Abstract
Pharyngocutaneous fistula (PCF) is the most common complication which significantly increases morbidity. High-level evidence is lacking that determines the PCF rates in the primary laryngectomy. The main objective of this study was to systematically identify the factors leading to the PCF formation in primary laryngectomy. Human studies reporting at least one risk factor for developing PCF in patients undergoing primary total laryngectomy for laryngeal cancer were included. PubMed, EMBASE, and Cochrane databases were searched for the data extraction. Risk of bias assessment tool for non-randomized trial tool was used. Cochrane’s Q test and Higgin’s I2-heterogeneity was applied. The Mantel–Haenszel and DerSimonian Laird method was employed. Odds ratio was calculated for each risk factor, a P-value < 0.05 was considered as statistically significant. PROSPERO registration CRD42021248382. The meta-analysis comprised a total of 2446 patients in 14 included non-randomized studies. The among the analyzed risk factors—comorbidities (OR 2.781, R: 1.892–4.088, P < 0.001), site of tumor (OR 4.485, R: 3.003–6.699, P < 0.001), low pre-operative hemoglobin (OR 3.590, R: 2.130–6.050, P < 0.001), low pre-operative albumin (OR 2.833, R: 1.596–5.031, P < 0.001), utilization of surgical staplers (OR 0.172, R: 0.064–0.460, P < 0.001) (protective effect), positive mucosal margin (OR 4.92 R: 1.90–12.75, P = 0.001). The risk factors for PCF in patients undergoing primary TL included comorbidities, hypopharyngeal involvement, pre-operative hemoglobin and albumin, stapler usage, and positive mucosal margin.
Level of Evidence - III
Supplementary Information
The online version contains supplementary material available at 10.1007/s13193-022-01581-z.
Keywords: Laryngeal cancer, Total laryngectomy, Pharyngocutaneous fistula, Complications, Risk factors
Introduction
Laryngeal cancer is a common cancer in the world [1]. Since the last decade, there is a trend towards non-surgical treatment. However, in advanced cancers with large volume tumors, cartilage destruction, compromised laryngeal function, and recurrent or residual disease, surgery remains the treatment of choice [2].
Pharyngocutaneous fistula (PCF) is the most common complication following total laryngectomy, which significantly increases morbidity. PCF prolongs hospital stay, nasogastric feeding, delays adjuvant therapy, and could involve additional surgery for controlling vessel blow-outs or reconstructing the pharynx [3]. With technological evolution, newer solutions emerge that need to be explored to seek alternative methods. The focus of pharyngocutaneous fistula studies have been in the salvage settings; it is very well established that radiation, anemia, and hypoalbuminemia have a role in development of PCF.
Various factors affecting PCF such as suture material and method of pharyngeal closure have always been suspected but never shown to be significant. High-level evidence is lacking that determines the PCF rates in the primary laryngectomy. Here we intend to investigate the existing data to assess the risk factors predisposing to pharyngocutaneous fistula in patient undergoing primary total laryngectomy for laryngeal cancer.
Methodology
Search Strategy
Based on the AMSTAR 2 guidelines, at least two databases must be included in the search strategy to get adequate literature coverage [4]. We have included PubMed, EMBASE, and Cochrane databases to get a comprehensive coverage of published literature [5]. The published literature in English between 1970 and 2020 was considered.
Search Syntax
“Laryngeal cancer,” “Hypopharyngeal cancer,” “Total laryngectomy,” “Primary laryngectomy,” “Laryngectomy,” “Pharyngocutaneous fistula,” “Salivary fistula,” “Salivary leak,” “Pharyngeal leak,” “Complications,” “Outcomes.” Boolean operators (NOT, AND, OR) were used in succession to obtain the results. The data was last retrieved on 10 April 2021.
Data Screening and Selection
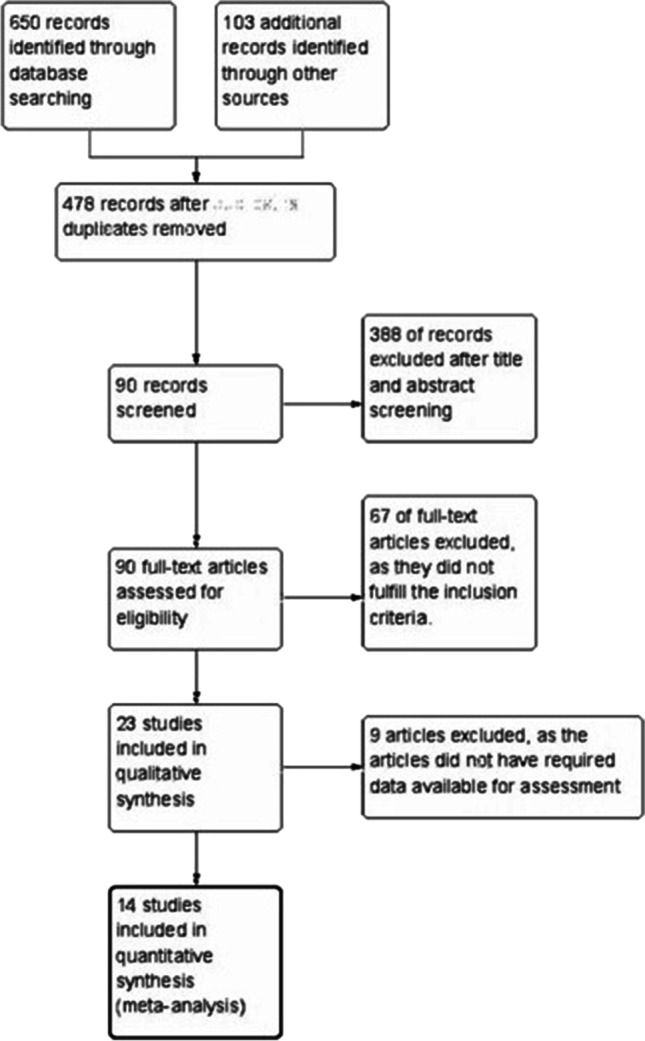
The retrieved articles were initially screened independently by two investigators KNR and AS, based to type of article, title, and abstract. The eligible articles were pooled; a thorough full-text analysis and references in the relevant articles were further assessed by hand-searching (Fig. 1). The articles were selected by K. N. R., A. A., and A. S.; any disagreement on inclusion of articles was resolved by the senior authors R. D. A. and N. M. N.
Fig. 1.
Study flow diagram
Inclusion Criteria
Primary laryngectomy/laryngopharyngectomy for laryngeal and hypopharyngeal cancer
Laryngeal or hypopharyngeal cancers with total laryngectomy/laryngopharyngectomy (with or without neck dissection) as a primary modality of therapy
Original articles published in peer-reviewed journals.
Study must report at least one risk factor for pharyngocutaneous fistula.
