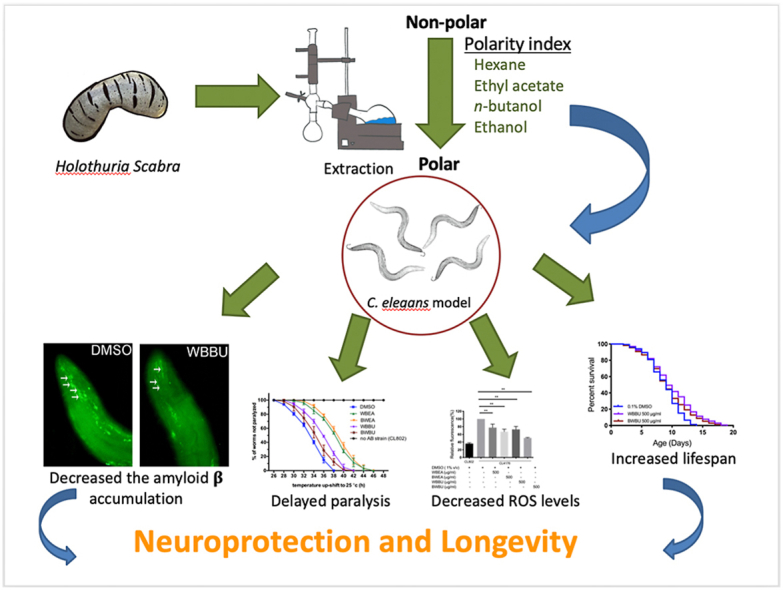
Abstract
Background and aim
Alzheimer's disease (AD) is the most common aged-related neurodegenerative disorder that is associated with the toxic amyloid-β (Aβ) aggregation in the brain. While the efficacies of available drugs against AD are still limited, natural products have been shown to possess neuroprotective potential for prevention and therapy of AD. This study aimed to investigate the neuroprotective effects of H. scabra extracts against Aβ aggregation and proteotoxicity in C. elegans model of Alzheimer's diseases.
Experimental procedure
Whole bodies (WB) and body wall (BW) of H. scabra were extracted and fractionated into ethyl acetate (WBEA, BWEA), butanol (WBBU, BWBU), and ethanol (BWET). Then C. elegans AD models were treated with these fractions and investigated for Aβ aggregation and polymerization, biochemical and behavioral changes, and level of oxidative stress, as well as lifespan extension.
Results and conclusion
C. elegans AD model treated with H. scabra extracts, especially triterpene glycoside-rich ethyl acetate and butanol fractions, exhibited significant reduction of Aβ deposition. These H. scabra extracts also attenuated the paralysis behavior and improved the neurological defects in chemotaxis caused by Aβ aggregation. Immunoblot analysis revealed decreased level of Aβ oligomeric forms and the increased level of Aβ monomers after treatments with H. scabra extracts. In addition, H. scabra extracts reduced reactive oxygen species and increased the mean lifespan of the treated AD worms. In conclusion, this study demonstrated strong evidence of anti-Alzheimer effects by H. scabra extracts, implying that these extracts can potentially be applied as natural preventive and therapeutic agents for AD.
Taxonomy (classification by EVISE)
Alzheimer's disease, Neurodegenerative disorder, Traditional medicine, Experimental model systems, Molecular biology.
Keywords: Alzheimer's disease, Amyloid-β, Neuroprotection, Sea cucumber, Caenorhabditis elegans
Graphical abstract
Highlights
-
•
H. scabra extracts significantly reduced amyloid-β deposition in transgenic C. elegans model of Alzheimer's disease.
-
•
H. scabra extracts attenuated the paralysis and improved the chemotaxis behavior caused by amyloid-β aggregation.
-
•
H. scabra extracts decreased levels of amyloid-β oligomeric and high molecular weight forms.
-
•
H.scabra extracts reduced the reactive oxygen species and increased the lifespan of transgenic C. elegans AD models.
1. Introduction
Alzheimer's disease (AD) is the most progressive neurodegenerative disease associated with aging. AD is clinically characterized by progressive impairment of cognitive function and degeneration of brain neurons due to aggregation of toxic amyloid-β (Aβ1-42) protein that can deposit either intracellularly in neurons or extracellularly in nearby areas. The Aβ1-42 peptides are derived from amyloid precursor protein (APP) through sequentially cleavage by β- and γ-secretases and self-polymerized into dimers or oligomers, which finally aggregate into Aβ plaques that cause oxidative damage and disrupt neuronal functions.1, 2, 3 From the World Alzheimer Report in 2016, more than 46 million people are suffering from AD worldwide. The number of AD patients are estimated to increase and reach 74.7 million in 2030. And if there is no effective preventive and therapeutic measures the number of AD patients is estimated to reach more than 130 million by 2050. Thus, AD is now becoming a major public health concern around the world,3 and the third major cause of disability and death of elderly population. Nowadays, there are only few drugs approved for the treatment of AD, and these drugs only relief the symptoms and are unable to directly eliminate the cause of AD or slow the rate of its progression.4 Therefore, research and discoveries of more effective anti-AD drugs are urgently needed.
Recently, certain natural products from plants and animals with anti-AD property have been discovered. Saponin or chemicals with similar structure from plant extracts including EGb761 from Ginkgo biloba,5 ginsenosides from Panax ginseng,6 and frondoside A from the sea cucumber, Cucumaria frondose,7 have been shown to protect against Aβ-induced toxicity and inhibited Aβ deposition in Caenorhabditis elegans model.8,9 Moreover, natural polyphenols from some edible plants were shown to possess neuroprotective properties in AD nematode models, including a stilbenoid, resveratrol, from grapes,10 and curcuminoid from curcumin.11 Interestingly, some of these compounds especially triterpene glycosides and polyphenols were also presented in the marine animals such as sea cucumbers.12,13 Sea cucumbers have long been used as traditional food and folk medicine by Asian and Middle Eastern communities since the ancient time.14, 15, 16 In Chinese historical accounts, sea cucumbers are recognized as tonics and traditional remedies because of their effectiveness against hypertension, asthma, rheumatism, cuts and burns, impotence, and constipation.17 The bioactive extracts/compounds of sea cucumber have also been shown to possess a wide-range of pharmacological benefits including anti-oxidant, anti-tumor, anti-viral, anti-inflammation, anti-bacterial, and anti-fungal activities.12 The white sea cucumber, Holothuria scabra, is the most plentiful species of sea cucumber found in the Indo-Pacific region including Thailand. This species of sea cucumber contains high amounts of triterpene glycosides in its body wall.18 Our group has previously studied and found that H. scabra extracts promote longevity and anti-aging effects in C. elegans model.19 Moreover, we have reported an anti-Parkinson potential of H. scabra extracts against α-synuclein protein aggregation and 6-hydroxydopamine (6-OHDA) – induced dopaminergic neurodegeneration in C. elegans model of Parkinson's disease.20
AD is a complex neurodegenerative disease. Presently, there is still no satisfactory in vitro cellular models for studying AD pathogenesis and therapeutics, while using transgenic mice models of AD for such studies are costly and time consuming. Simple invertebrate models of neurodegenerative disease offer experimental advantages for pharmacological evaluation and screening.6 C. elegans is a small, free-living nematode that has many biological advantages, such as short and reproducible lifespan and life cycle, ease of maintenance, tractable genetic manipulation, and significant conservation of genes with human; thus, it is widely accepted as a suitable animal model for studying aging and neurodegenerative diseases.21, 22, 23 Particularly, in case of AD multiple transgenic strains have been generated to express amyloid-β aggregation and utilized as an in vivo AD model for testing the efficacy of extracts/compounds, as well as their potential mechanism against Aβ-induced neurotoxicity.6,24,25
Therefore, our study was carried out to evaluate the potential of H. scabra extracts on Aβ deposition and proteotoxicity in transgenic C. elegans AD models, including the muscle-expressing Aβ CL4176 and CL2006 strains and pan-neuronal cell-expressing Aβ CL2355 strain. We hypothesized that H. scabra extracts could delay Aβ-mediated paralysis, reduce Aβ aggregation, attenuate Aβ-induced pathological behaviors, and extend the lifespan.
2. Materials and methods
2.1. Preparation of H. scabra extracts
The sea cucumber, H. scabra, were provided by the Coastal Fisheries Research and Development Center, Prachuap Khiri Khan, Thailand. They were euthanized with clove oil and cut midline at the dorsal part to open the body. Whole body (WB) and body wall (BW) of H. scabra were collected, chopped into small pieces and stored at −80 °C for subsequent usage. To obtain the natural components, sea cucumber samples were solvent-extracted using low to high polarity solvents, i.e., ethanol, ethyl acetate, and butanol, respectively, using the extraction procedure described in our previous studies.19,20 The extraction of H. scabra used in this study were composed of ethanol extract of body wall (BWET), ethyl acetate extracts of whole body and body wall (WBEA and BWEA) and butanol extracts of whole body and body wall (WBBU and BWBU). The stock solutions of these extracts were dissolved in dimethyl sulfoxide (DMSO) and stored at −20 °C. A final concentration of 0.1% DMSO was applied in all experiments. Furthermore, H. scabra extracts were analyzed to determine the chemical compounds by using proton nuclear magnetic resonance (1H NMR). The 1H NMR was recorded on a Bruker Ascend™ 400 MHz or Bruker Advance 500 MHz in CDCL3 using TMS as an internal standard, or otherwise instructed.20,26 The handling and extraction protocol of H. scabra were approved by Faculty of Science, Mahidol University Animal Care and Use Committee (MUSC-ACUC), permit no. MUSC 60-049-399.
