Abstract
Stunting (<-2SD of length- or height-for-age on WHO growth curves) is the most used predictor of child neurodevelopmental (ND) risk. Occipitofrontal head circumference (OFC) may be an equally feasible, but more direct and robust predictor. We explored association of the two measurements with ND outcome, separately and combined, and examined if cutoffs are more efficacious than continuous measures in predicting ND risk. Infants and young children in rural Guatemala (n=642; age range=0.1 to 35.9 months) were enrolled in a prospective natural history study, and their neurodevelopment was tested using the Mullen Scales of Early Learning (MSEL) longitudinally. Length- or height-for-age and OFC-for-age were calculated. We performed age-adjusted multivariable regression analyses to explore the association between 1) length or height and ND, 2) OFC and ND, and 3) both length or height and OFC combined, with ND; concurrently, predictively, and longitudinally, as continuous variables and using WHO z-score cut-offs. Continuous length- or height-for-age and OFC z-scores were more strongly associated with MSEL than the traditional −2SD WHO cutoff. The combination of height-for-age z-score and OFC z-score was consistently, strongly associated with the MSEL Early Learning Composite concurrently (p-values 0.0004–0.11), predictively (p-value 0.001–0.07), with the exception of the 18-24 months age group which had very few records, and in the longitudinal model (p-value <0.0001–0.004). The combination of continuous length- or height-for-age and OFC shows additional utility in estimating ND risk in infants and young children. Measurement of OFC may improve precision of prediction of ND risk in infants and young children.
Keywords: head circumference, stunting, neurodevelopment, young children, low resource settings
Introduction
Direct measurement of child neurodevelopment (ND) through performance-based assessment is often not feasible in low resource settings (LRSs; settings defined by financial, healthcare, and infrastructure constraints)1 because it is resource-intensive and there is a scarcity of validated and adapted tools that can be used worldwide with comparable results across populations2,3. Stunting, defined as >2SD below the mean in length- or height-for-age on WHO growth charts, a highly prevalent condition among children living in LRSs4–7 is the most frequently correlate used to estimate childhood ND risk, and many studies across the globe support its use5,8,9.
While stunting is the most broadly accepted and widely used proxy for ND risk, growing evidence suggests that it may be an incomplete correlate to predict ND risk in children10. In a large meta-analysis, Prado et al. (2019) noted that nutritional interventions were associated with changes in linear growth but only small improvements in child ND. Meanwhile, interventions focused on child stimulation and caregiving resulted in positive changes in ND but not in linear growth. Therefore, the interrelationships between stunting and ND risk are likely shared but incompletely overlap8,11,12.
Occipitofrontal head circumference (OFC) may be a more logical and accurate correlate of child ND risk13,14 than stunting. OFC is an anthropometric surrogate of brain volume as demonstrated by several studies using neuroimaging15 and therefore, is conceptually correlated with ND16,17. Furthermore, in high-income countries, research has repeatedly shown an association between microcephaly and poor ND outcome, yet OFC has been under-studied in LRSs, potentially due to the belief that the growth of the head was spared under conditions of poverty and nutritional stress, and few data exist15,17,18. Additionally, no studies have evaluated both stunting and OFC in the same cohort to evaluate whether they can be combined to create an even stronger predictor of ND risk than either measure alone.
In this secondary analysis of an infant cohort evaluating post-natal Zika infection (DMID 16-0057, PIs: Asturias/Munoz), we analyzed the association between linear growth and OFC with ND outcome, both separately and combined, to identify the best correlate for ND risk in infants and young children. We hypothesize that the relationship between OFC and ND risk is stronger than between stunting and ND risk, but that a combination of both metrics would improve the prediction of ND risk in children from LRSs.
Methods
Study and Setting:
From June 2017 through August 2019, a cohort of infants and children were prospectively enrolled in a natural history study (‘The Study’) of the incidence and sequelae of postnatally acquired Zika virus (ZIKV) infection at the Center for Human Development research and clinic site in southwest Guatemala. No acute ZIKV cases were confirmed during the observation period. Located in the lowlands, the site ecompasses 22 rural communities with approximately 30,000 residents. These communities are monolingual Spanish-speaking, and they suffer from high rates of food insecurity and child undernutrition, diarrheal disease, and maternal and child morbidity and mortality19,20. The Study was funded by the National Institutes of Health through the Baylor College of Medicine Vaccine and Treatments Evaluation Unit (VTEU). The Study was approved by the Institutional Review Board at Baylor College of Medicine, the Colorado Multiple Institutional Review Board, the National Ethics Committee of the Ministry of Public Health in Guatemala and the Trifinio Community Advisory Board.
Two groups of children were included in the Study: infants enrolled from birth to 3 months of age (837 screened, 500 enrolled, 431 completed the Study), and young children one to five years of age (521 screened, 374 enrolled, 327 completed the Study). Screening and enrollment was conducted in-person at the subject’s home by trained nurses that lived in the region. All Study recruitment, enrollment and visits were conducted in Spanish, the local language. All subjects were prospectively followed for one year using the Mullen Scales of Early Learning (MSEL) as it has been described previously21–23. An Early Learning Composite (ELC) score is created from the sum of the scores for Fine Motor, Expressive and Receptive Language and Visual Reception and was used as the ND outcome in all analyses 24. Infants were administered the MSEL at enrollment, six months and 12 months after study enrollment. Older children were administered the MSEL at enrollment and 12 months after study enrollment. Test adminstration was done by local psychologists trained and supervised by Study neuropsychologists from the University of Colorado.
Measurements of length or height and head circumference were carried out at all study visits. Duplicate height measurements were obtained using Seca Infantometers (Seca GmbH, Hamburg, Germany) to measure length for infants and using stadiometers to measure height for children that could stand up. Length and height were recorded to the nearest 0.1 cm. A third measurement was obtained if the difference between the 2 duplicate measurements was >0.4 cm. A Seca 211 Head Circumference Measuring Tape (12 – 59 cm) was used to measure head circumference. Duplicate head circumference measurements were recorded to the nearest 0.1 cm. A third measurement was carried out if a difference > 0.2 cm was observed with the first 2 measurements. In the case of a third measurement for any of these growth parameters, the 2 closest values were averaged for the final data.
