Learning objectives.
By reading this article you should be able to:
-
•
Recall the epidemiology of systemic sclerosis relevant to anaesthesia.
-
•
Describe the features of systemic sclerosis and which features affect postoperative outcomes.
-
•
Explain the pathophysiology and the impact of modern treatments on clinical outcomes.
-
•
Discuss the importance of multidisciplinary management and the benefit of specialist input during perioperative period.
Key points.
-
•
Systemic sclerosis is a rare, multisystem, immune-mediated, inflammatory disease characterised by fibrosis and vasculopathy.
-
•
Associated pulmonary arterial hypertension is an independent predictor for 30-day postoperative pneumonia, congestive heart failure and all-cause mortality.
-
•
Of all major adverse cardiac events, only myocardial infarction is independently associated with systemic sclerosis.
-
•
Postoperative pneumonia accounts for more than one-third of 30-day mortality.
-
•
Involvement of a rheumatology specialist is recommended to estimate the extent of disease severity, optimise therapy and contribute to managing postoperative complications.
Systemic sclerosis (SSc) is a rare autoimmune, inflammatory and progressive multisystem connective tissue disease (CTD), characterised by small vessel vasculopathy and abnormal collagen deposition, leading to fibrosis of the skin and internal organs.
This, the first of a two-part review, focuses on its epidemiology, pathophysiology, diagnosis and medical therapies. In a forthcoming article, we will address the perioperative management of patients with SSc.
The hallmark of SSc is scleroderma (Greek, meaning ‘hard skin’) and sclerodactyly (finger involvement). The condition is perhaps best conceptualised as a disease spectrum. Two main phenotypes are recognised in clinical practice, defined by the extent of cutaneous involvement: limited cutaneous systemic sclerosis (lcSSc; skin involvement limited to distal to knees or elbows) and diffuse cutaneous systemic sclerosis (dcSSc; skin involvement extending proximal to the knees and elbows) (Table 1). It is important to highlight that ‘limited’ does not mean limited to skin, as lcSSc may involve many internal organs. A third phenotype (SSc sine scleroderma, without scleroderma) is described in a minority of patients.1 In addition, a subgroup of patients (up to 20%) may have overlap features with other CTDs such as systemic lupus erythematosus or polymyositis (SSc overlap).1
Table 1.
Clinical subtypes of systemic sclerosis. PAH, pulmonary arterial hypertension; GI, gastrointestinal; CTD, connective tissue disease.
| Prevalence | Features | |
|---|---|---|
|
Limited cutaneous systemic sclerosis (lcSSc) Previously known as knows as CREST syndrome: Calcinosis, Raynaud's phenomenon, (O)Esophageal dysmotility, Sclerodactyly, Telangiectasia |
Up to 70–80% |
Onset Often there is a long interval between onset of Raynaud's and skin changes. Cutaneous features Early ‘puffy hands’ followed by skin thickening and tightening on the limbs, distal to the elbows and knees but may include neck and face. The limited skin pattern should not underestimate internal organ involvement (see text). The limited skin involvement may be locally severe (i.e. susceptible to digital ischaemia and autoamputation, soft tissue calcification, telangiectasia). Predominant antibodies Anti-centromere autoantibody. Other features Higher risk of PAH and severe GI disease. Late-stage complications are common. |
| Diffuse cutaneous systemic sclerosis (dcSSc) | Up to 20–30% |
Onset Usually there is a short interval (<1 yr) between onset of Raynaud's and skin changes. Cutaneous features Skin sclerosis extends proximal to elbows and knees with or without trunk. Skin involvement may recede as disease progresses. Predominant antibodies Mainly anti-Scl-70/anti-topoisomerase 1 and anti-U3-RNP autoantibodies. Other features It is not synonym with organ internal organ involvement but rather associated. Higher risk of certain internal organ involvement: cardiac disease, interstitial lung disease, renal crisis. |
| Overlap syndromes | Up to 20% | Presents features of SSc (e.g. skin changes, vasculopathy) plus manifestations of other CTD (e.g. myositis, Sjogren's syndrome or undifferentiated/mixed CTD). Autoantibodies not specific for SSc (e.g. anti RNP) or specific for overlap syndrome (polymyositis/SSc overlap) |
| SSc sine scleroderma | Rare (<5%) | Characterised by Raynaud's phenomenon, typical SSc serology, organ-based vascular involvement but no skin thickening. |
Although terms such as scleroderma or CREST (calcinosis, Raynaud syndrome, [o]esophageal dysmotility, sclerodactyly and telangiectasia) syndrome have been used in the past, in this review, the term systemic sclerosis will be used, in keeping with the nomenclature recommended by the American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR).2
Because of its multisystem involvement, these patients can find themselves under the care of anaesthetist in a variety of environments: for disease-specific elective surgery (e.g. debulking of soft tissue calcinosis, peripheral or proximal sympathectomy, lung transplantation); for elective surgery or emergency care unrelated to the disease process (e.g. emergency surgery, out of operating theatre resuscitation, obstetric delivery). Systemic sclerosis has implications for all aspects of anaesthesia. Features such as pulmonary arterial hypertension (PAH), cardiac dysfunction, renal failure and severe interstitial lung disease (ILD) carry significant morbidity and present particular challenges for the anaesthetist. Subclinical organ involvement can cause unanticipated difficulties.