Exclusion Criteria
Non-human studies
Laryngectomy/laryngopharyngectomy for non-oncological reason
Neoadjuvant chemotherapy/radiation therapy prior to surgery
Any previous oncological treatment
Recurrent or second primary tumors
Not reported – reoperative outcomes
Review articles, meeting abstracts, case reports, editorial letters, and other forms of publication
Incomplete data or insufficient information
Overlapping study populations, shared dataset
Data Extraction
All included articles were independently hand screened by two authors K. N. R. and A. S. The following study characteristics were recorded: first author, year of publication, country of origin, sample size (treatment naïve primary total laryngectomy for malignancy), type of study, pharyngocutaneous fistula rate (among primary laryngectomy), age, sex, comorbidities, smoking, alcohol consumption, subsite, T staging, N staging, pre-operative hemoglobin, pre-operative albumin, type of pharyngeal closure (vertical/T type and horizontal closure), stapler use, suture material use, tracheo-esophageal prosthesis insertion, mucosal margin, and previous tracheostomy data were searched and documented (Table 1).
Table 1.
Characteristics of included studies
| Sl No | Author | Year of publication | Country | Sample size | Type of study | PCF | % | Level of evidence | Newcastle–Ottawa Scale |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Thompson | 2020 | UK | 114 | Retrospective | 7 | 6.14 | 3b | 6 |
| 2 | Nitassi | 2016 | Morocco | 136 | Retrospective | 37 | 27.21 | 3b | 6 |
| 3 | Aydin | 2014 | Turkey | 47 | Prospective | 14 | 29.79 | 3b | 7 |
| 4 | Stankovic | 2012 | Serbia | 316 | Retrospective | 37 | 11.71 | 3b | 5 |
| 5 | Calli | 2011 | Turkey | 182 | Prospective | 27 | 14.84 | 3b | 5 |
| 6 | Tsou | 2010 | China | 112 | Retrospective | 24 | 21.43 | 3b | 7 |
| 7 | Goncalves | 2009 | Brazil | 60 | Prospective | 13 | 21.67 | 3b | 5 |
| 8 | Akduman | 2008 | Turkey | 17 | Retrospective | 6 | 35.29 | 3b | 6 |
| 9 | Wakisaka | 2008 | Japan | 40 | Retrospective | 7 | 17.50 | 3b | 5 |
| 10 | Galli | 2005 | Italy | 190 | Retrospective | 25 | 13.16 | 3b | 6 |
| 11 | Markou | 2004 | Greece | 291 | Retrospective | 36 | 12.37 | 3b | 7 |
| 12 | Cavalot | 2000 | Italy | 265 | Retrospective | 22 | 8.30 | 3b | 6 |
| 13 | Herranz | 2000 | Spain | 471 | Retrospective | 99 | 21.02 | 3b | 5 |
| 14 | Papazoglou | 1994 | Greece | 205 | Retrospective | 10 | 4.88 | 3b | 5 |
| Total | 2446 | 364 | 14.88 |
PCF, pharyngocutaneous fistula
Quality Assessment
Level of Evidence
The level of evidence of the eligible studies was performed independently by two authors, as per the Oxford Centre for Evidence- Based Medicine (OCEBM) criteria.
Methodology Quality
Methodological quality was assessed by two authors, the Newcastle–Ottawa Scale was used for quality assessment of the included studies. The score ranged from 0 to 9. The articles with score > 5 was selected for meta-analysis.
Risk of Bias Assessment
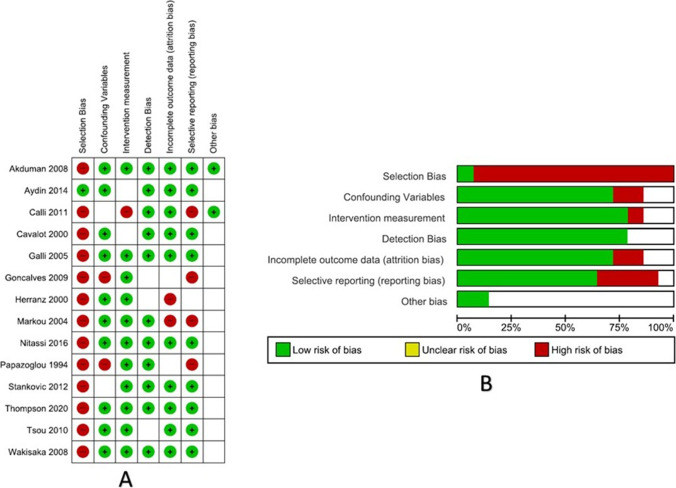
Risk of bias assessment tool for non-randomized trial tool from AMSTAR guidelines was used to determine the bias [4]. The following domains were assessed—selection bias, confounding variables, intervention measurement, detection bias, attrition, reporting, and other bias. The studies were graded as low risk, unclear risk, and high risk using QUADAS-2 tool on RevMan v.5.4 (Cochrane collaboration, Copenhagen, Denmark) (Figs. 2 and 3).
Fig. 2.
A Risk of bias summary; B risk of bias graph
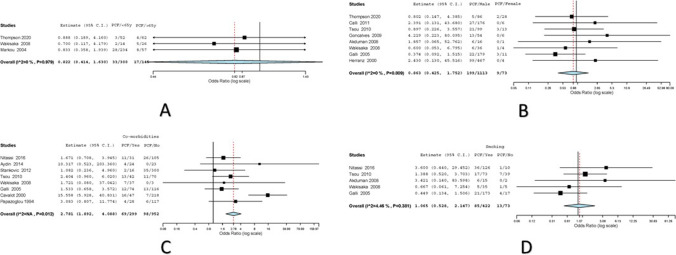
Fig. 3.
A Forest plot for age as risk factor for PCF; B forest plot for gender as risk factor for PCF; C forest plot for comorbidities as risk factor for PCF; D forest plot for smoking as risk factor for PCF
Statistical Analysis
Cochrane’s Q test and Higgin’s I2-heterogeneity of the included studies by using OpenMeta and STATA software. A P-value for heterogeneity (Ph) > 0.1 and I2 < 50% indicated nonsignificant heterogeneity, and therefore, the fixed-effects model (Mantel–Haenszel method) was applied. Otherwise, the random effects model (DerSimonian Laird method) was employed. Odds ratio was calculated for each risk factor; a P-value < 0.05 was considered as statistically significant.
Reporting and Registration
The meta-analysis was registered in International prospective register of systematic reviews (PROSPERO), registration no CRD42021248382. This work has been reported in concordance with the PRISMA [6] (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) and AMSTAR (Assessing the methodological quality of systematic reviews) guidelines.
Results
Literature Retrieval and Data Extraction
The initial literature search using the predefined search syntax identified a total of 753 papers. Of these, 478 remained after deleting 275 duplicates. Upon title and abstract screening, 388 articles were removed due to nonconformity with our study. After full-text analysis of the remaining 90 articles, 67 papers were rejected due to lack of necessary information needed for analysis, not meeting the criteria for inclusion, or coinciding with exclusion criteria. Finally, 14 studies were considered eligible and chosen for meta-analysis.
Quality of Included Studies
The main characteristics of the included studies are summarized in Table 1. Overall, the meta-analysis included a total of 2446 patients in 14 included studies. Eligible studies were either a prospective (3) or retrospective (11) cohort study design; none of the studies was randomized controlled trial. The studies were level 3b evidence as per the OCEBM levels of evidence guidelines. The Newcastle–Ottawa score ranged from 5 to 7 (Table 1). Based on the RoBANS risk of Bias assessment, the included studies had highest risk of selection bias with least risk of intervention or detection bias (Fig. 3).