Only visits for children under 36 months of age for whom a valid OFC (−5 ≤ OFC WHO z-score ≤ 5) and Length or Height (−6 ≤ Length or Height WHO z-score ≤ 6) was measured were included in this analysis. Five records were excluded for improbable OFC measurements, and six records were excluded for improbable or missing length or height measurements, per WHO growth chart guidelines 25. There were 651 infants and young children with 1,492 visits in the analysis dataset. World Health Organization (WHO) growth standards were used to calculate z-scores and determine microcephaly and stunting status by age and gender 13. For the purposes of the Study, microcephaly was defined as OFC >2SD below the mean and stunting was defined as >2SD below the mean in length- or height-for-age. Stunting and microcephaly status (yes/no) was determined at every visit. Records were divided into 6 age groups: 0-5.99 months, 6-11.99 months, 12-17.99 months, 18-23.99 months, 24-29.99 months, and 30-35.99 months. If a child had more than one visit in a given age group, only the first record per person in each age group was retained in the analysis cohort. We used length- or height-for-age and OFC-for-age both as continuous exposures, and as dichotomized exposures according to the WHO z-score cutoff of 2SD below the mean for age and gender.
Statistical Analysis
First, we determined the percentage of children in each age group that were classified as having stunting, microcephaly, and both stunting and microcephaly. We then conducted three separate multivariable regression analyses to explore the association between concurrent measures of head circumference, length, and ND. We analyzed the association between concurrent length or height and ELC scores (Table 1), concurrent head size and ELC score (Table 2) and linear growth and head size both included in the same model as separate independent variables (Table 3) and ELC score (dependent variable), by 6-month age strata. Table 3 also includes an analysis of the sum of the length- or height-for-age z-score and the OFC z-score as the independent variable. This analysis was limited to children that had an OFC measurement at most recent visit (minimum age at most recent visit > 11 months), and since OFC was measured up to age 36 months, the children in this analysis were age >11 – 36 months.
Table 1:
Concurrent Associations* Between Continuous Length or Height WHO z-Score and Stunting Status with MSEL ELC Score in Infants and Young Children in Guatemala 2017-2019
| N | Mean (Standard Deviation, Range) of Length- or Height-for-age z-score | Beta Estimate (SE) for association with MSEL ELC score | p-value | |
|---|---|---|---|---|
| Continuous length- or height-for-age z-score | ||||
| Age 0-5.99 months | 456 | −0.63 (1.20, −5.41, 4.57) | 0.30 (0.12) | 0.01 |
| Age 6-11.99 months | 421 | −0.92 (1.02, −5.05, 2.28) | 0.33 (0.19) | 0.09 |
| Age 12-17.99 months | 411 | −1.55 (0.99, −4.93-2.03) | 1.05 (0.31) | 0.0007 |
| Age 18-23.99 months | 40 | −2.11 (1.34, −4.34-1.90) | 0.49 (1.28) | 0.71 |
| Age 24-29.99 months | 74 | −1.94 (11.27, −4.70-3.09) | 1.90 (0.80) | 0.02 |
| Age 30-35.99 months | 90 | −1.84 (1.11, −4.87-1.52) | 3.50 (1.41) | 0.01 |
| Stunting | N (%) with Stunting | |||
| Age 0-5.99 months | 456 | 53 (11.6%) | −1.29 (0.45) | 0.0047 |
| Age 6-11.99 months | 421 | 60 (14.3%) | −0.83 (0.57) | 0.14 |
| Age 12-17.99 months | 411 | 136 (33.1%) | −1.21 (0.65) | 0.06 |
| Age 18-23.99 months | 40 | 21 (52.5%) | −0.72 (3.41) | 0.83 |
| Age 24-29.99 months | 74 | 36 (48.7%) | −0.47 (2.10) | 0.82 |
| Age 30-35.99 months | 90 | 39 (43.3%) | −6.25 (3.20) | 0.054 |
If a child had more than one visit in a given age group, the analysis only included data collected at the first visit in the age group for that child. All analyses adjusted for age.
Table 2:
Concurrent Associations* Between Continuous OFC WHO z-Score and Microcephaly Status with MSEL ELC Score in Infants and Young Children in Guatemala 2017-2019
| N | Mean (Standard Deviation, Range) of OFC z-score | Beta Estimate (SE) for association with MSEL ELC score | p-value | |
|---|---|---|---|---|
| Continuous OFC z-score | ||||
| Age 0-5.99 months | 456 | −0.60 (1.12, −4.94-2.91) | 0.22 (0.13) | 0.09 |
| Age 6-11.99 months | 421 | −0.82 (0.96, −3.83-1.88) | 0.54 (0.20) | 0.008 |
| Age 12-17.99 months | 411 | −1.07 (0.94, −4.62-1.85) | 0.82 (0.32) | 0.01 |
| Age 18-23.99 months | 40 | −1.31 (1.17, −3.95-1.07) | 2.51 (1.43) | 0.09 |
| Age 24-29.99 months | 74 | −1.08 (1.01, −4.85-0.80) | 0.18 (1.05) | 0.87 |
| Age 30-35.99 months | 90 | −1.09 (0.92, −3.78-1.41) | 5.15 (1.68) | 0.003 |
| Microcephaly | N (%) with Microcephaly | |||
| Age 0-5.99 months | 456 | 46 (10.1%) | −0.80 (0.49) | 0.0997 |
| Age 6-11.99 months | 421 | 53 (12.6%) | −1.80 (0.59) | 0.002 |
| Age 12-17.99 months | 411 | 66 (16.1%) | −1.03 (0.83) | 0.21 |
| Age 18-23.99 months | 40 | 10 (25.0%) | −6.00 (3.83) | 0.13 |
| Age 24-29.99 months | 74 | 11 (14.9%) | −2.99 (2.93) | 0.31 |
| Age 30-35.99 months | 90 | 16 (17.8%) | −12.75 (3.98) | 0.002 |
If a child had more than one visit in a given age group, the analysis only included data collected at the first visit in the age group for that child. All analyses adjusted for age.