Epidemiology
The overall global incidence is 1:10,000.3 In Europe the prevalence ranges from seven to 33 per 100,000 individuals, being almost four times higher in women.4 It most commonly presents in the third to fifth decade, and has the highest mortality among rheumatic diseases, with 10-yr survival of only 66%.5,6 Risk factors for SSc include female sex, a history of silicone breast implants, Native American or African American race, previous chemotherapy (bleomycin, taxanes, gemcitabine) radiotherapy, exposure to gadolinium (in patients with renal failure), silica dust, vinyl chloride or organic solvents.7,8
Scleroderma renal crisis (SRC) was the main cause of mortality in the past, but this has been attenuated with the use of angiotensin-converting enzyme inhibitors (ACEi). The main causes of death in patients with SSc are now pulmonary fibrosis/ILD (19%), myocardial disease (14%) and PAH (14%).9 In the past 10 yrs, improved understanding, screening for subclinical involvement (e.g. for PAH and ILD) and targeted management of associated organ impairment have resulted in improved outcomes, including increased survival duration and quality of life.
Cardiac involvement and interstitial lung fibrosis emerge in approximately 50% patients within the first 5 yrs.10 Overall, SSc is associated with a three-fold increased risk of myocardial ischaemia, and symptomatic cardiac involvement is associated with 75% mortality at 5 yrs.11,12 Occult cardiac disease has been suspected in patients with intraoperative cardiac conduction defects leading to low output state and sinus arrest, despite unremarkable preoperative cardiac investigations.13,14 The prevalence of mild PAH can be as high as 64%, and progression may be rapid, over a median observation time of 12 months.15 The presence of PAH carries a three-times higher risk of mortality than SSc alone.16 In addition, up to 31% of patients with SSc may have sleep disordered breathing.17
Overall, patients with SSc seem to require more high-risk surgeries than patients without SSc (36% vs 21%, p<0.001).18 When compared with the general population, patients with SSc undergo twice the number of thoracic, breast and vascular surgeries, but fewer urological and gynaecological/obstetric procedures.18 Although transplant surgery is required in <2% of cases, this is six times higher than that in patients without SSc (1.9% vs 0.3%, p<0.001).18 Isolated heart transplantation is performed rarely in those with SSc because of multisystem (particularly lung) involvement, with only a few cases described.12
Perioperative outcomes
There are few specific data on perioperative outcomes. Risk factors reported for adverse outcomes include: advanced age, male sex, rapidly progressing disease, extensive involvement of internal organs, extensive skin involvement, the presence of PAH and ILD, documented cardiac disease, renal dysfunction, and treatment with immunosuppressant drugs and steroids. Although some of these features may theoretically influence perioperative outcomes, the literature is not consistent.
Although there is a quoted general non-operative risk for myocardial infarction (MI) and stroke of three and two times higher, respectively, in patients with SSc, the perioperative data are inconclusive.11,18 In one study of 4385 patients undergoing non-cardiac surgery, univariate analysis found that there was a higher risk of major adverse cardiac events (MACE) (odds ratio [OR]=2.45, p<0.001) including MI (OR=3.33; p<0.001), ischaemic stroke (OR=2.02; p<0.001), cardiac arrest (OR=2.11; p<0.001) and in-hospital all-cause death (OR=3.07; p<0.001). However, on multivariate analysis, only MI was independently associated with SSc (model 1 OR=1.85, P=0.048; model 2 OR=1.94, P=0.031) with no associations with ischaemic stroke, cardiac arrest or all-cause death. The typical patient with SSc in this study was an older female (mean age, 63 yrs) of Caucasian background with significantly (p<0.001) more cardiac and systemic comorbidities (hypertension 43%, dyslipidaemia 30%, coronary artery disease 17%, pulmonary hypertension 17%, tachyarrhythmias 16%, chronic kidney disease 16%, ILD 14%, congestive heart failure 11%, cerebrovascular disease 10%, and cardiac conduction disorders 2%) than patients without SSc. Patients with SSc required significantly more (p<0.001) orthopaedic (44%), gastrointestinal (GI) (22%), vascular (11%), breast (5.5%), thoracic (3.5%), and non-cardiac transplant surgeries (1.9%).
