years of education, years since diagnosis, and naïve CD4+ cell counts (Perez-Valero et al., 2014). Valcour et al. adjusted for baseline CD4+ count (Valcour et al., 2015). Cross et al. did not mention a specific method of adjusting for bias (Cross et al., 2013). Ellis and Valcour were the only two randomized controlled trials included in the study (Ellis et al., 2014; Valcour et al., 2015).
NPZ-4 and GDS scores were pooled across the four studies. The pooled studies included 186 patients in high CPE groups and 130 patients in low CPE groups. In the fixed effects model, there was a standardized mean difference of 0.06 (95% confidence interval (CI): −0.17, 0.30, p=0.60, I2 = 26%) indicating that HIV regimens with a high CPE score were not significantly associated with the NPZ-4 scores. The findings remained consistent in the random effects model as well with a standardized mean difference of 0.10 (95% CI: −0.19, 0.38, p=0.26, I2=26%). Valcour et al was the only study that utilized NPZ-4 rather than GDS in this analysis (Valcour et al., 2015). A sub-group of only GDS studies was completed after excluding Valcour et al. from the analysis. A similar result was found, with a standardized mean difference attenuated towards the null (0.01, 95% CI: −0.25, 0.28, I2=22%) in the fixed effects model and a standardized mean difference of 0.02 (95% CI: −0.34, 0.30, p=0.28; I2=22%) in the random effects model.
Discussion:
Our systematic review examined the association of ART with high CNS penetration on HIV-related cognitive impairment in patients with HIV. Eight studies including six observational studies and two RCTs were included in the systematic review. In this meta-analysis, we found no evidence that suggests higher CPE cART regimens were associated with neurocognitive function in PLWHA at risk for or with HAND. While the meta-analysis may have been underpowered to detect significant differences due to only four studies being included, the standardized mean difference was close to the null, thus adding more support to the findings that higher CPE cART regimens may have no effect on neurocognitive function.
Most studies did not report any significant association, while two reported a positive correlation between higher CPE and cognitive performance, and one reported a negative correlation between CPE and cognitive performance. The ALLRT cohort did find a positive effect with patients taking >3 ARVs, thus having a higher CPE, but the estimated association was small, and numerically similar to the combined estimated association reported here (Smurzynski et al., 2011). However, the ALLRT cohort reported the correlation between CPE score and change in NPZ-3 rather than absolute change in score and thus could not be included in the meta-analysis (Smurzynski et al., 2011). Adhering to a regimen with a higher CPE can treat or prevent HAND remains controversial, and while this analysis did not evaluate diagnoses of HAND, these tests are often used to diagnose HAND include NPZ and GDS scores.
This study has several limitations. This analysis relies heavily on data from observational trials which lack blinding, may have unmeasured confounding, such as variations in family history of dementia, and hence could be prone to confounding bias. Specific instruments for cognitive functioning also introduce measurement error, especially due to inter-clinician variation in rating PLWHA based on subjective ratings scales. The wide variety of cART regimens chosen by providers and not by study design fails to allow for individual drug effects to be elucidated. Different ARVs have different side effect profiles, such as efavirenz’s neuropsychiatric effects, and this cannot be ruled out as a factor in this analysis. Patients were also included from countries across the globe. There is likely variation by site for different standards of care or demographics impact cognitive function. The small sample size of the included studies creates the possibility that this study was underpowered to detect the differences. The use of the NPZ and GDS scores as outcomes may introduce some selection bias in this meta-analysis because patients who undergo neurocognitive performance testing are a unique population composed of those in research studies or needing neurocognitive testing rather than the general HIV population. The follow-up time for the included studies of the systematic review varied greatly from 16 weeks to 4.7 years. For the meta-analysis specifically, follow-up time ranged from 16 to 52 weeks in studies. If longer exposure to higher CPE regimens is needed for cognitive protection, having varying follow-up times for included studies may bias the results towards the null if not enough follow-up time was allowed to see meaningful improvement.