Overall Rate of PCF
Among the 2446 patients undergoing primary total laryngectomy, 346 patients had pharyngocutaneous fistula (14.88%). We systematically evaluated 16 risk factors to identify the factors significantly associated with PCF. Table 2 shows the main results of the meta-analysis.
Table 2.
Summary of results from the meta-analysis
| Risk factor | Number of studies reporting | Number of laryngectomies | Number of patients with PCF | PCF % | Cochran Q value | Odds ratio | Statistical test | P value | |
|---|---|---|---|---|---|---|---|---|---|
| Age | < 65 years | 3 | 300 | 33 | 11.00% | 0.041 | 0.822 (0.414–1.630) | BREF | 0.575 |
| > 65 years | 145 | 17 | 11.72% | ||||||
| Sex | Male | 8 | 1113 | 199 | 17.88% | 3.745 | 0.863 (0.425–1.752) | BREF | 0.684 |
| Female | 73 | 9 | 12.33% | ||||||
| Comorbidities | Yes | 8 | 299 | 69 | 23.08% | 17.92 | 2.781 (1.892–4.088) | BFEM | < 0.001 |
| No | 952 | 98 | 10.29% | ||||||
| Smoking | Yes | 5 | 422 | 85 | 20.14% | 4.187 | 1.065 (0.528–2.147) | BREF | 0.86 |
| No | 73 | 13 | 17.80% | ||||||
| Alcohol | Yes | 5 | 275 | 46 | 16.73% | 2.447 | 0.765 (0.471–1.243) | BREF | 0.279 |
| No | 227 | 60 | 26.43% | ||||||
| Site | Larynx | 3 | 569 | 76 | 13.36% | 0.266 | 4.485 (3.003–6.699) | BREF | < 0.001 |
| Hypopharynx | 166 | 67 | 40.36% | ||||||
| T stage | T1 and T2 | 8 | 131 | 8 | 6.11% | 3.62 | 0.769 (0.370–1.598) | BREF | 0.481 |
| T3 and T4 | 1054 | 140 | 13.28% | ||||||
| N stage | N0 | 3 | 70 | 13 | 18.57% | 0.044 | 0.794 (0.345–1.828) | BREF | 0.588 |
| N + | 333 | 45 | 13.51% | ||||||
| Pre-operative hemoglobin | < 12 | 4 | 174 | 40 | 22.99% | 2.809 | 3.590 (2.130–6.050) | BREF | < 0.001 |
| > 12 | 455 | 48 | 10.55% | ||||||
| Pre-operative albumin | < 4 | 3 | 81 | 32 | 39.51% | 10.999 | 2.833 (1.596–5.031) | BFEM | < 0.001 |
| > 4 | 207 | 36 | 17.39% | ||||||
| Type of closure | Vertical/T type | 2 | 504 | 112 | 22.22% | 3.548 | 0.762 (0.412–1.408) | BFEM | 0.385 |
| Horizontal | 55 | 16 | 29.09% | ||||||
| Stapler use | Yes | 2 | 91 | 5 | 5.49% | 0.257 | 0.172 (0.064–0.460) | BREF | < 0.001 |
| No | 151 | 35 | 23.18% | ||||||
| Suture material | Vicryl | 3 | 600 | 105 | 17.50% | 3.08 | 0.781 (0.428–1.425) | BREF | 0.42 |
| Others | 326 | 39 | 11.96% | ||||||
| TEP insertion | Yes | 3 | 322 | 46 | 14.29% | 0.694 | 0.880 (0.594–1.305) | BREF | 0.526 |
| No | 554 | 96 | 17.33% | ||||||
| Mucosal margin | Positive | 1 | 22 | 8 | 36.36% | NA | 4.92 (1.90–12.75) | BFEM | 0.001 |
| Negative | 269 | 28 | 10.41% | ||||||
| Pre-operative tracheostomy | Yes | 6 | 163 | 33 | 20.25% | 11.757 | 0.714 (0.456–1.118) | BFEM | 0.141 |
| No | 832 | 158 | 18.99% | ||||||
PCF, Pharyngocutaneous fistula; BREF, binary random effects model; BFEM, binary fixed-effects model; TEP, tracheo-esophageal prosthesis; NA, not applicable; red colored, statistically significant
Age
Three studies had reported the effects of age on development of PCF in 445 laryngectomies [7–9]. Variations were attributed to heterogeneity in the included studies (I2 = 0%, Cochran Q = 0.041, Het. P value = 0.979). In age < 65 years, numbers of total laryngectomy (TL) performed were 300; 33/300 (11%) developed PCF. Among patients who were > 65 years, 145 TLs were performed with 17/145 (11.72%) developed PCF. The overall (odds ratio) OR values with the corresponding 95% CI were 0.822 (0.414–1.630) with P = 0.575 on random effects model (Fig. 3).
Sex
Eight studies had reported the effect of gender on PCF development among 1186 patients [7, 8, 10–15]. Variations were attributed to heterogeneity in the included studies (I2 = 0%, Cochran Q = 3.74, Het. P value = 0.809). One thousand one hundred thirteen males had undergone TL; 119/1113 (17.88%) developed PCF. Only 73 female TL were reported among the pooled dataset; 9/73 (12.3%) developed PCF. The overall (odds ratio) OR values with the corresponding 95% CI were 0.863 (0.425–1.752) with P = 0.684 on random effects model (Fig. 3).
Comorbidities
Many articles had described various comorbidities as a factor leading to PCF, diabetes, hypertension, and cardiac illness were most reported. Eight articles among the included studies reported on comorbidities as a factor among 1251 patients in developing post-operative PCF [8, 11, 14, 16–20]. Not many variations were attributed to heterogeneity in the included studies (Cochran Q = 17.920, Het. P value = 0.012). Two hundred ninety-nine patients had comorbidities; 69/299 (23.08%) developed PCF. Ninety-eight out of nine hundred fifty-two (10.29%) patients without comorbidities developed PCF. The overall (odds ratio) OR values with the corresponding 95% CI were 2.781 (1.892–4.088) with P ≤ 0.001 on fixed-effects model (Fig. 3).
Tobacco Smoking
Five studies had reported the effect of smoking on PCF development among 495 patients [8, 11, 13, 14, 16]. Variations were attributed to heterogeneity in the included studies (I2 = 4.45%, Cochran Q = 4.18, Het. P value = 0.38). Four hundred forty-two previous or active tobacco smokers had undergone TL; 85/422 (20.14%) developed PCF. Only 73 patients had no history of tobacco smoking in the pooled dataset; 13/73 (17.8%) developed PCF. The Overall (odds ratio) OR values with the corresponding 95% CI were 1.065 (0.528–2.147) with P = 0.86 on Random effects model (Fig. 3).