Patients with SSc who required lung transplantation had a multivariate-adjusted 48% relative increased risk of death at 1 yr compared with those without SSc who received lung transplantation for ILD, but similar to those who had a diagnosis of non-SSc-associated PAH.19
There are few data on ICU outcomes after surgery, most information being provided by small non-operative studies or pooled data of patients with systemic rheumatic disease (SDR), which includes SSc. Exacerbation of SDR (43%) and isolated infections (34%) seem to be the main current causes for ICU admission in patients with SDR.20 For those with SSc, respiratory failure (66%) and acute renal failure (15%) are the main organ dysfunctions requiring critical care support.21 Interestingly, 72% of the patients had pulmonary fibrosis on baseline imaging.21 Associated PAH and ILD were also common (32% and 72% respectively).21 The dcSSc phenotype seem to be more prevalent among those requiring critical care.21,22 A high proportion of patients with SDR require renal replacement support (39%).20 Those with SSc requiring ICU admission had a 30-day mortality of 31%, increasing at 6 months and 1 yr.21 Respiratory complications, infection, or both are the main causes of ICU mortality. Invasive mechanical ventilation in ICU was associated with an in-hospital mortality of 85%.21
Chronic pain is common in patients with SSc. A study of 42 patients revealed a high incidence of pain (93%).23 Almost half (45%) experienced pain daily. Joints and hand pain, Raynaud's phenomenon pain and back pain are common sites.23,24 Although neuropathic pain was present in 26% of patients, this was a feature of more severe disease, though pain intensity does not seem to correlate with disease severity.23,24 Low back pain was present even in early disease stages and was associated with anxiety and depression.24
Pathophysiology
The pathophysiology of SSc is complex and as yet incompletely understood (Fig 1). Systemic sclerosis is most likely initiated by unknown triggers in an individual with a susceptible genetic background. Initiation sets off a complex pathological interplay between the key triad of endothelial cells, circulating immune cells (and their inflammatory mediators) and fibroblasts. Overall, this leads to several interacting processes: immune system dysfunction leading to production of autoantibodies and cell-mediated autoimmunity, fibroproliferative vasculopathy in small vessels, and fibroblast dysfunction causing excessive collagen and matrix components accumulation.25
Fig 1.
Schematic representation of SSc pathophysiology. SSc, systemic sclerosis; ET1, endothelin 1; TGF β, transforming growth factor beta; PDGF, platelet derived growth factor.
Although traditionally SSc was regarded as a prototypical fibrotic disease, recent data suggest dysregulated tissue healing, rather than primary fibrosis as core concept.7 Microvascular endothelial cell injury and apoptosis activate an immune response (T and B lymphocytes, monocytes and macrophages) leading to an inappropriate and sustained overproduction of cytokines (e.g. endothelin 1 [ET-1], transforming growth factor beta [TGF-β], and platelet derived growth factor [PDGF]). These amplify and promote inflammation and the transformation of fibroblasts into activated myofibroblasts. Myofibroblasts secrete extracellular matrix proteins and collagen resulting in fibrosis, and profibrotic cytokines (TGF-β) which further enhance the profibrotic milieu. Persistent immune activation leads to diffuse microvascular injury and tissue fibrosis which becomes systemic and progressive. Amplification and persistence of this activated profibrotic state is mediated via (i) tissue hypoxia, which promotes further endothelial cell dysfunction and amplification of the pathological processes; (ii) mechanical tissue stress arising in affected tissues which further promotes fibroblast activation; and (iii) dysregulated production of cytokine mediators (TGF-β, ET-1 and PDGF). The continuous ischaemia–reperfusion small-vessel injury leads to pathological changes involving the skin (e.g. Raynaud's phenomenon, digital ulceration and gangrene), stomach (e.g. gastric antrum vascular ectasia [GAVE] – which may be responsible for silent blood loss), kidneys (e.g. renal crisis) and pulmonary vasculature (e.g. PAH).26 Raynaud's phenomenon is likely to be triggered by endothelial cell injury and perpetuated by ensuing small vessel vasculopathy and arterial vasospasm.26 Interestingly, a so-called ‘myocardial Raynaud's phenomenon’ with small vessel disease and coronary vasospasm has been described to explain the MIs occurring in patients with no evidence of epicardial atherosclerotic changes.27
