Abstract
The development of refractory ascites in approximately 10% of patients with decompensated cirrhosis heralds the progression to a more advanced stage of cirrhosis. Its pathogenesis is related to significant hemodynamic changes, initiated by portal hypertension, but ultimately leading to renal hypoperfusion and avid sodium retention. Inflammation can also contribute to the pathogenesis of refractory ascites by causing portal microthrombi, perpetuating the portal hypertension. Many complications accompany the development of refractory ascites, but renal dysfunction is most common. Management starts with continuation of sodium restriction, which needs frequent reviews for adherence; and regular large volume paracentesis of 5 L or more with albumin infusions to prevent the development of paracentesisinduced circulatory dysfunction. Albumin infusions independent of paracentesis may have a role in the management of these patients. The insertion of a covered, smaller diameter, transjugular intrahepatic porto-systemic stent shunt (TIPS) in the appropriate patients with reasonable liver reserve can bring about improvement in quality of life and improved survival after ascites clearance. Devices such as an automated low-flow ascites pump may be available in the future for ascites treatment. Patients with refractory ascites should be referred for liver transplant, as their prognosis is poor. In patients with refractory ascites and concomitant chronic kidney disease of more than stage 3b, assessment should be referred for dual liver-kidney transplants. In patients with very advanced cirrhosis not suitable for any definitive treatment for ascites control, palliative care should be involved to improve the quality of life of these patients.
Keywords: Ascites, Transjugular intrahepatic portosystemic stent shunt, Liver transplantation, Albumin, Paracentesis
INTRODUCTION
The development of ascites in the natural history of cirrhosis heralds the onset of decompensation. More contemporary data from the United Kingdom suggest that decompensation occurs at the rate of 31% in the first year after the diagnosis of cirrhosis, and thereafter at the rate of 5–7% per annum, with ascites being the most common mode of decompensation [1]. Ascites is usually responsive to diuretic therapy at the initial stage. However, with the progression of the cirrhotic process, renal sodium retention becomes more avid and increasing diuretic doses are required to control the ascites. Ultimately, the patient either develops complications to the diuretics or the ascites is no longer responsive to the diuretics. The patient is said to have refractory ascites (RA) and some form of second-line therapy will need to be instituted. Approximately 10% of patients with cirrhosis and ascites have RA at any given time. In addition to the usual complications associated with the presence of ascites such as the risk for the development of spontaneous bacterial peritonitis, electrolyte abnormalities, or renal dysfunction, the presence of RA is associated with its own unique problems such as a constant sense of fullness, decreased appetite, the development of various hernias, nutritional deficiencies, and sarcopenia. Therefore, patients with RA have a very poor quality of life [2]. Older literature has indicated a 1-year mortality for patients with RA to be at 50% [3], although more recent reports have indicated a slightly improved prognosis, but the mortality is still in excess of 20% at 1 year [4].
DEFINITION OF RA
Tense ascites can be recurrent or refractory. Recurrent ascites is ascites that recurs at least three times a year despite dietary sodium restriction and diuretic therapy. It may be a forerunner of RA [5]. RA is defined as ascites that cannot be mobilized or the early recurrence of which (after a large volume paracentesis [LVP]) cannot be prevented by medical therapy [5]. RA can be divided into two subtypes: diuretic resistant or diuretic intolerant. Table 1 details the diagnostic criteria for both subtypes.
Table 1.
Diagnostic criteria for refractory ascites
| Criteria | ||
|---|---|---|
| Refractory ascites | Ascites that cannot be mobilized or the early recurrence of which (i.e., after therapeutic paracentesis) cannot be satisfactorily prevented by medical therapy | |
| A) diuretic resistant | The development of refractory ascites is due to lack of response to dietary sodium restriction and maximal doses of diuretics | |
| B) diuretic intractable | The development of refractory ascites is due to the development of diuretic-induced complications* that precludes the use of effective doses of diuretics | |
| Duration of treatment | Maximum doses of diuretic + adherence to a low sodium diet of ≤88 mmol/day for ≥1 week | |
| Maximum diuretic doses | Either spironolactone 400 mg/day or amiloride 30 mg/day plus furosemide 160 mg/day | |
| Lack of response | Mean weight loss of <0.8 kg over 4 days and daily urinary sodium excretion less than the daily sodium intake | |
| Early ascites recurrence | Re-appearance of grade 2 or moderate ascites with moderate symmetrical abdominal distention, or grade 3 with massive ascites with marked abdominal distention within 4 weeks of initial mobilization | |
| Diuretic induced complications* | Renal impairment, hyponatremia, hypo- or hyperkalemia, hepatic encephalopathy | |
Adapted from Salerno et al. [5]
Renal impairment: increase of serum creatinine by >100% to a value >133 μmol/L (2 mg/dL) in patients with ascites responding to treatment. Hyponatremia: decrease of serum sodium by >10 mmol/L to a serum sodium of <125 mmol/L. Hypo- or hyperkalemia: change in serum potassium to <3 mmol/L or >6 mmol/L despite appropriate measures. Hepatic encephalopathy: development of encephalopathy in the absence of any other precipitating factor.
PATHOPHYSIOLOGY OF RA
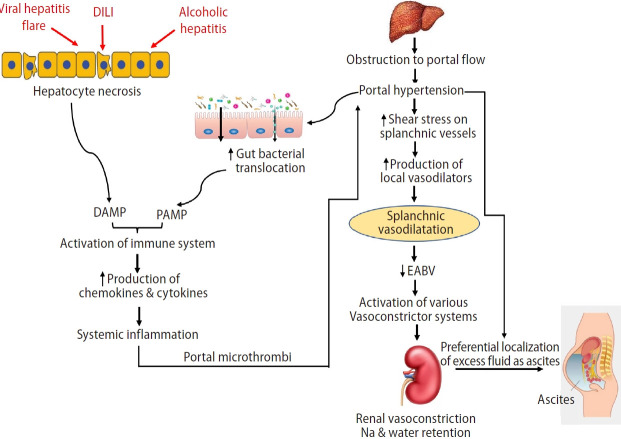
Patients with decompensated cirrhosis have significant hemodynamic changes, initiated by architectural distortion of the liver related to cirrhosis. The laying down of fibrous scar tissues and nodular formation within the liver provides the fixed component of obstruction to portal flow, while stellate cell activation furnishes the dynamic component of increased resistance to portal flow. Stellate cells themselves also produce extracellular matrix and collagen, adding to the fixed component of the increase in intrahepatic resistance as the liver cirrhosis progresses. Microthrombi formation within the intrahepatic vasculature can add to the distortion of the liver architecture by causing areas of parenchymal extinction [6]. Another process that contributes to the progressive increase in portal hypertension in cirrhosis is the development of collateral vessels. There is angiogenesis driven by vascular endothelial growth factor, augmenting the splanchnic capacitance, leading to increased portal flow [7], perpetuating the portal hypertension.
The development of portal hypertension has many downstream effects. Firstly, the distension of the splanchnic vessels increases the shear stress on the vessels, and this leads to the production of various vasodilators including nitric oxide. As a result, splanchnic vasodilatation occurs. Some of these excess splanchnic vasodilators can be transferred to the systemic circulation via portosystemic shunts, causing systemic vasodilation. The resultant relative insufficient effective arterial blood volume (EABV) leads to the activation of various vasoconstrictor systems in an attempt to reduce the extent of the splanchnic and systemic vasodilation and to stimulate renal sodium and water retention to increase the intravascular volume. However, the presence of portal hypertension will preferentially localize the excess fluid into the peritoneal cavity as ascites, leaving the central circulation relatively deficient in EABV.
Another downstream effect of portal hypertension is the disruption of the gut vascular barrier related to venous congestion from splanchnic vasodilatation and splanchnic neo-angiogenesis. The increased permeability of the gut results in a rise in the translocation of gut bacteria. Many of these bacterial products have vasodilatory properties themselves; contributing to the splanchnic vasodilatation. Other components of bacterial products can stimulate the innate immune system, leading to systemic inflammation. Within the liver, the pro-inflammatory milieu promotes further fibrosis; within the splanchnic circulation, inflammation promotes splanchnic thrombosis [7,8], further aggravating the portal hypertension, thereby perpetuating the above-mentioned portal hypertension-related hemodynamic changes (Fig. 1).
Figure 1.
Pathophysiology of ascites formation. DILI, drug induced liver injury; DAMP, damage associated molecular pattern; PAMP, pathogen associated molecular pattern; EABV, effective arterial blood volume.
As the cirrhotic process progresses, and the portal hypertension increases, the above changes become more severe, and the sodium retention becomes more avid, while the renal circulation becomes more vasoconstricted. Ultimately, altered renal blood flow sets in [9], renal hypoperfusion ensues, leading to the development of chronic renal insufficiency, or what was previously known as type 2 hepatorenal syndrome, and the ascites becomes refractory to diuretic therapy.
MANAGEMENT OF RA
The management of patients with RA should follow a stepwise approach, starting with sodium restriction, and LVP. Judicious use of medications could avoid further complications. In the appropriate patients, the insertion of a transjugular intrahepatic portosystemic stent shunt (TIPS) should be considered. All patients with RA should be assessed for liver transplant (Fig. 2).
