Objective:
To determine the association between olfactory function and cognition in patients and rodents.
Background:
Perioperative neurocognitive disorders include delayed neurocognitive recovery (dNCR). The contribution of olfactory function to dNCR remains undetermined. It is unknown whether odor enrichment could mitigate dNCR.
Methods:
We performed a prospective observational cohort study to determine potential association between olfactory impairment and dNCR in patients. We assessed the effects of anesthesia/surgery on olfactory and cognitive function in mice using the block test and Barnes maze. We measured interleukin-6 (IL-6), olfactory mature protein, growth-associated protein 43, mature and premature olfactory neurons, postsynaptic density 95, and synaptophysin in blood, nasal epithelium, and hippocampus of mice. Odor enrichment, IL-6 antibody, and knockout of IL-6 were used in the interaction experiments.
Results:
Patients with dNCR had worse odor identification than the patients without dNCR [preoperative: 7 (1.25, 9) vs 10 (8, 11), median (interquartile range), P<0.001; postoperative: 8 (2.25, 10) vs 10 (8, 11), P<0.001]. Olfactory impairment associated with dNCR in patients before and after adjusting age, sex, education, preoperative mini-mental state examination score, and days of the neuropsychological tests. Anesthesia/surgery induced olfactory and cognitive impairment, increased levels of IL-6 in blood and nasal epithelium, decreased amounts of olfactory receptor neurons and their markers in the nasal epithelium, and reduced amounts of synapse markers in the hippocampus of mice. These changes were attenuated by odor enrichment and IL-6 antibody.
Conclusion:
The anesthesia/surgery-induced olfactory impairment may contribute to dNCR in patients and postoperative cognitive impairment in mice. Odor enrichment could be a potential intervention.
Keywords: anesthesia/surgery, cognition, interleukin-6, odor enrichment, olfactory function
Perioperative neurocognitive disorder (PND), one of the most common postoperative complications in older adults, includes postoperative delirium, delayed neurocognitive recovery (dNCR), mild neurocognitive disorders, and major neurocognitive disorders.1 Among them, dNCR occurs about 1 to 4 weeks after anesthesia/surgery.1 We determined dNCR by assessing cognitive function in the participants about 1 week after the anesthesia/surgery in patients. We targeted dNCR in animal studies by evaluating the effects of anesthesia/surgery on cognitive function at postoperative 7 to 11 days.
Olfactory impairment contributes to cognitive impairment in humans,2,3 occurs following anesthesia/surgery (reviewed in Elterman et al4), and is associated with Alzheimer’s disease (AD) neuropathogenesis (reviewed in5) and long-term cognitive impairment in Parkinson’s disease patients.6 Better olfactory function is associated with better cognitive function in humans7 and AD transgenic mice.8 Finally, odor enrichment may improve olfactory function in rodents.9,10
Mature olfactory receptor neurons in the olfactory epithelium send projections to the olfactory bulb, communicating with higher brain regions, including the cortex, amygdala (reviewed in11), and potentially hippocampus (reviewed in12). Olfactory marker protein (OMP)13 and growth-associated protein 43 (GAP43)14,15 are markers of mature and premature olfactory receptor neurons, respectively. Anesthesia/surgery reduces amounts of synaptophysin, a presynaptic marker, and postsynaptic density 95 (PSD95), an excitatory postsynaptic marker, and induces cognitive impairment in mice.16
However, no studies have determined whether there is an association between olfactory function and dNCR in patients or postoperative cognitive impairment in rodents and whether odor enrichment, a nonpharmacological approach, mitigates postoperative cognitive impairment. We, therefore, performed both clinical and animal studies to test a hypothesis that olfactory function is associated with dNCR in patients and postoperative cognitive impairment in mice, and odor enrichment mitigates postoperative cognitive impairment in mice.
Interleukin-6 (IL-6), a proinflammatory cytokine that can be released from muscle and brown adipocytes,17 contributes to cognitive impairment (reviewed in18) and is associated with olfactory impairment.19 IL-6 has been shown to contribute to postoperative cognitive impairment in patients20–25 and rodents.26–30 However, whether anesthesia/surgery could induce olfactory impairment by increasing IL-6 amounts in blood and nasal olfactory epithelium, leading to cognitive impairment, has not been studied. Thus, although there could be many mechanisms of postoperative cognitive impairment, the mechanistic hypothesis in the present study was that anesthesia/surgery induces elevation of IL-6 levels in the blood and olfactory epithelium of mice, which reduces numbers of olfactory receptor neurons and causes olfactory impairment, leading to reduced hippocampus synapses and cognitive impairment in mice.
METHODS
Clinical Investigation
Study Design
We performed a prospective observational study at the Affiliated Hospital of Xuzhou Medical University, Xuzhou, China, from December 2016 to July 2017. The study (XYFY2016-KL018-01) was approved by the Clinical Research Ethics Committee of the Hospital and registered at ClinicalTrial.gov (NCT02992600). Written informed consent was obtained from all participants. We measured the incidence of dNCR, olfactory identification (OI), and olfactory threshold (OT) in participants. There were no protocol deviations, missing data, and immediate postoperative adverse events in the study. This manuscript was written according to the applicable STROBE guideline.
Subject Enrollment
Please see supplemental information (Supplemental Digital Content 1, http://links.lww.com/SLA/E49) for the details.
Neuropsychological Tests
The neuropsychological tests included Short Story Module of the Randy Memory Test, Verbal Fluency Test, Trail Making Test Parts A, Digit-Symbol Substitution Test, Digit Span Subtest of Wechsler Adult Intelligence Scale-Revised (WAIS), Finger Tapping Test, Grooved Pegboard Test (dominant and nondominant), and Block Test as performed in a previous study31 with modifications. The participants received the neuropsychological tests 1 day before and then 5 to 10 days (average of 7 days) after the anesthesia/surgery when they were ready to receive it. Researchers were trained to perform the tests using established protocols. These tests were only performed in the hospital. Participants were defined as having dNCR when their Z-scores were negative with absolute values ≥1.96 on at least 2 different tests among 10 neuropsychological tests.31
Olfactory Function Test
Olfactory function was assessed 1 day before and then 5 to 10 days (average of 7 days) after the anesthesia/surgery at the same time as the neuropsychological tests by using odor threshold (score: 1–16) and odor identification (score: 0–16) tests developed by Sniffin’ Sticks (Burghardt, Wedel, Germany), which uses nasal chemosensory performance based on pen-like odor dispensing devices.32
Anesthesia/Surgery
Surgeries included urological surgery (N=88), general surgery (N=67), orthopedic surgery (N=49), and thoracic surgery (N=2). The anesthesia care was following American Society of Anesthesiology guidelines, hospital policy, and at the discretion of anesthesiologists.