The rate of progression, generation of autoantibodies and organ involvement vary widely between individual patients, resulting in a heterogenous clinical picture. Improved understanding of disease pathogenesis has allowed identification of molecular targets for therapeutic intervention, with promising results in clinical trials. Interleukin 6 (IL-6) overexpression, produced mainly by activated macrophages, was reported to be associated with skin progression, and worse long-term survival.28
Diagnosis and treatment
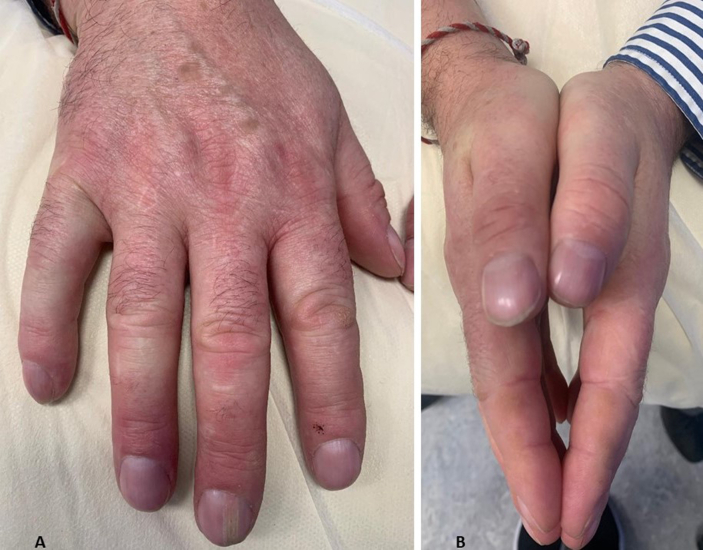
Patients may present with constitutional symptoms or a constellation of symptoms and signs suggestive of the diagnosis. Pathognomonic signs include scleroderma, sclerodactyly (Fig 2), calcinosis (Fig 3) and widespread telangiectasia. These changes eventually lead to terminal tuft resorption (Fig 4). The subjective sensation of ‘puffy’ hands (in early disease) prevents pressing palms and fingers tightly together (Fig 5). The early ‘puffy hands’ phase is usually followed by progressive distal to proximal sclerodactyly. Some may present with clinically manifest organ specific dysfunction or failure (e.g. lung fibrosis, PAH or renal failure).7 Raynaud's phenomenon is a consistent finding and can precede other features by years. Characteristics of ‘pathological Raynaud's’ (Fig 6) include new adult onset, unusual triggers (spicy food, emotion, anxiety), asymmetrical finger or hand involvement, severe or prolonged episodes and the presence of digital scars, pits or dystrophic changes on finger pulps.
Fig 2.
Sclerodactyly, Raynaud's phenomenon and digital necrosis.
Fig 3.
Cutaneous calcinosis.
Fig 4.
Terminal tuft resorption.
Fig 5.
(A) Early illness ‘puffy’ hands. (B) Inability to join palms together.
Fig 6.
Asymmetric Raynaud's.
The diagnosis is challenging, and any suspicion – particularly for cases presenting out of hours – should involve a rheumatologist. The autoantibody profile in SSc is broadly predictive of the clinical phenotype or associated complications. Most patients have antinuclear (ANA) autoantibodies, and specific SSc autoantibodies are found on extractable nuclear antigen (ENA) autoantibody screening. The presence of overlapping syndromes, the negative autoantibodies screen and the variable disease presentations adds to the complexity of diagnosis.
No specific, molecular targeted treatment is yet available as disease modifying therapy for SSc, although several promising agents are being investigated.29 Tocilizumab, a monoclonal antibody against IL-6 receptor, has showed promising results in SSc ILD in phase 3 trials.30,31 Historically, treatment has been oriented towards specific organ dysfunction (e.g. ACEi for renal crisis, proton pump inhibitors [PPI] for gastro-oesophageal reflux, etc.; Table 2). Recent additions to this include nintedanib and tocilizumab for ILD and evidence-based management strategies for PAH.15 Traditionally there was no role for immunosuppression in SSc. This paradigm is changing as understanding of disease pathogenesis evolves. Immunosuppressants (e.g. mycophenolate mofetil or rituximab) are now used in the early phases of dcSSc, with evidence for improved pulmonary function tests and reduction of the ILD extent.32 As evidence and opinions change regarding steroids, those with evidence of inflammation and at highest risk of poor outcomes (e.g. rapidly progressive skin involvement dcSSc) may be taking medium-term moderate-dose regimens and require additional ‘stress’ doses perioperatively.33
Table 2.
Supportive treatment and perioperative significance. SIADH, syndrome of inappropriate antidiuretic hormone secretion; ACEi, angiotensin-converting enzyme inhibitors; TGF β, transforming growth factor beta; PDGF, platelet derived growth factor; TNF α, tumour necrosis factor alpha; cGMP, cyclic guanosine monophosphate; cAMP, cyclic adenosine monophosphate.