Alcohol Consumption
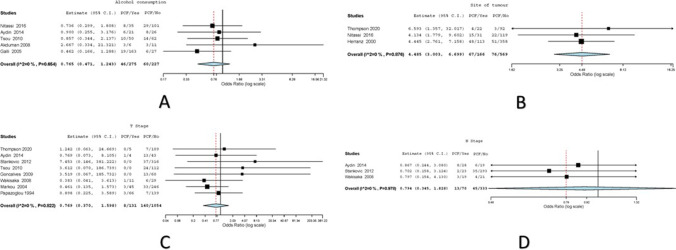
Five studies had reported the effect of smoking on PCF development among 495 patients [13,14,16,17,19. Variations were attributed to heterogeneity in the included studies (I2 = 0, Cochran Q = 2.44, Het. P value = 0.654). Two hundred seventy-five previous or active tobacco smokers had undergone TL; 46/275 (16.73%) developed PCF. Two hundred twenty-seven patients had no history of alcohol consumption in the pooled dataset; 60/227 (17.8%) developed PCF. The overall (odds ratio) OR values with the corresponding 95% CI were 0.765 (0.471–1.147) with P = 0.86 on random effects model (Fig. 4).
Fig. 4.
A Forest plot for alcohol as risk factor for PCF; B forest plot for site as risk factor for PCF; C forest plot for the T stage as risk factor for PCF; D forest plot for the N stage as risk factor for PCF
Site
Three studies had reported the effect of site of tumor on PCF development among 735 patients [7, 15, 16]. Variations were attributed to heterogeneity in the included studies (I2 = 0, Cochran Q = 0.266, Het. P value = 0.876). Five hundred sixty-nine patients with laryngeal involvement underwent TL; 76/569 (13.36%) developed PCF. One hundred sixty-six patients with hypopharyngeal involvement had undergone TL in the pooled dataset; 67/166 (40.36%) developed PCF. The overall (odds ratio) OR values with the corresponding 95% CI were 4.485 (3.003–6.699) with P ≤ 0.001 on random effects model (Fig. 4).
T Stage
Eight studies had reported the effect of the tumor stage on PCF development among 1185 patients [7–9, 11, 12, 17, 18, 20]. Variations were attributed to heterogeneity in the included studies (I2 = 0, Cochran Q = 3.62, Het. P value = 0.822). One hundred thirty-one patients with T1 and T2 tumor had undergone TL; 8/131 (6.11%) developed PCF. One thousand fifty-four patients with T3 and T4 tumor had undergone TL in the pooled dataset; 140/1054 (13.28%) developed PCF. The overall (odds ratio) OR values with the corresponding 95% CI were 0.769 (0.37–1.598) with P = 0.481 on random effects model (Fig. 4).
N Stage
Three studies had reported the effect of nodal involvement on PCF development among 403 patients [8, 17, 18]. Variations were attributed to heterogeneity in the included studies (I2 = 0, Cochran Q = 0.044, Het. P value = 0.978). Seventy patients with N0 nodal involvement had undergone TL; 13/70 (18.57%) developed PCF. Three hundred thirty-three patients with N + nodal involvement had undergone TL in the pooled dataset; 45/333 (13.51%) developed PCF. The overall (odds ratio) OR values with the corresponding 95% CI were 0.794 (0.345–1.828) with P = 0.588 on random effects model (Fig. 4).
Pre-operative Hemoglobin
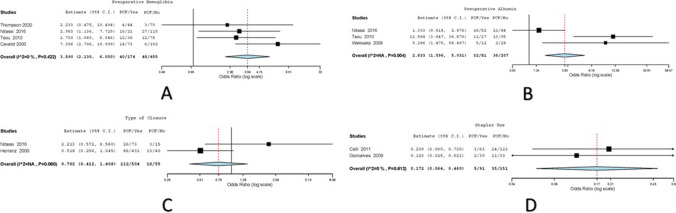
Four studies had reported the effect of pre-operative hemoglobin on PCF development among 629 patients [7, 11, 16, 19]. Variations were attributed to heterogeneity in the included studies (I2 = 0, Cochran Q = 2.809, Het. P value = 0.422). One hundred seventy-four patients with hemoglobin < 12 had undergone TL; 40/174 (22.99%) developed PCF. Four hundred fifty-five patients with hemoglobin > 12 had undergone TL in the pooled dataset; 48/455 (10.55%) developed PCF. The overall (odds ratio) OR values with the corresponding 95% CI were 3.590 (2.130–6.050) with P ≤ 0.001 on random effects model (Fig. 5).
Fig. 5.
A Forest plot for pre-operative hemoglobin as risk factor for PCF; B forest plot for pre-operative albumin as risk factor for PCF; C forest plot for type of closure as risk factor for PCF; D forest plot for stapler use as risk factor for PCF
Pre-operative Albumin
Three studies had reported the effect of pre-operative albumin on PCF development among 288 patients [8, 11, 16]. Variations were attributed to heterogeneity in the included studies (Cochran Q = 10.999, Het. P value = 0.004). Eighty-one patients with albumin < 4 had undergone TL; 32/81 (39.51%) developed PCF. Two hundred seven patients with albumin > 4 had undergone TL in the pooled dataset, 36/207 (17.39%) developed PCF. The overall (odds ratio) OR values with the corresponding 95% CI were 2.833 (1.596–5.031) with P ≤ 0.001 on random effects model (Fig. 5).
Type of Closure
Only two studies had reported the effect of type of closure on PCF development among 559 patients [15, 16]. Variations were attributed to heterogeneity in the included studies (Cochran Q = 3.548, Het. P value = 0.06). Five hundred four patients had undergone TL with vertical/T type closure; 112/504 (22.22%) developed PCF. Fifty-five patients had undergone TL with horizontal closure; 16/55 (29.09%) developed PCF. The overall (odds ratio) OR values with the corresponding 95% CI were 0.762 (0.412–1.408) with P = 0.385 on random effects model (Fig. 5).
Stapler Use
Two studies had reported the effect of stapler use on PCF development among 242 patients [10, 12]. Variations were attributed to heterogeneity in the included studies (I2 = 0, Cochran Q = 0.257, Het. P value = 0.612). Ninety-one patients had undergone TL with use of staplers; 5/91 (5.49%) developed PCF. One hundred fifty-one patients had undergone TL without use of staplers; 35/151 (23.18%) developed PCF. The overall (odds ratio) OR values with the corresponding 95% CI were 0.172 (0.064–0.46) with P ≤ 0.001 on random effects model (Fig. 5).
Suture Material
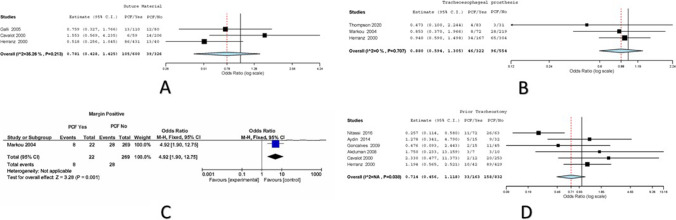
Three studies had reported the effect of different suture materials on PCF development among 926 patients [14, 15, 19]. Variations were attributed to heterogeneity in the included studies (I2 = 35.256, Cochran Q = 3.089, Het. P value = 0.213). Six hundred patients had undergone TL with use of vicryl; 105/600 (17.5%) developed PCF. Three hundred twenty-six patients had undergone TL with use of other suture materials; 39/326 (11.96%) developed PCF. The overall (odds ratio) OR values with the corresponding 95% CI were 0.781 (0.428–1.425) with P = 0.42 on random effects model (Fig. 6).
