Abstract
Background
Fibromyalgia (FM) is a multifunctional chronic musculoskeletal pain condition characterised by sensory hypersensitivity. Photobiomodulation (PBM) has shown a positive impact on relieving pain; however, no studies to our knowledge have analysed a whole-body PBM intervention in subjects with FM. The aims of the study were to compare the effects of whole-body PBM with placebo PBM on pain, functionality and psychological symptoms in patients suffering from FM.
Methods
Forty-two subjects were recruited from a private care practice. The design of the study is a randomised, triple-blinded, placebo-controlled clinical trial. Participants received 12 treatment sessions. Pain, quality of life, level of physical activity and psychological factors were assessed at baseline (T0), after session 6 (T1), after treatment (T2) and at 2-week (T3) follow-up.
Results
There were statistically significant differences in pain at 4 weeks (p ≤ 0.001) (T2) and the 2-week follow-up (T3) (p ≤ 0.001). In relation to the quality of life, there were statistically significant improvements after session 6 (p ≤ 0.001) (T1), immediately after treatment (p ≤ 0.001) (T2) and at the 2-week (T3) follow-up (p ≤ 0.001). Kinesiophobia presented significant differences between groups immediately after treatment (p ≤ 0.001) (T2) and at the 2-week (T3) follow-up (p ≤ 0.001), with self-efficacy only showing significant differences between groups 2 weeks after the treatment (p = 0.01) (T2). There were no differences between groups when comparing pain catastrophising at any time.
Conclusion
Whole-body PBM resulted in a significant reduction in pain and an improvement in quality of life in those participants suffering from FM after receiving 4 weeks of treatment. Furthermore, psychological factors such as kinesiophobia and self-efficacy were also improved. Thus, a whole-body PBM treatment is presented as a possible new multifactorial treatment with potential benefits for those with FM and more studies are needed to corroborate our findings.
Trial registration
ClinicalTrials.gov (NCT0424897).
Keywords: Photobiomodulation, Fibromyalgia, Pain, Chronic pain, Quality of life, Psychological factors, Kinesiophobia, Pain catastrophising, Self-efficacy
Key Summary Points
| Why carry out this study? |
| Fibromyalgia is a multifunctional condition characterised by sensory hypersensitivity and widespread pain, as well as the presence of fatigue, degenerative or inflammatory disorders, neurological problems, cognitive behaviour disorders, loss of memory and restless sleep; nevertheless, there is a lack of evidence to confirm the efficacy of treatments due to the multi-casual aetiology of this condition |
| Whole-body photobiomodulation appears to be a potential multifactorial treatment |
| What was the hypothesis of the study? |
| We hypothesise that a whole-body application of PBM will improve pain and quality of life, as well as psychological factors, in patients suffering from FM |
| What was learned from the study? |
| Whole-body PBM treatment is presented as a possible new multifactorial treatment with potential benefits in pain, quality of life and psychological factors for those with FM |
| The presented findings permit a better understanding of FM and present whole-body PBM intervention as a potential benefit for those with FM, opening new possibilities of both treatment and research |
Introduction
Fibromyalgia (FM) is a multifunctional condition and one of the most common causes of chronic widespread pain [1]. The aetiology and diagnosis of FM are still unclear because of the presence of several symptoms and comorbidities associated with this condition [2]. The American College of Rheumatology (ACR) defines different criteria in the diagnosis of FM; these include pain pressure sensitivity of 4 kg of pressure and widespread pain, pain which is widespread in at least four of five regions, symptoms with the same intensity level of pain for at least 3 months, Widespread Pain Index (WPI) score ≥ 7 and Symptom Severity Scale (SSS) score ≥ 5 or WPI score between 4 and 6 points and SSS score ≥ 9, and in addition the diagnosis of FM is completely valid regardless of other previous diseases present in the patient [1]. Furthermore, the presence of degenerative or inflammatory disorders, cognitive behaviour disorders, restless sleep, fatigue and somatic symptoms have been described in those with FM [1, 3].
The prevalence of FM in the general population is 2.7%. It varies from 0.4% to 9.3% and can be up to 15.7% in a clinical setting, being 4.2% in women and 0.2% in men [4, 5]. Although this syndrome can appear in all age groups, the prevalence of FM increases with age, rising in middle age (50–59 years) and decreasing in the elderly population (80+ years) [5, 6].
Current evidence proposes different treatments, such as exercise and cognitive behavioural therapy for those suffering from FM, with both appearing to be beneficial [3, 7–9]. However, there is a lack of evidence to confirm the efficacy of these treatments due to the multi-casual aetiology of this condition. Therefore, it is crucial to find a multifactorial and definitive treatment [3, 8, 10].
New treatments such as photobiomodulation (PBM) are showing positive effects by improving musculoskeletal and neuropathic pain and also improving quality of life [10]. PBM typically uses wavelengths of light ranging from 600 to 1070 nm, with a fluence (energy density) range of between 1 and 150 J/cm2. In this range, the effective tissue penetration is maximal because the principal tissue chromophores (haemoglobin and melanin) have high absorption bands at wavelengths shorter than 600 nm. For the treatment of superficial tissue (skin, subcutaneous tissue, superficial fascia and muscles), wavelengths in the range of 600–700 nm are used, while wavelengths in the range of 780–950 nm are used to treat deeper tissue(deep fascia and muscles, bone, brain) [11, 12]. Recently, the use of whole-body PBM has been shown to offer not only a local but also a systemic response, including a brain PBM treatment [13]. Improvements of neuronal bioenergetic functions, cerebral blood flow, oxidative stress, neuroinflammation, neural apoptosis, neurotrophic factors, neurogenesis and effects on intrinsic brain networks have been proposed [11]. PBM has been investigated by many institutions on a wide range of neurological and psychological conditions [11] and on patients suffering from FM, showing that psychological factors are altered [10, 13, 14]; therefore, it is important to include these factors in both FM assessment and treatment programmes.