Control Group
We recruited 30 nonanesthesia/surgery volunteers with the same criteria. They received the same neuropsychological tests at the same time intervals, i.e., there were 5 to 10 days between the first and second neuropsychological tests in these volunteers.
Animal Studies
Mice and Treatment
The animal protocol was approved by the Massachusetts General Hospital (Boston, MA) Standing Committee on the Use of Animals in Research and Teaching (Protocol number: 2006N000219). Efforts were made to minimize the number of animals used. C57BL/6J mice (4-month-old female, Charles River Laboratories, Wilmington, MA) and 4-month-old IL-6 knockout (B6.129S1-Il6 tm1Kopf, stock number: 002650, Jackson Lab, Bar Harbor, ME) mice were used in the present study and were housed in a controlled environment (20–22°C; 12 hours of light/dark on a reversed light cycle) with free access to food and water for 7 days before the experiments. In the interventional studies, the IL-6 antibody (Catalog number: MAB206; R&D Systems, Minneapolis, MN) was administrated as described previously33 and per the manufacturer’s protocol. Specifically, each mouse received 1500 ng IL-6 antibody 18 hours before the anesthesia/surgery using tail vein injection under brief anesthesia (1.4% isoflurane for 3 minutes). Please see supplemental information (Supplemental Digital Content 1, http://links.lww.com/SLA/E49) for details.
Anesthesia/Surgery in Mice. Mice were randomly assigned to anesthesia/surgery or control group. We studied the effects of anesthesia/surgery (laparotomy under 1.4% isoflurane) on cognitive function using our established animal model34 (Supplemental Figure 1, Supplemental Digital Content 1, http://links.lww.com/SLA/E49). We did not determine how long the anesthesia/surgery-induced cognitive impairment could last because the time-course study of postoperative cognitive impairment was not the objective of the present study and will be investigated in the future.
Odor Enrichment. Odor enrichment was performed in mice for 21 days (starting 22 days before the anesthesia/surgery) as described in a previous study.9
Olfaction Test (Block Test). The olfaction block test was performed 1 day before anesthesia/surgery (-D1) and then on day 1 (D1), 2 (D2), 3 (D3), and 11 (D11; using a different group of mice) after anesthesia/surgery as described previously.35 Sniffing time was defined as the percentage of the mouse sniffed the novel block to the time the mouse sniffed all blocks. Note that the novel block contains the scent of a different mouse chosen from a different cage. Olfactory impairment was defined when the mouse spent reduced time sniffing novel block.36
Barnes Maze. The Barnes maze test was performed 7 to 11 days after the anesthesia/surgery using the methods described before.34
Open-field Test. The open-field test was performed as described before.34
Nasal Epithelium and Brain Tissue Samples. Different mice were used to harvest nasal and brain tissues to measure OMP and GAP43 (nasal epithelium) and PSD95 and synaptophysin amounts (hippocampus). Mouse nasal epithelium was dissected 12 hours after the anesthesia/surgery.37 Mouse hippocampus was dissected 11 days after anesthesia/surgery.34 We chose 11 days to determine the effects of anesthesia/surgery on PSD95 and synaptophysin amounts in mouse hippocampus because we would evaluate the impact of anesthesia/surgery on cognitive function in mice at 11 days postoperatively. Each mouse was anesthetized with 1.4% isoflurane for 2 minutes and then decapitated, and tissues were immediately harvested. Harvested nasal epithelium37 and hippocampus34 were processed using the method described before.
Western Blots. We performed the western blot as described before34 using the following antibodies: anti-PSD95 (1:1000; molecular weight: 95 kDa; Cell Signaling, Danvers, MA), anti-synaptophysin (1:1000; molecular weight: 38 kDa; Cell Signaling), anti-OMP (1:1000; molecular weight: 19 kDa; Sigma, St Louis, MO), anti-GAP43 (1:1000, molecular weight: 43 kDa, Abcam, Cambridge, MA), anti-GAPDH (1:2500, molecular weight: 37 kDa, Abcam), and anti-β-actin (1:1000; molecular weight: 42 kDa; nontargeted protein control; Sigma).
IL-6 Enzyme-linked Immunosorbent Assay. The mouse IL-6 immunoassay kit (catalog number: M6000B; R&D Systems) was used to determine IL-6 amounts in mouse blood per the manufacturer’s protocol.
Immunohistochemistry. Immunohistochemistry was carried out using methods described previously.15 Mice were anesthetized with 1.4% isoflurane for 3 minutes and perfused transcardially with phosphate buffer saline, followed by 4% cold, buffered paraformaldehyde. The mouse nasal epithelium was then dissected. OMP antibody (ab183947, 1:500 dilution, Abcam) or GAP43 (ab16053, 1:200 dilution) was used. During image acquisition, the exposure time was kept consistent. The number of OMP-positive and GAP43-positive neurons was assessed under the same ×40 microscope field (3 fields per slide, and all of the cells in the field were counted) in a double-blind manner.
Statistical Analysis
The normality of the continuous variables was tested using the Shapiro-Wilk test. Normally distributed continuous variables are presented as a mean and SD; and were compared using Student t test. Nonnormally distributed continuous variables are presented as median and interquartile ranges; and were compared using Mann-Whitney U test. Categorical variables are presented as count and percentage of the total; and were compared using the χ2 test or Fisher’s exact test, as appropriate.
On the basis of the multiple predictor variables (eg, age, sex, and education levels) and given the incidence of dNCR is 20%,31 we should at least recruit 200 (4×10/0.2) patients.
Logistic regression models were used to assess the associations between baseline OI or OT score, postoperative OI or postoperative OT score and the incidence of dNCR. Multiple variables logistic regressions were used to assess the associations after adjusting age, sex, education level, the preoperative cognition mini-mental state examination (MMSE) score, and the postoperative days of the neuropsychological tests. Odds ratios and their 95% confidence intervals were reported.
