Abstract
Background/Aims
The utility of Baveno-VII criteria of clinically significant portal hypertension (CSPH) to predict decompensation in compensated advanced chronic liver disease (cACLD) patient needs validation. We aim to validate the performance of CSPH criteria to predict the risk of decompensation in an international real-world cohort of cACLD patients.
Methods
cACLD patients were stratified into three categories (CSPH excluded, grey zone, and CSPH). The risks of decompensation across different CSPH categories were estimated using competing risk regression for clustered data, with death and hepatocellular carcinoma as competing events. The performance of “treating definite CSPH” strategy to prevent decompensation using non-selective beta-blocker (NSBB) was compared against other strategies in decision curve analysis.
Results
One thousand one hundred fifty-nine cACLD patients (36.8% had CSPH) were included; 7.2% experienced decompensation over a median follow-up of 40 months. Non-invasive assessment of CSPH predicts a 5-fold higher risk of liver decompensation in cACLD patients (subdistribution hazard ratio, 5.5; 95% confidence interval, 4.0–7.4). “Probable CSPH” is suboptimal to predict decompensation risk in cACLD patients. CSPH exclusion criteria reliably exclude cACLD patients at risk of decompensation, regardless of etiology. Among the grey zone, the decompensation risk was negligible among viral-related cACLD, but was substantially higher among the non-viral cACLD group. Decision curve analysis showed that “treating definite CSPH” strategy is superior to “treating all varices” or “treating probable CSPH” strategy to prevent decompensation using NSBB.
Conclusions
Non-invasive assessment of CSPH may stratify decompensation risk and the need for NSBB in cACLD patients.
Keywords: Portal hypertension; Hypertension, portal; Liver cirrhosis
Graphical Abstract
INTRODUCTION
Compensated advanced chronic liver disease (cACLD) patients can be risk-stratified based on the presence of clinically significant portal hypertension (CSPH) [1], which is defined as hepatic venous pressure gradient (HVPG) measurement beyond 10 mmHg [2]. In a randomized trial, Villanueva et al. [3] showed that non-selective beta-blockers (NSBBs) prevent decompensation and improve survival in cACLD patients with HVPG ≥10 mmHg. Although the Baveno-VII consensus recommends NSBB for cACLD patients with CSPH [4], controversies remain, especially among virologically-suppressed cACLD patients, where the decompensation risk is generally low [5,6].
Given the invasive nature and logistic challenges to measuring HVPG in every cACLD patient, the non-invasive assessment of CSPH is an important unmet need [7]. A unifying, noninvasive diagnosis for CSPH was lacking until the recent Baveno-VII consensus [4]. While the combination of baseline liver stiffness measurement (LSM) and platelet count (Baveno-VII criteria) correlates with baseline CSPH [8,9], this proposed non-invasive assessment of CSPH has not been validated to predict liver decompensation. In this study, we aimed to validate the performance of the Baveno-VII criteria of CSPH to predict liver decompensation and the need for NSBB in an international real-world cohort of cACLD patients.
MATERIALS AND METHODS
This is a retrospective analysis of cACLD patients identified via institutional registries of cACLD patients from four countries (Italy, India, China, and Singapore) between January 2014 and December 2017. We identified cACLD patients based on institutional transient elastography database from Singapore, India, and China, regardless of cirrhosis etiology. The Italy cohort included consecutively treated HCV patients with available transient elastography results consistent with the diagnosis of cACLD (LSM ≥10 kPa). The study was approved by the respective institutional ethics committees with waiver of consent granted, and conducted in compliance with the 1975 Helsinki declaration.
The diagnosis of cACLD was made based on LSM ≥10 kPa with supportive features of cirrhosis such as 1) radiological (nodular liver or irregular liver margin or splenomegaly), 2) histological features of advanced fibrosis or established cirrhosis, 3) presence of gastroesophageal varices or 4) HVPG >5 mmHg [4]. Individual chart review was performed for all patients to confirm the diagnosis of cACLD [4] and relevant clinical data were collected using a unified data template. We excluded patients with a history of liver decompensating events such as ascites, variceal bleeding or hepatic encephalopathy [4], baseline hepatocellular carcinoma (HCC), invalid LSM, or missing data. Given that treatment of virus-related cirrhosis is the standard of care for cirrhosis patients, we excluded patients with untreated virus-related cirrhosis, which was defined as hepatitis B virus-related cirrhosis without virological suppression or hepatitis C virus-related cirrhosis without sustained virological response [4]. We also excluded patients with significant alcohol intake (30 g/day in males or 20 g/day in females) identified based on electronic medical records. Given that there is no approved specific treatment for non-alcoholic steatohepatitis (NASH) cirrhosis, we included all NASH cirrhosis in our cohort. Finally, we excluded patients with NSBB usage because NSBB use can reduce decompensating events in compensated cirrhosis patients with CSPH, as shown in the PREDESCI (β blockers to Prevent Decompensation of Cirrhosis in Patients with Clinically Significant Portal Hypertension) trial. Given that our study period predated the recent Baveno-VII consensus which recommended the widespread use of empiric NSBB to prevent decompensation in cACLD patients, the treatment of high-risk varices (HRV) was intended to prevent variceal bleeding (rather than decompensation). The decisions between endoscopic variceal ligation versus NSBB were physician-dependent.