Recent research has shown that the use of PBM improves symptoms in FM patients, resulting in a reduction in pain and improvements in sleep disorders, tiredness, muscle spasm, morning stiffness and tender points [10, 15, 16]. People suffering from FM usually present with tender points in different areas because of a nonspecific response of the central nervous system in its interaction with the autonomic nervous system [17]. This is caused by a central sensitisation phenomenon characterised by the dysfunction of neuro-circuits, which involves the perception, transmission and processing of afferent nociceptive stimuli [18].
Whole-body PBM could offer a new treatment possibility for people with FM due to its multifactorial potential. However, to date only one whole-body PBM study has been conducted. It took place in a sports population and showed short-term effects without conclusive changes [19]; hence, more research is needed.
We hypothesise that a whole-body application of PBM will primarily improve pain and quality of life. Secondarily, psychological symptoms are expected to improve in patients suffering from FM. To our knowledge, this is the first study to use whole-body PBM in FM patients. The protocol study of the present research has been previously published [20].
The goal of the study is to analyse short-term changes in pain, quality of life and psychological factors after a whole-body PBM treatment programme compared to a placebo group.
Methods
Design
This is a triple-blinded, randomised, placebo-controlled clinical trial with blinding of participants, therapists, evaluators and statistician to active or placebo whole-body PBM.
Setting
Participants were recruited in a private care practice in Malaga, Spain. Potential referrals were informed of the trial through formal meetings and trial information sheets.The study was registered in ClinicalTrials.gov (NCT04248972) and conducted in accordance with the Declaration of Helsinki. This study is reported in line with the CONSORT Statement [21, 22]. The present study has received ethical approval by the Ethics Committee of Human Research of the University of Granada, Spain (1044/CEIH/2020). All the participants accepted and signed an informed consent before beginning the study.
Patients Involvement
Patients were involved in the design and conduct of this research. During the feasibility stage, the priority of the research question, choice of outcome measures and methods of recruitment were discussed with patients in a focus group session.
Participants
Participants were screened by a physiotherapist to determine whether they met the following inclusion and exclusion criteria.
Inclusion Criteria
Aged between 34 and 64 years.
FM diagnosis from a rheumatologist according to the ACR classification criteria [1]. To make a diagnosis of fibromyalgia in adults, it is necessary for all of the following criteria to be met: (a) present generalised pain, i.e., in at least four of five regions, (b) present symptoms with the same intensity level for at least 3 months, (c) Symptom Severity Scale (SSS) score ≥ 5 and Widespread Pain Index (WPI) ≥ 7, or SSS score ≥ 9 and WPI between 4 and 6, and (d) A diagnosis of fibromyalgia does not exclude the presence of other illnesses and is valid irrespective of other diagnoses.
Exclusion Criteria
The presence of any inflammatory, neurological or orthopaedic disease which can alter balance, hearing or vision, or any cognitive impairment which might impact the ability to answer questions. Furthermore, fascial muscle disorders such as trigger points, myofascial syndrome pain and neck pain.
Participants were randomised to receive either a whole-body PBM therapy or placebo.
Patients were required not to receive or participate in any other FM study or treatment during the study period. Any changes in medication type or dosage during the study period were recorded, and prescribed medications from medical doctors were stored. Therefore, the placebo related only to the use of PBM. Patients who had already undergone treatments before the trial started were accepted since FM patients need continuous care. In addition, these previously received treatments were those related to manual therapy and physical activity.
PBM Therapy Programme
Participants randomised to this treatment will receive a whole-body PBM treatment using a NovoTHOR® whole-body light bed (see Fig. 1). For each treatment session, participants will lie supine in the treatment bed for 20 min, with no or minimal attire (underwear). Treatment sessions will be three times weekly for a 4-week period, resulting in a total of 12 treatment sessions. The parameters of the equipment are shown in Table 1.
Fig. 1.

NovoTHOR bed
Table 1.
NovoTHOR parameters
| NovoTHOR XL parameters | Unit | |
|---|---|---|
| Wavelengths of red and near-infrared (NIR) LEDs 50:50 ratio |
660 850 |
nm nm |
| Number of LEDs | 2880 | |
| Power emitted per LED | 0.336 | W |
| Beam area per LED (at the lens/skin contact surface) | 12.0 | cm2 |
| Total power emitted | 967 | W |
| Total area of NovoTHOR emitting surfaces | 34,544 | cm2 |
| Treatment time | 1200 | s |
| Continuous wave (CW) (not pulsed) | CW | |
| Irradiance | 0.028 | W/cm2 |
| Fluence | 25.2 | J/cm2 |
Placebo Feature
The placebo feature of the whole-body PBM bed provides controls that select active or placebo (sham) treatments in a way that is undetectable by the participant, operator or observers, such that no one is aware whether the participant is receiving an active or placebo treatment. There is a switch box (see Fig. 2) that randomises participants to active or placebo group; no other randomisation is necessary. With this system, if the operator becomes unblinded they will only see which treatment that particular participant is getting and hence only that particular participant would be excluded from the trial and not the operator. A blocked randomisation system (randomly varying the block size) to ensure that comparison groups are generated in a 1:1 ratio of approximately the same size will be used. For every block of ten participants, five will be allocated to each arm of the trial. In the worst case scenario, the allocation could be unbalanced by as much as two.
Fig. 2.

NovoTHOR randomising switch box
Furthermore, special goggles that block the PBM light are worn by the participant, operator and observers. These emit LED light inside (behind the lenses, so that the wearer sees some red light) to make it harder for the participants, operator or observers to detect whether the PBM bed is in the active or placebo mode. The goggles are designed to accommodate spectacles.
Also, heating elements are activated in the NovoTHOR bed even when the PBM bed is in the placebo mode, so that participants feel like they are receiving an active treatment.
PBM is safe and easy to administer, is non invasive and has no known side effects, with few reported contraindications [23].
For each treatment session, participants lie supine in the treatment bed for 20 min, with no or minimal attire (underwear). Treatment sessions were three times weekly for a 4-week period, resulting in 12 treatment sessions.
Data Collection
The assessments of primary and secondary outcome measures were at baseline, after treatment 6, immediately following the last treatment (4 weeks) and then at the follow-up 2 weeks after completion of the treatment. Figure 3 illustrates these assessment times through a flow diagram.
