Abstract
We have previously shown that specific-pathogen-free interleukin-10 (IL-10)-deficient (IL-10 KO) mice reconstituted with Helicobacter hepaticus develop severe colitis associated with a Th1-type cytokine response. In the present study, we formally demonstrate that IL-12 is crucial for disease induction, because mice deficient for both IL-10 and IL-12 p40 show no intestinal pathology following H. hepaticus infection. By using monoclonal antibodies (MAbs) to IL-12, gamma interferon (IFN-γ), and tumor necrosis factor alpha (TNF-α), we have further analyzed the role of these cytokines in the maintenance of the Th1 response and inflammation in IL-10 KO mice with established H. hepaticus-induced colitis. Treatment of infected colitic IL-10 KO mice with anti-IL-12 p40 resulted in markedly reduced intestinal inflammation, colonic IFN-γ, TNF-α, and inducible nitric oxide synthase (iNOS) mRNA levels, and H. hepaticus-specific IFN-γ secretion by mesenteric lymph node (MLN) cells compared to the findings in control MAb-treated mice. Moreover, the diminished pathology was associated with decreased numbers of colonic CD3+ T cells and significantly reduced frequencies of Helicobacter-reactive CD4+ Th1 cells in MLN. In contrast, anti-IFN-γ and/or anti-TNF-α had no effect on intestinal inflammation in IL-10 KO mice with established colitis. Using IL-10/IFN-γ double-deficient mice, we further show that IFN-γ is not required for the development of colitis follwing H. hepaticus infection. MLN cells from infected IL-10/IFN-γ KO animals secreted elevated amounts of IL-12 and TNF-α following bacterial antigen stimulation, indicating alternative pathways of disease induction. Taken together, our results demonstrate a crucial role for IL-12 in both inducing and sustaining intestinal inflammation through recruitment and maintenance of a pool of pathogenic Th1 cells.
Inflammatory bowel disease (IBD) is thought to be the consequence of an aberrant mucosal immune response that damages tissues of the intestinal tract (29, 35, 39). From experiments with different animal models, it has become clear that the intestinal flora play an essential role in triggering the disease (2, 13, 33, 36, 42). This is also true for the enterocolitis that spontaneously develops in interleukin-10 (IL-10)-deficient (IL-10 KO) mice in conventional animal facilities (24), because these animals display less severe or no disease when reared under specific-pathogen-free (SPF) or germfree conditions (5, 37). That gut flora also play a role in human IBD has been suggested by studies demonstrating associations between various bacterial species and disease, either by direct detection or by disease-associated antimicrobial immune responses (6, 16, 35, 41, 43), as well as diminished inflammation following antibiotic or probiotic treatment of patients with disease (8, 20, 21, 32, 44).
To study how the gut flora may influence the development of intestinal pathology in IL-10 KO mice, we analyzed SPF-reared IL-10-deficient mice on the C57BL/10SgSnAi background following reconstitution with a defined microbial agent, Helicobacter hepaticus. As early as 2 to 4 weeks after inoculation with this gram-negative bacterium, IL-10 KO animals displayed moderate to severe inflammation of the large bowel that was associated with a Th1-type cytokine response by mesenteric lymph node (MLN) cells stimulated in vitro with SHelAg, a soluble H. hepaticus antigen (Ag) preparation (25). These intestinal lesions were absent in uninfected IL-10 KO controls as well as in simultaneously infected wild-type (WT) mice, the latter instead mounting an IL-10-dominated cytokine response to the bacterium (25). The H. hepaticus-induced colitis in IL-10 KO mice was prevented by administration of monoclonal antibody (MAb) to either IL-12 p40 or gamma interferon (IFN-γ) from the start of the infection, suggesting that interference with the development of the Th1 response to the bacterium or inhibition of the Th1-effector phase can effectively block disease induction (25). Subsequent studies (14) analyzing germfree IL-10 KO mice on a C57BL/6 × 129/Ola background 9 weeks after bacterial exposure have indicated that H. hepaticus may not be sufficient for colitis induction and suggest the contribution of resident background flora to the pathological response. Moreover, it is clear that other bacterial species in the absence of H. hepaticus can also trigger intestinal inflammation in IL-10-deficient mice (18, 37).
An important goal of IBD research is the development of effective therapies for patients with Crohn's disease and ulcerative colitis. Because a dysregulated cytokine response has been implicated in the pathogenesis of IBD, it is important to know which of these factors are critical for the maintenance of disease as they may offer new approaches for therapy. Previous studies with murine colitis models have suggested that the continuous presence of IL-12 is crucial for sustaining the inflammatory response (10, 27). However, its downstream IFN-γ effector molecule does not appear to play as important a role in disease maintenance (10, 19). The latter observation seemed somewhat surprising, because preventive treatment with anti-IFN-γ MAb blocks the development of disease in both spontaneous enterocolitis and the H. hepaticus-induced inflammation observed in IL-10 KO mice (5, 25).
In the present study, we have addressed the requirements for IL-12, IFN-γ, and TNF-α in disease maintenance in the H. hepaticus/IL-10 KO colitis model by performing in vivo neutralization of cytokines. In addition, for the first time in studies of IBD, we have employed double-cytokine-deficient mice to formally address the roles of IL-12, IFN-γ, and IL-4 in disease development. Our data demonstrate that IL-12 is crucial for both induction and maintenance of colitis following H. hepaticus inoculation of IL-10-deficient mice. Moreover, while IFN-γ may play a role in disease induction, this cytokine is not required for the development of colitis or for the ongoing inflammatory process after H. hepaticus infection. Instead, neutralization of IL-12 correlates with reduced numbers of T cells infiltrating the intestine as well as diminished frequencies of SHelAg-specific Th1 cells in MLN, suggesting an important role for this cytokine in maintaining the pool of pathogenic cells.
MATERIALS AND METHODS
Experimental animals and infections.
Six- to 12-week old, female SPF C57BL/6NAi IL-4 KO, C57BL/10SgSnAi IL-10 KO, C57BL/6 IL-12 p40 KO (backcrossed to the 12th, 10th, and 5th generations, respectively), C57BL/10SgSnAi WT, and double-deficient IL-10/IL-4 KO and IL-10/IL-12 p40 KO mice (generated by crossing the above-mentioned single-cytokine-deficient mice as described previously (22, 48) were obtained from Taconic Farms (Germantown, N.Y.). The animals employed tested negative for antibodies to specific murine viruses and were free of Helicobacter species as assessed by PCR. The IL-4 KO and IL-10 KO lines were originally obtained from R. Kühn and W. Müller (University of Cologne, Cologne, Germany), and the IL-12-deficient animals were obtained from J. Magram (Hoffmann-La Roche, Inc., Nutley, N.J.). IL-10/IFN-γ double-deficient mice were generated by crossing C57BL/10Sg SnAi IL-10 KO males with C57BL/6Ai IFN-γ KO females (Taconic Farms), and the progeny were intercrossed to generate IL-10/IFN-γ KO offspring. All animals were housed in sterile microisolator cages with autoclaved bedding, food, and water at the animal facility at the National Institute of Allergy and Infectious Diseases in accordance with the procedure outlined in the Guide for the Care and Use of Laboratory Animals (26a) under an animal study proposal approved by the National Institute of Allergy and Infectious Diseases Animal Care and Use Committee.
Mice were inoculated intraperitoneally (i.p.) or intragastrically (i.g.) with 0.5 ml of an H. hepaticus suspension (standard Frederick isolate 1A) (17, 45) prepared to a McFarland turbidity standard of 1.0 in phosphate-buffered saline (PBS), representing 2.45 × 109 CFU/ml. We have previously demonstrated that these two routes of inoculation with H. hepaticus result in the same intestinal histological changes in IL-10 KO mice (25). The bacteria were confirmed to be H. hepaticus by PCR before injection (4). Age-matched uninfected animals were included as controls.
In vivo MAb treatment.
Four weeks after bacterial inoculation, by which time colitis is established in IL-10 KO animals (25), mice were injected i.p. every 3 to 4 days for 4 weeks with 1 mg of anti-IL-12 (MAb C17.8; initially provided by G. Trinchieri) (49), anti-IFN-γ (XMG-6) (9), anti-TNF-α (XT22–11) (1), a combination of anti-IFN-γ and anti-TNF-α, or control MAb GL113 (anti-β-galactosidase) in 0.5 ml of PBS. All MAbs were purified from ascites fluid by two sequential 50% ammonium sulfate precipitations. Four days after the last MAb injection, mice were sacrificed, MLN cells were collected for in vitro culture, and intestinal tissues were collected for histology and reverse transcriptase (RT)-PCR analysis.
Pathology and immunohistochemistry.