Figure 2.
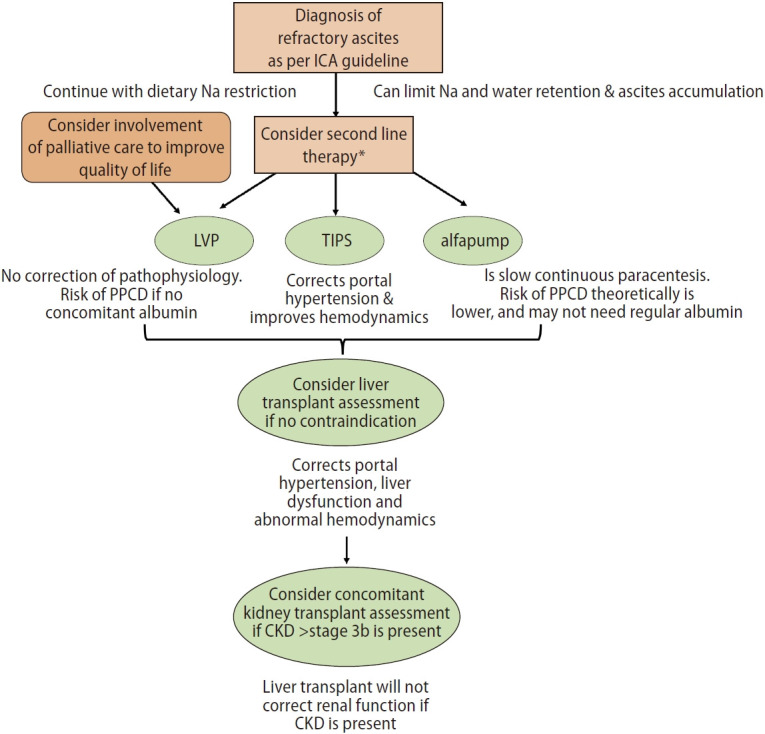
Suggested treatment algorithm of refractory ascites. ICA, International Ascites Club; LVP, large volume paracentesis; PPCD, post paracentesis circulatory dysfunction; TIPS, transjugular intrahepatic portosystemic stent shunt; alfapump, automated low flow ascites pump; CKD, chronic kidney disease. *Second line therapies are: LVP, TIPS or alfapump.
Dietary sodium and fluid restriction
Dietary sodium restriction is required at all stages of ascites including those with RA, as it reduces the rate of ascites accumulation. It is recommended that daily sodium intake should be limited to 88 mmol or 2 g per day [10]. Counselling with a dietitian is helpful, as is frequent reviews of food diary, especially in patients who are accumulating ascites at a rapid rate. Information on where to purchase low sodium food items and advice on low sodium recipes are other measures that can improve compliance with sodium restriction. Some patients who have been labeled as having RA can lose their ascites and start responding to diuretics again once they adhere to their sodium restriction, especially in patients whose daily renal sodium excretion is more than 88 mmol/day.
Fluid restriction is not required in patients with RA. It is difficult to enforce and is not practical. Fluid restriction is only useful when the fluid intake is less than the urine output, which in patients with RA is often around 500 mL/day. In patients who have hyponatremia with serum sodium of ≤125 mmol/L, it is recommended that some fluid restriction be instituted [11]. However, the level of serum sodium that should initiate fluid restriction has not been well defined.
Calculating the sodium balance
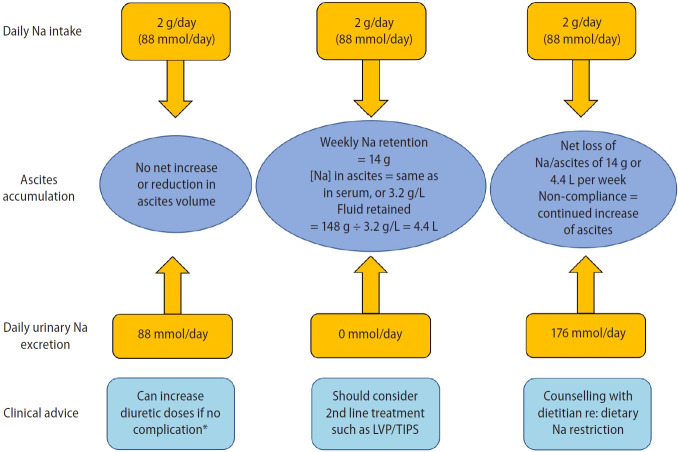
This is important in determining compliance with dietary sodium restriction, especially in patients who are rapidly gaining weight after a LVP. A 24-hour urine collection to measure the renal sodium output and a weight chart are required. A 24-hour urine collection is preferred to a spot urine sample as it is more accurate. In patients who are prescribed an 88 mmol daily sodium restriction diet, and who are excreting no urinary sodium at all, the daily sodium accumulation is 88 mmol/day or 616 mmol/week. Since the ascitic sodium concentration is the same as serum sodium concentration, the weekly ascites accumulation is 616 mmol/week ÷ 140 mmol/L or 4.4 L/week. Any patient who is requesting a weekly LVP of more than 4.4 L is clearly non-compliant with dietary sodium restriction, and repeat dietary counselling is needed (Fig. 3). The accumulation of ascites is usually a little less, as there is insensible loss of sodium through the respiratory tract. Frequently, a food record is very revealing, as many patients regard sodium restriction as “just not adding salt at the table” without realizing that many prepared food items are high in sodium. Patients who are excreting more than 88 mmol of sodium per day should be losing weight while on an 88 mmol sodium intake per day, as they should be in a negative sodium balance. If this is not happening, the dietary reeducation is needed [12].
Figure 3.
Calculating the sodium balance. LVP, large volume paracentesis; Na, sodium; TIPS, transjugular intrahepatic portosystemic stent shunt. *Renal impairment as indicated by increase of serum creatinine by >100% to a value of >133 μmol/L or 2 mg/dL; or hyponatreamia with a decrease in serum sodium of >10 mmol/L to a value of <125 mmol/L; or hypokalemia to a value of <3.0 mmol/L; or hyperkalemia to a value of >6.0 mmol/L; or the development of hepatic encephalopathy in the absence of any other precipitating factors.
Albumin infusions
Regular albumin infusions have been advocated for the management of patients with decompensated cirrhosis. Indeed, in patients with uncomplicated ascites who were still responding to diuretic therapy, the use of regular albumin infusions, initially 40 g twice weekly for 2 weeks, then 40 g weekly for a total of 18 months, has been shown to improve their overall survival [13], especially in patients whose serum albumin was maintained at a minimum of 40 g/L [14]. However, for patients with more advanced cirrhosis who were on the liver transplant waiting list, the use of regular albumin infusions at a dose of 40 g every 2 weeks plus midodrine did not impact their probability of developing complications nor their survival [15]. In the only randomized controlled trial including 70 patients with cirrhosis and RA, 45 patients were randomized to receive 40 g of albumin twice weekly [16]. There was a significant reduction in the 24-month hospital admissions for complications of cirrhosis and mortality. This suggests that regular albumin infusions may be beneficial for these patients. However, further supportive randomized controlled trials are needed before regular albumin infusions can be recommended as the standard of care for patients with cirrhosis and RA. It also appears that the dosing and frequency of infusions may be important to achieve positive results.
The use of non-selective beta-blockers (NSBBs)
NSBBs are the cornerstone in the management of portal hypertension in cirrhosis. The blocking of β1 adrenergic action reduces heart rate and hence cardiac output by 20%; the blocking of β2 adrenergic action in the splanchnic vasculature allows unopposed adrenergic action, causing splanchnic vasoconstriction, and hence reduced portal inflow including that from collateral vessels by about 15%. Therefore, the total reduction in portal venous flow with NSBB use is approximately 35%. Labetalol and carvedilol are 2 NSBBs that also have α1 adrenergic blocking effects, and therefore can cause intra-hepatic vasodilatation, with further reduction in portal pressure. The use of NSBB in patients with compensated cirrhosis and clinically significant portal hypertension (hepatic venous pressure gradient ≥10 mmHg) has been shown to significantly reduce the likelihood of decompensation or death [17]. However, the use of NABBs in patients with ascites is more controversial. The initial studies certainly indicated that the use of NSBBs in patients with ascites, especially those with RA, was associated with increased complications and mortality [18-21]. Subsequent studies showed that NSBB use in patients with ascites, including those with RA had no impact on the development of renal dysfunction or mortality [22,23]. There were also less bacterial infections with NSBB use [24]. In fact, the withdrawal of NSBB was associated with an increase in the likelihood of variceal bleeding, bacterial infections, and the development of renal dysfunction, as well as an increase in hospitalization rate and mortality [25]. In patients with acute-on-chronic liver failure (ACLF), many of whom had RA, the use of NSBB was thought to be associated with a reduction in ACLF grade [26]. These seemingly contradictory findings may be related to significant heterogeneity among the various studies.