LSM
All transient elastography were performed by certified operators using either M or XL probe, based on the manufacturer’s instruction. LSM was measured as the median of at least 10 successful measurements, expressed in kilopascal (kPa). LSM was considered unreliable when the interquartile range (IQR) was beyond 30% of the median LSM value, or when there were less than 10 successful measurements.
Definition of CSPH
We used the Baveno-VII criteria to define CSPH (LSM ≥25 kPa) and exclude CSPH (LSM <15 kPa and platelet ≥150×109/L) [4]. Patients who did not fulfil the inclusion or exclusion CSPH criteria were classified as grey zone. Patients within the grey zone were further categorized into high probability of CSPH (defined as LSM between 20–25 kPa and platelet <150×109/L, or LSM between 15–20 kPa and platelet <110×109/L), or low probability of CSPH (defined as the remaining patients within the grey zone) [4].
Study outcomes
Patients were followed-up every 3 to 6 months from the diagnosis of cACLD to the onset of first liver decompensation (variceal bleeding, clinically overt ascites and overt hepatic encephalopathy), HCC or death, whichever occurred earlier. Variceal bleeding was confirmed from the endoscopy. Ascites was defined as clinically overt ascites requiring diuretic treatment. Overt hepatic encephalopathy was defined by West Haven Classification grade 2 and beyond. We defined liver-related events as the presence of either liver decompensation, HCC, or death.
Statistical analysis
Baseline data were summarized based on CSPH criteria into three categories, namely CSPH excluded, grey zone and CSPH (LSM ≥25 kPa) [4]. Continuous data were reported in mean±standard deviation or median with IQR based on normality of data distribution. Categorical data were summarized by frequency (percentage). Numerical baseline variables comparisons across the three groups were performed using the one-way analysis of variance/Kruskal-Wallis rank test and chi-square/Fisher’s exact tests for categorical variables. The log-rank test was used to compare the median follow-up times of the three groups.
The risk of liver decompensation was estimated using the competing risk regression for clustered data, with HCC and death as competing risks. The corresponding subdistribution hazard ratio (sHR), 95% confidence interval (95% CI) and cumulative incidence were reported [10]. Cumulative incidences of liver-related events and death were obtained by survival analysis. Subgroup analysis was performed to determine if the presence of HRV and etiology influenced the performance of CSPH to predict decompensation, liver-related events and death among cACLD patients.
Univariable and multivariable competing risk regression for clustered data were conducted to select predictors of liver decompensation regarding HCC and death as competing events. Optimal cut-offs of continuous predictors in the final model were chosen based on the Youden and Liu criteria. All statistical tests were two-sided with a 5% significance level. Statistical analysis was performed using STATA/SE version 17.0 (StataCorp LLC, College Station, TX, USA). Decision curve analysis was performed by R version 4.0.5 (R Foundation for Statistical Computing, Vienna, Austria).
Decision curve analysis (DCA)
DCA was used to assess the application of various screening strategies to stratify patients for NSBB to prevent decompensation in real-life settings [11]. DCA evaluates various screening strategies including (1) treating only HRV, (2) treating all esophageal varices (given that varices are manifestations of CSPH), and (3) treating CSPH (diagnosed based on the Baveno-VII non-invasive criteria), in comparison with default strategies of either treating everyone with NSBB, or treating no patients with NSBB. The net benefit of each strategy was assessed across a range of threshold probabilities, with the area under the curve corresponding to the estimated benefit of each strategy to prevent decompensation. Overall, DCA allows objective assessment of the number of additional patients experiencing decompensation for every patient treated with NSBB using various screening strategies. Further details of DCA are described in Supplementary Material 1.
RESULTS
Baseline characteristics
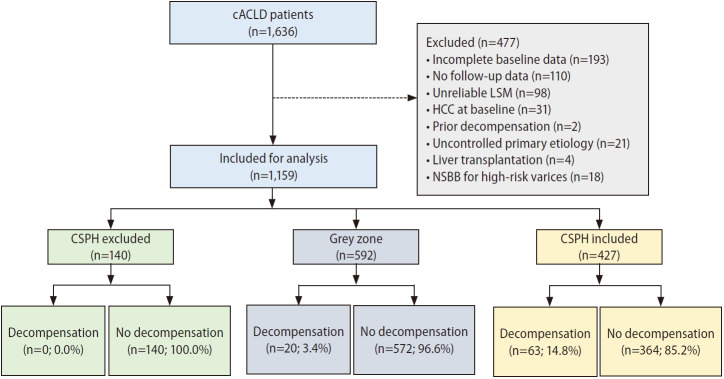
A total of 1,159 cACLD patients were included and the Consolidated Standards of Reporting Trials diagram was summarized in Figure 1. The cohort was predominantly male with virus-related cirrhosis (77.4%) with Child-Turcott-Pugh (CTP) class A (95.0%); 10.3% had body mass index >30 kg/m2. Baveno-VII criteria stratified cACLD patients into three categories, namely CSPH (36.8%), grey zone (51.1%) and CSPH excluded (12.1%). Patients with CSPH had more advanced liver disease (higher CTP score, higher Model of End-stage Liver Disease score, higher bilirubin, lower albumin and lower platelet count), higher mean LSM and Fibrosis-4 score than those without CSPH (P<0.001 for all) (Table 1). The details of each cohort stratified by study sites were summarized in Supplementary Table 1.
Figure 1.