Table 3:
Concurrent Associations* Between Length- or Height-for-age and OFC z-Score, and Stunting and Microcephaly Status, with MSEL ELC Score in Infants and Young Children in Guatemala 2017-2019
| Continuous measure of growth: | N | Beta Estimate (SE) for continuous length- or height-for-age z-score | p-value | Beta Estimate (SE) for continuous OFC z-score | p-value |
|---|---|---|---|---|---|
| Age 0-5.99 months | 456 | 0.26 (0.14) | 0.06 | 0.08 (0.15) | 0.59 |
| Age 6-11.99 months | 421 | 0.16 (0.21) | 0.46 | 0.47 (0.23) | 0.04 |
| Age 12-17.99 months | 411 | 0.88 (0.33) | 0.009 | 0.44 (0.35) | 0.21 |
| Age 18-23.99 months | 40 | 0.27 (1.26) | 0.83 | 2.48 (1.45) | 0.097 |
| Age 24-29.99 months | 74 | 2.38 (0.91) | 0.01 | −1.26 (1.15) | 0.28 |
| Age 30-35.99 months | 90 | 2.38 (1.45) | 0.10 | 4.21 (1.76) | 0.02 |
| Growth cutoffs: | Beta Estimate for Stunting | p-value | Beta Estimate for Microcephaly | p-value | |
| Age 0-5.99 months | 456 | −1.00 (0.49) | 0.04 | −0.60 (0.37) | 0.11 |
| Age 6-11.99 months | 421 | −0.30 (0.59) | 0.61 | −1.35 (.46) | 0.004 |
| Age 12-17.99 months | 411 | −0.98 (0.66) | 0.14 | −1.15 (0.66) | 0.07 |
| Age 18-23.99 months | 40 | −0.16 (3.50) | 0.96 | −2.80 (3.50) | 0.43 |
| Age 24-29.99 months | 74 | −0.68 (2.17) | 0.75 | 0.91 (2.21) | 0.68 |
| Age 30-35.99 months | 90 | −4.84 (3.27) | 0.14 | −5.52 (3.26) | 0.09 |
| Length- or Height-for-age and OFC-for-age z-scores combined | Beta Estimate for Length- or Height-for-age z-score + OFC-for-age z-score | p-value | |||
| Age 0-5.99 months | 456 | 0.18 (0.07) | 0.016 | ||
| Age 6-11.99 months | 421 | 0.31 (0.12) | 0.009 | ||
| Age 12-17.99 months | 411 | 0.67 (0.19) | 0.0004 | ||
| Age 18-23.99 months | 40 | 1.23 (0.90) | 0.18 | ||
| Age 24-29.99 months | 74 | 0.85 (0.53) | 0.11 | ||
| Age 30-35.99 months | 90 | 3.16 (0.92) | 0.0009 |
If a child had more than one visit in a given age group, the analysis only included data collected at the first visit in the age group for that child. All analyses adjusted for age.
Next, we analyzed the association between linear growth at enrollment (Table 4), head size at enrollment (Table 5), and both linear growth and head size at enrollment (Table 6) and ELC scores at most recent Study visit (dependent variable), in order to examine the association between linear growth and/or head size and subsequent ND, by 6-month enrollment age strata. Similar to Table 3, Table 6 includes an analysis of the sum of the length- or height z-score and the OFC z-score at enrollment as the independent variable. The analyses presented in Tables 4–6 gives the ND effects of prior low linear growth and small head size the maximum amount of time to emerge within the confines of the 1-year length of the study. The length of time between anthropometric data collection and ELC data collection was 11.04 – 13.83 months.
Table 4:
Associations*,** Between Length- or Height-for-age and Stunting Status at Enrollment with MSEL ELC at Most Recent Study Visit (collected at least 11 months later) in Infants and Young Children in Guatemala 2017-2019
| N | Mean (Standard Deviation, Range) of Length- or Height-for-age z-score | Beta Estimate (SE) for Association with MSEL ELC | p-value | |
|---|---|---|---|---|
| Continuous Length- or Height-for-age z-score | ||||
| Age 0-5.99 months | 424 | −0.64 (1.21, −5.41-4.57) | 0.79 (0.25) | 0.002 |
| Age 18-23.99 months | 33 | −2.02 (1.37, −4.34-1.90) | −0.05 (2.006) | 0.98 |
| Age 24-29.99 months | 62 | −2.01 (1.34, −4.70-3.09) | 2.64 (0.85) | 0.003 |
| Age 30-35.99 months | 49 | −1.82 (1.23, −4.87-1.52) | 4.18 (1.99) | 0.04 |
| Stunting | N (%) with Stunting | |||
| Age 0-5.99 months | 424 | 48 (11.3%) | −2.27 (0.97) | 0.02 |
| Age 18-23.99 months | 33 | 16 (48.5%) | 2.41 (5.59) | 0.67 |
| Age 24-29.99 months | 62 | 32 (51.6%) | −4.43 (2.37) | 0.07 |
| Age 30-35.99 months | 49 | 23 (46.9%) | −8.65 (4.92) | 0.09 |
All analyses adjusted for age
No data was available for children ages 6-11.99 months and data were available for only 8 children from ages 12-17.99 due to age at study enrollment algorithm. Therefore, these age groups were not included in this analysis.
Table 5:
Associations*,** Between Continuous OFC-for-age and Microcephaly Status at Enrollment with MSEL ELC at Most Recent Study Visit (collected at least 11 months later) in Infants and Young Children in Guatemala 2017-2019
| N | Mean (Standard Deviation, Range) of OFC-for-age | Beta Estimate (SE) for Association with MSEL ELC | p-value | |
|---|---|---|---|---|
| Continuous OFC-for-age z-score | ||||
| Age 0-5.99 months | 424 | −1.60 (1.10, −4.74-2.91) | 0.65 (0.28) | 0.02 |
| Age 18-23.99 months | 33 | −1.29 (1.26, −3.95-1.07) | 1.81 (2.23) | 0.42 |
| Age 24-29.99 months | 62 | −1.17 (1.00, −4.85-0.80) | 2.98 (1.16) | 0.01 |
| Age 30-35.99 months | 49 | −0.98 (0.87, −2.92-1.41) | 1.94 (3.00) | 0.52 |
| Microcephaly | N (%) with Microcephaly | |||
| Age 0-5.99 months | 424 | 39 (9.2%) | −1.25 (1.06) | 0.24 |
| Age 18-23.99 months | 33 | 9 (27.3%) | −11.68 (5.89) | 0.056 |
| Age 24-29.99 months | 62 | 10 (16.1%) | −6.49 (3.20) | 0.047 |
| Age 30-35.99 months | 49 | 6 (12.2%) | −5.27 (7.78) | 0.50 |
All analyses adjusted for age
No data was available for children ages 6-11.99 months and data were available for only 8 children from ages 12-17.99 due to age at study enrollment algorithm. Therefore, these age groups were not included in this analysis.