The number of mice was 10 per group in behavioral studies and 6–10 per group in biochemistry studies, per results from our pilot and previous studies. We used a 2-way analysis of variance (repeated measurements) followed by the Bonferroni test to evaluate the interaction of treatment (control and anesthesia/surgery) and days 7–10 on the latency for the mouse to identify and enter the escape box in Barnes maze test. We used Student t test to determine the potential difference in the measurements of the Block test, Open-field test, and biochemistry studies between the control condition and the anesthesia/surgery. We used the Mann-Whitney U test to determine the potential difference in the measurements of the Barnes maze test at 11 days after the anesthesia/surgery between the control condition and the anesthesia/surgery. The nature of the hypothesis testing was 2-tailed. P value <0.05 was considered statistically significant. Statistical analysis was conducted using GraphPad Prism software (version 8.0) (SPSS version 25, SPSS Inc, Chicago, IL) and R for Windows, version 4.1.2.
RESULTS
Olfactory Function was Associated With dNCR in Patients
We assessed 3574 participants for eligibility; 2822 participants did not meet the inclusion criteria. Among 752 participants screened, 452 were excluded, leading to the enrollment of 300 participants. We further excluded 94 participants due to refusing or being unable to complete the postoperative olfactory or neuropsychological tests (N=69) and earlier discharge (N=25), leading to 206 participants in the final data analysis (Fig. 1). There were no significant differences in demographic characteristics between the participants with dNCR (N=42) and the participants without dNCR (N=164) except for age and preoperative MMSE scores (Table 1). The participants with dNCR had lower OI or OT than those without dNCR (Table 1). Logistic regression demonstrated the associations between baseline or postoperative follow-up OT or OI and the incidence of dNCR in the participants before and after adjusting age, sex, education, preoperative MMSE score, and days of postoperative neuropsychological tests (Table 2).
FIGURE 1.
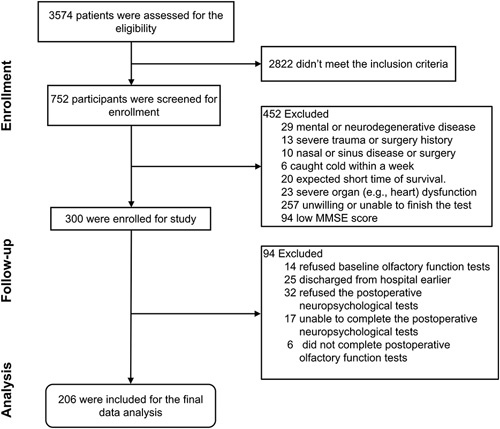
Flow diagram for the study to assess the association of olfactory and cognitive impairment in participants from Xuzhou Medical University. We initially evaluated 3574 participants for eligibility; 752 were screened for the study, and 206 were included in the data analysis for baseline and postoperative tests.
TABLE 1.
Demographic Characteristics of Participants
| Participants (N=206) | |||
|---|---|---|---|
| Characteristics | dNCR (N=42) | Non-dNCR (N=164) | P |
| Age, M (IQR) (y) | 69.00 (66.00, 77.00) | 67.00 (63.00, 72.00) | 0.028 |
| Male, n (%) | 28 (0.67) | 112 (0.68) | 0.987 |
| Education, M (IQR) (y) | 9.00 (6.00, 9.00) | 9.00 (6.00, 10.50) | 0.266 |
| BMI, M (IQR), (kg/m2) | 24.22 (21.80, 26.46) | 24.16 (22.10, 26.57) | 0.756 |
| Follow-up, M (IQR), (d) | 7.00 (6.25, 9.00) | 7.00 (6.00, 8.00) | 0.294 |
| Preoperative MMSE, M (IQR), score | 26.50 (24.00, 28.00) | 27.00 (25.00, 29.00) | 0.040 |
| Stroke, n (%) | 6 (0.14) | 16 (0.09) | 0.570 |
| Hypertension, n (%) | 18 (0.43) | 70 (0.43) | 1 |
| Diabetes mellitus, n (%) | 7 (0.17) | 19 (0.12) | 0.532 |
| Coronary disease, n (%) | 2 (0.05) | 23 (0.14) | 0.169 |
| Alcohol problem, n (%) | 5 (0.12) | 20 (0.12) | 1 |
| Smoking, n (%) | 8 (0.19) | 35 (0.21) | 0.910 |
| Length of anesthesia, M (IQR) (min) | 185.00 (160.00, 250.00) | 190.00 (140.00, 245.00) | 0.451 |
| Length of surgery, M (IQR) (min) | 150.00 (130.00, 223.75) | 160.00 (118.75, 210.00) | 0.532 |
| OI at baseline (M, IQR), score | 7.00 (1.25, 9.00) | 10.00 (8.00, 11.00) | <0.001 |
| OI at 1 wk (M, IQR), score | 8.00 (2.25, 10.00) | 10.00 (8.00, 11.00) | <0.001 |
| OT at baseline (M, IQR), score | 6.00 (3.25, 8.00) | 7.00 (5.00, 9.00) | 0.015 |
| OT at 1 wk (M, IQR), score | 5.00 (3.00, 6.75) | 6.00 (4.00, 9.00) | 0.001 |
Mann-Whitney U test was used to compare the nonnormally distributed continuous variables and the Pearson χ2 test was used to compare categorical variables.
P values refer the difference between dNCR cohort and non-dNCR cohort.
BMI indicates body mass index; IQR, interquartile range, 25%–75%; M, median.
TABLE 2.
Olfactory Function is Associated With Delayed Neurocognitive Recovery in Patients
| Baseline (N=206) | ||||
|---|---|---|---|---|
| Unadjusted | Adjusted for Age, Sex, Education, Preoperative MMSE Score, and Days of Postoperative Neuropsychological Tests | |||
| Smell Test Score | Odds Ratio (95% CI) | P | Odds Ratio (95% CI) | P |
| OI | 0.80 (0.73, 0.88) | <0.001 | 0.79 (0.72, 0.88) | <0.001 |
| OT | 0.89 (0.80, 0.98) | 0.021 | 0.87 (0.78, 0.98) | 0.016 |
| Postoperative follow-up (N=206) | ||||
| Smell test score | ||||
| OI | 0.81 (0.74, 0.89) | <0.001 | 0.81 (0.73, 0.90) | <0.001 |
| OT | 0.82 (0.73, 0.93) | 0.002 | 0.82 (0.72, 0.93) | 0.003 |
Baseline: 1 day before the anesthesia/surgery.