Consolidated Standards of Reporting Trials diagram. cACLD, compensated advanced chronic liver disease; HCC, hepatocellular carcinoma; LSM, liver stiffness measurement; NSBB, non-selective beta-blocker; CSPH, clinically significant portal hypertension.
Table 1.
Baseline demographic of study subjects stratified based on the non-invasive diagnosis of clinically significant portal hypertension
| Variable | CSPH not fulfilled |
P-value | ||||
|---|---|---|---|---|---|---|
| Total cohort (n=1,159) | CSPH excluded (n=140) | Grey zone (n=592) | CSPH fulfilled (n=427) | |||
| Age (years) | 55±13 | 57±14 | 57±14 | 53±12 | <0.001 | |
| Gender, male | 776 (67.0) | 89 (63.6) | 374 (63.2) | 313 (73.3) | 0.002 | |
| Ethnicity | <0.001 | |||||
| Caucasian | 357 (30.8) | 66 (47.1) | 207 (35.0) | 84 (19.7) | ||
| Chinese | 328 (28.3) | 47 (33.6) | 165 (27.9) | 116 (27.2) | ||
| Indian | 310 (26.8) | 2 (1.4) | 128 (21.6) | 180 (42.2) | ||
| Malay | 111 (9.6) | 20 (14.3) | 62 (10.5) | 29 (6.8) | ||
| Arabic | 28 (2.4) | 2 (1.4) | 18 (3.0) | 8 (1.9) | ||
| Others | 25 (2.2) | 3 (2.1) | 12 (2.0) | 10 (2.3) | ||
| Etiology | <0.001 | |||||
| Hepatitis B | 247 (21.3) | 34 (24.3) | 127 (21.5) | 86 (20.1) | ||
| Hepatitis C | 650 (56.1) | 95 (67.9) | 374 (63.2) | 181 (42.4) | ||
| Alcohol | 105 (9.1) | 1 (0.7) | 28 (4.7) | 76 (17.8) | ||
| NASH | 102 (8.8) | 4 (2.9) | 41 (6.9) | 57 (13.4) | ||
| Others | 55 (4.7) | 6 (4.3) | 22 (3.7) | 27 (6.3) | ||
| MELD score | 8±3 | 7±1 | 8±3 | 9±3 | <0.001 | |
| Child-Turcott-Pugh score | 5.2±0.6 | 5.0±0.2 | 5.1±0.4 | 5.4±0.7 | <0.001 | |
| LSM (kPa) | 23.8±12.2 | 12.3±1.3 | 17.9±3.7 | 35.7±12.4 | <0.001 | |
| Fibrosis-4 | 4.4±3.6 | 2.1±1.1 | 4.4±3.4 | 5.3±4.1 | <0.001 | |
| Laboratory parameters | ||||||
| Albumin (g/L) | 40±5 | 43±4 | 41±5 | 38±6 | <0.001 | |
| Bilirubin (μmol/L) | 19±15 | 14±8 | 17±11 | 24±19 | <0.001 | |
| ALT (μmol/L) | 77±63 | 75±62 | 79±65 | 73±62 | 0.368 | |
| Platelets (×103/μL) | 141±66 | 205±49 | 138±64 | 125±62 | <0.001 | |
| Platelet count (×103/μL) | <0.001 | |||||
| <150 | 708 (61.1) | 0 (0.0) | 408 (68.9) | 300 (70.3) | ||
| ≥150 | 451 (38.9) | 140 (100.0) | 184 (31.1) | 127 (29.7) | ||
| INR | 1.09±0.14 | 1.03±0.08 | 1.07±0.13 | 1.14±0.14 | <0.001 | |
| Creatinine (μmol/L) | 68 (57–80) | 69 (58–80) | 69 (58–83) | 66 (56–80) | 0.162 | |
| Esophageal varices | ||||||
| No varices | 641 (60.7) | 88 (82.2) | 367 (69.0) | 186 (44.6) | <0.001 | |
| Low-risk varices | 357 (33.8) | 19 (17.8) | 140 (26.3) | 198 (47.5) | <0.001 | |
| High-risk varices | 58 (5.5) | 0 (0.0) | 25 (4.7) | 33 (7.9) | 0.003 | |
| Follow-up time (months) | 40 (30–52) | 44 (34–53) | 40 (31–52) | 39 (30–50) | 0.010 | |
Values are presented as mean±standard deviation, median (interquartile range), or frequency (%).
CSPH, clinically significant portal hypertension; MELD, Model of End-stage Liver Disease; LSM, liver stiffness measurement; ALT, alanine aminotransferase; INR, international normalized ratio.
Non-invasive diagnosis of CSPH and decompensation
Over a median (IQR) follow-up of 40 months (30–52), 83 patients (7.2%) developed liver decompensation, 67 patients (5.8%) had de-novo HCC, 51 patients (4.4%) died, and none received liver transplantation. The commonest decompensating event was ascites, followed by variceal bleeding and hepatic encephalopathy (Supplementary Table 2). None of the 140 patients (12.1%) fulfilling the exclusion criteria for CSPH developed liver decompensation.
