Learning objectives.
By reading this article you should be able to:
-
•
Recall the mechanisms of action of antiarrhythmic drugs.
-
•
Discuss the modified Singh–Vaughan Williams classification and its limitations.
-
•
Outline different classes of antiarrhythmic drugs and their relevant pharmacological profiles.
-
•
Explain the role of pharmacotherapy in managing perioperative cardiac arrhythmias.
Key points.
-
•
Antiarrhythmic agents suppress cardiac arrhythmias by inhibiting automaticity, triggered activity and re-entry.
-
•
Most antiarrhythmic agents show use dependency, with greater efficacy in abnormal tissue and at high heart rates.
-
•
The modified Singh–Vaughan Williams classification system provides a useful framework for classifying antiarrhythmic drugs.
-
•
Treatment goals must be clarified before starting pharmacotherapy.
Knowledge of the electrophysiology of arrhythmia generation at the molecular and genetic levels is expanding, contributing to the development of safer antiarrhythmic drugs. Most arrhythmias that occur during the perioperative period do not require treatment. Antiarrhythmic pharmacotherapy may be necessary in patients with persistent arrhythmias accompanied by haemodynamic disturbance.
In Part 1 of this series, we reviewed normal cardiac electrophysiology and the mechanisms of arrhythmogenesis.1 In this article, we discuss the pharmacological principles of arrhythmia treatment, the classification of antiarrhythmic agents and their use in perioperative practice. It is intended that this article should be read in conjunction with Part 1, in which the relevant ion channels and currents are discussed in detail.
Pharmacological principles
Arrhythmias are caused by abnormal impulse generation and propagation. Antiarrhythmic drug therapy involves (i) suppression of enhanced pacemaker activity, (ii) prevention of triggered automaticity and (iii) modification of conduction and refractoriness to prevent re-entry.2 Drug classes mentioned below refer to the modified Singh–Vaughan Williams classification system, which is discussed later in the article.
Suppression of enhanced automaticity
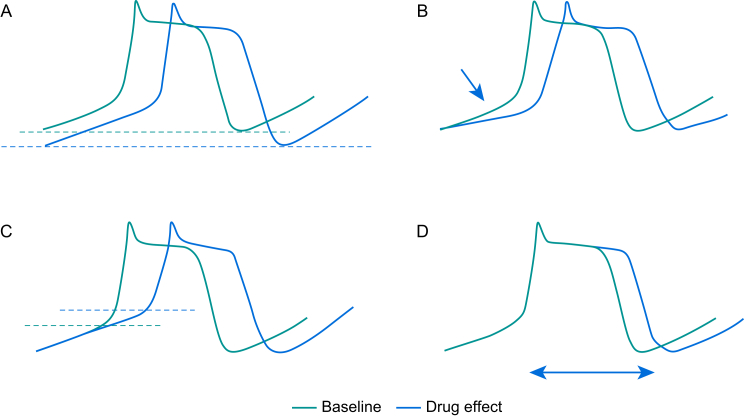
Figure 1 illustrates the ways in which enhanced automaticity may be suppressed. First, suppression occurs when the resting membrane potential (RMP) becomes more negative. Adenosine increases the inwardly rectifier K+ current (IK1) during phase 4 of the action potential, hyperpolarising the RMP.3 Second, suppression occurs by decreasing the slope of spontaneous pacemaker potentials. β-Adrenergic receptor blockers (β-blockers, class II activity) indirectly reduce the funny currents (If), thereby decreasing the slope of phase 4 within pacemaker cells. Third, Na+ or Ca2+ channel blockade (class I and class IV activity, respectively), increases the threshold potential. Finally, inhibition of enhanced automaticity results from increasing the action potential duration (class III activity) by blocking rapid and slow delayed rectifier currents (IKr, IKs).
Fig 1.
Automaticity can be suppressed by (A) hyperpolarisation of the RMP, (B) reducing the slope of pacemaker potential (phase 4), (C) increasing the threshold potential, (D) increasing the action potential duration.
Inhibition of triggered activity
Delayed afterdepolarisations (DADs) can be prevented by reducing intracellular Ca2+ via reducing the activity of Ca2+ channels, either directly using calcium channel blockers (class IV activity) or indirectly using β-blockers (class II activity). Consequently, activity of the 3Na+/Ca2+ exchanger is decreased, thereby reducing the risk of the membrane reaching its threshold potential. Early afterdepolarisations (EADs) can be prevented by shortening the action potential duration with β-blockers (class II activity), calcium channel blockers (class IV activity) or Na+ channel blockers (class Ib activity).