Table 6:
Associations*,** Between Length- or Height-for-age and OFC-for-age z-Score, and Stunting and Microcephaly Status, at Enrollment, with MSEL ELC Score at Most Recent Study Visit (collected at least 11 months later) in Infants and Young Children in Guatemala 2017-2019
| Continuous measures of growth: | N | Beta Estimate (SE) for continuous Length- or Height-for-age z-score | p-value | Beta Estimate (SE) for continuous OFC z-score | p-value |
|---|---|---|---|---|---|
| Age 0-5.99 months | 424 | 0.67 (0.30) | 0.03 | 0.25 (0.33) | 0.45 |
| Age 18-23.99 months | 33 | −0.13 (2.08) | 0.95 | 1.82 (2.27) | 0.43 |
| Age 24-29.99 months | 62 | 2.05 (0.95) | 0.04 | 1.75 (1.26) | 0.17 |
| Age 30-35.99 months | 49 | 4.18 (2.12) | 0.055 | 0.001 (3.08) | 0.99 |
| Growth Cutoffs: | Beta Estimate for Stunting | p-value | Beta Estimate for Microcephaly | p-value | |
| Age 0-5.99 months | 424 | −1.66 (1.04) | 0.11 | −1.17(0.77) | 0.13 |
| Age 18-23.99 months | 33 | 3.07 (5.69) | 0.59 | −4.36 (5.66) | 0.45 |
| Age 24-29.99 months | 62 | −4.25 (2.40) | 0.08 | −1.41 (2.41) | 0.56 |
| Age 30-35.99 months | 49 | −9.89 (5.25) | 0.07 | 3.87 (5.55) | 0.49 |
| Length- or Height-for-age and OFC-for-age z-scores combined | Beta Estimate for Length- or Height-for-age z-score + OFC-for-age z-score | p-value | |||
| Age 0-5.99 months | 424 | 0.47 (0.15) | 0.002 | ||
| Age 18-23.99 months | 33 | 0.76 (1.48) | 0.61 | ||
| Age 24-29.99 months | 62 | 1.93 (0.56) | 0.001 | ||
| Age 30-35.99 months | 49 | 2.66 (1.45) | 0.07 |
All analyses adjusted for age
No data was available for children ages 6-11.99 months and data were available for only 8 children from ages 12-17.99 due to age at study enrollment algorithm. Therefore, these age groups were not included in this analysis.
Finally, we analyzed the association between linear growth, head size (independent variables) and ELC score (dependent variable) at every visit for which both measures were collected, in order to incorporate all longitudinal data available (Table 7). This mixed model included multiple records per child and accounted for within-subject correlations. All analyses were adjusted for age. Sex was explored as a potential confounder, but sex and ND outcome were not correlated (Pearson correlation coefficient = −0.01, p = 0.70), and the addition of sex to the models did not appreciably change the associations between the anthropometric measures and ND. Thus, sex was not acting as a confounder, and was not retained in the final models. All analyses conducted in SAS version 9.4 (Cary, NC). As we were exploring the relative strength of different anthropometric measures to predict / indicate poor ND, no statistical adjustment for multiple comparisons was performed.
Table 7:
Mixed Models of the Association between Length or Height and OFC, as well as stunting and microcephaly with MSEL scores, using data from all visits at which length or height, OFC and MSEL were collected in Infants and Young Children in Guatemala 2017-2019
| N of subjects (N of visits) | Beta Estimate (SE) | p-value | |
|---|---|---|---|
| Length or Height continuous z-score only * | |||
| Infants: ELC | 484 (1354) | 0.37 (0.13) | 0.006 |
| Older children: ELC | 167 (213) | 2.20 (0.66) | 0.002 |
| OFC continuous z-score only * | |||
| Infants: ELC | 484 (1354) | 0.37 (0.15) | 0.01 |
| Older children: ELC | 167 (213) | 2.63 (0.89) | 0.005 |
| Length- or Height-for-age and OFC-for-age included as separate independent variables * | |||
| Infants: ELC | |||
| Length or Height | 484 (1354) | 0.29 (0.15) | 0.06 |
| OFC | 484 (1354) | 0.25 (0.15) | 0.11 |
| Older children: ELC | |||
| Length or Height | 167 (213) | 1.63 (0.66) | 0.02 |
| OFC | 167 (213) | 2.39 (0.84) | nc |
| Stunting (<−2 z-score) only * | |||
| Infants: ELC | 484 (1354) | −1.44 (0.43) | 0.001 |
| Older children: ELC | 167 (213) | dnc | nc |
| Microcephaly (<−2 z-score) only ** | |||
| Infants: ELC | 484 (1354) | −1.29 (0.45) | 0.005 |
| Older children: ELC | 167 (213) | −7.11 (2.65) | 0.055 |
| Stunting and microcephaly included as separate independent variables * | |||
| Infants: ELC | |||
| Stunting | 484 (1279) | −1.18 (0.39) | 0.003 |
| Microcephaly | 484 (1279) | −0.75 (0.44) | 0.09 |
| Older children: ELC | |||
| Stunting | 167 (213) | −2.40 (1.66) | nc |
| Microcephaly | 167 (213) | −6.28 (2.19) | nc |
| Length- or Height-for-age and OFC-for-age Z-scores combined | |||
| Infants: ELC | 484 (1354) | 0.24 (0.08) | 0.004 |
| Older children: ELC | 167 (213) | 1.97 (0.46) | <0.0001 |
intercept and stunting status included as random effects in the mixed model with the fao(3) structure
intercept and head circumference included as random effects in the mixed model with the fao(3) structure
nc = p-value not calculated
dnc = model did not converge
Results
There were 642 infants and young children in the Study with sufficient growth and ND data to be included in this analysis. The analysis cohort was 46.6% female, had a mean age of 8 months at enrollment (range 0.1-35.9 months), and the large majority did not report an ethnicity (73.1%)25. Figure 1 shows the increase in stunting, microcephaly, and both conditions as infants and young children age from 0–3 years, with the highest percentage of children with adverse growth measurements occurring in the 18-24 month age group.