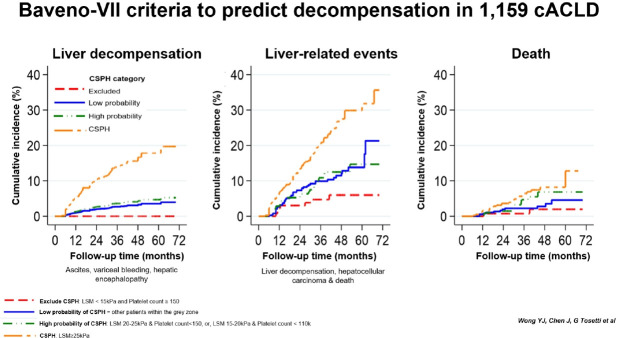
CSPH patients had a higher risk of liver decompensation, liver-related events and death when compared to those without CSPH (Fig. 2, Supplementary Table 3). The risk of liver decompensation was low among patients within the grey zone, regardless of whether they had a high or low probability of CSPH based on non-invasive criteria (Table 2). After excluding subjects with HRVs, these findings remained the same (Supplementary Fig. 1).
Figure 2.
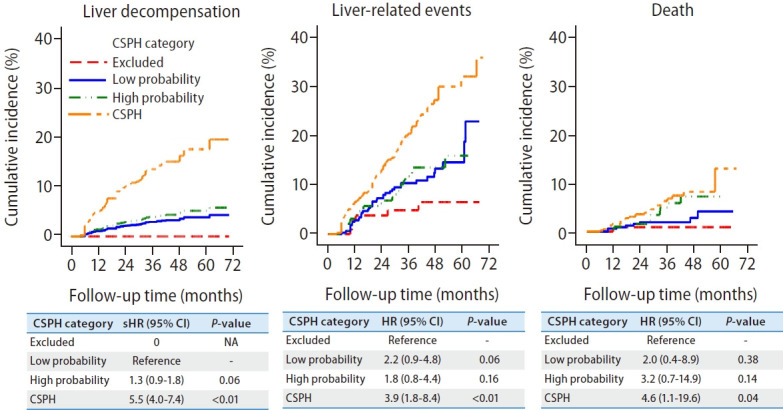
Clinical outcomes according to the non-invasive diagnosis of clinically significant portal hypertension in compensated advanced chronic liver disease patients. Liver decompensation was defined as the presence of ascites, variceal bleeding and hepatic encephalopathy. Liver-related events was defined as the presence of liver decompensation, hepatocellular carcinoma or death. CSPH, clinically significant portal hypertension; sHR, subdistribution hazard ratio; CI, confidence interval; NA, not applicable.
Table 2.
Cumulative incidence of liver-related events stratified based on non-invasively assessed clinically significant portal hypertension status
| Category | No. of events (cumulative incidence %) at 3-year |
||
|---|---|---|---|
| Liver decompensation* | Liver-related events† | All-cause death† | |
| CSPH excluded (n=140) | 0 (0.0) | 7 (5.0) | 2 (1.4) |
| Grey-zone (n=592) | 10 (2.6) | 43 (11.3) | 11 (2.9) |
| Low probability of CSPH | 8 (3.7) | 21 (10.0) | 9 (4.2) |
| High probability of CSPH | |||
| CSPH (n=427) | 59 (13.8) | 82 (19.2) | 25 (5.8) |
Definition of CSPH category: CSPH, defined as liver stiffness measurement (LSM) ≥25 kPa; CSPH excluded, defined as LSM <15 kPa and platelet count ≥150; grey-zone, patients who did not fulfilled non-invasive criteria to diagnose or exclude CSPH; high probability of CSPH, defined as LSM 20–25 kPa & platelet count <150, or LSM 15–20 kPa & platelet count <110×109/L; low probability of CSPH, other patients within the grey zone.
CSPH, clinically significant portal hypertension.
Cumulative incidence was calculated based on competing risks regression for clustered data with hepatocellular carcinoma and death as competing risks.
Cumulative incidence was calculated based on Cox regression with shared frailty.
Predictors of liver decompensation were the presence of CSPH (sHR, 2.48 [1.35–4.55]; P=0.003), non-viral related cirrhosis (sHR, 3.25 [1.83–5.76]; P<0.001], international normalized ratio (INR) >1.1 (sHR, 2.08 [1.14–3.80]; P=0.017), and albumin <37 g/L (sHR, 3.38 [1.83–6.25]; P<0.001) (Supplementary Table 4). Application of the “Rule-of-five” in our cohort demonstrate an incremental risk of liver decompensation, with LSM >25 kPa significantly associated with a higher risk of liver decompensation (Supplementary Table 5).
Subgroup analysis by etiology
Given that non-virus-related cirrhosis has a higher risk of decompensation, we performed a subgroup analysis based on etiology. The exclusion criteria of CSPH performed well in excluding patients at risk of decompensation, regardless of the underlying etiology of cirrhosis. Similarly, the presence of CSPH also predicts a higher risk of decompensation compared to those with CSPH excluded (Fig. 3). The 3-year cumulative incidence of decompensation among non-virus-related cACLD (CSPH excluded, 0%; low probability, 15.0%; high probability, 14.3%; CSPH, 22.2%) was higher than virus-related cACLD patients (CSPH excluded, 0%; low probability, 0.3%; high probability, 1.8%; CSPH, 9.0%).
Figure 3.