Fig. 3.
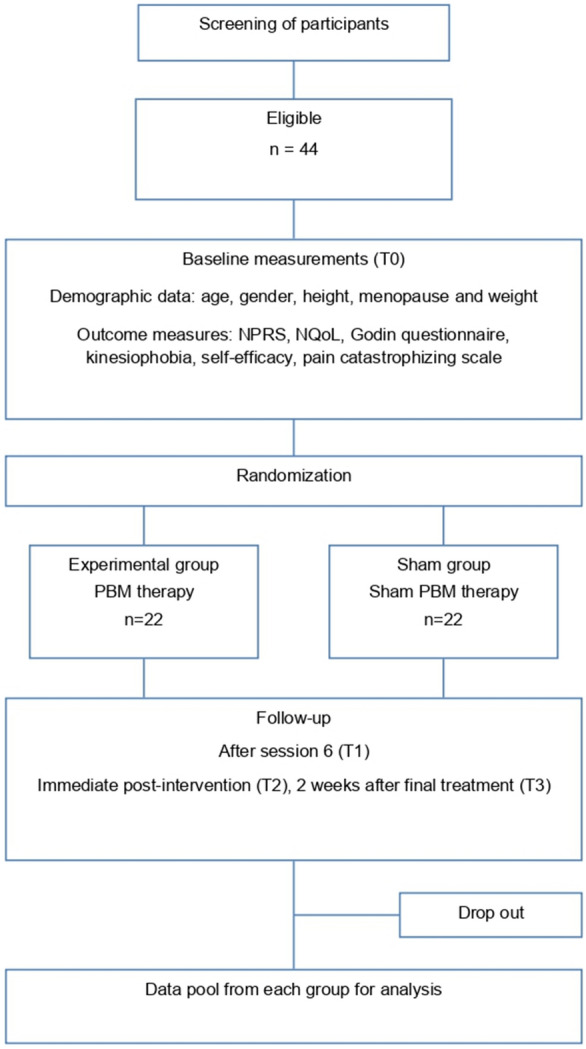
Flow diagram illustrating the assessment times
Outcome Measures
Primary outcome measures:
The Numeric Pain Rating Scale (NPRS), where 0 indicates “no pain” and 10 indicates “worst possible pain.” At each measurement point of the study, patients of both groups were asked to rate the average intensity of their pain over the past 7 days. This procedure has demonstrated a high degree of validity and reliability [24].
Secondary outcome measures:
Quality of life (QoL). Health-related quality of life (HRQL) measured by the VAS, where 0 indicates “no quality of life” and 10 indicates “the best possible quality of life.” At each measurement point of the study, patients of both groups were asked to rate the average QoL over the past 7 days. This procedure has been demonstrated to be easier for patients to respond to and reliable [25].
The Leisure Time Physical Activity Instrument (LTPAI) is used to measure the physical activity of patients. This has four components, each with three levels of activity: light, medium and vigorous. Scores indicate the number of hours these activity levels had been carried out each week in the last 4 weeks, with a sum showing the total number of hours of physical activity [26]. This tool has shown satisfactory test-retest reliability for the total score, i.e., ICC = 0.86 (CI 0.79–0.93) [26].
The Pain Catastrophising Scale, a validated questionnaire to assess the mechanism by which catastrophising impacts pain experience [27]. This is a validated questionnaire where the mechanism by which the pain experience is affected by catastrophism is evaluated. It was first developed in 1995 and has three different aspects. The first part, called “helplessness,” corresponds to questions 1–5 and 12 and refers to what the person believes they have been able to do to influence their pain [28].
The second part, called “magnification,” corresponds to questions 6, 7 and 13 and refers to the exaggeration of the threatening properties of the painful stimulus.
Lastly, “rumination” corresponds to questions 8–11 and refers to the fact that the patient is unable to stop thinking about the pain and cannot get away from the idea [28].
Therefore, the questionnaire consists of 13 items divided into three subsections. The scoring scale is from 1 to 5 with the final scores ranging from 0 to 52. Higher scores correspond to higher levels of catastrophism [28].
-
4.
The Spanish version of the Tampa Scale of Kinesiophobia, a valid and reliable measure of fear of movement [29]. It consists of 11 items each with one of four response options, where “strongly disagree” scores 1 point and “strongly agree” scores 4 points. Hence, the total score will vary between a minimum of 11 and a maximum of 44. A high score translates as a greater fear of movement/injury, that is, high levels of kinesiophobia [28, 30]. The Tampa Scale for Kinesiophobia-11 has been shown to be consistent, reliable and appropriate to assess fear of movement in patients with FM within a clinical context [31].
-
5.
The self-efficacy questionnaire assesses personal confidence to carry out an activity with the aim of successfully achieving the desired outcome [32]. It consists of ten items with a response scale of four points [28, 33]. The final score ranges from 0 to 44, with higher scores meaning higher perception of competence to handle a stressful situation efficiently. The self-efficacy scale has shown adequate psychometric properties and is considered a useful tool to help health professionals monitor patients' self-efficacy perception and programme both physical activity and walking exercise intervention goals and their implementation [34].
Recruitment Procedures
Participants were recruited from a private clinic and rehabilitation service in Malaga, Spain. In addition, advertisements were placed on social media to increase the potential number of participants in the study. The physiotherapist who carried out the recruitment of the participants provided information about the study, including details of eligibility criteria. After giving informed consent, participants were randomised to an active or placebo whole-body treatment.
To improve adherence to the treatment, the physiotherapist administering the treatment was in regular contact with the participants to remind them of their time schedule and to follow-up with them.
Statistical Analysis
SPSS® Statistics version 21.0 (IBM, Chicago, IL, USA) will be used for all analyses. The Shapiro-Wilk test will be used to verify data distribution normality. To study intragroup mean differences for all the outcomes between the four assessment times [baseline (T1), after session 6 (T2), immediate post-intervention (T3) and 2 weeks after the final treatment (T4)] repeated-measures analysis of variance (ANOVA) will be used. To compare the two groups (PBM intervention and placebo groups) at baseline and at follow-ups regarding clinical characteristics, a multivariate ANOVA (MANOVA) was conducted, with four levels corresponding to each time assessment (T1, T2, T3 and T4) and with the two intervention groups as independent factors. A value of p < 0.05 was considered statistically significant. Between- and within-group effect sizes for all quantitative variables were measured with the Cohen d coefficient. An effect size > 0.8 was considered large, around 0.5 moderate and < 0.2 small [35].