Figure 1:
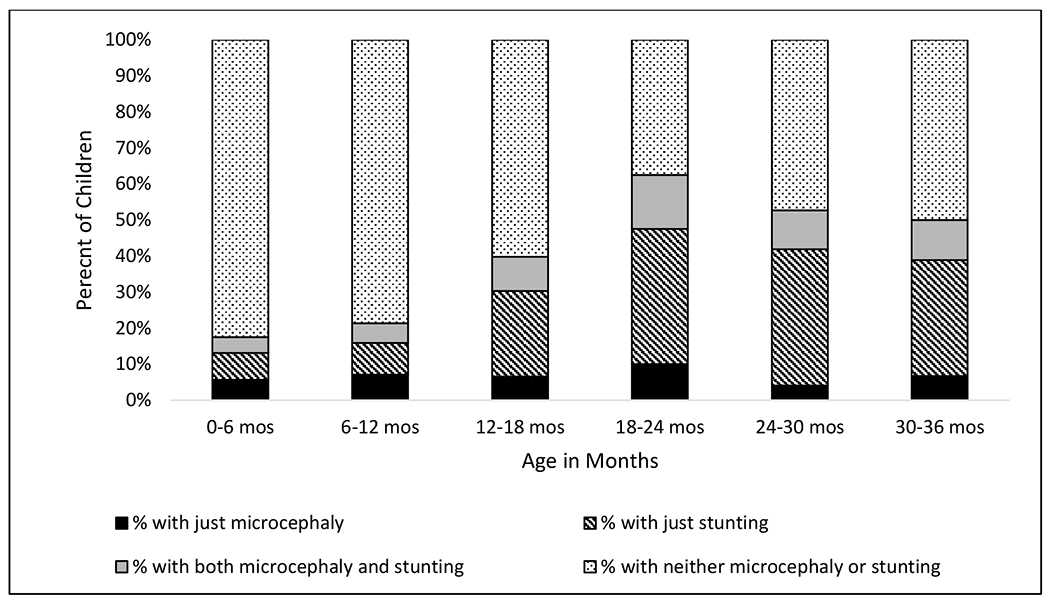
Percentage of Children with Stunting*, Microcephaly*, Both Conditions, and Neither Condition by Age Group in a Cohort of Infants and Young Children in Rural Southwest Guatemala.
*> 2SD below the mean on the WHO growth chart
In concurrent analysis of continuous length or height and stunting with continuous ELC score at most recent study visit, length- or height-for-age was significantly associated with lower ELC score in almost all age groups examined. This association was stronger when length- or height-for-age was analyzed continuously rather than categorizing the child with stunting or not (Table 1). In concurrent analysis of OFC z-score and microcephaly designation with ELC score at most recent study visit, smaller OFC was significantly associated with lower ELC score in most age groups. Again, this association was equally strong or stronger when the OFC data were analyzed continuously rather than categorized by microcephaly status (Table 2). In analyses of concurrent length- or height-for-age and OFC-for-age (independent variables both included in the same model) and ELC score (dependent variable) at most recent visit, neither the trend nor the cutoff point was significantly associated with ELC score for either length- or height-for-age or OFC-for-age. However, the combined measure (length- or height-for-age z-score + OFC-for-age z-score) was significantly associated with ELC score at age 0–18 months and age 30-36 months (Table 3).
Children with lower length- or height-for-age z-score at enrollment had lower MSEL ELC score 11+ months later. This association was seen in both infants age < 6 months at enrollment and children age 24-36 months at enrollment. These associations were stronger when using continuous length- or height-for-age z-score measurements compared to cutoff value for stunting (Table 4). Children with lower OFC-for-age z-score at enrollment had lower MSEL ELC score 11+ months later. This association was seen in both infants age < 6 months at enrollment and children age 24-30 months at enrollment. The associations were stronger with the continuous OFC-for-age z-score compared to microcephaly status established using the z-score cut-off (Table 5). In analysis of length or height and OFC at enrollment (independent variables both included in the same model) and ELC score 11+ months later (dependent variable), there seemed to be no clear pattern of significant association with ELC score for either length or height, or OFC, analyzed continuously or using cutoffs. However, the combined measure (length- or height-for-age z-score + OFC z-score) at enrollment age 0 – 6 months and enrollment age 24-36 months was significantly associated with ELC score at most recent Study visit for the children in those enrollment age groups (Table 6).
In the mixed model analysis of all records collected for which length- or height-for-age, OFC-for-age, and MSEL ELC score were available, lower length- or height-for-age z-scores and smaller OFC-for-age z-scores were significantly associated with lower MSEL ELC scores, both continuously and using WHO z-score cut-offs. Length- or height -for-age tended to have a stronger association with ELC scores than OFC-for-age did when both were included in the model as separate independent variables. Likewise, stunting and microcephaly were both associated with lower MSEL ELC scores. Lower summed length- or height-for-age z-score + OFC-for-age z-score variable was significantly associated with lower MSEL ELC score in both infants and young children (Table 7).
Discussion
In this prospective study of anthropometric and ND data from a cohort of infants and young children living in a LRS in rural Guatemala, the length- or height-for-age and OFC-for-age were predictive of early childhood ND risk both concurrently and one year later. Continuous anthropometric measurements had a stronger association with ND risk than the more traditional cutoff points recommended by WHO (stunting, microcephaly). Head size was not shown to be more robust compared to linear growth in their association with ND risk in these children. Most importantly, the summed length- or height-for-age z-score + OFC-for-age z-score consistently had a stronger association (smaller p-values) with ND outcome than either growth measure alone in concurrent, predictive, and longitudinal models.