The recent detailed evaluation of the cardiovascular effects of NSBB use in advanced cirrhosis has shed some light to guide the use of NSBB in patients with RA [27]. These patients with their significant arterial vasodilatation are critically dependent on adequate cardiac systolic function and sympathetic hyper-activity to maintain renal perfusion. Therefore, the use of NSBB may impair cardiac systolic function and reduce the renal perfusion pressure to the point that is below the threshold of renal blood flow autoregulation. That is, the kidneys are no longer able to adjust the renal perfusion in response to a fall in the perfusion pressure. Therefore, patients with RA who are taking NSBB are at risk for the development of renal dysfunction including hepatorenal syndrome [28]. The guidance from the 2021 American Association for the Study of the Liver on the management of ascites suggests that NSBBs may be withheld in patients with hemodynamic abnormalities as indicated by low systolic blood pressure <90 mmHg, hyponatremia with serum sodium <130 mmol/L, or the presence of acute kidney injury. NSBBs might be reintroduced if circulatory dysfunction improves with improvement of these parameters [11]. Carvedilol is not recommended for patients with RA as it causes more systemic hypotension due to its additional adrenergic blocking effects [29].
Large volume paracentesis (LVP)
LVP arbitrarily has been defined as removal of ascites of >5 L. Because LVP does not correct the underlying pathophysiology of ascites formation, ascites recurs soon after a session of LVP. This is because the loss of ascites through LVP is associated with a reduction in the intra-abdominal pressure, and this tends to exaggerate the pressure difference between the cirrhotic liver and the abdominal cavity, which encourages the rapid refilling of the abdominal cavity. Therefore, repeat LVPs are usually required in the management of these patients. Repeat LVPs have been shown to be safe and effective in the management of RA in cirrhosis, and is associated with lower incidence of electrolyte abnormalities, renal dysfunction, and hemodynamic instability when compared to continued diuretic use [30]. However, the redistribution of the circulatory volume to refill the abdominal cavity can lead to a further reduction in the EABV, increasing the likelihood of developing further renal dysfunction, dilutional hyponatremia, and risk for mortality, a condition known as post-paracentesis circulatory dysfunction (PPCD) [31]. Therefore, volume replacement with colloid solutions such as albumin has been recommended following LVP to prevent PPCD [32]. In general, the higher the volume of LVP, the more likely the patient is to develop PPCD. There has never been a dose response study for albumin use for LVP and the literature has reported various doses of albumin being used with LVP. Expert opinion suggests an albumin dose of 6–8 g/L of ascites removed [11], although reduced dose of 4 g of albumin/L of ascites removed was equally effective in the prevention of PPCD [33]. A further study showed that by providing a higher amount of albumin of 9.0±2.5 g/L of ascites removed and limiting LVP to 8 L can prevent the development of renal dysfunction despite the presence of PPCD [34]. Survival was also not affected over a mean follow-up of 2 years in those who developed PPCD.
It has been suggested that paracenteses of <5 L do not require any intravascular volume replacement, as these small paracenteses are not associated with significant disturbance of systemic and renal hemodynamics [35]. However, in patients with ACLF, albumin use with small volume paracentesis has been shown to reduce the incidence of PPCD together with its attendant complications such as acute kidney injury, hyponatremia, and high mortality [36]. This is because albumin, with its volume expanding, anti-inflammatory and immune modulatory properties, can significantly reduce the heightened inflammation and severely deranged hemodynamics that are commonly observed in patients with ACLF.
Finally, the presence of coagulopathy should not be a contra-indication to LVP, as minimal bleeding was reported with LVP even in patients who had an PT-INR of >1.5 and a platelet count of <50×109/L [37]. Therefore, the infusion of platelets or clotting factors are not necessary for LVP.
TIPS
A TIPS is a prosthesis that bridges a branch of the portal vein with a branch of the hepatic vein and is very effective in reducing the portal pressure. Physiologically, the lowering of portal pressure allows the gradual return of the splanchnic volume to the central circulation, thereby slowly filling the EABV [38]. This is associated with the gradual suppression of the activated renin-angiotensin-aldosterone and the sympathetic nervous systems [39], accompanied by a gradual reduction in the severity of renal sodium retention in these patients. When the activities of these neurohormonal systems have fallen to below their sodium retaining thresholds, we can expect ascites clearance. This usually takes about 3 to 6 months [40]. Six months post TIPS placement in patients with RA, 45% of patients show complete response, whilst 63% show partial response [41]. Eventually, TIPS is effective in controlling ascites in approximately 80% of patients. Therefore, it is important to manage patient expectation, as TIPS does not clear the ascites instantly; rather, the ascites will gradually diminish until it eventually disappears. While the ascites is still present, it is important to continue dietary sodium restriction until total ascites clearance. The use of diuretics post-TIPS is controversial, as this tends to reduce the EABV, and theoretically can delay the clearance of ascites.
Several randomized controlled trials have compared TIPS vs. LVP in the management of RA (Table 2) [42-47], and all shown that TIPS is significantly better than LVP in the control of ascites. However, the survival advantage of TIPS over LVP in patients with RA was not established until recently [48], and this is dependent on careful patient selection [49,50]. In general, younger patients who have a low Model of End-stage Liver Disease (MELD) score tend to do well with excellent transplant-free survival at 3 years [50]. This is especially true if the patient’s major problem is related to portal hypertension and not liver dysfunction. A small increase in patient’s age, MELD score, or hemodynamic parameters can decrease transplant-free survival significantly [50]. For the former patient, TIPS can be used as a definitive treatment for the RA, while for the latter patient, TIPS is used as a bridge therapy while waiting for a liver transplant. A recent study suggests that a TIPS inserted in patients with recurrent ascites (the need for at least three LVPs within 12 months with a time interval of >4 weeks between LVPs) could result in fewer side effects and improved survival when compared to LVP (93% vs. 52%, P=0.003) [51]; in particular, the post-TIPS incidence of hepatic encephalopathy (HE) (see below) was similar between the two groups. However, this study has not been replicated, and therefore, TIPS insertion at the stage of recurrent ascites cannot be recommended as standard of care yet.
Table 2.
Randomized controlled trials of TIPS vs. LVP as treatment for refractory ascites
| Study | No. of assessed/enrolled | Inclusion criteria | Exclusion criteria | Safety | Efficacy |
|---|---|---|---|---|---|
| Lebrec et al. [42] (1996) | 25/25 | Cirrhosis & RA defined as: no response to maximum diuretics for 5 days while in hospital, or ≥2 admissions for tense ascites in ≤4 months | Age >70 years | 1) HE: TIPS group, severe recurrent grade 3 HE in 2 patients, mild HE in 1 patient; LVP group, no HE | Minimum or no ascites at 4 months of all surviving patients: TIPS group, 5/6; paracentesis group, 1/10 |
| HE ≥ grade 2 | |||||
| PVT | |||||
| Biliary obstruction | |||||
| sCr >1.7 mg/dL | 2) Survival at 2 years: TIPS group, 29%±13%; paracentesis group, 60%±16% | ||||
| HCC | |||||
| Active bacterial infection | |||||
| Severe extra-hepatic disease | |||||
| Pulmonary hypertension | |||||
| Rössle et al. [43] (2000) | 150/60 | Cirrhosis & RA as defined by IAC criteria*: 55% | HE ≥ grade 2 | 1) HE at mean follow-up of approximately 44 months: TIPS group, 58%; LVP group, 48% | Complete response at 6 months: TIPS group, 79%; paracentesis group, 24% (P=0.001) |
| PVT | |||||
| Cirrhosis & recurrent ascites: 45% | Bilirubin >5 mg/dL | ||||
| sCr >3.0 mg/dL | |||||
| Advanced HCC | 2) Survival at 2 years: TIPS group, 58%; paracentesis group, 32% (P=0.11) | Partial response at 6 months: TIPS group, 5%; paracentesis group, 19% (P=N.S.) | |||
| Hepatic hydrothorax | |||||
| Technical failure of paracentesis | |||||
| Ginès et al. [44] (2002) | 119/70 | Cirrhosis & RA as defined by IAC criteria* | Age <18 or >75 years | 1) Severe HE at mean followup of 282–325 days: TIPS group, 60%; LVP group, 34% (P=0.03) | Median time to ascites recurrence: TIPS group, 171 days; paracentesis group, 20 days (P<0.0001) |
| HE ≥ grade 2 | |||||
| PVT | |||||
| Bilirubin >10 mg/dL | |||||
| sCr >3.0mg/dL | |||||
| Prothrombin index <40% | 2) Transplant free survival at 2 years: TIPS group, 26%; paracentesis group, 30% (P=0.51) | ||||
| Platelet <40×109/L | |||||
| HCC | |||||
| CHF | |||||
| Parenchymal renal disease | |||||
| Sanyal et al. [45] (2003) | 525/109 | Cirrhosis & RA as defined by IAC criteria* | HE ≥ grade 2 | 1) Severe HE: TIPS group, 38%; LVP group, 21% (P=0.058) | Recurrent ascites at 180 days: TIPS group, 6/57 (10.5%); paracentesis group, 26/52 (50%) (P<0.0001) |
| PVT | |||||
| sCr <1.5 mg/dL | Bilirubin >10 mg/dL | ||||
| INR >2.0 | |||||
| HCC | 2) Transplant free survival: TIPS group, 19.6 months; paracentesis group, 12.4 months (P=0.77) | ||||
| Bacterial infection | |||||
| Alcoholic hepatitis | |||||
| Cardiopulmonary failure | |||||
| Pulmonary hypertension | |||||
| Parenchymal renal disease | |||||
| Recent GI bleed | |||||
| Life limiting extra-hepatic disease | |||||
| Salerno et al. [49] (2007) | 137/66 | Cirrhosis & RA as defined by IAC criteria*: 68% | Age >72 years | 1) HE: TIPS group, 61%; LVP group, 39% (P=0.086) | Failure of treatment: TIPS group, 7/33 (21.2%); paracentesis group, 19/33 (57.6%) (P=0.0012) |
| HE ≥ grade 2 | |||||
| Cirrhosis & recidivant ascites: 32% | PVT | ||||
| Bilirubin >6 mg/dL | |||||
| sCr >3.0 mg/dL | 2) Transplant free survival at 2 years: TIPS group, 59%; paracentesis group, 29% (P=0.021) | ||||
| Child-Pugh score >11 | |||||
| Advanced HCC | |||||
| Bacterial infection | |||||
| Cardiopulmonary failure | |||||
| Recent GI bleed | |||||
| Narahara et al. [47] (2011) | 78/60 | Cirrhosis & RA as defined by IAC criteria* | Age >70 years | 1) Severe HE: TIPS group, 67%; LVP group, 17% (P<0.01) | Complete or partial response at 1 year: TIPS group, 20/30 (67%); paracentesis group, 8/30 (17%) (P<0.005) |
| Episodes of HE | |||||
| Bilirubin <3.0 mg/dL | PV cavernoma | ||||
| sCr <1.9 mg/dL | HCC or other Malignancy | 2) Overall survival at 2 years: TIPS group, 64%; paracentesis group, 35% (P<0.005) | |||
| Child-Pugh score <11 | Active infection | ||||
| Active severe | |||||
| Cardiac or pulmonary disease | |||||
| Organic kidney disease |
TIPS, transjugular intrahepatic portosystemic stent shunt; RA, refractory ascites; HE, hepatic encephalopathy; PVT, portal vein thrombosis; sCr, serum creatinine; HCC, hepatocellular carcinoma; LVP, large volume paracentesis; IAC, International Ascites Club; N.S., not significant; CHF, congestive heart failure; INR, international normalized ratio; GI, gastrointestinal; PV, portal vein.
