Abstract
Haemophilus ducreyi is the etiologic agent of chancroid, a sexually transmitted genital ulcer disease that facilitates the transmission of human immunodeficiency virus. In the human model of infection, the histopathology of infected sites in part resembles a delayed-type hypersensitivity (DTH) response. In this study, T cells were isolated from skin biopsy specimens obtained from 24 subjects who were infected for 7 to 14 days. One clone and 12 lines that responded to H. ducreyi antigens were obtained from 12 of the subjects. Fluorescence-activated cell sorter analysis showed that the antigen-responsive lines and clone were predominantly CD3+ and CD4+. The lines and clone responded to H. ducreyi antigen in a dose-dependent manner and produced gamma interferon (IFN-γ) alone or IFN-γ and interleukin-10 (IL-10) but no IL-4 or IL-5 in response to H. ducreyi. Proliferation of T cells was dependent on the presence of autologous antigen-presenting cells. The lines showed little response to antigens prepared from other members of the Pasteurellaceae and responded to different fractions of H. ducreyi separated by preparative sodium dodecyl sulfate-polyacrylamide gel electrophoresis. We conclude that T cells that recognize H. ducreyi antigens are recruited to sites experimentally infected with the organism. The lack of cross-reactivity to the Pasteurellaceae and the response of the lines to different antigen fractions suggest that subjects are sensitized to H. ducreyi during the course of infection.
Haemophilus ducreyi causes chancroid, a genital ulcer disease that facilitates human immunodeficiency virus transmission (20, 56). Although rare in the United States (14), chancroid is common in Africa and Asia, where it accounts for up to 56% of genital ulcer disease (9, 10, 16, 39, 45, 53). H. ducreyi readily acquires antimicrobial resistance factors (38) and remains uniformly susceptible only to macrolides, quinolones, and broad-spectrum cephalosporins (33). Understanding the immune response to H. ducreyi infection may facilitate the development of alternative strategies to control chancroid.
Patients with chancroid do not seek medical attention until they have painful ulcers, 3 to 6 weeks after initiation of infection (24, 38). Naturally occurring ulcers are characterized by an influx of polymorphonuclear leukocytes (PMNs) which line the ulcer base and by a perivascular and interstitial infiltrate of macrophages, CD45RO+ T cells, and relatively few B cells (1, 27, 28, 32). Patients with ulcers of several weeks duration have serum antibody and blastogenic responses to H. ducreyi (15, 55), as well as increased levels of soluble interleukin-2 (IL-2) receptors in their urine and serum (2), suggesting the generation of a cell-mediated host response. Although natural infection may not reliably induce protective immunity on subsequent exposure, infection is confined to the skin, mucous membranes, and regional lymph nodes (11, 24, 38, 54).
Our laboratory developed an experimental model of H. ducreyi infection in human subjects that mimics the initial papular and pustular stages of natural infection (6, 48, 49). In the model, subjects are infected for up to 2 weeks and do not develop serum antibody or blastogenic responses to H. ducreyi (6, 48) even on rechallenge (5), probably due to the limited duration of infection. The cutaneous immune response to experimental infection is similar to that seen in natural infection and includes two major components: an infiltrate of PMNs that form epidermal pustules and a dermal infiltrate that consists of CD45RO+ T cells, macrophages, and some B cells (41, 48). The mononuclear cell infiltrate and the presence of mRNAs for gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α) resembles a delayed-type hypersensitivity (DTH) response (41, 48), even though the subjects have no history of chancroid. H. ducreyi and other members of the Pasteurellaceae family share multiple antigenic determinants on outer membrane proteins, heat shock proteins, and secreted products (18, 19, 40, 42, 46, 47, 54). Thus, we have hypothesized that previous colonization with the Pasteurellaceae may have sensitized subjects so that cross-reactive memory cells are recruited to the skin on their first exposure to H. ducreyi (41).
Here we describe the isolation of T-cell lines harvested from lesions of experimentally infected human volunteers. We characterized their antigen responsiveness and cytokine production and examined their cross-reactivity with related bacterial species and their response to fractionated H. ducreyi whole cells.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
Actinobacillus actinomycetemcomitans (ATCC 29522) was kindly provided by Dominique Galli of the Indiana University School of Dentistry. Haemophilus parahaemolyticus (ATCC 10014), Haemophilus paraphrohaemolyticus (ATCC 29237), Haemophilus parainfluenzae (ATCC 33392), and Haemophilus parainfluenzae (paraphrophilus) (ATCC 29242) were purchased from the American Type Culture Collection, Rockville, Md. Nontypeable Haemophilus influenzae 1479 and H. ducreyi 35000 and 35000HP (where HP indicates human passaged) were described previously (6, 46, 48). Bacterial strains were maintained on chocolate agar plates or grown in brain heart infusion broth containing hemin (50 μg/ml), 1% IsoVitaleX, and 5% fetal bovine serum as described previously (25).
Preparation of bacterial antigens.
Bacteria were grown to mid-log phase, collected by centrifugation at 10,000 × g, and washed three times with sterile saline. Freeze-thawed whole cells (FTWC) were suspended in 10 mM HEPES as described previously (23). For some experiments, bacterial pellets were suspended in 10 mM HEPES and lysed in a French pressure cell. A portion of the lysate was centrifuged at 100,000 × g for 1 h at 4°C. The soluble fraction (So1) was harvested, and the total membrane pellet was suspended in HEPES buffer. The protein concentration was determined by a protein assay (Bio-Rad Laboratories, Hercules, Calif.) using bovine serum albumin as a standard. Each preparation was adjusted to a final concentration of 1 mg/ml. For some experiments, the preparations were heat treated at 60°C for 45 min.
H. ducreyi FTWC preparations were separated by preparative sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on a 10% polyacrylamide gel, which was cut into nine slices (0.5 by 5 cm) horizontally. Each segment was eluted in phosphate-buffered saline (PBS) overnight at 4°C. Eluted proteins were concentrated (Centricon- 10; Amicon) and examined by SDS-PAGE. Eluted proteins were mixed 1:1 with latex microspheres (diameter, 0.8 μm; Sigma) in 0.1 M glycine (pH 8.6) and incubated at 4°C with agitation overnight. The suspensions were pelleted by centrifugation at 13,000 × g, incubated in blocking buffer (PBS with 10% human AB serum; Sigma Chemical Co., St. Louis, Mo.) for 6 h at 4°C, washed twice in RPMI 1640 (Gibco-BRL, Gaithersburg, Md.) supplemented with 2% human serum, and suspended in 100 μl of RPMI 1640 supplemented with 10% human serum.
Human subjects.