Sample Size Calculation
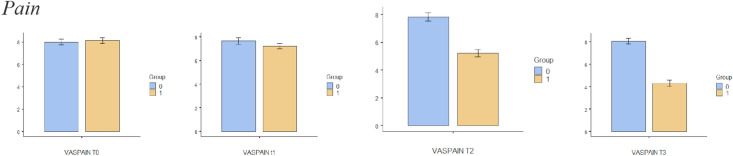
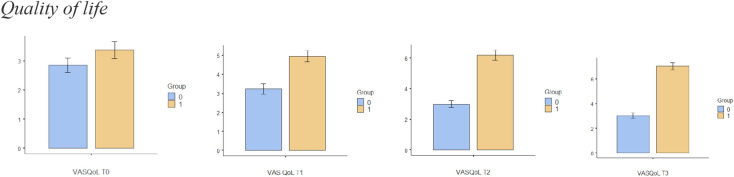
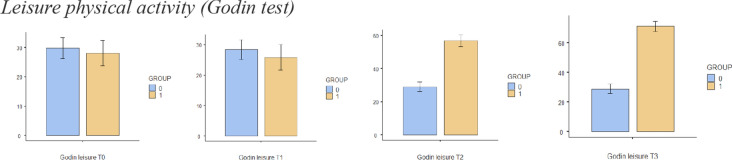
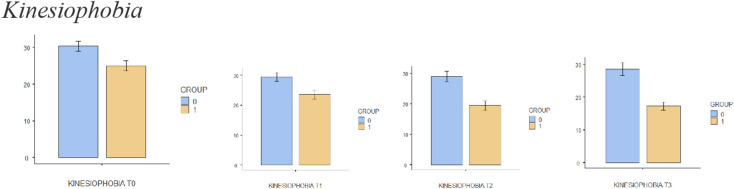
The sample size for this trial is based on an expected mean difference between groups of 2 points on the NPRS, which is the minimum clinically important difference [36]. Based on the results of other randomised clinical trials [32, 37] and previous reviews [38], and assuming (1) a standard deviation of 2.0 units on the NPRS to detect the difference between the intervention and placebo groups, (2) a value of α = 0.05 and (3) a statistical power of 90%, a minimum of 22 patients per group is needed. Differences in the different variables between groups are shown in Figs. 4, 5, 6, 7, 8 and 9.
Fig. 4.
Bar plots showing differences in pain between different assessment times.Group 0: Placebo group. Group 1: Intervention group
Fig. 5.
Bar plots showing differences in quality of life between different assessment times. Group 0: Placebo group. Group 1: Intervention group
Fig. 6.
Bar plots showing differences in leisure activity between different assessment times
Fig. 7.
Bar plots showing differences in kinesiophobia between different assessment times
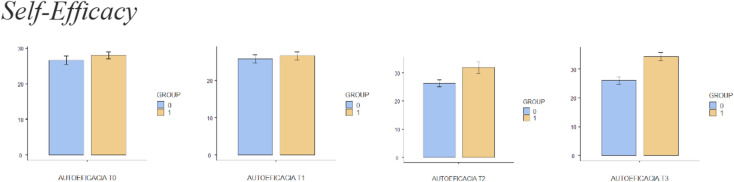
Fig. 8.
Bar plots showing differences in self-efficacy between different assessment times
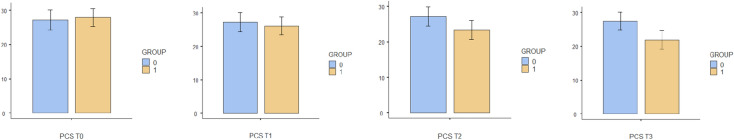
Fig. 9.
Bar plots showing differences in PCS between different assessment times. Group 0: Placebo group. Group 1: Intervention group
Results
Sample characteristics are shown in Table 2.
Table 2.
Summary of sociodemographic data of the women diagnosed with fibromyalgia
| Variable | Women with FM (n = 44) | |
|---|---|---|
| Mean ± SD/frequency (%) | 95% CI | |
| Age (years) | 52.83 ± 8.04 | [50.33, 55.34] |
| Height (m) | 1.63 ± 0.05 | [1.62, 1.65] |
| Weight (kg) | 78.19 ± 18.03 | [72.57, 83.81] |
| BMI (kg/m2) | 29.32 ± 6.21 | [27.38, 31.25] |
| Menopause status | ||
| Premenopausal | 29 (69.05) | |
| Postmenopausal | 13 (30.95) | |
Data are expressed as mean ± SD for quantitative variables and as frequency (%) for qualitative variables
CI confidence interval, BMI body mass index
Between-group difference variables at baseline (T0), after session 6 (T1), immediately after the treatment programme (T2) and at the 2-week follow-up (T3) are shown in Table 3.
Table 3.