The insertion of TIPS is associated with many complications. Immediate complications related to the procedure include arrhythmia, hemoperitoneum, and liver capsule rupture, which in experienced hands are rare. Other complications in the early post-TIPS period include shunt migration, shunt kinking, and ischemic hepatitis as evidenced by a significant rise in liver enzymes and hemolytic anemia. Therefore, patients may remain jaundiced for several weeks to months post TIPS. When bare stents were used in earlier times, shunt stenosis occurred frequently. These are now relatively uncommon with the polytetrafluoroethylene (PTFE) covered stents [52], related to reduction in the thickness of the neointima. The major clinical complication is HE, either newly onset or worsening of existing HE irrespectively of the type of stent used, estimated to occur in 30–50% of patients [53,54]. The risk factors for the development of HE includes advancing age, higher Child-Pugh and MELD scores, prior episodes of spontaneous HE, sarcopenia and lower portal systemic pressure gradient post-TIPS [55]. The latter is usually associated with a maximally dilated TIPS. A recent report confirmed a lower incidence of HE (27%) in patients who had their PTFE stent deliberately under-dilated to 6 mm compared to patients whose stent was dilated to 8–10 mm (54%) without any negative impact on variceal bleeding or ascites recurrence or on the incidence of stent thrombosis [56]. Therefore, it appears that either under-dilation or smaller diameter stents are more appropriate to reduce the likelihood of post-TIPS HE. The use of lactulose and rifaximin pre-emptively has also been shown to provide better HE control in the post-TIPS period [57,58]. Another potential complication of TIPS insertion is the development of cardiac failure post-TIPS. The placement of TIPS returns a significant volume from the splanchnic circulation to the systemic circulation, and the cardiac output can increase by up to 50% [38]. Therefore, patients with pre-existing cardiac dysfunction, whether systolic incompetence or abnormal diastolic relaxation, or the presence of pulmonary hypertension are at risk for post-TIPS cardiac decompensation. The appropriate pre-TIPS cardiac investigations include electrocardiogram, echocardiogram, and measurement of brain natriuretic peptide (BNP) [59]. A normal cardiac investigation with a BNP level of <40 pg/mL and a pro-N-terminal BNP of <125 pg/mL have been reported as indicators that will rule out cardiac decompensation post-TIPS [60].
Other pre-TIPS investigations include assessing for sites of infection, especially biliary and dental infections. Once the TIPS is inserted, any source of infection that can reach the TIPS via the blood stream may produce endotipsitis. Although this is a rare complication, the occurrence of endotipsitis will lead to recurrence of bacteremia which may not be eradicated even with prolonged courses of antibiotics [61].
Appropriate patient selection is very important to optimize patient response to TIPS with clearance of ascites and to reduce the likelihood for complications. Various academic societies have recommended against placing a TIPS in patients who are older than 70 years of age, with a MELD score of >18, who have had spontaneous HE ≥ grade 2, or the presence of cardiac failure, pulmonary hypertension, liver cancer, sepsis, or occlusive portal vein thrombosis [11,62]. Clearance of ascites with TIPS is associated with improved quality of life [63], better nitrogen balance [64], significant muscle gain [65], and improved survival [48,66,67]. A recently validated Freiburg Index of Post-TIPS survival (FIPS) included age, bilirubin, albumin, and creatinine in its prediction of high risk for mortality post-TIPS. Patients in the high-risk category had a median post-TIPS survival of 5 months vs. 48 months in the low-risk group (P<0.001) [68]. However, the predictive power of FIPS is not as accurate in patients who have received an early TIPS for ascites that has not yet reached the refractory stage. A further study showed improved post-TIPS survival in patients who received an 8 mm covered stent compared to those who received a 10 mm covered stent [69].
The automated low flow ascites pump (alfapump)
The alfapump is a programmable and rechargeable device that is implanted subcutaneously. It slowly pumps the ascites from the peritoneal cavity via a peritoneal catheter and discharges it via a bladder catheter into the bladder, from there it is discharged as urine (Fig. 4). Effectively, it is performing continuous small volume paracentesis. The device is programmed to pump ascites for up to 16 hours during awake hours, so not to disturb the patient’s sleep by requiring the patient to urinate the ascites at night. The device is fitted with various sensors in the peritoneum and in the bladder so that it will stop pumping if there is little or no ascites. The rate of ascites discharge can also be adjusted according to the patient’s dietary sodium consumption. Therefore, the management of ascites is individualized. Usually, the use of the alfapump system does not require the concomitant use of albumin infusions.
Figure 4.
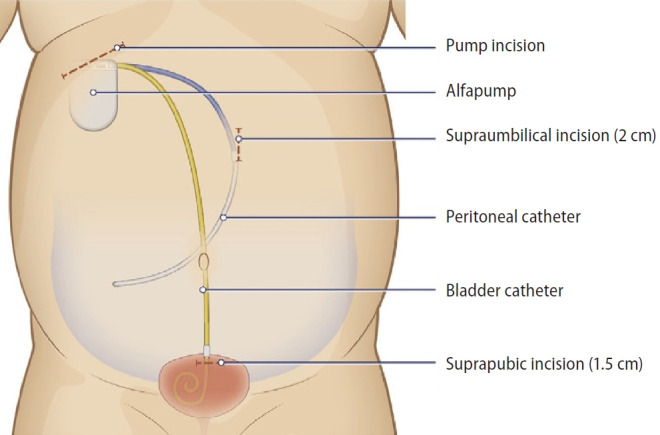
The automated low flow ascites pump (alfapump) system in situ.
A randomized controlled trial [70], several prospective [71-75], and retrospective [76] studies as well as a meta-analysis [77] have shown that the alfapump is effective in the control of ascites by reducing the frequency and volume of paracenteses. The initial study showed a high incidence of complications including infection of the alfapump system, pump malfunction, and dislodgement of catheters [71]. With the use of prophylactic antibiotics, refinement of pump design, and the implantation techniques, these complications have become less frequent. A proportion of patients still develop renal dysfunction despite the slow continuous discharge of ascites. A physiological study showed that there is still activation of the various vasoconstrictor systems with the small volume but continuous paracentesis [78]. It has been suggested that patients should be monitored for the development of renal dysfunction, and given intermittent albumin as required. Therefore, it is prudent to avoid alfapump insertion in patients with renal dysfunction with serum creatinine >132 μmol/L (1.5 mg/dL) or estimated glomerular filtration rate <30 mL/min/1.32 m2 [79]. Other contra-indications for alfapump insertion include at least 2 or more systemic or local abdominal infections in the previous 6 months, recent intra-abdominal surgery, history of bladder cancer, previous solid organ transplantation, and bilirubin level >85 μmol/L [79].
