Abstract
Osteosarcoma (OGS) is the most common primary bone tumor in children and adolescents which requires a multidisciplinary approach to management. Although chemotherapy and surgery can cure more than half of localized OGS cases, the unique challenges faced by resource-limited countries like India make this outcome difficult to achieve. Various questions in the management of OGS including role of high-dose methotrexate (HDMTX) in neoadjuvant setting, triplet vs doublet chemotherapy, intensification of chemotherapy based on response in setting of doublet, and indigenous prosthesis in setting of limb salvage need to be defined. Similarly, in the metastatic and recurrent setting, questions regarding intent of treatment, indications of chemotherapy, timing of surgery, and role of targeted therapies need clarification. Lack of randomized trials from India makes definite conclusions difficult, but an attempt can be made to define the best approach in the Indian scenario from available evidence. Hence, a critical review of literature from India and the West was done to define possible management approaches and highlight the lacuna for future research.
Keywords: Chemotherapy in osteosarcoma, Indian perspective, Limb salvage surgery, High-dose methotrexate, Relapsed osteosarcoma
Introduction
Osteosarcoma (OGS) is the most common primary malignant bone tumor in children and adolescents [1]. It is a systemic disease with 80% of non-metastatic patients having micrometastasis at presentation [2]. Presence of overt metastasis in 20–30% of patients at presentation and significant benefit of adjuvant chemotherapy in localized OGS demonstrated in the early studies (61% 6-year overall survival (OS) with chemotherapy compared to 11% without chemotherapy) has given further credence to the systemic nature of disease and need for systemic therapy [ 3–5]. However, the outcomes achieved in Western studies are often difficult to replicate in the Indian setting due to a variety of factors including advanced disease at presentation, limited facilities for providing supportive care making it difficult to administer intensive chemotherapy, and lack of surgical expertise. Management protocols are often institution-specific and adapted to availability of resources at a particular center. A limited number of studies on OGS have been published from India and are mostly retrospective in nature. A previous review by Ramaswamy et al. briefly highlighted Indian data on various bone and soft tissue sarcomas; however, a more comprehensive discussion on various aspects of epidemiology and management of OGS from an Indian perspective was warranted [6]. Hence, we did a review of published Indian studies on OGS and attempted to fill in the lacuna by appropriate Western literature to give a comprehensive picture on the best approach to management of OGS in the Indian scenario.
Is Epidemiology and Clinical Presentation of OGS different in India?
OGS has a bimodal age distribution with distinct epidemiology in children and adults. Several Indian studies have focused on epidemiology of bone tumors (Table 1) [7–13]. In the largest study by Gulia et al., looking at the profile of bone tumors registered at bone and soft tissue clinic of Tata Memorial Hospital (TMH) over one year, OGS was the most common primary malignant bone tumor with 60% of patients presenting in the second decade, 75% being localized at diagnosis, and 47% arising at the lower end of femur, which is the most common site followed by the upper end of tibia [27%] and humerus [10%] [9]. A striking male preponderance was also observed, which is not seen in Western studies. The lung was the most common site of metastasis [84%] followed by bone [14%]. OGS was also the most common primary bone tumor in studies by Swaminathan et al. and the Hospital Based Cancer Registry [HBCR] report of 2012–2014 [7, 14]. Although some studies have reported Ewing Sarcoma as the commonest primary bone malignancy, numbers are small. Peak in the second decade with the lower end of femur being the most common primary site has been consistently reported in all studies from India. [7–13] This data is consistent with previously published Western literature [15–17]. In a large study from our center of 237 OGS patients, 95% had appendicular skeleton involvement, 42% had symptom duration of more than 4 months, 44% had tumor size more than 10 cm, 5% had pathological fracture, 71% had elevated alkaline phosphatase (ALP), and 50% had an elevated lactate dehydrogenase (LDH) [18]. Higher proportion of patients with large tumor volume and metastasis at presentation (25% in data from TMH and 31% in a study from our institute) points towards advanced disease at diagnosis in our population, which might also explain the higher prevalence of laboratory abnormalities than reported in Western literature [9, 18–20] Overall, although age and site of OGS in Indian patients is similar to those in the West, male preponderance, longer symptom duration, and higher prevalence of laboratory abnormalities have been consistently observed in Indian studies.
Table 1.
Epidemiological data from India on bone sarcomas
| Study details | State from which study published | No of patients | Prevalence of OGS-AYA/adult | Comments |
|---|---|---|---|---|
| Swaminathan et al, International Journal of Cancer, 2008 [7] | Chennai | 1334 childhood cancers | OGS most common malignant bone tumor followed by Ewing sarcoma; CNS tumors were most common childhood solid tumors | |
| Jain et al, IJMPO, 2011 [8] | Karnataka | 117 (bone tumors) | Most common malignant bone tumor in this study accounting for 35% of all malignant bone tumors | Lower end of femur commonest site, peak age 11–20 years |
| Gulia et al, IJC, 2016 [9] | Mumbai | 1203 (bone tumors) | Most common primary malignant bone tumor | 60% patients aged 11–20 years, 75% localized at diagnosis, 47% lower end of femur |
| Sharma et al, IJMPO, 2017 [10] | Kashmir | 303 (pediatric solid tumors) | 2nd most common bone tumor after Ewing sarcoma, and 5th most common solid tumor overall | 80% diagnosed in adolescents, 90% localized at presentation |
| Singh et al, IJMPO, 2016 [11] | Delhi | 287 (AYA tumors) | Among bone tumors (28), Ewing sarcoma was the most common (13) followed by osteosarcoma (11) | |
| Kakkar et al, SAJC, 2017 [12] | Delhi | 1077 AYA cancers (15–39 years) | Ewing sarcoma was the most common primary malignant bone tumor followed by OGS | |
| Pandey et al, SAJC, 2019 [13] | Bihar | 105 (pediatric solid tumors) | Ewing sarcoma was the most common primary bone tumor, osteosarcoma was rare | Median age 13 years |
IJMPO Indian Journal of Medical and Pediatric Oncology, IJC Indian Journal of Cancer, SAJC South Asian Journal of Cancer, AYA adolescent and young adult, OGS osteosarcoma
What determines prognosis in Non-Metastatic OGS?
The two most important prognostic factors are stage at presentation and grade of response to neoadjuvant chemotherapy (NACT) [21]. A number of Indian studies have reported on prognostic factors in both non-metastatic and metastatic OGS; they are summarized in Table 2 [22–31]. In a large study from our center, duration of symptoms >4 months in patients whose disease remained localized and good performance status (PS) predicted for better event-free survival (EFS) and good PS and normal alkaline phosphatase (ALP) predicted for better OS in non-metastatic OGS. Interestingly, response to NACT did not predict survival [18]. Previous large Western datasets and a large study from India have established response to NACT as one of the important prognostic factors in OGS [22, 30, 31]. Intensification of chemotherapy done at our center in poor responders could have nullified the prognostic impact of response to NACT and this should be considered an outlier.
Table 2.
Indian studies evaluating prognostic factors in osteosarcoma
| Study details | Setting (localized vs metastatic) | No. of patients | Prognostic factors | Comments |
|---|---|---|---|---|
| Natarj et al, JSO, 2017 [18] | Localized | 237 |
Duration of symptoms >4 months and good PS predicted better EFS Good PS and normal ALP predicted better OS |
Response to NACT did not predict survival; all patients with poor response to NACT had treatment escalation |
| Puri et al, JSO, 2017 [22] | Both |
853 738—localized 115—metastatic |
Size >8cm, type of surgery (better OS in limb salvage), and chemotherapy induced necrosis (>90%) predicted for overall survival Site of tumor (long bones), limb salvage surgery and <90% necrosis predicted for higher local recurrence |
Metastatic OGS not analyzed |
|
Poudel et al, JSO 2017 [23] |
Localized | 95 | Biopsy from outside treating center and delay in surgery after NACT associated with local recurrence; high tumor volume >200cc does not predict local recurrence | |
| Bajpai et al, Pediatric blood and Cancer, 2013 [24] | Localized | 124 | Chemotherapy compliance was a predictor for good histological response, did not predict for survival | Longer follow-up may be needed to show significance on survival outcomes |
| Rastogi et al, International Orthopedics, 2012 [25] | Localized | 40 | Raised VEGF levels in OS patients compared with healthy controls, no correlation with tumor size, site, recurrence | |
|
Bajpai et al, JPHO, 2011 [26] |
Both (treated with curative intent) | 31 | PET-CT predicts histological response to NACT | |
|
Bajpai et al, Pediatric Radiology, 2011 [27] |
Both (treated with curative intent) | 31 | Pre and post chemotherapy size on MRI predicts necrosis, diffusion per unit volume can predict NACT response | |
|
Bajpai et al, Pediatric Blood and Cancer, 2009 [28] |
Both (treated with curative intent) | 31 | Post-NACT VEGF expression as well as VEGF change following NACT significantly correlated with histological necrosis. | |
| Bakhshi et al, JPHO, 2009 [29] | Both | 63 | Her2/neu or p53 expression not related to grade or stage of tumor; chondroblastic subtype associated with her2 positivity | |
| Kumar et al, JCRT, 2006 [30] | Both | 49 (20 cases where IHC was available) | Her2 expression by IHC did not correlate with prognosis | |
| Nataraj et al, Clinical and Translational Oncology, 2015 [31] | Metastatic | 102 |
Elevated ALP and number of metastasis >3 predicted lower EFS Elevated ALP, >3 metastasis, and margin positivity predicted for lower OS |
JSO Journal of Surgical Oncology, JPHO Journal of Pediatric Hematology and Oncology, JCRT Journal of Cancer Research and Therapeutics, PS performance status, EFS event-free survival, OS overall survival, NACT neoadjuvant chemotherapy, VEGF vascular endothelial growth factor, ALP alkaline phosphatase, IHC immunohistochemistry, OGS osteosarcoma
The effect of tumor volume on local recurrence is controversial. Although previous studies have suggested that larger size increases chances of local recurrence [32–34], both large studies from India did not find this association [22, 23]. Rather, delay in surgery after NACT is a more important prognostic factor as demonstrated in our study and is a major logistic issue in Indian scenario, which needs to be addressed [23]. Therefore, planning for surgery including logistics must begin as soon as chemotherapy is initiated. Newer markers including dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI), positron emission tomography-computed tomography (PET-CT), and change in tissue vascular endothelial growth factor [VEGF] expression post NACT can predict histologic necrosis, though this data needs prospective validation before incorporation in routine clinical practice [26–28]. Also, the prognostic significance of Her2/neu expression, CXCR4, p53, and micro RNA expression needs to be better defined before they can be used routinely [21, 29, 30].
Systemic Therapy in Non-metastatic OGS
Survival with OGS has improved significantly since the introduction of systemic therapy in the 1970s. A systematic review showed improvement in survival from 16% with local therapy alone to more than 70% with systemic chemotherapy, thus establishing the rationale for the same [35]. However, these improvements have been largely reported in developed nations, with a gross mismatch seen in low- and middle-income countries (LMICs). NACT is the standard of care in Indian scenario as it gives time to prepare for logistics for surgery including prosthesis and reduction in tumor size and vascularity may allow for limb sparing surgery (LSS), although data to support the second hypothesis are scarce. The only randomized trial comparing NACT to adjuvant chemotherapy (ACT) showed equivalent outcomes in both [36]. Multiagent protocols tested by various cooperative groups have reported good histological response in 40–60% cases with long-term EFS of 40–70% and OS of around 50–80% with no protocol clearly superior to other and no evidence to intensify treatment based on histological response [37–44] (Table 3). High-dose methotrexate (HDMTX) is an integral component of most Western protocols [37–41, 43, 44], with most large Indian centers adopting a HDMTX-free approach [18, 45–47] . We have summarized the evidence from these studies in Tables 3 and 4 and various unanswered questions are discussed below.