Peripheral blood and biopsy specimens from pustules were obtained from 24 subjects who participated in a reinfection trial or mutant-parent comparison trials (Table 1) (3–5, 12, 21, 52, 57, 58) (unpublished observations). All specimens were obtained at the clinical end point of infection, defined as the development of a painful pustule or a pustule that was present 14 days after inoculation, as described previously (3–5, 12, 21, 52, 57, 58). Informed consent was obtained from the subjects for participation, in accordance with the human experimentation guidelines of the U.S. Department of Health and Human Services and the Institutional Review Board of Indiana University—Purdue University at Indianapolis.
TABLE 1.
Sources of tissue samples
| Subject/sitea | Duration of infection (days) | Reference |
|---|---|---|
| H6R/P | 8 | 5 |
| 93/P | 10 | 3 |
| 95/P | 7 | 3 |
| 95/M | 7 | 3 |
| 96/P | 8 | 3 |
| 96/M | 8 | 3 |
| 98/M | 9 | 3 |
| 99/M | 14 | 3 |
| 101/P | 7 | 4 |
| 103/P | 9 | 4 |
| 105/P | 7 | 4 |
| 108/P | 7 | 4 |
| 111/M | 7 | 52 |
| 114/M | 8 | 52 |
| 117/M | 14 | 57 |
| 119/M | 7 | 57 |
| 134/P | 14 | 21 |
| 135/P | 14 | 21 |
| 136/P | 6 | 21 |
| 139/P | 9 | 21 |
| 140/P | 12 | 21 |
| 148/P+Mb | 8 | 58 |
| 150/P+Mb | 8 | 58 |
| 155/P | 14 | 12 |
| 158/P | 14 | 12 |
| 170/P | 14 | Unpublished |
Volunteer numbers are shown. P, sites inoculated with parent; M, sites inoculated with an isogenic mutant.
Cells from the parent and mutant sites were pooled.
Isolation of PBMCs and T-cell lines.
Peripheral blood mononuclear cells (PBMCs) were enriched from whole blood by Ficoll-Hypaque density gradient centrifugation and diluted in RPMI 1640 supplemented with penicillin-streptomycin, l-glutamine (BioWhittaker, Walkersville, Md.), and 10% heat-inactivated human AB serum as described previously (48).
Mononuclear cells were obtained from biopsy specimens by mincing tissue with a scalpel, extruding it through a 70-μm mesh filter, and subjecting it to density gradient centrifugation as described previously (41). The usual cell yield was 105 to 106 cells per biopsy specimen. T-cell lines or clones were propagated from these mononuclear cells. In initial experiments, the T cells were grown in bulk in the presence of phytohemagglutinin (PHA; 2 μg/ml), 106 γ-irradiated nonautologous PBMCs, and IL-2 (50 U/ml) (Biotest Diagnostics Corp., Danville, N.J.) and then cloned by limiting dilution as described by Koelle et al. (30). Antigen-responsive clones were expanded by a modification of a method utilizing autologous γ-irradiated PBMCs, allogeneic B lymphoblastoid cells (a gift of Peter A. Sieling, University of California Los Angeles), 30 ng of OKT3 (anti-CD3) per ml, IL-2 (45 U/ml), and heat-treated Sol (5 μg/ml) (44; P. A. Sieling, unpublished data). In all subsequent experiments, T-cell lines were grown in the presence of heat-inactivated Sol or FTWC (5 μg/ml), γ-irradiated autologous PBMCs, and IL-2 (50 U/ml) with repetitive cycles of rest and stimulation as described previously (17). Cells were cultivated in RPMI 1640 supplemented with antibiotics, glutamine, and human serum as described above at 37°C in a humid atmosphere containing 5% CO2.
Proliferation assays.
After being washed in RPMI 1640, 2 × 104 T cells and 105 γ-irradiated autologous PBMCs were seeded in wells of a 96-well plate with different amounts of bacterial preparations in triplicate. As controls for these assays, T cells and PBMCs were incubated with tetanus toxoid (2 μg/ml) or PHA (2 μg/ml) and γ-irradiated autologous PBMCs were incubated with H. ducreyi antigens in the absence of T cells. The cells were incubated for 96 h, and [3H]thymidine (0.5 μCi/well; Amersham Pharmacia Biotech, Piscataway, N.J.) was added for the last 8 h of incubation. The cells were harvested with a Filtermate Packard cell harvester (Packard Instrument Co., Inc., Rockville, Md.), and [3H]thymidine uptake was determined with a Direct Beta Counter Matrix 9600 (Packard). A stimulation index (SI) of proliferation was determined as E/C, where C (control) is defined as the cpm in the absence of antigen or mitogen and E (experimental) is defined as the cpm in the presence of antigen or mitogen. For each value, a standard error was calculated as an approximate estimate of the variance for the ratio of two means (29). The background cpm in the absence of antigen or mitogen was 43.0 ± 41.0 (mean ± standard deviation) for the proliferation assays reported in Table 2.
TABLE 2.
Characterization of H. ducreyi antigen-specific cell lines and clone
| Subject/sitea | Phenotype | SI in response to:
|
Cytokine response to H. ducreyi (pg/ml)
|
|||
|---|---|---|---|---|---|---|
| H. ducreyi antigenb | Tetanus toxoid | PHA | IFN-γ | IL-10 | ||
| Th1 | ||||||
| 139/P | CD3 95% (29)c | 1.3 (29)d | 0.4 | 120.0 | 880 (58)e | <8f |
| CD4 88% | ||||||
| 155/P | CD3 99% (99) | 12.5 (29) | 0.8 | 21.4 | 148,320 (42) | <8 |
| CD4 97% | ||||||
| 158/P | CD3 99% (29) | 93.6 (29) | 1.9 | 205.0 | 270,000 (29) | <8 |
| CD4 88% | ||||||
| CD8 8% | ||||||
| 170/P | CD3 99% (32) | 8.5 (22) | 3.4 | 49.7 | 16,300 (21) | <8 |
| CD4 95% | ||||||
| Presumptive T regulatory | ||||||
| H6R/P | CD3 99% (40) | 19.7 (72) | 2.3 | 54.6 | 500 (53) | 50 |
| CD4 99% | ||||||
| 99/M | CD4 92% (15) | 195.0 (44) | 1.0 | 97.0 | 67,450 (105) | 560 |
| 105/P | CD3 98% (82) | 3.0 (96) | 1.1 | 56.3 | 610,000 (118) | 40 |
| CD4 97% | ||||||
| 150/P+M | NDg | 0.6 (63) | ND | 108.0 | 4,440,000 (63) | 1,720 |
| Uncharacterized | ||||||
| 95/P | CD3 98% (67) | 2.3 (52) | ND | 12.0 | ND | ND |
| CD4 93% | ||||||
| 95/M | CD3 98% (67) | 8.9 (52) | ND | 6.0 | ND | ND |
| CD4 14% | ||||||
| CD8 17% | ||||||
| 98/M | CD3 97% (40) | 7.8 (29) | ND | 93.0 | ND | ND |
| CD4 39% | ||||||
| CD8 37% | ||||||
| 101/P | ND | 3.6 (43) | 0.8 | 165.0 | ND | ND |
| 103/P | CD3 89% (55) | 25.8 (41) | 8.0 | 180.0 | ND | ND |
| CD4 59% | ||||||
| CD8 24% | ||||||
Subject and site designations are identical to those in Table 1. Cell lines were designated Th1, presumptive T regulators, or uncharacterized based on the cytokine profile.