Postoperative follow-up: about 1 week after the anesthesia/surgery.
This table shows the results of logistic regression analysis before and after the adjustment of age, sex, education, preoperative MMSE score and days of postoperative neuropsychological tests.
Anesthesia/Surgery-induced Olfactory and Cognitive Impairment in Mice
In the olfaction test, mice with anesthesia/surgery spent less time sniffing a block with scent (novel block) compared with the control mice on days 1, 2, and 3 (Figs. 2A–C) but not on day 11 (Supplemental Figure 2, Supplemental Digital Content 1, http://links.lww.com/SLA/E49) after the anesthesia/surgery.
FIGURE 2.
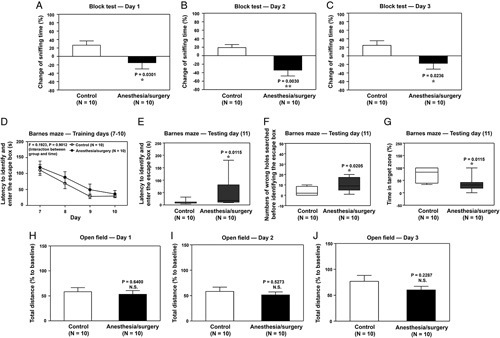
Anesthesia/surgery impairs olfactory and cognitive function in mice. Effects of anesthesia/surgery on olfactory function detected by block tests 1 (A), 2 (B), and 3 (C) days after the anesthesia/surgery. (D) Effects of anesthesia/surgery on latency to identify and enter the escape box on Barnes maze training days 7–10 days after anesthesia/surgery. Effects of anesthesia/surgery on latency to identify and enter the escape box (E), numbers of wrong holes searched before identifying the escape box (F), and time in the target zone (G) on Barnes maze testing day (day 11 after anesthesia/surgery). Effects of anesthesia/surgery on total distance in the open-field test 1 (H), 2 (I), and 3 (J) days after anesthesia/surgery. ANOVA, analysis of variance; SEM, standard error of the mean. N=10/group. Data are presented as means±SEM. The Student t test was used to analyze the data in A, B, C, H, I, and J; P values refer to the difference in sniffing time or total distance between the control condition and anesthesia/surgery. Data are presented as means±SEM, and 2-way ANOVA and post-hoc analysis with Bonferroni was used to analyze the data presented in D; P value refers to the interaction of treatment (control vs anesthesia/surgery) and time (days 7–10) on latency to identify and enter the escape box. Data are presented as the median and interquartile range (25%–75%). Mann-Whitney U test was used to analyze the data in panels E, F, and G; P values refer to the difference in behavioral changes between control and anesthesia/surgery groups. *P value <0.05; **P value <0.01.
There was no significant interaction of treatment (control vs anesthesia/surgery) and day (day 7–10) on latency for mice to identify and enter the escape box on training days for the Barnes maze (Fig. 2D). However, on the testing day of the Barnes maze (11 days after anesthesia/surgery), the anesthesia/surgery significantly increased the latency of identifying and entering the escape box (Fig. 2E), increased the number of wrong holes searched before identifying and entering the escape box (Fig. 2F) and decreased time spent in the target zone (containing escape box) of Barnes maze (Fig. 2G) compared with control condition in mice. Further, anesthesia/surgery did not significantly change mice’s moved distance in an open-field test on days 1, 2, and 3 (Figs. 2H–J) after the anesthesia/surgery compared with the control condition.
Anesthesia/Surgery Reduced OMP and GAP43 Amounts in the Olfactory Epithelium of the Nasal Cavity and PSD95 and Synaptophysin Amounts in the Hippocampus of Mice
Western blots and immunohistochemistry imaging showed that the anesthesia/surgery decreased OMP (Figs. 3A, B) and GAP43 amounts (Figs. 3E, F), the thickness of the OMP-positive cell layer (Figs. 3C, D), and expressions of GAP43-positive cells (Figs. 3G, H) in the olfactory epithelium of mouse nasal cavity compared with control condition at 12 hours after the anesthesia/surgery. Consistent with the behavioral changes, the anesthesia/surgery decreased amounts of PSD95 (Figs. 3I, J) and synaptophysin (Figs. 3K, L) in mice’s hippocampus at 11 days after the anesthesia/surgery compared with the control condition.
FIGURE 3.
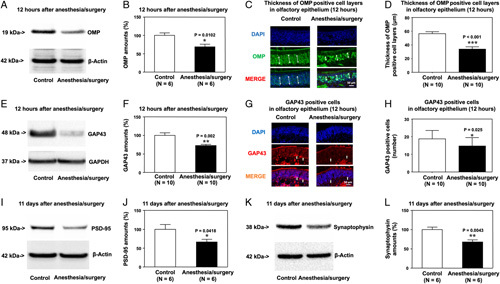
Anesthesia/surgery decreases OMP and GAP43 amounts in the olfactory epithelium and amounts of PSD95 and synaptophysin in the hippocampus of mice. Effects of anesthesia/surgery on OMP amounts detected by western blot (A, B) and expression of OMP-positive cells detected by immunohistochemistry (C, D) in the olfactory epithelium of the nasal cavity of mice 12 hours after anesthesia/surgery. Effects of anesthesia/surgery on GAP43 amounts detected by western blot (E, F) and expression of GAP43-positive cells detected by immunohistochemistry (G, H) in the olfactory epithelium of the nasal cavity of mice 12 hours after the anesthesia/surgery. Effects of anesthesia/surgery on amounts of PSD95 (I, J) and synaptophysin (K, L) in the hippocampus of mice 11 days after the anesthesia/surgery. SEM, standard error of the mean. N = 6–10/group. Data are presented as means±SEM, and Student t test was used to analyze the data in panels B, D, F, H, J, and L; P values refer to the difference in protein amounts or number of cells between control and anesthesia/surgery groups. *P value < 0.05; **P value < 0.01, ***P value < 0.001.
