Abstract
Anaplastic thyroid carcinoma (ATC) is a rare thyroid malignancy with a dire prognosis, nearly 100% disease specific mortality and a median overall survival less than 6 months. In view of the limited data from India on anaplastic thyroid cancer, we conducted this audit to analyze the treatment pattern, outcomes and factors influencing it. This is a retrospective analysis of outcomes of patients treated in a single institution between January 2008 and December 2020. Baseline characteristics, treatment received, and outcomes among adult patients with ATC were collected. Progression free survival (PFS) and overall survival (OS) were analyzed. SPSS version 20 and RStudio version 3.1.1 were used for analysis. In this cohort of 134 patients, the median age at diagnosis was 59 years, with 63.4% of them being females. At presentation, 70.9% of them had good performance status (PS 0–1). Only 38.8% received treatment with curative intent (either surgery fb adjuvant or neoadjuvant chemotherapy fb surgery and adjuvant or definitive chemoradiotherapy) while 61.2% patients received palliative treatment (either palliation alone or palliative chemotherapy or palliative surgery or palliative RT). Predominant pattern of progression was local progression (79.8%).
Median PFS and OS of the overall cohort were 58 days and 80 days respectively. PFS and OS were significantly better in patients treated with curative intent vs palliative intent (116 and 134 days vs 45 and 50 days; p = 0.00 and 0.00 respectively). Among patients treated with curative intent, OS was significantly better in patients undergoing surgery vs CTRT (155 vs 76 days; p = 0.03). Among patients treated with upfront surgery, both PFS and OS were better with the addition of adjuvant CTRT/RT vs no adjuvant (332 and 540 days vs 55 and 91 days; p = 0.00 and 0.003 respectively). ATC is a rare cancer with dismal prognosis. Local therapy with surgery followed adjuvant seems to be associated with the better outcomes. Systemic therapy seems to be a better option for palliation. Our data reflects the real world data of this rare cancer.
Keywords: Anaplastic carcinoma, Thyroid, Systemic therapy, Audit, Outcome
Background
Anaplastic thyroid carcinoma (ATC) is a rare thyroid malignancy accounting for around 2% of thyroid cancer [1]. However, it has a dire prognosis with nearly a 100% disease-specific mortality rate [2] and contributes to nearly 1/3rd of all mortalities caused by thyroid cancer [3]. The median overall survival in anaplastic thyroid cancer with or without metastasis is less than 6 months [4–7]. Due to the rare nature of the disease, large randomized studies are not feasible in ATC. In view of the dismal prognosis majority of patients, do not opt for any kind of treatment [8].
Nearly 20% of ATC have their origin as differentiated thyroid cancer (DTC) and in another 20%, they coexist with DTC [9, 10]. Hence, these tumors share molecular pathogenesis similar to that seen in DTC. Hence, these patients have alterations in BRAF gene [11], RAS gene, NTRK, etc. [12]. The targeting of these genes in DTC has led to an improvement in outcomes [12]. However, there is limited data regarding the use of tyrosine kinase inhibitors in these patients and these are beyond the reach of patients in low-middle income countries. Even the data for use of systemic chemotherapy is limited from low-middle income countries.
We are a tertiary care cancer center in India. In view of the limited data from India on anaplastic thyroid cancer, we conducted this audit to estimate the treatment pattern, outcomes and factors influencing it.
Methods
Patient Selection
The data was extracted from the electronic medical record system subjected to the selection criteria given below. All patients who fulfilled below mentioned criteria were selected:
Adult patient (Age ≥ 18 years)
Pathological proof of anaplastic thyroid cancer
Treated in the investigator’s center
Diagnosed between January 2008 and December 2020
Patients underwent routine blood investigations including complete blood counts, liver, and renal function tests. Imaging modalities include ultrasound or CT neck for locoregional evaluation, CT thorax, and abdomen or PET CT for evaluation of distant metastases. Histopathologic proof obtained either by FNAC or by biopsy. Treatment intent of either curative vs palliative was made depending on the extent of the disease and performance status of the patient. The following data were extracted from the electronic medical records — age, gender, comorbidities, performance status, T-grouping, N-grouping, stage grouping, treatment received, status at last follow-up and date of progression, and death, if occurred. Data was entered in Microsoft excel sheet and was cross-checked by two investigators. The data was censored for analysis on 22 May 2021.