Inhibition of re-entry
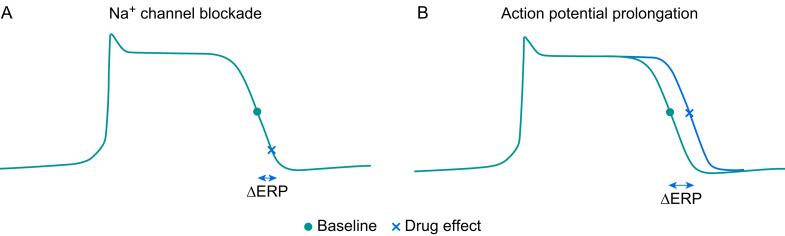
Inhibition of re-entry can be achieved by either directly blocking Na+ channels (INa) (class Ia and Ic activity) or prolonging the action potential (class III activity). The former slows Na+ channel recovery from an inactivated state and the latter can be achieved by blocking delayed rectifier K+ channels (Fig 2). Both strategies prolong the effective refractory period.
Fig 2.
(A) Na+ channel blockers delay the point at which Na+ channels recover from an inactivated state, thereby increasing the effective refractory period (ERP). (B) K+ channel blockers prolong the action potential duration, increasing the ERP.
Accelerating conduction through regions of slow conduction may also prevent re-entry. Lidocaine (class Ib) binds inactivated Na+ channels with very rapid dissociation. Consequently, the action potential duration is reduced and Na+ channels recover more rapidly, increasing conduction speed in regions of slow conduction.
Use dependency
Most antiarrhythmic drugs selectively block channels that are in an ‘active’ or ‘inactive’ state, but have minimal effect on channels in a ‘resting’ state—a phenomenon termed ‘use dependency’. Therefore, therapeutic effects are more pronounced with tachycardia or loss of a normal RMP, such as occurs with ischaemia. With increased doses, antiarrhythmic drugs may become proarrhythmic, as they start to affect ion channels in normal tissues.
Classification of antiarrhythmic drugs
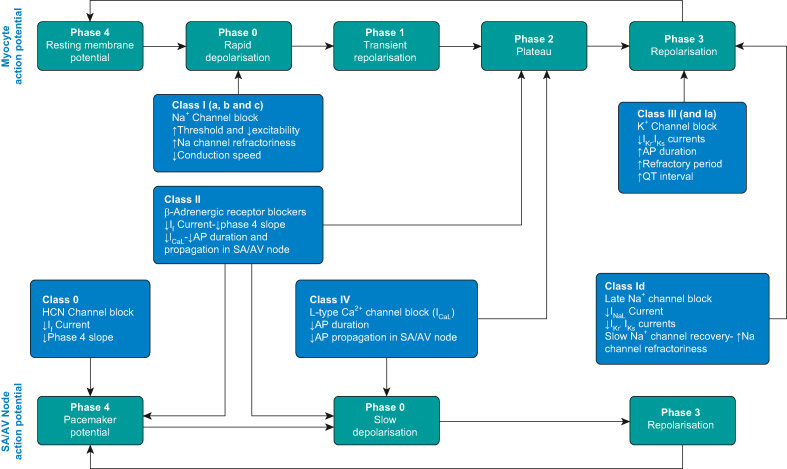
The Singh–Vaughan Williams classification categorises antiarrhythmic drugs into four classes based on their primary mechanisms of action.4,5 The main advantage of the Singh–Vaughan Williams classification is simplicity. However, there are numerous drawbacks. Firstly, a number of antiarrhythmic drugs do not neatly fit into any of the four classes. Secondly, the system oversimplifies the complex actions of antiarrhythmic drugs, as most agents have more than one mechanism of action. In 2018, the American Heart Association updated the Singh–Vaughan Williams classification to include drugs that do not fit into the four classes.6 Despite shortcomings, the modified classification system provides a good starting point for describing the mechanism of action and adverse effect effects of antiarrhythmic drugs. Table 1 summarises the different classes of antiarrhythmic drugs and their clinical use. Figure 3 illustrates their corresponding mechanisms of action.
Table 1.
Singh–Vaughan Williams classification of antiarrhythmic drugs and their clinical use. ACE, angiotensin-converting enzyme; AVNRT, atrioventricular node re-entry tachycardia; MI, myocardial infarction; PVC, premature ventricular contraction; SVT, supraventricular tachycardia.
| Antiarrhythmic class | Examples | Clinical use |
|---|---|---|
| Class 0—HCN channel modulators | Ivabradine | Heart rate control for tachycardia |
| Class 1—voltage-gated Na+ blockers | ||
| 1a—intermediate dissociation kinetics | Quinidine, procainamide | SVT, AF, VT/VF; not commonly used |
| 1b—fast dissociation kinetics | Lidocaine, mexiletine, phenytoin | VT, particularly after MI |
| 1c—slow dissociation kinetics | Flecainide | AF and atrial flutter |
| 1d—late Na+ channel (INaL) blocker | Ranolazine | AF treatment and prevention |
| Class 2—autonomic modulators | ||
| β-adrenergic receptor blockers | Non-selective: propranolol Selective: metoprolol, esmolol |
Prevention of supraventricular tachyarrhythmia Rate control of atrial and ventricular tachyarrhythmias |
| Muscarinic (M1) receptor blockers | Atropine, glycopyrrolate | Bradycardia, atrioventricular block |
| Muscarinic (M2) receptor activators | Digoxin | Rate control of AF |
| Adenosine – A1 receptor activators | Adenosine | SVT |
| Class 3—K+ channel blockers | ||
| Selective IKr blockers | Sotalol, ibutilide | Supraventricular arrhythmias, including AF, AVNRT, PVC, VF, VT; rate control of AF |
| Non-selective (IKr and IKs) blockers | Amiodarone | |
| Multi-ion channel blocker | Vernakalant | Termination of AF |
| Class 4—L-type Ca2+ channel blockers | Verapamil, diltiazem | Paroxysmal SVT, rate control of AF and atrial flutter |
| Class 5—mechanosensitive channel blockers | No drugs available clinically | |
| Class 6—gap junction channel blockers | No drugs available clinically | |
| Class 7—upstream target modulators | ACE inhibitors and angiotensin II receptor blocker | |
Fig 3.