2.2. Worm strains, maintenance, and synchronization
C. elegans strains used in this study are listed in supplementary data (Table S1). The C. elegans strains were composed of wild type strain (N2), CL802 (smg-1(cc546);rol-6(su1006); control strain for CL4176), CL2122 (mtl-2::GFP; control strain for CL2355), transgenic CL4176 (myo-3p::Aβ1-42::let-851 3′UTR + rol-6(su1006); human Aβ1–42 expression on muscle cells), transgenic CL2006 (unc-54::Aβ1-42 + rol-6(su1006); human Aβ1–42 expression on muscle cells), and transgenic CL2355 (snb-1::Aβ1-42::3′ UTR(long) + mtl-2::GFP; human Aβ1–42 expression on pan-neuronal cells) which were obtained from the Caenorhabditis Genetics Center (CGC; University of Minnesota, MN, USA). Experiments performed in C. elegans were approved by Faculty of Science, Mahidol University Animal Care and Use Committee (MUSC-ACUC), permit no. MUSC 60-051-401. The worms were cultured on solid nematode growth medium (NGM) plates and fed with Escherichia coli bacteria strain OP50. All C. elegans strains were maintained following standard protocols at 16 °C.27 The expressions of human Aβ1-42 in muscle cells of CL4176 or CL2006 worms and neuronal cells of CL2355 worms were induced by increasing temperature from 16 to 25 °C. The chunking method was used to preserve and maintain C. elegans population throughout the experimental periods.
Synchronously spawned eggs were isolated by using bleaching solution (0.5 ml of 1 M NaOH, 0.6 ml of NaClO and 3.9 ml of dH2O) and shaken for 7–10 min with a vortex shaker.19 The egg pellets were dropped on fresh NGM plate without bacteria OP50 at 16 °C overnight to obtain synchronous L1 larvae that were subsequently used in each experiment.
2.3. Amyloid β-induced paralysis assay
Paralysis assay was performed in CL4176 worms and its control CL802 to examine the effect of H. scabra extracts on delaying the onset of amyloid-β induced paralysis. Based on our previous experiments, H. scabra extracts at 500 μg/ml showed strongest effect on lifespan extension and reduction of α-synuclein aggregation in C. elegans models without negative effects to the worms,19,20 thus the dose at 500 μg/ml was selected for experiments in the present report. The synchronous L1 worms were prepared on NGM plate containing 50 μg/ml 5-fluorodeoxyuridine (FUDR; Sigma-Aldrich, St. Louis, MO, USA) to prevent growth of progeny28 and fed with OP50 mixed with 500 μg/ml of H. scabra extracts or 0.1% DMSO control at 16 °C for 24 h. Tetracycline hydrochloride (TET) has been shown to decrease amyloid-β deposition in in vitro29 and C. elegans model of AD,27,30 thus 50 μM TET was used as positive control. To stimulate the Aβ induced paralysis, the worms were temperature-upshifted from 16 to 25 °C for 36 h. The worms that failed to move their body or moved only their head when gently touched with a platinum loop were scored as paralyzed worms. The paralysis was individually scored at 2 h intervals until all worms were paralyzed.25,31 All paralysis assays were done in triplicate with 40 worms per treated condition.
2.4. Chemotaxis behavior assays
In addition to investigating the Aβ toxicity by paralysis assay on the muscle-expressed Aβ CL4176 strain, we performed chemotaxis assay to determine the recovery of Aβ-induced neuronal toxicity by H. scabra extracts. Chemotaxis assay was performed on CL2355 strain with human Aβ expression in neuronal cells, and CL2122 was used for control strain as described previously.5 Synchronized L1 larvae of CL2355 and its control CL2122 were treated with 500 μg/ml of H. scabra extracts or the vehicle alone. Tetracycline hydrochloride (50 μM) was used as positive control. They were maintained in 16 °C for 24 h and then transferred to 25 °C for another 36 h to induce Aβ1-42 expression in neurons. Then, forty worms were collected and washed with M9 buffer for three times. Then the worms were placed on the center of the 100 mm agar plates containing 1.9% agar, 1 mM CaCl2, 1 mM MgSO4, and 25 mM phosphate buffer, pH 6.0.25 Each assay plate was divided into four quadrants with two tests (B1&B2) and two controls (E1&E2). Then, 1 μl of attractant (0.1% benzaldehyde in 100% ethanol) (Sigma-Aldrich, St. Louis, MO, USA) was added onto a spot at 5 mm away from the edge of assay plate, along with 1 μl of 1 M sodium azide added on the original spot. On the opposite side of the attractant, 1 μl of control 100% ethanol and 1 μl of 1 M sodium azide were added. Assay plates containing the worms were incubated at 25 °C for 1 h and the chemotaxis index (CI) were scored. Chemotaxis index (CI) was calculated according to the following formula: CI = (number of worms at the attractant location-number of worms at the control location)/total number of worms on the plate. The experiment was performed independently three times.
2.5. Fluorescence quantification of amyloid-β protein accumulation
To elucidate the effect of H. scabra extracts on inhibiting Aβ deposition and aggregation which are the causative of neurotoxicity and onset of AD,32 CL2006 worms carrying human Aβ1-42 expression in muscle cells were investigated by staining with 4-bis(3-carboxy-hydroxy-phenylethenyl)- benzene (X-34) fluorescence dye which mark Aβ deposit. Thirty synchronous L1 larvae were placed onto solid NGM plates containing 500 μg/ml of H. scabra extracts or the vehicle. Tetracycline hydrochloride (50 μM) was used as positive control. The worms were maintained in 16 °C for 48 h. Subsequently, the temperature was shifted to 25 °C and the worms were continuously incubated for 48 h.33 After treatment, the worms were washed in M9 buffer, fixed in 4% paraformaldehyde/PBS, pH 7.4, for 24 h at 4 °C, and permeabilized in 5% β-mercaptoethanol, 1% triton X-100, 125 mM Tris, pH 7.4, at 37 °C for 24 h. The worms were washed three times in M9 buffer, stained with X-34, in 1% DMSO for 2 min at room temperature, and the samples were de-stained with 50% ethanol twice for 2 min.34,35 Stained samples were mounted onto glass slide with 2% agar pad for microscopy and the staining of Aβ accumulation in muscles was observed under BX53 fluorescence microscope (Olympus, Corp., Tokyo, Japan) with a digital camera. The quantity of deposits stained with X-34 in the anterior part of the pharyngeal bulb at the individual worm's head was measured and analyzed by ImageJ software (National Institute of Health, Bethesda, MD, USA). Wild-type N2 was used as control. The experiment was performed in triplicate independently.
2.6. Western blotting of Aβ species analysis
The Aβ species in transgenic C. elegans CL2006 and CL4176 strains were identified by immunoblotting after separation of proteins on a 15% Tris-Tricine gel following the standard Western blotting protocol.7,24 Synchronous L1 larvae were placed onto solid NGM plates containing 500 μg/ml of H. scabra extracts or the vehicle. Tetracycline hydrochloride (50 μM) was used as positive control. CL2006 worms were maintained in 16 °C for 48 h and then were incubated at 25 °C for 48 h. CL4176 worms were maintained at 16 °C for 24 h, then the temperature was increased from 16 to 25 °C for 36 h. C. elegans were collected and washed with M9 buffer for three times, quickly frozen in liquid nitrogen. The worms were homogenized in lysis buffer containing 1X RIPA buffer and 1X protease inhibitor cocktail (1X, Sigma-Aldrich, St. Louis, MO, USA) and then cooled on ice and centrifuged at 10,000 rpm for 15 min at 4 °C. The proteins in supernatant were quantified using Bicinchoninic acid assay (BCA protein assay; Thermo Fisher Scientific, Waltham, MA, USA). The protein (30 μg) was denatured prior to electrophoresis, protein samples were run at 120 V on SDS –Tris gel, and then transferred to Amersham™ Hybond®P 0.45 μm PVDF membrane for detecting molecular weights (MW) of Aβ species.7,24 The membrane was blocked for 2 h in Tris-buffered saline (TBS-tween® 20) + 5% bovine serum albumin (BSA) at room temperature to prevent non-specific binding of antibodies. The Aβ protein species were incubated with the mouse anti-Aβ monoclonal antibody 6E10 (1:500; MyBiosource, SanDiego, CA, USA) or rabbit anti-amyloid oligomers polyclonal antibody A11 (1:5,000; Thermo Fisher Scientific, Waltham, MA, USA) overnight at 4 °C and were detected with goat anti-mouse IgG-peroxidase conjugated H + L (1:5,000; Sigma-Aldrich, St. Louis, MO, USA) or goat anti-rabbit secondary antibody (1:2,000; Abcam, Cambridge, MA, USA), respectively. Mouse monoclonal anti-α smooth muscle actin antibody (1:1,000; Sigma-Aldrich, St. Louis, MO, USA) was used to detect actin as an internal control. Secondary antibody binding was carried out for 2 h at room temperature. The immunoreactive protein bands were detected by using PerkinElmer chemiluminescence detection kit assay (Thermo Fisher Scientific, Waltham, MA, USA) and imaged with chemiluminescent gel document (Alliance Q9 mini). Mean intensity of the Aβ-reactive bands were analyzed by using Image-J software (National Institute of Health, Bethesda, MD, USA).
2.7. Lifespan assay
Lifespan assay was performed in transgenic C. elegans strains, CL2006, CL4176 and CL2355, following the protocol described previously.19,20 Synchronized 50 worms of L1 larvae per group were transferred to NGM plates containing 50 μg/ml FUDR in absence or presence of 500 μg/ml of each fraction of H. scabra extracts. Then, transgene expression was induced by upshifting the temperature from 16 to 25 °C. The worms were counted as alive or dead every day until the last worm died. The dead worms were considered if they did not respond to gentle stimulation with a platinum wire or did not exhibit pharyngeal pumping movement. The exploding, protruding, or contaminated worms were counted as censored worms which were excluded in the lifespan calculation. The experiment was performed in triplicate. The number of survival and dead worms were analyzed statistically and plotted as a survival curve.