Progression-free survival (PFS) was defined as the time in months from the date of diagnosis to the date of the first evidence of progression. Overall survival (OS) was defined as the time in months from the date of diagnosis to the date of death.
SPSS version 20 and R Studio version 3.1.1 were used for analysis. Descriptive statistics were performed. Continuous variables were expressed in terms of the median with the range while non-continuous variables were expressed in terms of percentages with its 95% confidence intervals (CI). Kaplan Meier method was used for estimation of PFS and OS. The median with its 95% CI interval was estimated. Log-rank test was performed for univariate analysis and COX regression method with tie handling was used for multivariate analysis.
Results
Baseline Characteristics
In our cohort of 134 patients with anaplastic thyroid carcinoma, treated between January 2008 and December 2020, the median age at diagnosis was 59 years (range 29–83), with 63.4% (85) of them being females. Most of them, 70.9% (95) was in good PS (of 0–1) at presentation. On histopathology, 118 (88.05%) had de novo anaplastic carcinoma, whereas, 12% (16) had coexistent differentiated component (13 papillary carcinoma, 2 follicular carcinoma; 1 hurthle cell carcinoma). Few patients had coexistent features, which include multi nodular goiter in eight patients (6.78%), lymphocytic thyroiditis in four (3.39%), and follicular neoplasm in four (3.39%). Most of the patients were euthyroid whereas two (1.7%) had hyperthyroidism and one (0.85%) had hypothyroidism. Baseline characteristics are shown in Table 1.
Table 1.
Baseline characteristics. * Acute presentation was defined as symptoms being shorter than 30 days. Leukocytosis was defined as the presence of leucocyte count of more than 10,000/mm.3 at presentation. Hypercalcemia was defined as the serum level of corrected calcium of more than 10 mg/dl at presentation. ECOG PS, Eastern Cooperative Oncology Group performance status
| Variable | Value (n = 134) |
|---|---|
|
Age in years Median (range) |
59 (29–83) |
|
Gender no.(%) Female Male |
85 (63.4) 49 (36.6) |
|
ECOG PS-no(%) 0–1 2–4 |
95 (70.9) 39 (29.1) |
|
Comorbidities no. (%) Nil Diabetes mellitus Hypertension Others |
90 (67.1) 14 (10.4) 20 (14.9) 10 (7.4) |
|
Presentation no. (%) Acute presentation* Presence of leucocytosis** Presence of hypercalcemia*** |
40 (29.8) 43 (32.0) 4 (2.9) |
|
T grouping no. (%) T3 T4 |
23 (17.1) 97 (82.8) |
|
T size no. (%) More than 5 cms |
107(79.8) |
|
N stage no. (%) N0 N1 |
57 (42.5) 77 (57.4) |
|
M stage no. (%) M0 M1 |
78 (58.2) 56 (41.7) |
|
Stage no. (%) IVA IVB IVC |
8 (5.9) 70 (52.3) 56 (41.8) |
|
Distant metastasis sites no. (%) Lung Bone Liver |
(Few had more than one site) 49 (36.6) 10 (7.4) 4 (3.5) |
Pattern of Treatment
Based on the extent of the disease and performance status of the patient, treatment decisions were made. Surgery (total thyroidectomy with nodal dissection and central compartment clearance) was done in loco regional resectable disease with good performance status. In patients with unresectable loco regional disease, CTRT was given. Very few patients received chemotherapy with neoadjuvant intent followed by surgery. In patients with distant metastases with good performance status, palliative chemotherapy was given. In patients with poor performance status, supportive care alone was given.