Table 3.
Various international neoadjuvant protocols for osteosarcoma with outcomes
| Study details | N | Chemotherapy | Good histological response rate (>90% necrosis) | EFS | OS |
|---|---|---|---|---|---|
| MSKCC T10 [37] | 153 |
Preop-Mtx Post op- GR-MTX, BCD PR-APBCD |
34% | 72% (5years) | 82% (5years) |
| MSKCC T12 [38] | 73 |
Preop-MTX+BCD Post op GR-MBCD PR-AP |
44% | 73% (5years) | 78% (5years) |
|
IOR OS-2 39 |
164 |
Preop-MAP Post op GR-MAP PR-MAP + IE |
71% | 75% (5years) | |
| EOI study 2 [40] | 391 |
Arm A-AP ARM B MA + BCP/AP, similar to T10, not response tailored |
30% | 44% (5years) | 55% (5years) |
| EOI study 3 [41] | 504 | DP with g-CSF | 51% | 37% (5years) | 58% (5years) |
| COSS 86 [42] | 171 | MAP, I in high risk | 68% | 66% (10years) | 75% (10years) |
| INT0133 [43] | 617 | MAP ± I and mifamurtide | 45% | 64% (6years) | 74% (6years) |
| EURAMOS 1 [44] | 716 |
Preop-MAP Post OP GR-MAP PR-MAP/IE |
72.6% with MAP | 60% vs 57% at 3 years | OS 77% vs 72% at 3 years (no difference) |
MTX/M methotrexate, A adriamycin, P cisplatin, I ifosfamide, E etoposide, B bleomycin, EFS event-free survival, OS overall survival, GR good response, PR poor response, g-CSF granulocyte colony-stimulating factor
Table 4.
Indian studies evaluating chemotherapy protocols and outcomes is non-metastatic osteosarcoma
| study details | No. of patients of OS | Protocol used | Time interval | Type of surgery | Outcomes and factors predicting survival | Toxicity |
|---|---|---|---|---|---|---|
| Nataraj et al, JSO, 2015 [18] | 237 |
3# NACT-AP ACT GR-3# AP PR-IE alternate with AP (6#) Cumulative dose of A—450mg/m2 P—720mg/m2 I—15g/m2 |
2003–2012 | 70% limb salvage |
GR—25% 5-year EFS—36% 5-year OS—50% Duration of symptoms >4 months and good PS predicted better EFS Good PS and normal ALP predicted better OS |
Not reported |
| Bajpai et al, Pediatric Blood and Cancer, 2019 [45, 56] |
41 94 385 |
OGS 99—dyads of IAP alternate, total 6# (A—60mg/m2; P—100mg/m2) Cumulative doses were A—240 mg/m2; P—400 mg/m2; and I—40 g/m2. OGS99 enhanced-3# NACT and 6# ACT, etoposide in ACT, increased dose of P to 120mg/m2, doxorubicin to 75mg/m2 The cumulative doses of these drugs were A—375 mg/m2; p—480 mg/m2; I—45 g/m2; and E—1800 mg/m2. OGS 12 4# NACT and 4# ACT, removed etoposide The cumulative doses were P—600 mg/m2; A—300 mg/m2; and I—54 g/m2 |
2000–2005 2006–2010 2011–2016 |
GR—61% 5-year EFS 38% (95% CI, 21.248–55.352) 5-year OS—not evaluable (med f/up—19 months) GR—63% 5-year EFS—50% (95% CI, 39.216–60.384) 5-year OS—60% (95% CI, 49.812–70.588) Med f/up—86 months GR—58% 5-year EFS—62% (95% CI, 50.624–62.776) 5-year OS 73% (95% CI, 66.816–78.184) Median f/up—34 months |
FN—51% FN—63% (with primary g-CSF) FN—40% (with primary g-CSF) |
|
| Sukumaran et al, Indian Journal of Orthopedics, 2018 [46] | 62 (all pediatric <14 years), 44 localized | IAP | 2008–2013 | 87% limb salvage |
GR-72% 3-year DFS—88.2% 3-year OS—54.9% Localized disease an female sex associated with better DFS |
40% grade 3/4 toxicity, 1 treatment related death, 90% completed planned 6 cycles |
| Shanmugam et al, IJMPO, 2019 [47] | 20 | AP±I | 60% limb salvage |
Median OS—17 months (not well reported) Completion of chemotherapy associated with better survival |
Not reported |
IJMPO Indian Journal of Medical and Pediatric Oncology, JSO Journal of Surgical Oncology, A adriamycin, P cisplatin, I ifosfamide, E etoposide, DFS disease-free survival, EFS event-free survival, OS overall survival, GR good response, PR poor response, NACT neoadjuvant chemotherapy, ACT adjuvant chemotherapy, FN febrile neutropenia, PS performance status, ALP alkaline phosphatase
Controversies and Challenges in Indian Setting—Why is HDMTX not standard?
Currently, the three-drug regimen of HDMTX at 12g/m2 with cisplatin and doxorubicin used in the control arm of EURAMOS-1 trial is the standard of care in developed countries. However, the data for benefit of HDMTX is uncertain and comes mainly for phase II trials and retrospective analysis [34, 37, 38]. A study by the Co-operative German-Austrian-Swiss Osteosarcoma study group (COSS-82 study) established the importance of early administration of doxorubicin and cisplatin (AP) in combination with HDMTX making MAP (methotrexate, adriamycin, cisplatin) one of the standard regimens for OGS [48]. The benefit of HDMTX was questioned after randomized trials by the European Osteoarcoma Intergroup (EOI). A phase III study by EOI compared HDMTX at 8g/m 2 with AP to AP alone. Majority of patients received NACT with surgery planned after 9 weeks of chemotherapy. Five-year disease-free survival (DFS) was better in the AP arm (57% vs 41%) with no significant difference in OS. However, the dose intensity of P and A in the three-drug arm was significantly lower compared to the two-drug arm (66%) with AP cycles at 31-day intervals instead of planned 21 days in the triple-drug arm. The HDMTX dose was also suboptimal (8g/m2 compared to standard of 12g/m2) thus limiting the power of the study to make definite conclusions [49]. The second EOI study failed to show superiority of an intensive protocol similar to the T10 protocol developed at Memorial Sloan Kettering Cancer Center (MSKCC) over AP. This study was not powered to detect the benefit of methotrexate. Moreover, only 50% of patients were able to complete the intensive protocol compared to 94% completion rates in AP arm and this likely contributed to lack of benefit of intensive therapy. [40] A subsequent meta-analysis by Anninga et al. showed superiority of three- or four- (MAP or MAP plus ifosfamide (I)) vs the two-drug regimen of AP in terms of both EFS and OS. However, the meta-analysis had only two above mentioned randomized trials, thus limiting the conclusions regarding the benefit of methotrexate [35]. A second Cochrane meta-analysis on this subject was also inconclusive [50]. Despite the limited prospective evidence for HDMTX, it has been accepted as a part of standard chemotherapy protocol for OGS all around the world. The recently reported EURAMOS-1 study which was a multicentric trial that recruited patients from 326 centers across 17 countries also used MAP in the control arm reflecting global practice [44]. However, administration of HDMTX requires hospital admission and therapeutic drug monitoring (TDM) of methotrexate levels. Logistic issues including lack of availability of TDM, unavailability of glucarpidase, and shortage of admission beds have limited the use of HDMTX in Indian centers, especially in the first-line setting. Various alternative approaches have been used, which are subsequently discussed.
What Are the Alternative Approaches to HDMTX?
To circumvent the issues with delivery of HDMTX and prevent possible undertreatment of patients with doublet chemotherapy, various three-drug approaches have been tried including combination of ifosfamide (I) with AP in all cycles, alternating dyads of IAP ± etoposide (E) and treatment guided by histological response (Table 5) [45–47, 51–55]. Most of the evidence for these approaches comes from small phase II studies with best outcomes having been achieved with alternate regimen of the above drugs as reported by Daw et al. [55] However, a total of twelve cycles were used and toxicity was significant with almost 100% patients having one episode of grade four neutropenia. A similar but modified regimen has been adapted by TMH in India [44]. Their initial protocol consisted of six cycles of alternating IAP (OGS 99), three as NACT, and three as ACT with 5-year EFS of 38% in 41 evaluable patients. This protocol was further enhanced by increasing the number of cycles to nine (six in adjuvant), increasing dose of P and adding etoposide (E) to adjuvant chemotherapy (OGS 99 enhanced). Five-year EFS of 50% was reported with this protocol; however, 63% patents developed febrile neutropenia even with primary growth factor prophylaxis. To further improve outcomes, cumulative doses of IAP were further increased in OGS 12 protocol and a total of eight cycles were delivered as alternating dyads. Five-year EFS of 62% was achieved in 385 evaluable patients which is quite similar to Daw protocol and historical methotrexate regimens [55, 56]. However, both hematological and non-hematological toxicity was significant. Around 40% patients had febrile neutropenia, 36% and 51% had grade 3/4 thrombocytopenia and anemia respectively, 14% had grade 3/4 mucositis, and 9% had grade 3/4 diarrhea. Therefore, triplet chemotherapy without HDMTX is attractive but comes with its own set of complications. Another alternative is intensification based on histological response and both these approaches have not been directly compared in a randomized trial. Although the largest trial to test intensification of chemotherapy based on histological necrosis did not show any benefit of intensification (EURAMOS-1) [44], the results of this trial are not directly applicable to our setting as control arm used a triplet HDMTX containing chemotherapy regimen. No randomized trial has proven or refuted the benefit of intensification of adjuvant chemotherapy on the basis of response to NACT after a doublet non-HDMTX induction. In a study from our institute using uniform doublet (AP) chemotherapy as NACT with addition of IE alternating to AP in poor responders, 5-year EFS of 36% and OS of 50% in 237 patients were demonstrated [18]. These outcomes are similar to those achieved in EOI studies using a doublet regimen [57]. Although outcomes with triplet therapy may appear to be superior based on cross-study comparisons, their toxicity is significant, randomized trials have not shown superiority of HDMTX based triplet therapy over AP, and no randomized evidence exists to support superiority of non-HDMTX triplet therapy over doublet. However, if resources for administration and monitoring of HDMTX are available or if a center has experience in managing toxicities of three drug protocols while maintaining treatment intensity, they can be considered acceptable strategies.
Table 5.