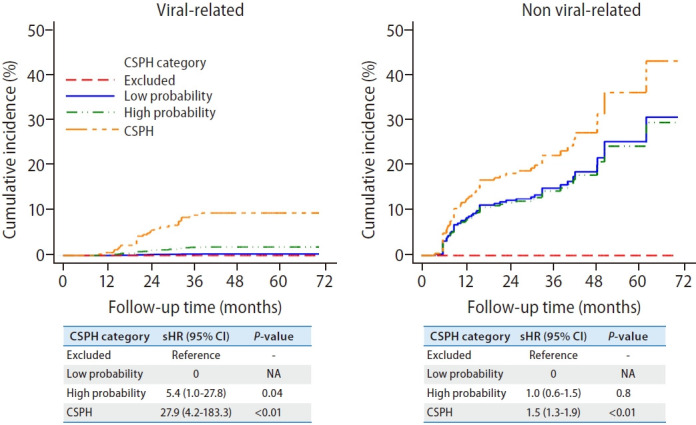
Cumulative incidence of decompensation based on etiology (virus-related vs. non-viral related). The 3-year cumulative incidence of decompensation among non-virus-related cACLD (CSPH excluded, 0%; low probability, 15.0%; high probability, 14.3%; CSPH, 22.2%) was higher than virus-related cACLD patients (CSPH excluded, 0%; low probability, 0.3%; high probability, 1.8%; CSPH, 9.0%). CSPH, clinically significant portal hypertension; sHR, subdistribution hazard ratio; CI, confidence interval; NA, not applicable; cACLD, compensated advanced chronic liver disease.
While the decompensation risk between the grey zone and those with CSPH excluded were similar among virus-related cACLD patients, such risk was substantially higher among non-viral cACLD patients within the grey zone. Among cACLD patients within the grey zone, there were no differences observed between patients with a low or high probability of CSPH, regardless of the underlying etiology (Fig. 3). The predictors of decompensation among patients in grey zone were the etiology of liver cirrhosis, INR, albumin and bilirubin (Supplementary Table 6).
Decision curve analysis
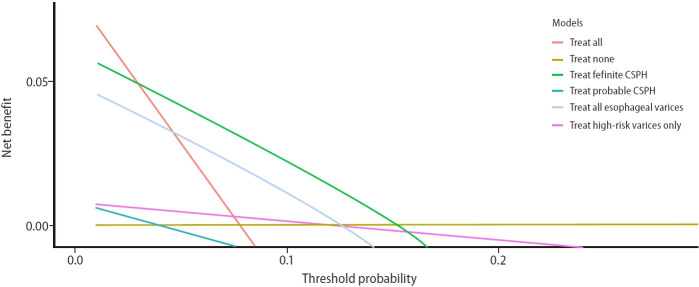
Using the treatment threshold derived from our cohort (between 5–10% decompensation rate at 5 years), “treating definite CSPH” strategy is superior to “treating probable CSPH” and “treating any varices” strategy to initiate NSBB. This is demonstrated by the largest area under the curve by adopting “treating definite CSPH” strategy. The number needed to treat for CSPH-based strategy was 27 and 50, at treatment thresholds of 5% and 10%, respectively (Fig. 4, Supplementary Table 7).
Figure 4.
Decision curve analysis demonstrating the benefit of initiating non-selective beta-blocker based on various strategies such as treating “high-risk varices” (pink), “all esophageal varices” (red), “treat definite CSPH” (green), “treat probable CSPH” (turquoise) and “treat none” (brown), across different threshold risk of annual decompensation. The area under the curve between different lines and the brown line (treat none) reflect the estimated benefit of each treatment strategy. At a treatment threshold between 5–10% of decompensation rate, treating “definite CSPH” is the best strategy to initiate non-selective beta-blocker to prevent decompensation. CSPH, clinically significant portal hypertension.
DISCUSSION
This international multicenter study demonstrates that non-invasive assessment of CSPH predicted liver decompensation in a large cohort of cACLD patients. Baveno-VII criteria reliably exclude CSPH, complementing earlier study by Ripoll et al. [2] showing a negligible risk of liver decompensation in patients with HVPG below 10 mmHg. CSPH was present in one-third of cACLD patients, and was associated with a five-fold higher risk of liver decompensation. Moreover, our DCA further supports the strategy of initiating NSBB in patients with CSPH to prevent liver decompensation, which is in line with an earlier randomized trial [3] and meta-analysis [12] supporting the use of carvedilol in preventing liver decompensation.
Compared to the seminal PREDESCI study, our cohort had a lower 5-year decompensation risk than the seminar PREDESCI study (7.9% vs. 20%, P<0.001) because the majority of the HCV-related cirrhosis patients from the PREDESCI cohort were untreated, and only 37% of our cohort had CSPH [2]. While one may argue that virological suppression would have modified the natural course of CSPH in virus-related cirrhosis, our findings reflect the real-world setting where virological suppression is now an achievable standard of care in virus-related cACLD patients following the introduction of high-efficacious, pangenotypic direct-acting antiviral treatment [13]. Moreover, our decompensation rate was consistent with recent studies demonstrating a relatively low risk of decompensation among virologically-suppressed cACLD patients [5,6,14].