The details of the pattern of treatment are shown in Fig. 1. The intent of treatment was curative in 52 (38.8%) patients. The initial modality of treatment received in curative patients was surgery in 43 (32.1%), chemo radiation in 7 (5.2%), and neoadjuvant chemotherapy followed by surgery in 2 (1.5%). In 2 patients who received neoadjuvant chemotherapy, the regimens used were doxorubicin + cisplatin and paclitaxel + cisplatin in 1 patient each. Among the patients who underwent curative surgical resection, the procedure performed was R0, R1, and R2 in 23 (53.4%), 18 (41.8%), and 2 (4.6%) patients respectively. Emergency tracheostomy was performed in fifteen patients (11.2%) who came with stridor. Adjuvant treatment was offered to 17 patients (39.5% n = 43) and 10 received radiation while 7 received concurrent chemo radiation. Dose of adjuvant RT ranged from 60 to 64 Gy. Among patients treated with curative chemo radiation, the concurrent regimen was weekly doxorubicin in 3 patients and cisplatin based regimen in 4.
Fig. 1.
Patterns of treatment. BSC, best supportive care
Palliative treatment was offered to 82 (61.1%) patients among which 41 (30.5%) received supportive care, 30 (22.4%) palliative chemotherapy, 8 (5.9%) palliative RT, and 3 (2.2%) underwent palliative surgery. Dose of palliative intent RT ranged from 20 to 30 Gy.
Radiation therapy was offered to 37 patients (27.6%) at some point of time. Among them, 15 patients received RT as upfront treatment (7 CTRT and 8 palliative RT alone). Seventeen patients received in an adjuvant setting (7 of them CTRT and 10 RT alone). The rest of 5 of them received palliative therapy in a second line setting.
Systemic therapy was administered to 39 patients. Thirty (22.3%) patients received palliative chemotherapy upfront, 7 received in subsequent lines, and 2 had received neoadjuvant therapy. Most common systemic regimen administered was a combination of taxane and platinum. Only one patient received tyrosine kinase inhibitor therapy. It was pazopanib used in this patient, post-progression on weekly paclitaxel and carboplatin. The regimens administered are shown in Table 2.
Table 2.
Chemotherapy regimens administered. The total number is more than 39 as few patients received more than 1 line
| Regimen | N = 39 (%) |
|---|---|
| Paclitaxel + carboplatin weekly | 24 (61.5) |
| Paclitaxel + carboplatin 3 weekly | 5 (12.8) |
| Paclitaxel alone | 4 (10.2) |
| Paclitaxel + cisplatin | 2 (5.1) |
| Doxorubicin + cisplatin | 2 (5.1) |
| Endoxan | 1 (2.5) |
| Carboplatin alone | 1 (2.5) |
| Pazopanib | 1 (2.5) |
Outcomes
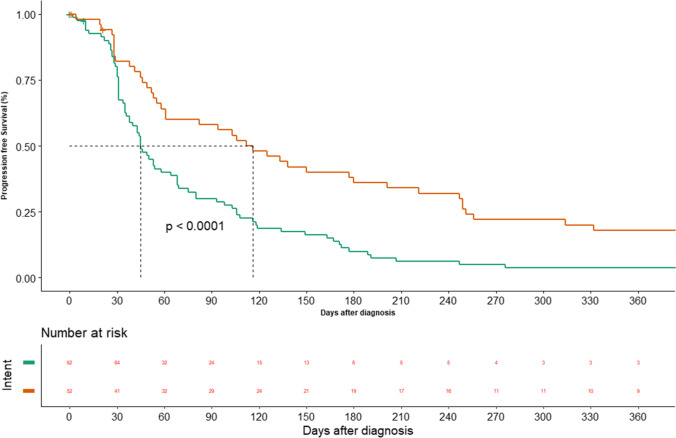
Out of 134 patients, 124 patients had progression. The median progression free survival was 58 days (95% CI: 43.6–72.3) (Fig. 2). The sites of progression were loco-regional in 107 (79.8%) and distant in 17 (12.6%) patients respectively. The median PFS in palliative and curative patients was 45 days (95% CI: 36.2–53.7) vs 116 days (95% CI: 71.0–160.9) respectively (P = 0.00) (Fig. 3).