2.8. Measurement of intracellular reactive oxygen species (ROS) in C. elegans
The intracellular reactive oxygen species (ROS) level in worms was determined by using 2′,7′- dichlorodihydrofluorescin diacetate (H2DCF-DA) (Molecular Probes, Carlsbad, CA, USA) following the protocol previously described.6,7 Briefly, the age-synchronized L1 transgenic C. elegans CL2006 and CL4176 strains were treated with 500 μg/ml of H. scabra extracts or the vehicle. Tetracycline hydrochloride (50 μM) was used as positive control. To initiate the amyloid-β expression, the worms were temperature upshifted from 16 °C to 25 °C. Then, C. elegans were collected and washed with M9 buffer three times to remove the bacteria. The worms were homogenized using sonication and subsequently centrifuged at 2,000 rpm at 4 °C for 3 min. The supernatant was collected and incubated with DCF-DA solution (50 μM in M9) at 37 °C for 30 min. The fluorescent DCF was quantified in a fluorescence spectrophotometer (Spark ® 10 M microplate reader, TECAN, USA) with excitation at 485 nm and emission at 530 nm. The fluorescence was measured every 10 min for 2 h.
2.9. Statistical analysis
Each experiment was performed independently three times. All data were statistically analyzed using GraphPad Prism software (GraphPad software, Inc., Jolla, CA). For paralysis assays, a two-way ANOVA was used to determine significant difference. Analysis of lifespan was performed on Kaplan-Meier curve and p values was calculated using the log-rank test. Survival curves resulting in p-values of <0.01 relative to control was considered to be significantly different. All results were presented as mean ± standard deviation (SD). The significant difference at the p < 0.01 level was calculated by one-way ANOVA analysis following the Tukey-Kramer test for multiple comparison results.
3. Results
3.1. H. scabra extracts delayed the progression of Aβ-induced paralysis
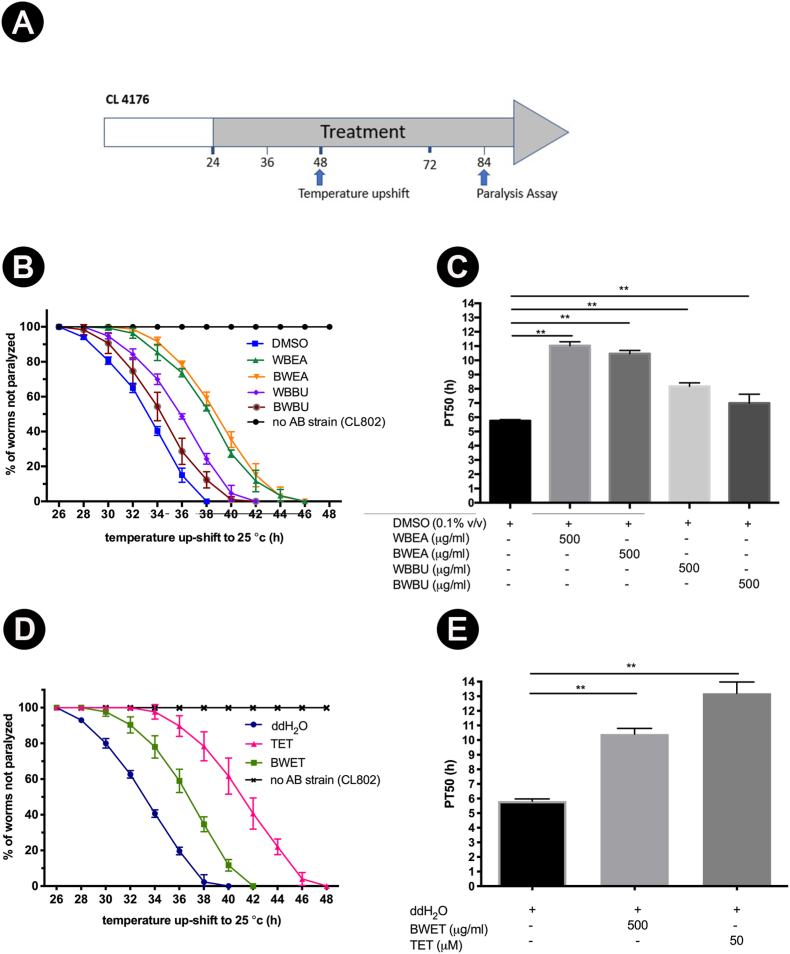
To determine whether H. scabra extracts could delay the Aβ-induced toxicity, the paralysis assays were performed using CL4176 strain that expresses human Aβ1-42 and CL802 strain was used as a control. CL4176 worms fed with all fractions of H. scabra extracts at 500 μg/ml or 50 μM TET exhibited significant delay of paralysis induced by human Aβ1-42 expression in muscle cells (Fig. 1B, D), with WBEA and BWEA fractions being the most effective when compared to the control. The delay of paralysis by WBEA and BWEA appeared to be statistically significant at 28–44 h after temperature upshift, whereas the worms treated with WBBU and BWBU showed significant delay of paralysis at 30–38 h after temperature upshift (p < 0.01; Fig. 1B). BWET-treated worms also exhibited significant delay of paralysis at 28–40 h after temperature upshift when compared to ddH2O -treated control group (p < 0.01; Fig. 1D).
Fig. 1.
Effects of H. scabra extracts on delaying paralysis in transgenic Aβ-expressing C. elegans, strain CL4176. Diagram illustrating the paralysis assay of CL4176 and CL802 (control) worms, showing the time at which the temperature was raised from 16 °C to 25 °C, when the H. scabra extracts were administered, and when the paralysis assay was done (A). Time duration of Aβ-induced paralysis in the transgenic CL4176 strain treated with 500 μg/ml of WBEA, BWEA, WBBU, BWBU fractions (B) and BWET fraction (D). Tetracycline treatment served as positive control, whereas 0.1% DMSO and ddH2O were used as negative controls (B, D). Synchronous L1 worms were placed for 24 h at 16 °C on NGM plates fed with H. scabra extracts, tetracycline, or vehicle. The numbers of the paralyzed worms were counted at 26 h following temperature upshift to 25 °C then at 2 h intervals thereafter, and plotted as percent of unparalyzed worms from three independent experiments. CL802 which does not express Aβ transgene was used as transgenic control. Paralysis assays were quantified as PT50 which is the duration at which 50% of worms were paralyzed after temperature upshift (C, E). An asterisk (∗∗) indicates the significant difference in paralysis curves at p < 0.01 compared with control.
For quantitative analysis, PT50 value of each group was determined as a time interval when half of the worms were paralyzed starting from the onset of paralysis.5,25 The duration of temperature upshifted to induce paralysis was 27 h. Thus, PT50 of 5.75 h for DMSO-treated control group was obtained by subtracting the onset paralysis time at 27 h from the time when 50% of the worms were paralyzed at 32.75 h (Supplementary table S2). CL4176 worms treated with WBEA, BWEA, WBBU, and BWBU exhibited statistically significant lengthening of PT50 (delayed paralysis time) at 11.02 ± 0.27, 10.47 ± 0.22, 8.17 ± 0.25, 6.99 ± 0.62 h compared to DMSO-treated group 5.75 ± 0.08 h (p < 0.01; Fig. 1C). Additionally, BWET-treated, and TET-treated positive control exhibited significant delay of PT50 at 10.42 ± 0.38, 13.20 ± 0.77, compared to ddH2O-treated negative control at 5.83 ± 0.14 (p < 0.01; Fig. 1E). In CL802 worms treated with DMSO- or ddH2O no paralysis was detected. Thus, these results suggested that all fractions of H. scabra extracts, especially WBEA and BWEA, could protect the transgenic AD worms from Aβ-induced paralysis.
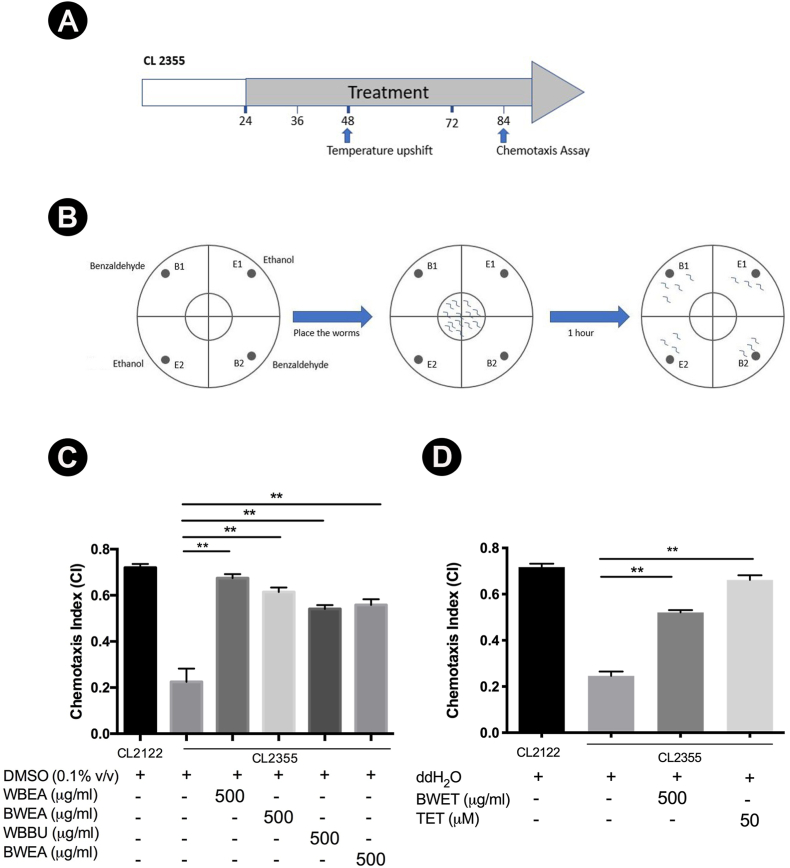
3.2. H. scabra extracts ameliorated Aβ-induced neuronal defect in chemotaxis behavior
Chemotaxis assay was performed using CL2355 strain containing neurons that express human Aβ and CL2122 as a control strain. Since the chemotaxis response in worms is mediated by interneurons to stimulate motor neurons, the Aβ expression in neurons leads to defect in chemotaxis response. In this assay, a chemical, benzaldehyde, was used as an attractant and ethanol as a control, both of which were mixed with sodium azide to paralyze the worms on contact (Fig. 2B). Quantitatively, the response was reported as chemotaxis index (CI) which was measured as a fraction of worms able to reach the location of attractant. The results revealed that CL2355 strain exhibited a significant decrease of CI compared with CL2122 control strain, when both were treated with DMSO (CICL2122 0.72 ± 0.01 vs CICL2355 0.23 ± 0.05; p < 0.01; Fig. 2C). CL2355 worms treated with WBEA, BWEA, WBBU, and BWBU showed significantly increased CI when compared with DMSO-treated group (p < 0.01; Fig. 2C). Interestingly, CL2355 treated with WBEA and BWEA showed markedly high CI almost equal to that of CL2122 (Fig. 2C). By contrast, CL2355 worms treated ddH2O also exhibited a significant decrease of CI compared with CL2122 control worms (CICL2122 0.71 ± 0.01 vs CICL2355 0.24 ± 0.01; p < 0.01; Fig. 2D). Furthermore, CL2355 worms treated with BWET fraction showed significant increase of CI compared with ddH2O-treated group (p < 0.01; Fig. 2D). The positive control, TET-treated worms at concentration of 50 μM, also showed significantly increase of CI compared to control ddH2O-treated group (Fig. 2D). These findings indicated that H. scabra extracts could ameliorate Aβ-induced toxicity on neural control of chemotaxis.