Tissues were fixed in Bouin's fixative or 10% neutral buffered formalin, embedded in paraffin, sectioned at 5 μm, and stained with hematoxylin and eosin (H&E). A longitudinal section of the entire cecum was made together with a cross section of the ascending colon about 1 cm from the cecum. Sections were evaluated in a blinded fashion by the same pathologist (A.W.C.), and an average score of what was seen throughout the whole section was assigned based on a four-scale scoring system with emphasis on the number of infiltrating cells in the lamina propria and the number of crypt abscesses. Hyperplasia was evaluated by microscopic examination of mucosal thickness with an ocular micrometer. A score of 0 denotes no inflammation with only a few infiltrating lymphocytes; 1 corresponds to a mild lesion equivalent to a one-cell-thick layer in the lamina propria; 2 is roughly equivalent to an average two-cell thickness of lymphocytes; 3 implies roughly a three-to-four-cell thickness; and 4 is equivalent to a more-than-four-cell thickness or the presence of severe lesions, such as ulcers or crypt abscesses. Results are presented as the mean score ± standard error (SE) of 3 to 5 mice/group. The absence of an error bar indicates that all animals within a group had identical scores. In certain cases, histopathologic median and range values are also given.
Colonic tissues fixed in Bouin's fixative were used for immunohistochemical staining of T cells with a polyclonal rabbit anti-human CD3 antibody (which cross-reacts with mouse CD3; DAKO Corp., Carpinteria, Calif.) and an ABC Vectastain Elite kit (Vector Laboratories, Inc., Burlingame, Calif.) (25).
Antigen preparation.
SHelAg was prepared from cultures of H. hepaticus as described previously (25). Briefly, the organisms were harvested and washed extensively in PBS followed by sonication at 4°C to lyse the bacteria. Cell debris was removed by centrifugation at 8,000 × g (Sorvall RC2-B, SS-34 rotor) for 30 min at 4°C. The supernatant was sterile filtered, protein content was determined by Bradford's technique (Pierce, Rockford, Ill.), and the Ag was stored at −40°C until use.
Cell cultures and cytokine assays.
Single-cell suspensions were prepared from MLN, and cells were resuspended in tissue culture medium (RPMI 1640 supplemented with 10% heat-inactivated fetal calf serum [FCS], 100 U of penicillin per ml, 100 μg of streptomycin per ml, 2 mM glutamine, 20 mM HEPES, 1 mM sodium pyruvate, 0.1 mM nonessential amino acids, and 50 μM 2-mercaptoethanol). Experiments were performed with MLN suspensions from individual mice or with cells pooled from 3 to 5 mice per group.
To measure cytokine responses, MLN cells (3 × 106/ml) were cultured in medium alone or with 0.3 μg of SHelAg per ml or plate-bound anti-CD3 (2.5 μg/well; MAb 145–2C11; PharMingen, San Diego, Calif.) in 24-well plates in a total volume of 1 ml/well. In certain experiments, anti-CD4 (GK1.5; rat immunoglobulin G2b [IgG2b]; 10 μg/ml) (12), anti-CD8 (2.43; rat IgG2b; 10 μg/ml) (34), or anti-IL-12 (C17.8; rat IgG2a; 20 μg/ml) (49) MAbs were added to parallel SHelAg cultures. Supernatants were collected 72 h later, and IFN-γ and IL-12 were measured by enzyme-linked immunosorbent assay (ELISA) with MAb from PharMingen, while TNF-α was detected with a kit from R&D Systems (Minneapolis, Minn.).
Intracellular staining for IFN-γ.
Analysis of intracellular cytokine expression was performed with cells from the same SHelAg-stimulated cultures used in the cytokine secretion assays according to a previously described protocol (23). Briefly, MLN cells were incubated for an additional 18 h in fresh medium added to replace the culture supernatant collected at 72 h. Thereafter, cells were stimulated in 1-ml cultures in 24-well plates coated overnight with 2.5 μg of anti-CD3 per well (145–2C11; PharMingen). Nine hours later, brefeldin A (10 μg/ml; Sigma, St Louis, Mo.) was added, and after 5 h, cells were washed once in RPMI, stained with Cy-chrome-labeled anti-CD4 (RM4–5; PharMingen), and fixed for 15 min in 2% paraformaldehyde (Sigma) at room temperature. After 30 min of incubation in permeabilization buffer (PBS containing 0.1% saponin [Calbiochem-Novabiochem. Corp., La Jolla, Calif.], 0.1% FCS, and 20 mM HEPES) with 10% normal mouse serum and anti-FcγRII/III (2.4G2; 5 μg/ml; PharMingen) at 4°C, cells were stained for 30 min with pretitrated phycoerythrin (PE)-labeled anti-IFN-γ (XMG1.2; PharMingen) at 4°C, washed twice with permeabilization buffer, and resuspended in PBS plus 0.5% FCS. Cell fluorescence was measured with a FACScan flow cytometer, and data were analyzed with CellQuest software (Becton Dickinson).
RT-PCR and Southern blotting for detection of cytokine mRNA.
Since in general, the degree of inflammation in the colon was found to proportionally reflect that observed in the cecum, the colon was chosen for RT-PCR analyses to avoid lymphocyte patches present irregularly throughout the cecum while preserving the intact cecum for histological examination. Colonic tissue (3 to 5 mm of ascending colon) was homogenized in RNA STAT-60 (Tel-Test, Friendswood, Tex.) with a tissue Polytron (Omni, Waterbury, Conn.), and total RNA was isolated as recommended by the manufacturer. An RT-PCR procedure was performed to determine relative quantities of mRNA for various cytokines (46, 47). Briefly, 1 μg of RNA was reverse transcribed with random hexamer oligonucleotides (Boehringer Mannheim, Indianapolis, Ind.) and Superscript II RT (GIBCO BRL, Gaithersburg, Md.). cDNA was then amplified with specific primer pairs for IFN-γ (32 cycles), TNF-α (28 cycles), inducible nitric oxide synthase (iNOS) (26 cycles), or the housekeeping gene hypoxanthine phosphoribosyltransferase (HPRT; 24 cycles) by using Taq DNA polymerase and deoxynucleotide triphosphates (both from GIBCO BRL). After an initial incubation at 95°C for 3 min, temperature cycling was initiated as follows: 94°C for 2 min, 54°C for 2 min, and 72°C for 3 min. An additional extension for 5 min was performed at the end of the last cycle. For the experiment shown in Fig. 8C to E, 1 min was used at the 94 and 54°C steps, and 30 cycles were used for TNF-α. PCR products were electrophoresed in a 1.5% agarose gel and then transferred onto Hybond-N+ nylon membranes (Amersham, Little Chalfont, United Kingdom), followed by hybridization with specific fluorescein-labeled probes (46, 47). Blots were developed with the ECL enhanced chemiluminescence detection system (Amersham) and exposed to autoradiographic film (ECL-Hyperfilm; Amersham). The signal intensities of bands were scanned, and the optical density was quantified with NIH Image sortware (National Institutes of Health, Bethesda, Md.). The primers and probes were as follows: HPRT sense, GTTGGATACAGGCCAGACTTTGTTG; HPRT antisense, GATTCAACTTGCGCTCATCTTAGGC; HPRT probe, GTTGTTGGATATGCCCTTGAC; IFN-γ sense, TACTGCCACGGCACAGTCATTGAA; IFN-γ antisense, GCAGCGACTCCTTTTCCGCTTCCT; IFN-γ probe, GGAGGAACTGGCAAAAGGA; TNF-α sense, GATCTCAAAGACAACCAACTAGTG; TNF-α antisense, CTCCAGCTGGAAGACTCCTCCCAG; TNF-α probe, CCCGACTACGTGCTCCTCACC; iNOS sense, CCCTTCCGAAGTTTCTGGCAGCAGC; iNOS antisense, GGCTGTCAGAGCCTCGTGGCTTTGG; iNOS probe, CAAGGTCTACGTTCAGGACATC.