Fig. 2.
Progression free survival — overall
Fig. 3.
Progression free survival — according to intent
Among curative patients, the median PFS in patients undergoing surgery was 112 days (95% CI 51.3–172.6) vs 58 days (95% CI 0–163.6) in patients undergoing curative chemoradiation (P = 0.127). Among patients undergoing surgery, the median PFS among those receiving adjuvant therapy was 332 days (95% CI: 178.3–681.5) vs 55 days (95% CI: 43.5–66.4) in those not receiving it (P = 0.000).
Among patients treated with palliative intent, the median PFS among patients undergoing palliative chemotherapy was 103 days (95% CI 76.6–129) and among palliative radiation was 45 days (95% CI 31.1–58.8) and palliative surgery was 106 days (95% CI: 45.1–166.8). Median PFS was better when chemotherapy was administered (p = 0.00). The baseline characteristics influencing PFS are shown in Table 3. Age < 60 and good PS (0/1) were the factors associated with better PFS.
Table 3.
Factors affecting progression free survival. ECOG PS, Eastern Cooperative Oncology Group performance status
| Variable | Subgroup | Median PFS(95% CI) | P-value logrank |
|---|---|---|---|
| Age | Elderly | 46.0 (27.3–64.4) | 0.092 |
| Nonelderly | 68 (26.1–109.8) | ||
| Gender | Male | 64.0 (38.0–89.9) | 0.308 |
| Female | 55.0 (36.4–73.5) | ||
| ECOG PS | 0–1 | 98 (66.4–129.5) | 0.00 |
| 2–4 | 35 (27.4–42.5) | ||
| T size | < 5 cm | 68 (2.8–133.1) | 0.068 |
| ≥ 5 cm | 58.0 (42.1–73.8) | ||
| N stage | N0 | 61 (27.9–94.0) | 0.271 |
| N1 | 54 (36.2–71.7 | ||
| M stage | M0 | 61 (28.9–93.0) | 0.121 |
| M1 | 54 (31.4–76.5) | ||
| Hyperleukocytosis | No | 64 (37.3–90.6) | 0.436 |
| Yes | 45 (25.9–64.0) |
The median follow-up was 76 days. We had 124 deaths and the median overall survival was 80 days (95% CI: 58.9–101.23) (Fig. 4). The median OS in patients treated with curative intent was 134 days (95% CI 90.0–177.9) vs 50 days (95% CI 29.5–70.4) in patients treated with palliative intent (p = 0.000) (Fig. 5). Among curative treated patients, the median OS in patients undergoing surgery was 155 days (95% CI 73.0–236.9) vs 76 days (95% CI 0–181.6) in patients undergoing curative chemoradiation (P = 0.032). Among patients undergoing surgery, the median OS among those receiving adjuvant therapy was 540 days (95% CI 174.2–905.7) vs 91 days (95% CI 76.7–105.2) in those not receiving it (P = 0.003).
Fig. 4.
Overall survival
Fig. 5.
Overall survival — according to intent
Among palliative treated patients, the median OS among patients undergoing supportive care alone was 34 days (95% CI 31.1–36.8), palliative chemotherapy was 129 days (95% CI 65.7–192.2), among palliative radiation was 45 days (95% CI 0–89.3), and palliative surgery was 106 days (95% CI 45.1–166.8). Median OS was better when chemotherapy was administered (p = 0.002). The baseline characteristics influencing OS are shown in Table 4. Age < 60 and good PS (0/1) were the factors associated with better OS.
Table 4.