In agreement with our findings, recent research has suggested that continuous growth measurements may better predict which children are at ND risk compared to categorized growth measurements (i.e. stunted, microcephalic)10. While z-score cut-offs have clinical and research utility, categorizing a child with a z-score of −1.9 with healthy growth and one with a z-score of −2.1 with adverse growth may be arbitrary and inappropriately reduce concern for a child with a z-score slightly above the cut-off.
Of the few studies that have looked at the association between OFC and ND outcome in LRSs, the results have been equivocal16,26–28 potentially suggesting that, like stunting, the relationship between OFC and ND risk incompletely overlaps. The finding that length or height would prove slightly more strongly associated with ND outcome than OFC was unexpected. It is possible that adverse body growth and adverse head growth occur at different times in childhood due to different challenges and exposures, and thus may have different associations with ND risk across time. The relatively short one year timeline of our study may have complicated our ability to capture such nuances. It is also possible that it was easier to detect changes in ND functioning among children with stunting because the rates of stunting were much higher, rising to almost half of all children in the oldest groups, compared to the rates of microcephaly among children in our study. Lastly, our unequal representation of ages across the 0-3 year early childhood period and the lack of OFC data for children over age 3 years, weakened our ability to compare their associations with ND risk.
The literature suggests that there is an association between linear growth and head size. Several risk factors associated with living in poverty, including malnutrition, enteric infections and other repeated illness, preterm birth, and intrauterine growth restriction, have been associated with lower z-scores on both growth measures29–32. Much is still unknown about this relationship in regards to OFC, as nutritional interventions and growth monitoring programs, which measure linear growth longitudinally, have not routinely included OFC16,28,33,34 likely due to the belief that OFC is spared under conditions of nutritional stress, and possibly due to an aversion to measuring OFC based on historical harmful misinterpretation of such knowledge. However, several studies have supported the interrelationship of linear growth and head size. Sanitation programs and prenatal and early childhood nutritional supplementation interventions have been shown to positively impact both linear growth and head size35–37. Like stunting, rates of microcephaly seem to increase as children age, which may also implicate the adverse cumulative effects of prolonged exposures to infection and undernutrition for children living in poverty on all child growth9,37,38.
Because many studies over the years have linked length or height to ND outcome in LRSs and OFC to ND outcome in high-income countries, we anticipated our very interesting finding that length or height and OFC combined would be more strongly associated with ND risk than either would be alone. Notably, in the literature from HICs, children who have both a small body and small head size are described as having proportionate, or relative microcephaly. It has been suggested that this may confer less ND risk than is present in children who have a small head size in relation to the body (i.e., disproportionate, or absolute microcephaly)40,41. However, common causes of microcephaly may differ between HICs and LMICs, and our data contradict this belief that proportionate microcephaly is less concerning. Instead, our data suggest that a small body and small OFC is indicative of a “double growth challenge” and the child with both conditions is at greater ND risk than if either condition is present alone. Additionally, we found an association between combined length or height and OFC with poor ND performance when examining repeated assessments collected over time. This finding suggests the need to better understand the continuum of adverse growth, define how many total adverse growth measurements would qualify a child as being at ND risk, and specify a timeframe for when intervention should occur. A global growth algorithm should be developed to incorporate growth trajectories of both length or height and OFC against a healthy standard for application in LRSs.
The strengths of our study were the large number of children for whom multiple growth records were available across infancy and early childhood. Performance-based ND assessment administered by highly trained personnel with an established tool validated for use at the study site allowed us to objectively and rigorously measure infant and early childhood ND. Lastly, the location of our study site in Guatemala, which has the highest rate of stunting in Latin America42,43 and where we have also documented high rates of microcephaly44 made this an optimal setting to explore these research questions.
Limitations of the study included uneven distribution of age at enrollment due to the design of the Study, resulting in a much larger group of infants than young children. While children up to age 5 years were enrolled in the Parent Study, we did not collect OFC measurements after 36 months, in line with clinical practice at US well child medical visits. However, because the growth of children living in this LRS may be on a very different trajectory than the growth of children living in more optimal conditions, this lack of OFC collection after 3 years of age may have led us to miss important data points through the preschool years45. In addition, the lack of neurodevelopment measures beyond 3 years of age limits our ability to determine the extent to which early life growth affects neurodevelopment throughout childhood, especially when the child reaches the school years. While gestational age may impact OFC measurements and determination of ‘catch-up’ OFC growth in infants, the Study did not collect or calculate gestational age as these data are only available by caregiver report due to lack of access to prenatal care for the majority of mothers. Lastly, we did not have a “healthy” normative sample for ND testing at the study site. Comparing children within this small community, with many shared ND risk factors, potentially made it difficult to isolate the specific effects of adverse length or height or head circumference growth.
The identification of children at increased ND risk has important implications for the prevention of the loss of human potential in both high and low resource settings. Performance-based assessment is not always feasible, so easily measured proxies are needed for population-based estimations of ND risk. Public health workers, clinicians, and research groups should collect OFC, along with the most commonly used proxy, length or height, to strengthen their ability to identify children most at risk of ND faltering. Based on our findings and comparison of the relative strength of associations between continuous and categorical measures, as well as height and OFC separately vs combined, we recommend utilizing continuous growth measurements, rather than categorizing children as having stunting or microcephaly. Furthermore, the development and validation of a ‘combined’ measure of head size + body size as a more accurate proxy of ND risk in early childhood should be pursued in populations living in LRSs, along with the development of a simple digital tool that clinicians and public health practitioners throughout the world can easily access, understand, and apply this combined measure to determine a child’s neurodevelopmental risk.
Acknowledgements
The authors would like to thank Dr. Walla Dempsey, Dr. Gail Tauscher, Dr. Kay Tomashek, and Dr. Wendy Keitel for their guidance of the parent study as DMID and VTEU project officers and investigators. We thank Paola Arroyave, Sara Hernández, and Alejandra Martínez for their tireless work to collect the performance-based ND measurements in difficult conditions. We also wish to thank the families who participated in this study, and all of the research nurses and personnel from FUNSALUD who have worked on the parent study.