Between-group difference variables at baseline (T0), after session 6 (T1), immediately after the treatment programme (T2) and at the 2-week follow-up (T3) (95% CI)
| T1 (baseline) | T2 (after session 6) | T3 (immediate post-intervention) | T4 (2 weeks after final treatment) | |
|---|---|---|---|---|
| VAS pain (mean) |
− 8.72 p = 0.78 (− 1.00; 1.00)a − 0.18b 0.37 c |
1.00 p = 0.154 (− 1.13; 1.00)a 0.37b 0.36 c |
3.00 p = < 0.001 (2.00; 3.00)a 2.06b 0.392c |
4.00 p = < 0.001 (3.00; 5.00)a 2.87b 0.40c |
| HRQL |
− 5.56 p = 0.21 (− 1.00; 4.63)a − 0.42b 0.37c |
− 2.00 p = < 0.001 (− 3.00; − 1.00)a − 0.129b 0.41c |
− 3.00 p = < 0.001 (− 4.00; − 3.00)a − 2.49b 0.39c |
− 4.00 p = < 0.001 (− 5.00; − 4.00)a − 3.26b 0.37c |
| LTPAI |
1.00 p = 0.93 (− 13.00; 15.00) a 0.09 b 5.62 c |
1.00 p = 0.68 (1.00; 10.00)a 0.15b 5.30c |
− 28.00 p = < 0.001 − 38.00; − 19.00)a − 1.90b 4.52c |
− 43.00 p = < 0.001 (− 52.00; − 32.00)a − 2.70b 4.82c |
| Kinesiophobia |
5.00 p = 0.01 (1.00; 10.00)a 0.83b 2.00c |
6.00 p = 0.008 (2.00; 11.00)a 0.87b 2.08c |
10.00 p = < 0.001 (5.00; 15.00)a 1.25b 2.34c |
12.00 p = < 0.001 (7.00; 18.00)a 1.49b 2.35c |
| Self-efficacy |
− 1.00 p = 0.512 (− 4.00; 2.00)a − 0.28b 1.52c |
− 7.00 p = 0.67 (− 4.00; 3.00)a − 0.16b 1.56c |
− 7.00 p = 0.034 (− 11.00; − 6.73)a − 0.73b 2.34c |
− 8.00 p = < 0.001 (− 12.00; − 5.00)a − 1.33b 1.94c |
| Pain catastrophising |
1.00 p = 0.859 (− 8.78; 7.35)a − 0.05b 3.99c |
1.00 p = 0.81 (− 8.00; 9.00)a 0.08b 3.92c |
4.00 p = 0.32 (− 4.00; 11.00)a 0.30b 3.83c |
6.00 p = 0.14 (− 200; 14.00)a 0.44b 3.85c |
SE size effect, LTPAI The Leisure Time Physical Activity Instrument
a95% CI
bCohen‘s d
cSE difference
Between-group Differences in Pain and Quality of Life
Comparisons between groups are shown in detail in Table 3 and Figs. 2 and 3. There were statistically significant differences in pain immediately after treatment (T2) and at the 2-week (T3) follow-up. In relation to quality of life, there were statistically significant changes after session 6 (T1), immediately after treatment (T2) and at the 2-week (T3) follow-up.
Between-group Differences in Leisure Physical Activity and Psychological Factors
Comparisons between groups are shown in detail in Table 3. There were statistically significant differences in leisure activity immediately after treatment (T2) and at the 2-week (T3) follow-up. In relation to quality of life, there were statistically significant changes after session 6 (T1), immediately after treatment (T2) and at the 2-week (T3) follow-up. Kinesiophobia was shown to present significant differences between groups immediately after treatment (T2) and at the 2-week (T3) follow-up, with self-efficacy only showing significant differences between groups 2 weeks after the treatment (T3). There were no differences between groups when comparing pain catastrophising at any time.
Discussion
The goal of the study is to analyse changes in pain, quality of life and psychological factors after a whole-body PBM treatment programme compared to a placebo group.
There were statistically significant differences in pain immediately after treatment (T2) and at the 2-week (T3) follow-up. In relation to quality of life, there were statistically significant changes after session 6 (T1), immediately after treatment (T2) and at the 2-week (T3) follow-up. There were statistically significant differences in leisure activity immediately after treatment (T2) and at the 2-week (T3) follow-up. Kinesiophobia presented significant differences between groups immediately after treatment (T2) and at the 2-week (T3) follow-up, with self-efficacy only showing significant differences between groups 2 weeks after the treatment (T3). There were no differences between groups at any time when comparing pain catastrophising.
This is the first study analysing the effects of whole-body PBM in a population suffering from chronic pain; therefore, comparison with other studies is difficult. Ghigiarelliet et al. showed short-term effects of whole-body PBM in a sports population, without showing any conclusive change [19]. The effects of local PBM on pain are well known and established [39], and its usefulness has been shown in different conditions such as knee osteoarthritis [40], oral mucositis [41], idiopathic burning mouth syndrome [42], neck disability and chronic neck pain [43], pain and function in tendinopathy [44], myofascial temporomandibular disorder [45], fractures [46], central nervous diseases [47] and even in the microbiome [48]. On the other hand, other studies show that PBM does not decrease pain and disability, specifically in people with non-specific low back pain, although those studies analysed the use of local PBM and not whole-body PBM [49].
The results obtained in terms of decreased pain and improvement in the quality of life may be explained by different mechanisms. PBM is proposed to stimulate an upregulation of mitochondrial activity by acting on the mitochondrial respiratory chain, which consequently increases ATP production into muscle cells and decreases oxidative stress and reactive oxygen species production [20]. Furthermore, PBM increases NADH, protein and RNA as well as a reciprocal augmentation in oxygen consumption [50]. Low-intensity light stimulates cytochrome c oxidase, which enhances nitric oxide synthesis. This signalling molecule can then function in both intra- and extracellular signalling pathways, specifically in skeletal muscle tissue 24 h after irradiation [50, 51]. PBM acts on the mitochondria, specifically on photoreceptors within the mitochondrial respiratory chain. Those suffering from FM how a lack of energy in response to any physical activity; therefore, compromised mitochondrial respiration and decreased ATP synthesis are the proposed mechanisms [52–54]. This is related to chronic pain and fatigue as well as a psychological impact [54]. In this regard, our results showed a significant decrease in kinesiophobia and self-efficacy in those treated with PBM. This may be explained not only by the decreased level of pain in patients but also by the possibility of PBM of the brain when the treatment stimulates the whole body; this has been used in a wide range of neurological and psychological conditions [11]. However, there were no significant changes in relation to pain catastrophism when comparing both groups. Recent knowledge shows that subjects suffering from FM need a multidisciplinary approach. Most non-pharmacological treatments consist of physical exercise programmes or a cognitive-behavioural focus or a combination of both. It has been suggested that the effectiveness of the treatment plan is increased by the inclusion of patient education; therefore, a lack of pain education in the treatment may explain the absence of changes in pain catastrophism [8]. In addition, the maladaptive coping strategy of pain catastrophising is believed to play a key role in FM pathology, which may increase pain perception [13]. However, our results showed decreased pain even without decreasing pain catastrophising.