International data evaluating non-high-dose methotrexate three drug approaches in osteosarcoma
| Study details | N | Chemotherapy | Good path response rate | OS (5years) % | EFS (5years) % |
|---|---|---|---|---|---|
| Patel et al [51], American Journal of Clinical Oncology, 2002 | 12 | IAP | 63% | 83 | 75 |
| Piperno Neumann et al [52], JCO, 2006 | 32 | IAP±E for poor risk | 37% | 86 | 74 |
| Tunn and Reichardt et al [53], Onkologie, 2007 | 53 | AP+VCr+CYC | NA | 71 | 60.4 |
| Assi et al [54], Current Oncology, 2010 | 32 | IAP | 47% | 69 | 65 |
| Daw et al [55], Cancer, 2010 | 75 | CARBO AI | 61% | 79 | 66.7 |
I ifosfamide, A adriamycin, P cisplatin, CYC cyclophosphamide, Carbo carboplatin, E etoposide, EFS event-free survival, OS overall Survival, JCO Journal of Clinical Oncology
Surgical Management of Localized OGS—Is Limb Salvage Feasible in Indian setting?
Surgical resection is the treatment of choice for local control and should be a wide excision with a cuff of normal tissue including previously done biopsy tract, drain tract, or contaminated tissue. Oncological outcome must always take precedence over functional outcome. When oncological outcomes are expected to be the same, limb salvage should be preferred. There is convincing evidence from multiple Indian and international studies that LSS improves survival albeit with a slightly higher risk of local recurrence and is the procedure of choice where expertise is available [18, 22, 23, 58, 59]. High tumor volume is not a contra-indication for limb salvage surgery [58]. The specific issues that need to be dealt with in Indian scenario include waiting time for surgery, cost of implants, large tumor volume at presentation, and lack of expertise in oncological surgery [23, 62]. In a retrospective study from our center, every week, delay in surgery after NACT increased the risk of local recurrence [23]. The delay can be attributed to a variety of factors including financial difficulties of the patient, delay in release of funds from welfare schemes, and long waiting lists in crowded government hospitals. While tumor volume plays a significant role in the complexity of surgical procedure, the relation to local recurrence is controversial [60, 61]. If adequate oncological margins can be achieved, tumor volume is not a contraindication for limb salvage surgery. 58 60,61 In our institutional experience of non-metastatic OGS undergoing LSS, 47.3% (45/95) patients presented with a tumor volume >200ml [23]. As far as cost is concerned, the type of surgery and implant used is heavily dependent on the paying capacity of the patient due to lack of insurance or centralized health schemes available to most patients. Cost of indigenous endoprosthetic (>1 lakh rupees) and expandable implants (>5 lakh rupees) remains a major obstacle to their routine use with autograft being the most widely practiced mode of reconstruction in India [62]. The lack of availability of well-trained orthopedic oncologists at peripheral centers also contributes to late presentation of these patients to central government hospitals and long waiting list for surgeries thus contributing to poor outcomes of these patients [62] [63],
.
What Are the Reconstruction Options Available After Limb Salvage?
There are various types of reconstruction options available after either LSS or amputation and can be broadly divided into biological and non-biological options. Various Indian studies looking at outcomes with different surgical approaches are summarized in Table 6 [64–71].
Table 6.
Indian studies evaluating surgical (limb salvage/amputation/ECRT) outcomes in osteosarcoma
| Study details | No. of OS patients | Procedure type | Delay in presentation | Metastasis | Local recurrence | Other adverse factors | Outcomes |
|---|---|---|---|---|---|---|---|
| Tiwari et al, Journal of Clinical Orthopedics and Trauma, 2017 [64] | 25 | Biological-vascularized fibular graft ± ECRT (8 patients) | N/A | Non-metastatic | 2 patients | Complication rate—14.2% in ECRT group vs 62.5% in no ECRT | Limb salvage achieved in 24/25 patients |
| Puri et al, Journal of Surgical Oncology, 2018 [65] | 38 | ECRT—50gy for diaphyseal tumors | N/A | Non-metastatic | 10%—all soft tissue |
Infection—12% Fracture through ECRT graft—6% Removal of ECRT required—25% |
5 year OS—74% 5-year ECRT graft survival—79% |
| Nayak et al, Sarcoma, 2020 [66] | 61 |
ECRT vs no ECRT for diaphyseal OS 22—ECRT Rest-other forms of reconstruction, 7 vascularized bone transfer and 18 endoprosthesis |
N/A | Non-metastatic | 3 in ECRT and vs 1 in non ECRT |
5-year LRFS 85% vs 97% 5-year OS 70% vs 58% None of above statistically significant, non-randomized comparison |
|
| Natarajan et al, International Orthopedics, 2003 [67] | 82 | Limb Salvage surgery with Indigenous prosthesis in Proximal tibia OS | N/A | Non-metastatic | 4.8% | Infection—16 patients and fracture 12 patients | 5-year prosthesis survival 84% in overall cohort |
| Natarajan et al, International orthopedics, 2005 [68] | 165 (total 246) | Limb salvage surgery with custom made indigenous prosthesis | N/A | N/A | N/A | 16.6% overall complication rate, aseptic loosening 22 patients, and fracture-related failure 22 patients | Overall 10 year OS—76.9% (separate for OS N/A), 10-year prosthesis survival 74.2% |
| Agarwal et al, Clinical Orthopedics and Related research, 2007 [69] | 135 | Limb salvage surgery; 92 non-biological reconstruction with Indian made prosthesis, 23 rotationplasty, 20 no reconstruction | N/A | Non-metastatic | 15% | 25% pulmonary metastasis without local recurrence, 32% complication rate, 7% infection | Response to chemotherapy predicted survival |
| Chauhan et al, Indian Journal of Surgical Oncology, 2013 [70] | 21 | Limb Salvage Surgery, Indian made modular segmental replacement prosthesis in all patients | N/A | Non-metastatic | 3 patients of OS | 3 patients had infection, 3 patients had LR, 1 fracture, 5 pulmonary metastasis | 5-year prosthesis survival 84% |
| Tiwari et al, Indian Journal of Orthopedics, 2014 [71] | 51 | Limb Salvage surgery; 35—non biological reconstruction; 12—biological reconstruction | N/A |
46—non-metastatic 5—metastatic |
4 patients |
Early complications—5 patients Late complications—10 patients 1 patient with positive margin underwent amputation |
3-year EFS 61.8%, 3-year OS 66% Age <14 years and GR after NACT predicted for better EFS |
ECRT extracorporeal radiotherapy, EFS event-free survival, OS overall, LRFS locoregional recurrence-free survival, GR good response, LR local recurrence, NACT neoadjuvant chemotherapy
Biological Reconstruction
Fibula is the most common bone used as an autograft and can be either vascularized/non-vascularized depending upon whether the vascular pedicle was anastomosed or not. Defects >10cm usually require a vascularized fibular graft. One study added a single dose of radiation with vascularized fibula graft and showed decreased local recurrence and this concept should be explored in future studies [64]. Allograft reconstruction entails use of non-vascularized cadaveric bone. The graft can be chosen according to the patient’s anatomy. However, the use of allograft in India is limited by lack of availability at most Indian centers and limitations including non-union and fracture due to its non-vascularized nature.
Reimplantation of processed tumor done after extra corporeal radiation therapy (ECRT) is gaining popularity as one of the most common biological processes used in India. This involves killing the tumor cells within the affected bone following resection by a single radiation dose, usually 50 gray [72, 73]. The bone is then prepared with antiseptic solution and replaced into the defect. Irradiating tumor bone has the advantage of providing a size- and shape-matched graft with properties of an allograft [74]. The irradiated bone behaves like a dead elastic bone and provides just structural support while union happens by creeping substitution from the host end [75]. Re-implantation of processed bone presents some advantages over allograft implantation including availability of graft material, better osteotomy site match, better graft size match, possibility of soft tissue reconstruction, and no disease transmission problems. A large international study by Hong et al. evaluating ECRT for malignant bone tumors (37 with OGS) showed no local recurrence with 5-year OS of 85% [72]. Two studies from TMH and our institute have demonstrated the safety of this technique in bone sarcomas with 5-year OS of around 70% [65, 66, 74], although data are retrospective and superiority in functional outcome over customized prosthesis has not been proven. Other methods of extracorporeal tumor killing include pasteurization, alcohol, liquid nitrogen, and autoclaving. A recent retrospective study reported similar outcomes with radiation- and alcohol-induced tumor killing; however, more data is needed to establish equivalence [76].
Van Ness rotationplasty is another type of biological reconstruction that converts an above-the-knee amputation into a below-the-knee amputation. This is accomplished by resecting the tumor, rotating the lower leg 180 degrees, and reattaching the remaining distal tibia to the remaining proximal femur. It is indicated as a reconstruction option in skeletally immature children where endoprosthetic fitting may not be feasible. It is more commonly performed for lower extremity juxta-articular bone sarcomas which require resection of either distal femoral or proximal tibial physis. The major contraindication for rotationplasty include a dysfunctional sciatic nerve or extensive neurovascular encasement which precludes successful surgery [77].
Compared to amputation, it gives better functional outcome and reduces energy expenditure during gait. However, the main disadvantage of this technique is the cosmetic disfigurement of the resulting limb making it unacceptable to some patients [78]. The only study evaluating rotationplasty in Indian setting reported on 23 OGS patients. Although functional outcomes were acceptable, complication rates were high and psychological impact of the deformity was not assessed. Although rotationplasty remains an option in patients who cannot afford a prosthesis, its use is restricted and thorough preoperative counseling and post-operative physiotherapy are absolutely essential [79].
Non-biological Reconstruction
It involves replacing the defect with material such as metal (endoprosthesis) or bone cement (nail-cement-plate composite). Endoprosthetic reconstruction is commonly performed for intra-articular resections. However, the cost of these implants is a major obstacle to their usage in Indian scenario. Expandable prosthesis are even costlier and are an option when limb length discrepancy is expected to be >5cm. This has led to the use of endogenous custom-made prosthesis in a majority of centers. The two largest studies that have reported on the use of Indian indigenous prosthesis analyzed 135 and 165 OGS patients respectively [71, 72] with few other small studies supporting their use [73, 74]. Varying complication rates of 16.6–32% have been reported with aseptic loosening, infection, and periprosthetic fracture being the most common. However, prosthesis survival of around 80–90% at 5 years and 70–80% at 10 years has been consistently seen, which is similar to Western literature [80, 81]. No direct comparisons exist between endoprosthesis vs ECRT/fibular grafts and decisions are often individualized based on patients tumor and financial resources.
When Is Radiation Therapy Useful in OGS?
OGS has been conventionally thought to be radioresistant except for the small cell variety and no prospective trials have tested the utility of radiation in OGS. Although no head-to-head trials exist, surgery is preferred for local control whenever possible. However, a subset of patients may benefit from radiation in the definite and adjuvant setting. Traditionally, patients with OGS at unusual locations like the skull base, spine, and pelvis, where adequate surgical margins are difficult have been treated with definite radiation. Machak et al. reported one of the largest series of extremity OGS treated with definite RT. [82] A total of 31 patients were treated with a median 60gy of external beam radiation (EBRT) after induction chemotherapy with 5-year OS of 61%. Caceres et al. reported pathological complete response in 80% of patients treated with chemotherapy and 60gy EBRT in a series of 13 patients with 3-year OS of 75% [83]. For pelvic and unresectable head and neck osteosarcomas, 60–70gy EBRT is conventionally used. Use of other modalities of radiation have also been reported with one series that reported local control rates of 72% using proton-based RT in such locations [84]. However, two small studies from India have demonstrated that with proper patient selection, surgical management with limb sparing surgery is feasible in such locations with acceptable long-term functional outcome and survival. Local recurrence rates were high (23%) and limited number of OGS patients were treated [85, 86]. Hence, the decision should be based on expertise and experience of the surgical team after discussion in a multidisciplinary tumor board.