There is no defined effective treatment for HAND, so the identification of safe and effective prevention is still needed. Our study suggests that CPE may not be strongly correlated with treatment success in HIV-associated cognitive impairment. Based on the results of this study, a cART regimen with a high CPE cannot be recommended over a regimen with a low CPE for a patient with HAND solely for the reason of preventing progression of neurocognitive decline, although maximization of therapy should always be pursued to achieve virologic suppression. Further research, particularly randomized, controlled trials comparing early initiation of specific cART regimens with high and low CPE, is warranted to better understand their role in preventing neurocognitive decline.
Figure 2:
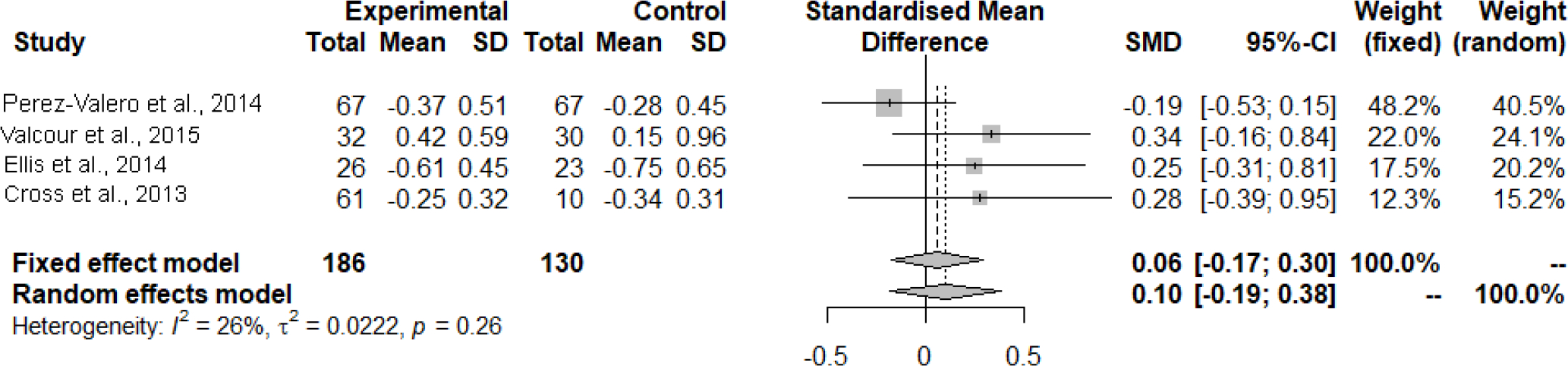
Mean difference in change of neurocognitive performance comparing groups on high-CPE cART regimens versus low-CPE cART regimens
Figure 3:
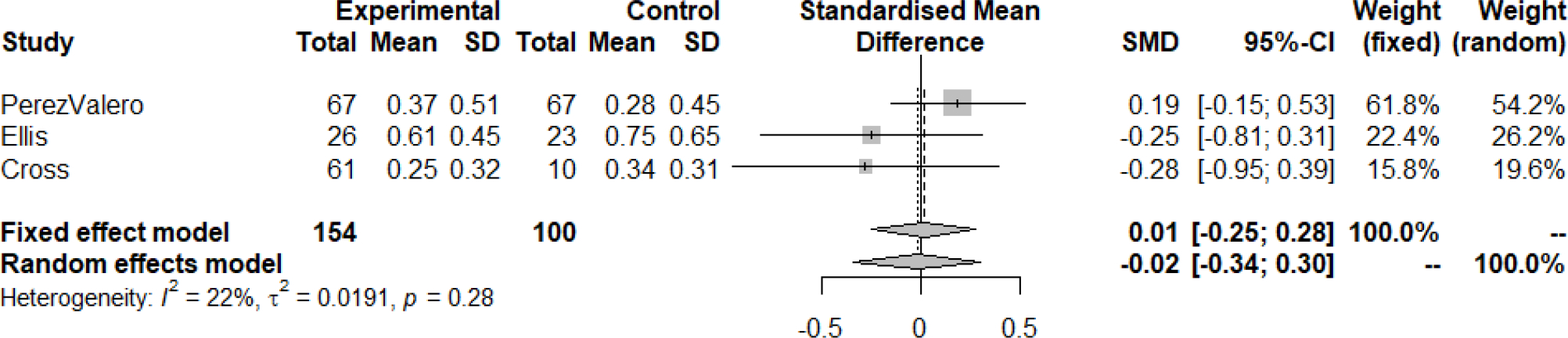
Mean difference in change of neurocognitive performance comparing groups on high-CPE cART regimens versus low-CPE cART regimens, including only trials that evaluated GDS cART: combined antiretroviral therapy; CI: confidence interval; CPE: central nervous system penetration effectiveness; GDS: global deficit score; SD: standard deviation; SMD: standardized mean difference
Acknowledgments:
Contents of this study was presented as a poster at the ACCP Global Conference on Clinical Pharmacy from October 20–23, 2018 in Seattle, WA. Cross et al provided their data set for use in this analysis. Their study was funded by the South African Research Foundation, Medical Research Council of South Africa, Biological Psychiatry Special Interest Group of SASOP and the NIMH (R01 Grant MH085604). We would like to thank all of the study participants, investigators, and data management teams of the included studies. Buchanan was partially supported by the NIH Avenir grant 1DP2DA046856-01. Buchanan and Vyas were partially supported by Institutional Development Award Number U54GM115677 from the National Institute of General Medical Sciences of the National Institutes of Health, which funds Advance Clinical and Translational Research (Advance-CTR). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding:
No funding was provided for this study.
APPENDIX TABLES
Table A1.
PubMed/Medline Search Strategy
| ID | Search | Hits |
|---|---|---|
| 1 | (“Anti-HIV Agents/adverse effects”[Mesh] OR “Antiretroviral Therapy, Highly Active/adverse effects”[Mesh] OR “HIV Infections/drug therapy”[Mesh]) | 76,115 |
| 2 | (“Cognition/drug effects”[Mesh] OR “AIDS Dementia complex”[Mesh] OR “HIV associated dementia”[All Fields]) | 18,531 |