Financial Support
This project has been funded in whole or in part with Federal funds from the National Institute of Allergy and Infectious Diseases (NIAID). Research was supported by a NIAID DMID Vaccine and Treatment Evaluation Unit (VTEU) award to Baylor College of Medicine (Contract No. HHSN27220130015I) and EMMES (Contract No. 75N93021C00012).
Footnotes
Conflicts of Interest
None.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation (Institutional Review Board at Baylor College of Medicine, the Colorado Multiple Institutional Review Board) and with the Helsinki Declaration of 1975, as revised in 2008, and has been approved by the institutional committees, the Institutional Review Board at Baylor College of Medicine, the Colorado Multiple Institutional Review Board, and the Ethics Review Committee of the Ministry of Public Health in Guatemala.
References
- 1.Van Zyl C, Badenhorst M, Hanekom S, Heine M. Unravelling ‘low-resource settings’: A systematic scoping review with qualitative content analysis. BMJ Glob Heal. 2021;6(6). doi: 10.1136/bmjgh-2021-005190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fernald LCH, Prado E, Kariger P, Raikes A. A Toolkit for Measuring Early Childhood Development in Low and Middle-Income Countries. 2017. https://ideas.repec.org/b/wbk/wbpubs/29000.html.
- 3.Villagomez AN, Muñoz FM, Peterson RL, et al. Neurodevelopmental delay: Case definition & guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine. 2019. doi: 10.1016/j.vaccine.2019.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards : Length/Height-for-Age, Weight-for-Age, Weight-for-Length, Weight-Forheight and Body Mass Index-for-Age : Methods and Development.; 2006. doi: 10.4067/S0370-41062009000400012 [DOI] [Google Scholar]
- 5.Prado EL, Abbeddou S, Adu-Afarwuah S, et al. Linear Growth and Child Development in Burkina Faso, Ghana, and Malawi. Pediatrics. 2016;138(2). doi: 10.1542/peds.2015-4698 [DOI] [PubMed] [Google Scholar]
- 6.Black RE, Allen LH, Bhutta ZA, et al. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet. 2008. doi: 10.1016/S0140-6736(07)61690-0 [DOI] [PubMed] [Google Scholar]
- 7.UNICEF. The State of the World’s Children 2016: A fair chance for every child. Publ Home Page. 2016. [Google Scholar]
- 8.Sudfeld CR, Charles McCoy D, Danaei G, et al. Linear Growth and Child Development in Low- and Middle-Income Countries: A Meta-Analysis. Pediatrics. 2015;135(5):e1266–e1275. doi: 10.1542/peds.2014-3111 [DOI] [PubMed] [Google Scholar]
- 9.Meeks Gardner JM, Grantham-McGregor SM, Himes J, Chang S. Behaviour and development of stunted and nonstunted Jamaican children. J Child Psychol Psychiatry Allied Discip. 1999. doi: 10.1017/S0021963099004035 [DOI] [PubMed] [Google Scholar]
- 10.Perumal N, Bassani DG, Roth DE. Use and misuse of stunting as a measure of child health. J Nutr. 2018;148(3). doi: 10.1093/jn/nxx064 [DOI] [PubMed] [Google Scholar]
- 11.Prado EL, Larson LM, Cox K, Bettencourt K, Kubes JN, Shankar AH. Do effects of early life interventions on linear growth correspond to effects on neurobehavioural development? A systematic review and meta-analysis. Lancet Glob Heal. 2019;7(10). doi: 10.1016/S2214-109X(19)30361-4 [DOI] [PubMed] [Google Scholar]
- 12.Leroy JL, Frongillo EA. Perspective: What Does Stunting Really Mean? A Critical Review of the Evidence. Adv Nutr. 2019;10(2). doi: 10.1093/advances/nmy101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization. WHO Child Growth Standards: Head Circumference-for-Age, Arm Circumference-for-Age, Triceps Skinfold-for-Age and Subscapular Skinfold-for-Age: Methods and Development.; 2007. [Google Scholar]
- 14.Connery A, Colbert A, Lamb M. Head circumference may be the best proxy for neurodevelopmental risk in children in low-resource settings. Arch Dis Child. 2022. [DOI] [PubMed] [Google Scholar]
- 15.Gale CR, O’Callaghan FJ, Bredow M, Martyn CN. The influence of head growth in fetal life, infancy, and childhood on intelligence at the ages of 4 and 8 years. Pediatrics. 2006. doi: 10.1542/peds.2005-2629 [DOI] [PubMed] [Google Scholar]
- 16.Ivanovic DM, Leiva BP, Pérez HT, et al. Head size and intelligence, learning, nutritional status and brain development: Head, IQ, learning, nutrition and brain. Neuropsychologia. 2004. doi: 10.1016/j.neuropsychologia.2003.11.022 [DOI] [PubMed] [Google Scholar]
- 17.Reiss AL, Abrams MT, Singer HS, Ross JL, Denckla MB. Brain development, gender and IQ in children. A volumetric imaging study. Brain. 1996;119(5). doi: 10.1093/brain/119.5.1763 [DOI] [PubMed] [Google Scholar]
- 18.Von der Hagen M, Pivarcsi M, Liebe J, et al. Diagnostic approach to microcephaly in childhood: A two-center study and review of the literature. Dev Med Child Neurol. 2014;56(8). doi: 10.1111/dmcn.12425 [DOI] [PubMed] [Google Scholar]
- 19.Olson D, Lamb MM, Lopez MR, et al. A rapid epidemiological tool to measure the burden of norovirus infection and disease in resource-limited settings. Open Forum Infect Dis. 2017;4(2). doi: 10.1093/ofid/ofx049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Asturias EJ, Heinrichs G, Domek G, et al. The Center for Human Development in Guatemala: an innovative model for global population health. Adv Pediatr. 2015;Manuscript. [DOI] [PubMed] [Google Scholar]
- 21.Colbert AM, Lamb MM, Asturias EJ, et al. Reliability and Validity of an Adapted and Translated Version of the Mullen Scales of Early Learning (AT-MSEL) in Rural Guatemala. Child Care Health Dev. 2020. doi: 10.1111/cch.12748 [DOI] [PubMed] [Google Scholar]
- 22.Connery AK, Colbert AM, Lamb MM, et al. Receptive Language Skills Among Young Children in Rural Guatemala: The Relationship Between the Test de Vocabulario en Imagenes Peabody and a Translated and Adapted Version of the Mullen Scales of Early Learning. Child Care Health Dev. July 2019:cch.12702. doi: 10.1111/cch.12702 [DOI] [PubMed] [Google Scholar]
- 23.Connery A, Berrios-Siervo G, Arroyave P, et al. Responding to the Zika Epidemic: Preparation of a Neurodevelopmental Testing Protocol to Evaluate Young Children in Rural Guatemala. Am J Trop Med Hyg. December 2018:tpmd180713. doi: 10.4269/ajtmh.18-0713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mullen EM. Mullen Scales of Early Learning. Bloomington, MN: Pearson [Google Scholar]
- 25. https://www.who.int/childgrowth/software/readme_sas.pdf.