Fig. 2.
Effects of H. scabra extracts on neuronal functions of Aβ-expressed C. elegans CL2355 strain. Diagram illustrating the chemotaxis assay indicating the time of treatment in CL2355 and CL2122 (control) worms, when the H. scabra extracts were administered, and chemotaxis assay was done (A). Schematic illustration of chemotaxis assay (B). Chemotaxis assay was performed by using benzaldehyde as an attractant and 100% ethanol as a control odorant. Synchronized L1 worms were treated with either vehicle (DMSO or ddH2O) or fractions of H. scabra extracts or TET (positive control). After treatments, worms were placed on the center of the assay plate and incubated at 25 °C for 1 h and CI was scored. The chemotaxis index in the control strain (CL2122) and the transgenic strain (CL2355) were scored (C, D). Data showed the mean ± SD value of CI from triplicated independent experiments. ∗∗ indicates statistically significant difference between treated and untreated groups at p < 0.01.
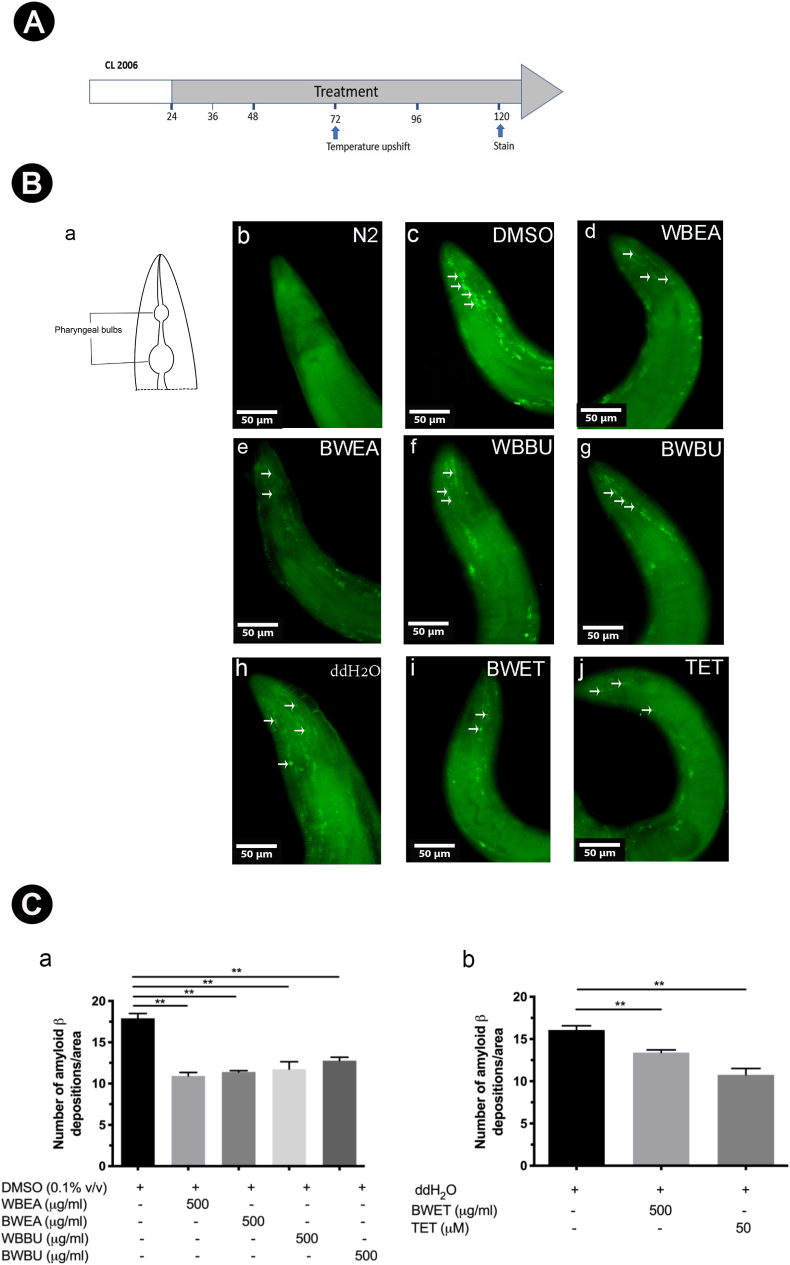
3.3. H. scabra extracts decreased Aβ protein accumulation in transgenic CL2006 worms
The effect of H. scabra extracts on inhibiting Aβ deposition and aggregation was examined in CL2006 worms by staining with X-34 fluorescence dye to quantify the amount of Aβ deposition (Fig. 3B, white arrows). The amount of Aβ deposition was scored in the worm's head region at the level of pharyngeal bulb. The number of Aβ deposition was markedly reduced in worms treated with WBEA, BWEA, WBBU, and BWBU when compared with DMSO-treated worms (Fig. 3B). The result showed that the mean number of Aβ deposits in each group was significantly decreased in CL2006 worms treated with WBEA, BWEA, WBBU, and BWBU fractions compared with DMSO-treated worms (p < 0.01; Fig. 3C). Similarly, the number of Aβ deposition was distinctly reduced in worms treated with BWET and TET when compared with ddH2O treated worms (Fig. 3B). The mean number of Aβ deposits in CL2006 worms treated with BWET and TET was also significantly decreased compared with ddH2O-treated worms (p < 0.01; Fig. 3C). These results suggested that the protection of H. scabra extracts against Aβ toxicity may result from their abilities to inhibit Aβ oligomerization, which lead to a reduction of toxic Aβ fibrils and an increase of monomeric form which is not toxic.
Fig. 3.
Effects of H. scabra extracts in decreasing Aβ deposits in transgenic CL2006 worms. An illustration indicating the time duration of treatment and Aβ deposit detection (A). A schematic diagram indicates position of pharyngeal bulbs (Ba) and representative images of X-34 staining in wild-type N2 (Bb), in transgenic CL2006 fed with DMSO (control) (Bc), and ddH2O (control) (Bh), with 500 μg/ml of WBEA (Bd), BWEA (Be), WBBU (Bf), BWBU (Bg), BWET (Bi), and TET at 50 μM (Bj). White arrows indicate Aβ deposits in the worm head. Quantitative analysis of Aβ deposits in the transgenic CL2006 fed with H. scabra extracts compared with control (DMSO or ddH2O) (C). The Aβ deposits was quantified by ImageJ software and expressed as mean number ± SD of Aβ deposits/anterior area of a worm (n = 30 for each analysis) (C). ∗∗ indicates statistically significant difference between treated and untreated groups at p < 0.01.