| 3 | (“CPE”[All Fields] OR “CNS-Penetration Effectiveness”[All Fields] OR “CNS penetration effectiveness”[All Fields] OR “CNS penetration”[All Fields] OR “central nervous system penetration”[All Fields]) | 7,434 |
| 4 | 1 AND 2 AND 3 | 50 |
| 5 | (“AIDS Dementia Complex”[Mesh] OR “HIV dementia”[All Fields] OR “HIV Associated Dementia”[All Fields] OR “HIV Cognitive impairment”[All Fields] OR (“HIV”[All Fields] AND “Cognitive impairment”[All Fields]) OR (“HIV”[All Fields] AND “Dementia”[All Fields]) OR (“HIV”[All Fields] AND “Cognitive”[All Fields]) AND (“CPE”[All Fields] OR “CNS Penetration Effectiveness”[All Fields] OR “CNS-Penetration effectiveness”[All Fields] OR “Cognition/drug effects”[MeSH]) | 137 |
| 6 | 4 OR 5 | 140 |
| 7 | 6 AND “humans”[MeSH Terms] | 138 |
Table A2:
EMBASE Search Strategy
| ID | Search | Hits |
|---|---|---|
| 1 | (‘haart’/exp OR ‘haart’ OR ‘cart’ OR ‘highly active antiretroviral therapy’/exp OR ‘highly active antiretroviral therapy’ OR ‘combined antiretroviral therapy’/exp OR ‘combined antiretroviral therapy’ OR ‘hiv therapy’ OR ‘antiretroviral therapy’/exp OR ‘antiretroviral therapy’) | 96,887 |
| 2 | (‘cpe’ OR ‘cns penetration effectiveness’ OR ‘cns penetration effectiveness’) | 9,309 |
| 3 | 1 AND 2 | 136 |
| 4 | 3 NOT ‘prevalence’ | 106 |
| 5 | (‘cognitive function’ OR ‘cognition’ OR ‘neurocognitive impairment’ OR ‘npz4’ OR ‘gds’) | 378,794 |
| 6 | 4 AND 5 | 46 |
Table A3:
Cochrane Risk of Bias for Randomized Controlled Trials Quality Assessment
| Quality Indicator | Ellis 2014 | Valcour 2015 |
|---|---|---|
| Selection Bias: Random Sequence Generation | Low | Unclear |
| Selection Bias: Allocation Concealment | Unclear | Unclear |
| Reporting Bias: Selective Reporting | Low | Low |
| Other Bias: Other Sources of Bias | Low | Low |
| Performance Bias: Blinding (Participants and Personnel) | High | High |
| Detection Bias: Blinding (Outcome Assessment) | High | High |
| Attrition Bias: Incomplete Outcome Data | Low | Low |
Table A4:
Strobe Checklist for Observational Studies Quality Assessment
| Item No. | Recommendation | Cross 2013 | Cysique 2009 | Marra 2009 | Perez-Valero 2014 | Smurzynski 2011 | Tozzi 2009 | |
|---|---|---|---|---|---|---|---|---|
| Title and abstract | 1 | (a) Indicate the study’s design with a commonly used term in the title or the abstract | No | No | Yes | Yes | No | No |
| (b) Provide in the abstract an informative and balanced summary of what was done and what was found | Yes | Yes | Yes | Yes | Yes | Yes | ||
| Introduction | ||||||||
| Background/rationale | 2 | Explain the scientific background and rationale for the investigation being reported | Yes | Yes | Yes | Yes | Yes | Yes |
| Objectives | 3 | State specific objectives, including any prespecified hypotheses | Yes | Yes | Yes | Yes | Yes | Yes |
| Methods | ||||||||