For lines 95 to 99, proliferation in response to Sol; for the remainder of the lines and the clone, proliferation in response to FTWC.
Age of culture (days) at the time of the FACS analysis is shown in parentheses.
Age of culture (days) at the time of the proliferation assay is shown in parentheses.
Age of culture (days) at the time of the cytokine analysis is shown in parentheses.
<8, lower than the limits of detection.
ND, not done due to an insufficient number of cells.
Cytokine production.
T cells (106/ml) and γ-irradiated autologous PBMCs (4 × 106/ml) were cultured with or without the H. ducreyi preparations. As a control, γ-irradiated autologous PBMCs alone were cultured under the same conditions. The supernatants were harvested after a 24-h incubation, and cytokine production was determined by enzyme-linked immunosorbent assay (PharMingen). The limits of detection were 98 pg/ml for IFN-γ and 8 pg/ml for IL-4, IL-5, and IL-10. Control supernatants did not contain any IFN-γ, but γ-irradiated PBMCs from a few volunteers produced IL-10 in response to H. ducreyi. Thus, IL-10 production is reported as the difference between IL-10 produced by T cells plus feeder cells and IL-10 produced by feeder cells alone in the presence of antigen (See Table 2).
Flow cytometry.
To determine the phenotype of the T-cell lines, approximately 105 cells were stained with fluorescent antibodies (BD Biosciences, San Jose, Calif.) as described previously (41). The following sets of antibodies were used: CD3-FITC plus CD16-PE plus CD56-PE, CD3-FITC plus CD19-PE or CD4-FITC plus CD8-PE. As controls, isotype-matched antibodies were used to detect nonspecific staining. Cells were washed, suspended in PBS plus 2% paraformaldehyde, and analyzed using a FACScan flow cytometer (BD Biosciences). A gate was placed around live cells, and percentages are reported as the proportion of gated cells.
RESULTS
Generation of an H. ducreyi antigen-specific human T-cell clone and lines.
Twenty-nine punch biopsy specimens of pustules were obtained from 24 volunteers who participated in a reinfection trial or several mutant-parent comparison trials (Table 1). Samples were obtained from sites inoculated with the parent H. ducreyi strains 35000 or 35000HP or isogenic mutants derived from these strains. All samples were obtained at the clinical end point in the human challenge model, defined as development of a painful pustule or 14 days of infection.
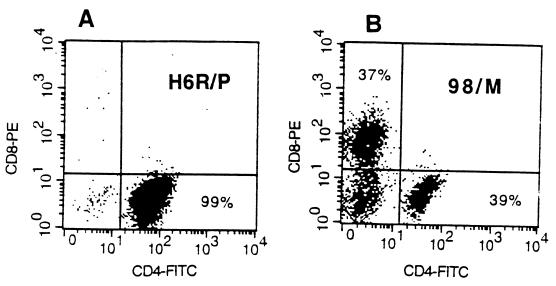
H. ducreyi expresses a heat-labile cytolethal distending toxin that inhibits the growth of primary human T cells and causes apoptosis of Jurkat T cells in vitro (23). Therefore, we first attempted to propagate T cells in the absence of H. ducreyi antigens. Two biopsy specimens were obtained from a subject (H6R), who participated in a reinfection trial (5). One sample was obtained from an infected site, and another was obtained from a control site that was inoculated with heat-killed bacteria and appeared clinically normal. Cells were expanded in bulk in the presence of PHA, IL-2 and γ-irradiated nonautologous PBMCs. No T cells were obtained in culture from the control biopsy specimen, while T cells from the pustule expanded and were cloned using a limiting-dilution technique (30). From 386 seeded wells, 64 clones were recovered and 1 responded to heat-treated H. ducreyi Sol (optimal antigen concentration, 1 to 10 μg/ml; SI = 10) but did not respond to tetanus toxoid. The clone was expanded in the presence of heat-treated antigen. The expanded cells proliferated in response to heat-treated H. ducreyi Sol (optimal antigen concentration, 1 μg/ml; SI = 59) and FTWC (Table 2). Fluorescence-activated cell sorter (FACS) analysis showed that the clone consisted only of CD4+ cells (Fig. 1). By enzyme-linked immunosorbent assay, the clone produced IFN-γ (500 pg/ml) and IL-10 (50 pg/ml) in response to antigen stimulation (Table 2). The data suggested that primary T cells could proliferate in the presence of heat-treated H. ducreyi antigens and that antigen-responsive T cells were recruited to sites that were actively infected but were not present in skin that was not infected.
FIG. 1.
FACS analysis of the H6R/P clone (A) and line 98/M (B) for CD4 and CD8 after 40 days of expansion in vitro.
The limitation of the non-antigen-dependent methods of isolating T cell lines is that antigen-responsive cells, IL-2-responsive cells, and cells responsive to alloantigens will all be expanded. Thus, we subsequently expanded T cell lines from 27 biopsy specimens in the presence of heat-inactivated H. ducreyi antigens (Sol or FTWC, 5 μg/ml), γ-irradiated autologous PBMCs, and IL-2. For two subjects (subjects 148 and 150), cells from parent and mutant inoculated sites were pooled. No lines were obtained from four biopsy specimens; 21 biopsy specimens yielded T-cell lines. Of these, 12 repeatedly had SIs of >2 or produced cytokines in response to antigen stimulation during in vitro expansion for a range of 15 days to 6 months (Table 2). These lines were further characterized.
Characterization of antigen-responsive lines.