Summary of mechanism of actions of antiarrhythmic drug. AP, action potential.
Class 0: hyperpolarisation-activated cyclic nucleotide-gated channel blockers
Class 0 drugs block hyperpolarisation-activated cyclic nucleotide-gated (HCN) channels in the sinoatrial (SA) node, consequently reducing the ‘funny current’ (If) during phase 4 of the action potential. Ivabradine is the only drug in this class that is currently approved for clinical use. Ivabradine is a novel drug with negative chronotropic actions and no other haemodynamic effects. Ivabradine is useful in patients with impaired cardiac function—with or without ischaemic heart disease—who have suboptimal heart rate control despite treatment with β-blockers. In a clinical trial, a single dose of ivabradine before surgery was associated with an attenuated heart rate response to intubation and surgical incision without causing hypotension, suggesting the drug has a role in myocardial protection in selected patients.7 As ivabradine is only available in oral formulation, it has limited utility for treating tachycardia during surgery. Anaesthetists are likely to see an increasing number of patients taking ivabradine who are presenting for surgery.
Class I: voltage-gated sodium channel blockers
Class I drugs block Na+ channels, reduce excitability and increase the threshold potential. Class I drugs are subdivided into types a-d, according to their Na+ channel binding kinetics.4,8,9.
Class Ia: intermediate dissociation kinetics
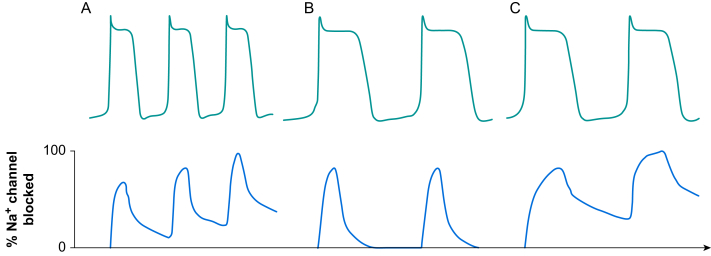
Class Ia drugs bind to Na+ channels in the open state with dissociation kinetics intermediate between class Ib and Ic drugs. These agents demonstrate use dependency, with enhanced channel blocking properties at high heart rates (Fig 4A). Class Ia drugs reduce the slope of phase 0 of the action potential and, therefore, decrease conduction speed but not to the same extent as class Ic agents. Class Ia drugs also block delayed rectifier K+ channels causing prolongation of the action potential (class III activity).
Fig 4.
Dissociation kinetics of Na+ channel blockers. Na+ channel blockers bind to Na+ channels in the open or inactivated states. Dissociation kinetics of the blockers and recovery of channels during diastole determines the extent of Na+ channel blockade. (A) Class Ia drugs have an intermediate dissociation rate and Na+ channel blockade is influenced by the heart rate. As the heart rate increases, the time available for drugs to dissociate from the channels during diastole is reduced. This increases level of Na+ channel blockade and provides heart rate-dependent steady state Na+ blockade. (B) Class Ib drugs have rapid dissociation kinetics allowing full recovery of Na+ channels before the next action potential. (C) Class Ic drugs have slow dissociation kinetics. The drugs do not completely dissociate from the Na+ channels even at low heart rate. This results in steady-state Na+ channel blockade even when the cell membrane is fully repolarised.
Quinidine and procainamide are representative class Ia drugs, and are effective in the treatment of supraventricular arrhythmias, including atrial fibrillation (AF), and ventricular tachyarrhythmias attributable to re-entry. Despite their efficacy, class Ia drugs cause haemodynamic instability and are associated with proarrhythmia. Consequently, class Ia agents are now rarely used.
Class Ib: rapid dissociation kinetics
Class Ib drugs preferentially bind to partially depolarised cells (e.g. because of myocardial ischaemia), in which the majority of Na+ channels are in an inactivated state. Dissociation is rapid, leaving most Na+ channels ready for the next action potential (Fig 4B). The action potential is shorter and recovery of Na+ channels is fast, which leads to accelerated conduction in regions of slow conduction.8 In healthy myocytes, conduction speed is normal and phase 0 of the action potential is unaffected.