| Study design | 4 | Present key elements of study design early in the paper | Yes | Yes | Yes | Yes | Yes | Yes |
| Setting | 5 | Describe the setting, locations, and relevant dates, including periods of recruitment, exposure, follow-up, and data collection | Yes | Yes | No | Yes | Yes | Yes |
| Participants | 6 | (a) Cohort study—Give the eligibility criteria, and the sources and methods of selection of participants. Describe methods of follow-up Case-control study—Give the eligibility criteria, and the sources and methods of case ascertainment and control selection. Give the rationale for the choice of cases and controls Cross-sectional study—Give the eligibility criteria, and the sources and methods of selection of participants |
Yes | Yes | Yes | Yes | Yes | Yes |
| (b) Cohort study—For matched studies, give matching criteria and number of exposed and unexposed Case-control study—For matched studies, give matching criteria and the number of controls per case |
N/A | N/A | N/A | N/A | N/A | N/A | ||
| aVariables | 7 | Clearly define all outcomes, exposures, predictors, potential confounders, and effect modifiers. Give diagnostic criteria, if applicable | Yes | Yes | Yes | Yes | Yes | Yes |
| Data sources/measurement | 8* | For each variable of interest, give sources of data and details of methods of assessment (measurement). Describe comparability of assessment methods if there is more than one group | Yes | Yes | Yes | Yes | Yes | Yes |
| Bias | 9 | Describe any efforts to address potential sources of bias | No | Yes | No | Yes | Yes | No |
| Study size | 10 | Explain how the study size was arrived at | No | No | No | No | No | No |
| Quantitative variables | 11 | Explain how quantitative variables were handled in the analyses. If applicable, describe which groupings were chosen and why | Yes | Yes | Yes | Yes | Yes | Yes |
| Statistical methods | 12 | (a) Describe all statistical methods, including those used to control for confounding | Yes | Yes | Yes | Yes | Yes | No |
| (b) Describe any methods used to examine subgroups and interactions | Yes | Yes | Yes | Yes | Yes | Yes | ||
| (c) Explain how missing data were addressed | No | No | No | No | No | No | ||
| (d) Cohort study—If applicable, explain how loss to follow-up was addressed Case-control study—If applicable, explain how matching of cases and controls was addressed Cross-sectional study—If applicable, describe analytical methods taking account of sampling strategy |
No | Yes | No | N/A | No | No | ||
| (e) Describe any sensitivity analyses | No | Yes | No | Yes | No | No | ||
| Results | ||||||||
| Participants | 13* | (a) Report numbers of individuals at each stage of study—eg numbers potentially eligible, examined for eligibility, confirmed eligible, included in the study, completing follow-up, and analysed | Yes | No | Yes | Yes | Yes | Yes |