Odor Enrichment Mitigated the Anesthesia/Surgery-induced Behavioral and Cellular Changes in Mice
Mice exposed to anesthesia/surgery plus 21-day odor enrichment (Supplemental Table 1, Supplemental Digital Content 1, http://links.lww.com/SLA/E49) did not show impairment of olfaction (Supplemental Figure 3a–c, Supplemental Digital Content 1, http://links.lww.com/SLA/E49), cognition (Supplemental Figure 3d–g, Supplemental Digital Content 1, http://links.lww.com/SLA/E49), or locomotion (Supplemental Figure 3h–j, Supplemental Digital Content 1, http://links.lww.com/SLA/E49) compared with control condition plus 21-day odor enrichment.
Consistently, anesthesia/surgery did not significantly decrease amounts of OMP and GAP43, numbers of OMP-positive or GAP43-positive cells in the olfactory epithelium of the nasal cavity, and PSD95 and synaptophysin amount in the hippocampus as compared with the control condition in the mice that received odor enrichment for 21 days (Figs. 4A–J).
FIGURE 4.
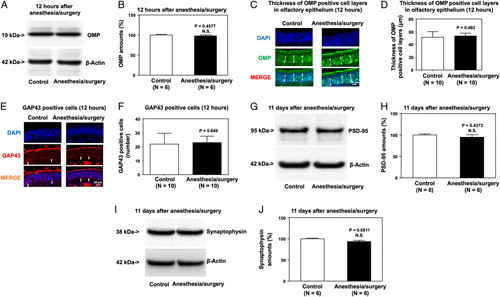
Odor enrichment mitigates the anesthesia/surgery-induced reductions in OMP and GAP43 amounts in the olfactory epithelium and decreases in PSD95 and synaptophysin amounts in the hippocampus of mice. Effects of anesthesia/surgery plus odor enrichment on amounts of OMP detected by western blot (A, B) and expression of OMP-positive cells detected by immunohistochemistry (C, D) olfactory epithelium of the nasal cavity of mice at 12 hours after the anesthesia/surgery. Effects of anesthesia/surgery plus odor enrichment on the expression of GAP43-positive cells detected by immunohistochemistry (E, F) in the olfactory epithelium of the nasal cavity of mice 12 hours after the anesthesia/surgery. Effects of anesthesia/surgery plus odor enrichment on amounts of PSD95 (G, H) and synaptophysin (I, J) in the hippocampus of mice at 11 days after the anesthesia/surgery. SEM, standard error of mean. Data are presented as means±SEM, and Student test was used to analyze the data in panels B, D, F, H, and J; P values indicate the difference in protein amounts between control and anesthesia/surgery plus odor enrichment groups.
IL-6 Antibody Attenuated the Anesthesia/Surgery-induced Behavioral and Cellular Changes in Mice
Two-way analysis of variance showed a significant interaction of group (control vs anesthesia/surgery) and treatment (saline vs IL-6 antibody) on blood IL-6 amounts 6 hours after anesthesia/surgery (Fig. 5A). Specifically, anesthesia/surgery significantly increased blood IL-6 amounts in mice, and treatment with IL-6 antibody attenuated this elevation.
FIGURE 5.
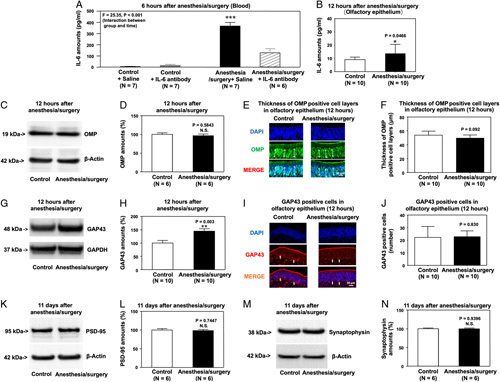
IL-6 antibody treatment attenuates the anesthesia/surgery-induced increases in blood IL-6 amounts, reductions in OMP and GAP43 amounts in the olfactory epithelium, and reductions in PSD95 and synaptophysin amounts in the hippocampus of mice. (A) IL-6 antibody attenuates the anesthesia/surgery-induced increases in blood IL-6 amounts of mice. (B) Effects of anesthesia/surgery on IL-6 amounts in olfactory epithelium determined by ELISA. Effects of anesthesia/surgery plus IL-6 antibody treatment on OMP amounts detected by western blot (C, D) and expression of OMP-positive cells detected by immunohistochemistry (E, F) in the olfactory epithelium of the nasal cavity of mice at 12 hours after the anesthesia/surgery. Effects of anesthesia/surgery plus IL-6 antibody on amounts of GAP43 detected by western blot (G, H) and expression of GAP43-positive cells detected by immunohistochemistry (I, J) in the olfactory epithelium of the nasal cavity of mice at 12 hours after anesthesia/surgery. Effects of anesthesia/surgery plus IL-6 antibody treatment on amounts of PSD95 (K, L) and synaptophysin (M, N) in the hippocampus of mice at 11 days after the anesthesia/surgery. N = 6–10/group. Data are presented as means±SEM. Two-way ANOVA was used to analyze the data in panel A; P value refers to the interaction of group (control vs anesthesia/surgery) and treatment (saline vs IL-6 antibody). Data are presented as means±SEM, and Student t test was used to analyze the data in panels B, D, F, H, J, L, and N; P values refer to the difference in protein amounts between control and anesthesia/surgery plus IL-6 antibody groups. ANOVA indicates analysis of variance; ELISA, enzyme-linked immunosorbent assay; IL-6, interleukin-6; SEM, standard error of mean. *P value <0.05; **P value <0.01.
The anesthesia/surgery significantly increased IL-6 amounts in the olfactory epithelium of mice’s nasal cavity 12 hours after the anesthesia/surgery (Fig. 5B). IL-6 antibody treatment also attenuated the anesthesia/surgery-induced reduction in OMP, GAP43, PSD95, synaptophysin amounts (Figs. 5C–N), and the anesthesia/surgery-induced olfactory and cognitive impairment (Supplemental Figure 4, Supplemental Digital Content 1, http://links.lww.com/SLA/E49). The anesthesia/surgery did not decrease OMP and GAP43 amounts compared with controls in the IL-6 knockout mice pretreated (Supplemental Figure 5, Supplemental Digital Content 1, http://links.lww.com/SLA/E49).