Strengths and Limitations
The present study shows strengths that must be highlighted. This is the first study analysing whole-body PBM effects in subjects suffering from FM. There were no missing data during the treatment or follow-up assessments. The design of the study is a triple-blinded trial; thus, results are more reliable, which decreases potential bias. On the other hand, some limitations should be mentioned. As the goal was to specifically assess the effects of PBM on those with FM, other interventions which may be used, such as pain education, were not included. Sample size was calculated based on the primary outcome; thus, other variables were not considered. This is a first clinical trial in subjects with FM, and results should be interpreted with caution and not extrapolated to other populations.
Future Research
Future PBM studies for the treatment of those with FM should include pain education programmes to assess changes in pain, quality of life and psychological factors, specifically pain catastrophising. Furthermore, to assess other biomarkers, such as cortisol and melatonin, assessing patient stress and circadian rhythms, the composition of the gastrointestinal tract microbiota, its metabolites, luminal neurotransmitters and brain neurochemistry in addition to nutritional and lifestyle factors in people with fibromyalgia is necessary before and after the treatment. Finally, longitudinal studies analysing the long-term effects of PBM in subjects with FM, as well as in other populations with chronic pain, are necessary.
Conclusion
Whole-body PBM decreases pain and improves the quality of life in those suffering from FM. Furthermore, psychological factors such as kinesiophobia and self-efficacy are also improved.
However, this is a first step in this line of research and more studies are needed to corroborate our findings.
Acknowledgements
Funding
The study and Rapid Service Fee was supported by James Carroll.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole and have given their approval for this version to be published.
Author Contributions
Conceptualization: SNL, PB, JC, AGM; methodology: SNL, PB, JC, AGM; formal analysis and investigation: SNL, AGM; writing-original draft preparation: SNL, PB, JC, AGM; writing-review and editing: SNL, PB, JC, AGM; resources: SNL, PB, JC, AGM; supervision: SNL, PB, JC, AGM. All authors reviewed the manuscript.
Disclosures
Santiago Navarro Ledesma and Ana Gonzalez-Muñoz have nothing to disclose. James Carroll and Patricia Burton's affiliation is THOR Photomedicine.
Compliance with Ethics Guidelines
The present study has received ethical approval by the Ethics Committee of Human Research of the University of Granada, Spain (1044/CEIH/2020). The trial was conducted following the Declaration of Helsinki (revised in 2013).
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
- 1.Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Häuser W, Katz RL, et al. 2016 revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin Arthritis Rheum Semin Arthritis Rheum. 2016;46:319–329. doi: 10.1016/j.semarthrit.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 2.Arnold LM, Bennett RM, Crofford LJ, Dean LE, Clauw DJ, Goldenberg DL, et al. AAPT diagnostic criteria for fibromyalgia. J Pain. 2019;20:611–628. doi: 10.1016/j.jpain.2018.10.008. [DOI] [PubMed] [Google Scholar]
- 3.Bellato E, Marini E, Castoldi F, Barbasetti N, Mattei L, Bonasia DE, et al. Fibromyalgia syndrome: etiology, pathogenesis, diagnosis, and treatment. Pain Res Treat. 2012;2012. [DOI] [PMC free article] [PubMed]
- 4.Mas AJ, Carmona L, Valverde M, Ribas B, Navarro F, Ortiz AM, et al. Prevalence and impact of fibromyalgia on function and quality of life in individuals from the general population: results from a natiowide study in Spain. Clin Exp Rheumatol. 2008;26:519–526. [PubMed] [Google Scholar]
- 5.Queiroz LP. Worldwide epidemiology of fibromyalgia. Curr Pain Headache Rep. 2013;17. [DOI] [PubMed]
- 6.Vos T, Abajobir AA, Abbafati C, Abbas KM, Abate KH, Abd-Allah F, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1211–1259. doi: 10.1016/S0140-6736(17)32154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clauw DJ. Fibromyalgia: a clinical review. JAMA J Am Med Assoc. 2014;311:1547–1555. doi: 10.1001/jama.2014.3266. [DOI] [PubMed] [Google Scholar]
- 8.García-Ríos MC, Navarro-Ledesma S, Tapia-Haro RM, Toledano-Moreno S, Casas-Barragán A, Correa-Rodríguez M, et al. Effectiveness of health education in patients with fibromyalgia: a systematic review. Eur J Phys Rehabil Med. 2019;55:301–313. doi: 10.23736/S1973-9087.19.05524-2. [DOI] [PubMed] [Google Scholar]
- 9.Sosa-Reina MD, Nunez-Nagy S, Gallego-Izquierdo T, Pecos-Martín D, Monserrat J, Álvarez-Mon M. Effectiveness of therapeutic exercise in fibromyalgia syndrome: a systematic review and meta-analysis of randomized clinical trials. Biomed Res Int. 2017;2017. [DOI] [PMC free article] [PubMed]
- 10.Yeh SW, Hong CH, Shih MC, Tam KW, Huang YH, Kuan YC. Low-level laser therapy for fibromyalgia: a systematic review and meta-analysis. Pain Physician. 2019;22:241–254. [PubMed] [Google Scholar]
- 11.Salehpour F, Mahmoudi J, Kamari F, Sadigh-eteghad S, Rasta H, Hamblin MR, et al. Brain photobiomodulation therapy: a narrative review. Mol Neurobiol. 2019;55:6601–6636. doi: 10.1007/s12035-017-0852-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karu TI, Pyatibrat LV, Kolyakov SF, Afanasyeva NI. Absorption measurements of a cell monolayer relevant to phototherapy: reduction of cytochrome c oxidase under near IR radiation. J Photochem Photobiol B. 2005;81:98–106. doi: 10.1016/j.jphotobiol.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 13.Galvez-Sánchez CM, Reyes del Paso GA, Duschek S. Cognitive impairments in fibromyalgia syndrome: associations with positive and negative affect, alexithymia, pain catastrophizing and self-esteem. Front Psychol. 2018;9. [DOI] [PMC free article] [PubMed]