Data for adjuvant radiation in OGS are scarce. Delaney et al. reported on 41 patients with OGS of various sites who underwent gross tumor resection, subtotal resection, or biopsy only. All patients were treated with EBRT with a median dose of 66gy. Local control rates of 78.4% in patients with complete resection and 77% in those undergoing subtotal resection were reported thus showing utility of RT in preventing recurrence in patients with a margin positive resection and unable to undergo re-surgery [87]. In rare patients with positive margins, it is usually difficult to go for re-resection in our setting due to various logistic constraints. Therefore, our institutional policy is to go for adjuvant radiation in such cases based on the above evidence.
In patients with head and neck osteosarcoma, adjuvant radiation improves local control especially in patients with close margins or high-risk features including large tumor size, lymphovascular invasion, or soft tissue infiltration [88, 89].
Upfront Metastatic Osteosarcoma
What Determines Prognosis in Metastatic OGS?
Isolated lung metastases have better outcomes than patients with other bone/visceral metastasis [90]. No. of metastasis (≤3 vs >3), unilateral vs bilateral lung involvement, ALP, lactate dehydrogenase (LDH), and bone involvement are other important prognostic factors in metastatic OS [ 31, 91]. Two largest studies to evaluate metastatic OGS in Indian setting reported >3 lung metastasis, elevated ALP, and poor response to induction chemotherapy as predictors for poor outcome [31, 92].
Approach to Metastatic OGS
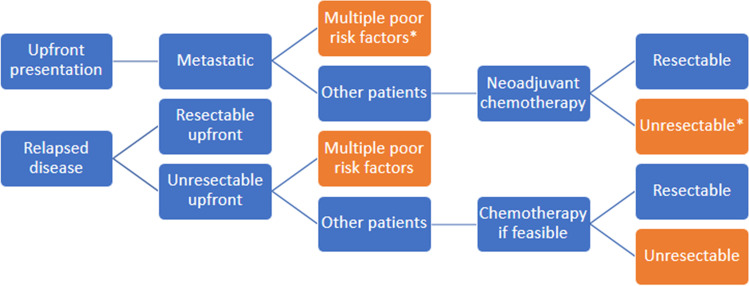
In patients who are candidates for aggressive therapy, neoadjuvant chemotherapy (same drugs as localized) and surgery of local site plus all resectable metastatic sites and adjuvant chemotherapy is the standard of care. Patients with multiple poor prognostic factors and extensive unresectable disease are treated with palliative intent therapy (Figure 1). We evaluated addition of IE post local site surgery to AP (alternate AP and IE post-surgery, maximum 11 cycles, 8# in those who underwent upfront local surgery outside) in 95 patients with upfront metastatic OGS. Lung metastasectomy was done after completion of all planned chemotherapy in patients whose disease persisted but did not progress during therapy. With this approach, 5-year OS was 28%, which is similar to Western literature [30, 31, 93, 94]. More than three lung metastasis and elevated ALP were prognostic for poor survival. Further intensification with higher cumulative doses of IAP did not improve outcomes [92]. Role of metronomic chemotherapy in bone sarcomas was refuted in a randomized trial from our center [95].
Fig. 1.
Management tree for metastatic/relapsed osteosarcoma; all patients with resectable disease should go for surgery at primary and metastatic sites; * poor risk factors include short remission duration <2 years, bilateral multiple metastases, extensive non-pulmonary metastases—these patients go for palliative intent therapy
Pulmonary Metastasectomy—For Whom and When?
There have been no randomized trials regarding the efficacy of pulmonary metastasectomy in OGS; however, for a selected subgroup of patients, it is regarded as a standard approach to treatment [96]. Relative contraindications for metastasectomy include poor PS, inadequate pulmonary reserve, multiple metastasis (≥4), time to relapse <12 months, synchronous metastasis, uncontrolled extrathoracic disease, pleural infiltration, mediastinal involvement, bilateral or central location, and poor response to second-line chemotherapy [93, 97–100]. Five-year survival of up to 40% has been reported in patients without any unfavorable features who are able to undergo complete resection [96, 101].
Metastasectomy can be done at interim assessment with limb surgery or as a separate surgery at the end of chemotherapy. In a recent retrospective study from India, 37 patients underwent delayed metastasectomy for resectable lung metastasis. Completion of systemic chemotherapy, disease-free interval (DFI) more than 2 years after surgery, and no post metastasectomy recurrence predicted better survival [102]. In our institute data reported above, 5-year survival was comparable to Western literature when a uniform delayed metastasectomy approach was employed. As it avoids futile surgery in patients who progress while on chemotherapy, this approach is particularly prudent in resource-limited settings like ours and in patients who have a stable disease after NACT [31, 92]. Indian data on metastatic OGS are summarized in Table 7.
Table 7.
Indian studies evaluating chemotherapy protocols and outcomes is metastatic osteosarcoma
| Study details | No. of patients of metastatic OS | Protocol used | Site of metastasis | Surgery | Outcomes and factors predicting survival | Toxicity (grade 3/4) |
|---|---|---|---|---|---|---|
| Bajpai et al, JGO, 2018 [92] | 80 |
OGS 12 4# NACT and 4# ACT, removed etoposide The cumulative doses were CDDP 600 mg/m2 , Dox 300 mg/m2, and Ifo 54 g/m2 |
83% lung metastasis, 44% bilateral, 3% bone only metastasis | 69 patients underwent surgery, 50 limb salvage and 40 metastasectomy |
GR—57% 4-year EFS—24% 4-year OS—27% Median f/up—32 months |
FN—51% Anemia—54% Thrombocytopenia—36% |
| Nataraj et al, Clinical and Translational Oncology, 2016 31 | 102 |
3# NACT—AP ACT IE alternate with AP (6#) Cumulative dose of A—450mg/m2 P—720mg/m2 I—15g/m2 |
67.6% lung only metastasis, 22.5% both lung and bone, 9% bone only | 56—local site surgery, limb salvage 50%, delayed metastasectomy at end of ACT in 5 patients who did not progress or did not have CR at lung lesion |
5-year EFS—12.7% 5-year OS—28.1% Median F/up—23 months |
Total—31.4% Neutropenia—20.6% Thrombocytopenia—3.9% Mucositis—3.9% Toxic deaths—3 (2.9%) |
| Ramanujan et al, Indian Journal of Cancer, 2020 [102] | 37 | IAP | Synchronous or metachronous resectable lung metastasis in all | Delayed metastasectomy after completion of chemotherapy in all patients, 22% needed two procedures |
GR—23% Median OS—38 ± 2.7 months Median post-metastasis survival was 23 ± 5.7 months 2-, 3-, and 5-year OS were 86 ± 5.8%, 60.8 ± 8.6%, and 20.7 ± 7.4%, respectively Completion of systemic chemotherapy, disease-free interval (DFI) of >2 years, and absence of pulmonary recurrence postmetastasectomy were predictors of survival |
|
| Pramanik et al, JAMA Oncology, 2018 [95] | 72 (bone sarcomas), progressive after at least 2 lines of chemotherapy | Thalidomide, celecoxib, etoposide, cyclophosphamide |
No benefit of oral metronomic therapy, 100% vs 94.6% progression at 6 months in placebo vs OMT group Median PFS—48 days; median OS—80 days |
JAMA Journal of American Medical Association, JGO Journal of Global Oncology, A adriamycin, P cisplatin, I ifosfamide, E etoposide, DFS disease-free survival, EFS event-free survival, OS overall survival, GR good response, PR poor response, NACT neoadjuvant chemotherapy, ACT adjuvant chemotherapy, FN febrile neutropenia, PS performance status, DFI disease-free interval, OMT oral metronomic therapy
Relapsed Osteosarcoma
Despite improvement in outcomes of localized and metastatic OS with use of multimodality therapy, 30–40% of patients eventually relapse and generally have poor long-term outcome ranging from 13 to 40% [103]. The lung is the most common site of recurrence (80%) followed by bone (10%). Isolated local recurrence is uncommon (4–10%) [104, 105]. Due to lack of Indian data on management of recurrent OS, management principles are extrapolated from Western literature.
Management of Lung Only Recurrence
The patterns of lung involvement at relapse depend on disease-free interval with extensive and bilateral involvement more common with early relapse [ 97]. Complete surgical resection has been shown to improve survival [101, 103]. Benefit of chemotherapy in this setting is controversial and does not seem to benefit patients who undergo complete surgical resection [106, 107]. Chemotherapy delays progression in unresectable disease but there is no evidence to suggest that it can convert unresectable disease to resectable [106, 107]. There is no universal standard chemotherapy regimen in this setting. Combinations of drugs not used in first line like etoposide with ifosfamide with or without carboplatin are most commonly used [108]. Other second-line options include cyclophosphamide topotecan, gemcitabine docetaxel, high-dose ifosfamide, single-agent gemcitabine, and HDMTX (if not used upfront) [108–113].
Management of Local Recurrence
Local recurrence is more common in axial OGS [114]. Initial concerns about higher local recurrence with limb salvage surgeries have been allayed with multiple institutional studies showing comparable outcomes to amputation [115]. For patients with local recurrence, surgery with adjuvant chemotherapy is generally practiced though evidence from randomized trials is lacking. Preoperative chemotherapy can be used if systemic recurrence is there in addition to local recurrence. General practice at most centers is to offer amputation as the standard oncological procedure for local recurrence as limb salvage in this setting is controversial [114]. However, local resection and limb salvage can be considered and depends on the resectability of the recurrence. Relapse more than 2 years after initial treatment and complete surgical resection is associated with improved outcomes [116].
Management of Bone Metastasis/Recurrence
Treatment of choice is surgical resection if disease is limited and resectable. Adjuvant chemotherapy is generally administered [117]. For patients with multiple bone lesions, palliative radiation either external beam or with radio-isotopes including samarium 153, strontium 89, or radium 223 based can be used [118].
Is There Any Role of Targeted therapy and Immunotherapy in Advanced OGS?
Activity of sorafenib has been demonstrated in two phase II trials with relapsed refractory OGS with 46% progression-free survival at 4 months [119]. Activity of regorafenib has been demonstrated in two double-blind placebo-controlled studies with response rates of 8–13%; however, stable disease was seen in 64% patients for 8 weeks. [120, 121] Cabozantanib was recently shown to have activity in relapsed Ewing’s and OGS in the phase II CABONE trial [122]. An objective response rate of 12% was demonstrated in the OGS cohort with a 6-month PFS of 33% These data provide proof of concept of antiangiogenic activity in OGS and form a basis for larger trials. Mifamurtide, an agent derived from Bacillus Calmette-Guerin (BCG), has been shown to be active in OGS but with discordant results in adjuvant and metastatic setting [ 123, 124]. Hence, it has not gained widespread acceptance and is not yet available in India. Data for immune checkpoint inhibitors is scarce with only 1/22 patients of OGS enrolled in SARC028 trial showing an objective response [ 125]. Other than limited efficacy as described, high cost limits the widespread use of targeted and immunotherapy in OGS in the Indian setting.