Fig. 6.
A Forest plot for suture material as risk factor for PCF; B forest plot for TEP insertion as risk factor for PCF; C forest plot for mucosal margin as risk factor for PCF; D forest plot for pre-operative tracheostomy as risk factor for PCF
TEP Insertion
Three studies had reported the effect of TEP insertion on PCF development among 876 patients [7, 9, 15]. Variations were attributed to heterogeneity in the included studies (I2 = 0, Cochran Q = 0.694, Het. P value = 0.707). Three hundred twenty-two patients had undergone TL with TEP insertion; 46/322 (14.29%) developed PCF. Five hundred fifty-four patients had undergone TL without insertion of TEP; 96/554 (17.33%) developed PCF. The overall (odds ratio) OR values with the corresponding 95% CI were 0.88 (0.594–1.305) with P = 0.526 on random effects model (Fig. 6).
Mucosal Margin
One study reported the effect of mucosal margins on PCF development among 292 patients [9]. Variations were attributed to heterogeneity in the included studies. Twenty-two patients had undergone TL with positive mucosal margins; 8/22 (36.36%) developed PCF. Two hundred sixty-nine patients had undergone TL without use of staplers; 28/269 (10.41%) developed PCF. The overall (odds ratio) OR values with the corresponding 95% CI were 4.92 (1.9–12.75) with P ≤ 0.001 on random effects model (Fig. 6).
Pre-operative Tracheostomy
Six studies had reported the effect of pre-operative tracheostomy on PCF development among 995 patients [12, 13, 15–17, 19]. Variations were attributed to heterogeneity in the included studies. One hundred sixty-three patients had undergone TL with pre-operative tracheostomy; 33/163 (20.25%) developed PCF. Eight hundred thirty-two patients had undergone TL without pre-operative tracheostomy; 158/832 (18.99%) developed PCF. The overall (odds ratio) OR values with the corresponding 95% CI were 0.714 (0.456–1.118) with P = 0.141 on random effects model (Fig. 6).
Discussion
In last two decades, the laryngeal cancer treatment had a paradigm shift from the surgical treatment to non-surgical therapy based on some well-performed trials [21–24]. Functional organ preservation may not be feasible in certain intermediate and advanced cases; hence, a primary total laryngectomy is still an initial treatment in such cases, especially in patients who do not want or are physically unable to undergo the ordeal of chemoradiation and follow-up, which may benefit from TL as a primary treatment option. Alternative treatment options, such as combined therapy with concurrent chemoradiotherapy and salvage surgery (including its morbidity), could be too costly for in certain patient subset. Total laryngectomy is commonly used primary treatment in many developing nations including India and Brazil, mainly due to cost concerns [25, 26]. There has been multiple level I and II evidence generated in the past decade pertaining to the risk factors leading to the development of pharyngocutaneous fistula following salvage laryngectomy [27–32], but none of them has addressed it for primary total laryngectomy alone.
In our study, we did not find the age to be the risk factor for the development of PCF in patients undergoing primary TL. A recent meta-analysis by Wang and colleagues, which included patients undergoing salvage and primary TL, showed that the age of patient to be a significant risk factor leading to development of PCF [32]. Similarly, there was no statistically significant difference in the PCF rates among males and females.
Multiple studies have suggested that comorbidities such as diabetes mellitus, pulmonary disorders, cardiopathy, and hypothyroidism may contribute to the occurrence of PCF. Unfortunately, pinpointing the exact comorbidity leading to development of PCF is difficult to assess due to heterogeneity and lack of consensus in data collection and reporting. In our meta-analysis, we found that having an associated comorbidity significantly impacted towards the development of PCF. An Italian paper in 2008 demonstrated that diabetes mellitus lead to increased risk of development of PCF [33]. Just having a comorbidity does not mean that the individual will develop a PCF; it is vital to understand that all comorbidities must be well controlled, and patient must be optimized before surgery to reduce the risk of PCF.
It is very difficult to ascertain the role of tobacco smoking and alcohol consumption on the development of PCF; the previous history of habits alone may not be sufficient to determine the causal relationship. We emphasize the clinicians to note the major variables pertaining to tobacco smoking and alcohol consumption; this may help to determine time-dose–response relationship. Based on the current available data, a tangible conclusion cannot be derived due to inadequacy of variables to demonstrate the effect in primary TL. Due to this discrepancy in data reporting, there is a controversy in discerning the role of habits on development of PCF, with studies showing both causal [32–34] and noncausal [35, 36] effects.
The subsite of the tumor plays a vital role in determining the extent of surgical resection. Involvement of the hypopharynx usually entails the removal of pharyngeal mucosa along with the Larynx. Primary closure of pharyngeal mucosa can be attempted if the width of the unstretched and non-devascularized pharyngeal mucosa is over 3.5 cm [37]; else, this may lead to neopharyngeal stenosis or neopharyngeal breakdown if augmentation pharyngoplasty is not performed [32, 37, 38]. The hypopharyngeal involvement leads to higher chances of PCF; this corroborates with our study. Further subsite stratification was not feasible due to non-uniformity in reporting.
Higher T stage of the disease leads to higher chances of PCF in combined salvage and primary laryngectomy dataset [32]; higher T stage was not seen as an independent risk factor for the development of PCF in patients undergoing primary TL. Further individual subset analysis of each T stage is necessary especially between T3 and T4a and by stage matching it to the latest AJCC staging system. The N stage of the disease has not been shown to be risk factor to the development of PCF in both salvage and in primary TL [27, 29, 31, 32].
The nutritional status of the patient is determined by pre-operative hemoglobin and albumin levels as surrogate markers [39]. This directly affects the ability of the body to handle surgical stress and wound healing. Our results are in line with the meta-analysis conducted by the Brazilian group [27]. Ensuring adequate nutritional status of the patient is of utmost essential to prevent the development of PCF.
The effect of type of pharyngeal closure has been hypothesized to be a factor in PCF development but has never been proven, mainly due to overlapping surgical techniques, non-standardized nomenclature, and reporting. Only two studies were included in his analysis based on the inclusion and exclusion criteria; in our study, we did not find any statistically significant difference PCF rates among different types of pharyngeal closure. The trifurcation in the T-shaped closure could theoretically increase the risk of fistula development, according to some studies [16, 40]. On the other hand, other studies have found that vertical closure increases the risk of a fistula [41, 42]. In some defect shapes, the T-shaped closure is thought to cause less tension than the vertical closure. However, horizontal closure may not be appropriate for vertically extended pharyngeal defects.
There has been a recent increase in the trend to use staplers for the creation of neopharynx. Only two studies were included in our analysis; both the studies showed a statistically significant difference in the development of PCF [10, 12]. This result is compounded by a recent meta-analysis from Taiwan, which shows a reduction of operative time and reduced complications following the use of staplers [43]. We must highlight that the use of staplers for creating neopharynx requires considerable expertise and may lead to non-satisfactory results if the staplers are not applied in a designated manner. There is a considerable selection, and reporting bias found among the included studies as seen in surgical technique articles [44]. The result of PCF reduction with stapler use must be further strengthened with well-controlled randomized studies.