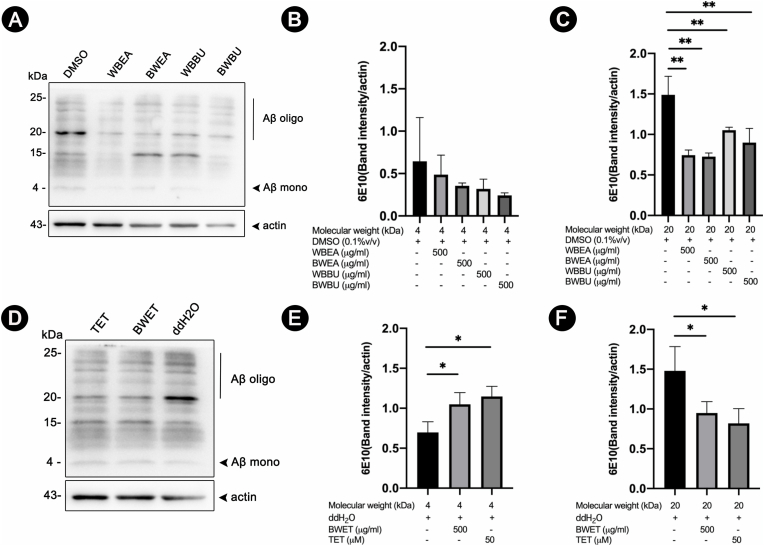
3.4. The effect of H. scabra extracts on Aβ oligomerization in transgenic C. elegans
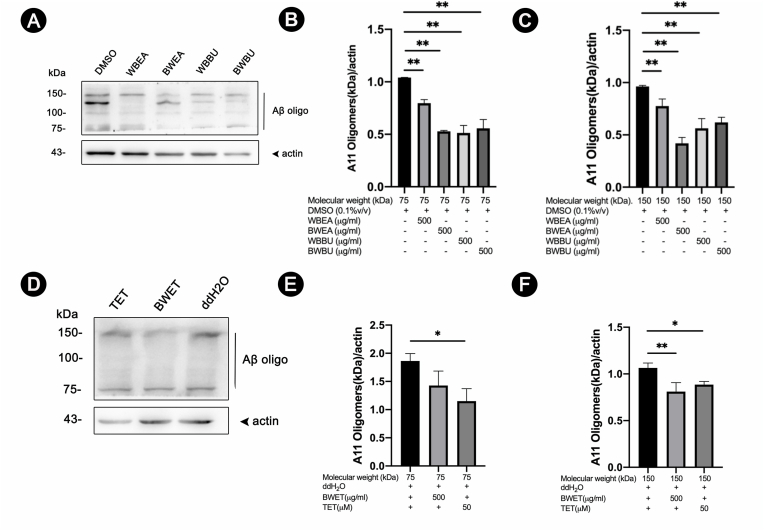
To support the findings mentioned in the preceding sections that the decrease in Aβ oligomerization appeared to be protective against Aβ-induced paralysis and deficit in chemotaxis behavior, we investigated whether H. scabra extracts could reduce Aβ deposition by decreasing Aβ oligomerization by Western blotting. Anti-Aβ 6E10 antibody for detecting Aβ species (Fig. 4 and S1) and anti-oligomer A11 antibody for detecting high molecular weight Aβ oligomers were used to detect Aβ variations in transgenic CL2006 and CL4176 worms treated with H. scabra extracts (Fig. 5 and S2). The results demonstrated that the level of Aβ oligomer band with molecular weight (MW) ∼20 kDa was markedly reduced in both strains when fed with H. scabra extracts and TET (positive control) as compared with the negative control (Fig. 4 and S1 (A, D)). The mean density of this Aβ oligomer band was significantly reduced in both extract-treated CL2006 and CL4176 worms (p < 0.01) (Fig. 4 and S1 (C, F)). Interestingly, a concurrent and significant increase of the Aβ monomer band at 4 kDa was detected in CL4176 worms fed with the extracts and TET (positive control) when compared with the negative control (p < 0.01) (Fig. S1 (A-E). However, density of the Aβ monomers band at 4 kDa in the treated CL2006 worms did not differ significantly from that of the negative control (Fig. 4 (A, B)).
Fig. 4.
Effects of H. scabra extracts on Aβ species in transgenic CL2006 strain detected by anti-Aβ 6E10 antibody. Representative images of Western blotting of Aβ species in worms fed with or without H. scabra extracts at dose 500 μg/ml (A, D), or 50 μM TET (positive control) (D), and detected by anti- Aβ (6E10) or anti-actin antibodies. Quantification of intensities of Aβ bands at 4 kDa an d 20 kDa were carried out with ImageJ software (B–C, E-F). Vertical line indicated Aβ oligomer band at MW higher than 20 kDa, whereas arrow heads indicated Aβ monomer band at MW 4 kDa and actin band (43 kDa). Data are expressed as mean ± SD of relative band intensity normalized against actin from three independent experiments. ∗, ∗∗ indicate statistically significant difference between treated and untreated groups at p < 0.05 and 0.01, respectively.
Fig. 5.
Effects of H. scabra extracts on Aβ species in transgenic CL2006 strains as detected by anti- Aβ oligomer A11 antibody. Representative images of Western blotting of Aβ species in worms fed with or without H. scabra extracts at dose 500 μg/ml (A, D), or 50 μM TET (positive control) (D), and detected by anti-Aβ (A11) or anti-actin antibodies. Quantification of intensities of high molecular weight Aβ oligomer bands at 75 kDa (B, E) and 150 kDa (C, F) were carried out with ImageJ software. Vertical line indicated high molecular Aβ oligomer bands at 75–150 kDa, while arrow head indicated actin band (43 kDa). Data are expressed as mean ± SD of relative band intensity against actin from three independent experiments. ∗, ∗∗ indicate statistically significant difference between treated and untreated groups at p < 0.05 and 0.01, respectively.
When using A11 antibody to detect high MW Aβ oligomers, both C. elegans worms that were treated with the extracts from H. scabra and TET showed reduction of high MW Aβ oligomer band as compared with the control (Fig. 5 and S2). The mean densities of Aβ oligomer bands at MW ∼75 kDa and ∼150 kDa were significantly decreased in all extract-treated groups compared to untreated groups (p < 0.01) (Fig. 5 and S2 (B–F)). The results revealed that the Aβ species detected by 6E10 and A11 did not overlap in term of MW, suggesting that Aβ oligomerizations were attenuated by H. scabra extracts. Hence, these findings indicated that H. scabra extracts mediated the protective effects against Aβ toxicity by reducing the formation of Aβ oligomers.
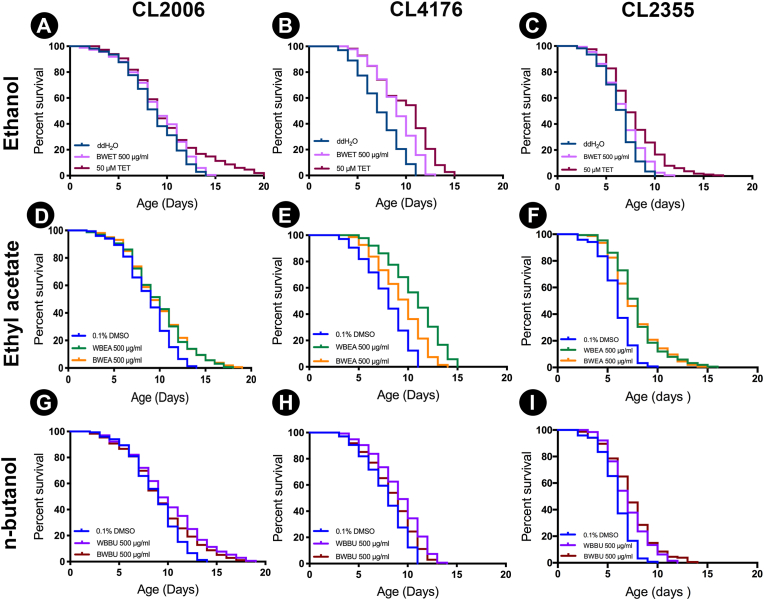
3.5. The effect of H. scabra extracts on C. elegans lifespan
Positive correlation was found between lifespan extension and protection against Aβ toxicity,36,37 thus we investigated the effect of H. scabra extracts on lifespan extension by performing survival assay using CL4176 and CL2006 strains. CL2006 worms treated with WBEA, BWEA, WBBU, BWBU, BWET, and TET showed significant increase of the mean lifespan to 9.75 ± 3.37, 9.82 ± 3.37, 9.89 ± 3.7, 9.29 ± 3.5, 9.13 ± 3.07, and 9.91 ± 3.94 days, respectively, when compared to their corresponding control groups (p < 0.01) (Fig. 6 A, D, G)) (Table 1). The mean lifespan of CL4176 worms treated with WBEA, BWEA, WBBU, BWET and TET also significantly increased to 10.75 ± 2.65, 9.22 ± 2.4, 9.17 ± 2.50, 9.04 ± 2.20, and 9.92 ± 2.90 days, respectively, when compared to control worms (p < 0.01) (Fig. 6 (B, E, H)) (Table 1). However, the mean lifespan of BWBU-treated group was 8.49 ± 2.53 days, which was increased but not significantly different from that of the control group (Fig. 6H) (Table 1). These results indicated that H. scabra extracts were capable of extending mean lifespan of transgenic Aβ-expressed worms. Moreover, to evaluate the effect of neuronal Aβ expression on overall survival, we performed a lifespan assay in CL2355 strain. The mean lifespans of the worms treated with WBEA, BWEA, WBBU, BWBU and TET significantly increased to 7.85 ± 2.39, 7.76 ± 2.48, 7.04 ± 2.00, 7.27 ± 2.36, and 7.60 ± 2.81 days, respectively when compared to control worms (p < 0.01) (Fig. 6 (C, F, I)) (Table 1). However, the mean lifespan of BWET-treated group was 6.81 ± 2.03 days, which was not significantly different from that of the control group (Fig. 6C) (Table 1).
Fig. 6.
Effect of H. scabra extracts on lifespan of transgenic Aβ-expressing worms. The survival curves show the lifespans of transgenic worms CL2006 (A), CL4176 (B), and CL2355 (C) treated with BWET compared to the control; CL2006 (D), CL4176 (E), and CL2355 (F) treated with WBEA and BWEA compared to the control; and CL2006 (G), CL4176 (H), and CL2355 (I) treated with WBBU and BWBU compared to the control.
Table 1.