DISCUSSION
This study in animals and humans demonstrated that olfactory function was associated with dNCR in patients and postoperative cognitive impairment in mice. Moreover, odor enrichment mitigated the anesthesia/surgery-induced olfactory and cognitive impairment in mice. These results highlight the need for more clinical studies to assess whether odor enrichment can reduce the incidence and severity of PND in patients.
The data showed that patients who developed dNCR had a worse olfactory function at baseline and 1 week after anesthesia/surgery than patients who did not develop dNCR, suggesting an association between olfactory impairment and dNCR in patients. Further studies in mice showed olfactory impairment up to 3 days after anesthesia/surgery and cognitive impairment at 11 days after anesthesia/surgery, potentially mediated by increased IL-6 amounts in blood and olfactory epithelium of the nasal cavity, decreased OMP and GAP43 amounts in the olfactory epithelium of nasal cavity, and reduced PSD95 and synaptophysin amounts in the hippocampus of mice.
The mice in the olfactory function test on day 11 were different from those on the Barnes Maze test on day 11. Anesthesia/surgery might initially impair olfactory function by decreasing numbers of olfactory receptor neurons (as evidenced by reduced OMP and GAP43 amounts) 12 hours after the anesthesia/surgery, which leads to cognitive impairment by reducing synaptic markers in the hippocampus 11 days after the anesthesia/surgery. We do not know why there was a time gap between the onset of postoperative olfactory impairment and the onset of postoperative cognitive impairment at present. There could be other changes between postoperative olfactory impairment at 12 hours (and the associated reductions in OMP and GAP43 amounts) and cognitive impairment at 11 days (and the associated decreases in synaptic markers). Future studies are needed to reveal such changes.
Odor enrichment and treatment with IL-6 antibody rescued anesthesia/surgery-induced olfactory and cognitive impairment and the associated changes in IL-6, OMP, GAP43, PSD95, and synaptophysin amounts in mice. These data further suggest the contribution of olfactory impairment to cognitive impairment and reveal, at least partially, the underlying mechanism by which anesthesia/surgery induces olfactory and cognitive impairment in mice.
IL-6 is a proinflammatory cytokine and may contribute to cognitive impairment.18 IL-6 can be released from muscle and brown adipocytes17 and is associated with olfactory impairment.19 Our results suggest that anesthesia/surgery can increase IL-6 amounts in blood and olfactory epithelium, reducing the amounts of olfactory receptor neurons in the olfactory epithelium in the nasal cavity, and leading to olfactory impairment. The olfactory impairment then causes synaptic loss in the hippocampus, leading to cognitive impairment.
AD neuropathogenesis (eg, neurofibrillary tangles) appears in brain regions, including the olfactory bulb, prepiriform cortex, entorhinal cortex, and amygdala, which are involved in olfactory processing (reviewed in5). However, no studies have shown changes in olfactory receptor neurons in the olfactory epithelium of the nasal cavity, the peripheral nerve system, after anesthesia/surgery. Here, we demonstrated the IL-6-associated reductions in expression of mature and premature olfactory receptor neurons in mice after anesthesia/surgery and that odor enrichment, a nonpharmacological approach, attenuated these changes.
Odorants interact with olfactory receptors located on the surface of olfactory sensory neurons in the olfactory epithelium of the nasal cavity. Activated olfactory receptors initiate signal transduction by increasing the generation of cAMP, which generates action potentials to convey odor information to the olfactory bulb.38 OMP can promote signal transduction by the increasing generation of cAMP,39 and knockout of OMP leads to deficits in detecting and discriminating odorants.40,41 The present study demonstrated that anesthesia/surgery decreased OMP and OMP-positive neurons, part of the peripheral nerve system, in the olfactory epithelium of mice’s nasal cavity. These data suggest that anesthesia/surgery can cause olfactory impairment by decreasing OMP and olfactory sensory neurons. Anesthesia/surgery also reduced amounts of GAP43, a marker of premature olfactory sensory neurons.14,15 Thus, it is conceivable that anesthesia/surgery could reduce olfactory sensory neurons by regulating premature olfactory sensory neurons. Finally, anesthesia/surgery also decreased synaptophysin, a presynaptic marker,42,43 and PSD95, a postsynaptic marker.44 Taken together, findings from the current proof-of-concept and intervention study suggest a potential signaling pathway in the blood (IL-6), the olfactory epithelium (IL-6), premature olfactory sensory neurons (GAP43), olfactory sensory neurons (OMP), and hippocampus (synaptophysin and PSD95) (Supplemental Figure 6, Supplemental Digital Content 1, http://links.lww.com/SLA/E49). Future mechanistic investigations are needed to confirm and dissect the pathway.
The current study has limitations. First, the sample size of the human study was small and relatively lacking in diversity. We recruited participants from only 1 large hospital, anesthesia depth was not monitored in the participants, and some important perioperative information (eg, amounts of blood loss) was missing. Future systematic studies should confirm our findings in more extensive and diverse samples. Second, we used 4-month-old mice rather than aged mice, and dNCR occurs more frequently in older adults. However, this study has established a system for future research that can compare the effects of anesthesia/surgery on olfactory and cognitive function between adult and aged mice. Third, the study demonstrated that anesthesia/surgery decreased the markers of olfactory neurons at 12 hours, induced olfactory impairment for up to 3 days, and caused cognitive impairment on day 11 after the anesthesia/surgery compared with the control condition. Notably, assessing these changes at different times was a limitation of the present study.
In conclusion, these clinical and preclinical studies suggest that anesthesia/surgery-induced olfactory impairment could contribute, at least partially, to postoperative cognitive impairment. We demonstrated an IL-6-associated mechanism by which an external stressor (anesthesia/surgery) induced olfactory and cognitive impairment through the interaction of the peripheral nerve system (olfactory receptor neurons) and the central nervous system (synapses in the hippocampus). Moreover, odor enrichment could be a potential intervention for dNCR by preventing or treating anesthesia/surgery-induced olfactory impairment. These findings should promote future preclinical and clinical investigation to reveal underlying mechanisms and interventions, including nonpharmacological approaches, for dNCR and other types of PND.
Supplementary Material
Footnotes
Present address: Deborah J. Culley, MD, Department of Anesthesiology and Critical Care, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA.