| Drug and mechanism of action | Adverse effects |
|---|---|
| Skin and musculoskeletal | |
| Skin thickening/restriction of joints | |
|
Methotrexate Mechanism Immunomodulator; inhibits dihydropholic acid reductase leading to impaired DNA synthesis and immune cells replication impairment. |
Hepatic fibrosis (rare), bone marrow suppression (rare), acute pneumonitis (rare). |
|
Mycophenolate mofetil Mechanism Immunomodulator; antimetabolite with antifibrotic role via lymphocytes (T and B) activation suppression and antibody production impairment. |
Myelosuppression, pancytopenia, hepatotoxicity, increased risk infection. |
|
Cyclophosphamide Mechanism Immunomodulator; cytotoxic effect on both resting and active lymphocytes (particularly T-helper and B cells). |
Drug-induced cardiomyopathy, immunosuppression, haemorrhagic cystitis, pneumonitis, SIADH. |
|
Steroids (e.g. prednisolone) Mechanism Immunomodulator; supresses lymphocytes, fibroblasts and antibody production. |
Hypertension, diabetes, electrolyte imbalances, peptic ulcer disease, skin frailty, adrenal suppression. |
|
Rituximab Mechanism Immunomodulator; induces killing of the CD20+ cells (lymphocytes B depletion). |
Progressive multi-focal leucoencephalopathy, increased risk of infection including serious infections. |
| Finger ulcers | |
|
Phosphodiesterase 5 inhibitor (PDEI) (e.g. sildenafil) Mechanism Vasodilator; increases intracellular cGMP leading to vasodilatation. |
Ischaemic optic neuropathy, ventricular function reduction, cerebral venous thrombosis. Risk of severe hypotension with gram-negative sepsis. Unpredictable effects when nitrates are co-administered. |
|
Endothelin-1 receptor antagonists (ERAs) (e.g. bosentan) Mechanism Vasodilator and (?) antifibrotic role via competitive inhibition of endothelin-1 receptors; endothelin also plays a role in cell proliferation, fibrosis and inflammation. |
May increase blood loss (vasoconstriction inhibition), negative lusitropic effect. |
| Reynaud's phenomenon | |
| Calcium channel blockers | Reduces cardiac morbidity in non-cardiac surgery. |
|
Fluoxetine Mechanism Vasodilator; selective serotonin uptake inhibitor; serotonin causes direct vasodilation through 5HT7 and 5HT2B receptors. |
Bradycardia, coronary vasospasm, SIADH, platelet inhibition, prolonged bleeding times, cytochrome P450 inhibition |
|
Angiotensin receptor blockers Mechanism Vasodilator; displacement of angiotensin II via competitive antagonism of the angiotensin II receptors. |
Hyperkalaemia (dysrhythmias), renal impairment |
|
Prostacyclin receptors analogues (e.g. epoprostenol) Mechanism Vasodilator and platelet aggregation/adhesion inhibitor; increases intracellular cAMP via action on the prostacyclin IP receptor. |
May increase blood loss through vasodilation. |
| Interstitial lung disease | |
| Mycophenolate mofetil | See above. |
| Cyclophosphamide | See above. |
|
Azathioprine Mechanism Immunomodulator; purine synthesis inhibition leading to DNA/RNA synthesise inhibition affecting B and T cells. |
Reduces the effect of atracurium, vecuronium and pancuronium. |
|
Nintedanib Mechanism Antifibrotic and anti-inflammatory; reduces fibroblasts activity via inhibition of profibrotic mediators (PDGF, fibroblast growth factor, TGF-β and vascular endothelia growth factor). |
Vomiting, gastrointestinal perforation, weight loss, arterial thromboembolism, myocardial infarction, bleeding, hypothyroidism, increased liver enzymes. |
|
Pirfenidone Mechanism Antifibrotic and anti-inflammatory; reduces fibroblasts proliferation, collagen production and reduces the production of mediators such as TGF-β, TNF-α and IL-1β. |
Gastroesophageal reflux disease, vomiting, photosensitivity, increase hepatic enzyme concentrations (high risk if a CYP1A2 inhibitor is being used concomitantly). |
| Rituximab | See above. |
|
Tocilizumab Mechanism Immunomodulator; monoclonal antibody that inhibits competitively the IL-6 receptor leading to failure of inflammatory recruitment of B and T cells. |
Mouth ulcers, gastrointestinal perforation, hypertension, increased risk of infections, malignancy, liver dysfunction. |
|
Calcineurin inhibitors (e.g. cyclosporine, tacrolimus) Mechanism Immunomodulator; inhibits calcineurin enzyme leading to decreased T cell activation and signalling. |
Used post lung transplant in some SSc patients. Associated with renal vasoconstriction and SRC. |
| Cardiac disease | |
| Systolic dysfunction: ACEi | Hyperkalaemia (dysrhythmias), renal impairment. |
| Diastolic dysfunction: diuretics | Hypovolemia, electrolyte losses, dysrhythmias. |
| Cardiac resynchronisation therapy/pacemakers | May require preoperative reprogramming to avoid interference from diathermy. |
| Pulmonary arterial hypertension | |
| ERAs, PDE5i, Prostacyclin analogues | See above. |
|
Prostacyclin receptor agonists (e.g. selexipag) Mechanism Vasodilator and platelet aggregation/adhesion inhibitor; prostacyclin IP receptor agonism leading to vasodilatation, decreased cell proliferation and platelet aggregation inhibition. |
Myalgia, anaemia, nasopharyngitis. |
| Anticoagulation | Ensure timely discontinuation or reversal. |
| Oesophageal disease | |
| Proton pomp inhibitors | Hypomagnesaemia, hypocalcaemia. |
| H2 blockers | Confusion, pancreatitis, hepatitis, seizures. |
| Antiacids | Hypermagnesemia, milk-alkali syndrome, hypophosphataemia. |
| Gastric disease | |
| Prokinetics | Risk of aspiration in patients who missed prokinetics or are not on them. |
| Scleroderma renal crisis | |
| ACEi | See above. |
Although spanning various classes, different mechanisms of action and treating various SSc complications, the drugs used in SSc belong largely to five main pathways:
-
(i)
Immunomodulation (e.g. steroids, rituximab, mycophenolate mofetil, tocilizumab, etc.)
