Abstract
Calprotectin, an S100 calcium-binding protein with broad-spectrum antimicrobial activity in vitro, is expressed in neutrophils, monocytes, and gingival keratinocytes. In periodontitis, calprotectin appears upregulated and is detected at higher levels in gingival crevicular fluid and tissue specimens. How calprotectin contributes to the pathogenesis of periodontal diseases is unknown. To isolate the effects of calprotectin, a calprotectin-negative oral epithelial cell line was transfected with calprotectin genes to enable expression. Porphyromonas gingivalis was permitted to bind and invade transfected cells expressing calprotectin and sham transfectants. Rates of invasion into both cell lines were compared using the antibiotic protection assay. Transfected cells expressing calprotectin showed 40 to 50% fewer internalized P. gingivalis than sham transfectants. Similarly, binding to calprotectin expressing cells was reduced approximately twofold at all time points (15, 30, 45, and 60 min) as estimated by immunofluorescence analysis. Independent of invasion, however, prolonged exposure to P. gingivalis induced epithelial cell rounding and detachment from the substratum. These morphological changes were delayed, however, in cells expressing calprotectin. Using P. gingivalis protease-deficient mutants, we found that Arg-gingipain and Lys-gingipain contributed to epithelial cell rounding and detachment. In conclusion, expression of calprotectin appears to protect epithelial cells in culture against binding and invasion by P. gingivalis. In addition, cells expressing calprotectin are more resistant to detachment mediated by Arg-gingipain and Lys-gingipain. In periodontal disease, calprotectin may augment both the barrier protection and innate immune functions of the gingival epithelium to promote resistance to P. gingivalis infection.
Calprotectin is a noncovalently complexed heterodimer of two small anionic proteins, MRP8 and MRP14 (20, 37). Constitutively expressed in the cytosol of neutrophils, monocytes (8), activated macrophages (35), and squamous mucosal epithelia (3, 50), calprotectin is a member of the S100 family of calcium binding proteins (24). With its broad spectrum of in vitro antimicrobial effects (28, 29, 46, 47), calprotectin has been proposed to play a role in innate host defense of epithelia (4, 13). In candidiasis, keratinocytes subjacent to the infected superficial layer exhibit intense staining of calprotectin, which appears as a limiting barrier to penetration by candida mycelia (13, 14). Similarly, increased calprotectin expression is also observed in herpes simplex virus-and Epstein-Barr virus-infected and neighboring oral keratinocytes (13).
Levels of calprotectin in body fluids, including plasma, saliva, and synovial fluid, elevate markedly in various infections and inflammatory diseases and serve as a marker for disease activity (6, 17, 38, 40). In periodontitis, calprotectin levels in gingival crevicular fluid (GCF) from diseased sites are significantly higher than those in GCF from healthy sites and show positive correlations with several clinical and biochemical markers, including probing depth, interleukin 1β, prostaglandin E2, collagenase, and aspartate aminotransferase activity (23, 30). In GCF, calprotectin probably originates from pocket epithelia and inflammatory cells, including neutrophils and monocytes. Calprotectin expression in pocket epithelia and oral epithelia is increased in periodontitis, as suggested by immunohistochemistry (43).
Porphyromonas gingivalis, a gram-negative anaerobe, is considered an important periodontal pathogen (52). In vitro, P. gingivalis invades gingival epithelial cells, pocket epithelial cells, and endothelial cells (11, 25, 41, 42). Invasion into epithelial cells is fimbria-dependent, mediating adherence of P. gingivalis to host cells (34, 49). P. gingivalis also expresses proteases which are able to degrade epithelial adhesion proteins and induce detachment of epithelial cells from the substratum (21, 22, 48). Together, these virulence factors may allow P. gingivalis to invade deeper in the connective tissue and contribute to tissue destruction in periodontal diseases.
We hypothesize that calprotectin expression may protect epithelia against infection by P. gingivalis. In a previous study, we generated stable oral epithelial cell lines expressing calprotectin (KB-MRP8/14) and sham-control transfectants (KB-EGFP) from KB cells, a calprotectin-negative oral epithelial cell line (K. Nisapakultorn, K. F. Ross, and M. C. Herzberg, submitted for publication). Using these cell lines, we showed that calprotectin expression reduces invasion and binding of Listeria monocytogenes and salmonellae to epithelial cells (32; Nisapakultorn et al., submitted). The aim of this study was to assess the effect of epithelial calprotectin expression on an invasive periodontal pathogen, P. gingivalis, and to provide evidence for the role of calprotectin in innate host defense in periodontal disease. Expression of calprotectin appeared to reduce binding and invasion by P. gingivalis and protect keratinocyte monolayers from Arg- and Lys-gingipain protease-mediated cellular detachment.