C.Z., Y.H., and X.L. contributed equally to the study.
Y.S., J.-L.C., and Z.X. jointly supervised the study.
Study concept and design: Z.X., Y.S. J.-L.C., E.R.M., G.C., D.J.C., and Y.Z. Acquisition of data: C.Z., Y.H., X.L., H.T., Y.D., F.L. Analysis and interpretation of data: C.Z., Y.H., X.L., H.Z., E.R.M., and Z.X. Drafting of the manuscript: Z.X., Y.D., and Y.H. Critical revision of the manuscript for important intellectual content: Z.X. Obtained funding: Z.X., Y.S., J.-L.C., E.R.M., and G.C. Administrative, technical, and material support: Z.X., Y.S., and J.-L.C. Study supervision: Z.X., Y.S., and J.-L.C.
Z.X. provided consulting services to Shanghai 9th and 10th hospital and Baxter (invited speaker) in the last 36 months. The remaining authors report no conflicts of interest.
This work was supported by the National Institutes of Health (R01AG041274 and RF1AG070761 to Z.X.; P01AG031720, R01AG030618, K24AG035075, R01AG051658, to E.M.; and R21AG048637 and R01AG051812, to G.C.); the National Natural Science Foundation of China (81720108012 and to Y.S.) and Ministry of Science and Technology of China (2021ZD0202003 to Y.S.); the Sci-Tech Innovation 2030 Agenda (2021ZD0203100 to JLC) and the National Natural Science Foundation of China (81720108013 and 82130033 to J.C.).
The sponsors have no involvement in study design, data collection and interpretation, writing of the manuscript, and the decision to submit the manuscript for publication. Notably, there are 2 parts of independent studies in this manuscript. The animal studies were performed at Massachusetts General Hospital and Harvard University. The clinical investigation to study the association between olfactory function and perioperative neurocognitive disorder was performed at Xuzhou Medical University. There were no collaborations between the 2 institutes. We only merged the data in this manuscript to demonstrate the effects of anesthesia/surgery and odor enrichment on olfactory and cognitive function in patients and rodents.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal's website, www.annalsofsurgery.com.
Contributor Information
Ce Zhang, Email: zhangcemelody88@163.com.
Yuan Han, Email: yuan.han@fdeent.org.
Xiaojun Liu, Email: lxjmzk@163.com.
Hong Tan, Email: honggg20040317@126.com.
Yuanlin Dong, Email: YDONG@mgh.harvard.edu.
Yiying Zhang, Email: YZHANG37@mgh.harvard.edu.
Feng Liang, Email: FLIANG2@mgh.harvard.edu.
Hui Zheng, Email: HZHENG1@mgh.harvard.edu.
Gregory Crosby, Email: GCROSBY@BWH.HARVARD.EDU.
Deborah J. Culley, Email: Deborah.culley@pennmedicine.upenn.edu.
Edward R. Marcantonio, Email: emarcant@bidmc.harvard.edu.
Yuan Shen, Email: kmshy@tongji.edu.cn.
Jun-Li Cao, Email: caojl0310@aliyun.com.
Zhongcong Xie, Email: zxie@mgh.harvard.edu.
REFERENCES
- 1. Evered L, Silbert B, Knopman DS, et al. Recommendations for the nomenclature of cognitive change associated with anaesthesia and surgery-2018. Anesthesiology. 2018;129:872–879. [DOI] [PubMed] [Google Scholar]
- 2. Vyhnalek M, Magerova H, Andel R, et al. Olfactory identification in amnestic and non-amnestic mild cognitive impairment and its neuropsychological correlates. J Neurol Sci. 2015;349:179–184. [DOI] [PubMed] [Google Scholar]
- 3. Wilson RS, Schneider JA, Arnold SE, et al. Olfactory identification and incidence of mild cognitive impairment in older age. Arch Gen Psychiatry. 2007;64:802–808. [DOI] [PubMed] [Google Scholar]
- 4. Elterman KG, Mallampati SR, Kaye AD, et al. Postoperative alterations in taste and smell. Anesth Pain Med. 2014;4:e18527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Murphy C. Olfactory and other sensory impairments in Alzheimer disease. Nat Rev Neurol. 2019;15:11–24. [DOI] [PubMed] [Google Scholar]
- 6. Fullard ME, Tran B, Xie SX, et al. Olfactory impairment predicts cognitive decline in early Parkinson’s disease. Parkinsonism Relat Disord. 2016;25:45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yahiaoui-Doktor M, Luck T, Riedel-Heller SG, et al. Olfactory function is associated with cognitive performance: results from the population-based LIFE-Adult-Study. Alzheimers Res Ther. 2019;11:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Young JW, Sharkey J, Finlayson K. Progressive impairment in olfactory working memory in a mouse model of mild cognitive impairment. Neurobiol Aging. 2009;30:1430–1443. [DOI] [PubMed] [Google Scholar]
- 9. Khodosevich K, Lazarini F, von Engelhardt J, et al. Connective tissue growth factor regulates interneuron survival and information processing in the olfactory bulb. Neuron. 2013;79:1136–1151. [DOI] [PubMed] [Google Scholar]
- 10. Rochefort C, Gheusi G, Vincent JD, et al. Enriched odor exposure increases the number of newborn neurons in the adult olfactory bulb and improves odor memory. J Neurosci. 2002;22:2679–2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Su CY, Menuz K, Carlson JR. Olfactory perception: receptors, cells, and circuits. Cell. 2009;139:45–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Compston A. The hippocampus and the sense of smell. A review, by Alf Brodal. Brain 1947: 70; 179-222. Brain. 2010;133:2509–2513. [DOI] [PubMed] [Google Scholar]
- 13. Dibattista M, Reisert J. The odorant receptor-dependent role of olfactory marker protein in olfactory receptor neurons. J Neurosci. 2016;36:2995–3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brann JH, Firestein SJ. A lifetime of neurogenesis in the olfactory system. Front Neurosci. 2014;8:182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Turner JH, May L, Reed RR, et al. Reversible loss of neuronal marker protein expression in a transgenic mouse model for sinusitis-associated olfactory dysfunction. Am J Rhinol Allergy. 2010;24:192–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang C, Zhang Y, Shen Y, et al. Anesthesia/surgery induces cognitive impairment in female Alzheimer’s disease transgenic mice. J Alzheimers Dis. 2017;57:505–518. [DOI] [PubMed] [Google Scholar]