Effect of H. scabra extracts on lifespan of CL2006, CL4176 and CL2355 worms.
| Strain + treatment | % increase | Mean ± SD | Max | P value |
|---|---|---|---|---|
| CL2006 + 0.1% DMSO | – | 8.75 ± 2.60 | 14 | – |
| CL2006 + 500 μg/ml WBEA | 11.40 | 9.75 ± 3.37∗∗ | 18 | <0.01 |
| CL2006 + 500 μg/ml WBBU | 13.03 | 9.89 ± 3.70∗∗ | 19 | <0.01 |
| CL2006 + 500 μg/ml BWEA | 12.22 | 9.82 ± 3.37∗∗ | 19 | <0.01 |
| CL2006 + 500 μg/ml BWBU | 6.17 | 9.29 ± 3.50∗∗ | 18 | <0.01 |
| CL2006+ ddH2O | – | 8.72 ± 2.70 | 14 | – |
| CL2006 + 500 μg/ml BWET | 4.82 | 9.13 ± 3.07∗∗ | 15 | <0.01 |
| CL2006 + 50 μM TET | 13.77 | 9.91 ± 3.94∗∗ | 20 | <0.01 |
| CL4176 + 0.1%DMSO | – | 7.85 ± 2.22 | 11 | – |
| CL4176 + 500 μg/ml WBEA | 36.94 | 10.75 ± 2.65∗∗ | 15 | <0.01 |
| CL4176 + 500 μg/ml WBBU | 16.81 | 9.17 ± 2.50∗∗ | 14 | <0.01 |
| CL4176 + 500 μg/ml BWEA | 17.45 | 9.22 ± 2.40∗∗ | 13 | <0.01 |
| CL4176 + 500 μg/ml BWBU | 8.15 | 8.49 ± 2.53 | 13 | 0.1806 |
| CL4176+ ddH2O | – | 7.40 ± 2.20 | 11 | – |
| CL4176 + 500 μg/ml BWET | 22.16 | 9.04 ± 2.20∗∗ | 13 | <0.01 |
| CL4176 + 50 μM TET | 34.05 | 9.92 ± 2.90∗∗ | 15 | <0.01 |
| CL2355 + 0.1%DMSO | – | 5.96 ± 1.6 | 10 | – |
| CL2355 + 500 μg/ml WBEA | 31.70 | 7.85 ± 2.39∗∗ | 16 | <0.01 |
| CL2355 + 500 μg/ml WBBU | 18.12 | 7.04 ± 2.00∗∗ | 12 | <0.01 |
| CL2355 + 500 μg/ml BWEA | 28.69 | 7.67 ± 2.48∗∗ | 15 | <0.01 |
| CL2355 + 500 μg/ml BWBU | 21.97 | 7.27 ± 2.36∗∗ | 14 | <0.01 |
| CL2355+ ddH2O | – | 6.38 ± 1.78 | 10 | – |
| CL2355 + 500 μg/ml BWET | 6.73 | 6.81 ± 2.03 | 12 | 0.0147 |
| CL2355 + 50 μM TET | 19.12 | 7.60 ± 2.81∗∗ | 17 | <0.01 |
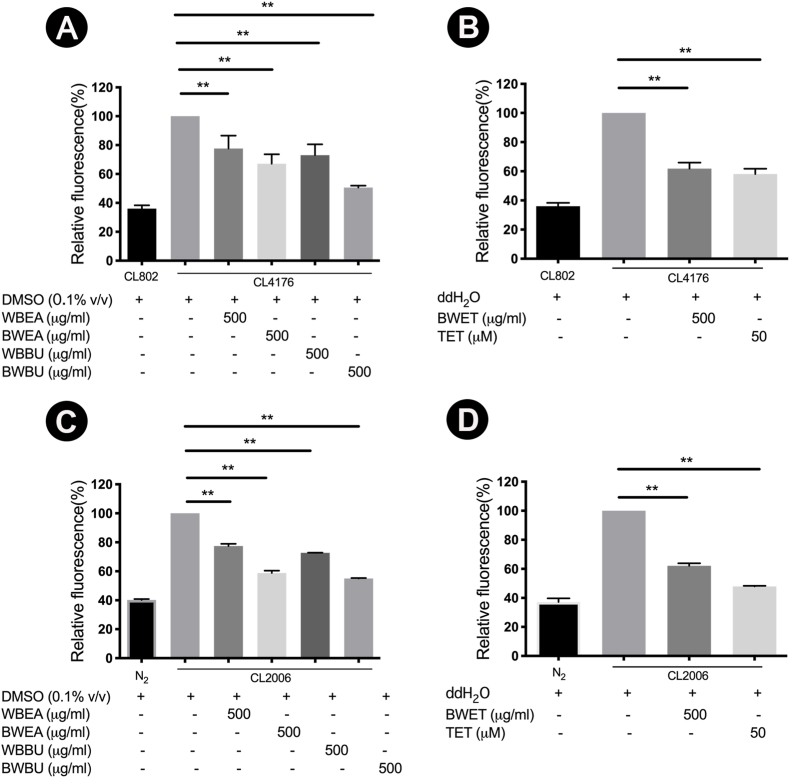
3.6. H. scabra extracts reduced reactive oxygen species (ROS) in C. elegans
Numerous evidences have shown an association of oxidative stress with Aβ-induced toxicity and AD.5,6 We, therefore, tested the effect of H. scabra extracts on the ROS production in transgenic CL4176 and CL2006 strains and their control strains (CL802 or N2 wild type). The untreated transgenic CL4176 and CL2006 strains exhibited increased levels of ROS compared with the corresponding control strains (Fig. 7). Treatment with all fractions of H. scabra extracts before the induction of Aβ expression significantly prevented the elevation of ROS levels in both CL4176 and CL2006 worms. Interestingly, no significant difference was observed in CL802 (control strain) worm and CL4176 worm treated with 500 μg/ml WBEA fraction (Fig. 7A), indicating the most effective extracts to reduce ROS. These results indicated that H. scabra extracts could attenuate the levels of ROS in both CL4176 and CL2006 worms.
Fig. 7.
Effect of H. scabra extracts on the reduction of ROS levels in the transgenic CL4176 (A–B) and CL2006 (C–D) worms fed with vehicle or H. scabra extracts at a dose 500 μg/ml or TET 50 μM (positive control). Results are expressed as percentage of fluorescence (%DCF) relative to vehicle-treated transgenic CL4176 and CL2006 (untreated control) worms, which is set as 100%. Bars represent the standard error (SD). ∗∗indicates statistical significance at p < 0.01 compared with control groups.
4. Discussion
Alzheimer's disease (AD) is a common neurodegenerative disease associated with aging. The major pathogenic factor of AD is the misfolding and formation of Aβ oligomers, leading to insoluble aggregates and finally neuronal damage. C. elegans is an ideal model organism for screening and discovering anti-AD drugs because of its simple yet representative nervous system, short life cycle, well-established genetic profile, and low dosage of drug consumption. Furthermore, the absence of endogenous Aβ production in the worms enables researchers to find direct effect of Aβ involvement in pathological behaviors,38 as well as providing convenience in utilizing a variety of transgenic strains for AD models of drug discovery studies.6,24,25 Based on these advantages, we chose to investigate the potential anti-AD effect of H. scabra extracts in C. elegans model of AD, which we believe to be a pioneering study to demonstrate strong evidences for in vivo anti-Aβ toxicity effects of H. scabra extracts. Our results showed that WBEA, BWEA, WBBU, BWBU, and BWET fractions of H. scabra extracts at 500 μg/ml could alleviate pathological features of AD by attenuating Aβ oligomerization and aggregation in muscles and neurons in similar fashion as tetracycline which was used as a control. These results indicated that the bioactive compounds in these fractions mediate anti-Aβ toxicity to prevent pathological defects in both muscle- and neuron-expressed Aβ strains. In addition, the dose at 500 μg/ml was selected for the study since previous studies have shown the negative effects of the extracts at high dose, whereas the low dose showed no protective effect.19,20 This could be from the beneficial effect of the compounds mediated through the mechanism known as hormesis.39,40 Although all extracts have been shown to significantly improve behavioral features of AD pathology, WBEA and BWEA fractions appeared to be the most effective fractions with anti-Aβ proteotoxicity. These groups of extracts have also been shown to reduce α-synuclein aggregation in Parkinson's disease.20 Recent studies by our group showed that triterpene glycosides or saponins were the major components in H. scabra extracts.19,20 In addition, our previous studies also showed that BWEA,41 WBEA20 and WBBU19 fractions contain triterpene glycoside and phenolic compounds. Thus, it is possible that the reduction of Aβ deposition in C. elegans treated with the H. scabra extracts may result from the actions of triterpene glycosides or saponins and phenolic compounds in the extracts. In addition, the phenolic compounds in the extracts are strong antioxidants that may attenuate oxidative stress from Aβ as well as diminish the proteotoxicity caused by Aβ aggregation. Furthermore, accumulated evidences suggested that saponins have significant neuroprotective effects on attenuation of central nervous system disorders.42 Therefore, our next goal is to purify the bioactive compounds from the most effective extracts and test their efficacies and mechanisms of actions against AD, and compare with an available standard AD drug. If the anti-AD effect of these compounds is strong then attempt will be made to chemically synthesize them for further long-term usage and application.
Based on the “amyloid cascade hypothesis”, Aβ deposits lead to a series of AD features.43 In C. elegans, the paralysis phenotype and chemotaxis defect are resulted from Aβ depositions. From our results, the mean number of Aβ deposition per nematode was significantly decreased in CL2006 worms treated with WBEA, BWEA, WBBU, BWBU, BWET as well as TET-treated worms as compared with untreated worms. This suggested that H. scabra extracts, especially WBEA and BWEA fractions, can protect against AD-like symptoms through directly inhibiting Aβ accumulation. To further examine whether H. scabra extracts reduced the formation of Aβ oligomer, immunoblotting was performed. It was revealed that H. scabra extracts could significantly decrease of oligomeric form of Aβ at 20 kDa, which is the main toxic species,44 as well as the larger oligomers at approximately 75 and 150 kDa in both CL4176 and CL2006 transgenic worms. Previous study has shown that the reduction of Aβ oligomerization may be mediated through Aβ degradation, alteration of its aggregated forms, or clearance of the aggregated proteins.27 Moreover, upregulation of autophagic vesicles has been detected in ginsenosides-treated C. elegans, indicating that ginsenosides, a plant triterpene glycoside, may promote autophagy-lysosomal process to eliminate Aβ aggregation.45 The sea cucumber triterpene glycosides, the main components in the ethyl acetate fractions of H. scabra may act by the same mechanism.19,20 Additionally, recent study from our group has shown that 2-butoxytetrahydrofuran (2-BTHF), a cyclic ether isolated from H. scabra, can interact with heat shock factor-1 (HSF-1) transcription factor to upregulate the expression of autophagic genes which mediate the clearance of Aβ aggregates through autophagy-lysosomal pathway.46 Moreover, in addition to 2-BTHF there may be other bioactive substances in the extracts that exert this activity; for instance, diterpene glycosides or holothurins from H. scabra ethyl acetate extracts has been shown to reduce α-synuclein aggregation by activating the degradation process of autophagy-lysosomal pathway.47 Therefore, it is likely that the bioactive substances included in the H. scabra extracts may exercise synergistic effects in either directly eliminating Aβ aggregates or indirectly reducing Aβ toxicity as demonstrated in other studies.48 In addition to autophagy pathway, insulin/insulin-like growth factor-1 (IGF-1) signaling (IIS) pathway may also participate in the regulation of Aβ aggregate in multiple ways to protect worms from the toxicity of Aβ oligomers in C. elegans. It was found that DAF-16 and HSF-1 upregulations due to decrease of IIS signaling could negatively regulate protein disaggregate.49 Previous study also showed an upregulation of DAF-16 in worms treated with H. scabra extracts.19 Therefore, it is possible that reduction of Aβ oligomers by H. scabra extracts may be mediated partly through HSF-1 and DAF-16.