Once the ascites is under control, patients show significant improvement in their mobility and quality of life [75,80]. The effects of the alfapump on the survival of these patients has not been formally studied but has been shown to be at least the same as patients who undergo regular LVP [66].
Liver transplantation
Liver transplantation remains the definitive treatment for patients with RA and concomitant liver dysfunction. However, for patients whose major complications of liver cirrhosis are related to portal hypertension alone without significant liver dysfunction, their priority for liver transplantation remains low. A recent publication has shown that patients with ascites and a MELD score of <15 still had a mortality risk of 47.5% at 1 year without a liver transplant, related to infectious causes [81]. The presence of persistent ascites is equivalent to adding 4.5 MELD [82] or 3.5 MELD-Na [83] score points to the patient’s calculated MELD, especially in patients with a lower calculated MELD score of less than 21 [83,84]. Therefore, patients with RA as the only manifestation of cirrhosis should still be considered for liver transplantation despite fairly low MELD score. Patients with RA and concomitant hyponatremia will have higher priority for liver transplant as their ranking will be captured by a higher MELD-Na score. After liver transplantation, ascites may persist for weeks to months, as it takes time for the systemic and renal hemodynamics to adjust back to normal, especially if high portal inflow persists after liver transplantation [85]. Therefore, patients are advised to remain on sodium-restricted diet in the post-transplant period until ascites disappears.
Treatment of RA in patients with chronic kidney disease (CKD)
CKD has always been known to be associated with RA. It used to be known as type 2 hepatorenal syndrome [86]. However, the prevalence of CKD in cirrhosis is increasing, related to the increased prevalence of nonalcoholic steatohepatitis and its associated conditions such as diabetes mellitus and systemic hypertension [87]. Furthermore, it is now also recognized that CKD can develop after repeat episodes of acute kidney injury [88]. There is very little published literature specifically on the management of ascites in patients with CKD. In general, patients with RA and CKD should have their ascites managed the same way as patients without CKD. However, the volume of ascites removed at paracentesis should not be excessive, as the risk for acute kidney injury post-paracentesis is proportional to the volume of ascites removed [89]. The insertion of a TIPS to treat RA in patients with CKD also appears to be safe [90]. When these patients are being evaluated for liver transplantation, consideration should be given for combined liver kidney transplant, especially in patients with stage ≥3b CKD with their GFR at ≤44 mL/min/1.73 m2 for more than 3 months.
Palliative care in patients with RA
There remains a significant number of patients with RA who are not liver transplant candidates. They are not appropriate as TIPS recipients because of comorbid conditions, and the alfapump system is not widely available. Therefore, LVP remains the only option available to these patients as a treatment for their RA. Recently, there has been a push for these patients to receive palliative care as their survival is rather limited [91]. There is some interest in the use of a tunnelled catheter to provide long-term ascites drainage at home rather than having regular hospital visits for LVPs [92,93]. Some patients reported preference for the tunnelled catheter as this avoids repeat LVPs in hospital, and therefore improved quality of life [94]. However, bacterial peritonitis, ascites leakage, and local cellulitis remain concerns [95]. Therefore, until there are well-designed randomized controlled trials to confirm its safety and efficacy, this cannot be recommended as standard of care for patients with advanced liver disease and RA [96].
CONCLUSION
RA represents further deterioration of the patient with ascites when the ascites is no longer responsive to diuretic therapy. Despite this, sodium restriction remains an integral part of the management of these patients. LVP remains the cornerstone of ascites management, but care needs to be taken to avoid inducing the development of PPCD. Regular infusions of albumin may be of benefits but remain to be proven. In the appropriate patients, TIPS insertion can provide permanent relief of ascites. The use of an alfapump system in patients who are not TIPS candidates can provide slow and continuous ascites removal, therefore eliminating abdominal bloating with associated benefits of increased appetite, and eventual improved mobility. This requires long-term use of antibiotics as prophylaxis against infection of the alfapump system. As the prognosis of patients with cirrhosis is negatively impacted by the presence of RA, these patients need to be assessed for liver transplant. In patients with RA and CKD, consideration should be given for combined liver-kidney transplant.
Abbreviations
- ACLF
acute-on-chronic liver failure
- alfapump
automated low flow ascites pump
- BNP
brain natriuretic peptide
- CKD
chronic kidney disease
- EABV
effective arterial blood volume
- FIPS
Freiburg Index of Post-TIPS survival
- HE
hepatic encephalopathy
- LVP
large volume paracentesis
- MELD
Model of End-stage Liver Disease
- NSBB
non-selective beta-blocker
- PPCD
post-paracentesis circulatory dysfunction
- PTFE
polytetrafluoroethylene
- RA
refractory ascites
- TIPS
transjugular intrahepatic portosystemic stent shunt
Footnotes
Conflicts of Interest
Sequana Medical: Consultant, and grant support to institution.
REFERENCES
- 1.Fleming KM, Aithal GP, Card TR, West J. The rate of decompensation and clinical progression of disease in people with cirrhosis: a cohort study. Aliment Pharmacol Ther. 2010;32:1343–1350. doi: 10.1111/j.1365-2036.2010.04473.x. [DOI] [PubMed] [Google Scholar]
- 2.Macdonald S, Jepsen P, Alrubaiy L, Watson H, Vilstrup H, Jalan R. Quality of life measures predict mortality in patients with cirrhosis and severe ascites. Aliment Pharmacol Ther. 2019;49:321–330. doi: 10.1111/apt.15084. [DOI] [PubMed] [Google Scholar]
- 3.Moreau R, Delègue P, Pessione F, Hillaire S, Durand F, Lebrec D, et al. Clinical characteristics and outcome of patients with cirrhosis and refractory ascites. Liver Int. 2004;24:457–464. doi: 10.1111/j.1478-3231.2004.0991.x. [DOI] [PubMed] [Google Scholar]
- 4.Jepsen P, Watson H, Macdonald S, Vilstrup H, Jalan R. MELD remains the best predictor of mortality in outpatients with cirrhosis and severe ascites. Aliment Pharmacol Ther. 2020;52:492–499. doi: 10.1111/apt.15882. [DOI] [PubMed] [Google Scholar]
- 5.Salerno F, Guevara M, Bernardi M, Moreau R, Wong F, Angeli P, et al. Refractory ascites: pathogenesis, definition and therapy of a severe complication in patients with cirrhosis. Liver Int. 2010;30:937–947. doi: 10.1111/j.1478-3231.2010.02272.x. [DOI] [PubMed] [Google Scholar]
- 6.Gracia-Sancho J, Marrone G, Fernández-Iglesias A. Hepatic microcirculation and mechanisms of portal hypertension. Nat Rev Gastroenterol Hepatol. 2019;16:221–234. doi: 10.1038/s41575-018-0097-3. [DOI] [PubMed] [Google Scholar]
- 7.Iwakiri Y, Trebicka J. Portal hypertension in cirrhosis: pathophysiological mechanisms and therapy. JHEP Rep. 2021;3:100316. doi: 10.1016/j.jhepr.2021.100316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Engelmann C, Clària J, Szabo G, Bosch J, Bernardi M. Pathophysiology of decompensated cirrhosis: portal hypertension, circulatory dysfunction, inflammation, metabolism and mitochondrial dysfunction. J Hepatol. 2021;75 Suppl 1:S49–S66. doi: 10.1016/j.jhep.2021.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stadlbauer V, Wright GA, Banaji M, Mukhopadhya A, Mookerjee RP, Moore K, et al. Relationship between activation of the sympathetic nervous system and renal blood flow autoregulation in cirrhosis. Gastroenterology. 2008;134:111–119. doi: 10.1053/j.gastro.2007.10.055. [DOI] [PubMed] [Google Scholar]