-
(ii)
ILD antifibrotics (e.g. nintedanib, pirfenidone)
-
(iii)
Vasodilators (calcium channel blockers, phosphodiesterase 5 inhibitors [PDEI], prostacyclin analogues, etc.)
-
(iv)
General cardiac disease (e.g. ACEI, diuretics, antihypertensives, etc.)
-
(v)
General GI disease (e.g. antacids, PPIs, prokinetics, etc.)
Of major importance for the anaesthetist are the vasodilators used for the management of PAH (e.g. endothelin-1 receptor antagonists, PDEIs, prostacyclin analogues) and their perioperative management (see Part 2). In addition to PAH, these vasodilators may be used on specialist advice for the perioperative management of Raynaud's phenomenon or finger ulceration.
Role of the rheumatologist
The SSc perioperative literature to guide evidence-based care is scarce. Reflecting this, there are no guidelines to guide management of anaesthesia or perioperative care. Most evidence is observational and derived from case reports or series, and expert opinion. With exception of some perioperative cardiovascular outcomes, the care of these patients relies on extrapolating data from the rheumatology literature.18 Although there are multiple specialty guidelines for the management of the disease and its complications (e.g. SRC, antiphospholipid syndrome [APS]), only one European guideline to date includes advice on anaesthesia.34 It also includes a concise summary of some of the associated emergencies relevant to the anaesthetist such as SRC, PAH and right heart decompensation.
In the absence of robust data and guidelines, the rheumatologist has an essential role in the perioperative period (Table 3). Their input bridges the current gap between specialty guidelines and perioperative care. In our experience, most perioperative requests for specialist input are for (i) immunosuppression queries (including steroids and infection), (ii) use of beta blockers and (iii) development of new complications (e.g. is this postoperative creatinine increase a precursor of SRC or not?).
Table 3.
The rheumatologist's role in the perioperative period. SRC, scleroderma renal crisis; VTE, venous thromboembolism; ACEi, angiotensin-converting enzyme inhibitors.
|
Conclusions
Systemic sclerosis is a rare progressive autoimmune CTD. Its multisystem involvement and the need for more high-risk surgical procedures present a major clinical challenge for the anaesthetist. Pulmonary hypertension is present in more than half of patients and is a major perioperative risk factor. In addition, postoperative MI is strongly associated with systemic sclerosis. Specific issues for the anaesthetist, such as difficult direct laryngoscopy and intraoperative aspiration, add to the risks of perioperative morbidity. Although there is no specific treatment, the overall outcome and survival have improved in recent decades as a result of better understanding of the disease and progress in providing specific organs support. This increases the likelihood of encountering older patients with significant comorbidities requiring anaesthesia and critical care. Unfortunately, the lack of guidelines to drive evidence-based perioperative care adds to the complexity of management. Hence, the involvement of the rheumatology specialist is paramount for successful perioperative outcomes.
Declaration of interests
The authors declare that they have no conflicts of interest.
Biographies
Catalin Iulian Efrimescu FCAI is a post-CST fellow in anaesthesiology at Mater Misericordiae University Hospital.
Suzanne Donnelly MD MRCPI is a consultant rheumatologist at Mater Misericordiae Hospital. She provides clinical expertise for the patients with systemic sclerosis at the Irish national centres for pulmonary hypertension and heart/lung transplant. Associate Professor Donnelly is also associate dean (education) and programme director at the School of Medicine, University College Dublin.
Donal Buggy DSc MD MSc FRCPI FFSEM FRCA FESA-IC FCAI is a consultant anaesthesiologist at Mater Misericordiae Hospital and professor of anaesthesiology and perioperative medicine at University College Dublin. He is an active clinician, scientist and mentor and has published extensively on diverse topics across the specialty.
Matrix codes: 1A02, 1C01, 1H02, 2A03, 2A07, 3A01, 3I00
MCQs
The associated MCQs (to support CME/CPD activity) will be accessible at www.bjaed.org/cme/home by subscribers to BJA Education.