Different suture materials like catgut, silk, polydioxanone, polyglycolic acid (monofilament and braided), and polypropylene have been used for pharyngeal closure [45–47]. The sutures are usually chosen based on the surgeon’s comfort and experience with it. Also, the techniques of pharyngeal mucosal approximation by various techniques were simple continuous interlocking sutures [45], interrupted sutures with extraluminal or intraluminal knots, Connell [48], Lembert [49], and Gambee [50]. The literature lacks information of leak rates with these different suturing techniques and materials. In our analysis, suture materials did not have a bearing on the development of PCF. A well-controlled blinded study must be conducted among the surgeons of various expertise by keeping certain patient parameters constant to accurately determine the effect of suture materials on development of PCF.
Rehabilitation following total laryngectomy is crucial to restore the functionality. TEP is now a standard of care for speech rehabilitation due to its in speech intelligibility, better acquisition, and fluency. Patients do not require a second surgery for speech acquisition when primary TEP is inserted. Furthermore, after a laryngectomy, patients can begin speech therapy within 2 weeks. In our study, preforming a primary TEP following a primary TL showed no statistically increase in the PCF rates. In a systematic review by Neto and colleagues, inserting a primary TEP during the TL and total laryngopharyngectomy leads to increase in the peri-TEP leak, wound infection, and stomal stenosis [51]. This study has also included patients undergoing primary TL, salvage procedures, and augmentation pharyngoplasty. A primary TEP is now a standard of care if there is no contraindication.
The presence of positive infiltrated margins may explain the reason for PCF occurring more frequently, mainly due to poor healing process occurring locally at the surgical wound. The presence of tumor cells can alter or obstruct the healing process and wound closure. In addition, the surgeon’s attempts to avoid positive margins by further and deeper pharyngeal mucosal excision into healthy tissue may result in insufficient tissue to perform the pharyngoplasty, resulting in tension wound closure which is predisposed to complications.
Our study did not find any difference in the PCF rates among the previously tracheostomized patents and non-tracheostomized patients undergoing primary TL. The tracheostoma is and exteriorized tracheal wound, which is almost always contaminated and colonized with respiratory flora. In a paper by Asher and colleagues, they hypothesized that the duration of tracheostomy has an effect on the post-operative complications including PCF formation, mainly due to migration of tracheostoma wound from clean contaminated to contaminated wound with the increase in duration. This data was not analyzed due to non-availability of the parameters among the included articles.
Our meta-analysis, however, has some limitations. In the few articles included, and the research was insufficient, in articles needing clarification, the missing data was sought from the respective corresponding authors. Some studies had fewer cases, and the negative results may not have been published. All of these factors could have contributed to the bias, as it cannot be completely ruled out. In the future, more high-quality studies will be needed to confirm these findings.
Conclusion
In conclusion, PCF after total laryngectomy is caused by a multitude of factors. The risk factors for PCF are currently debatable, and many studies are required to determine the most important risk factor. In this meta-analysis, we were able to determine the risk factors for PCF in patients undergoing primary TL, which included comorbidities, hypopharyngeal involvement, pre-operative hemoglobin and albumin, stapler usage, and positive mucosal margin. In clinical practice, our study provides important evidence-based medical evidence for the prevention and reduction of the development of PCF for primary TL.
Supplementary Information
Below is the link to the electronic supplementary material.
Author Contribution
All authors have contributed equally towards the development of the manuscript.
Data Availability
Data is available with the corresponding author.
Declarations
Provenance and Peer review
Not commissioned, externally peer-reviewed.
Conflict of Interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Karthik Nagaraja Rao, Email: Karthik.nag.rao@gmail.com.
Ripu Daman Arora, Email: neelripu@gmail.com.
Ambesh Singh, Email: ambesh.singh26@gmail.com.
Nitin M. Nagarkar, Email: directoroffice@aiimsraipur.edu.in
Aakash Aggarwal, Email: aggarwal.aakash3@gmail.com.
References
- 1.Bobdey S, Jain A, Balasubramanium G. Epidemiological review of laryngeal cancer: an Indian perspective. Indian J Med Paediatr Oncol Off J Indian Soc Med Paediatr Oncol. 2015;36(3):154–160. doi: 10.4103/0971-5851.166721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahn S-H, Hong HJ, Kwon SY, Kwon KH, Roh J-L, Ryu J, et al. Guidelines for the surgical management of laryngeal cancer: Korean Society of Thyroid-Head and Neck Surgery. Clin Exp Otorhinolaryngol. 2017;10(1):1–43. doi: 10.21053/ceo.2016.01389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Molteni G, Sacchetto A, Sacchetto L, Marchioni D. <p>Optimal management of post-laryngectomy pharyngo-cutaneous fistula</p>. Open Access Surg. 2020;13:11–25. doi: 10.2147/OAS.S198038. [DOI] [Google Scholar]
- 4.Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. doi: 10.1136/bmj.j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bramer WM, Rethlefsen ML, Kleijnen J, Franco OH. Optimal database combinations for literature searches in systematic reviews: a prospective exploratory study. Syst Rev. 2017;6(1):245. doi: 10.1186/s13643-017-0644-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg. 2021;88:105906. doi: 10.1016/j.ijsu.2021.105906. [DOI] [PubMed] [Google Scholar]
- 7.Thompson CSG, Asimakopoulos P, Evans A, Vernham G, Hay AJ, Nixon IJ. Complications and predisposing factors from a decade of total laryngectomy. J Laryngol Otol. 2020;134(3):256–262. doi: 10.1017/S0022215120000341. [DOI] [PubMed] [Google Scholar]