- 14.Galvez-Sánchez CM, Duschek S, Reyes GA. Psychological impact of fibromyalgia. Psychol Res Behav Manag. 2019;12:117–127. doi: 10.2147/PRBM.S178240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gur A, Karakoc M, Cevik R, Nas K, Sarac AJ, Karakoc M. Efficacy of low power laser therapy and exercise on pain and functions in chronic low back pain. Lasers Surg Med. 2003;32:233–238. doi: 10.1002/lsm.10134. [DOI] [PubMed] [Google Scholar]
- 16.Honda Y, Sakamoto J, Hamaue Y, Kataoka H, Kondo Y, Sasabe R, et al. Effects of physical-agent pain relief modalities for fibromyalgia patients: a systematic review and meta-analysis of randomized controlled trials. Pain Res Manag. 2018;2018. [DOI] [PMC free article] [PubMed]
- 17.Kisselev SB, Moskvin SV. The use of laser therapy for patients with fibromyalgia: a critical literary review. J Lasers Med Sci. 2019;10:12–20. doi: 10.15171/jlms.2019.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siracusa R, Di Paola R, Cuzzocrea S, Impellizzeri D. Fibromyalgia: pathogenesis, mechanisms, diagnosis and treatment options update. Int J Mol Sci. 2021. [DOI] [PMC free article] [PubMed]
- 19.Ghigiarelli JJ, Fulop AM, Burke AA, Ferrara AJ, Sell KM, Gonzalez AM, et al. The effects of whole-body photobiomodulation light-bed therapy on creatine kinase and salivary interleukin-6 in a sample of trained males: a randomized, crossover study. Front Sports Act Living. 2020;2:1–13. doi: 10.3389/fspor.2020.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Navarro-Ledesma S, Gonzalez-Muñoz A, Carroll J, Burton P. Short- and long-term effects of whole-body photobiomodulation on pain, functionality, tissue quality, central sensitisation and psychological factors in a population suffering from fibromyalgia: protocol for a triple-blinded randomised clinical trial. Ther Adv Chronic Dis. 2022;13:204062232210780. doi: 10.1177/20406223221078095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, Devereaux PJ, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. J Clin Epidemiol. 2010;63:e1–37. doi: 10.1016/j.jclinepi.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 22.Eldridge SM, Chan CL, Campbell MJ, Bond CM, Hopewell S, Thabane L, et al. CONSORT 2010 statement: extension to randomised pilot and feasibility trials. Pilot Feasibility Stud. 2016;2:1–32. doi: 10.1186/s40814-016-0105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamblin MR, Huang YY, Sharma SK, Carroll J. Biphasic dose response in low level light therapy—an update. Dose-Response. 2011;9:602–618. doi: 10.2203/dose-response.11-009.Hamblin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jensen MP, Turner JA, Romano JM, Fisher LD. Comparative reliability and validity of chronic pain intensity measures. Pain. 1999;83:157–162. doi: 10.1016/s0304-3959(99)00101-3. [DOI] [PubMed] [Google Scholar]
- 25.Shmueli A. The visual analogy rating scale of health-related quality of life: an examination of end-digit preferences. Health Qual Life Outcomes. 2005;3:2–6. doi: 10.1186/1477-7525-3-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mannerkorpi K, Hernelid C. Leisure time physical activity instrument and physical activity at home and work instrument. Development, face validity, construct validity and test-retest reliability for subjects with fibromyalgia. Disabil Rehabil. 2005;27:695–701. doi: 10.1080/09638280400009063. [DOI] [PubMed] [Google Scholar]
- 27.Darnall BD, Sturgeon JA, Cook KF, Taub CJ, Roy A, Burns JW, et al. Development and validation of a daily pain catastrophizing scale. J Pain. 2017;18:1139–1149. doi: 10.1016/j.jpain.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Navarro-Ledesma S, Gonzalez-Muñoz A, Carroll J, Burton P. Short- and long-term effects of whole-body photobiomodulation on pain, functionality, tissue quality, central sensitisation and psychological factors in a population suffering from fibromyalgia: protocol for a triple-blinded randomised clinical trial. Ther Adv Chronic Dis. 2022;13. [DOI] [PMC free article] [PubMed]
- 29.Gómez-Pérez L, López-Martínez AE, Ruiz-Párraga GT. Psychometric properties of the spanish version of the Tampa Scale for Kinesiophobia (TSK) J Pain. 2011;12:425–435. doi: 10.1016/j.jpain.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 30.Larsson C, Hansson EE, Sundquist K, Jakobsson U. Kinesiophobia and its relation to pain characteristics and cognitive affective variables in older adults with chronic pain. BMC Geriatr. 2016;1–7. [DOI] [PMC free article] [PubMed]
- 31.Salvador EMES, Franco KFM, Miyamoto GC, Franco YRDS, Cabral CMN. Analysis of the measurement properties of the Brazilian-Portuguese version of the Tampa Scale for Kinesiophobia-11 in patients with fibromyalgia. Braz J Phys Ther Revista Brasileira de Fisioterapia. 2021;25:168–174. doi: 10.1016/j.bjpt.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bandura A. Self-efficacy: toward a unifying theory of behavioral change. Psychol Rev. 1997;84:191–215. doi: 10.1037//0033-295x.84.2.191. [DOI] [PubMed] [Google Scholar]
- 33.Jurado M del MM, Pérez-Fuentes M del C, Ruiz NFO, Márquez MDMS, Linares JJG. Self-efficacy and emotional intelligence as predictors of perceived stress in nursing professionals. Medicina (Lithuania). 2019;55. [DOI] [PMC free article] [PubMed]
- 34.López-Roig S, Pastor-Mira MÁ, Núñez R, Nardi A, Ivorra S, León E, et al. Assessing self-efficacy for physical activity and walking exercise in women with fibromyalgia. Pain Manag Nurs. 2021;22:571–578. doi: 10.1016/j.pmn.2021.05.007. [DOI] [PubMed] [Google Scholar]
- 35.J C. Statistical power analysis for the behavioral sciences, 2nd edn. Hillsdale: Lawrence Erlbaum Associates; 1988. p. 1390.