References
- 1.Moinzadeh P., Aberer E., Ahmadi-Simab K., et al. Disease progression in systemic sclerosis-overlap syndrome is significantly different from limited and diffuse cutaneous systemic sclerosis. Ann Rheum Dis. 2015;74(4):730–737. doi: 10.1136/annrheumdis-2013-204487. [published Online First: 2014/01/07] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van den Hoogen F., Khanna D., Fransen J., et al. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum. 2013;65(11):2737–2747. doi: 10.1002/art.38098. [published Online First: 2013/10/15] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnes J.K., Mouthon L., Mayes M.D. In: Scleroderma: From Pathogenesis to Comprehensive Management. Varga J., Denton C.P., Wigley F.M., et al., editors. Springer International Publishing; Cham: 2017. Epidemiology, environmental, and infectious risk factors; pp. 11–24. [Google Scholar]
- 4.Bergamasco A., Hartmann N., Wallace L., et al. Epidemiology of systemic sclerosis and systemic sclerosis-associated interstitial lung disease. Clin Epidemiol. 2019;11:257–273. doi: 10.2147/CLEP.S191418. [published Online First: 2019/05/23] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yazdany J., Manno R., Hellmann D.B., et al. In: Current Medical Diagnosis & Treatment 2021. Papadakis M.A., McPhee S.J., Rabow M.W., editors. McGraw-Hill Education; New York, NY: 2021. Scleroderma (Systemic sclerosis) [Google Scholar]
- 6.Steen V.D., Medsger T.A. Changes in causes of death in systemic sclerosis, 1972-2002. Ann Rheum Dis. 2007;66(7):940–944. doi: 10.1136/ard.2006.066068. [published Online First: 2007/03/03] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Denton C.P., Khanna D. Systemic sclerosis. Lancet. 2017;390(10103):1685–1699. doi: 10.1016/S0140-6736(17)30933-9. [published Online First: 2017/04/18] [DOI] [PubMed] [Google Scholar]
- 8.Coroneos C.J., Selber J.C., Offodile A.C., 2nd, et al. US FDA breast implant postapproval studies: long-term outcomes in 99,993 patients. Ann Surg. 2019;269(1):30–36. doi: 10.1097/SLA.0000000000002990. [published Online First: 2018/09/18] [DOI] [PubMed] [Google Scholar]
- 9.Tyndall A.J., Bannert B., Vonk M., et al. Causes and risk factors for death in systemic sclerosis: a study from the EULAR Scleroderma Trials and Research (EUSTAR) database. Ann Rheum Dis. 2010;69(10):1809–1815. doi: 10.1136/ard.2009.114264. [published Online First: 2010/06/17] [DOI] [PubMed] [Google Scholar]
- 10.Jaeger V.K., Wirz E.G., Allanore Y., et al. Incidences and risk factors of organ manifestations in the early course of systemic sclerosis: A longitudinal EUSTAR study. PLoS One. 2016;11(10) doi: 10.1371/journal.pone.0163894. [published Online First: 2016/10/06] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Avina-Zubieta J.A., Man A., Yurkovich M., et al. Early cardiovascular disease after the diagnosis of systemic sclerosis. Am J Med. 2016;129(3):324–331. doi: 10.1016/j.amjmed.2015.10.037. [published Online First: 2015/11/26] [DOI] [PubMed] [Google Scholar]
- 12.Turcotte-Gosselin F., Turgeon P.Y., Ikic A., et al. Is heart transplantation a valuable option in patients with diffuse systemic sclerosis and primary cardiac involvement? Clin Case Rep. 2020;8(1):137–141. doi: 10.1002/ccr3.2600. [published Online First: 2020/01/31] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nikai T., Kanata K., Ozasa H., et al. Suddenly developing low output syndrome during acute thoracic aortic dissection surgery in a patient with progressive systemic sclerosis. Masui. 2002;51(2):182–185. [published Online First: 2002/03/14] [PubMed] [Google Scholar]
- 14.Kihira C., Mizutani H., Shimizu M. Sinus arrest developed during gastric cancer operation in a progressive systemic sclerosis patient. J Dermatol. 1995;22(5):357–359. doi: 10.1111/j.1346-8138.1995.tb03404.x. [published Online First: 1995/05/01] [DOI] [PubMed] [Google Scholar]
- 15.Coghlan J.G., Denton C.P., Grunig E., et al. Evidence-based detection of pulmonary arterial hypertension in systemic sclerosis: the DETECT study. Ann Rheum Dis. 2014;73(7):1340–1349. doi: 10.1136/annrheumdis-2013-203301. [published Online First: 2013/05/21] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiong A., Liu Q., Zhong J., et al. Increased risk of mortality in systemic sclerosis-associated pulmonary hypertension: a systemic review and meta-analysis. Adv Rheumatol. 2022;62(1):10. doi: 10.1186/s42358-022-00239-2. [published Online First: 20220330] [DOI] [PubMed] [Google Scholar]