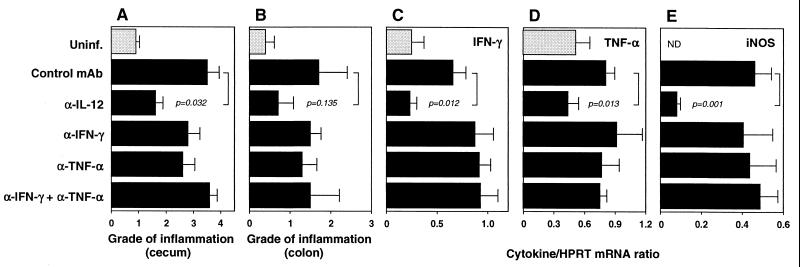
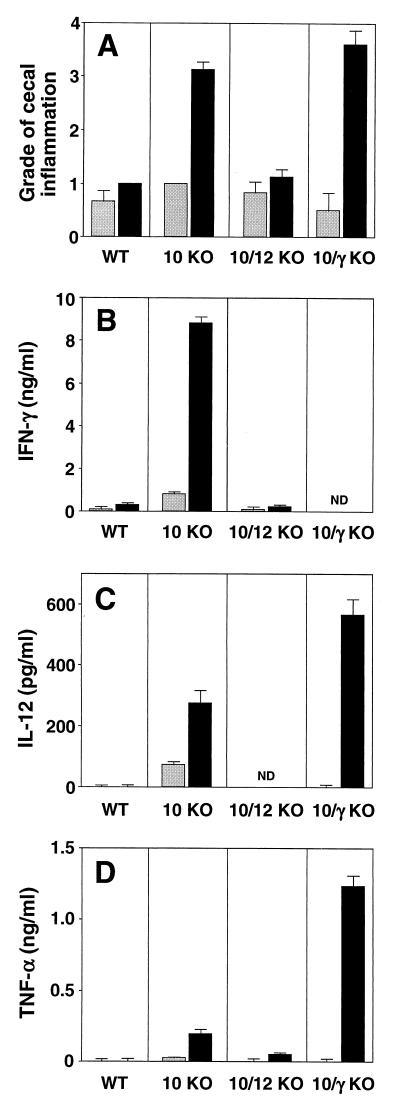
FIG. 8.
Treatment with anti-IFN-γ and/or anti-TNF-α has no effect on established H. hepaticus-induced intestinal inflammation in IL-10 KO mice. IL-10 KO mice were infected with H. hepaticus and treated with the indicated MAb between weeks 4 and 8 of infection. Uninfected (Uninf.) animals were included as controls. Four days after the last MAb injection, mice were sacrificed, and intestinal inflammation and colonic cytokine mRNA levels were determined. (A and B) Pathology in the cecum (A) and ascending colon (B). Bars represent mean histology scores ± SE of 5 mice/group. Median scores for the cecum and colon for the different groups were as follows: uninfected = 1 (range, 0.5 to 1) and 0.5 (range, 0 to 1), infected plus control MAb = 4 (range, 2 to 4) and 1 (range, 0.5 to 4), infected plus anti-IL-12 = 2 (range, 1 to 2) and 0.5 (range, 0 to 2), infected plus anti-IFN-γ = 3 (range, 2 to 4) and 1.5 (range, 1 to 2), infected plus anti-TNF-α = 2 (range, 2 to 4) and 1 (range, 1 to 2.5), and infected plus anti-IFN-γ plus anti-TNF-α = 4 (range, 3 to 4) and 1 (range, 0.5 to 4). (C to E) RT-PCR analysis of IFN-γ (C), TNF-α (D), and iNOS (E) mRNA levels in colon expressed as a cytokine/HPRT ratio for each mouse as described in the legend to Fig. 4. Bars represent mean values ± SE of 5 mice/group. α, anti; ND, not detected.
Statistical analysis.
The statistical significance of differences in cytokine expression between groups was evaluated by using Student's two-tailed t test. Comparison of grades of inflammation between the groups was performed by nonparametric Wilcoxon-Mann-Whitney test.
RESULTS
IL-10/IL-12 double-deficient mice fail to develop colitis after H. hepaticus infection, while IL-10/IL-4 KO animals are as susceptible to disease as IL-10 KO mice.
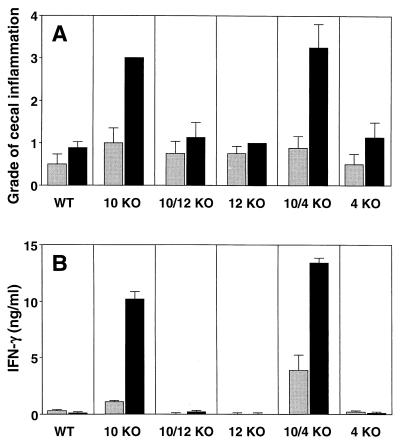
We have previously shown that SPF-reared IL-10 KO mice develop colitis after experimental infection with H. hepaticus and that the disease is dramatically reduced when the animals are treated with anti-IL-12 or anti-IFN-γ MAb from the start of the infection (25). To formally investigate the role of IL-12 in disease induction, we here analyzed intestinal pathology in mice doubly deficient for IL-10 and IL-12 that were infected with H. hepaticus for 5 weeks. As shown in Fig. 1A, while infected IL-10 KO mice displayed severe intestinal pathology, IL-10/IL-12 double-deficient animals showed no disease after bacterial inoculation, and their cecal histological scores were comparable to those of WT controls as well as single IL-12 KO mice. While these data obtained from a single time point postinfection do not rule out the possibility of later compensatory mechanisms, they indicate at the very minimum that IL-12 is crucial for initial disease induction.
FIG. 1.
H. hepaticus does not trigger colitis in IL-10/IL-12 double-deficient mice. WT, IL-10 KO, IL-10/IL-12 KO, IL-12 KO, IL-10/IL-4 KO, and IL-4 KO animals were infected i.g. with H. hepaticus (black bars), and intestinal pathology and cytokine responses were analyzed 5 weeks later. Gray bars represent uninfected controls. (A) Inflammation in the cecum (typhlitis). Bars represent mean histology scores ± SE of four mice/group, with a median value for infected IL-10 KO of 3 (all mice scored as 3) and that of infected IL-10/IL-4 KO of 3.5 (range, 2 to 4). Similar results were observed for the colon, although scores were lower (not shown). (B) IFN-γ levels detected in 72-h supernatants from MLN cells (3 × 106/ml) stimulated with 0.3 μg of SHelAg per ml. Bars represent means ± standard deviations of duplicate ELISA values from MLN cells pooled from 4 mice/group. No IFN-γ was detected from cells cultured in medium alone.
To examine if IL-4 plays a protective role in our model of colitis, mice deficient in this cytokine were analyzed in parallel. In contrast to IL-10-deficient mice, however, IL-4 KO mice showed no intestinal pathology upon H. hepaticus infection, and as expected, only IL-4-deficient mice also lacking IL-10 developed colitis after inoculation with the bacterium (Fig. 1A). Our previous findings demonstrated a strong association between Helicobacter-induced colitis in IL-10 KO mice and a Th1-cytokine response to SHelAg (25). This pattern was confirmed in the present study, because only MLN cells from cytokine-deficient mice with disease responded with significant IFN-γ production to SHelAg stimulation (Fig. 1B).
Anti-IL-12 treatment of H. hepaticus-infected IL-10 KO mice with established disease reduces intestinal inflammation.
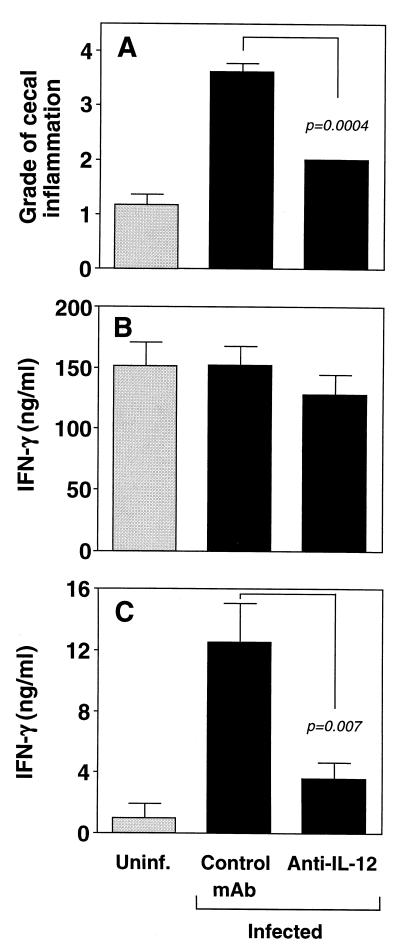
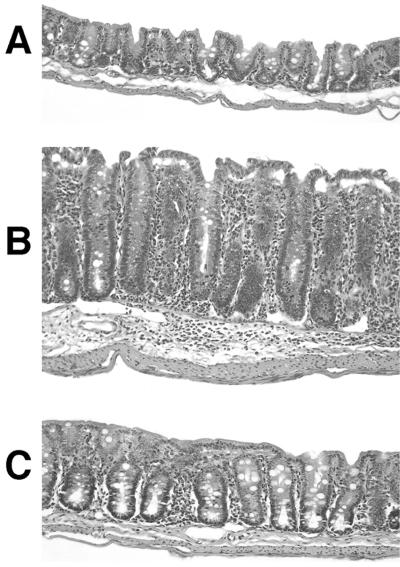
To investigate the role of IL-12 in the maintenance of established H. hepaticus-induced colitis, IL-10 KO mice infected 4 weeks earlier were treated every 3 to 4 days with 1 mg of neutralizing anti-IL-12 p40 MAb. After 4 weeks of treatment, mice were sacrificed, and large bowel inflammation was scored. Infected animals treated with anti-IL-12 showed a marked reduction in cecal inflammation (typhlitis) compared to mice receiving control MAb (Fig. 2A). The diminished inflammation observed in anti-IL-12-treated infected mice was characterized by diminished hyperplasia and reduced numbers of infiltrating cells in the lamina propria compared to those in animals receiving control MAb (Fig. 3).