- 8.Wakisaka N, Murono S, Kondo S, Furukawa M, Yoshizaki T. Post-operative pharyngocutaneous fistula after laryngectomy. Auris Nasus Larynx. 2008;35(2):203–208. doi: 10.1016/j.anl.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 9.Markou KD, Vlachtsis KC, Nikolaou AC, Petridis DG, Kouloulas AI, Daniilidis IC. Incidence and predisposing factors of pharyngocutaneous fistula formation after total laryngectomy. Is there a relationship with tumor recurrence? Eur Arch Oto-Rhino-Laryngol Off J Eur Fed Oto-Rhino-Laryngol Soc EUFOS Affil Ger Soc Oto-Rhino-Laryngol - Head Neck Surg. 2004;261(2):61–7. doi: 10.1007/s00405-003-0643-6. [DOI] [PubMed] [Google Scholar]
- 10.Calli C, Pinar E, Oncel S. Pharyngocutaneous fistula after total laryngectomy: less common with mechanical stapler closure. Ann Otol Rhinol Laryngol. 2011;120(5):339–344. doi: 10.1177/000348941112000510. [DOI] [PubMed] [Google Scholar]
- 11.Tsou Y-A, Hua C-H, Lin M-H, Tseng H-C, Tsai M-H, Shaha A. Comparison of pharyngocutaneous fistula between patients followed by primary laryngopharyngectomy and salvage laryngopharyngectomy for advanced hypopharyngeal cancer. Head Neck. 2010;32(11):1494–1500. doi: 10.1002/hed.21352. [DOI] [PubMed] [Google Scholar]
- 12.Gonçalves AJ, de Souza J a L, Menezes MB, Kavabata NK, Suehara AB, Lehn CN. Pharyngocutaneous fistulae following total laryngectomy comparison between manual and mechanical sutures. Eur Arch Oto-Rhino-Laryngol Off J Eur Fed Oto-Rhino-Laryngol Soc EUFOS Affil Ger Soc Oto-Rhino-Laryngol - Head Neck Surg. 2009;266(11):1793–8. doi: 10.1007/s00405-009-0945-4. [DOI] [PubMed] [Google Scholar]
- 13.Akduman D, Naiboğlu B, Uslu C, Oysu C, Tek A, Sürmeli M, et al. Pharyngocutaneous fistula after total laryngectomy: incidence, predisposing factors, and treatment. Kulak Burun Bogaz Ihtis Derg KBB J Ear Nose Throat. 2008;18(6):349–354. [PubMed] [Google Scholar]
- 14.Galli J, Valenza V, Parrilla C, Galla S, Marchese MR, Castaldi P, et al. Pharyngocutaneous fistula onset after total laryngectomy: scintigraphic analysis. Acta Otorhinolaryngol Ital Organo Uff Della Soc Ital Otorinolaringol E Chir Cerv-facc. 2009;29(5):242–244. [PMC free article] [PubMed] [Google Scholar]
- 15.Herranz J, Sarandeses A, Fernández MF, Barro CV, Vidal JM, Gavilán J. Complications after total laryngectomy in nonradiated laryngeal and hypopharyngeal carcinomas. Otolaryngol-Head Neck Surg Off J Am Acad Otolaryngol-Head Neck Surg. 2000;122(6):892–898. doi: 10.1016/S0194-59980070020-9. [DOI] [PubMed] [Google Scholar]
- 16.Nitassi S, Belayachi J, Chihab M, Rkain I, Benayad J, Benbouzid MA, et al. Evaluation of post laryngectomy pharyngocutaneous fistula risk factors. Iran J Otorhinolaryngol. 2016;28(85):141–147. [PMC free article] [PubMed] [Google Scholar]
- 17.Aydin S, Taskin U, Orhan I, Altas B, Ege SS, Yucebas K, et al. The impact of pharyngeal repair time and suture frequency on the development of pharyngocutaneous fistula after total laryngectomy. J Craniofac Surg. 2014;25(3):775–779. doi: 10.1097/SCS.0000000000000826. [DOI] [PubMed] [Google Scholar]
- 18.Stanković M, Milisavljević D, Stojanov D, Zivić M, Zivaljević S, Stanković I, et al. Influential factors, complications and survival rate of primary and salvage total laryngectomy for advanced laryngeal cancer. Coll Antropol. 2012;36(Suppl 2):7–12. [PubMed] [Google Scholar]
- 19.Cavalot AL, Gervasio CF, Nazionale G, Albera R, Bussi M, Staffieri A, et al. Pharyngocutaneous fistula as a complication of total laryngectomy: review of the literature and analysis of case records. Otolaryngol-Head Neck Surg Off J Am Acad Otolaryngol-Head Neck Surg. 2000;123(5):587–592. doi: 10.1067/mhn.2000.110617. [DOI] [PubMed] [Google Scholar]
- 20.Papazoglou G, Doundoulakis G, Terzakis G, Dokianakis G. Pharyngocutaneous fistula after total laryngectomy: incidence, cause, and treatment. Ann Otol Rhinol Laryngol. 1994;103(10):801–805. doi: 10.1177/000348949410301010. [DOI] [PubMed] [Google Scholar]
- 21.Department of Veterans Affairs Laryngeal Cancer Study Group. Wolf GT, Fisher SG, Hong WK, Hillman R, Spaulding M, et al. Induction chemotherapy plus radiation compared with surgery plus radiation in patients with advanced laryngeal cancer. N Engl J Med. 1991;324(24):1685–90. doi: 10.1056/NEJM199106133242402. [DOI] [PubMed] [Google Scholar]
- 22.Wolf GT, Forastiere A, Ang K, Brockstein B, Conley B, Goepfert H, et al. Workshop report: organ preservation strategies in advanced head and neck cancer–current status and future directions. Head Neck. 1999;21(8):689–693. doi: 10.1002/(SICI)1097-0347(199912)21:8<689::AID-HED2>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 23.Forastiere AA, Goepfert H, Maor M, Pajak TF, Weber R, Morrison W, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003;349(22):2091–2098. doi: 10.1056/NEJMoa031317. [DOI] [PubMed] [Google Scholar]
- 24.Pointreau Y, Garaud P, Chapet S, Sire C, Tuchais C, Tortochaux J, et al. Randomized trial of induction chemotherapy with cisplatin and 5-fluorouracil with or without docetaxel for larynx preservation. J Natl Cancer Inst. 2009;101(7):498–506. doi: 10.1093/jnci/djp007. [DOI] [PubMed] [Google Scholar]
- 25.Silver CE, Beitler JJ, Shaha AR, Rinaldo A, Ferlito A. Current trends in initial management of laryngeal cancer: the declining use of open surgery. Eur Arch Otorhinolaryngol. 2009;266(9):1333–1352. doi: 10.1007/s00405-009-1028-2. [DOI] [PubMed] [Google Scholar]
- 26.Terrell JE, Fisher SG, Wolf GT, for the Veterans Affairs Laryngeal Cancer Study Group Long-term quality of life after treatment of laryngeal cancer. Arch Otolaryngol Neck Surg. 1998;124(9):964–71. doi: 10.1001/archotol.124.9.964. [DOI] [PubMed] [Google Scholar]
- 27.Dedivitis RA, Aires FT, Cernea CR, Brandão LG. Pharyngocutaneous fistula after total laryngectomy: systematic review of risk factors. Head Neck. 2015;37(11):1691–1697. doi: 10.1002/hed.23804. [DOI] [PubMed] [Google Scholar]
- 28.Hasan Z, Dwivedi RC, Gunaratne DA, Virk SA, Palme CE, Riffat F. Systematic review and meta-analysis of the complications of salvage total laryngectomy. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol. 2017;43(1):42–51. doi: 10.1016/j.ejso.2016.05.017. [DOI] [PubMed] [Google Scholar]