- 17.Nokes B.T., Raza H.A., Cartin-Ceba R., et al. Individuals with scleroderma may have increased risk of sleep-disordered breathing. J Clin Sleep Med. 2019;15(11):1665–1669. doi: 10.5664/jcsm.8036. [published Online First: 2019/11/20] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luo Y., Jiang C., Krittanawong C., et al. Systemic sclerosis and the risk of perioperative major adverse cardiovascular events for inpatient non-cardiac surgery. Int J Rheum Dis. 2019;22(6):1023–1028. doi: 10.1111/1756-185X.13537. [published Online First: 2019/03/06] [DOI] [PubMed] [Google Scholar]
- 19.Bernstein E.J., Peterson E.R., Sell J.L., et al. Survival of adults with systemic sclerosis following lung transplantation: a nationwide cohort study. Arthritis Rheumatol. 2015;67(5):1314–1322. doi: 10.1002/art.39021. [published Online First: 2015/01/13] [DOI] [PubMed] [Google Scholar]
- 20.Malézieux-Picard A., Hivernat H., Martis N., Fuzibet J.-G., Bernardin G., Queyrel V. Outcome of patients with systemic rheumatic diseases admitted in intensive care unit: a prognostic study of 98 patients. Annals of the Rheumatic Diseases. suppl Supplement 2 2017:76. doi: 10.1136/annrheumdis-2017-eular.3692. [DOI] [Google Scholar]
- 21.Pene F., Hissem T., Berezne A., et al. Outcome of patients with systemic sclerosis in the intensive care unit. J Rheumatol. 2015;42(8):1406–1412. doi: 10.3899/jrheum.141617. [published Online First: 2015/07/03] [DOI] [PubMed] [Google Scholar]
- 22.Shalev T., Haviv Y., Segal E., et al. Outcome of patients with scleroderma admitted to intensive care unit. A report of nine cases. Clin Exp Rheumatol. 2006;24(4):380–386. [published Online First: 2006/09/08] [PubMed] [Google Scholar]
- 23.Ostojic P., Jankovic K., Djurovic N., et al. Common causes of pain in systemic sclerosis: frequency, severity, and relationship to disease status, depression, and quality of life. Pain Manag Nurs. 2019;20(4):331–336. doi: 10.1016/j.pmn.2019.02.006. [published Online First: 2019/05/20] [DOI] [PubMed] [Google Scholar]
- 24.Evers C., Jordan S., Maurer B., et al. Pain chronification and the important role of non-disease-specific symptoms in patients with systemic sclerosis. Arthritis Res Ther. 2021;23(1):34. doi: 10.1186/s13075-021-02421-1. [published Online First: 2021/01/21] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pattanaik D., Brown M., Postlethwaite B.C., Postlethwaite A.E. Pathogenesis of systemic sclerosis. Front Immunol. 2015 doi: 10.3389/fimmu.2015.00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carr Z.J., Klick J., McDowell B.J., et al. An update on systemic sclerosis and its perioperative management. Curr Anesthesiol Rep. 2020;10(4):512–521. doi: 10.1007/s40140-020-00411-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cannarile F., Valentini V., Mirabelli G., et al. Cardiovascular disease in systemic sclerosis. Ann Transl Med. 2015;3(1):8. doi: 10.3978/j.issn.2305-5839.2014.12.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khan K., Xu S., Nihtyanova S., et al. Clinical and pathological significance of interleukin 6 overexpression in systemic sclerosis. Ann Rheum Dis. 2012;71(7):1235–1242. doi: 10.1136/annrheumdis-2011-200955. [published Online First: 2012/05/16] [DOI] [PubMed] [Google Scholar]
- 29.Chung M.P., Chung L. Drugs in phase I and phase II clinical trials for systemic sclerosis. Expert Opin Investig Drugs. 2020;29(4):349–362. doi: 10.1080/13543784.2020.1743973. [published Online First: 2020/03/18] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khanna D., Lin C.J.F., Furst D.E., et al. Tocilizumab in systemic sclerosis: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med. 2020;8(10):963–974. doi: 10.1016/S2213-2600(20)30318-0. [published Online First: 2020/09/01] [DOI] [PubMed] [Google Scholar]
- 31.Roofeh D., Lin C.J.F., Goldin J., et al. Tocilizumab prevents progression of early systemic sclerosis-associated interstitial lung disease. Arthritis Rheumatol. 2021;73(7):1301–1310. doi: 10.1002/art.41668. [published Online First: 2021/02/05] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fraticelli P., Fischetti C., Salaffi F., et al. Combination therapy with rituximab and mycophenolate mofetil in systemic sclerosis. A single-centre case series study. Clin Exp Rheumatol. 2018;36 Suppl 113(4):142–145. [published Online First: 20180930] [PubMed] [Google Scholar]
- 33.Herrick Controversies on the use of steroids in systemic sclerosis. J Scleroderma Relat Disord. 2017;2(2):84–91. doi: 10.5301/jsrd.5000234. [DOI] [Google Scholar]
- 34.Hachulla E., Agard C., Allanore Y., et al. French recommendations for the management of systemic sclerosis. Orphanet J Rare Dis. 2021;16(Suppl 2):322. doi: 10.1186/s13023-021-01844-y. [published Online First: 2021/07/27] [DOI] [PMC free article] [PubMed] [Google Scholar]