| (b) Give reasons for non-participation at each stage | Yes | No | Yes | Yes | Yes | Yes | ||
| (c) Consider use of a flow diagram | Yes | No | Yes | No | Yes | No | ||
| Descriptive data | 14* | (a) Give characteristics of study participants (eg demographic, clinical, social) and information on exposures and potential confounders | No | Yes | Yes | Yes | Yes | Yes |
| (b) Indicate number of participants with missing data for each variable of interest | No | No | No | No | No | No | ||
| (c) Cohort study—Summarise follow-up time (eg, average and total amount) | Yes | Yes | Yes | N/A | Yes | Yes | ||
| Outcome data | 15* | Cohort study—Report numbers of outcome events or summary measures over time | Yes | Yes | Yes | Yes | Yes | Yes |
| Case-control study—Report numbers in each exposure category, or summary measures of exposure | ||||||||
| Cross-sectional study—Report numbers of outcome events or summary measures | ||||||||
| Main results | 16 | (a) Give unadjusted estimates and, if applicable, confounder-adjusted estimates and their precision (eg, 95% confidence interval). Make clear which confounders were adjusted for and why they were included | Yes | Yes | Yes | Yes | Yes | Yes |
| (b) Report category boundaries when continuous variables were categorized | N/A | N/A | N/A | N/A | N/A | N/A | ||
| (c) If relevant, consider translating estimates of relative risk into absolute risk for a meaningful time period | ||||||||
| Other analyses | 17 | Report other analyses done—eg analyses of subgroups and interactions, and sensitivity analyses | N/A | Yes | Yes | Yes | Yes | Yes |
| Discussion | ||||||||
| Key results | 18 | Summarise key results with reference to study objectives | Yes | Yes | Yes | Yes | Yes | Yes |
| Limitations | 19 | Discuss limitations of the study, taking into account sources of potential bias or imprecision. Discuss both direction and magnitude of any potential bias | Yes | Yes | Yes | Yes | Yes | Yes |
| Interpretation | 20 | Give a cautious overall interpretation of results considering objectives, limitations, multiplicity of analyses, results from similar studies, and other relevant evidence | Yes | Yes | Yes | Yes | Yes | Yes |
| Generalisability | 21 | Discuss the generalisability (external validity) of the study results | Yes | Yes | Yes | Yes | Yes | Yes |
| Other information | ||||||||
| Funding | 22 | Give the source of funding and the role of the funders for the present study and, if applicable, for the original study on which the present article is based | Yes | Yes | Yes | Yes | Yes | Yes |
Footnotes
Disclosure Statement:
No potential conflict of interest was reported by the authors.
Institutional Review Board Status: This study was deemed exempt by the Investigational Review Board at the University of Rhode Island.