Factors affecting overall survival. ECOG PS, Eastern Cooperative Oncology Group performance status
| Variable | Subgroup | Median OS (95% CI) | P-value logrank |
|---|---|---|---|
| Age | Elderly | 59.0 (25.7–92.2) | 0.047 |
| Nonelderly | 94.0 (57.1–130.8) | ||
| Gender | Male | 92 (49.0–134.9) | 0.341 |
| Female | 77.0 (50.22–103.7) | ||
| ECOG PS | 0–1 | 111 (85.0–136.9) | 0.00 |
| 2–4 | 38 (28.9–47.0) | ||
| T size | < 5 cm | 85 (23.0–146.9) | 0.128 |
| ≥ 5 cm | 77 (49.2–104.7) | ||
| N stage | N0 | 80 (38.7–121.2) | 0.339 |
| N1 | 83 (56.6–109.3) | ||
| M stage | M0 | 92 (58.5–125.4) | 0.138 |
| M1 | 69 (43.3–94.6) | ||
| Hyperleukocytosis | No | 94.0 (72.8–115.1) | 0.135 |
| Yes | 45 (9.0–80.9) |
Discussion
To the best of our knowledge, this is one of the largest series from India on audit and outcomes of anaplastic carcinoma. The incidence of ATC in our institute was low (Fig. 1). This is lower than the incidence reported in literature where ATC generally accounts for 1–2% of all thyroid cancer patients [1]. We do not have a population based registry data on ATC in India and hence, we cannot speculate that the incidence of ATC is low in India. Our finding have a referral bias since our center is a premier academic referral center for cancer treatment and > 50% of the patients seen are outstation. It is possible that differentiated thyroid cancer which have long-term disease control would be referred to our center, while ATC in whom the outcomes are poor won't be referred with the same enthusiasm.
The baseline characteristics in the current study are similar to those reported from previous studies in China and India [6, 8]. The median age in the current study was in the 6th decade as opposed to the 7th decade in the western world [13–16]. This seems to be a general finding in low and middle income countries (LMIC) where there is an apparent early presentation of cancer by a decade. This is as a result of pyramidal shape of population pyramid in LMIC, as opposed to dumbbell in high-income countries [17]. There was a significant proportion of female patients. This is observed in ATC and is in stark contrast to the sex ratio in squamous cell carcinoma of head and neck cancer where male population largely predominates [18, 19]. Most of the patients (> 85%) present in the advanced stage with nearly 1/3rd having distant metastasis. A high proportion of patients presented poor performance status (> 25%) and thus explaining the high proportion of best supportive care rates seen in the current study. This again is similar to the rate of best supportive care seen across various studies.
Minimum work up included complete blood counts, liver and renal function tests, thyroid function tests, imaging with ultrasound or CT neck for locoregional evaluation, CT thorax, and abdomen or PET CT for evaluation of distant metastases. Histopathologic proof obtained either by FNAC or by biopsy. Treatment intent of either curative vs palliative was made depending on the extent of the disease and performance status of the patient. This practice is similar to that of prior studies [5, 6].
The treatment pattern suggested that surgical treatment seems to have a favorable impact on PFS and OS in patients eligible for curative treatment. However, the outcomes of patients treated only with surgery were far from satisfactory and administration of adjuvant treatment was associated with a trend towards improved PFS and OS. In our cohort, neoadjuvant chemotherapy was given to a few patients with extensive disease burden and post-chemotherapy they could undergo surgery. Considering the favorable impact of surgery on outcomes, this strategy might be preferred in patients with extensive local disease as opposed to treating them with palliative therapies.
For concurrent therapy, both doxorubicin and cisplatin were used. This is in consensus with the literature suggesting that both these regimens have activity in ATC [20]. A variety of systemic therapies were used for palliation. Thus suggesting a lack of consensus among medical oncologists about which is the appropriate regimen. This reflects the dismal results with systemic therapy in this cancer. However, these results are better than those treated with best supportive care or palliative radiation, thus suggesting that palliative chemotherapy probably should be considered if the patient is fit for it. Interesting aspect of the current audit is the lack of testing for driver mutations. Nearly 20–40% of ATC harbor actionable mutations [21]. However, the access to these therapies in LMIC is below 1% and hence, frequently, these tests are not ordered.