- 29.Liang J-W, Li Z-D, Li S-C, Fang F-Q, Zhao Y-J, Li Y-G. Pharyngocutaneous fistula after total laryngectomy: a systematic review and meta-analysis of risk factors. Auris Nasus Larynx. 2015;42(5):353–359. doi: 10.1016/j.anl.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 30.Paleri V, Drinnan M, van den Brekel MWM, Hinni ML, Bradley PJ, Wolf GT, et al. Vascularized tissue to reduce fistula following salvage total laryngectomy: a systematic review. Laryngoscope. 2014;124(8):1848–1853. doi: 10.1002/lary.24619. [DOI] [PubMed] [Google Scholar]
- 31.Sayles M, Grant DG. Preventing pharyngo-cutaneous fistula in total laryngectomy: a systematic review and meta-analysis. Laryngoscope. 2014;124(5):1150–1163. doi: 10.1002/lary.24448. [DOI] [PubMed] [Google Scholar]
- 32.Wang M, Xun Y, Wang K, Lu L, Yu A, Guan B, et al. Risk factors of pharyngocutaneous fistula after total laryngectomy: a systematic review and meta-analysis. Eur Arch Oto-Rhino-Laryngol Off J Eur Fed Oto-Rhino-Laryngol Soc EUFOS Affil Ger Soc Oto-Rhino-Laryngol - Head Neck Surg. 2020;277(2):585–599. doi: 10.1007/s00405-019-05718-9. [DOI] [PubMed] [Google Scholar]
- 33.Boscolo-Rizzo P, De Cillis G, Marchiori C, Carpenè S, Da Mosto MC. Multivariate analysis of risk factors for pharyngocutaneous fistula after total laryngectomy. Eur Arch Oto-Rhino-Laryngol Off J Eur Fed Oto-Rhino-Laryngol Soc EUFOS Affil Ger Soc Oto-Rhino-Laryngol - Head Neck Surg. 2008;265(8):929–936. doi: 10.1007/s00405-007-0562-z. [DOI] [PubMed] [Google Scholar]
- 34.Aslıer NGY, Doğan E, Aslıer M, İkiz AÖ. Pharyngocutaneous fistula after total laryngectomy: risk factors with emphasis on previous radiotherapy and heavy smoking. Turk Arch Otorhinolaryngol. 2016;54(3):91–98. doi: 10.5152/tao.2016.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patel UA, Moore BA, Wax M, Rosenthal E, Sweeny L, Militsakh ON, et al. Impact of pharyngeal closure technique on fistula after salvage laryngectomy. JAMA Otolaryngol- Head Neck Surg. 2013;139(11):1156–62. doi: 10.1001/jamaoto.2013.2761. [DOI] [PubMed] [Google Scholar]
- 36.Erdag MA, Arslanoglu S, Onal K, Songu M, Tuylu AO. Pharyngocutaneous fistula following total laryngectomy: multivariate analysis of risk factors. Eur Arch Oto-Rhino-Laryngol Off J Eur Fed Oto-Rhino-Laryngol Soc EUFOS Affil Ger Soc Oto-Rhino-Laryngol - Head Neck Surg. 2013;270(1):173–179. doi: 10.1007/s00405-012-2111-7. [DOI] [PubMed] [Google Scholar]
- 37.Hui Y, Wei WI, Yuen PW, Lam LK, Ho WK. Primary closure of pharyngeal remnant after total laryngectomy and partial pharyngectomy: how much residual mucosa is sufficient? Laryngoscope. 1996;106(4):490–494. doi: 10.1097/00005537-199604000-00018. [DOI] [PubMed] [Google Scholar]
- 38.Chotipanich A (2021) Total laryngectomy: a review of surgical techniques. Cureus [Internet]. [cited 2021 Oct 13]. Available from: https://www.cureus.com/articles/69113-total-laryngectomy-a-review-of-surgical-techniques [DOI] [PMC free article] [PubMed]
- 39.Corona LP, de Oliveira Duarte YA, Lebrão ML. Markers of nutritional status and mortality in older adults: the role of anemia and hypoalbuminemia. Geriatr Gerontol Int. 2018;18(1):177–182. doi: 10.1111/ggi.13137. [DOI] [PubMed] [Google Scholar]
- 40.Deniz M, Ciftci Z, Gultekin E. Pharyngoesophageal suturing technique may decrease the incidence of pharyngocutaneous fistula following total laryngectomy. Surg Res Pract. 2015;2015:363640. doi: 10.1155/2015/363640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walton B, Vellucci J, Patel PB, Jennings K, McCammon S, Underbrink MP. Post-Laryngectomy stricture and pharyngocutaneous fistula: review of techniques in primary pharyngeal reconstruction in laryngectomy. Clin Otolaryngol Off J ENT-UK Off J Neth Soc Oto-Rhino-Laryngol Cervico-Facial Surg. 2018;43(1):109–116. doi: 10.1111/coa.12905. [DOI] [PubMed] [Google Scholar]
- 42.van der Kamp MF, Rinkel RNPM, Eerenstein SEJ. The influence of closure technique in total laryngectomy on the development of a pseudo-diverticulum and dysphagia. Eur Arch Oto-Rhino-Laryngol Off J Eur Fed Oto-Rhino-Laryngol Soc EUFOS Affil Ger Soc Oto-Rhino-Laryngol - Head Neck Surg. 2017;274(4):1967–1973. doi: 10.1007/s00405-016-4424-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee Y-C, Fang T-J, Kuo I-C, Tsai Y-T, Hsin L-J. Stapler closure versus manual closure in total laryngectomy for laryngeal cancer: a systematic review and meta-analysis. Clin Otolaryngol Off J ENT-UK Off J Neth Soc Oto-Rhino-Laryngol Cervico-Facial Surg. 2021;46(4):692–698. doi: 10.1111/coa.13702. [DOI] [PubMed] [Google Scholar]
- 44.Paradis C. Bias in surgical research. Ann Surg. 2008;248(2):180–188. doi: 10.1097/SLA.0b013e318176bf4b. [DOI] [PubMed] [Google Scholar]
- 45.Haksever M, Akduman D, Aslan S, Solmaz F, Ozmen S. Modified continuous mucosal Connell suture for the pharyngeal closure after total laryngectomy: zipper suture. Clin Exp Otorhinolaryngol. 2015;8(3):281–288. doi: 10.3342/ceo.2015.8.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Altissimi G, Frenguelli A. Linear stapler closure of the pharynx during total laryngectomy: a 15-year experience (from closed technique to semi-closed technique) Acta Otorhinolaryngol Ital. 2007;27(3):118–122. [PMC free article] [PubMed] [Google Scholar]
- 47.Soylu L, Kıroğlu M, Aydoğan B, Çetik F, Kıroğlu F, Akçalı Ç, et al. Pharyngocutaneous fistula following laryngectomy. Head Neck J Sci Spec Head Neck. 1998;20(1):22–25. doi: 10.1002/(SICI)1097-0347(199801)20:1<22::AID-HED4>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 48.Huddleston HT, Dunnihoo DR. Connell Suture. J Gynecol Surg. 1998;14(3):149–149. doi: 10.1089/gyn.1998.14.149. [DOI] [Google Scholar]
- 49.Hardy KJ. A View of the development of intestinal suture. Part Ii. Principles and Techniques. Aust N Z J Surg. 1990;60(5):377–84. doi: 10.1111/j.1445-2197.1990.tb07388.x. [DOI] [PubMed] [Google Scholar]
- 50.Hirata K, Konishi T, Ueda Y, Kurosaki S, Tomisaki I, Nasu K, et al. Healing in the intestinal anastomosis–comparison of the Albert-Lembert and Gambee methods. J UOEH. 2000;22(1):1–6. doi: 10.7888/juoeh.22.1. [DOI] [PubMed] [Google Scholar]
- 51.BaraunaNeto JC, Dedivitis RA, Aires FT, Pfann RZ, Matos LL, Cernea CR. Comparison between primary and secondary tracheoesophageal puncture prosthesis: a systematic review. ORL. 2017;79(4):222–229. doi: 10.1159/000477970. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is available with the corresponding author.