References:
- Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, Clifford DB, Cinque P, Epstein LG, Goodkin K, Gisslen M, Grant I, Heaton RK, Joseph J, Marder K, Marra CM, McArthur JC, Nunn M, Price RW, Pulliam L, Robertson KR, Sacktor N, Valcour V, & Wojna VE (2007). Updated research nosology for HIV-associated neurocognitive disorders. Neurology, 69(18),1789–99. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caniglia EC, Cain LE, Justice A, Tate J, Logan R, Sabin C, Winston A, van Sighem A, Miro JM, Podzamczer D, Olson A, Arribas JR, Moreno S, Meyer L, del Romero J, Dabis F, Bucher HC, Wandeler G, Vourli G, Skoutelis A, Lanoy E, Gasnault J, Costagliola D, & Hernán MA; HIV-CAUSAL Collaboration et al; HIV-CAUSAL Collaboration. (2014). Antiretroviral penetration into the CNS and incidence of AIDS-defining neurologic conditions. Neurology, 83(2),134–41. doi: 10.1212/WNL.0000000000000564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (2021). Estimated HIV incidence and prevalence in the United States, 2015–2019. HIV Surveillance Supplemental Report. 26(No. 1). http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html. Published May 2021. Accessed [07/06/2021]. [Google Scholar]
- Clifford DB, & Ances BM (2013). HIV-associated neurocognitive disorder. Lancet Infect Dis, 13(11), 976–86. doi: 10.1016/S1473-3099(13)70269-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross HM, Combrinck MI, & Joska JA (2013). HIV-associated neurocognitive disorders: antiretroviral regimen, central nervous system penetration effectiveness, and cognitive outcomes. S Afr Med J, 103(10), 758–62. doi: 10.7196/samj.6677. [DOI] [PubMed] [Google Scholar]
- Cysique LA, Vaida F, Letendre S, Gibson S, Cherner M, Woods SP, McCutchan JA, Heaton RK, & Ellis RJ (2009). Dynamics of cognitive change in impaired HIV-positive patients initiating antiretroviral therapy. Neurology, 73(5), 342–8. doi: 10.1212/WNL.0b013e3181ab2b3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeks SG, Lewin SR, & Havlir DV (2013). The end of AIDS: HIV infection as a chronic disease. Lancet, 382(9903), 1525–33. doi: 10.1016/S0140-6736(13)61809-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis RJ, Letendre S, Vaida F, Haubrich R, Heaton RK, Sacktor N, Clifford DB, Best BM, May S, Umlauf A, Cherner M, Sanders C, Ballard C, Simpson DM, Jay C, & McCutchan JA (2014). Randomized trial of central nervous system-targeted antiretrovirals for HIV-associated neurocognitive disorder. Clin Infect Dis, 58(7), 1015–22. doi: 10.1093/cid/cit921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR, Woods SP, Ake C, Vaida F, Ellis RJ, Letendre SL, Marcotte TD, Atkinson JH, Rivera-Mindt M, Vigil OR, Taylor MJ, Collier AC, Marra CM, Gelman BB, McArthur JC, Morgello S, Simpson DM, McCutchan JA, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, & Grant I; CHARTER Group. (2010). HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology, 75(23), 2087–96. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, Corkran SH, Duarte NA, Clifford DB, Woods SP, Collier AC, Marra CM, Morgello S, Mindt MR, Taylor MJ, Marcotte TD, Atkinson JH, Wolfson T, Gelman BB, McArthur JC, Simpson DM, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, & Grant I; et al. ; CHARTER Group; HNRC Group. (2011). HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol, 17(1), 3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, & Sterne JA; Cochrane Bias Methods Group; Cochrane Statistical Methods Group. (2011). The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ, 343, d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letendre S, Marquie-Beck J, Capparelli E, Best B, Clifford D, Collier AC, Gelman BB, McArthur JC, McCutchan JA, Morgello S, Simpson D, Grant I, & Ellis RJ; CHARTER Group. (2008). Validation of the CNS Penetration-Effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Arch Neurol, 65(1), 65–70. doi: 10.1001/archneurol.2007.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letendre SL, FitzSimons C, Ellis RJ, Clifford D, Collier AC, Gelman B, Marra C, McArthur J, McCutchan JA, Morgello S, Simpson D, Vaida F, & Heaton R (2010). Correlates of CSF Viral Loads in 1,221 volunteers of the CHARTER cohort. | Charter. In: Conference on Retroviruses and Opportunistic Infections. 2010 Available at: https://charternntc.org/content/correlates-csf-viral-loads-1221-volunteers-charter-cohort. (accessed 26 Jul2019). [Google Scholar]