- 26.Connery AK, Lamb MM, Colbert AM, et al. A Prospective Cohort Study of Head Circumference and its Association with Neurodevelopmental Outcomes in Infants and Young Children in Rural Guatemala. J Dev Orig Health Dis. 2022:1–8. [DOI] [PubMed] [Google Scholar]
- 27.Scharf RJ, Rogawski ET, Murray-Kolb LE, et al. Early childhood growth and cognitive outcomes: Findings from the MAL-ED study. Matern Child Nutr. 2018. doi: 10.1111/mcn.12584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sindhu KN, Ramamurthy P, Ramanujam K, et al. Low head circumference during early childhood and its predictors in a semi-urban settlement of Vellore, Southern India. BMC Pediatr. 2019. doi: 10.1186/s12887-019-1553-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prado EL, Dewey KG. Nutrition and brain development in early life. Nutr Rev. 2014;72(4):267–284. doi: 10.1111/nure.12102 [DOI] [PubMed] [Google Scholar]
- 30.Stoch MB, Smythe PM. 15-year developmental study on effects of severe undernutrition during infancy on subsequent physical growth and intellectual functioning. Arch Dis Child. 1976;51(5). doi: 10.1136/adc.51.5.327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Devakumar D, Bamford A, Ferreira MU, et al. Infectious causes of microcephaly: epidemiology, pathogenesis, diagnosis, and management. Lancet Infect Dis. 2018;18(1):e1–e13. doi: 10.1016/s1473-3099(17)30398-5 [DOI] [PubMed] [Google Scholar]
- 32.DeSilva M, Munoz FM, Sell E, et al. Congenital microcephaly: Case definition & guidelines for data collection, analysis, and presentation of safety data after maternal immunisation. Vaccine. 2017;35(48). doi: 10.1016/j.vaccine.2017.01.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grantham-McGregor SM, Fernald LC, Sethuraman K. Effects of health and nutrition on cognitive and behavioural development in children in the first three years of life Part 1: Low birthweight, breastfeeding, and proteinenergy malnutrition. Food Nutr Bull. 1999. doi: 10.1177/156482659902000107 [DOI] [Google Scholar]
- 34.Ivanovic DM, Leiva BP, Pérez HT, et al. Nutritional status, brain development and scholastic achievement of Chilean high-school graduates from high and low intellectual quotient and socio-economic status. Br J Nutr. 2002. doi: 10.1079/bjn2001485 [DOI] [PubMed] [Google Scholar]
- 35.Vaidya A, Saville N, Shrestha BP, de L Costello AM, Manandhar DS, Osrin D. Effects of antenatal multiple micronutrient supplementation on children’s weight and size at 2 years of age in Nepal: follow-up of a double-blind randomised controlled trial. Lancet. 2008;371(9611). doi: 10.1016/S0140-6736(08)60172-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Surkan PJ, Shankar M, Katz J, et al. Beneficial effects of zinc supplementation on head circumference of Nepalese infants and toddlers: A randomized controlled trial. Eur J Clin Nutr. 2012;66(7). doi: 10.1038/ejcn.2012.42 [DOI] [PubMed] [Google Scholar]
- 37.Grembi JA, Lin A, Karim MA, et al. Effect of Water, Sanitation, Handwashing, and Nutrition Interventions on Enteropathogens in Children 14 Months Old: A Cluster-Randomized Controlled Trial in Rural Bangladesh. J Infect Dis. 2020. doi: 10.1093/infdis/jiaa549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Waterlow JC. Classification and Definition of Protein-Calorie Malnutrition. Br Med J. 1972;3(5826). doi: 10.1136/bmj.3.5826.566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Colombo M, de la Parra A, López I. Intellectual and physical outcome of children undernourished in early life is influenced by later environmental conditions. Dev Med Child Neurol. 1992;34(7). doi: 10.1111/j.1469-8749.1992.tb11492.x [DOI] [PubMed] [Google Scholar]
- 40.Shen S, Xiao W, Zhang L, et al. Prevalence and clinical profiles of disproportionate and proportionate microcephaly in China: a population-based cross-sectional study. Lancet. 2018;392. doi: 10.1016/s0140-6736(18)32680-1 [DOI] [Google Scholar]
- 41.Opitz JM, Holt MC. Microcephaly: General considerations and aids to nosology. In: Journal of Craniofacial Genetics and Developmental Biology. Vol 10. ; 1990. [PubMed] [Google Scholar]
- 42.World Health Organization. Countdown to 2015 decade report (2000-2010) with country profiles: taking stock of maternal, newborn and child survival. Matern Newborn Child Surviv Countdown to 2015. 2015. [Google Scholar]
- 43.Gatica-Domínguez G, Victora C, Barros AJD. Ethnic inequalities and trends in stunting prevalence among Guatemalan children: an analysis using national health surveys 1995-2014. Int J Equity Health. 2019;18(1). doi: 10.1186/s12939-019-1016-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rick AM, Domek G, Cunningham M, et al. High background congenital microcephaly in rural Guatemala: Implications for neonatal congenital Zika virus infection screening. Glob Heal Sci Pract. 2017. doi: 10.9745/GHSP-D-17-00116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miller LC, Joshi N, Lohani M, et al. Head growth of undernourished children in rural Nepal: association with demographics, health and diet. Paediatr Int Child Health. 2016. doi: 10.1080/20469047.2015.1133517 [DOI] [PubMed] [Google Scholar]