MATERIALS AND METHODS
Stable epithelial cell lines expressing calprotectin.
KB epithelial cells expressing calprotectin (KB-MRP8/14) and KB sham-control transfectants (KB-EGFP) were generated from KB cells (ATCC CCL-17), a calprotectin-negative buccal carcinoma cell line, as previously described (Nisapakultorn et al., submitted). Briefly, KB cells were cotransfected with the mammalian expression vector, pIRES-EGFP (Clontech, Palo Alto, Calif.), containing MRP8 and MRP14 genes, and the selectable marker pSV2-neo, to generate KB-MRP8/14. A sham-control transfectant, KB-EGFP, was generated by cotransfection of insertless pIRES-EGFP and pSV2-neo. Cytosolic calprotectin (MRP8-MRP14) expression in transfected cells was verified both by sandwich enzyme-linked immunosorbent assay and indirect immunofluorescence using the MRP8-MRP14 heterodimer-specific (2) monoclonal antibody 27E10 (Bachem, King of Prussia, Pa.).
Epithelial cell culture.
KB-EGFP and KB-MRP8/14 were maintained in modified Eagle medium supplemented with 10% fetal bovine serum and G418 sulfate (700 μg/ml; Geneticin; Mediatech, Herndon, Va.). To avoid an unwanted antimicrobial affect from G418 sulfate, KB transfectants were grown in media without G418 sulfate for 4 days before experiments.
Bacteria strain and culture conditions.
P. gingivalis strains used in this study (Table 1) were grown anaerobically at 37°C in Todd-Hewitt broth (Difco, Detroit, Mich.) supplemented with hemin (5 μg/ml; Sigma, St. Louis, Mo.) and menadione (1 μg/ml; Sigma) or on Todd-Hewitt agar supplemented with hemin, menadione, and 5% defribinated sheep blood.
TABLE 1.
P. gingivalis strains used in this study
| Strain | Phenotype | Genotype | Source |
|---|---|---|---|
| FDC381 | Wild type | C. A. Genco | |
| DPG3 | Fimbria-deficient mutant of FDC381 | ΔfimA | C. A. Genco (26) |
| ATCC 33277 | Wild type | American Type Culture Collection | |
| KDP129 | Kgpa-deficient mutant of ATCC 33277 | Δkgp | K. Nakayama (36) |
| KDP133 | Rgpb-deficient mutant of ATCC 33277 | ΔrgpA ΔrgpB | K. Nakayama (31) |
| KDP136 | Kgp- and Rgp-deficient mutant of ATCC 33277 | Δkgp ΔrgpA ΔrgpB | K. Nakayama (45) |
Kgp, lysine-specific cysteine protease or Lys-gingipain.
Rgp, arginine-specific cysteine protease or Arg-gingipain.
Bacterial invasion assays.
Bacterial invasion into KB transfected cells was determined by an antibiotic protection assay (12). KB-EGFP and KB-MRP8/14 (1.2 × 105 cells) were grown overnight in 24-well cell culture plates. P. gingivalis ATCC 33277 or FDC381 was added at a multiplicity of infection (MOI) of 100 and incubated for 1.5 h at 37°C in an atmosphere of 5% CO2–95% air. The monolayers were washed twice with Dulbecco's phosphate-buffered saline (DPBS), and incubated with medium containing metronidazole (200 μg/ml; Sigma) and gentamicin (300 μg/ml; Sigma) for 1 h to kill extracellular bacteria. The monolayers were washed again with DPBS and lysed with distilled water for 15 min at room temperature. Released intracellular bacteria were diluted and plated with a spiral plater (Spiral Biotech, Bethesda, Md.). Internalized bacteria were enumerated by colony counting after 5 to 7 days. Each invasion assay was performed in triplicate wells and repeated in at least three independent experiments.
Bacterial adhesion assay.