A Peek Into the Future—Role of NGS and Genomic Profiling
With few advancements in treatment and the dismal prognosis for patients with relapsed, refractory disease, OGS patients may benefit from deep molecular, genomic sequencing and comprehensive molecular profiling. NGS and array comparative genomic hybridization (aCGH) are the most promising approaches although the best sample, timing of testing, and technology are yet to be optimally defined [ 126]. Moreover, cost and availability of these technologies are major barriers to their adoption in routine clinical practice. Targetable mutations are rare in OGS with VEGFA, platelet-derived growth factor receptor (PDGFR), cyclin-dependent kinase 4 (CDK4), and mouse double minute 2 homolog (MDM2) being the most common potential targets seen in about 20% of patients [127]. Suehara et al. analyzed 71 OGS samples and proposed an algorithm for targeted therapy in relapsed OGS. Patients with 4q12 amplification involving PDGFRA/KDR could be targeted by pazopanib/regorafenib (15–20% of OGS), patients with 6q12 amplification causing VEGFA overexpression may be candidates for bevacizumab/sorafenib/pazopanib (20–25% of OGS), and patients with 12q13 amplification involving MDM2 and CDK4 can be potentially sensitive to palbociclib (10–15%) with the remaining 50–60% patients treated by conventional chemotherapy [128]. However, it must be remembered that evidence for use of these drugs is limited and cost is a major hurdle to their routine use. Therefore, NGS-guided therapy is still experimental and should be offered only in the context of a clinical trial.
Future Research Questions Relevant to Indian Scenario
Although outcomes have improved with advent of multimodality therapy especially in localized OGS, there are significant challenges in the Indian setting which need to be addressed. First, there needs to be more systematic recording of data to define the extent of the problem especially in population-based cancer registries (PBCRs). Most of the data on management of OGS from India is in the form of single institutional retrospective analysis with no randomized trials reported to date. The question of utility HDMTX and intensification of chemotherapy after doublet induction are particularly relevant to LMIC setting and can only be answered by well-designed randomized trials. With advent of newer radiation modalities like proton beam therapy, intensity modulated radiation therapy, and ECRT, role of radiation therapy for local control and adjuvant therapy needs to be revisited. Finally, targeted therapies and precision medicine hold promise in relapsed refractory setting but need more evidence. Such data may further revolutionize management of OGS.
Conclusion
The purpose of this article was to provide a comprehensive review of Indian literature on osteosarcoma and highlight the unique challenges faced while managing these patients. While majority of patients with localized OGS can be cured with multimodality therapy, delay in diagnosis, advanced disease at presentation, poor performance status, inability to complete planned chemotherapy, delay in surgery, lack of expertise for LSS at small centers, and high cost of prosthesis remain major challenges in the Indian scenario. Non-HDMTX-based doublet or triplet chemotherapy can be used with comparable outcomes to the West as shown in multiple Indian studies. HDMTX remains a standard approach where facilities for administration and monitoring are available. Early initiation of planning for surgery is essential to overcome logistic hurdles and provide optimal outcomes. LSS should be considered whenever feasible and indigenous prosthesis can be used to make reconstruction with a prosthesis affordable and within reach of more patients. For patients with metastatic osteosarcoma, disease burden and PS usually decide intent of therapy and outcomes are generally poor.
Author Contribution
A. M., D. P., and S. B. conceptualized the manuscript; A. M., S. G., and S. B. did the review of literature; A. M., D. P., S. G., and V. S. K. wrote the manuscript. S. B. and S. A. K. reviewed the manuscript and suggested revisions. A. M., D. P., V. S. K., S. G., S. A. K., and S. B. approved the final manuscript.
Declarations
Conflict of Interest
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Abhenil Mittal, Email: drabhenil@gmail.com.
Deepam Pushpam, Email: deepampushpam@gmail.com.
Shuvadeep Ganguly, Email: ganguly.shuvadeep@gmail.com.
Venkatesan Sampath Kumar, Email: venkatortho4@gmail.com.
Shah Alam Khan, Email: shahalamkhan70@gmail.com.
Sameer Bakhshi, Email: sambakh@hotmail.com.
References
- 1.Link MP, Eilber F. Paediatric oncology: OS. In: Pizzo PA, Poplack DG, editors. Principles and practice of paediatric oncology. Philadelphia, PA: Lippincott; 1989. [Google Scholar]
- 2.CADE S. Osteogenic sarcoma; a study based on 133 patients. J R Coll Surg Edinb 1955 Dec;1(2):79-111 [PubMed]
- 3.Link MP, Goorin AM, Miser AW, Green AA, Pratt CB, Belasco JB, et al. The effect of adjuvant chemotherapy on relapse-free survival in patients with OS of the extremity. N Engl J Med. 1986;314(25):1600–1606. doi: 10.1056/NEJM198606193142502. [DOI] [PubMed] [Google Scholar]
- 4.Link MP, Goorin AM, Horowitz M, Meyer WH, Belasco J, Baker A, et al. Adjuvant chemotherapy of high-grade osteosarcoma of the extremity. Updated results of the Multi-Institutional Osteosarcoma Study. Clin Orthop Relat Res. 1991;270:8–14. doi: 10.1097/00003086-199109000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Meyers PA, Schwartz CL, Krailo M, Kleinerman ES, Betcher D, Bernstein ML, et al. Osteosarcoma: a randomized, prospective trial of the addition of ifosfamide and/or muramyl tripeptide to cisplatin, doxorubicin, and high-dose methotrexate. J Clin Oncol. 2005;23(9):2004–2011. doi: 10.1200/JCO.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 6.Ramaswamy A, Rekhi B, Bakhshi S, Hingmire S, Agarwal M. Indian data on bone and soft tissue sarcomas: a summary of published study results. South Asian J Cancer. 2016;5(3):138–145. doi: 10.4103/2278-330X.187587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swaminathan R, Rama R, Shanta V. Childhood cancers in Chennai, India, 1990-2001: incidence and survival. Int J Cancer. 2008;122(11):2607–2611. doi: 10.1002/ijc.23428. [DOI] [PubMed] [Google Scholar]
- 8.Jain K, Sunila RR, Mruthyunjaya RCS, Gadiyar HB, et al. Bone tumors in a tertiary care hospital of south India: a review 117 cases. Indian J Med Paediatr. Oncol. 2011;32(2):82–85. doi: 10.4103/0971-5851.89778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gulia A, Puri A, Chorge S, Panda PK. Epidemiological data and case load spectrum of patients presenting to bone and soft tissue disease management group at a tertiary cancer center. Indian J Cancer. 2016;53(2):333–338. doi: 10.4103/0019-509X.197734. [DOI] [PubMed] [Google Scholar]
- 10.Sharma N, Ahmad A, Bhat GM, Aziz SA, Lone MM, Bhat NA. A profile of pediatric solid tumors: a single institution experience in Kashmir. Indian J Med Paediatr Oncol. 2017;38(4):471. doi: 10.4103/ijmpo.ijmpo_95_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh R, Shirali R, Chatterjee S, Adhana A, Arora RS. Epidemiology of cancers among adolescents and young adults from a tertiary cancer center in Delhi. Indian J Med Paediatr Oncol. 2016;37(2):90–94. doi: 10.4103/0971-5851.180135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kakkar N, Gupta A, Sharma NK, Agarwal P, Kaur J. Adolescents and young adults: a study of distribution of cancer at ages 15-39 years in a tertiary care hospital from North India: Epidemiological considerations. South Asian J Cancer. 2017;6(4):180–182. doi: 10.4103/sajc.sajc_263_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pandey A, Singh A, Kumar V, Prakash J, Runu R, Thakur V, et al. Pediatric cancers in Bihar: a retrospective tertiary cancer center study. South Asian J Cancer. 2020;9(1):53. doi: 10.4103/sajc.sajc_48_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.http://ncdirindia.org/NCRP/ALL_NCRP_REPORTS/PBCR_REPORT_2012_2014/index.htm
- 15.http://seer.cancer.gov/publications/childhood/introduction.pdf (Accessed on May 31, 2011).
- 16.Mirabello L, Troisi RJ, Savage SA. OS incidence and survival rates from 1973 to 2004: data from the Surveillance, Epidemiology, and End Results Program. Cancer. 2009;115(7):1531–1543. doi: 10.1002/cncr.24121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ries LAG, Smith MA, Gurney JG, Linet M, Tamra T, Young JL, Bunin GR (eds). Cancer incidence and survival among children and adolescents: United States SEER Program 1975-1995, National Cancer Institute, SEER Program. NIH Pub. No. 99-4649. Bethesda, MD, 1999.