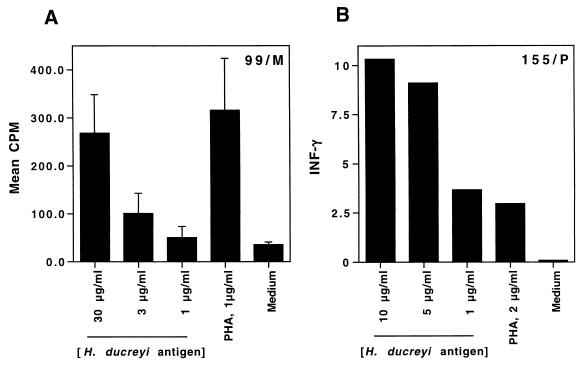
Of the T cell lines, 10 proliferated in response to H. ducreyi (Table 2). In general, the proliferative responses were dose dependent (Fig. 2). The T-cell lines proliferated in response to PHA and H. ducreyi FTWC but did not respond as well to tetanus toxoid (Fig. 2 and Table 2), suggesting that the lines were antigen specific. We also compared the proliferation of the T cells in the presence of γ-irradiated allogeneic or autologous PBMCs. Only autologous PBMCs supported the H. ducreyi-specific proliferation of T cells (data not shown), suggesting that the proliferative response to H. ducreyi was likely to be major histocompatibility complex restricted and not a nonspecific response.
FIG. 2.
Proliferation of (A) and IFN-γ production by (B) T cells from volunteers 99 (A) and 155 (B) in response to 10, 5, or 1 μg of heat-treated H. ducreyi FTWC per ml, 2 μg of PHA per ml, or medium alone. For the proliferation assays, the results are expressed as mean and standard error of triplicate wells; for the cytokine assays, triplicate wells were pooled and single measurements were obtained.
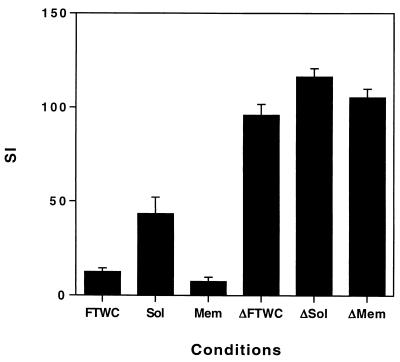
The cells responded equally well to heat-inactivated FTWC, Sol, and membrane preparations of H. ducreyi (Fig. 3). However, proliferation was lower for cells in the presence of antigens that were not heat inactivated (Fig. 3), which was consistent with our previous observations that CDT inhibited T-cell growth (23).
FIG. 3.
Proliferation of T cells from volunteer 99 in the presence of γ-irradiated autologous PBMCs in response to FTWC, Sol, or membrane (Mem) antigens that were heat treated (Δ) or not heat treated. Results are expressed as mean and standard error of triplicate wells. Similar results were obtained with clone H6R.
Seven of the lines produced cytokines in response to H. ducreyi (Fig. 2 and Table 2). Two cell lines (139/P and 150/P+M) that produced cytokines in an antigen-specific manner did not proliferate in the presence of H. ducreyi (Table 2). Three cell lines (99/M, 105/P, and 150/P+M) produced both IFN-γ and IL-10 in response to H. ducreyi, while four cell lines (139/P, 155/P, 158/P, and 170/P) produced only IFN-γ. No IL-4 or IL-5 was detected in 24-h culture supernatants (data not shown). Thus, some of the lines had characteristics of the Th1 phenotype, while others may have contained regulatory T cells (Table 2).
Sufficient cell growth was obtained from 10 of the lines for flow cytometry analysis; all values shown were obtained during the period when the lines were antigen responsive. The cell lines were 89 to 99% CD3+ (Table 2). After one to three rounds of antigen stimulation, some cell lines consisted of both CD4+ and CD8+ cells while others consisted primarily of CD4+ cells. Lines that could be maintained for months in culture (99/M, 105/P, 139/P, 155/P, 158/P, and 170/P) consisted primarily of CD4+ cells (Table 2 and data not shown). With the exception of 103/P, which had 9% CD16 and/or CD56+ cells, the cell lines had fewer than 0.5% CD16, CD56+, or CD19+ cells.
Response to the Pasteurellaceae.
Four lines and the clone were tested for proliferation in response to antigens prepared from related bacterial species (Table 3). Two cell lines, 155/P and 158/P, were strong responders to H. ducreyi (SIs = 109 and 85, respectively) and also responded to a few other species (10 > SI > 2). Other cell lines and the H6R/P clone showed no cross-reactivity with other members of the Pasteurellaceae, suggesting that these subjects developed an H. ducreyi-specific memory response during the course of experimental infection.
TABLE 3.
Cross-reactivity of T-cell lines with members of the Pasteurellaceae
| Species | SI for line or clonea:
|
||||
|---|---|---|---|---|---|
| H6R/P | 99/M | 155/P | 158/P | 170/P | |
| H. ducreyi | 13.3 ± 6.4 | 15.7 ± 4.4 | 108.7 ± 24.8 | 85.4 ± 24.2 | 11.4 ± 3.1 |
| H. influenzae | 1.4 ± 0.8 | 0.6 ± 0.2 | 1.7 ± 0.6 | 4.8 ± 1.3 | 0.6 ± 0.2 |
| H. parahaemolyticus | 1.3 ± 0.6 | 0.6 ± 0.1 | 9.3 ± 2.3 | 1.5 ± 0.6 | 1.9 ± 0.6 |
| H. paraphrohaemolyticus | 1.0 ± 0.5 | 0.7 ± 0.2 | 3.9 ± 1.1 | 1.7 ± 0.5 | 0.1 ± 0.5 |
| H. parainfluenzae | 1.2 ± 0.6 | 0.7 ± 0.2 | 0.9 ± 0.4 | 1.9 ± 0.5 | 0.9 ± 0.3 |
| H. paraphrophilus | 1.0 ± 0.5 | 0.7 ± 0.3 | 1.1 ± 0.4 | 3.2 ± 1.7 | 1.1 ± 0.3 |
| A. actinomycetemcomitans | ND | ND | 0.9 ± 0.4 | 4.0 ± 1.2 | ND |
ND, not done. Values are means ± standard errors.
Response to crude fractionated antigens.