- 17. Qing H, Desrouleaux R, Israni-Winger K, et al. Origin and function of stress-induced IL-6 in murine models. Cell. 2020;182:372–387 e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Heppner FL, Ransohoff RM, Becher B. Immune attack: the role of inflammation in Alzheimer disease. Nat Rev Neurosci. 2015;16:358–372. [DOI] [PubMed] [Google Scholar]
- 19. Henkin RI, Schmidt L, Velicu I. Interleukin 6 in hyposmia. JAMA Otolaryngol Head Neck Surg. 2013;139:728–734. [DOI] [PubMed] [Google Scholar]
- 20. Patanella AK, Zinno M, Quaranta D, et al. Correlations between peripheral blood mononuclear cell production of BDNF, TNF-alpha, IL-6, IL-10 and cognitive performances in multiple sclerosis patients. J Neurosci Res. 2010;88:1106–1112. [DOI] [PubMed] [Google Scholar]
- 21. Schuitemaker A, Dik MG, Veerhuis R, et al. Inflammatory markers in AD and MCI patients with different biomarker profiles. Neurobiol Aging. 2009;30:1885–1889. [DOI] [PubMed] [Google Scholar]
- 22. Hudetz JA, Gandhi SD, Iqbal Z, et al. Elevated postoperative inflammatory biomarkers are associated with short- and medium-term cognitive dysfunction after coronary artery surgery. J Anesth. 2011;25:1–9. [DOI] [PubMed] [Google Scholar]
- 23. Li Y, Chen D, Wang H, et al. Intravenous versus volatile anesthetic effects on postoperative cognition in elderly patients undergoing laparoscopic abdominal surgery. Anesthesiology. 2021;134:381–394. [DOI] [PubMed] [Google Scholar]
- 24. Vasunilashorn SM, Ngo L, Inouye SK, et al. Cytokines and postoperative delirium in older patients undergoing major elective surgery. J Gerontol A Biol Sci Med Sci. 2015;70:1289–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lv XC, Lin Y, Wu QS, et al. Plasma interleukin-6 is a potential predictive biomarker for postoperative delirium among acute type a aortic dissection patients treated with open surgical repair. J Cardiothorac Surg. 2021;16:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cibelli M, Fidalgo AR, Terrando N, et al. Role of interleukin-1beta in postoperative cognitive dysfunction. Ann Neurol. 2010;68:360–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lai IK, Valdearcos M, Morioka K, et al. Blocking Kv1.3 potassium channels prevents postoperative neuroinflammation and cognitive decline without impairing wound healing in mice. Br J Anaesth. 2020;125:298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xiang X, Yu Y, Tang X, et al. Transcriptome profile in hippocampus during acute inflammatory response to surgery: toward early stage of PND. Front Immunol. 2019;10:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hu J, Feng X, Valdearcos M, et al. Interleukin-6 is both necessary and sufficient to produce perioperative neurocognitive disorder in mice. Br J Anaesth. 2018;120:537–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rashid MH, Sparrow NA, Anwar F, et al. Interleukin-6 mediates delirium-like phenotypes in a murine model of urinary tract infection. J Neuroinflammation. 2021;18:247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Moller JT, Cluitmans P, Rasmussen LS, et al. Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. International Study of Post-Operative Cognitive Dysfunction. Lancet. 1998;351:857–861. [DOI] [PubMed] [Google Scholar]
- 32. Hummel T, Sekinger B, Wolf SR, et al. ‘Sniffin’ sticks’: olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem Senses. 1997;22:39–52. [DOI] [PubMed] [Google Scholar]
- 33. Pallua N, Low JF, von Heimburg D. Pathogenic role of interleukin-6 in the development of sepsis. Part II: Significance of anti-interleukin-6 and anti-soluble interleukin-6 receptor-alpha antibodies in a standardized murine contact burn model. Crit Care Med. 2003;31:1495–1501. [DOI] [PubMed] [Google Scholar]
- 34. Liufu N, Liu L, Shen S, et al. Anesthesia and surgery induce age-dependent changes in behaviors and microbiota. Aging (Albany NY). 2020;12:1965–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lehmkuhl AM, Dirr ER, Fleming SM. Olfactory assays for mouse models of neurodegenerative disease. J Vis Exp. 2014;90:e51804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tillerson JL, Caudle WM, Parent JM, et al. Olfactory discrimination deficits in mice lacking the dopamine transporter or the D2 dopamine receptor. Behav Brain Res. 2006;172:97–105. [DOI] [PubMed] [Google Scholar]
- 37. Oberland S, Neuhaus EM. Whole mount labeling of cilia in the main olfactory system of mice. J Vis Exp. 2014;94:52299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jones DT, Reed RR. Golf: an olfactory neuron specific-G protein involved in odorant signal transduction. Science. 1989;244:790–795. [DOI] [PubMed] [Google Scholar]
- 39. Reisert J, Yau KW, Margolis FL. Olfactory marker protein modulates the cAMP kinetics of the odour-induced response in cilia of mouse olfactory receptor neurons. J Physiol. 2007;585(Pt 3):731–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Youngentob SL, Margolis FL, Youngentob LM. OMP gene deletion results in an alteration in odorant quality perception. Behav Neurosci. 2001;115:626–631. [DOI] [PubMed] [Google Scholar]
- 41. Youngentob SL, Kent PF, Margolis FL. OMP gene deletion results in an alteration in odorant-induced mucosal activity patterns. J Neurophysiol. 2003;90:3864–3873. [DOI] [PubMed] [Google Scholar]
- 42. Chen Y, Wang B, Liu D, et al. Hsp90 chaperone inhibitor 17-AAG attenuates Abeta-induced synaptic toxicity and memory impairment. J Neurosci. 2014;34:2464–2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hooper PL, Durham HD, Torok Z, et al. The central role of heat shock factor 1 in synaptic fidelity and memory consolidation. Cell Stress Chaperones. 2016;21:745–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. El-Husseini AE, Schnell E, Chetkovich DM, et al. PSD-95 involvement in maturation of excitatory synapses. Science. 2000;290:1364–1368. [PubMed] [Google Scholar]