- 18.Nataraj V, Batra A, Rastogi S, Khan SA, Sharma MC, Vishnubhatla S, et al. Developing a prognostic model for patients with localized OS treated with uniform chemotherapy protocol without high dose methotrexate: a single center experience of 237 patients. J Surg Oncol. 2015;112:662–668. doi: 10.1002/jso.24045. [DOI] [PubMed] [Google Scholar]
- 19.Thorpe WP, Reilly JJ, Rosenberg SA. Prognostic significance of alkaline phosphatase measurements in patients with osteogenic sarcoma receiving chemotherapy. Cancer. 1979;43(6):2178–2181. doi: 10.1002/1097-0142(197906)43:6<2178::AID-CNCR2820430603>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 20.Link MP, Goorin AM, Horowitz M, Meyer WH, Belasco J, Baker A, et al. Adjuvant chemotherapy of high-grade OS of the extremity. Updated results of the Multi-Institutional OS Study. Clin Orthop Relat Res. 1991;270:8–14. doi: 10.1097/00003086-199109000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Bakhshi S, Radhakrishnan V. Prognostic markers in OS. Expert Rev Anticancer Ther. 2010;10:271–287. doi: 10.1586/era.09.186. [DOI] [PubMed] [Google Scholar]
- 22.Puri A, Byregowda S, Gulia A, Crasto S, Chinaswamy G. A study of 853 high grade OSs from a single institution-are outcomes in Indian patients different? J Surg Oncol. 2018;117(2):299–306. doi: 10.1002/jso.24809. [DOI] [PubMed] [Google Scholar]
- 23.Poudel RR, Tiwari V, Kumar VS, Bakhshi S, Gamanagatti S, Khan SA, et al. Factors associated with local recurrence in operated OSs: a retrospective evaluation of 95 cases from a tertiary care center in a resource challenged environment. J Surg Oncol. 2017;115(5):631–636. doi: 10.1002/jso.24602. [DOI] [PubMed] [Google Scholar]
- 24.Bajpai J, Puri A, Shah K, Susan D, Jambhekar N, Rekhi B, et al. Chemotherapy compliance in patients with OS. Pediatr Blood Cancer. 2013;60(1):41–44. doi: 10.1002/pbc.24155. [DOI] [PubMed] [Google Scholar]
- 25.Rastogi S, Kumar R, Sankineani SR, Marimuthu K, Rijal L, Prakash S, et al. Role of vascular endothelial growth factor as a tumour marker in OS: a prospective study. Int Orthop. 2012;36(11):2315–2321. doi: 10.1007/s00264-012-1663-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bajpai J, Kumar R, Sreenivas V, Sharma MC, Khan SA, Rastogi S, et al. Prediction of chemotherapy response by PET-CT in OS: correlation with histologic necrosis. J Pediatr Hematol Oncol. 2011;33(7):e271–e278. doi: 10.1097/MPH.0b013e31820ff29e. [DOI] [PubMed] [Google Scholar]
- 27.Bajpai J, Gamnagatti S, Kumar R, Sreenivas V, Sharma MC, Khan SA, et al. Role of MRI in OS for evaluation and prediction of chemotherapy response: correlation with histological necrosis. Pediatr Radiol. 2011;41(4):441–450. doi: 10.1007/s00247-010-1876-3. [DOI] [PubMed] [Google Scholar]
- 28.Bajpai J, Sharma M, Sreenivas V, Kumar R, Gamnagatti S, Khan SA, et al. VEGF expression as a prognostic marker in OS. Pediatr Blood Cancer. 2009;53(6):1035–1039. doi: 10.1002/pbc.22178. [DOI] [PubMed] [Google Scholar]
- 29.Bakhshi S, Gupta A, Sharma MC, Khan SA, Rastogi S. Her-2/neu, p-53, and their coexpression in OS. J Pediatr Hematol Oncol. 2009;31(4):245–251. doi: 10.1097/MPH.0b013e318197947e. [DOI] [PubMed] [Google Scholar]
- 30.Rakesh Kumar V, Gupta N, Kakkar N, Sharma SC. Prognostic and predictive value of c-erbB2 overexpression in osteogenic sarcoma. J Cancer Res Ther. 2006;2(1):20–23. doi: 10.4103/0973-1482.19770. [DOI] [PubMed] [Google Scholar]
- 31.Nataraj V, Rastogi S, Khan SA, Sharma MC, Agarwala S, Vishnubhatla S, et al. Prognosticating metastatic OS treated with uniform chemotherapy protocol without high dose methotrexate and delayed metastasectomy: a single center experience of 102 patients. Clin Transl Oncol. 2016;18(9):937–944. doi: 10.1007/s12094-015-1467-8. [DOI] [PubMed] [Google Scholar]
- 32.Bielack SS, Kempf-Bielack B, Delling G, Exner UG, Flege S, Helmke K, et al. Prognostic factors in high-grade OS of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative OS study group protocols. J Clin Oncol. 2002;20(3):776–790. doi: 10.1200/JCO.2002.20.3.776. [DOI] [PubMed] [Google Scholar]
- 33.Hauben EI, Weeden S, Pringle J, Van Marck EA, Hogendoorn PC. Does the histological subtype of high-grade central OS influence the response to treatment with chemotherapy and does it affect overall survival? A study on 570 patients of two consecutive trials of the European OS Intergroup. Eur J Cancer. 2002;38(9):1218–1225. doi: 10.1016/S0959-8049(02)00037-0. [DOI] [PubMed] [Google Scholar]
- 34.Bacci G, Longhi A, Versari M, Mercuri M, Briccoli A, Picci P. Prognostic factors for OS of the extremity treated with neoadjuvant chemotherapy: 15-year experience in 789 patients treated at a single institution. Cancer. 2006;106(5):1154–1161. doi: 10.1002/cncr.21724. [DOI] [PubMed] [Google Scholar]
- 35.Anninga JK, Gelderblom H, Fiocco M, Kroep JR, Taminiau AH, Hogendoorn PC, et al. Chemotherapeutic adjuvant treatment for OS: where do we stand? Eur J Cancer. 2011;47(16):2431–2445. doi: 10.1016/j.ejca.2011.05.030. [DOI] [PubMed] [Google Scholar]
- 36.Goorin AM, Schwartzentruber DJ, Devidas M, Gebhardt MC, Ayala AG, Harris MB, et al. Presurgical chemotherapy compared with immediate surgery and adjuvant chemotherapy for nonmetastatic OS: Pediatric Oncology Group Study POG-8651. J Clin Oncol. 2003;21(8):1574–1580. doi: 10.1200/JCO.2003.08.165. [DOI] [PubMed] [Google Scholar]
- 37.Rosen G, Caparros B, Huvos AG, et al. Preoperative chemotherapy for osteogenic sarcoma: selection of postoperative adjuvant chemotherapy based on the response of the primary tumor to preoperative chemotherapy. Cancer. 1982;49(6):1221–1230. doi: 10.1002/1097-0142(19820315)49:6<1221::AID-CNCR2820490625>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 38.Meyers PA, Gorlick R, Heller G, Casper E, Lane J, Huvos AG, et al. Intensification of preoperative chemotherapy for osteogenic sarcoma: results of the Memorial Sloan-Kettering (T12) protocol. J Clin Oncol. 1998;16(7):2452–2458. doi: 10.1200/JCO.1998.16.7.2452. [DOI] [PubMed] [Google Scholar]
- 39.Bacci G, Picci P, Pignatti G, De Cristofaro R, Dallari D, Avella M, et al. Neoadjuvant chemotherapy for nonmetastatic OS of the extremities. Clin Orthop Relat Res. 1991;270:87–89. doi: 10.1097/00003086-199109000-00014. [DOI] [PubMed] [Google Scholar]
- 40.Souhami RL, Craft AW, Eijken JW, Nooji M, Spooner D, Bramwell VH, et al. Randomised trial of two regimens of chemotherapy in operable OS: a study of the European OS Intergroup. Lancet. 1997;350:911–917. doi: 10.1016/S0140-6736(97)02307-6. [DOI] [PubMed] [Google Scholar]
- 41.Lewis IJ, Nooij MA, Whelan J, Sydes MR, Grimer R, Hogendoorn PC, et al. Improvement in histologic response but not survival in OS patients treated with intensified chemotherapy: a randomized phase III trial of the European OS Intergroup. J Natl Cancer Inst. 2007;99(2):112–128. doi: 10.1093/jnci/djk015. [DOI] [PubMed] [Google Scholar]
- 42.Fuchs N, Bielack SS, Epler D, Beiling P, Delling G, Korholz D, et al. Long-term results of the co-operative German-Austrian-Swiss OS study group’s protocol COSS-86 of intensive multidrug chemotherapy and surgery for OS of the limbs. Ann Oncol. 1998;9(8):893–899. doi: 10.1023/A:1008391103132. [DOI] [PubMed] [Google Scholar]
- 43.Meyers PA, Schwartz CL, Krailo M, Kleinerman ES, Betcher D, Bernstein ML, et al. OS: a randomized, prospective trial of the addition of ifosfamide and/or muramyl tripeptide to cisplatin, doxorubicin, and high-dose methotrexate. J Clin Oncol. 2005;23(9):2004–2011. doi: 10.1200/JCO.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 44.Marina NM, Smeland S, Bielack SS, Bernstein M, Jovic G, Kralio MD, et al. Comparison of MAPIE versus MAP in patients with a poor response to preoperative chemotherapy for newly diagnosed high-grade OS (EURAMOS-1): an open-label, international, randomised controlled trial. Lancet Oncol. 2016;17(10):1396–1408. doi: 10.1016/S1470-2045(16)30214-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bajpai J, Chandrasekharan A, Simha V, Mandal T, Shah K, Hingmare S, et al. OS journey over two decades in India: small steps, big changes. Pediatr Blood Cancer. 2019;66(9):e27877. doi: 10.1002/pbc.27877. [DOI] [PubMed] [Google Scholar]
- 46.Sukumaran RK, Rajeshwari B, Sugath S, Chellappan SG, Thankamony P, Parukuttyamma K. Methotrexate free chemotherapy and limb salvage surgery for paediatric OS in India. Indian J Orthop. 2018;52(1):58–64. doi: 10.4103/ortho.IJOrtho_195_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shanmugam S, Govindasamy G, Hussain SA, Fells SPS. Pediatric bone sarcomas: outcome of multimodality treatment in a single institution in South India over a decade. Indian J Med Paediat Oncol. 2019;40(5):38. [Google Scholar]
- 48.Winkler K, Beron G, Delling G, Heise U, Kabisch H, Purfürst C, et al. Neoadjuvant chemotherapy of osteosarcoma: results of a randomized cooperative trial (COSS-82) with salvage chemotherapy based on histological tumor response. J Clin Oncol. 1988;6(2):329–337. doi: 10.1200/JCO.1988.6.2.329. [DOI] [PubMed] [Google Scholar]
- 49.Bramwell VH, Burgers M, Sneath R, et al. A comparison of two short intensive adjuvant chemotherapy regimens in operable OS of limbs in children and young adults: the first study of the European OS Intergroup. J Clin Oncol. 1992;10:1579–1591. doi: 10.1200/JCO.1992.10.10.1579. [DOI] [PubMed] [Google Scholar]
- 50.van Dalen EC, van As JW, de Camargo B. Methotrexate for high-grade OS in children and young adults. Cochrane Database Syst Rev. 2011;5:CD006325. doi: 10.1002/14651858.CD006325.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Patel SJ, Lynch JW, Jr, Johnson T, Caroli RR, Schumacher C, Spanier S, et al. Dose-intense ifosfamide/doxorubicin/cisplatin based chemotherapy for OS in adults. Am J Clin Oncol. 2002;25:489–495. doi: 10.1097/00000421-200210000-00014. [DOI] [PubMed] [Google Scholar]
- 52.Piperno-Neumann S, Bui B, Blay J, et al. A multicentric prospective study of intensive induction chemotherapy (APIAI) in localized OS patients: results of a phase II trial coordinated by the French Sarcoma Group (FSG) and the FNCLCC BECT. J Clin Oncol. 2006;24:9521. doi: 10.1200/jco.2006.24.18_suppl.9521. [DOI] [Google Scholar]
- 53.Tunn PU, Reichardt P. Chemotherapy for OS without high-dose methotrexate: a 12-year follow-up on 53 patients. Onkologie. 2007;30:228–232. doi: 10.1159/000100776. [DOI] [PubMed] [Google Scholar]
- 54.Assi H, Missenard G, Terrier P, Le Pechoux C, Bonvalot S, Vanel D, et al. Intensive induction chemotherapy without methotrexate in adult patients with localized OS: results of the Institut Gustave-Roussy phase II trial. Curr Oncol. 2010;17:23–31. doi: 10.3747/co.v17i6.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Daw NC, Neel MD, Rao BN, Billups CA, Wu J, Jenkins JJ, et al. Frontline treatment of localized OS without methotrexate: results of the St. Jude Children’s Research Hospital OS99 trial. Cancer. 2011;117:2770–2778. doi: 10.1002/cncr.25715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bajpai J, Chandrasekharan A, Talreja V, Simha V, Chandrakanth MV, Rekhi B, et al. Outcomes in non-metastatic treatment naive extremity osteosarcoma patients treated with a novel non-high dosemethotrexate-based, dose-dense combination chemotherapy regimen 'OGS-12'. Eur J Cancer. 2017;85:49–58. doi: 10.1016/j.ejca.2017.08.013. [DOI] [PubMed] [Google Scholar]
- 57.Craft AW. OS: the European OS Intergroup (EOI) perspective. Cancer Treat Res. 2009;152:263–274. doi: 10.1007/978-1-4419-0284-9_13. [DOI] [PubMed] [Google Scholar]
- 58.Poudel RR, Kumar VS, Bakhshi S, Gamanagatti S, Rastogi S, Khan SA. High tumor volume and local recurrence following surgery in OS: a retrospective study. Indian J Orthop. 2014;48(3):285. doi: 10.4103/0019-5413.132520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li X, Zhang Y, Wan S, et al. A comparative study between limb-salvage and amputation for treating OS. J Bone Oncol. 2016;5(1):15–21. doi: 10.1016/j.jbo.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grimer RJ, Taminiau AM, Cannon SR. Surgical subcommitte of the European Osteosarcoma Intergroup. Surgical outcomes in osteosarcoma. J Bone Joint Surg (Br) 2002;84:395–400. doi: 10.1302/0301-620X.84B3.0840395. [DOI] [PubMed] [Google Scholar]
- 61.Bispo Júnior RZ, Camargo OP de. Prognostic factors in the survival of patients diagnosed with primary non-metastatic osteosarcoma with a poor response to neoadjuvant chemotherapy. Clin São Paulo Braz 2009;64:1177–1186. [DOI] [PMC free article] [PubMed]
- 62.Khan SA, Kumar VS, Poudel RR Limb salvage in India. In Sarcoma 2017 (pp. 483-510). Springer. Cham.