Aging is a naturally occurring biological process wherein physiological functions of organism gradually decline concurrently with the rises of age-associated disease, including Alzheimer's disease.37 Previous studies showed that H. scabra extracts are able to increase lifespan of both wild-type and transgenic C. elegans model of Parkinson's disease.19,20 In this study, we found that WBEA, BWEA, WBBU, BWBU, and BWET fractions could also extend lifespan and survival rates of the transgenic AD worms. Several factors have been shown to be associated with the lifespan extension in the treated C. elegans, including high amounts of anti-oxidants and the presence of active compounds in the extracts. Moreover, in AD and PD C. elegans models, the lifespan extension may also be resulted from the elimination of toxic protein aggregates, such as Aβ or α-synuclein as found in the studies of Oleuropein aglycone27 and n-butanol extract of Hedyotis diffusa (HDB).50 Thus, lifespan extension activity of H. scabra extracts in transgenic AD worms may be mediated through similar mechanisms, i.e., the anti-oxidant properties of chemicals in the extracts and their abilities to eliminate toxic protein aggregation.
Numerous studies demonstrated that oxidative stress plays a key role in Aβ-induced neurotoxicity and the progression of AD.51 To this end, elevated ROS production was found in transgenic C. elegans expressing constitutive and inducible Aβ.52 In this study, we found that the extracts derived from H. scabra could decrease the intracellular ROS in transgenic CL4176 and CL2006 worms. It is possible that H. scabra extracts decreased Aβ oligomerization, leading to reduced intracellular ROS in AD worms. Moreover, the extracts themselves may exert anti-oxidant properties to directly scavenging ROS.19,53 Taken together, the results suggested that H. scabra extracts could reduce Aβ oligomeric form and their toxicity, as well as exert anti-oxidant effect to neutralize oxidative stress, thereby improving the overall health span and lifespan of the organism. Therefore, using crude extracts of H. scabra to treat AD seems to be more advantageous than single compound as they may act synergistically to activate several targets to attenuate AD progression. In addition to demonstrating the therapeutic value of H. scabra extracts in protecting against AD, this study also highlighted the economic value of H. scabra, as the increasing demand will stimulate the production of this species, which in turn will generate income for farmers as well as overall economy of society.
5. Conclusions
The present study demonstrated the neuroprotective effects of H. scabra extracts against AD in C. elegans model. The extracts reduced the Aβ aggregation by inhibiting the generation of toxic Aβ oligomeric forms, thus ameliorate the deficits in physiological behaviors from Aβ-induced toxicity. In addition, H. scabra extracts could also extend the lifespan and reduced ROS level in AD worms. This study demonstrated possibilities that H. scabra extracts could be used for prevention as well as potential treatment for AD, which should be further tested in mammalian models as well as in clinical trial before application in humans could be adopted.
Declaration of competing interest
The authors report no conflict of interest in this study.
Acknowledgements
This study was supported by Mahidol University (Basic Research Fund: fiscal year 2021- BRF2-NDFR27/2564) to Krai Meemon, and a graduate research support grant (2020) from Mahidol Alumni Association, Mahidol University to Warannida Kleawyothatis. The research was partially supported by CIF grant, Faculty of Science, Mahidol University. C. elegans strains used in this study were provided by the Caenorhabditis Genetics Center (CGC), which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440).
Footnotes
Footnotes: Anti-Alzheimer effects of H. scabra extracts.
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.jtcme.2022.10.006.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Leiteritz A., Schmiedl T., Baumanns S., Wenzel U. Amyloid-beta induced paralysis is reduced by cholecalciferol through inhibition of the steroid-signaling pathway in an Alzheimer model of Caenorhabditis elegans. Nutr Neurosci. 2019:1–8. doi: 10.1080/1028415X.2019.1596371. [DOI] [PubMed] [Google Scholar]
- 2.LaFerla FM, Green KN. Animal models of Alzheimer disease. Cold Spring Harbor Perspect Med.2(11):a006320. [DOI] [PMC free article] [PubMed]
- 3.Udeochu J.C., Shea J.M., Villeda S.A. Microglia communication: parallels between aging and Alzheimer's disease. Clin Exp Neuroimmunol. 2016;7(2):114–125. doi: 10.1111/cen3.12307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cummings J., Aisen P.S., DuBois B., et al. Drug development in Alzheimer's disease: the path to 2025. Alzheimer's Res Ther. 2016;8(1):39. doi: 10.1186/s13195-016-0207-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu Y., Wu Z., Butko P., et al. Amyloid-beta-induced pathological behaviors are suppressed by Ginkgo biloba extract EGb 761 and ginkgolides in transgenic Caenorhabditis elegans. J Neurosci. 2006;26(50):13102–13113. doi: 10.1523/JNEUROSCI.3448-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang M., Qian F., Liu Q., et al. Evaluation of structure–activity relationships of ginsenosides against amyloid β induced pathological behaviours in transgenic Caenorhabditis elegans. RSC Adv. 2017;7(64):40095–40104. [Google Scholar]
- 7.Tangrodchanapong T., Sobhon P., Meemon K. Frondoside A attenuates amyloid-β proteotoxicity in transgenic Caenorhabditis elegans by suppressing its formation. Front Pharmacol. 2020;11:553579. doi: 10.3389/fphar.2020.553579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Griffin E.F., Scopel S.E., Stephen C.A., et al. ApoE-associated modulation of neuroprotection from Abeta-mediated neurodegeneration in transgenic Caenorhabditis elegans. Dis Model Mech. 2019;12(2) doi: 10.1242/dmm.037218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang C., Zheng C. Using Caenorhabditis elegans to model therapeutic interventions of neurodegenerative diseases targeting microbe-host interactions. Front Pharmacol. 2022;13 doi: 10.3389/fphar.2022.875349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Regitz C., Fitzenberger E., Mahn F.L., Dußling L.M., Wenzel U. Resveratrol reduces amyloid-beta (Aβ1–42)-induced paralysis through targeting proteostasis in an Alzheimer model of Caenorhabditis elegans. Eur J Nutr. 2016;55(2):741–747. doi: 10.1007/s00394-015-0894-1. [DOI] [PubMed] [Google Scholar]
- 11.Jagota S., Rajadas J. Effect of phenolic compounds against Aβ aggregation and aβ-induced toxicity in transgenic C. elegans. Neurochem Res. 2012;37(1):40–48. doi: 10.1007/s11064-011-0580-5. [DOI] [PubMed] [Google Scholar]
- 12.Bordbar S., Anwar F., Saari N. High-value components and bioactives from sea cucumbers for functional foods--a review. Mar Drugs. 2011;9(10):1761–1805. doi: 10.3390/md9101761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Phan H.T., Samarat K., Takamura Y., Azo-Oussou A.F., Nakazono Y., Vestergaard MdC. Polyphenols modulate alzheimeí s amyloid beta aggregation in a structure-dependent manner. Nutrients. 2019;11 doi: 10.3390/nu11040756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kiew P.L., Don M.M. Jewel of the seabed: sea cucumbers as nutritional and drug candidates. Int J Food Sci Nutr. 2012;63(5):616–636. doi: 10.3109/09637486.2011.641944. [DOI] [PubMed] [Google Scholar]
- 15.Ruggieri G.D. Drugs from the sea. Science. 1976;194(4264):491–497. doi: 10.1126/science.9691. [DOI] [PubMed] [Google Scholar]
- 16.Shinde P., Banerjee P., Mandhare A. Marine natural products as source of new drugs: a patent review (2015-2018) Expert Opin Ther Pat. 2019;29(4):283–309. doi: 10.1080/13543776.2019.1598972. [DOI] [PubMed] [Google Scholar]
- 17.Guo Y., Ding Y., Xu F., et al. Systems pharmacology-based drug discovery for marine resources: an example using sea cucumber (Holothurians) J Ethnopharmacol. 2015;165:61–72. doi: 10.1016/j.jep.2015.02.029. [DOI] [PubMed] [Google Scholar]
- 18.Morakot Sroyraya P.J.H., Siangcham Tanapan, Tinikul Ruchanok, Jattujan Prapaporn, Poomtong Tanate, Sobhon Prasert. Nutritional components of the sea cucumber Holothuria acabra. Funct Foods Health Dis. 2017;7(3):168–181. [Google Scholar]
- 19.Jattujan P., Chalorak P., Siangcham T., et al. Holothuria scabra extracts possess anti-oxidant activity and promote stress resistance and lifespan extension in Caenorhabditis elegans. Exp Gerontol. 2018;110:158–171. doi: 10.1016/j.exger.2018.06.006. [DOI] [PubMed] [Google Scholar]
- 20.Chalorak P., Jattujan P., Nobsathian S., Poomtong T., Sobhon P., Meemon K. Holothuria scabra extracts exhibit anti-Parkinson potential in C. elegans: a model for anti-Parkinson testing. Nutr Neurosci. 2018;21(6):427–438. doi: 10.1080/1028415X.2017.1299437. [DOI] [PubMed] [Google Scholar]