- 10.Moore KP, Wong F, Gines P, Bernardi M, Ochs A, Salerno F, et al. The management of ascites in cirrhosis: report on the consensus conference of the International Ascites Club. Hepatology. 2003;38:258–266. doi: 10.1053/jhep.2003.50315. [DOI] [PubMed] [Google Scholar]
- 11.Biggins SW, Angeli P, Garcia-Tsao G, Ginès P, Ling SC, Nadim MK, et al. Diagnosis, evaluation, and management of ascites, spontaneous bacterial peritonitis and hepatorenal syndrome: 2021 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;74:1014–1048. doi: 10.1002/hep.31884. [DOI] [PubMed] [Google Scholar]
- 12.Adebayo D, Neong SF, Wong F. Refractory ascites in liver cirrhosis. Am J Gastroenterol. 2019;114:40–47. doi: 10.1038/s41395-018-0185-6. [DOI] [PubMed] [Google Scholar]
- 13.Caraceni P, Riggio O, Angeli P, Alessandria C, Neri S, Foschi FG, et al. Long-term albumin administration in decompensated cirrhosis (ANSWER): an open-label randomised trial. Lancet. 2018;391:2417–2429. doi: 10.1016/S0140-6736(18)30840-7. [DOI] [PubMed] [Google Scholar]
- 14.Caraceni P, Tufoni M, Zaccherini G, Riggio O, Angeli P, Alessandria C, et al. On-treatment serum albumin level can guide longterm treatment in patients with cirrhosis and uncomplicated ascites. J Hepatol. 2021;74:340–349. doi: 10.1016/j.jhep.2020.08.021. [DOI] [PubMed] [Google Scholar]
- 15.Solà E, Solé C, Simón-Talero M, Martín-Llahí M, Castellote J, Garcia-Martínez R, et al. Midodrine and albumin for prevention of complications in patients with cirrhosis awaiting liver transplantation. A randomized placebo-controlled trial. J Hepatol. 2018;69:1250–1259. doi: 10.1016/j.jhep.2018.08.006. [DOI] [PubMed] [Google Scholar]
- 16.Di Pascoli M, Fasolato S, Piano S, Bolognesi M, Angeli P. Longterm administration of human albumin improves survival in patients with cirrhosis and refractory ascites. Liver Int. 2019;39:98–105. doi: 10.1111/liv.13968. [DOI] [PubMed] [Google Scholar]
- 17.Villanueva C, Albillos A, Genescà J, Garcia-Pagan JC, Calleja JL, Aracil C, et al. β blockers to prevent decompensation of cirrhosis in patients with clinically significant portal hypertension (PREDESCI): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2019;393:1597–1608. doi: 10.1016/S0140-6736(18)31875-0. [DOI] [PubMed] [Google Scholar]
- 18.Sersté T, Melot C, Francoz C, Durand F, Rautou PE, Valla D, et al. Deleterious effects of beta-blockers on survival in patients with cirrhosis and refractory ascites. Hepatology. 2010;52:1017–1022. doi: 10.1002/hep.23775. [DOI] [PubMed] [Google Scholar]
- 19.Sersté T, Francoz C, Durand F, Rautou PE, Melot C, Valla D, et al. Beta-blockers cause paracentesis-induced circulatory dysfunction in patients with cirrhosis and refractory ascites: a crossover study. J Hepatol. 2011;55:794–799. doi: 10.1016/j.jhep.2011.01.034. [DOI] [PubMed] [Google Scholar]
- 20.Kalambokis GN, Christodoulou D, Baltayiannis G, Christou L. Propranolol use beyond 6 months increases mortality in patients with Child-Pugh C cirrhosis and ascites. Hepatology. 2016;64:1806–1808. doi: 10.1002/hep.28575. [DOI] [PubMed] [Google Scholar]
- 21.Mandorfer M, Bota S, Schwabl P, Bucsics T, Pfisterer N, Kruzik M, et al. Nonselective β blockers increase risk for hepatorenal syndrome and death in patients with cirrhosis and spontaneous bacterial peritonitis. Gastroenterology. 2014;146:1680–1690. doi: 10.1053/j.gastro.2014.03.005. e1. [DOI] [PubMed] [Google Scholar]
- 22.Scheiner B, Parada-Rodriguez D, Bucsics T, Schwabl P, Mandorfer M, Pfisterer N, et al. Non-selective beta-blocker treatment does not impact on kidney function in cirrhotic patients with varices. Scand J Gastroenterol. 2017;52:1008–1015. doi: 10.1080/00365521.2017.1329456. [DOI] [PubMed] [Google Scholar]
- 23.Leithead JA, Rajoriya N, Tehami N, Hodson J, Gunson BK, Tripathi D, et al. Non-selective β-blockers are associated with improved survival in patients with ascites listed for liver transplantation. Gut. 2015;64:1111–1119. doi: 10.1136/gutjnl-2013-306502. [DOI] [PubMed] [Google Scholar]
- 24.Sasso R, Rockey DC. Non-selective beta-blocker use in cirrhotic patients is associated with a reduced likelihood of hospitalisation for infection. Aliment Pharmacol Ther. 2021;53:418–425. doi: 10.1111/apt.16156. [DOI] [PubMed] [Google Scholar]
- 25.Bossen L, Krag A, Vilstrup H, Watson H, Jepsen P. Nonselective β-blockers do not affect mortality in cirrhosis patients with ascites: post hoc analysis of three randomized controlled trials with 1198 patients. Hepatology. 2016;63:1968–1976. doi: 10.1002/hep.28352. [DOI] [PubMed] [Google Scholar]
- 26.Mookerjee RP, Pavesi M, Thomsen KL, Mehta G, Macnaughtan J, Bendtsen F, et al. Treatment with non-selective beta blockers is associated with reduced severity of systemic inflammation and improved survival of patients with acute-on-chronic liver failure. J Hepatol. 2016;64:574–582. doi: 10.1016/j.jhep.2015.10.018. [DOI] [PubMed] [Google Scholar]
- 27.Téllez L, Albillos A. Non-selective beta-blockers in patients with ascites: the complex interplay among the liver, kidney and heart. Liver Int. 2022;42:749–761. doi: 10.1111/liv.15166. [DOI] [PubMed] [Google Scholar]
- 28.Téllez L, Ibáñez-Samaniego L, Pérez Del Villar C, Yotti R, Martínez J, Carrión L, et al. Non-selective beta-blockers impair global circulatory homeostasis and renal function in cirrhotic patients with refractory ascites. J Hepatol. 2020;73:1404–1414. doi: 10.1016/j.jhep.2020.05.011. [DOI] [PubMed] [Google Scholar]
- 29.Reiberger T, Mandorfer M. Beta adrenergic blockade and decompensated cirrhosis. J Hepatol. 2017;66:849–859. doi: 10.1016/j.jhep.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 30.Ginés P, Arroyo V, Quintero E, Planas R, Bory F, Cabrera J, et al. Comparison of paracentesis and diuretics in the treatment of cirrhotics with tense ascites. Results of a randomized study. Gastroenterology. 1987;93:234–241. doi: 10.1016/0016-5085(87)91007-9. [DOI] [PubMed] [Google Scholar]
- 31.Ruiz-del-Arbol L, Monescillo A, Jimenéz W, Garcia-Plaza A, Arroyo V, Rodés J. Paracentesis-induced circulatory dysfunction: mechanism and effect on hepatic hemodynamics in cirrhosis. Gastroenterology. 1997;113:579–586. doi: 10.1053/gast.1997.v113.pm9247479. [DOI] [PubMed] [Google Scholar]
- 32.Bernardi M, Caraceni P, Navickis RJ, Wilkes MM. Albumin infusion in patients undergoing large-volume paracentesis: a metaanalysis of randomized trials. Hepatology. 2012;55:1172–1181. doi: 10.1002/hep.24786. [DOI] [PubMed] [Google Scholar]
- 33.Alessandria C, Elia C, Mezzabotta L, Risso A, Andrealli A, Spandre M, et al. Prevention of paracentesis-induced circulatory dysfunction in cirrhosis: standard vs half albumin doses. A prospective, randomized, unblinded pilot study. Dig Liver Dis. 2011;43:881–886. doi: 10.1016/j.dld.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 34.Tan HK, James PD, Wong F. Albumin may prevent the morbidity of paracentesis-induced circulatory dysfunction in cirrhosis and refractory ascites: a pilot study. Dig Dis Sci. 2016;61:3084–3092. doi: 10.1007/s10620-016-4140-3. [DOI] [PubMed] [Google Scholar]
- 35.Peltekian KM, Wong F, Liu PP, Logan AG, Sherman M, Blendis LM. Cardiovascular, renal, and neurohumoral responses to single large-volume paracentesis in patients with cirrhosis and diuretic-resistant ascites. Am J Gastroenterol. 1997;92:394–399. [PubMed] [Google Scholar]
- 36.Arora V, Vijayaraghavan R, Maiwall R, Sahney A, Thomas SS, Ali R, et al. Paracentesis-induced circulatory dysfunction with modest-volume paracentesis is partly ameliorated by albumin infusion in acute-on-chronic liver failure. Hepatology. 2020;72:1043–1055. doi: 10.1002/hep.31071. [DOI] [PubMed] [Google Scholar]
- 37.De Gottardi A, Thévenot T, Spahr L, Morard I, Bresson-Hadni S, Torres F, et al. Risk of complications after abdominal paracentesis in cirrhotic patients: a prospective study. Clin Gastroenterol Hepatol. 2009;7:906–909. doi: 10.1016/j.cgh.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 38.Wong F, Sniderman K, Liu P, Allidina Y, Sherman M, Blendis L. Transjugular intrahepatic portosystemic stent shunt: effects on hemodynamics and sodium homeostasis in cirrhosis and refractory ascites. Ann Intern Med. 1995;122:816–822. doi: 10.7326/0003-4819-122-11-199506010-00002. [DOI] [PubMed] [Google Scholar]