Lidocaine is a representative class Ib drug. Lidocaine is useful in treating ventricular arrhythmias, especially in the setting of myocardial ischaemia, but is ineffective for atrial arrhythmias.10 To achieve a steady state therapeutic level, an i.v. bolus does of 2 mg kg−1 should be administered followed by infusion of 1–4 mg min−1. Because lidocaine undergoes hepatic metabolism, the dose should be reduced when cardiac output is low (reduced liver blood flow) or in the presence of hepatic dysfunction.
Advantages of lidocaine over other class I drugs are rapid onset, wide therapeutic index and a favourable safety profile. At therapeutic doses, lidocaine has no effect on the QRS duration, QT interval or atrioventricular (AV) conduction and has minimal effect on myocardial contractility and vascular tone. The main adverse effects of lidocaine are central nervous system excitation, which can include seizures.
Class Ic: slow dissociation kinetics
Class Ic drugs bind to inactivated Na+ channels and dissociate very slowly, leading to steady state channel blockade (Fig 4C). The slope of phase 0 of the action potential and conduction speed are reduced.
Flecainide is a representative class 1c drug. Flecainide also blocks the delayed rectifier K+ currents but does not prolong the QT interval. Flecainide is effective in treating atrial and ventricular tachyarrhythmias attributable to re-entry, such as AF, atrial flutter, premature ventricular contractions (PVCs) and ventricular tachycardia (VT). Flecainide is particularly useful for treating paroxysmal AF with a ‘pill-in-the-pocket’ approach. However, in the Cardiac Arrhythmia Suppression Trial (CAST), flecainide was associated with an increased incidence of sudden cardiac death, likely to be attributed to the provocation of potentially lethal arrhythmias in patients with a history of myocardial infarction.11 Therefore, flecainide should only be used in patients with structurally normal hearts. Flecainide is available in i.v. and oral formulations.
Class id
The only class Id drug available for clinical use is ranolazine. Ranolazine inhibits late Na+ (INaL) and delayed rectifier K+ (IKr) channels, resulting in slight prolongation of the action potential, thereby increasing refractoriness and reducing intracellular Ca2+. Ranolazine is approved for treating chronic stable angina pectoris.12 However, there is emerging evidence for a role in preventing and treating AF after cardiac surgery, which occurs in up to 50% of patients.12, 13, 14, 15 Ranolazine is contraindicated in patients with creatinine clearance <30 ml min−1.16
Class II: autonomic modulators
β-Adrenergic receptor antagonists
The antiarrhythmic effect of β-blockers results from blockade of β1-receptors in the heart. Activation of β1-receptors leads to an increase in the inwards If current via HCN channels, which results in increased automaticity. Ca2+ entry via ICaL also increases, which results in Ca2+ overload, leading to DADs. Concomitant β2-receptor-mediated hypokalaemia further predisposes to arrhythmia.
β-Blockers are effective in treating sinus tachycardia, supraventricular tachyarrhythmia and ventricular tachyarrhythmias (e.g. VT, polymorphic VT, ventricular fibrillation [VF]).4 β-Blockers reduce the ventricular response rate in AF and atrial flutter. Decreasing heart rate reduces myocardial oxygen demand, which may prevent myocardial ischaemia in patients with coronary artery disease. There is evidence that β-blockers reduce arrhythmia-related mortality, although the mechanism of benefit is unclear.10
Adverse effects of β-blockers include myocardial depression, bradycardia, heart block, fatigue, aggravation of peripheral vascular symptoms and bronchospasm. The use of selective β1-blockers (metoprolol, bisoprolol, atenolol, esmolol) may partially alleviate some of these adverse effects.9 However, β1 selectivity is typically insufficient to completely prevent β2-mediated undesirable effects such as bronchospasm and peripheral vascular insufficiency.
Propranolol is a non-selective β-blocker with class I activity at high dose, which may contribute to its antiarrhythmic effect. Sotalol is a non-selective β-blocker with class III activity. Class III activity augments the antiarrhythmic effect of sotalol but also predisposes to long QT and proarrhythmia.
Esmolol is an ultrashort-acting selective β-blocker available for i.v. use. The rapid onset and offset of clinical effect make the drug appealing for use in the perioperative period. A loading dose of 0.5–1 mg kg−1 followed by 0.05–0.3 mg kg−1 min−1 infusion may be used. The clinical effect dissipates ∼20 min after discontinuing the drug.8
Atropine and glycopyrrolate: muscarinic receptor blockers
Bradyarrhythmias are common during the perioperative period. Causes include drug-induced decreased sympathetic tone and myocardial ischemia. Severe bradyarrhythmia, with or without AV block, can cause haemodynamic instability and potentially evolve into a more severe arrhythmia, such as re-entry-induced torsade de pointes (polymorphic) VT. Muscarinic (M2) receptor blockers (atropine, glycopyrrolate) increase automaticity in the SA node and increase conduction through the AV node.