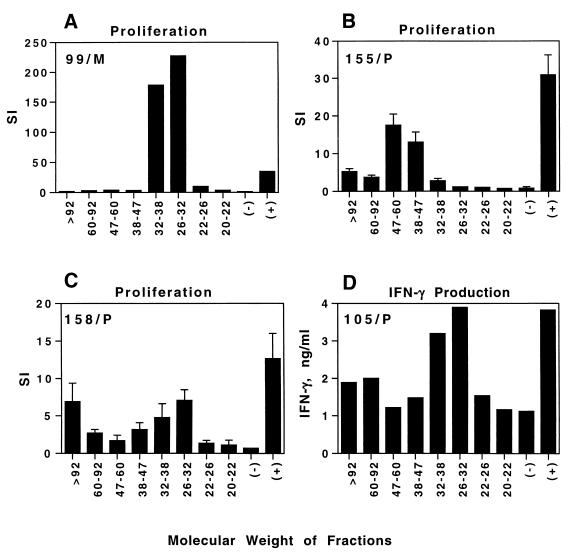
To determine whether an immunodominant protein was responsible for the antigen-specific responses of T cells, we separated H. ducreyi cells by preparative SDS-PAGE into nine fractions (data not shown). Proteins were eluted from the fractions and coupled to latex microspheres. The H6R/P clone proliferated only in response to a >83 kDa fraction (data not shown). The cell lines from different subjects proliferated in response to different and occasionally multiple fractions (Fig. 4), including those of >92 kDa (155/P and 158/P), 38 to 47 and 47 to 60 kDa (155/P), and 26 to 32 and 32 to 38 kDa (99/M and 158/P). Line 105/P produced IFN-γ in response to the 26- to 32-kDa and 32- to 38-kDa fractions (Fig. 4) but did not proliferate well in response to any fractions (data not shown). These data indicate that the cultured T cells did not respond to a single immunodominant antigen and suggest that different subjects were sensitized to different H. ducreyi antigens during the course of experimental infection.
FIG. 4.
Proliferation of T cells or IFN-γ production in the presence of γ-irradiated autologous PBMCs from lines 99/M (A), 155/P (B), 158/P (C), and 105/P (D) in response to heat-treated protein fractions of H. ducreyi of different molecular weights coupled to latex microspheres, microspheres alone (−), and microspheres coupled to H. ducreyi whole cells (+). Depending on the number of T cells available for an individual experiment, the results of the proliferation assays were obtained from single measurements or triplicate measurements for each fraction; error bars are shown for the latter experiments. The results are representative of two to four experiments done for each line at different times. To display the data, the y axis for SI varies in the figure.
DISCUSSION
In this study, we generated several H. ducreyi-specific T-cell lines and a clone from skin biopsy specimens from infected human volunteers. The T cells did not respond as well to related bacterial species as they did to H. ducreyi, and they responded to a variety of crude antigen fractions prepared from H. ducreyi. Taken together, the data suggest that subjects are specifically sensitized to H. ducreyi antigens and develop adaptive immunity during experimental infection.
The T cells were derived from biopsy specimens of sites infected with live H. ducreyi for 6 to 14 days. In the human model, sites inoculated with heat-killed bacteria do not contain an inflammatory infiltrate (51). PBMCs isolated on the day of infection, at end point, and 21 days after inoculation exhibit low levels of blastogenesis to H. ducreyi that do not vary over time, even in subjects who have been rechallenged (5, 48). In a reinfected subject (H6R), we did not recover T cells from a biopsy specimen of a site challenged with heat-killed control cells using PHA and IL-2 while a biopsy specimen of an infected site yielded an H. ducreyi-responsive clone. The remaining subjects who donated tissue to this study participated in mutant-parent comparison trials, and our protocols for these trials permit biopsies of infected sites only. Thus, although we attempted to grow T cells from noninfected skin in only one subject, it is likely that the antigen-responsive T-cell lines were cutaneous in origin and were not derived from peripheral blood, consistent with data reported for other cutaneous infections (30, 35).
The T-cell lines and clone were composed predominantly of CD4+ cells that secreted IFN-γ only or IFN-γ plus IL-10 in response to H. ducreyi. In murine systems, IL-10 down-regulates IFN-γ and monokine responses to intracellular infections and acts to prevent immunopathology (22). Thus, some lines had characteristics of Th1 cells while others may have contained regulatory T cells. The T-cell infiltrate in experimental lesions consists primarily of CD45RO+ cells that are 60 to 80% CD4+, 20 to 40% CD8+, and of the αβ lineage (41, 48). mRNAs for the Th1 cytokines IFN-γ and IL-2 are detected in all experimental lesions, while mRNAs for the Th2 cytokines IL-4 and IL-5 are present in about half of the lesions; mRNA for IL-10 was not measured in these studies (41). Some of the lines initially contained CD8+ cells, but the methods used to generate the lines are biased to expansion of CD4+ populations (30, 37), and eventually the lines became predominantly CD4+.
Although the mononuclear cell infiltrate in experimental infection develops within 24 h of inoculation and resembles a DTH response, the volunteers have no prior exposure to H. ducreyi, a requirement for a classic DTH response. We had hypothesized previously that epitopes shared by H. ducreyi and the Pasteurellaceae elicit an infiltrate of cross-reactive memory cells in the initial stages of infection and that a limited number of immunodominant antigens elicit the stereotypic infiltrate of memory cells seen in all subjects who have been infected (41). As reported by others (36), human T-cell lines are difficult to maintain in culture and the number of lines or clones that could be characterized for antigen responsiveness was limited. However, the lack of strong cross-reactivity of the T-cell lines to related bacterial species and the response of the different lines to different antigen fractions suggest that the subjects were sensitized to H. ducreyi during the course of experimental infection.
Based on these data and the immunohistopathology and cytokine analysis of experimental lesions (41, 48), we propose the following working model for the recruitment of T cells into the skin by H. ducreyi. Bacterial components such as lipoproteins and lipooligosaccharide initiate innate immunity through activation of toll-like receptors on macrophages (13, 31, 34), which secrete TNF-α, a potent inducer of E-selectin (ELAM-1) on the endothelium. In concert with other chemokines produced by the endothelial cells, macrophages, and CD1a cells, homing of memory/effector cells to the skin occurs within 24 h of inoculation (26, 43, 50). These memory effector cells may not initially be antigen specific, which may explain why about half the lines recovered from the biopsy specimens were not H. ducreyi responsive. During the course of infection in some subjects, T cells are sensitized by macrophages and CD1a cells presenting H. ducreyi antigens in the draining regional lymph nodes. These sensitized memory/effector cells also home to the lesion and expand, and the lesion becomes enriched in antigen-responsive cells. Thus, the immune response to H. ducreyi has features of both innate and adaptive T-cell immunity.
Recently, we have shown that H. ducreyi colocalizes primarily with PMNs and macrophages but remains extracellular throughout experimental infection (7, 8). We have found no evidence for an intracellular niche for the organism. The antigen-specific CD4+ cells may eventually provide help for the development of antibody responses that usually occur late in the ulcerative stage of disease (15). The possible function of CD8+ cells, which account for 20 to 40% of the T-cell infiltrate in experimental infection, in response to this extracellular pathogen is less clear. Given that subjects who are experimentally infected with H. ducreyi are not protected against rechallenge and that natural infection does not seem to reliably confer protection against subsequent exposure to the organism (5, 11, 24, 38), the DTH-like response may contribute more to pathology than bacterial clearance. Future studies will include a determination of the time course of the development of the antigen-specific response, an investigation of whether CD1-restricted T cells are also recruited to lesions, an investigation of the cytokines and/or chemokines responsible for homing, and a study of how the bacteria are orchestrating the host response.