- 63.Puri A. Orthopedic oncology - "the challenges ahead". Front Surg. 2014;1:27. doi: 10.3389/fsurg.2014.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tiwari A, Mehta S, Sharma SK, Chauhan V, Rohela H, Arora R. Vascularized fibula with and without extracorporeal radiotherapy for limb salvage surgery in Indian patients. J Clin Orthop Trauma. 2019;10(1):167–172. doi: 10.1016/j.jcot.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Puri A, Byregowda S, Gulia A, Patil V, Crasto S, Laskar S. Reconstructing diaphyseal tumors using radiated (50 Gy) autogenous tumor bone graft. J Surg Oncol. 2018;118(1):138–143. doi: 10.1002/jso.25092. [DOI] [PubMed] [Google Scholar]
- 66.Nayak P, Gulia A, Puri A. Does reconstruction with reimplantation of sterilized tumor bone provide survival benefit in diaphyseal OS? [Internet]. Vol. 2020, Sarcoma. Hindawi; 2020 [cited 2020 Aug 31]. p. e4092790 [DOI] [PMC free article] [PubMed]
- 67.Natarajan MV, Sivaseelam A, Rajkumar G, Hussain SHJ. Custom megaprosthetic replacement for proximal tibial tumours. Int Orthop. 2003;27(6):334–337. doi: 10.1007/s00264-003-0484-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Natarajan MV, Sivaseelam A, Ayyappan S, Bose JC, Sampath KM. Distal femoral tumours treated by resection and custom mega-prosthetic replacement. Int Orthop. 2005;29(5):309–313. doi: 10.1007/s00264-005-0677-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Agarwal M, Anchan C, Shah M, Puri A, Pai S. Limb salvage surgery for OS: effective low-cost treatment. Clin Orthop Relat Res. 2007;459:82–91. doi: 10.1097/BLO.0b013e31805d85c4. [DOI] [PubMed] [Google Scholar]
- 70.Chauhan A, Joshi GR, Chopra BK, Ganguly M, Reddy GR. Limb salvage surgery in bone tumors: a retrospective study of 50 cases in a single center. Indian J Surg Oncol. 2013;4(3):248–254. doi: 10.1007/s13193-013-0229-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tiwari A, Jain S, Mehta S, Kumar R, Kapoor G, Kumar K. Limb salvage surgery for OS: early results in Indian patients. Indian J Orthop. 2014;48(3):266–272. doi: 10.4103/0019-5413.132511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hong AM, Millington S, Ahern V, McCowage G, Boyle R, Tattersall M, et al. Limb preservation surgery with extracorporeal irradiation in the management of malignant bone tumor: the oncological outcomes of 101 patients. Ann Oncol. 2013;24(10):2676–2680. doi: 10.1093/annonc/mdt252. [DOI] [PubMed] [Google Scholar]
- 73.Pruksakorn D, Kongthavonskul J, Teeyakasem P, Phanphaisarn A, Chaiyawat P, Klangjorhor J, et al. Surgical outcomes of extracorporeal irradiation and re-implantation in extremities for high grade osteosarcoma: a retrospective cohort study and a systematic review of the literature. J Bone Oncol. 2018;14:100210. doi: 10.1016/j.jbo.2018.100210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sharma DN, Rastogi S, Bakhshi S, Rath GK, Julka PK, Laviraj MA, et al. Role of extracorporeal irradiation in malignant bone tumors. Indian J Cancer. 2013;50(4):306. doi: 10.4103/0019-509X.123601. [DOI] [PubMed] [Google Scholar]
- 75.Chauhan S, Khan SA, Prasad A. Irradiation-induced compositional effects on human bone after extracorporeal therapy for bone sarcoma. Calcif Tissue Int. 2018;103(2):175–188. doi: 10.1007/s00223-018-0408-2. [DOI] [PubMed] [Google Scholar]
- 76.Xu M, Xu M, Zhang S, Li H, Qiuchi AI, Yu X, et al. Comparative efficacy of intraoperative extracorporeal irradiated and alcohol-inactivated autograft reimplantation for the management of osteosarcomas-a multicentre retrospective study. World J Surg Oncol. 2021;19(1):157. doi: 10.1186/s12957-021-02271-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bernthal NM, Monument MJ, Randall RL, Jones KB. Rotationplasty: beauty is in the Eye of the Beholder. Oper Tech Orthop. 2014;24(2):103–110. doi: 10.1053/j.oto.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Winkelmann WW. Type-B-IIIa hip rotationplasty: an alternative operation for the treatment of malignant tumors of the femur in early childhood. J Bone Joint Surg Am. 2000;82:814–828. doi: 10.2106/00004623-200006000-00008. [DOI] [PubMed] [Google Scholar]
- 79.Agarwal M, Puri A, Anchan C, Shah M, Jambhekar N. Rotationplasty for bone tumors: is there still a role? Clin Orthop Relat Res. 2007;459:76–81. doi: 10.1097/BLO.0b013e31805470f0. [DOI] [PubMed] [Google Scholar]
- 80.Heisel C, Kinkel S, Bernd L, Ewerbeck V. Megaprostheses for the treatment of malignant bone tumours of the lower limbs. Int Orthop. 2006;30:452–457. doi: 10.1007/s00264-006-0207-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ahlmann ER, Menendez LR, Kermani C, Gotha H. Survivorship and clinical outcome of modular endoprosthetic reconstruction for neoplastic disease of the lower limb. J Bone Joint Surg (Br) 2006;88:790–795. doi: 10.1302/0301-620X.88B6.17519. [DOI] [PubMed] [Google Scholar]
- 82.Machak GN, Tkachev SI, Solovyev YN, Sinyukov PA, Ivanov SM, Kochergina NV, et al. Neoadjuvant chemotherapy and local radiotherapy for high-grade osteosarcoma of the extremities. Mayo Clin Proc. 2003;78(2):147–155. doi: 10.4065/78.2.147. [DOI] [PubMed] [Google Scholar]
- 83.Caceres E, Zaharia M, Valdivia S, Misad O, de la Flor J, Tejada F, et al. Local control of osteogenic sarcoma by radiation and chemotherapy. Int J Radiat Oncol Biol Phys. 1984;10(1):35–39. doi: 10.1016/0360-3016(84)90409-7. [DOI] [PubMed] [Google Scholar]
- 84.Ciernik IF, Niemierko A, Harmon DC, Kobayashi W, Chen YL, Yock TI, et al. Proton-based radiotherapy for unresectable or incompletely resected OS. Cancer. 2011;117(19):4522–4530. doi: 10.1002/cncr.26037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Salunke AA, Shah J, Warikoo V, Chakraborty A, Sahijwani H, Sharma M, et al. Surgical management of pelvic bone sarcoma with internal hemipelvectomy: oncologic and functional outcomes. J Clin Orthop Trauma. 2017;8(3):249–253. doi: 10.1016/j.jcot.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Puri A, Pruthi M, Gulia A. Outcomes after limb sparing resection in primary malignant pelvic tumors. Eur J Surg Oncol. 2014;40(1):27–33. doi: 10.1016/j.ejso.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 87.DeLaney TF, Park L, Goldberg SI, Hug EB, Liebsch NJ, Munzenrider JE, et al. Radiotherapy for local control of osteosarcoma. Int J Radiat Oncol Biol Phys. 2005;61(2):492–498. doi: 10.1016/j.ijrobp.2004.05.051. [DOI] [PubMed] [Google Scholar]
- 88.Guadagnolo BA, Zagars GK, Raymond AK, Benjamin RS, Sturgis EM. Osteosarcoma of the jaw/craniofacial region: outcomes after multimodality treatment. Cancer. 2009;115(14):3262–3270. doi: 10.1002/cncr.24297. [DOI] [PubMed] [Google Scholar]
- 89.Laskar S, Basu A, Muckaden MA, D'Cruz A, Pai S, Jambhekar N, et al. Osteosarcoma of the head and neck region: lessons learned from a single-institution experience of 50 patients. Head Neck. 2008;30(8):1020–1026. doi: 10.1002/hed.20820. [DOI] [PubMed] [Google Scholar]
- 90.Bacci G, Rocca M, Salone M, Balladelli A, Ferrari S, Palmerini E, et al. High grade OS of the extremities with lung metastases at presentation: treatment with neoadjuvant chemotherapy and simultaneous resection of primary and metastatic lesions. J Surg Oncol. 2008;98(6):415–420. doi: 10.1002/jso.21140. [DOI] [PubMed] [Google Scholar]
- 91.Bacci G, Ferrari S, Longhi A, Perin S, Forni C, Fabbri N, et al. Pattern of relapse in patients with OS of the extremities treated with neoadjuvant chemotherapy. Eur J Cancer. 2001;37(1):32–38. doi: 10.1016/S0959-8049(00)00361-0. [DOI] [PubMed] [Google Scholar]
- 92.Bajpai J, Chandrasekharan A, Simha V, Talreja V, Karpe A, Pandey N, et al. Outcomes in treatment-naïve patients with metastatic extremity OS treated with OGS-12, a novel non-high-dose methotrexate-based, dose-dense combination chemotherapy, in a tertiary care cancer center. J Glob Oncol. 2018;4:1–10. doi: 10.1200/JGO.17.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kager L, Zoubek A, Pötschger U, Flege S, Kempf-Bielack B, Branscheid D, et al. Primary metastatic OS: presentation and outcome of patients treated on neoadjuvant Cooperative OS Study Group protocols. J Clin Oncol. 2003;21:2011–2018. doi: 10.1200/JCO.2003.08.132. [DOI] [PubMed] [Google Scholar]
- 94.Ferguson WS, Harris MB, Goorin AM, Gebhardt MC, Link MP, Shochat SJ, et al. Presurgical window of carboplatin and surgery and multidrug chemotherapy for the treatment of newly diagnosed metastatic or unresectable OS: Pediatric Oncology Group trial. J Pediatr Hematol Oncol. 2001;23:340–348. doi: 10.1097/00043426-200108000-00004. [DOI] [PubMed] [Google Scholar]
- 95.Pramanik R, Agarwala S, Gupta YK, Thulkar S, Vishnubhatla S, Batra A, et al. Metronomic chemotherapy vs best supportive care in progressive pediatric solid malignant tumors: a randomized clinical trial. JAMA Oncol. 2017;3(9):1222–1227. doi: 10.1001/jamaoncol.2017.0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Petrella F, Diotti C, Rimessi A, Spaggiari L. Pulmonary metastasectomy: an overview. J Thorac Dis. 2017;9(Suppl 12):S1291–S1298. doi: 10.21037/jtd.2017.03.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kempf-Bielack B, Bielack SS, Jürgens H, Branscheid D, Berdel WE, Exner GU, et al. Osteosarcoma relapse after combined modality therapy: an analysis of unselected patients in the Cooperative Osteosarcoma Study Group (COSS) J Clin Oncol. 2005;23:559–568. doi: 10.1200/JCO.2005.04.063. [DOI] [PubMed] [Google Scholar]