- 39.Rössle M. TIPS: 25 years later. J Hepatol. 2013;59:1081–1093. doi: 10.1016/j.jhep.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 40.Tan HK, James PD, Sniderman KW, Wong F. Long-term clinical outcome of patients with cirrhosis and refractory ascites treated with transjugular intrahepatic portosystemic shunt insertion. J Gastroenterol Hepatol. 2015;30:389–395. doi: 10.1111/jgh.12725. [DOI] [PubMed] [Google Scholar]
- 41.Russo MW, Sood A, Jacobson IM, Brown RS., Jr Transjugular intrahepatic portosystemic shunt for refractory ascites: an analysis of the literature on efficacy, morbidity, and mortality. Am J Gastroenterol. 2003;98:2521–2527. doi: 10.1111/j.1572-0241.2003.08664.x. [DOI] [PubMed] [Google Scholar]
- 42.Lebrec D, Giuily N, Hadengue A, Vilgrain V, Moreau R, Poynard T, et al. Transjugular intrahepatic portosystemic shunts: comparison with paracentesis in patients with cirrhosis and refractory ascites: a randomized trial. French Group of Clinicians and a Group of Biologists. J Hepatol. 1996;25:135–144. doi: 10.1016/s0168-8278(96)80065-1. [DOI] [PubMed] [Google Scholar]
- 43.Rössle M, Ochs A, Gülberg V, Siegerstetter V, Holl J, Deibert P, et al. A comparison of paracentesis and transjugular intrahepatic portosystemic shunting in patients with ascites. N Engl J Med. 2000;342:1701–1707. doi: 10.1056/NEJM200006083422303. [DOI] [PubMed] [Google Scholar]
- 44.Ginès P, Uriz J, Calahorra B, Garcia-Tsao G, Kamath PS, Del Arbol LR, et al. Transjugular intrahepatic portosystemic shunting versus paracentesis plus albumin for refractory ascites in cirrhosis. Gastroenterology. 2002;123:1839–1847. doi: 10.1053/gast.2002.37073. [DOI] [PubMed] [Google Scholar]
- 45.Sanyal AJ, Genning C, Reddy KR, Wong F, Kowdley KV, Benner K, et al. The North American study for the treatment of refractory ascites. Gastroenterology. 2003;124:634–641. doi: 10.1053/gast.2003.50088. [DOI] [PubMed] [Google Scholar]
- 46.Salerno F, Merli M, Riggio O, Cazzaniga M, Valeriano V, Pozzi M, et al. Randomized controlled study of TIPS versus paracentesis plus albumin in cirrhosis with severe ascites. Hepatology. 2004;40:629–635. doi: 10.1002/hep.20364. [DOI] [PubMed] [Google Scholar]
- 47.Narahara Y, Kanazawa H, Fukuda T, Matsushita Y, Harimoto H, Kidokoro H, et al. Transjugular intrahepatic portosystemic shunt versus paracentesis plus albumin in patients with refractory ascites who have good hepatic and renal function: a prospective randomized trial. J Gastroenterol. 2011;46:78–85. doi: 10.1007/s00535-010-0282-9. [DOI] [PubMed] [Google Scholar]
- 48.Bai M, Qi XS, Yang ZP, Yang M, Fan DM, Han GH. TIPS improves liver transplantation-free survival in cirrhotic patients with refractory ascites: an updated meta-analysis. World J Gastroenterol. 2014;20:2704–2714. doi: 10.3748/wjg.v20.i10.2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Salerno F, Cammà C, Enea M, Rössle M, Wong F. Transjugular intrahepatic portosystemic shunt for refractory ascites: a meta-analysis of individual patient data. Gastroenterology. 2007;133:825–834. doi: 10.1053/j.gastro.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 50.Trebicka J. Does transjugular intrahepatic portosystemic shunt stent differentially improve survival in a subset of cirrhotic patients? Semin Liver Dis. 2018;38:87–96. doi: 10.1055/s-0038-1627457. [DOI] [PubMed] [Google Scholar]
- 51.Bureau C, Thabut D, Oberti F, Dharancy S, Carbonell N, Bouvier A, et al. Transjugular intrahepatic portosystemic shunts with covered stents increase transplant-free survival of patients with cirrhosis and recurrent ascites. Gastroenterology. 2017;152:157–163. doi: 10.1053/j.gastro.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 52.Barrio J, Ripoll C, Bañares R, Echenagusia A, Catalina MV, Camúñez F, et al. Comparison of transjugular intrahepatic portosystemic shunt dysfunction in PTFE-covered stent-grafts versus bare stents. Eur J Radiol. 2005;55:120–124. doi: 10.1016/j.ejrad.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 53.Casadaban LC, Parvinian A, Minocha J, Lakhoo J, Grant CW, Ray CE, Jr, et al. Clearing the confusion over hepatic encephalopathy after TIPS creation: incidence, prognostic factors, and clinical outcomes. Dig Dis Sci. 2015;60:1059–1066. doi: 10.1007/s10620-014-3391-0. [DOI] [PubMed] [Google Scholar]
- 54.Fonio P, Discalzi A, Calandri M, Doriguzzi Breatta A, Bergamasco L, Martini S, et al. Incidence of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt (TIPS) according to its severity and temporal grading classification. Radiol Med. 2017;122:713–721. doi: 10.1007/s11547-017-0770-6. [DOI] [PubMed] [Google Scholar]
- 55.Schindler P, Heinzow H, Trebicka J, Wildgruber M. Shunt-induced hepatic encephalopathy in tips: current approaches and clinical challenges. J Clin Med. 2020;9:3784. doi: 10.3390/jcm9113784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schepis F, Vizzutti F, Garcia-Tsao G, Marzocchi G, Rega L, De Maria N, et al. Under-dilated TIPS associate with efficacy and reduced encephalopathy in a prospective, non-randomized study of patients with cirrhosis. Clin Gastroenterol Hepatol. 2018;16:1153–1162. doi: 10.1016/j.cgh.2018.01.029. e7. [DOI] [PubMed] [Google Scholar]
- 57.Seifert LL, Schindler P, Schoster M, Weller JF, Wilms C, Schmidt HH, et al. Recurrence of hepatic encephalopathy after TIPS: effective prophylaxis with combination of lactulose and rifaximin. J Clin Med. 2021;10:4763. doi: 10.3390/jcm10204763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bureau C, Thabut D, Jezequel C, Archambeaud I, D’Alteroche L, Dharancy S, et al. The use of rifaximin in the prevention of overt hepatic encephalopathy after transjugular intrahepatic portosystemic shunt : a randomized controlled trial. Ann Intern Med. 2021;174:633–640. doi: 10.7326/M20-0202. [DOI] [PubMed] [Google Scholar]
- 59.García-Pagán JC, Saffo S, Mandorfer M, Garcia-Tsao G. Where does TIPS fit in the management of patients with cirrhosis? JHEP Rep. 2020;2:100122. doi: 10.1016/j.jhepr.2020.100122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Billey C, Billet S, Robic MA, Cognet T, Guillaume M, Vinel JP, et al. A prospective study identifying predictive factors of cardiac decompensation after transjugular intrahepatic portosystemic shunt: the toulouse algorithm. Hepatology. 2019;70:1928–1941. doi: 10.1002/hep.30934. [DOI] [PubMed] [Google Scholar]
- 61.Mizrahi M, Adar T, Shouval D, Bloom AI, Shibolet O. Endotipsitispersistent infection of transjugular intrahepatic portosystemic shunt: pathogenesis, clinical features and management. Liver Int. 2010;30:175–183. doi: 10.1111/j.1478-3231.2009.02158.x. [DOI] [PubMed] [Google Scholar]
- 62.European Association for the Study of the Liver EASL clinical practice guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406–460. doi: 10.1016/j.jhep.2018.03.024. [DOI] [PubMed] [Google Scholar]
- 63.Gülberg V, Liss I, Bilzer M, Waggershauser T, Reiser M, Gerbes AL. Improved quality of life in patients with refractory or recidivant ascites after insertion of transjugular intrahepatic portosystemic shunts. Digestion. 2002;66:127–130. doi: 10.1159/000065593. [DOI] [PubMed] [Google Scholar]
- 64.Allard JP, Chau J, Sandokji K, Blendis LM, Wong F. Effects of ascites resolution after successful TIPS on nutrition in cirrhotic patients with refractory ascites. Am J Gastroenterol. 2001;96:2442–2447. doi: 10.1111/j.1572-0241.2001.04051.x. [DOI] [PubMed] [Google Scholar]
- 65.Jahangiri Y, Pathak P, Tomozawa Y, Li L, Schlansky BL, Farsad K. Muscle gain after transjugular intrahepatic portosystemic shunt creation: time course and prognostic implications for survival in cirrhosis. J Vasc Interv Radiol. 2019;30:866–872. doi: 10.1016/j.jvir.2019.01.005. e4. [DOI] [PubMed] [Google Scholar]
- 66.Will V, Rodrigues SG, Berzigotti A. Current treatment options of refractory ascites in liver cirrhosis - a systematic review and meta-analysis. Dig Liver Dis. 2022;54:1007–1014. doi: 10.1016/j.dld.2021.12.007. [DOI] [PubMed] [Google Scholar]
- 67.Tsien C, Shah SN, McCullough AJ, Dasarathy S. Reversal of sarcopenia predicts survival after a transjugular intrahepatic portosystemic stent. Eur J Gastroenterol Hepatol. 2013;25:85–93. doi: 10.1097/MEG.0b013e328359a759. [DOI] [PubMed] [Google Scholar]