FIG. 2.
Treatment with anti-IL-12 diminishes established H. hepaticus-induced colitis and SHelAg-induced IFN-γ responses in IL-10 KO mice. IL-10 KO mice were infected i.p. with H. hepaticus (black bars). Four weeks later, animals were treated with anti-IL-12 or control MAb for an additional 4 weeks before analysis. Uninfected (Uninf.) IL-10 KO mice (gray bars) were included as controls. (A) Pathology in the cecum. Bars represent mean histology scores ± SE of individual mice from three separate experiments with uninfected (n = 6), infected control MAb-treated (n = 9), and infected anti-IL-12-treated (n = 9) IL-10 KO mice. Median scores for the different groups were as follows: uninfected = 1 (range, 1 to 2), infected plus control MAb = 4 (range, 3 to 4), and infected plus anti-IL-12 = 2 (all mice scored as 2). Similar results were observed for the colon, although scores were lower (data not shown) (Fig. 8). Comparable effects of anti-IL-12 treatment on pathology were seen in a separate experiment (not shown) in which mice were infected by the i.g. route. (B and C) IFN-γ levels detected in 72-h supernatants from MLN cells (3 × 106/ml) stimulated with plate-bound anti-CD3 (B) or 0.3 μg of SHelAg per ml (C). Bars represent means ± SE of ELISA values from individual MLN cell suspensions from mice in two of the three experiments shown in panel A in which anti-CD3 and SHelAg stimulations were performed. No IFN-γ was detected from cells cultured in medium alone.
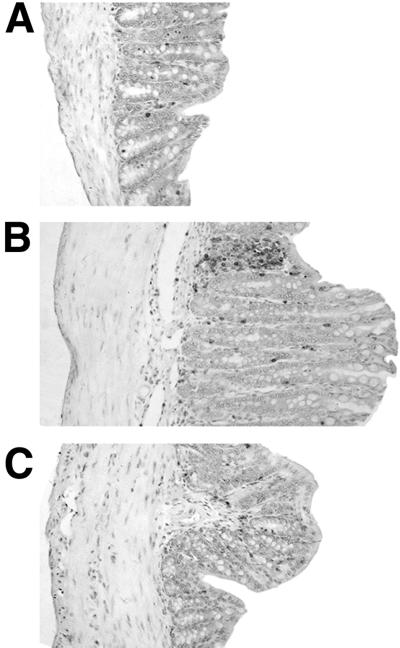
FIG. 3.
Reduced intestinal pathology after anti-IL-12 treatment of IL-10 KO mice with established H. hepaticus-induced cecal lesions. IL-10 KO mice were infected with H. hepaticus and treated with anti-IL-12 or control MAb between weeks 4 and 8 of infection. Shown are representative ceca of an uninfected IL-10 KO mouse (A) or infected IL-10 KO mouse treated with control MAb (B) or anti-IL-12 (C). All sections shown were located in the mid-cecum 1 to 1.5 cm from the cecal-colic junction on a longitudinal section of the entire cecum and represent scores of 1.0 (A), 3.5 (B), and 2.0 (C). Formalin-fixed tissues were stained with H&E. Magnification, ×110.
Diminished colitis in anti-IL-12-treated IL-10 KO mice with established disease correlates with reduced Helicobacter-associated Th1-cytokine responses in vitro and in vivo.
To further characterize the immune response in infected IL-10 KO mice with reduced intestinal inflammation after anti-IL-12 treatment of established disease, we measured IFN-γ as a marker of a Th1 response after in vitro stimulation of MLN cells with anti-CD3 or SHelAg. As previously reported for IL-10-deficient mice with spontaneous enterocolitis (10), MLN cells from anti-IL-12- and control MAb-treated IL-10 KO animals with established H. hepaticus-induced intestinal inflammation secreted comparable amounts of IFN-γ following anti-CD3 stimulation (Fig. 2B). Importantly, however, while infected control MAb-treated animals displayed a strong SHelAg-induced IFN-γ response, infected mice receiving anti-IL-12 secreted significantly reduced levels of this cytokine following bacterial Ag stimulation (Fig. 2C).
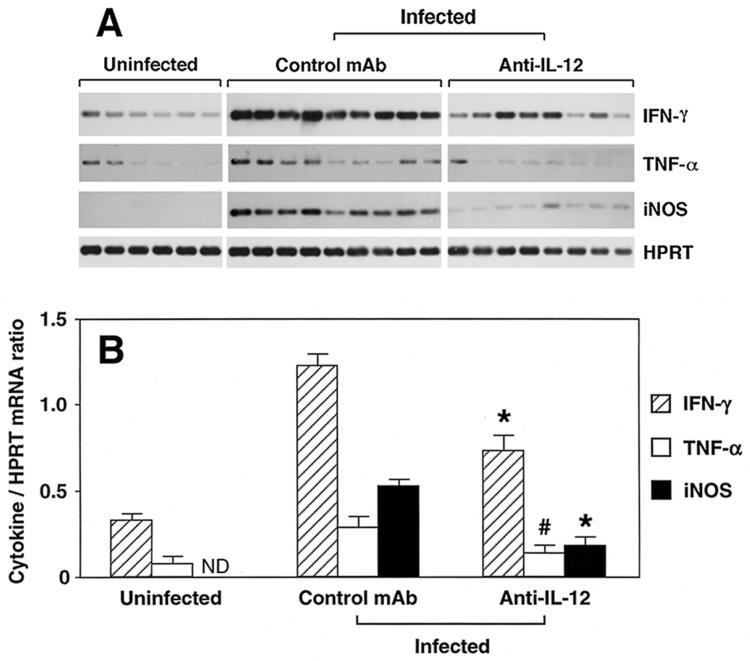
To analyze cytokine responses at the site of inflammation, ascending colon samples were collected, and various cytokines were analyzed by RT-PCR. Figure 4 shows pooled data from three independent experiments in which such analysis was performed. Compared to uninfected mice, infected animals treated with control MAb showed markedly increased mRNA levels of IFN-γ, TNF-α, and iNOS (Fig. 4). More importantly, however, infected IL-10 KO animals receiving anti-IL-12 once disease was established showed significantly reduced levels of mRNA for IFN-γ, TNF-α, and iNOS compared to the control MAb-treated animals (Fig. 4), suggesting a wholesale decrease in Th1-cell activity in these mice after anti-IL-12 treatment.
FIG. 4.
IFN-γ, TNF-α, and iNOS mRNA expression in colon of anti-IL-12-treated H. hepaticus-infected IL-10 KO mice. Colonic tissues from uninfected or H. hepaticus-infected IL-10 KO mice treated with control MAb or anti-IL-12 between weeks 4 and 8 of infection were collected and processed for RT-PCR analysis of IFN-γ, TNF-α, iNOS, and HPRT. (A) The results shown are autoradiographs after hybridization of PCR products with probes specific for IFN-γ, TNF-α, iNOS, or HPRT. (B) The intensities of the bands in panel A were measured, the cytokine/HPRT ratio was calculated for each mouse, and the mean ± SE for each group was determined. ND, not detected. Data are from the same individual mice in the three separate experiments shown in Fig. 2, with the exception of one anti-IL-12-treated mouse from which RNA preparation was not successful. * and #, P < 0.001 and P = 0.039, respectively, compared to control MAb-treated group. It should be noted that the data do not allow quantitative comparison between the cytokines, but only comparison of the same cytokine among groups of mice.
Anti-IL-12 treatment of IL-10 KO mice with established disease reduces the number of colonic CD3+ T cells and the frequency of Helicobacter-reactive IFN-γ-producing CD4+ Th1 cells.