ACKNOWLEDGMENTS
We thank Carol Schnizlein-Bick, Katy Palmer, Janet Arno, and Cong Ping Xie for their contributions to this work; Peter Sieling, Robert Modlin, and David Koelle for their advice on growing T cells; and Peter Sieling, Margaret Bauer, and Byron Batteiger for reviewing the manuscript. We also thank Jaffar Al-Tawfiq, Royden Young, and Clifton Bong for obtaining the clinical specimens, and we thank the volunteers who participated in the trials.
This work was supported by Public Health Service grants AI27863 and AI31494 from the National Institute of Allergy and Infectious Diseases (NIAID). The clinical samples used in this study were obtained from clinical trials supported by the Sexually Transmitted Diseases Clinical Trials Unit through contract NO1-AI175329 from the NIAID and by Public Health Service grant MO1RR00750 to the GCRC at Indiana University
REFERENCES
- 1.Abeck D, Freinkel A L, Korting H C, Szeimis R M, Ballard R C. Immunohistochemical investigations of genital ulcers caused by Haemophilus ducreyi. Int J STD AIDS. 1997;8:585–588. doi: 10.1258/0956462971920839. [DOI] [PubMed] [Google Scholar]
- 2.Abeck D, Korting H C, Zaba R, Dangor Y, Fehler G, Ballard R C. Soluble interleukin-2 receptors in serum and urine of patients with chancroid and their response to therapy. Int J STD AIDS. 1990;1:282–284. doi: 10.1177/095646249000100411. [DOI] [PubMed] [Google Scholar]
- 3.Al-Tawfiq J A, Bauer M E, Fortney K R, Katz B P, Hood A F, Ketterer M, Apicella M A, Spinola S M. A pilus-deficient mutant of Haemophilus ducreyi is virulent in the human model of experimental infection. J Infect Dis. 2000;181:1176–1179. doi: 10.1086/315310. [DOI] [PubMed] [Google Scholar]
- 4.Al-Tawfiq J A, Fortney K R, Katz B P, Elkins C, Spinola S M. An isogenic hemoglobin receptor-deficient mutant of Haemophilus ducreyi is attenuated in the human model of experimental infection. J Infect Dis. 2000;181:1049–1054. doi: 10.1086/315309. [DOI] [PubMed] [Google Scholar]
- 5.Al-Tawfiq J A, Palmer K L, Chen C-Y, Haley J C, Katz B P, Hood A F, Spinola S M. Experimental infection of human volunteers with Haemophilus ducreyi does not confer protection against subsequent challenge. J Infect Dis. 1999;179:1283–1287. doi: 10.1086/314732. [DOI] [PubMed] [Google Scholar]
- 6.Al-Tawfiq J A, Thornton A C, Katz B P, Fortney K R, Todd K D, Hood A F, Spinola S M. Standardization of the experimental model of Haemophilus ducreyi infection in human subjects. J Infect Dis. 1998;178:1684–1687. doi: 10.1086/314483. [DOI] [PubMed] [Google Scholar]
- 7.Bauer M E, Goheen M P, Townsend C A, Spinola S M. Haemophilus ducreyi associates with phagocytes, collagen, and fibrin and remains extracellular throughout infection of human volunteers. Infect Immun. 2001;69:2549–2557. doi: 10.1128/IAI.69.4.2549-2557.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bauer M E, Spinola S M. Localization of Haemophilus ducreyi at the pustular stage of disease in the human model of infection. Infect Immun. 2000;68:2309–2314. doi: 10.1128/iai.68.4.2309-2314.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Behets F M-T, Andriamiadana J, Randrianasolo D, Randriamanga R, Rasamilalao D, Chen C-Y, Weiss J B, Morse S A, Dallabetta G, Cohen M S. Chancroid, primary syphilis, genital herpes, and lymphogranuloma venereum in Antananarivo, Madagascar. J Infect Dis. 1999;180:1382–1385. doi: 10.1086/315005. [DOI] [PubMed] [Google Scholar]
- 10.Behets F M-T, Brathwaite A R, Hylton-Kong T, Chen C-Y, Hoffman I, Weiss J B, Morse S A, Dallabetta G, Cohen M S, Figueroa J P. Genital ulcers: etiology, clinical diagnosis, and associated human immunodeficiency virus infection in Kingston, Jamaica. Clin Infect Dis. 1999;28:1086–1090. doi: 10.1086/514751. [DOI] [PubMed] [Google Scholar]
- 11.Blackmore C A, Limpakarnjanarat K, Rigau-Perez J G, Albritton W L, Greenwood J R. An outbreak of chancroid in Orange County, California: descriptive epidemiology and disease-control measures. J Infect Dis. 1985;151:840–844. doi: 10.1093/infdis/151.5.840. [DOI] [PubMed] [Google Scholar]
- 12.Bong C T H, Throm R E, Fortney K R, Katz B P, Hood A F, Elkins C, Spinola S M. A DsrA deficient mutant of Haemophilus ducreyi is impaired in its ability to infect human volunteers. Infect Immun. 2001;69:1488–1491. doi: 10.1128/IAI.69.3.1488-1491.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brightbill H D, Libraty D H, Krutzik S R, Yang R-B, Belisle J T, Bleharski J R, Maitland M, Norgard M V, Plevy S E, Smale S T, Brennan P J, Bloom B R, Godowski P J, Modlin R L. Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science. 1999;285:732–736. doi: 10.1126/science.285.5428.732. [DOI] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention. Summary of notifiable diseases, United States. 1998. Morb Mortal Wkly Rep. 1999;47:78. [PubMed] [Google Scholar]
- 15.Chen C-Y, Mertz K J, Spinola S M, Morse S A. Comparison of enzyme immunoassays for antibodies to Haemophilus ducreyi in a community outbreak of chancroid in the United States. J Infect Dis. 1997;175:1390–1395. doi: 10.1086/516471. [DOI] [PubMed] [Google Scholar]
- 16.Chen C Y, Ballard R C, Beck-Sague C M, Dangor Y, Radebe F, Schmid S, Weiss J B, Tshabalala V, Fehler G, Htun Y, Morse S A. Human immunodeficiency virus infection and genital ulcer disease in South Africa. The herpetic connection. Sex Transm Dis. 2000;27:21–29. doi: 10.1097/00007435-200001000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Coligan J E, Kruisbeek A M, Margulies D H, Shevach E M, Strober W. Current protocols in immunology. New York, N.Y: John Wiley & Sons, Inc.; 1994. [Google Scholar]