Despite the heterogeneous risk of CSPH, we found the risk of liver decompensation in patients within the CSPH grey zone remained similar, irrespective of the underlying etiology of cirrhosis. The Baveno-VII consensus defined patients within CSPH “grey zone” as having either LSM between 20–25 kPa and platelet <150×109/L (defined as high probability of CSPH in our study), or LSM between 15–20 kPa and platelet <110×109/L (defined as low probability of CSPH in our study). While these patients had “at least” 60% predicted risk of CSPH, the observed risk of CSPH within the ANTICIPATE [15] cohort ranges from 57% to 78.5% in cACLD patients with low or high probability of CSPH, respectively. In other words, the diagnosis of CSPH grey zone may not be accurate in up to 43%. This heterogeneity in baseline decompensation risks can influence the treatment magnitude of NSBB in terms of absolute risk reduction and number needed to treat, thus making the diagnosis of “CSPH grey zone” unfavourable to be used as selection criteria to initiate NSBB in cACLD patients. In our cohort, the etiology of liver cirrhosis, serum INR, albumin and bilirubin correlates with the risk of liver decompensation in patients within CSPH grey zone (Supplementary Table 6). A recent study by Dajti and colleagues [16] demonstrated that spleen stiffness can reduce the proportion of patients within the CSPH grey zone. Further studies are required to validate the performance of spleen stiffness to stratify decompensation risk in patients with the CSPH grey zone.
Unlike virus-related cACLD whereby only patients with CSPH are at a higher risk of decompensation, a higher risk of decompensation was observed in non-virus-related cACLD patients with CSPH, as well as those within the grey zone. The exact reasons cannot be elucidated due to the relatively small proportion of non-virus-related cACLD in the current study. However there were several postulations: 1) the lack of definitive treatment for NASH cirrhosis may predispose these patients to an increased risk of disease progression and decompensation, 2) the potential difference in the natural history between NASH and virus-related cACLD, where the former may experience clinical decompensation at a lower portal pressure [17], and 3) unreported ongoing alcohol drinking, which may contribute to a higher risk of decompensation among alcoholic liver cirrhosis. Given the risk of decompensation is substantially higher in non-virus-related cACLD patients within the grey zone, one should remember that the decompensation risk is a continuous spectrum, therefore over-reliance on specific cut-off may potentially oversimplify the risk prediction in cACLD patients.
The expanding treatment indication of NSBB from preventing variceal bleeding to preventing decompensation represents a paradigm shift in the management of cACLD patients. While the Baveno-VI criteria could safely reduce the need for screening gastroscopy, it was unclear if this may represent a missing opportunity to identify cACLD patients with small esophageal varices for NSBB. While the risk of variceal bleeding is generally small in these patients, we demonstrated that the presence of small esophageal varices was associated with a higher risk of liver decompensation in our previous study [18]. In this regard, decision curve analysis showed that the “treating all esophageal varices” strategy is superior to “treating only HRV” strategy to prevent decompensation. However, even with routine endoscopy, CSPH may be present in those without esophageal varices. Indeed, decision curve analysis showed that the best strategy to prevent decompensation is not treating “probable CSPH” or “treating all esophageal varices”, but “treating definite CSPH strategy” instead (Supplementary Table 7). The strategy of treating definite CSPH strategy may prevent more decompensating events, at the same time mitigate the need for routine screening endoscopy among cACLD patients. In other words, the “treat definite CSPH” strategy may have significant impacts on resource allocation and carbon emission, given that endoscopy is the third-largest generator of carbon emission within healthcare [19].
The strengths of our study include the multicentre design with a relatively large sample size. We validated the performance of non-invasive Baveno-VII criteria using the competing risk analysis to account for multiple competing outcomes among cirrhosis patients, where the occurrence of an event could preclude (death) or modify (HCC or non-hepatic comorbidities) the probability of liver decompensation. Our findings provide a pragmatic estimation of the decompensation risk, given that most virus-related cirrhosis patients would have been treated with antiviral in a real-world setting [20]. Given the negligible risk of decompensation, NSBB is unlikely beneficial in patients whose CSPH were excluded based on Baveno-VII criteria. Prospective validation will help to understand if this criterion may be used to withdraw NSBB in cACLD patients with primary etiology controlled.
Our study has limitations. Due to the retrospective nature of our study, it is not possible to ensure alcohol abstinence in all subjects as we lack objective tests such as phosphatidylethanol to assess for alcohol intake. While the non-viral etiology was identified as one driving factor of liver decompensation, controlled or cured etiology may likely have contributed to a higher decompensation risk in alcohol-related cACLD (9.1%) as compared to virus-related cACLD patients. We acknowledged that variceal ligation may reduce the incidence of variceal bleeding in patients with HRVs. However, variceal ligation should not influence the incidence of other decompensating events such as ascites or hepatic encephalopathy [4], and our findings remained consistent after excluding subjects with HRVs (Supplementary Fig. 1). We did not account for radiological evidence of CSPH, such as portosystemic shunts, which may potentially under-estimate the prevalence of CSPH in this cohort. We acknowledge that there is variability in patients’ characteristics and clinical practice across different institutions, which may potentially influenced our results. Our findings remained robust for all key outcomes (liver decompensation, liver-related events and death) after adjusting our analysis by clusters (Fig. 2,Supplementary Table 7) [10]. Finally, the application of our findings should also consider the confounders of LSM (obesity, liver congestion or operator experience) and adverse effects of NSBB.
In summary, the non-invasive assessment of CSPH predicts a 5-fold higher decompensation risk in cACLD patients. Our findings support the use of Baveno-VII criteria of CSPH (i.e., LSM >25 kPa) to initiate NSBB in cACLD patients. While the risk of decompensation is low among virus-related cACLD fulfilling exclusion criteria of CSPH (LSM <15 kPa and platelet count ≥150), HCC surveillance should still be continued in these patients. Future studies should focus on identifying disease-specific thresholds to rule in or rule out CSPH following primary etiology suppression.