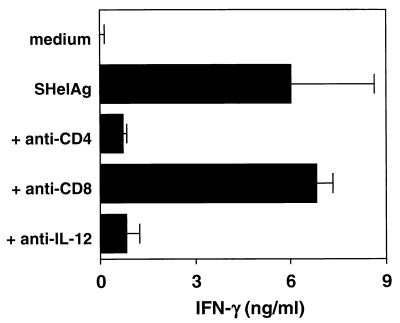
IL-12 is known to be required for optimal IFN-γ production by CD4+ T cells. Similarly, the SHelAg-induced IFN-γ response by MLN cells from infected IL-10 KO mice was shown to be dependent on CD4+ cells as well as IL-12, because addition of either anti-CD4 or anti-IL-12, but not anti-CD8 MAb to cultures completely abrogated the response (Fig. 5). Thus, to assess whether the reduced Th1-cell activity in animals treated in vivo with anti-IL-12 was due to blocking the ability of T cells to secrete cytokines and/or to a decrease in T-cell numbers, we performed immunohistochemical staining for CD3+ T cells on colonic tissue sections. Data from three independent experiments (n = 6 mice/group) showed that, compared to uninfected controls (12.5 ± 3.5 CD3+ cells/×40 field), infected IL-10 KO mice treated with control MAb showed an increase in the number of colonic T cells (38.0 ± 7.2 CD3+ cells/×40 field), and this number was significantly reduced in anti-IL-12-treated infected mice (20.3 ± 4.5 CD3+ cells/×40 field; P = 0.023 compared to control MAb-treated group). The colonic sections of infected control MAb-treated mice were characterized by hyperplasia and areas of higher T-cell density distributed irregularly throughout the lamina propria (Fig. 6). In colon sections of anti-IL-12-treated mice, these accumulations of CD3+ T cells were absent and reduced hyperplasia was observed (Fig. 6).
FIG. 5.
IL-12 and CD4 cell dependence of SHelAg-induced IFN-γ responses in H. hepaticus-infected IL-10 KO mice. IL-10 KO mice were infected i.p. with H. hepaticus, and cytokine responses were analyzed 7 weeks later. MLN cells (3 × 106/ml) were cultured in medium alone or with 0.3 μg of SHelAg per ml without or with the addition of anti-CD4, anti-CD8 (both at 10 μg/ml), or anti-IL-12 (20 μg/ml) as indicated. IFN-γ levels were measured in 72-h supernatants. Bars represent means ± standard deviations of duplicate ELISA values. The data shown are from one representative experiment of six performed (with 4- to 9-week-infected mice).
FIG. 6.
Immunostaining for CD3+ cells in colons of IL-10 KO mice, all with hematoxylin counterstain. IL-10 KO mice were infected with H. hepaticus and treated with anti-IL-12 or control MAb between weeks 4 and 8 of infection. Shown are representative colon sections of an uninfected IL-10 KO mouse (A) or infected IL-10 KO mouse treated with control MAb (B) or anti-IL-12 (C). Magnification, ×125.
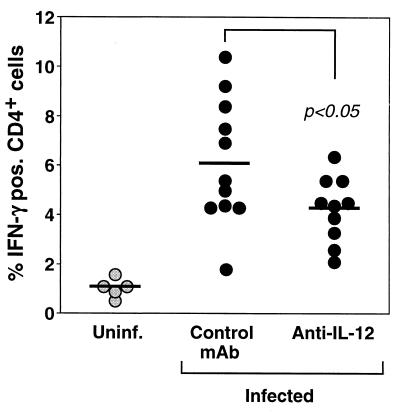
To analyze the effect of anti-IL-12 treatment on the generation of Helicobacter-reactive Th1 cells, the presence of IFN-γ-producing SHelAg-reactive CD4+ cells in MLN was analyzed by intracellular cytokine staining. After one round of in vitro stimulation of MLN cells with SHelAg, the frequency of IFN-γ-producing CD4+ cells in cultures from infected control MAb-treated mice was approximately sixfold increased compared to that of cells from uninfected animals (Fig. 7). Importantly, this frequency was significantly decreased in infected anti-IL-12-treated mice (Fig. 7).
FIG. 7.
Diminished frequency of SHelAg-induced IFN-γ-expressing CD4+ T cells in H. hepaticus-infected IL-10 KO mice treated with anti-IL-12. MLN cells from uninfected (Uninf.) or H. hepaticus-infected IL-10 KO mice treated with control MAb or anti-IL-12 between weeks 4 and 8 of infection were cultured for 72 h with SHelAg followed by overnight incubation in medium alone. Thereafter, cells were stimulated for 9 h with plate-bound anti-CD3 followed by an additional 5 h in the presence of brefeldin A. The cells were then stained with Cy-chrome-labeled anti-CD4 followed by PE-labeled anti-IFN-γ and analyzed by flow cytometry. Each dot indicates the percentage of CD4+ cells expressing IFN-γ from an individual mouse. The data are pooled from four separate experiments.
Anti-IFN-γ and/or anti-TNF-α treatment of IL-10 KO mice does not ameliorate established intestinal inflammation.
We have previously shown that treatment with neutralizing anti-IFN-γ from the start of the infection inhibits the development of colitis in IL-10 KO mice inoculated with H. hepaticus (25). To investigate the role of IFN-γ in disease maintenance, we treated colitic IL-10 KO mice with a MAb to this cytokine between weeks 4 and 8 of the infection. In contrast to anti-IL-12-treated animals, infected IL-10 KO mice treated in parallel with neutralizing anti-IFN-γ showed no or only minimal reduction in intestinal inflammation (cecal scores: uninfected, 1.5 ± 0.7; infected, 4.0 ± 0; infected plus control MAb, 3.8 ± 0.5; infected plus anti-IL-12, 2.0 ± 0; infected plus anti-IFN-γ, 3.0 ± 1.2; n = 4 mice/group). The anti-IFN-γ-treated mice also had similar or increased SHelAg-stimulated IFN-γ responses compared to control MAb-treated animals as measured in supernatants from MLN cultures or by intracellular staining (not shown). One possible explanation for these results could be that blockade of other Th1 cytokines, in particular TNF-α, is required to reduce pathology. Thus, to explore this possibility, a separate experiment was performed in which groups of five mice were treated with anti-IL-12, anti-IFN-γ, anti-TNF-α, or a combination of anti-IFN-γ and anti-TNF-α between weeks 4 and 8 of H. hepaticus infection. When histology scores were analyzed, only the group treated with anti-IL-12 showed a reduction in intestinal inflammation (Fig. 8A and B). The same pattern was observed when cytokine mRNA levels were analyzed at the site of inflammation. Thus, only the group given anti-IL-12 showed a significant reduction in colonic mRNA levels for IFN-γ, TNF-α, and iNOS, while anti-IFN-γ and/or anti-TNF-α treatment had no effect on the mRNA levels of these factors (Fig. 8C to E).
IFN-γ is not absolutely required for the development of H. hepaticus-induced colitis.
As shown in our original study, treatment with anti-IFN-γ blocks intestinal inflammation when given to IL-10 KO mice between weeks 0 and 4 of H. hepaticus infection (25). Our present data suggest a less important role for IFN-γ once disease is established, because IL-10-deficient mice given anti-IFN-γ between weeks 4 and 8 of infection showed no reduction in intestinal pathology. To analyze if Helicobacter-induced colitis can develop in the absence of IFN-γ, we next analyzed H. hepaticus infection in IL-10/IFN-γ double-deficient mice and found that these IL-10/IFN-γ KO animals were as susceptible to disease as IL-10 single-deficient mice (Fig. 9A). Histological examination revealed no evident differences in terms of cell types infiltrating the intestine in the two groups of colitic mice (not shown). These data suggested to us the existence of alternative pathways for the development of colitis following H. hepaticus infection. As expected, MLN cells from infected IL-10 KO mice secreted large amounts of IFN-γ when SHelAg-induced Th1-cytokine responses were analyzed (Fig. 9B). Importantly, MLN cells from infected IL-10/IFN-γ-deficient mice produced IL-12 following SHelAg stimulation in amounts comparable to those of infected IL-10 KO animals (Fig. 9C). Moreover, the IL-10/IFN-γ KO mice showed a dramatically increased SHelAg-specific TNF-α response compared to that of the IL-10-deficient animals (Fig. 9D).
FIG. 9.
IL-10/IFN-γ KO mice develop colitis following inoculation with H. hepaticus. WT, IL-10 KO, IL-10/IL-12 KO, and IL-10/IFN-γ KO animals were infected i.g. with H. hepaticus (black bars), and intestinal pathology and cytokine responses were analyzed 4 weeks later. Gray bars represent uninfected controls. (A) Pathology in the cecum. Bars represent mean histology scores ± SE of 3 to 5 mice/group, with the following median values for infected mice: WT = 1 (all mice scored as 1, n = 3), IL-10 KO = 3 (range, 3 to 3.5, n = 4), IL-10/IL-12 KO = 1 (range, 1 to 1.5, n = 4), and IL-10/IFN-γ KO = 4 (range, 3 to 4, n = 5). Similar results were observed for colon, although scores were lower (not shown). (B to D) IFN-γ (B), IL-12 (C), and TNF-α (D) levels detected in 72-h supernatants from MLN cell cultures stimulated with SHelAg. Bars represent means ± SD of duplicate ELISA values from MLN cells pooled from 3 to 5 mice/group. The data shown are from one representative experiment of two performed. ND, not detected. No IFN-γ, IL-12, or TNF-α was detected from cells cultured in medium alone, and no IL-4 was detected in supernatants from SHelAg-stimulated cultures.