Digoxin: M2-receptor activator
Digoxin has a prominent vagotonic effect, decreasing the Ca2+ current and increasing the K+ current in the AV node and atrial tissues, resulting in increased AV node refractoriness. Accordingly, digoxin is useful for treating re-entrant arrhythmias involving the AV node and for controlling the ventricular rate in patients with AF. However, digoxin must be avoided in patients with pre-excitation (e.g. Wolff–Parkinson–White syndrome), as slow AV conduction can enable conduction via an accessory pathway capable of rapid conduction, leading to an extremely rapid ventricular response and cardiovascular collapse.
Digoxin also inhibits Na+/K+-ATPase activity, resulting in increased intracellular Na+ concentration, leading to a reduction in 3Na+/Ca2+ exchanger activity and increased intracellular Ca2+. Thus, digoxin exerts a positive inotropic effect and is helpful in the treatment of AF associated with impaired cardiac function. The usual dose of digoxin is 0.25–0.5 mg as an i.v. bolus, which can be repeated up to 1 mg over 24 h.4 Digoxin has a narrow therapeutic index. Careful monitoring of digoxin level is mandatory, particularly in patients with renal impairment, as life-threatening toxicity can occur when plasma levels are increased. Atrial tachyarrhythmia with AV block is classically associated with digoxin toxicity, although virtually any arrhythmia is possible.
Adenosine
Adenosine activates adenosine receptors and increases K+ currents (IK1), thereby shortening the action potential and hyperpolarising the cell membrane in the SA and AV nodes, leading to decreased automaticity and slowed conduction through the AV node. Adenosine is highly effective in terminating AV re-entry tachycardia. The effects of adenosine are short-lived, with an elimination half-life of 10 s, because it is taken up rapidly into red blood cells. The usual initial dose is 6 mg i.v., followed rapidly by a saline flush, which may be repeated with further doses of 6–12 mg if necessary. Transient asystole is common. Occasionally, adenosine may cause AF and bronchospasm, and should be used with caution in patients with asthma.8,10
Class III: potassium channel blockers
Class III drugs primarily target rapid (IKr) and slow (IKs) delayed rectifier K+ channels. The main effects are increased action potential duration and refractoriness, which helps suppress atrial and ventricular arrhythmias caused by re-entry.17 However, class III drugs can cause life-threatening proarrhythmia.
The proarrhythmic effect of class III drugs is attributed to reverse use dependency. Drugs that block human ether-a-go-go-related gene (hERG) K+ channels, which are responsible for the IKr current, cause increased blockade at low heart rate. Hence, prolongation of action potential duration is most marked in patients with bradycardia and least marked with tachycardia. Marked prolongation of the action potential—as evidenced by a long QT interval on the ECG—predisposes to torsade de pointes VT. However, blockade of the channels responsible for the IKs current follows normal use dependency, and channel blockade is more effective at high heart rate. Therefore, inhibition of IKs acts as a safety mechanism, which explains why amiodarone (a non-selective IKr and IKs blocker) has fewer proarrhythmic effects than pure IKr blockers, such as sotalol or ibutilide.17
Amiodarone: non-selective potassium channel blocker
Although amiodarone is classified as a class III drug, it has activity across all classes. As a non-selective IKr and IKs blocker, amiodarone prolongs the action potential over a wide range of heart rates. Accordingly, amiodarone is effective in treating a broad range of supraventricular and ventricular arrhythmias. Amiodarone decreases the incidence of AF after cardiac surgery by 50–65%.18 Amiodarone depresses conduction through both the AV node and accessory pathways and is therefore effective in treating tachyarrhythmias associated with accessory pathways.4,10
Amiodarone is recommended in advanced cardiac life support guidelines.4,8 For VT and VF that are resistant to electrical cardioversion, the initial dose is 300 mg i.v. For other arrhythmias, the i.v. dosing recommendation is 300 mg over 10 min, followed by 900 mg over 23 h.5 A supplemental 150 mg i.v. dose may be administered for breakthrough arrhythmias. For patients able to take oral medication, 200 mg three times daily (after the 300 mg i.v. dose) is appropriate. Amiodarone has an elimination half-life of 29 days, and its effects can last more than 60 days after stopping the drug.10
Adverse effects of short-term perioperative use are limited to the cardiovascular system and include vasodilation and myocardial depression. However, these effects are less marked than with other comparable agents. The adverse effects of long-term therapy (several weeks) include atropine-resistant bradycardia and effects attributable to tissue deposition, such as pulmonary fibrosis, hepatic dysfunction, peripheral neuropathy, hypothyroidism and hyperthyroidism.8,10
Sotalol and ibutilide: selective potassium channel blockers
Sotalol and ibutilide are selective hERG K+ channel blockers, with similar indications to those of amiodarone. Sotalol is approved for treating life-threatening ventricular tachyarrhythmia and for maintaining sinus rhythm in patients with AF. However, owing to isolated IKr suppression, there is a dose-dependent prolongation of the QT interval with an associated risk of developing torsade de pointes VT. Sotalol is also associated with the adverse effects of non-selective β-blockade.8,17 Ibutilide can be given i.v. (1 mg over 10 min) for terminating acute AF and atrial flutter.10
Vernakalant: multi-ion channel blocker
Although vernakalant is categorised as a class III agent, it blocks multiple ion channels including Na+ channels (INaL) and various delayed rectifier K+ channels resulting in prolongation of the action potential and effective refractory period. Vernakalant is atrium specific and only causes mild QT prolongation, without increasing the risk of torsade de pointes VT.8 Vernakalant is used for terminating acute AF, which typically occurs within 90 min.4 It is administered as an i.v. bolus (3 mg kg−1). If necessary, a second dose of 2 mg kg−1 may be given after 10 min. Adverse effects include bradycardia and hypotension.