- 98.Harting MT, Blakely ML, Jaffe N, Cox C, Jr, Hayes-Jordan A, Benjamin RS, et al. Long-term survival after aggressive resection of pulmonary metastases among children and adolescents with osteosarcoma. J Pediatr Surg. 2006;41:194–199. doi: 10.1016/j.jpedsurg.2005.10.089. [DOI] [PubMed] [Google Scholar]
- 99.Letourneau PA, Xiao L, Harting MT, Lally KP, Cox C, Jr, Andrassy RJ, et al. Location of pulmonary metastasis in pediatric osteosarcoma is predictive of outcome. J Pediatr Surg. 2011;46:1333–1337. doi: 10.1016/j.jpedsurg.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Leary SE, Wozniak AW, Billups CA, Wu J, McPhearson V, Neel MD, et al. Survival of pediatric patients after relapsed osteosarcoma: the St. Jude Children’s Research Hospital experience. Cancer. 2013;119:2645–2653. doi: 10.1002/cncr.28111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Briccoli A, Rocca M, Salone M, Guzzardella GA, Balladelli A, Bacci G. High grade osteosarcoma of the extremities metastatic to the lung: long-term results in 323 patients treated combining surgery and chemotherapy, 1985-2005. Surg Oncol. 2010;19(4):193–199. doi: 10.1016/j.suronc.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 102.Ramanujan V, Krishnamurthy A, Venkataramani K, Kumar C. Pulmonary metastasectomy in primary extremity OS: choosing wisely, along with a brief review of literature. Indian J Cancer. 2020;57(2):172–181. doi: 10.4103/ijc.IJC_497_18. [DOI] [PubMed] [Google Scholar]
- 103.Putnam JB, Jr, Roth JA, Wesley MN, Johnston MR, Rosenberg SA. Survival following aggressive resection of pulmonary metastases from osteogenic sarcoma: analysis of prognostic factors. Ann Thorac Surg. 1983;36(5):516–523. doi: 10.1016/S0003-4975(10)60679-0. [DOI] [PubMed] [Google Scholar]
- 104.Goorin AM, Delorey MJ, Lack EE, Gelber RD, Price K, Cassady JR, et al. Prognostic significance of complete surgical resection of pulmonary metastases in patients with osteogenic sarcoma: analysis of 32 patients. J Clin Oncol. 1984;2(5):425–431. doi: 10.1200/JCO.1984.2.5.425. [DOI] [PubMed] [Google Scholar]
- 105.Ferrari S, Briccoli A, Mercuri M, Bertoni F, Picci P, Tienghi A, et al. Post relapse survival in OS of the extremities: prognostic factors for long-term survival. J Clin Oncol. 2003;21:710–715. doi: 10.1200/JCO.2003.03.141. [DOI] [PubMed] [Google Scholar]
- 106.Bielack SS, Kempf-Bielack B, Branscheid D, Carrie D, Friedel G, Helmke K, et al. Second and subsequent recurrences of OS: presentation, treatment, and outcomes of 249 consecutive cooperative OS study group patients. J Clin Oncol. 2009;27(4):557–565. doi: 10.1200/JCO.2008.16.2305. [DOI] [PubMed] [Google Scholar]
- 107.Chou AJ, Merola PR, Wexler LH, Gorlick RG, Vyas YM, Healey JH, et al. Treatment of OS at first recurrence after contemporary therapy: the Memorial Sloan-Kettering Cancer Center experience. Cancer. 2005;104(10):2214–2221. doi: 10.1002/cncr.21417. [DOI] [PubMed] [Google Scholar]
- 108.Berrak SG, Pearson M, Berberoğlu S, Ilhan IE, Jaffe N. High-dose ifosfamide in relapsed pediatric OS: therapeutic effects and renal toxicity. Pediatr Blood Cancer. 2005;44(3):215–219. doi: 10.1002/pbc.20228. [DOI] [PubMed] [Google Scholar]
- 109.Palmerini E, Jones RL, Marchesi E, Paioli A, Cesari M, Longhi A, et al. Gemcitabine and docetaxel in relapsed and unresectable high-grade osteosarcoma and spindle cell sarcoma of bone. BMC Cancer. 2016;20(16):280. doi: 10.1186/s12885-016-2312-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rodríguez-Galindo C, Daw NC, Kaste SC, Meyer WH, Dome JS, Pappo AS, et al. Treatment of refractory osteosarcoma with fractionated cyclophosphamide and etoposide. J Pediatr Hematol Oncol. 2002;24(4):250–255. doi: 10.1097/00043426-200205000-00006. [DOI] [PubMed] [Google Scholar]
- 111.Fox E, Patel S, Wathen JK, Schuetze S, Chawla S, Harmon D, et al. Phase II study of sequential gemcitabine followed by docetaxel for recurrent Ewing sarcoma, osteosarcoma, or unresectable or locally recurrent chondrosarcoma: results of Sarcoma Alliance for Research Through Collaboration Study 003. Oncologist. 2012;17(3):321. doi: 10.1634/theoncologist.2010-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Miser JS, Kinsella TJ, Triche TJ, Tsokos M, Jarosinski P, Forquer R, et al. Ifosfamide with mesna uroprotection and etoposide: an effective regimen in the treatment of recurrent sarcomas and other tumors of children and young adults. J Clin Oncol. 1987;5(8):1191–1198. doi: 10.1200/JCO.1987.5.8.1191. [DOI] [PubMed] [Google Scholar]
- 113.Cairo MS, Shen V, Krailo MD, Bauer M, Miser JS, Sato JK, et al. Prospective randomized trial between two doses of granulocyte colony-stimulating factor after ifosfamide, carboplatin, and etoposide in children with recurrent or refractory solid tumors: a children's cancer group report. J Pediatr Hematol Oncol. 2001;23(1):30–38. doi: 10.1097/00043426-200101000-00008. [DOI] [PubMed] [Google Scholar]
- 114.Nathan SS, Gorlick R, Bukata S, Chou A, Morris CD, Boland PJ, et al. Treatment algorithm for locally recurrent OS based on local disease-free interval and the presence of lung metastasis. Cancer. 2006;107(7):1607–1616. doi: 10.1002/cncr.22197. [DOI] [PubMed] [Google Scholar]
- 115.Picci P, Sangiorgi L, Rougraff BT, Neff JR, Casadei R, Campanacci M. Relationship of chemotherapy-induced necrosis and surgical margins to local recurrence in OS. J Clin Oncol. 1994;12(12):2699–2705. doi: 10.1200/JCO.1994.12.12.2699. [DOI] [PubMed] [Google Scholar]
- 116.Rodriguez-Galindo C, Shah N, McCarville MB, Billups CA, Neel MN, Rao BN, et al. Outcome after local recurrence of OS: the St. Jude Children’s Research Hospital experience (1970–2000) Cancer. 2004;100:1928–1935. doi: 10.1002/cncr.20214. [DOI] [PubMed] [Google Scholar]
- 117.Aung L, Gorlick R, Healey JH, Shi W, Thaler HT, Shorter NA, et al. Metachronous skeletal OS in patients treated with adjuvant and neoadjuvant chemotherapy for nonmetastatic OS. J Clin Oncol. 2003;21(2):342–348. doi: 10.1200/JCO.2003.06.177. [DOI] [PubMed] [Google Scholar]
- 118.Ozaki T, Flege S, Kevric M, Linder N, Maas R, Delling G, et al. OS of the pelvis: experience of the Cooperative OS Study Group. J Clin Oncol. 2003;21(2):334–341. doi: 10.1200/JCO.2003.01.142. [DOI] [PubMed] [Google Scholar]
- 119.Grignani G, Palmerini E, Pet D, Asaftei SD, D’Ambrosio L, Pignochino Y, et al. A phase II trial of sorafenib in relapsed and unresectable high-grade OS after failure of standard multimodal therapy: an Italian Sarcoma Group study. Ann Oncol. 2012;23(2):508–516. doi: 10.1093/annonc/mdr151. [DOI] [PubMed] [Google Scholar]
- 120.Davis LE, Bolejack V, Ryan CW, Ganjoo KN, Loggers ET, Chawla S, et al. Randomized double-blind phase II study of regorafenib in patients with metastatic OS. J Clin Oncol. 2019;37(16):1424–1431. doi: 10.1200/JCO.18.02374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Duffaud F, Mir O, Boudou-Rouquette P, Piperno-Neumann S, Penel N, Bompas E, et al. French Sarcoma Group. Efficacy and safety of regorafenib in adult patients with metastatic osteosarcoma: a non-comparative, randomised, double-blind, placebo-controlled, phase 2 study. Lancet Oncol. 2019;20(1):120–133. doi: 10.1016/S1470-2045(18)30742-3. [DOI] [PubMed] [Google Scholar]
- 122.Italiano A, Mir O, Mathoulin-Pelissier S, Penel N, Piperno-Neumann S, Bompas et al. Cabozantinib in patients with advanced Ewing sarcoma or osteosarcoma (CABONE): a multicentre, single-arm, phase 2 trial. Lancet Oncol 2020 Mar;21(3):446-455 [DOI] [PMC free article] [PubMed]
- 123.Meyers PA, Schwartz CL, Krailo MD, Healey JH, Bernstein ML, Betcher D, et al. Children's Oncology Group. OS: the addition of muramyl tripeptide to chemotherapy improves overall survival--a report from the Children's Oncology Group. J Clin Oncol. 2008;26(4):633–638. doi: 10.1200/JCO.2008.14.0095. [DOI] [PubMed] [Google Scholar]
- 124.Chou AJ, Kleinerman ES, Krailo MD, Chen Z, Betcher DL, Healey JH, et al. Children's Oncology Group. Addition of muramyl tripeptide to chemotherapy for patients with newly diagnosed metastatic OS: a report from the Children's Oncology Group. Cancer. 2009;115(22):5339–5348. doi: 10.1002/cncr.24566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tawbi HA, Burgess M, Bolejack V, Van Tine BA, Schuetze SM, Hu J, et al. Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): a multicentre, two-cohort, single-arm, open-label, phase 2 trial. Lancet Oncol. 2017;18(11):1493–1501. doi: 10.1016/S1470-2045(17)30624-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pincez T, Clément N, Lapouble E, Pierron G, Kamal M, Bieche I et al (2017) Feasibility and clinical integration of molecular profiling for target identification in pediatric solid tumors. Pediatr Blood Cancer 64(6) [DOI] [PubMed]
- 127.Weidenbusch B, Richter GHS, Kesper MS, Guggemoos M, Gall K, Prexler C, et al. Transcriptome based individualized therapy of refractory pediatric sarcomas: feasibility, tolerability and efficacy. Oncotarget. 2018;9(29):20747–20760. doi: 10.18632/oncotarget.25087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Suehara Y, Alex D, Bowman A, Middha S, Zehir A, Chakravarty D, et al. Clinical genomic sequencing of pediatric and adult osteosarcoma reveals distinct molecular subsets with potentially targetable alterations. Clin Cancer Res. 2019;25(21):6346–6356. doi: 10.1158/1078-0432.CCR-18-4032. [DOI] [PMC free article] [PubMed] [Google Scholar]