Acknowledgments
Dr. Wong YJ is supported by the Nurturing Clinician Scientist Scheme (NCCS) award by SingHealth Duke-NUS Academic Medical Centre.
We would like to thank Dr. Martin Putera and Dr. Garrett Kang for their contribution to this study.
Abbreviations
- cACLD
compensated advanced chronic liver disease
- CI
confidence interval
- CSPH
clinically significant portal hypertension
- CTP
Child-Turcott-Pugh
- DCA
decision curve analysis
- HCC
hepatocellular carcinoma
- HRV
high-risk varices
- HVPG
hepatic venous pressure gradient
- INR
international normalized ratio
- IQR
interquartile range
- LSM
liver stiffness measurement
- NASH
non-alcoholic steatohepatitis
- NSBB
non-selective beta-blocker
- sHR
subdistribution hazard ratio
Study Highlights
• Baveno-VII criteria can rule in or rule out CSPH among cACLD patients at baseline.
• Baveno-VII criteria have not been validated to predict decompensation and the need for NSBB in cACLD patients.
• The prevalence of CSPH among cACLD patients remained unclear.
• One-third of cACLD patients fulfilled the non-invasive criteria of CSPH.
• While non-invasive assessment of CSPH predicts decompensation risk and the need for NSBB in cACLD patients, “probable CSPH” is suboptimal to predict decompensation risk in cACLD patients.
• CSPH exclusion criteria might be used to stop NSBB in cACLD patients however further validations are required.
Footnotes
Authors’ contribution
Study conception: WYJ; Data acquisition: WYJ, SS, GT, ED, LC, LJ; Data analysis: WYJ, CZ, SS, SA, GT, CYH; Manuscript draft: WYJ, SS, SA, GT; Critical review of the manuscript and final review: All authors
Conflicts of Interest
The authors have no conflicts to disclose.
SUPPLEMENTARY MATERIAL
Supplementary material is available at Clinical and Molecular Hepatology website http://www.e-cmh.org.
Decision curve analysis
Baseline characteristics of study cohort based on study sites
Liver-related events based on study sites
The risk of liver decompensation, liver-related events and death according to the non-invasive diagnosis of clinically significant portal hypertension in cACLD patients with clustering effect
Predictors of liver decompensation in cACLD patients from proportional subdistribution hazards regression
Rule-of-5 in predicting liver decompensation in cACLD patients
Predictors of liver decompensation in cACLD patients (grey zone) from proportional subdistribution hazards regression
Decision curve analysis and number needed to treat/harm in comparison to treating only patients with high-risk varices
Cumulative incidence of clinical outcomes (liver decompensation, liver-related events and death) for compensated advanced chronic liver disease patients without high-risk varices. Liver decompensation was defined as the presence of ascites, variceal bleeding and hepatic encephalopathy. Liver-related events was defined as the presence of liver decompensation, hepatocellular carcinoma or death. CSPH, clinically significant portal hypertension; sHR, subdistribution hazard ratio; CI, confidence interval; NA, not applicable.
REFERENCES
- 1.D'Amico G, Morabito A, D'Amico M, Pasta L, Malizia G, Rebora P, et al. New concepts on the clinical course and stratification of compensated and decompensated cirrhosis. Hepatol Int. 2018;12(Suppl 1):34–43. doi: 10.1007/s12072-017-9808-z. [DOI] [PubMed] [Google Scholar]
- 2.Ripoll C, Groszmann R, Garcia-Tsao G, Grace N, Burroughs A, Planas R, et al. Hepatic venous pressure gradient predicts clinical decompensation in patients with compensated cirrhosis. Gastroenterology. 2007;133:481–488. doi: 10.1053/j.gastro.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 3.Villanueva C, Albillos A, Genescà J, Garcia-Pagan JC, Calleja JL, Aracil C, et al. β blockers to prevent decompensation of cirrhosis in patients with clinically significant portal hypertension (PREDESCI): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2019;393:1597–1608. doi: 10.1016/S0140-6736(18)31875-0. [DOI] [PubMed] [Google Scholar]
- 4.de Franchis R, Bosch J, Garcia-Tsao G, Reiberger T, Ripoll C, Baveno VII Faculty BavenoVII - renewing consensus in portalhypertension: report of the Baveno VII consensus workshop: personalized care in portal hypertension. J Hepatol. 2022;76:959–974. [Google Scholar]
- 5.Tosetti G, Degasperi E, Farina E, D'Ambrosio R, Soffredini R, Borghi M, et al. Decompensation in direct-acting antiviral cured hepatitis C virus compensated patients with clinically significant portal hypertension: too rare to warrant universal Β-blocker therapy. Am J Gastroenterol. 2021;116:1342–1344. doi: 10.14309/ajg.0000000000001158. [DOI] [PubMed] [Google Scholar]
- 6.Thabut D, Bureau C, Layese R, Bourcier V, Hammouche M, Cagnot C, et al. Validation of Baveno VI criteria for screening and surveillance of esophageal varices in patients with compensated cirrhosis and a sustained response to antiviral therapy. Gastroenterology. 2019;156:997–1009. doi: 10.1053/j.gastro.2018.11.053. e5. [DOI] [PubMed] [Google Scholar]