- 18.Cope L D, Lumbley S, Latimer J L, Klesney-Tait J, Stevens M K, Johnson L S, Purven M, Munson R S, Jr, Lagergard T, Radolf J D, Hansen E J. A diffusible cytotoxin of Haemophilus ducreyi. Proc Natl Acad Sci USA. 1997;94:4056–4061. doi: 10.1073/pnas.94.8.4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elkins C, Chen C-J, Thomas C E. Characterization of the hgbA locus encoding a hemoglobin receptor from Haemophilus ducreyi. Infect Immun. 1995;63:2194–2200. doi: 10.1128/iai.63.6.2194-2200.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fleming D T, Wasserheit J N. From epidemiological synergy to public health policy and practice: the contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sex Transm Infect. 1999;75:3–17. doi: 10.1136/sti.75.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fortney K R, Young R S, Bauer M E, Katz B P, Hood A F, Munson R S, Jr, Spinola S M. Expression of peptidoglycan-associated lipoprotein is required for virulence in the human model of Haemophilus ducreyi infection. Infect Immun. 2000;68:6441–6448. doi: 10.1128/iai.68.11.6441-6448.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gazzinelli R T, Wysoka M, Hieny S, Scharton-Kersten T, Cheever A, Kuhn R, Muller W, Trinchieri G, Sher A. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent on CD4+ T cells and accompanied by overproduction of IL-12, IFN-γ and TNF-α. J Immunol. 1996;157:798–805. [PubMed] [Google Scholar]
- 23.Gelfanova V, Hansen E J, Spinola S M. Cytolethal distending toxin of Haemophilus ducreyi induces apoptotic death of Jurkat T cells. Infect Immun. 1999;67:6394–6402. doi: 10.1128/iai.67.12.6394-6402.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hammond G W, Slutchuk M, Scatliff J, Sherman E, Wilt J C, Ronald A R. Epidemiologic, clinical, laboaratory, and therapeutic features of an urban outbreak of chancroid in North America. Rev Infect Dis. 1980;2:867–879. doi: 10.1093/clinids/2.6.867. [DOI] [PubMed] [Google Scholar]
- 25.Hiltke T J, Bauer M E, Klesney-Tait J, Hansen E J, Munson R S, Jr, Spinola S M. Effect of normal and immune sera on Haemophilus ducreyi 35000HP and its isogenic MOMP and LOS mutants. Microb Pathog. 1999;26:93–102. doi: 10.1006/mpat.1998.0250. [DOI] [PubMed] [Google Scholar]
- 26.Kelly K A. Modulation of leukocyte-endothelial cell interactions by infectious agents. Bull Inst Pasteur. 1997;95:147–159. [Google Scholar]
- 27.King R, Choudhri S H, Nasio J, Gough J, Nagelkerke N J D, Plummer F A, Ndinya-Achola J O, Ronald A R. Clinical and in situ cellular responses to Haemophilus ducreyi in the presence or absence of HIV infection. Int J STD AIDS. 1998;9:531–536. doi: 10.1258/0956462981922773. [DOI] [PubMed] [Google Scholar]
- 28.King R, Gough A, Nasio J, Ndinya-Achola F, Plummer F, Wilkins J. An immunohistochemical analysis of naturally occurring chancroid. J Infect Dis. 1996;174:427–430. doi: 10.1093/infdis/174.2.427. [DOI] [PubMed] [Google Scholar]
- 29.Kish L. Survey sampling. New York, N.Y: John Wiley & Sons, Inc.; 1965. [Google Scholar]
- 30.Koelle D M, Abbo H, Peck A, Ziegweid K, Corey L. Direct recovery of herpes simplex virus (HSV)-specific T lymphocyte clones from recurrent genital HSV-2 lesions. J Infect Dis. 1994;169:956–961. doi: 10.1093/infdis/169.5.956. [DOI] [PubMed] [Google Scholar]
- 31.Lien E, Sellati T J, Yoshimura A, Flo T H, Rawadi G, Finberg R W, Carroll J D, Espevik T, Ingalls R R, Radolf J D, Golenbock D T. Toll-like receptor 2 functions as a pattern recognition receptor for diverse bacterial products. J Biol Chem. 1999;274:33419–33425. doi: 10.1074/jbc.274.47.33419. [DOI] [PubMed] [Google Scholar]
- 32.Magro C M, Crowson A N, Alfa M, Nath A, Ronald A, Ndinya-Achola J O, Nasio J. A morphological study of penile chancroid lesions in human immunodeficiency virus (HIV)-positive and -negative African men with a hypothesis concerning the role of chancroid in HIV transmission. Hum Pathol. 1996;27:1066–1070. doi: 10.1016/s0046-8177(96)90285-3. [DOI] [PubMed] [Google Scholar]
- 33.Malonza I M, Tyndall M W, Ndinya-Achola J O, Maclean I, Omar S, MacDonald K S, Perriens J, Orle K, Plummer F A, Ronald A R, Moses S. A randomized, double-blind, placebo-controlled trial of single-dose ciprofloxacin versus erythromycin for the treatment of chancroid in Nairobi, Kenya. J Infect Dis. 1999;180:1886–1893. doi: 10.1086/315133. [DOI] [PubMed] [Google Scholar]
- 34.Modlin R L, Brightbill H D, Godowski P J. The toll of innate immunity on microbial pathogens. N Engl J Med. 1999;340:1834–1835. doi: 10.1056/NEJM199906103402312. [DOI] [PubMed] [Google Scholar]
- 35.Modlin R L, Kato H, Mehra V, Nelson P E, Bloom B R. Genetically restricted suppressor T cell clones derived from lepromatous leprosy lesions. Nature. 1986;322:459–461. doi: 10.1038/322459a0. [DOI] [PubMed] [Google Scholar]
- 36.Modlin R L, Mehra V, Wong L, Fujimiya Y, Chang W C, Horwitz D A, Bloom B R, Rea T H, Pattengale P K. Suppressor T lymphocytes from lepromatous leprosy skin lesions. J Immunol. 1986;137:2831–2834. [PubMed] [Google Scholar]
- 37.Modlin R L, Melancon-Kaplan J, Young S M M, Pirmez C, Kino H, Convit J, Rea T H, Bloom B R. Learning from lesions: patterns of tissue inflammation in leprosy. Proc Natl Acad Sci USA. 1988;85:1213–1217. doi: 10.1073/pnas.85.4.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morse S A. Chancroid and Haemophilus ducreyi. Clin Microbiol Rev. 1989;2:137–157. doi: 10.1128/cmr.2.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morse S A, Trees D L, Htun Y, Radebe F, Orle K A, Dangor Y, Beck-Sague C M, Schmid S, Fehler G, Weiss J B, Ballard R C. Comparison of clinical diagnosis and standard laboratory and molecular methods for the diagnosis of genital ulcer disease in Lesotho: association with human immunodeficiency virus infection. J Infect Dis. 1997;175:583–589. doi: 10.1093/infdis/175.3.583. [DOI] [PubMed] [Google Scholar]