- 36.Farrar JT, Young JP, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94:149–158. doi: 10.1016/S0304-3959(01)00349-9. [DOI] [PubMed] [Google Scholar]
- 37.Bourgault P, Lacasse A, Marchand S, Courtemanche-Harel R, Charest J, Gaumond I, et al. Multicomponent interdisciplinary group intervention for self-management of fibromyalgia: a mixed-methods randomized controlled trial. PLoS ONE. 2015;10:1–26. doi: 10.1371/journal.pone.0126324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9:105–121. doi: 10.1016/j.jpain.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 39.Cheng K, Martin LF, Slepian MJ, Patwardhan AM, Ibrahim MM. Mechanisms and pathways of pain photobiomodulation: a narrative review. J Pain. 2021;22:763–777. doi: 10.1016/j.jpain.2021.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vassão PG, de Souza ACF, da Silveira Campos RM, Garcia LA, Tucci HT, Renno ACM. Effects of photobiomodulation and a physical exercise program on the expression of inflammatory and cartilage degradation biomarkers and functional capacity in women with knee osteoarthritis: a randomized blinded study. Adv Rheumatol. 2021;61. [DOI] [PubMed]
- 41.Logan RM, Al-Azri AR, Bossi P, Stringer AM, Joy JK, Soga Y, et al. Systematic review of growth factors and cytokines for the management of oral mucositis in cancer patients and clinical practice guidelines. Support Care Cancer. 2020;28:2485–2498. doi: 10.1007/s00520-019-05170-9. [DOI] [PubMed] [Google Scholar]
- 42.Camolesi GCV, Marichalar-Mendía X, Padín-Iruegas ME, Spanemberg JC, López-López J, Blanco-Carrión A, et al. Efficacy of photobiomodulation in reducing pain and improving the quality of life in patients with idiopathic burning mouth syndrome. A systematic review and meta-analysis. Lasers Med Sci. 2022:2123–33. [DOI] [PMC free article] [PubMed]
- 43.Gross AR, Dziengo S, Boers O, Goldsmith CH, Graham N, Lilge L, et al. Low level laser therapy (LLLT) for neck pain: a systematic review and meta-regression. Open Orthop J. 2013;7:396–419. doi: 10.2174/1874325001307010396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tripodi N, Feehan J, Husaric M, Sidiroglou F, Apostolopoulos V. The effect of low-level red and near-infrared photobiomodulation on pain and function in tendinopathy: a systematic review and meta-analysis of randomized control trials. BMC Sports Sci Med Rehabil. 2021;13:1–13. doi: 10.1186/s13102-021-00306-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sobral APT, Sobral SDS, Campos TM, Horliana ACRT, Fernandes KPS, Bussadori SK, et al. Photobiomodulation and myofascial temporomandibular disorder: systematic review and meta-analysis followed by cost-effectiveness analysis. J Clin Exp Dent. 2021;13:e724–e732. doi: 10.4317/jced.58084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Neto FCJ, Martimbianco ALC, de Andrade RP, Bussadori SK, Mesquita-Ferrari RA, Fernandes KPS. Effects of photobiomodulation in the treatment of fractures: a systematic review and meta-analysis of randomized clinical trials. Lasers Med Sci. 2020;35:513–522. doi: 10.1007/s10103-019-02779-4. [DOI] [PubMed] [Google Scholar]
- 47.Yang M, Yang Z, Wang P, Sun Z. Current application and future directions of photobiomodulation in central nervous diseases. Neural Regen Res. 2021;16:1177–1185. doi: 10.4103/1673-5374.300486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liebert A, Bicknell B, Johnstone DM, Gordon LC, Kiat H, Hamblin MR. “Photobiomics”: can light, including photobiomodulation, alter the microbiome? Photobiomodul Photomed Laser Surg. 2019;37:681–693. doi: 10.1089/photob.2019.4628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tomazoni SS, Almeida MO, Bjordal JM, Stausholm MB, Machado CDSM, Leal-Junior ECP, et al. Photobiomodulation therapy does not decrease pain and disability in people with non-specific low back pain: a systematic review. J Physiother. 2020;66:155–165. doi: 10.1016/j.jphys.2020.06.010. [DOI] [PubMed] [Google Scholar]
- 50.Passarella S, Casamassima E, Molinari S, Pastore D, Quagliariello E, Catalano IM, et al. Increase of proton electrochemical potential and ATP synthesis in rat liver mitochondria irradiated in vitro by helium-neon laser. FEBS Lett. 1984;175:95–99. doi: 10.1016/0014-5793(84)80577-3. [DOI] [PubMed] [Google Scholar]
- 51.Poyton RO, Ball KA. Therapeutic photobiomodulation: nitric oxide and a novel function of mitochondrial cytochrome c oxidase. Discov Med. 2011;11:154–159. [PubMed] [Google Scholar]
- 52.Chung H, Dai T, Sharma SK, Huang Y-Y, Carrol JD, Hamblin MR. The nuts and bolts of low-level laser (light) therapy. Ann Biomed Eng. 2012;40:516–533. doi: 10.1007/s10439-011-0454-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Antonialli FC, De Marchi T, Tomazoni SS, Vanin AA, dos Santos GV, de Paiva PRV, et al. Phototherapy in skeletal muscle performance and recovery after exercise: effect of combination of super-pulsed laser and light-emitting diodes. Lasers Med Sci. 2014;29:1967–1976. doi: 10.1007/s10103-014-1611-7. [DOI] [PubMed] [Google Scholar]
- 54.Jonsjö MA, Åström J, Jones MP, Karshikoff B, Lodin K, Holmström L, et al. Patients with ME/CFS (myalgic encephalomyelitis/chronic fatigue syndrome) and chronic pain report similar level of sickness behavior as individuals injected with bacterial endotoxin at peak inflammation. Brain Behav Immun Health. 2019;100028. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.