The study has its own strengths and limitations. The study has all consecutive patients taken over a decade and hence provides a real world scenario. This is probably the largest series of ATC from a single center. The limitation is that the data was retrospectively collected which has its own bias. For instance, specific clinical presentation and duration of symptoms of some patients are not available. The data about molecular features was not available and as in routine care, none of the patients had undergone molecular testing. Very few patients have received second-line or third-line treatment.
Conclusion
ATC is a rare cancer with dismal prognosis. Local therapy with surgery followed adjuvant seems to be associated with the best outcomes. Palliative systemic therapy seems to be a better option for palliation. Our data reflects the real world data of this rare cancer.
Declarations
Ethics Approval
The study involves a retrospective analysis of data and in compliance with ethical standards.
Consent to Participate
This is a retrospective study hence for this type of study formal consent is not required
Consent for Publication
This is a retrospective study hence for this type of study formal consent is not required.
Conflict of Interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Aditya Pavan Kumar Kanteti, Joy Ghose, Vijay Maruti Patil, Anup Sunil Tamhankar, and George Abraham contributed equally and are co-authors.
Contributor Information
Prathamesh.S. Pai, Email: kumarprabhashtmh@gmail.com, Email: paips@tmc.gov.in
Kumar Prabhash, Email: kumarprabhashtmh@gmail.com, Email: paips@tmc.gov.in.
References
- 1.Hundahl SA, Fleming ID, Fremgen AM, Menck HR. A National Cancer Data Base report on 53,856 cases of thyroid carcinoma treated in the U.S., 1985–1995 [see commetns] Cancer. 1998;83:2638–2648. doi: 10.1002/(SICI)1097-0142(19981215)83:12<2638::AID-CNCR31>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 2.Neff RL, Farrar WB, Kloos RT, Burman KD. Anaplastic thyroid cancer. Endocrinol Metab Clin North Am. 2008;37(525–38):xi. doi: 10.1016/j.ecl.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Kitamura Y, Shimizu K, Nagahama M, et al. Immediate causes of death in thyroid carcinoma: clinicopathological analysis of 161 fatal cases. J Clin Endocrinol Metab. 1999;84:4043–4049. doi: 10.1210/jcem.84.11.6115. [DOI] [PubMed] [Google Scholar]
- 4.Kim JH, Leeper RD. Treatment of locally advanced thyroid carcinoma with combination doxorubicin and radiation therapy. Cancer. 1987;60:2372–2375. doi: 10.1002/1097-0142(19871115)60:10<2372::AID-CNCR2820601004>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 5.Yau T, Lo CY, Epstein RJ, et al. Treatment outcomes in anaplastic thyroid carcinoma: survival improvement in young patients with localized disease treated by combination of surgery and radiotherapy. Ann Surg Oncol. 2008;15:2500–2505. doi: 10.1245/s10434-008-0005-0. [DOI] [PubMed] [Google Scholar]
- 6.Sun C, Li Q, Hu Z, et al. Treatment and prognosis of anaplastic thyroid carcinoma: experience from a single institution in China. PLoS ONE. 2013;8:e80011. doi: 10.1371/journal.pone.0080011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ito K-I, Hanamura T, Murayama K, et al. Multimodality therapeutic outcomes in anaplastic thyroid carcinoma: improved survival in subgroups of patients with localized primary tumors. Head Neck. 2012;34:230–237. doi: 10.1002/hed.21721. [DOI] [PubMed] [Google Scholar]