- 40.Palmer K L, Munson R S., Jr Cloning and characterization of the genes encoding the haemolysin of Haemophilus ducreyi. Mol Microbiol. 1995;18:821–830. doi: 10.1111/j.1365-2958.1995.18050821.x. [DOI] [PubMed] [Google Scholar]
- 41.Palmer K L, Schnizlein-Bick C T, Orazi A, John K, Chen C-Y, Hood A F, Spinola S M. The immune response to Haemophilus ducreyi resembles a delayed-type hypersensitivity reaction throughout experimental infection of human subjects. J Infect Dis. 1998;178:1688–1697. doi: 10.1086/314489. [DOI] [PubMed] [Google Scholar]
- 42.Parsons L M, Waring A L, Shayegani M. Molecular analysis of the Haemophilus ducreyi groE heat shock operon. Infect Immun. 1992;60:4111–4118. doi: 10.1128/iai.60.10.4111-4118.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Picker L J, Treer J R, Ferguson-Darnell B, Collins P A, Bergstresser P R, Terstappen L W M M. Control of lymphocyte recirculation in man. J Immunol. 1993;150:1122–1136. [PubMed] [Google Scholar]
- 44.Riddell S R, Rabin M, Geballe A P, Britt W J, Greenberg P D. Class I MHC-restricted cytotoxic T lymphocyte recognition of cells infected with human cytomegalovirus does not require endogenous viral gene expression. J Immunol. 1991;146:2795–2804. [PubMed] [Google Scholar]
- 45.Risbud A, Chan-Tack K, Gadkari D, Gangakhedkar R R, Shepherd M E, Bollinger R, Mehendale S, Gaydos C, Divekar A, Rompalo A, Quinn T C. The etiology of genital ulcer disease by multiplex polymerase chain reaction and relationship to HIV infection among patients attending sexually transmitted disease clinics in Pune, India. Sex Transm Dis. 1999;26:55–62. doi: 10.1097/00007435-199901000-00009. [DOI] [PubMed] [Google Scholar]
- 46.Spinola S M, Griffiths G E, Bogdan J A, Menegus M A. Characterization of an 18,000 molecular-weight outer membrane protein of Haemophilus ducreyi that contains a conserved surface-exposed epitope. Infect Immun. 1992;60:385–391. doi: 10.1128/iai.60.2.385-391.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spinola S M, Griffiths G E, Shanks K L, Blake M S. The major outer membrane protein of Haemophilus ducreyi is a member of the OmpA family of proteins. Infect Immun. 1993;61:1346–1351. doi: 10.1128/iai.61.4.1346-1351.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spinola S M, Orazi A, Arno J N, Fortney K, Kotylo P, Chen C-Y, Campagnari A A, Hood A F. Haemophilus ducreyi elicits a cutaneous infiltrate of CD4 cells during experimental human infection. J Infect Dis. 1996;173:394–402. doi: 10.1093/infdis/173.2.394. [DOI] [PubMed] [Google Scholar]
- 49.Spinola S M, Wild L M, Apicella M A, Gaspari A A, Campagnari A A. Experimental human infection with Haemophilus ducreyi. J Infect Dis. 1994;169:1146–1150. doi: 10.1093/infdis/169.5.1146. [DOI] [PubMed] [Google Scholar]
- 50.Springer T A. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 51.Thornton A C, O'Mara E M, Jr, Sorensen S J, Hiltke T J, Fortney K, Katz B, Shoup R E, Hood A F, Spinola S M. Prevention of experimental Haemophilus ducreyi infection: a randomized, controlled clinical trial. J Infect Dis. 1998;177:1608–1613. doi: 10.1086/515320. [DOI] [PubMed] [Google Scholar]
- 52.Throm R E, Al-Tawfiq J A, Fortney K R, Katz B P, Hood A F, Hansen E J, Spinola S M. Evaluation of an isogenic MOMP-deficient mutant in the human model of Haemophilus ducreyi infection. Infect Immun. 2000;68:2602–2607. doi: 10.1128/iai.68.5.2602-2607.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Totten P A, Kuypers J M, Chen C-Y, Alfa M J, Parsons L M, Dutro S M, Morse S A, Kiviat N B. Etiology of genital ulcer disease in Dakar, Senegal, and comparison of PCR and serologic assays for detection of Haemophilus ducreyi. J Clin Microbiol. 2000;38:268–273. doi: 10.1128/jcm.38.1.268-273.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Trees D L, Morse S A. Chancroid and Haemophilus ducreyi: an update. Clin Microbiol Rev. 1995;8:357–375. doi: 10.1128/cmr.8.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Van Laer L, Vingerhoets J, Vanham G, Kestens L, Bwayo J, Otido J, Piot P, Roggen E. In vitro stimulation of peripheral blood mononuclear cells (PBMC) from HIV− and HIV+ chancroid patients by Haemophilus ducreyi antigens. Clin Exp Immunol. 1995;102:243–250. doi: 10.1111/j.1365-2249.1995.tb03772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wasserheit J N. Epidemiological synergy. Interrelationships between human immunodeficiency virus infection and other sexually transmitted diseases. Sex Transm Dis. 1992;19:61–77. [PubMed] [Google Scholar]
- 57.Young R S, Fortney K, Haley J C, Hood A F, Campagnari A A, Wang J, Bozue J A, Munson R S, Jr, Spinola S M. Expression of sialylated or paragloboside-like lipooligosaccharides are not required for pustule formation by Haemophilus ducreyi in human volunteers. Infect Immun. 1999;67:6335–6340. doi: 10.1128/iai.67.12.6335-6340.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Young R S, Fortney K R, Gelfanova V, Phillips C L, Katz B P, Hood A F, Latimer J L, Munson R S, Jr, Hansen E J, Spinola S M. Expression of cytolethal distending toxin and hemolysin are not required for pustule formation by Haemophilus ducreyi in human volunteers. Infect Immun. 2001;69:1938–1942. doi: 10.1128/IAI.69.3.1938-1942.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]