- 68.Bettinger D, Sturm L, Pfaff L, Hahn F, Kloeckner R, Volkwein L, et al. Refining prediction of survival after TIPS with the novel Freiburg index of post-TIPS survival. J Hepatol. 2021;74:1362–1372. doi: 10.1016/j.jhep.2021.01.023. [DOI] [PubMed] [Google Scholar]
- 69.Trebicka J, Bastgen D, Byrtus J, Praktiknjo M, Terstiegen S, Meyer C, et al. Smaller-diameter covered transjugular intrahepatic portosystemic shunt stents are associated with increased survival. Clin Gastroenterol Hepatol. 2019;17:2793–2799. doi: 10.1016/j.cgh.2019.03.042. e1. [DOI] [PubMed] [Google Scholar]
- 70.Bureau C, Adebayo D, Chalret de Rieu M, Elkrief L, Valla D, PeckRadosavljevic M, et al. Alfapump® system vs. large volume paracentesis for refractory ascites: a multicenter randomized controlled study. J Hepatol. 2017;67:940–949. doi: 10.1016/j.jhep.2017.06.010. [DOI] [PubMed] [Google Scholar]
- 71.Bellot P, Welker MW, Soriano G, von Schaewen M, Appenrodt B, Wiest R, et al. Automated low flow pump system for the treatment of refractory ascites: a multi-center safety and efficacy study. J Hepatol. 2013;58:922–927. doi: 10.1016/j.jhep.2012.12.020. [DOI] [PubMed] [Google Scholar]
- 72.Thomas MN, Sauter GH, Gerbes AL, Stangl M, Schiergens TS, Angele M, et al. Automated low flow pump system for the treatment of refractory ascites: a single-center experience. Langenbecks Arch Surg. 2015;400:979–983. doi: 10.1007/s00423-015-1356-1. [DOI] [PubMed] [Google Scholar]
- 73.Stirnimann G, Berg T, Spahr L, Zeuzem S, McPherson S, Lammert F, et al. Treatment of refractory ascites with an automated lowflow ascites pump in patients with cirrhosis. Aliment Pharmacol Ther. 2017;46:981–991. doi: 10.1111/apt.14331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Solbach P, Höner Zu Siederdissen C, Wellhöner F, Richter N, Heidrich B, Lenzen H, et al. Automated low-flow ascites pump in a real-world setting: complications and outcomes. Eur J Gastroenterol Hepatol. 2018;30:1082–1089. doi: 10.1097/MEG.0000000000001149. [DOI] [PubMed] [Google Scholar]
- 75.Wong F, Bendel E, Sniderman K, Frederick T, Haskal ZJ, Sanyal A, et al. Improvement in quality of life and decrease in largevolume paracentesis requirements with the automated lowflow ascites pump. Liver Transpl. 2020;26:651–661. doi: 10.1002/lt.25724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Will V, Rodrigues SG, Stirnimann G, Gottardi A, Bosch J, Berzigotti A. Transjugular intrahepatic portosystemic shunt and alfapump® system for refractory ascites in liver cirrhosis: outcomes and complications. United European Gastroenterol J. 2020;8:961–969. doi: 10.1177/2050640620938525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lepida A, Marot A, Trépo E, Degré D, Moreno C, Deltenre P. Systematic review with meta-analysis: automated low-flow ascites pump therapy for refractory ascites. Aliment Pharmacol Ther. 2019;50:978–987. doi: 10.1111/apt.15502. [DOI] [PubMed] [Google Scholar]
- 78.Solà E, Sanchez-Cabús S, Rodriguez E, Elia C, Cela R, Moreira R, et al. Effects of alfapump™ system on kidney and circulatory function in patients with cirrhosis and refractory ascites. Liver Transpl. 2017;23:583–593. doi: 10.1002/lt.24763. [DOI] [PubMed] [Google Scholar]
- 79.Aagaard NK, Malago M, De Gottardi A, Thomas M, Sauter G, Engelmann C, et al. Consensus care recommendations for alfapump® in cirrhotic patients with refractory or recurrent ascites. BMC Gastroenterol. 2022;22:111. doi: 10.1186/s12876-022-02173-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stepanova M, Nader F, Bureau C, Adebayo D, Elkrief L, Valla D, et al. Patients with refractory ascites treated with alfapump® system have better health-related quality of life as compared to those treated with large volume paracentesis: the results of a multicenter randomized controlled study. Qual Life Res. 2018;27:1513–1520. doi: 10.1007/s11136-018-1813-8. [DOI] [PubMed] [Google Scholar]
- 81.Mazumder NR, Atiemo K, Daud A, Kho A, Abecassis M, Levitsky J, et al. Patients with persistently low MELD-Na scores continue to be at risk of liver-related death. Transplantation. 2020;104:1413–1418. doi: 10.1097/TP.0000000000002997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Heuman DM, Abou-Assi SG, Habib A, Williams LM, Stravitz RT, Sanyal AJ, et al. Persistent ascites and low serum sodium identify patients with cirrhosis and low MELD scores who are at high risk for early death. Hepatology. 2004;40:802–810. doi: 10.1002/hep.20405. [DOI] [PubMed] [Google Scholar]
- 83.Somsouk M, Kornfield R, Vittinghoff E, Inadomi JM, Biggins SW. Moderate ascites identifies patients with low model for endstage liver disease scores awaiting liver transplantation who have a high mortality risk. Liver Transpl. 2011;17:129–136. doi: 10.1002/lt.22218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Prohic D, Mesihovic R, Vanis N, Puhalovic A. Prognostic significance of ascites and serum sodium in patients with low meld scores. Med Arch. 2016;70:48–52. doi: 10.5455/medarh.2016.70.48-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hadengue A, Lebrec D, Moreau R, Sogni P, Durand F, Gaudin C, et al. Persistence of systemic and splanchnic hyperkinetic circulation in liver transplant patients. Hepatology. 1993;17:175–178. [PubMed] [Google Scholar]
- 86.Salerno F, Gerbes A, Ginès P, Wong F, Arroyo V. Diagnosis, prevention and treatment of hepatorenal syndrome in cirrhosis. Gut. 2007;56:1310–1318. doi: 10.1136/gut.2006.107789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kumar R, Priyadarshi RN, Anand U. Chronic renal dysfunction in cirrhosis: a new frontier in hepatology. World J Gastroenterol. 2021;27:990–1005. doi: 10.3748/wjg.v27.i11.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bassegoda O, Huelin P, Ariza X, Solé C, Juanola A, Gratacós-Ginès J, et al. Development of chronic kidney disease after acute kidney injury in patients with cirrhosis is common and impairs clinical outcomes. J Hepatol. 2020;72:1132–1139. doi: 10.1016/j.jhep.2019.12.020. [DOI] [PubMed] [Google Scholar]
- 89.Patil V, Jain M, Venkataraman J. Paracentesis-induced acute kidney injury in decompensated cirrhosis - prevalence and predictors. Clin Exp Hepatol. 2019;5:55–59. doi: 10.5114/ceh.2019.83157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ponzo P, Campion D, Rizzo M, Roma M, Caviglia GP, Giovo I, et al. Transjugular intrahepatic porto-systemic shunt in cirrhotic patients with hepatorenal syndrome - chronic kidney disease: Impact on renal function. Dig Liver Dis. 2022;54:1101–1108. doi: 10.1016/j.dld.2021.09.008. [DOI] [PubMed] [Google Scholar]
- 91.D’Amico G, Morabito A, D’Amico M, Pasta L, Malizia G, Rebora P, et al. Clinical states of cirrhosis and competing risks. J Hepatol. 2018;68:563–576. doi: 10.1016/j.jhep.2017.10.020. [DOI] [PubMed] [Google Scholar]
- 92.Macken L, Bremner S, Gage H, Touray M, Williams P, Crook D, et al. Randomised clinical trial: palliative long-term abdominal drains vs large-volume paracentesis in refractory ascites due to cirrhosis. Aliment Pharmacol Ther. 2020;52:107–122. doi: 10.1111/apt.15802. [DOI] [PubMed] [Google Scholar]
- 93.Corrigan M, Thomas R, McDonagh J, Speakman J, Abbas N, Bardell S, et al. Tunnelled peritoneal drainage catheter placement for the palliative management of refractory ascites in patients with liver cirrhosis. Frontline Gastroenterol. 2020;12:108–112. doi: 10.1136/flgastro-2019-101332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cooper M, Pollard A, Pandey A, Bremner S, Macken L, Evans CJ, et al. Palliative long-term abdominal drains versus large volume paracentesis in refractory ascites due to cirrhosis (REDUCe study): qualitative outcomes. J Pain Symptom Manage. 2021;62:312–325. doi: 10.1016/j.jpainsymman.2020.12.007. e2. [DOI] [PubMed] [Google Scholar]
- 95.Roy A, Taneja S, Duseja AK, Singh V. Letter: long-term abdominal drains in refractory ascites-evolving concept of palliative care in decompensated cirrhosis. Aliment Pharmacol Ther. 2020;52:1266–1267. doi: 10.1111/apt.15944. [DOI] [PubMed] [Google Scholar]
- 96.Macken L, Hashim A, Mason L, Verma S. Permanent indwelling peritoneal catheters for palliation of refractory ascites in end-stage liver disease: a systematic review. Liver Int. 2019;39:1594–1607. doi: 10.1111/liv.14162. [DOI] [PubMed] [Google Scholar]