- 8.Pradhan R, Agarwal A, Lal P, et al. Clinico-Pathological profile of anaplastic thyroid carcinoma in an endemic goiter area. Indian J Endocrinol Metab. 2018;22:793–797. doi: 10.4103/ijem.IJEM_264_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nel CJ, van Heerden JA, Goellner JR, et al. Anaplastic carcinoma of the thyroid: a clinicopathologic study of 82 cases. Mayo Clin Proc. 1985;60:51–58. doi: 10.1016/S0025-6196(12)65285-9. [DOI] [PubMed] [Google Scholar]
- 10.McIver B, Hay ID, Giuffrida DF, et al. Anaplastic thyroid carcinoma: a 50-year experience at a single institution. Surgery. 2001;130:1028–1034. doi: 10.1067/msy.2001.118266. [DOI] [PubMed] [Google Scholar]
- 11.Quiros RM, Ding HG, Gattuso P, et al. Evidence that one subset of anaplastic thyroid carcinomas are derived from papillary carcinomas due to BRAF and p53 mutations. Cancer. 2005;103:2261–2268. doi: 10.1002/cncr.21073. [DOI] [PubMed] [Google Scholar]
- 12.Cabanillas ME, Zafereo M, Williams MD, et al (2018) Recent advances and emerging therapies in anaplastic thyroid carcinoma. F1000Res 7.: 10.12688/f1000research.13124.1 [DOI] [PMC free article] [PubMed]
- 13.Iyer PC, Dadu R, Ferrarotto R, et al. Real-world experience with targeted therapy for the treatment of anaplastic thyroid carcinoma. Thyroid. 2018;28:79–87. doi: 10.1089/thy.2017.0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mohebati A, Dilorenzo M, Palmer F, et al. Anaplastic thyroid carcinoma: a 25-year single-institution experience. Ann Surg Oncol. 2014;21:1665–1670. doi: 10.1245/s10434-014-3545-5. [DOI] [PubMed] [Google Scholar]
- 15.Prasongsook N, Kumar A, Chintakuntlawar AV, et al. Survival in response to multimodal therapy in anaplastic thyroid cancer. J Clin Endocrinol Metab. 2017;102:4506–4514. doi: 10.1210/jc.2017-01180. [DOI] [PubMed] [Google Scholar]
- 16.Deeken-Draisey A, Yang G-Y, Gao J, Alexiev BA. Anaplastic thyroid carcinoma: an epidemiologic, histologic, immunohistochemical, and molecular single-institution study. Hum Pathol. 2018;82:140–148. doi: 10.1016/j.humpath.2018.07.027. [DOI] [PubMed] [Google Scholar]
- 17.Kc S, Wurzer M, Speringer M, Lutz W. Future population and human capital in heterogeneous India. Proc Natl Acad Sci U S A. 2018;115:8328–8333. doi: 10.1073/pnas.1722359115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patil V, Singhai P, Noronha V, et al Effect of early palliative care on quality of life of advanced head and neck cancer patients: a phase III trial. J Natl Cancer Inst. 10.1093/jnci/djab020 [DOI] [PubMed]
- 19.Patil V, Noronha V, Dhumal SB, et al. Low-cost oral metronomic chemotherapy versus intravenous cisplatin in patients with recurrent, metastatic, inoperable head and neck carcinoma: an open-label, parallel-group, non-inferiority, randomised, phase 3 trial. Lancet Glob Health. 2020;8:e1213–e1222. doi: 10.1016/S2214-109X(20)30275-8. [DOI] [PubMed] [Google Scholar]
- 20.Haddad RI, Nasr C, Bischoff L, et al. NCCN Guidelines insights: thyroid carcinoma, version 2.2018. J Natl Compr Canc Netw. 2018;16:1429–1440. doi: 10.6004/jnccn.2018.0089. [DOI] [PubMed] [Google Scholar]
- 21.Tiedje V, Ting S, Herold T, et al. NGS based identification of mutational hotspots for targeted therapy in anaplastic thyroid carcinoma. Oncotarget. 2017;8:42613–42620. doi: 10.18632/oncotarget.17300. [DOI] [PMC free article] [PubMed] [Google Scholar]