DISCUSSION
In the present study, we have demonstrated a crucial role for IL-12 in both disease induction and maintenance in the murine model of IBD involving H. hepaticus infection of IL-10 KO mice. The mechanism by which IL-12 exerts its function involves the generation and maintenance of pathogenic Th1 cells as well as sustaining their numbers at the site of inflammation.
We have previously shown that administration of anti-IL-12 or anti-IFN-γ MAb from the start of the infection prevents colitis in IL-10 KO animals receiving H. hepaticus (25). To further investigate the requirement for these cytokines in disease induction, we have utilized for the first time in studies of IBD mice doubly deficient for IL-10 and IL-12, IL-10 and IFN-γ, and IL-10 and IL-4. The IL-10/IL-12 double-deficient mice showed no intestinal pathology after H. hepaticus inoculation, and levels of IFN-γ were undetectable in culture supernatants from MLN cells stimulated with SHelAg. In contrast, both IL-10/IFN-γ- and IL-10/IL-4-deficient animals developed colitis following H. hepaticus challenge. Importantly, H. hepaticus infection of IL-4 KO mice did not result in intestinal inflammation, confirming that IL-10, and not IL-4, is the critical cytokine for protection against Helicobacter-induced colitis, as well as demonstrating that IL-4 is not required for the generation of this IL-10-dependent mechanism.
To evaluate the importance of IL-12 for disease maintenance, 4-week H. hepaticus-infected IL-10 KO animals were treated with MAb to this cytokine for an additional 4 weeks before intestinal pathology was analyzed. Following this protocol, anti-IL-12-treated infected IL-10 KO mice showed a significant reduction in intestinal inflammation compared to control MAb-treated animals. These results agree with those of Neurath et al. (27) and Davidson et al. (10), who demonstrated a role for anti-IL-12 in reversing 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced colitis in BALB/c mice and the spontaneous enterocolitis of IL-10 KO mice, respectively. The reduced pathology observed after anti-IL-12 treatment of H. hepaticus-infected IL-10 KO mice with established colitis correlated with reduced levels of mRNA for IFN-γ, TNF-α, and iNOS in the colon. That anti-IL-12 was not merely acting by blocking cytokine secretion by Th1 cells was confirmed by immunohistochemical staining of colon showing reduced numbers of lamina propria-infiltrating CD3+ cells in the mice receiving anti-IL-12 MAb. An additional piece of evidence linking the presence of H. hepaticus-reactive pathogenic Th1 cells with intestinal disease was the finding of significantly reduced frequencies of SHelAg-specific IFN-γ-secreting CD4+ T lymphocytes in MLN of anti-IL-12-treated infected mice recovering from colitis. Importantly, analysis of culture supernatants from these MLN cells stimulated in vitro with SHelAg showed a significantly diminished IFN-γ response to the bacterium, but not to anti-CD3 stimulation, implying selective loss of H. hepaticus-specific CD4+ T lymphocytes. Fuss et al. (19) have reported that the main effect of anti-IL-12 on established TNBS colitis is the induction of Fas-mediated apoptosis of the Th1 cells causing inflammation. Although we cannot rule out that anti-IL-12 induced apoptosis in our model as well, attempts to detect terminal deoxynucleotidyltransferase-mediated deoxyuridine triphosphate nick end-labeling (TUNEL)-positive cells in the anti-IL-12-treated mice in the present study have so far been unsuccessful. It is also important to point out that the recently described cytokine IL-23, composed of the p19 protein in combination with the p40 subunit of IL-12, induces strong proliferation of mouse memory CD4+ T cells (28). Thus, it is possible that the beneficial effect of the anti-IL-12 p40 MAb used in these and previous colitis studies is associated with its ability to neutralize IL-23.
In the case of TNBS colitis, anti-IL-12 treatment frequently abrogated the established inflammation completely (27). In contrast, we observed that Helicobacter-infected anti-IL-12-treated IL-10 KO mice showed some degree of residual inflammation compared to uninfected controls. Even if the anti-IL-12 treatment was continued for six additional injections (total of 7 weeks of MAb treatment of established colitis), no further reduction in pathology was observed (not shown). Similar results have been reported for the spontaneous enterocolitis in IL-10 KO mice in which anti-IL-12 treatment, either alone or combined with daily administrations of recombinant IL-10, did not reverse disease completely (10). One important difference between the colitis models triggered by TNBS versus those triggered by bacterial flora is the continuous presence of the agent provoking disease. Thus, in the case of colitis induced by TNBS, this molecule is given at a single time and subsequently disappears from the body. In contrast, in the IL-10 KO colitis model, H. hepaticus or other flora are continuously present, thereby constantly recruiting new bacterium-specific Th cells from the pool of naïve cells. Consequently, it may not be surprising that, although ameliorating disease, anti-IL-12 administration will not reduce the inflammation to levels observed in uninfected mice, because this treatment preferentially affects activated T cells.
To further dissect the mechanism behind disease reversal following anti-IL-12 treatment, we used neutralizing MAbs to IFN-γ and/or TNF-α in vivo. However, neither MAb, alone or in combination, reduced the inflammation in infected IL-10 KO mice with established disease. Although we cannot exclude the possibility that IFN-γ and TNF-α were not completely neutralized in these experiments, the inability of anti-IFN-γ and anti-TNF-α to reverse disease is consistent with findings from other colitis models in which these MAbs were tested separately (5, 10, 19, 31). Similar to our data obtained with anti-IFN-γ, we have found that treatment of IL-10 KO mice with one of the iNOS inhibitors aminoguanidine or l-N6-(1-iminoethyl)-lysine (L-NIL) from the start of H. hepaticus infection blocked the development of colitis, whereas administration of aminoguanidine to IL-10 KO mice with established disease had no effect (A.G.R. and M.C.K., unpublished results). Thus, it appears that neutralization of cytokines produced by T cells (such as IFN-γ and TNF-α) and their downstream effectors (e.g., NO) is not enough to block the inflammatory process once established. Rather, the absolute number of pathogenic Th1 cells would have to be diminished in order to reduce the inflammation. The precise mechanism by which these intestinal T cells sustain the inflammation remains unknown. Using the TNBS colitis model, Stüber et al. (40) have demonstrated that the CD40-CD40L interaction is crucial for the in vivo priming of Th1 cells via the stimulation of IL-12 secretion by antigen-presenting cells (APCs). Recent data have shown that blockade of the CD40-CD40L interaction by anti-CD154 MAb is efficient also in reducing established colitis in T-cell-reconstituted SCID or RAG KO mice and in bone marrow-transplanted Tgε26 mice (11, 26). The authors reported reduced IL-12 production and speculate that the anti-CD154 treatment blocks T cells in their ability to activate APC, thereby impairing the development of colitis, possibly through interference with T-cell-dependent macrophage activation and/or cytokine secretion.
Our finding that IL-10/IFN-γ double-deficient mice were as susceptible to Helicobacter-induced colitis as IL-10 KO animals indicates that IFN-γ is not required for disease induction. Similarly, in the two colitis models of Tgε26 mice and CD45RBhi cell transfer into RAG KO mice, recipients reconstituted with T cells from IFN-γ KO animals developed intestinal inflammation comparable in severity to that observed in mice receiving wild-type cells (38). IFN-γ also appears to be dispensable for disease induction in the TNBS colitis model, because both BALB/c IFN-γ KO and 129/Sv/Ev IFN-γR1 KO mice developed disease following administration of this hapten (7, 15). Despite the absence of IFN-γ, the intestinal inflammation in H. hepaticus-infected IL-10/IFN-γ KO animals was characterized by the same type of cell infiltrates as those of single IL-10-deficient mice. In addition, both strains of mice displayed a Th1 response to the bacterium. The discrepancy between the IL-10/IFN-γ KO animal experiments and our initial observation that anti-IFN-γ MAb given to IL-10 KO mice blocks disease when given from the start of the infection could possibly be explained by the qualitatively different cytokine profiles mounted by these two mouse strains. Thus, in IL-10 KO mice, IFN-γ may be the crucial cytokine for disease development, while in IL-10/IFN-γ KO animals, TNF-α may play the important role, because this is the dominant cytokine produced after encounter with the bacterium. Importantly, MLN cells from both colitic IL-10 KO and IL-10/IFN-γ KO mice infected with H. hepaticus secreted IL-12 following SHelAg stimulation, suggesting an important function for this cytokine in disease induction regardless of the absence or presence of IFN-γ.