- Marra CM, Zhao Y, Clifford DB, Letendre S, Evans S, Henry K, Ellis RJ, Rodriguez B, Coombs RW, Schifitto G, McArthur JC, & Robertson K; AIDS Clinical Trials Group 736 Study Team. (2009). Impact of combination antiretroviral therapy on cerebrospinal fluid HIV RNA and neurocognitive performance. AIDS, 23(11), 1359–66. doi: 10.1097/QAD.0b013e32832c4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, & Altman DG; PRISMA Group. (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ, 339, b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Valero I, González-Baeza A, Estébanez M, Monge S, Montes-Ramírez ML, Bayón C, Pulido F, Bernardino JI, Zamora FX, González-García JJ, Lagarde M, Hernando A, Arnalich F, & Arribas JR (2014). A prospective cohort study of neurocognitive function in aviremic HIV-infected patients treated with 1 or 3 antiretrovirals. Clin Infect Dis, 59(11), 1627–1634. doi: 10.1093/cid/ciu640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson K, Lama JR, Pilcher CD, Rios J, Brandes P, Ruiz E, Appelmans E, Spudich S, & Duerr A (2017). Can we afford to wait? ART and the CNS. In: Conference on Retroviruses and Opportunistic Infections. Seattle, WA. Available at: http://www.croiconference.org/sessions/can-we-afford-wait-art-and-cns (accessed 26 Jul2019). [Google Scholar]
- Rumbaugh JA, & Tyor W (2015). HIV-associated neurocognitive disorders: Five new things. Neurol Clin Pract, 5(3), 224–231. doi: 10.1212/CPJ.0000000000000117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzer G (2007). meta: An R package for meta-analysis. R news, 7(3), 40–5. [Google Scholar]
- Smurzynski M, Wu K, Letendre S, Robertson K, Bosch RJ, Clifford DB, Evans S, Collier AC, Taylor M, & Ellis R (2011). Effects of central nervous system antiretroviral penetration on cognitive functioning in the ALLRT cohort. AIDS, 25(3), 357–65. doi: 10.1097/QAD.0b013e32834171f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozzi V, Balestra P, Salvatori MF, Vlassi C, Liuzzi G, Giancola ML, Giulianelli M, Narciso P, & Antinori Al. (2009). Changes in cognition during antiretroviral therapy: comparison of 2 different ranking systems to measure antiretroviral drug efficacy on HIV-associated neurocognitive disorders. J Acquir Immune Defic Syndr, 52(1), 56–63. doi: 10.1097/qai.0b013e3181af83d6. [DOI] [PubMed] [Google Scholar]
- UNAIDS. 2020 Global AIDS Update: Seizing the Moment; July 2020. UNAIDS. AIDSinfo website; accessed July 2020, http://aidsinfo.unaids.org/. UNAIDS. Core Epidemiology Slides; July 2020. UNAIDS. Global HIV & AIDS statistics – 2020 fact sheet; July 2020; UNAIDS. UNAIDS data 2020, July 2020. [Google Scholar]
- Valcour VG, Spudich SS, Sailasuta N, Phanuphak N, Lerdlum S, Fletcher JL, Kroon ED, Jagodzinski LL, Allen IE, Adams CL, Prueksakaew P, Slike BM, Hellmuth JM, Kim JH, & Ananworanich J; SEARCH 010/RV 254 Study Group. (2015). Neurological Response to cART vs. cART plus Integrase Inhibitor and CCR5 Antagonist Initiated during Acute HIV. PLoS One, 10(11), e0142600. doi: 10.1371/journal.pone.0142600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, & Vandenbroucke JP; STROBE Initiative. (2007). The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med, 4(10), e296. doi: 10.1371/journal.pmed.0040296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Hou J, Su B, Jiang T, Guo C, Wang W, Zhang Y, Chang B, Wu H, Zhang T The Prevalence of Frascati-Criteria-Based HIV-Associated Neurocognitive Disorder (HAND) in HIV-Infected Adults: A Systematic Review and Meta-Analysis. Front Neurol. 2020. Dec 1;11:581346. doi: 10.3389/fneur.2020.581346 [DOI] [PMC free article] [PubMed] [Google Scholar]