Class IV: calcium channel blockers
The antiarrhythmic effects of class IV drugs are attributed to blockade of L-type Ca2+ channels in the SA and AV nodes, which depresses automaticity and decreases conduction speed. Verapamil and diltiazem are representative drugs.
Class IV drugs are effective in reducing the ventricular response rate in AF and atrial flutter and for terminating AV re-entry tachycardia.8 Verapamil reduces AV conduction in a heart rate-dependent manner and has limited effect when the heart rate is low.17 The usual dose of verapamil for terminating AV node re-entry tachycardia is 5–10 mg i.v. followed by an infusion of 5 μg kg−1 min−1. Diltiazem at a dose of 20 mg i.v. has a similar effect to verapamil.10 Oral therapy is usually effective in suppressing paroxysmal AV node re-entry tachycardia and achieving heart rate control in patients with AF and atrial flutter. Calcium channel blockers do not suppress conduction via accessory pathways and are therefore contraindicated in patients with pre-excitation syndromes.
Unlike β-blockers, calcium channel blockers have not been shown to reduce mortality after myocardial infarction.19 The major adverse consequence of calcium channel blockers is myocardial depression, and these agents should be used cautiously in patients with heart failure.
Magnesium
Magnesium is indicated for preventing and treating torsade de pointes VT and is effective even if the serum concentration is normal. The mechanism of its antiarrhythmic effect is unknown, as magnesium does not shorten the QT interval. The mechanism may involve a membrane-stabilising effect as a result of blocking Ca2+ and K+ channels. Magnesium is also effective in treating arrhythmias associated with digoxin toxicity. Patients with AF have been shown to have a lower serum Mg2+ concentration than healthy individuals.20 Therefore, magnesium may have a role in controlling the ventricular response in patients with AF. The usual dose is 1–2 g i.v.
Pharmacological management of perioperative arrhythmias
Before commencing antiarrhythmic drug therapy, the risks and benefits should be carefully considered. Where possible, the underlying precipitant(s) for the arrhythmia should be corrected before initiating drug treatment (see Part 1).1 It is important to identify the goal of treatment. For example, for acute onset AF, the goal might be restoration of sinus rhythm with amiodarone with or without electrical cardioversion, whereas for chronic AF the goal might be controlling the ventricular response rate with β-blockers or calcium channel blockers, with or without digoxin.21
It is important to minimise risk. Flecainide should be avoided in patients with myocardial ischaemia or structural heart disease. Sotalol should be avoided in patients with prolonged QT. The risk–benefit of using amiodarone should be carefully considered in patients with severe pulmonary disease, although the drug is not specifically contraindicated in this circumstance. No specific pharmacotherapy is indicated for PVCs and non-sustained VT apart from electrolyte replacement and magnesium supplementation.4 An exception is in patients with myocardial ischaemia or after reperfusion of myocardial infarction, when non-sustained VT may progress to sustained VT.