- 21.Alvarez J., Alvarez-Illera P., Santo-Domingo J., Fonteriz R.I., Montero M. Modeling alzheimer's disease in Caenorhabditis elegans. Biomedicines. 2022;10(2) doi: 10.3390/biomedicines10020288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen X., Barclay J.W., Burgoyne R.D., Morgan A. Using C. elegans to discover therapeutic compounds for ageing-associated neurodegenerative diseases. Chem Cent J. 2015;9(1):65. doi: 10.1186/s13065-015-0143-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharma N., Khurana N., Muthuraman A. Lower vertebrate and invertebrate models of Alzheimer's disease - a review. Eur J Pharmacol. 2017;815:312–323. doi: 10.1016/j.ejphar.2017.09.017. [DOI] [PubMed] [Google Scholar]
- 24.Sangha J.S., Sun X., Wally O.S.D., et al. Liuwei Dihuang (LWDH), a traditional Chinese medicinal formula, protects against β-amyloid toxicity in transgenic Caenorhabditis elegans. PLoS One. 2012;7(8):e43990. doi: 10.1371/journal.pone.0043990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang W., Zhi D., Ren H., et al. Shengmai formula ameliorates pathological characteristics in AD C. elegans. Cell Mol Neurobiol. 2016;36(8):1291–1302. doi: 10.1007/s10571-015-0326-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pranweerapaiboon K., Apisawetakan S., Nobsathian S., Itharat A., Sobhon P., Chaithirayanon K. An ethyl-acetate fraction of Holothuria scabra modulates inflammation in vitro through inhibiting the production of nitric oxide and pro-inflammatory cytokines via NF-κB and JNK pathways. Inflammopharmacology. 2020;28(4):1027–1037. doi: 10.1007/s10787-019-00677-3. [DOI] [PubMed] [Google Scholar]
- 27.Diomede L., Rigacci S., Romeo M., Stefani M., Salmona M. Oleuropein aglycone protects transgenic C. elegans strains expressing Aβ42 by reducing plaque load and motor deficit. PLoS One. 2013;8(3) doi: 10.1371/journal.pone.0058893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kitisin T., Suphamungmee W., Meemon K. Saponin-rich extracts from Holothuria leucospilota mediate lifespan extension and stress resistance in Caenorhabditis elegans via daf-16. J Food Biochem. 2019;43(12) doi: 10.1111/jfbc.13075. [DOI] [PubMed] [Google Scholar]
- 29.Forloni G., Colombo L., Girola L., Tagliavini F., Salmona M. Anti-amyloidogenic activity of tetracyclines: studies in vitro. FEBS Lett. 2001;487(3):404–407. doi: 10.1016/s0014-5793(00)02380-2. [DOI] [PubMed] [Google Scholar]
- 30.Diomede L., Cassata G., Fiordaliso F., et al. Tetracycline and its analogues protect Caenorhabditis elegans from β amyloid-induced toxicity by targeting oligomers. Neurobiol Dis. 2010;40(2):424–431. doi: 10.1016/j.nbd.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 31.Yang J., Huang X.-B., Wan Q.-L., et al. Otophylloside B protects against Aβ toxicity in Caenorhabditis elegans models of Alzheimer's disease. Nat Product Bioprospect. 2017;7(2):207–214. doi: 10.1007/s13659-017-0122-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DaSilva K.A., Shaw J.E., McLaurin J. Amyloid-β fibrillogenesis: structural insight and therapeutic intervention. Exp Neurol. 2010;223(2):311–321. doi: 10.1016/j.expneurol.2009.08.032. [DOI] [PubMed] [Google Scholar]
- 33.Fernández-Moriano C., González-Burgos E., Iglesias I., Lozano R., Gómez-Serranillos M.P. Evaluation of the adaptogenic potential exerted by ginsenosides Rb1 and Rg1 against oxidative stress-mediated neurotoxicity in an in vitro neuronal model. PLoS One. 2017;12(8):e0182933. doi: 10.1371/journal.pone.0182933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Styren S.D., Hamilton R.L., Styren G.C., Klunk W.E. X-34, a fluorescent derivative of Congo red: a novel histochemical stain for Alzheimer's disease pathology. J Histochem Cytochem : Off J Histochem Soc. 2000;48(9):1223–1232. doi: 10.1177/002215540004800906. [DOI] [PubMed] [Google Scholar]
- 35.Link C.D., Johnson C.J., Fonte V., et al. Visualization of fibrillar amyloid deposits in living, transgenic Caenorhabditis elegans animals using the sensitive amyloid dye, X-34. Neurobiol Aging. 2001;22(2):217–226. doi: 10.1016/s0197-4580(00)00237-2. [DOI] [PubMed] [Google Scholar]
- 36.Cohen E., Bieschke J., Perciavalle R.M., Kelly J.W., Dillin A. Opposing activities protect against age-onset proteotoxicity. Science. 2006;313(5793):1604–1610. doi: 10.1126/science.1124646. [DOI] [PubMed] [Google Scholar]
- 37.Keowkase R., Shoomarom N., Bunargin W., Sitthithaworn W., Weerapreeyakul N. Sesamin and sesamolin reduce amyloid-β toxicity in a transgenic Caenorhabditis elegans. Biomed Pharmacother. 2018;107:656–664. doi: 10.1016/j.biopha.2018.08.037. [DOI] [PubMed] [Google Scholar]
- 38.Alexander A.G., Marfil V., Li C. Use of Caenorhabditis elegans as a model to study Alzheimer's disease and other neurodegenerative diseases. Front Genet. 2014;5:279. doi: 10.3389/fgene.2014.00279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Govindan S., Amirthalingam M., Duraisamy K., Govindhan T., Sundararaj N., Palanisamy S. Phytochemicals-induced hormesis protects Caenorhabditis elegans against alpha-synuclein protein aggregation and stress through modulating HSF-1 and SKN-1/Nrf2 signaling pathways. Biomed Pharmacother. 2018;102:812–822. doi: 10.1016/j.biopha.2018.03.128. [DOI] [PubMed] [Google Scholar]
- 40.Son T.G., Camandola S., Mattson M.P. Hormetic dietary phytochemicals. NeuroMolecular Med. 2008;10(4):236–246. doi: 10.1007/s12017-008-8037-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sangpairoj K., Chaithirayanon K., Vivithanaporn P., et al. Extract of the sea cucumber, Holothuria scabra, induces apoptosis in human glioblastoma cell lines. Funct Foods Health Dis. 2016;6:452. [Google Scholar]
- 42.Mitu S.A., Bose U., Suwansa-Ard S., et al. Evidence for a saponin biosynthesis pathway in the body wall of the commercially significant sea cucumber Holothuria scabra. Mar Drugs. 2017;15(11) doi: 10.3390/md15110349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hardy J., Selkoe D.J. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297(5580):353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 44.Wolff M., Zhang-Haagen B., Decker C., et al. Aβ42 pentamers/hexamers are the smallest detectable oligomers in solution. Sci Rep. 2017;7 doi: 10.1038/s41598-017-02370-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang W.Q.F., Lui Q., Qian c., et al. Evaluation of structure-activity relationships of ginsenosides against amyloid beta induced pathological behaviours in transgenic Caenorhabditis elegans. Royal Soci Chem Adv. 2017;7:40095–40104. [Google Scholar]
- 46.Tangrodchanapong T., Sornkaew N., Yurasakpong L., et al. Beneficial effects of cyclic ether 2-butoxytetrahydrofuran from sea cucumber Holothuria scabra against Aβ aggregate toxicity in transgenic Caenorhabditis elegans and potential chemical interaction. Molecules. 2021;26(8) doi: 10.3390/molecules26082195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chalorak P., Sornkaew N., Manohong P., et al. Diterpene glycosides from Holothuria scabra exert the alpha-synuclein degradation and neuroprotection against alpha-synuclein-Mediated neurodegeneration in C. elegans model. J Ethnopharmacol. 2021;279 doi: 10.1016/j.jep.2021.114347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grinan-Ferre C., Bellver-Sanchis A., Olivares-Martin M., Banuelos-Hortiguela O., Pallas M. Synergistic neuroprotective effects of a natural product mixture against AD hallmarks and cognitive decline in Caenorhabditis elegans and an SAMP8 mice model. Nutrients. 2021;13(7) doi: 10.3390/nu13072411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao L., Duan Z., Wang Y., et al. Protective effect of Terminalia chebula Retz. extract against Aβ aggregation and Aβ-induced toxicity in Caenorhabditis elegans. J Ethnopharmacol. 2021;268 doi: 10.1016/j.jep.2020.113640. [DOI] [PubMed] [Google Scholar]
- 50.DanQing L., YuJie G., ChengPeng Z., et al. N-butanol extract of Hedyotis diffusa protects transgenic Caenorhabditis elegans from Aβ-induced toxicity. Phytother Res. 2021;35(2):1048–1061. doi: 10.1002/ptr.6871. [DOI] [PubMed] [Google Scholar]
- 51.Pohanka M. Alzheimer's disease and oxidative stress: a review. Curr Med Chem. 2014;21(3):356–364. doi: 10.2174/09298673113206660258. [DOI] [PubMed] [Google Scholar]
- 52.Drake J., Link C.D., Butterfield D.A. Oxidative stress precedes fibrillar deposition of Alzheimer's disease amyloid beta-peptide (1-42) in a transgenic Caenorhabditis elegans model. Neurobiol Aging. 2003;24(3):415–420. doi: 10.1016/s0197-4580(02)00225-7. [DOI] [PubMed] [Google Scholar]
- 53.Wang Y., Cheng Q., Su Q., et al. Aesculin offers increased resistance against oxidative stress and protective effects against Abeta-induced neurotoxicity in Caenorhabditis elegans. Eur J Pharmacol. 2022;917 doi: 10.1016/j.ejphar.2022.174755. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.