In humans, anti-TNF-α therapy appears to be the most effective treatment to date for patients with Crohn's disease and ulcerative colitis (3). Interestingly, in a recent report, the beneficial effects of anti-TNF-α treatment were described as being attributed not merely to TNF neutralization, but rather to a more general effect on T lymphocytes, raising new possibilities of combined anticytokine and anti-T-cell strategies to treat these chronic diseases (30). Similarly, data from several different colitis models have now demonstrated that neutralization of cytokines produced by T cells may not be sufficient to block an ongoing inflammatory process in the gut. Rather, it appears crucial to reduce the number of pathogenic cells at the site of inflammation to see improvement of disease. Our data are consistent with these conclusions and add a further dimension to the scenario by demonstrating a reduction in H. hepaticus-reactive, presumably pathogenic CD4+ Th1 cells in MLNs of mice recovering from colitis. Further studies on the nature and mechanism of action of these bacteria-reactive T lymphocytes may lead to new strategies for therapeutic interventions involving targeting of microbe-specific T cells in the treatment of patients with IBD.
ACKNOWLEDGMENTS
We are grateful to Sara Hieny for preparation of anti-cytokine MAbs used for in vivo neutralizations, Barbara Kasprzak for assistance with immunohistochemical stainings, Ricardo Dreyfuss for help with photomicrographs, and Shyla Jagannatha for helpful assistance with statistical evaluation of our data. We also thank Ivan Fuss, Warren Strober, Jerrold Ward, and George Yap for helpful discussions and for critical reading of the manuscript.
REFERENCES
- 1.Abrams J S, Roncarolo M G, Yssel H, Andersson U, Gleich G J, Silver J E. Strategies of anti-cytokine monoclonal antibody development: immunoassay of IL-10 and IL-5 in clinical samples. Immunol Rev. 1992;127:5–24. doi: 10.1111/j.1600-065x.1992.tb01406.x. [DOI] [PubMed] [Google Scholar]
- 2.Aranda R, Sydora B C, McAllister P L, Binder S W, Yang H Y, Targan S R, Kronenberg M. Analysis of intestinal lymphocytes in mouse colitis mediated by transfer of CD4+, CD45RBhigh T cells to SCID recipients. J Immunol. 1997;158:3464–3473. [PubMed] [Google Scholar]
- 3.Baert F J, Rutgeerts P J. Medical therapies for ulcerative colitis and Crohn's disease. Curr Gastroenterol Rep. 2000;2:446–450. doi: 10.1007/s11894-000-0006-z. [DOI] [PubMed] [Google Scholar]
- 4.Battles J K, Williamson J C, Pike K M, Gorelick P L, Ward J M, Gonda M A. Diagnostic assay for Helicobacter hepaticus based on nucleotide sequence of its 16S rRNA gene. J Clin Microbiol. 1995;33:1344–1347. doi: 10.1128/jcm.33.5.1344-1347.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berg D J, Davidson N, Kühn R, Müller W, Menon S, Holland G, Thompson-Snipes L, Leach M W, Rennick D. Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4+ TH1-like responses. J Clin Investig. 1996;98:1010–1020. doi: 10.1172/JCI118861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blaser M J, Miller R A, Lacher J, Singleton J W. Patients with active Crohn's disease have elevated serum antibodies to antigens of seven enteric bacterial pathogens. Gastroenterology. 1984;87:888–894. [PubMed] [Google Scholar]
- 7.Camoglio L, te Velde A A, de Boer A, ten Kate F J, Kopf M, van Deventer S J. Hapten-induced colitis associated with maintained Th1 and inflammatory responses in IFN-γ receptor-deficient mice. Eur J Immunol. 2000;30:1486–1495. doi: 10.1002/(SICI)1521-4141(200005)30:5<1486::AID-IMMU1486>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 8.Campieri M, Gionchetti P. Probiotics in inflammatory bowel disease: new insight to pathogenesis or a possible therapeutic alternative? Gastroenterology. 1999;116:1246–1249. doi: 10.1016/s0016-5085(99)70029-6. [DOI] [PubMed] [Google Scholar]
- 9.Cherwinski H M, Schumacher J H, Brown K D, Mosmann T R. Two types of mouse helper T cell clone. III. Further differences in lymphokine synthesis between Th1 and Th2 clones revealed by RNA hybridization, functionally monospecific bioassays, and monoclonal antibodies. J Exp Med. 1987;166:1229–1244. doi: 10.1084/jem.166.5.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davidson N J, Hudak S A, Lesley R E, Menon S, Leach M W, Rennick D M. IL-12, but not IFN-γ, plays a major role in sustaining the chronic phase of colitis in IL-10-deficient mice. J Immunol. 1998;161:3143–3149. [PubMed] [Google Scholar]
- 11.De Jong Y P, Comiskey M, Kalled S L, Mizoguchi E, Flavell R A, Bhan A K, Terhorst C. Chronic murine colitis is dependent on the CD154/CD40 pathway and can be attenuated by anti-CD154 administration. Gastroenterology. 2000;119:715–723. doi: 10.1053/gast.2000.16485. [DOI] [PubMed] [Google Scholar]
- 12.Dialynas D P, Quan Z S, Wall K A, Pierres A, Quintans J, Loken M R, Pierres M, Fitch F W. Characterization of the murine T cell surface molecule, designated L3T4, identified by monoclonal antibody GK1.5: similarity of L3T4 to the human Leu-3/T4 molecule. J Immunol. 1983;131:2445–2451. [PubMed] [Google Scholar]
- 13.Dianda L, Hanby A M, Wright N A, Sebesteny A, Hayday A C, Owen M J. T cell receptor-αβ-deficient mice fail to develop colitis in the absence of a microbial environment. Am J Pathol. 1997;150:91–97. [PMC free article] [PubMed] [Google Scholar]
- 14.Dieleman L A, Arends A, Tonkonogy S L, Goerres M S, Craft D W, Grenther W, Sellon R K, Balish E, Sartor R B. Helicobacter hepaticus does not induce or potentiate colitis in interleukin-10-deficient mice. Infect Immun. 2000;68:5107–5113. doi: 10.1128/iai.68.9.5107-5113.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dohi T, Fujihashi K, Rennert P D, Iwatani K, Kiyono H, McGhee J R. Hapten-induced colitis is associated with colonic patch hypertrophy and T helper cell 2-type responses. J Exp Med. 1999;189:1169–1180. doi: 10.1084/jem.189.8.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elsaghier A, Prantera C, Moreno C, Ivanyi J. Antibodies to Mycobacterium paratuberculosis-specific protein antigens in Crohn's disease. Clin Exp Immunol. 1992;90:503–508. doi: 10.1111/j.1365-2249.1992.tb05874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fox J G, Dewhirst F E, Tully J G, Paster B J, Yan L, Taylor N S, Collins M J, Jr, Gorelick P L, Ward J M. Helicobacter hepaticus sp. nov., a microaerophilic bacterium isolated from livers and intestinal mucosal scrapings from mice. J Clin Microbiol. 1994;32:1238–1245. doi: 10.1128/jcm.32.5.1238-1245.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fox J G, Gorelick P L, Kullberg M C, Ge Z, Dewhirst F E, Ward J M. A novel urease-negative Helicobacter species associated with colitis and typhlitis in IL-10-deficient mice. Infect Immun. 1999;67:1757–1762. doi: 10.1128/iai.67.4.1757-1762.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fuss I J, Marth T, Neurath M F, Pearlstein G R, Jain A, Strober W. Anti-interleukin 12 treatment regulates apoptosis of Th1 T cells in experimental colitis in mice. Gastroenterology. 1999;117:1078–1088. doi: 10.1016/s0016-5085(99)70392-6. [DOI] [PubMed] [Google Scholar]
- 20.Gionchetti P, Rizzello F, Venturi A, Brigidi P, Matteuzzi D, Bazzocchi G, Poggioli G, Miglioli M, Campieri M. Oral bacteriotherapy as maintenance treatment in patients with chronic pouchitis: a double-blind, placebo-controlled trial. Gastroenterology. 2000;119:305–309. doi: 10.1053/gast.2000.9370. [DOI] [PubMed] [Google Scholar]
- 21.Gionchetti P, Rizzello F, Venturi A, Ugolini F, Rossi M, Brigidi P, Johansson R, Ferrieri A, Poggioli G, Campieri M. Review—antibiotic treatment in inflammatory bowel disease: rifaximin, a new possible approach. Eur Rev Med Pharmacol Sci. 1999;3:27–30. [PubMed] [Google Scholar]
- 22.Hoffmann K F, James S L, Cheever A W, Wynn T A. Studies with double cytokine-deficient mice reveal that highly polarized Th1- and Th2-type cytokine and antibody responses contribute equally to vaccine-induced immunity to Schistosoma mansoni. J Immunol. 1999;163:927–938. [PubMed] [Google Scholar]