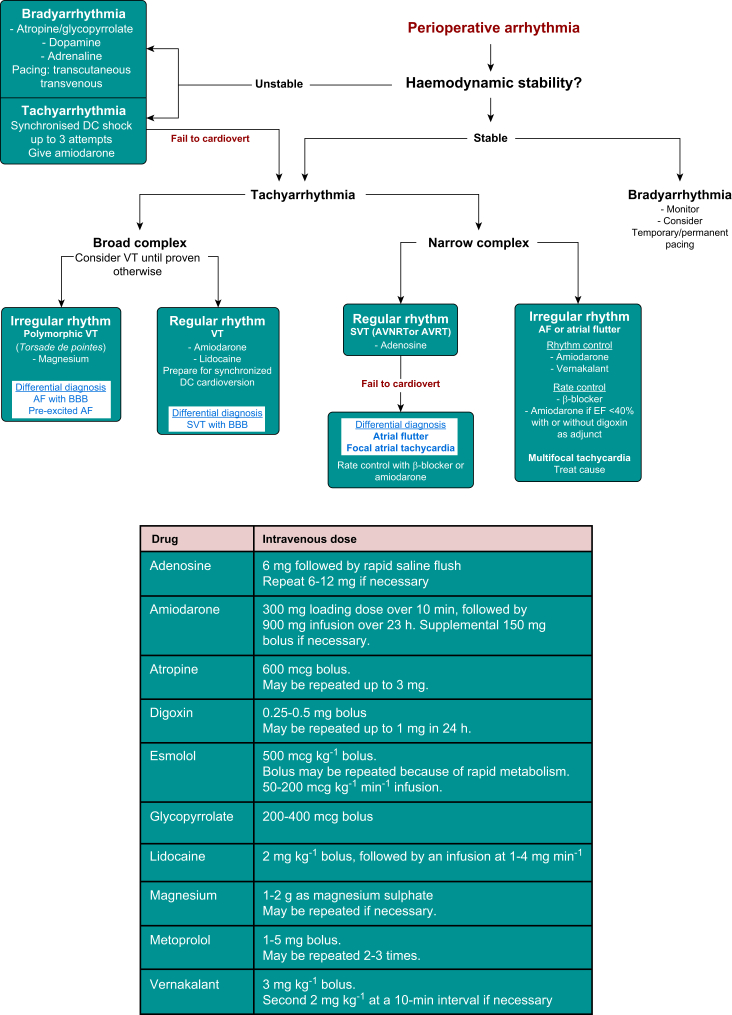
Figure 5 provides an algorithm for treating the common arrhythmias encountered during the perioperative period. Unstable tachyarrhythmias should be treated with synchronised electrical cardioversion (up to three shocks), usually in combination with i.v. amiodarone. Amiodarone is effective against most perioperative arrhythmias. As noted above, haemodynamic instability is less pronounced than with other comparable agents and the risk of proarrhythmia is relatively low.
Fig 5.
Common perioperative arrhythmias and management algorithm. Doses of commonly used antiarrhythmic drugs. AVNRT, atrioventricular node re-entry tachycardia; AVRT, atrioventricular re-entry tachycardia; BBB, bundle branch block; EF, ejection fraction; SVT, supraventricular tachycardia; VT, ventricular tachycardia.
Atrial fibrillation is the most common arrhythmia encountered in the perioperative period, with an incidence up to 50% in patients undergoing cardiac surgery.18 Although the majority of AF episodes are self-limiting, persistent AF may require intervention. If patients are haemodynamically stable, electrical cardioversion should be deferred, as there is a high recurrence rate after immediate cardioversion. In most circumstances, AF spontaneously reverts to sinus rhythm within 24 h. β-Blockers are the first-line choice in both cardiac and non-cardiac surgical patients. Esmolol is particularly useful during the perioperative period because of its ultrashort acting profile and the convenience of administering as a bolus or i.v. infusion. Digoxin may be considered for patients with impaired ventricular function, often in combination with β-blockers or calcium channel blockers. If restoration of sinus rhythm is required without electrical cardioversion (e.g. to avoid general anaesthesia), i.v. amiodarone or vernakalant are the preferred agents.4
Conclusions
Antiarrhythmic drugs are commonly used in the perioperative period. The risk balance of antiarrhythmic drugs should be carefully considered before initiating treatment. The modified Singh–Vaughan Williams classification system provides a useful framework for classifying antiarrhythmic drugs and helps to understand their mechanism of action and potential adverse effects.
Declaration of interest
The authors declare that they have no conflicts of interest.
Biographies
Chang Kim FANZCA is a consultant anaesthetist at Auckland City Hospital. He routinely provides anaesthesia care for patients requiring cardiac, thoracic, interventional cardiology procedures including TAVI, catheter ablations and PCIs, and non-cardiac procedures. He is a primary examiner for Australian and New Zealand College of Anaesthetists.
Nigel Lever FRACP FCSANZ is a consultant cardiologist at Auckland City Hospital and associate professor at the University of Auckland, with clinical expertise in cardiac rhythm disorders and cardiac ablation procedures. He is involved in translational research with cardiac mapping, heart failure and novel pacing therapies; and is also an examiner for the Royal Australasian College of Physicians.
Jeremy Ormond Cooper FANZCA is a consultant anaesthetist at Auckland City Hospital. He has expertise in preoperative patient assessment, cardiothoracic anaesthesia and an extensive interest and involvement in education.
Matrix codes: 1A02, 2A03, 3I00
MCQs
The associated MCQs (to support CME/CPD activity) will be accessible at www.bjaed.org/cme/home by subscribers to BJA Education.