- 7.European Association for the Study of the Liver. Clinical Practice Guideline Panel EASL clinical practice guidelines on non-invasive tests for evaluation of liver disease severity and prognosis - 2021 update. J Hepatol. 2021;75:659–689. doi: 10.1016/j.jhep.2021.05.025. [DOI] [PubMed] [Google Scholar]
- 8.Pons M, Augustin S, Scheiner B, Guillaume M, Rosselli M, Rodrigues SG, et al. Noninvasive diagnosis of portal hypertension in patients with compensated advanced chronic liver disease. Am J Gastroenterol. 2021;116:723–732. doi: 10.14309/ajg.0000000000000994. [DOI] [PubMed] [Google Scholar]
- 9.Podrug K, Trkulja V, Zelenika M, Bokun T, Madir A, Kanizaj TF, et al. Validation of the new diagnostic criteria for clinically significant portal hypertension by platelets and elastography. Dig Dis Sci. 2021;67:3327–3332. doi: 10.1007/s10620-021-07277-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou B, Fine J, Latouche A, Labopin M. Competing risks regression for clustered data. Biostatistics. 2012;13:371–383. doi: 10.1093/biostatistics/kxr032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vickers AJ, van Calster B, Steyerberg EW. A simple, step-by-step guide to interpreting decision curve analysis. Diagn Progn Res. 2019;3:18. doi: 10.1186/s41512-019-0064-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Villanueva C, Torres F, Sarin SK, Shah HA, Tripathi D, Brujats A, et al. Carvedilol reduces the risk of decompensation and mortality in patients with compensated cirrhosis in a competing-risk meta-analysis. J Hepatology. 2022;77:1014–1025. doi: 10.1016/j.jhep.2022.05.021. [DOI] [PubMed] [Google Scholar]
- 13.Wong YJ, Thurairajah PH, Kumar R, Tan J, Fock KM, Law NM, et al. Efficacy and safety of sofosbuvir/velpatasvir in a real-world chronic hepatitis C genotype 3 cohort. J Gastroenterol Hepatol. 2021;36:1300–1308. doi: 10.1111/jgh.15324. [DOI] [PubMed] [Google Scholar]
- 14.Asesio N, Pollo-Flores P, Caliez O, Munteanu M, Ngo A, Ngo Y, et al. Baveno VI criteria as a prognostic factor for clinical complications in patients with compensated cirrhosis. Dig Liver Dis. 2021;54:645–653. doi: 10.1016/j.dld.2021.09.004. [DOI] [PubMed] [Google Scholar]
- 15.Abraldes JG, Bureau C, Stefanescu H, Augustin S, Ney M, Blasco H, et al. Noninvasive tools and risk of clinically significant portal hypertension and varices in compensated cirrhosis: the “ANTICIPATE” study. Hepatology. 2016;64:2173–2184. doi: 10.1002/hep.28824. [DOI] [PubMed] [Google Scholar]
- 16.Dajti E, Ravaioli F, Marasco G, Alemanni LV, Clecchia L, Ferrarese A, et al. A combined Baveno VII and spleen stiffness algorithm to improve the non-invasive diagnosis of clinically significant portal hypertension in patients with compensated advanced chronic liver disease. Am J Gastroenterol. 2022;117:1825–1833. doi: 10.14309/ajg.0000000000001887. [DOI] [PubMed] [Google Scholar]
- 17.Sanyal AJ, Van Natta ML, Clark J, Neuschwander-Tetri BA, Diehl A, Dasarathy S, et al. Prospective study of outcomes in adults with nonalcoholic fatty liver disease. N Engl J Med. 2021;385:1559–1569. doi: 10.1056/NEJMoa2029349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Putera M, Teh KB, Kumar R, Wong YJ. Small esophageal varices in compensated cirrhosis patients: to treat or not to treat? J Hepatol. 2021;75:491–492. doi: 10.1016/j.jhep.2021.03.003. [DOI] [PubMed] [Google Scholar]
- 19.Maurice JB, Siau K, Sebastian S, Ahuja N, Wesley E, Stableforth W, et al. Green endoscopy network. Green endoscopy: a call for sustainability in the midst of COVID-19. Lancet Gastroenterol Hepatol. 2020;5:636–638. doi: 10.1016/S2468-1253(20)30157-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wong YJ, Thurairajah PH, Kumar R, Fock KM, Law NM, Chong SY, et al. The impact of unrestricted access to direct-acting antiviral among incarcerated hepatitis C virus-infected patients. Clin Mol Hepatol. 2021;27:474–485. doi: 10.3350/cmh.2021.0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Decision curve analysis
Baseline characteristics of study cohort based on study sites
Liver-related events based on study sites
The risk of liver decompensation, liver-related events and death according to the non-invasive diagnosis of clinically significant portal hypertension in cACLD patients with clustering effect
Predictors of liver decompensation in cACLD patients from proportional subdistribution hazards regression
Rule-of-5 in predicting liver decompensation in cACLD patients
Predictors of liver decompensation in cACLD patients (grey zone) from proportional subdistribution hazards regression
Decision curve analysis and number needed to treat/harm in comparison to treating only patients with high-risk varices
Cumulative incidence of clinical outcomes (liver decompensation, liver-related events and death) for compensated advanced chronic liver disease patients without high-risk varices. Liver decompensation was defined as the presence of ascites, variceal bleeding and hepatic encephalopathy. Liver-related events was defined as the presence of liver decompensation, hepatocellular carcinoma or death. CSPH, clinically significant portal hypertension; sHR, subdistribution hazard ratio; CI, confidence interval; NA, not applicable.