- 23.Jankovic D, Kullberg M C, Noben-Trauth N, Caspar P, Paul W E, Sher A. Single cell analysis reveals that IL-4 receptor/Stat6 signaling is not required for the in vivo or in vitro development of CD4+ lymphocytes with a Th2 cytokine profile. J Immunol. 2000;164:3047–3055. doi: 10.4049/jimmunol.164.6.3047. [DOI] [PubMed] [Google Scholar]
- 24.Kühn R, Löhler J, Rennick D, Rajewsky K, Müller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 25.Kullberg M C, Ward J M, Gorelick P L, Caspar P, Hieny S, Cheever A, Jankovic D, Sher A. Helicobacter hepaticus triggers colitis in specific-pathogen-free interleukin-10 (IL-10)-deficient mice through an IL-12- and gamma interferon-dependent mechanism. Infect Immun. 1998;66:5157–5166. doi: 10.1128/iai.66.11.5157-5166.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Z, Geboes K, Colpaert S, Overbergh L, Mathieu C, Heremans H, de Boer M, Boon L, D'Haens G, Rutgeerts P, Ceuppens J L. Prevention of experimental colitis in SCID mice reconstituted with CD45RBhigh CD4+ T cells by blocking the CD40-CD154 interactions. J Immunol. 2000;164:6005–6014. doi: 10.4049/jimmunol.164.11.6005. [DOI] [PubMed] [Google Scholar]
- 26a.National Institutes of Health. Guide for the care and use of laboratory animals. Bethesda, Md: National Institute of Allergy and Infectious Diseases, National Institutes of Health; 1996. [Google Scholar]
- 27.Neurath M F, Fuss I, Kelsall B L, Stuber E, Strober W. Antibodies to interleukin 12 abrogate established experimental colitis in mice. J Exp Med. 1995;182:1281–1290. doi: 10.1084/jem.182.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oppmann B, Lesley R, Blom B, Timans J C, Xu Y, Hunte B, Vega F, Yu N, Wang J, Singh K, Zonin F, Vaisberg E, Churakova T, Liu M, Gorman D, Wagner J, Zurawski S, Liu Y, Abrams J S, Moore K W, Rennick D, de Waal-Malefyt R, Hannum C, Bazan J F, Kastelein R A. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13:715–725. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- 29.Powrie F. T cells in inflammatory bowel disease: protective and pathogenic roles. Immunity. 1995;3:171–174. doi: 10.1016/1074-7613(95)90086-1. [DOI] [PubMed] [Google Scholar]
- 30.Raza A. Anti-TNF therapies in rheumatoid arthritis, Crohn's disease, sepsis, and myelodysplastic syndromes. Microsc Res Tech. 2000;50:229–235. doi: 10.1002/1097-0029(20000801)50:3<229::AID-JEMT6>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 31.Rennick D M, Fort M M, Davidson N J. Studies with IL-10−/− mice: an overview. J Leukoc Biol. 1997;61:389–396. doi: 10.1002/jlb.61.4.389. [DOI] [PubMed] [Google Scholar]
- 32.Rutgeerts P, Hiele M, Geboes K, Peeters M, Penninckx F, Aerts R, Kerremans R. Controlled trial of metronidazole treatment for prevention of Crohn's recurrence after ileal resection. Gastroenterology. 1995;108:1617–1621. doi: 10.1016/0016-5085(95)90121-3. [DOI] [PubMed] [Google Scholar]
- 33.Sadlack B, Merz H, Schorle H, Schimpl A, Feller A C, Horak I. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell. 1993;75:253–261. doi: 10.1016/0092-8674(93)80067-o. [DOI] [PubMed] [Google Scholar]
- 34.Sarmiento M, Glasebrook A L, Fitch F W. IgG or IgM monoclonal antibodies reactive with different determinants on the molecular complex bearing Lyt 2 antigen block T cell-mediated cytolysis in the absence of complement. J Immunol. 1980;125:2665–2672. [PubMed] [Google Scholar]
- 35.Sartor R B. Pathogenesis and immune mechanisms of chronic inflammatory bowel diseases. Am J Gastroenterol. 1997;92:5.S–11.S. [PubMed] [Google Scholar]
- 36.Schultz M, Tonkonogy S L, Sellon R K, Veltkamp C, Godfrey V L, Kwon J, Grenther W B, Balish E, Horak I, Sartor R B. IL-2-deficient mice raised under germfree conditions develop delayed mild focal intestinal inflammation. Am J Physiol. 1999;276:G1461–G1472. doi: 10.1152/ajpgi.1999.276.6.G1461. [DOI] [PubMed] [Google Scholar]
- 37.Sellon R K, Tonkonogy S, Schultz M, Dieleman L A, Grenther W, Balish E, Rennick D M, Sartor R B. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect Immun. 1998;66:5224–5231. doi: 10.1128/iai.66.11.5224-5231.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simpson S J, Shah S, Comiskey M, de Jong Y P, Wang B, Mizoguchi E, Bhan A K, Terhorst C. T cell-mediated pathology in two models of experimental colitis depends predominantly on the interleukin 12/signal transducer and activator of transcription (Stat)-4 pathway, but is not conditional on interferon γ expression by T cells. J Exp Med. 1998;187:1225–1234. doi: 10.1084/jem.187.8.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Strober W, Ehrhardt R O. Chronic intestinal inflammation: an unexpected outcome in cytokine or T cell receptor mutant mice. Cell. 1993;75:203–205. doi: 10.1016/0092-8674(93)80062-j. [DOI] [PubMed] [Google Scholar]
- 40.Stüber E, Strober W, Neurath M. Blocking the CD40L-CD40 interaction in vivo specifically prevents the priming of T helper 1 cells through the inhibition of interleukin 12 secretion. J Exp Med. 1996;183:693–698. doi: 10.1084/jem.183.2.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sutton C L, Kim J, Yamane A, Dalwadi H, Wei B, Landers C, Targan S R, Braun J. Identification of a novel bacterial sequence associated with Crohn's disease. Gastroenterology. 2000;119:23–31. doi: 10.1053/gast.2000.8519. [DOI] [PubMed] [Google Scholar]
- 42.Taurog J D, Richardson J A, Croft J T, Simmons W A, Zhou M, Fernandez-Sueiro J L, Balish E, Hammer R E. The germfree state prevents development of gut and joint inflammatory disease in HLA-B27 transgenic rats. J Exp Med. 1994;180:2359–2364. doi: 10.1084/jem.180.6.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tiveljung A, Soderholm J D, Olaison G, Jonasson J, Monstein H J. Presence of eubacteria in biopsies from Crohn's disease inflammatory lesions as determined by 16S rRNA gene-based PCR. J Med Microbiol. 1999;48:263–268. doi: 10.1099/00222615-48-3-263. [DOI] [PubMed] [Google Scholar]
- 44.Venturi A, Gionchetti P, Rizzello F, Johansson R, Zucconi E, Brigidi P, Matteuzzi D, Campieri M. Impact on the composition of the faecal flora by a new probiotic preparation: preliminary data on maintenance treatment of patients with ulcerative colitis. Aliment Pharmacol Ther. 1999;13:1103–1108. doi: 10.1046/j.1365-2036.1999.00560.x. [DOI] [PubMed] [Google Scholar]
- 45.Ward J M, Fox J G, Anver M R, Haines D C, George C V, Collins M J, Jr, Gorelick P L, Nagashima K, Gonda M A, Gilden R V, Tully J G, Russell R J, Benveniste R E, Paster B J, Dewhirst F E, Donovan J C, Anderson L M, Rice J M. Chronic active hepatitis and associated liver tumors in mice caused by a persistent bacterial infection with a novel Helicobacter species. J Natl Cancer Inst. 1994;86:1222–1227. doi: 10.1093/jnci/86.16.1222. [DOI] [PubMed] [Google Scholar]
- 46.Wynn T A, Eltoum I, Cheever A W, Lewis F A, Gause W C, Sher A. Analysis of cytokine mRNA expression during primary granuloma formation induced by eggs of Schistosoma mansoni. J Immunol. 1993;151:1430–1440. [PubMed] [Google Scholar]
- 47.Wynn T A, Eltoum I, Oswald I P, Cheever A W, Sher A. Endogenous interleukin 12 (IL-12) regulates granuloma formation induced by eggs of Schistosoma mansoni and exogenous IL-12 both inhibits and prophylactically immunizes against egg pathology. J Exp Med. 1994;179:1551–1561. doi: 10.1084/jem.179.5.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wynn T A, Morawetz R, Scharton-Kersten T, Hieny S, Morse III H C, Kuhn R, Muller W, Cheever A W, Sher A. Analysis of granuloma formation in double cytokine-deficient mice reveals a central role for IL-10 in polarizing both T helper cell 1- and T helper cell 2-type cytokine responses in vivo. J Immunol. 1997;159:5014–5023. [PubMed] [Google Scholar]
- 49.Wysocka M, Kubin M, Vieira L Q, Ozmen L, Garotta G, Scott P, Trinchieri G. Interleukin-12 is required for interferon-γ production and lethality in lipopolysaccharide-induced shock in mice. Eur J Immunol. 1995;25:672–676. doi: 10.1002/eji.1830250307. [DOI] [PubMed] [Google Scholar]