References
- 1.Kim C, Lever N, Cooper JO. Antiarrhythmic drugs and anaesthesia. Part 1: mechanisms of cardiac arrhythmias. BJA Educ 2023; 23:8–16 [DOI] [PMC free article] [PubMed]
- 2.Harvey R.D., Grant A.O. In: Basic & clinical pharmacology. 15th Edn. Katzung B.G., Vanderah T.W., editors. McGraw-Hill; New York: 2021. Agents used in cardiac arrhythmias. [Google Scholar]
- 3.Grant A.O. Cardiac ion channels. Circ Arrhythm Electrophysiol. 2009;2:185–194. doi: 10.1161/CIRCEP.108.789081. [DOI] [PubMed] [Google Scholar]
- 4.Dan G.A., Martinez-Rubio A., Agewall S., et al. Antiarrhythmic drugs – clinical use and clinical decision making: a consensus document from the EHRA and ESC Working Group on Cardiovascular Pharmacology, endorsed by the HRS, APHRS and ISCP. Europace. 2018;20:731–732. doi: 10.1093/europace/eux373. [DOI] [PubMed] [Google Scholar]
- 5.Stewart A.M., Greaves K., Bromilow J. Supraventricular tachyarrhythmias and their management in the perioperative period. Cont Educ Anaesth Crit Care Pain. 2015;15:90–97. [Google Scholar]
- 6.Lei M., Wu L., Terrar D.A., Huang C.L.H. Modernized classification of cardiac antiarrhythmic drugs. Circulation. 2018;138:1879–1896. doi: 10.1161/CIRCULATIONAHA.118.035455. [DOI] [PubMed] [Google Scholar]
- 7.Banerjee A., Mishra S. Use of preoperative single dose ivabradine for perioperative hemodynamic stabilization during non-cardiac elective surgery under general anaesthesia: a pilot study. J Clin Med Res. 2021;13:343–354. doi: 10.14740/jocmr4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knollmann B.C., Roden D.M. In: Goodman & gilman’s the pharmacological basis of therapeutics. 13th Edn. Brunton L.L., Hilal-Dandan R., Knollmann B.C., editors. McGraw-Hill; New York: 2017. Antiarrhythmic drugs. [Google Scholar]
- 9.Ritter J., Flower R., Henderson G., Loke Y.K., MacEwan D., Rang H. In: Rang & dale’s pharmacology. 9th Edn. Ritter J., Flower R., Henderson G., Loke Y.K., MacEwan D., Rang H., editors. Elsevier; Amsterdam: 2020. The heart. [Google Scholar]
- 10.Smith W., Ramsay J. In: Stoelting’s pharmacology & physiology in anesthetic practice. 6th Edn. Flood P., Rathmell J.P., Irman R.D., editors. Wolters Kluwer Health; Alphen aan den Rijn: 2021. Antiarrhythmic drugs. [Google Scholar]
- 11.Jones B., Burnand C. Antiarrhythmic drugs. Anaesth Intensive Care Med. 2021;22:319–323. [Google Scholar]
- 12.Rayner-Hartley E, Sedlak T. Ranolazine: a contemporary review. J Am Heart Assoc 20; 5: e003196. [DOI] [PMC free article] [PubMed]
- 13.Tagarakis G.I., Aidonidis I., Daskalopoulou S.S., et al. Effect of ranolazine in preventing postoperative atrial fibrillation in patients undergoing coronary revascularization surgery. Curr Vasc Pharmacol. 2013;11:988–991. doi: 10.2174/157016111106140128123506. [DOI] [PubMed] [Google Scholar]
- 14.Bekeith S., Meghani M., Shariff M.A., et al. Abstract 13387: effect of ranolazine on the incidence of atrial fibrillation following cardiac surgery. Circulation. 2015;132 [Google Scholar]
- 15.Bhave P.D., Goldman L.E., Vittinghoff E., Maselli J., Auerbach A. Incidence, predictors, and outcomes associated with postoperative atrial fibrillation after major noncardiac surgery. Am Heart J. 2012;164:918–924. doi: 10.1016/j.ahj.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koren M.J., Crager M.R., Sweeney M. Long-term safety of a novel antianginal agent in patients with severe chronic stable angina: the ranolazine open label experience (ROLE) J Am Coll Cardiol. 2007;49:1027–1034. doi: 10.1016/j.jacc.2006.10.067. [DOI] [PubMed] [Google Scholar]
- 17.Abbott G.W., Levi R. In: Pharmacology and physiology for anesthesia foundations and clinical application. 2nd Edn. Hemmings H.C., Egan T.D., editors. Elsevier; Amsterdam: 2019. Antiarrhythmic drugs. [Google Scholar]
- 18.Mitchell L.B., Crystal E., Heilbron B., Pagé P. Atrial fibrillation following cardiac surgery. Can J Cardiol. 2005;21(Suppl B):45B–50B. [PubMed] [Google Scholar]
- 19.Singh B.N. Advantages of beta blockers versus antiarrhythmic agents and calcium antagonists in secondary prevention after myocardial infarction. Am J Cardiol. 1990;66:9C–20C. doi: 10.1016/0002-9149(90)90757-r. [DOI] [PubMed] [Google Scholar]
- 20.Singh R.B., Manmohan M.D., Dube K.P., Singh V.P. Serum magnesium concentrations in atrial fibrillation. Acta Cardiol. 1976;3:221–226. [PubMed] [Google Scholar]
- 21.Bajpai A., Rowland E. Atrial fibrillation. Cont Educ Anaesth Crit Care Pain. 2006;6:219–224. [Google Scholar]