KB-EGFP and KB-MRP8/14 were grown to confluence on gelatin-coated coverslips. KB transfectants were incubated with P. gingivalis ATCC 33277 (1.2 × 107 CFU) for 15, 30, 45, and 60 min at 37°C. The monolayers were washed twice with DPBS and fixed for 10 min with 4% paraformaldehyde. Adherent bacteria were stained with rabbit anti-P. gingivalis serum (dilution, 1:10,000; kindly provided by A. Sharma, State University of New York at Buffalo), followed by goat anti-rabbit Alexa 568 (dilution, 1:500; Molecular Probes, Eugene, Oreg.). Since KB cells were not permeabilized, the antibody detected only adherent bacteria. At each time point, digital images from 10 random microscopic fields viewed at a × 200 magnification were captured with a Spot camera (Diagnostic Instruments Inc., Sterling Heights, Mich.), and the adherent bacteria in each field were counted.
Prolonged incubation with P. gingivalis.
KB, KB-EGFP, and KB-MRP8/14 (3 × 105 cells/well) were grown in six-well cell culture plates for 48 h before assay. P. gingivalis were resuspended in modified Eagle medium and added to the monolayers at an MOI of 100. Strains used in the experiments included P. gingivalis FDC381, DPG3, ATCC 33277, KDP129, KDP133, KDP136, and heat-killed (60°C for 1 h) FDC381 (Table 1). The monolayers without P. gingivalis served as controls. Epithelial monolayers were monitored with a phase-contrast microscope (Nikon, Tokyo, Japan), and images of cell monolayers were taken at 0, 5, 9, and 24 h after exposure to P. gingivalis.
Statistical analysis.
Data are presented as the means ± standard deviations (SD). Statistically significant differences between KB-EGFP and KB-MRP8/14 were determined by using a repeated-measure analysis of variance for bacterial invasion assay and two-way analysis of variance for bacterial adhesion assay.
RESULTS
Reduced P. gingivalis invasion into KB cells expressing calprotectin.
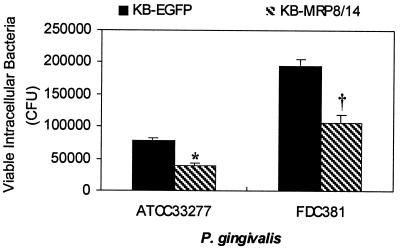
To determine whether calprotectin expression affects P. gingivalis invasion, the numbers of internalized P. gingivalis in KB-EGFP and KB-MRP8/14 were compared using the antibiotic protection assay. Approximately 40 to 50% fewer viable intracellular P. gingivalis from each of the two strains was recovered from KB-MRP8/14 than was recovered from KB-EGFP (Fig. 1).
FIG. 1.
P. gingivalis invasion into KB-EGFP and KB-MRP8/14. KB-EGFP or KB-MRP8/14 was incubated with P. gingivalis at an MOI of 100 for 1.5 h, followed by 1 h with antibiotics. The cell monolayers were then washed and lysed with distilled H2O to release intracellular bacteria. Internalized bacteria were plated and counted. Values are means ± SD from a representative experiment. Each experiment was performed in triplicate wells. The experiments were repeated at least three times with similar results for each strain of P. gingivalis. The differences between KB-EGFP and KB-MRP8/14 from three experiments were statistically significant (∗, P < 0.05; †, P < 0.01).
Reduced P. gingivalis binding to KB cells expressing calprotectin.
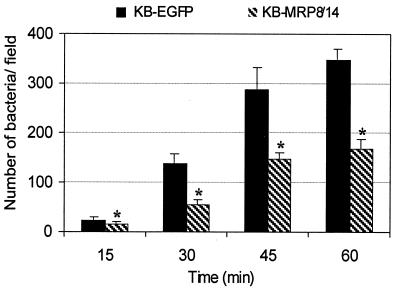
By immunofluorescence analysis (Fig. 2), the number of P. gingivalis that bound to KB-transfected cells increased over time. At all time points, approximately twofold fewer P. gingivalis cells bound to KB-MRP8/14 than bound to KB-EGFP.
FIG. 2.
Binding of P. gingivalis ATCC 33277 to KB-EGFP and KB-MRP8/14. KB-EGFP and KB-MRP8/14 cell monolayers were incubated with P. gingivalis for 15, 30, 45, or 60 min; washed; fixed; and stained for adherent P. gingivalis by indirect immunofluorescence. At each time point, images from 10 random microscopic fields at a magnification of ×200 were captured and the adherent bacteria in each field were counted. The differences between KB-EGFP and KB-MRP8/14 were significant (∗, P < 0.001). Error bars, SD.
KB cells expressing calprotectin are resistant to cell detachment induced by prolonged exposure to P. gingivalis.
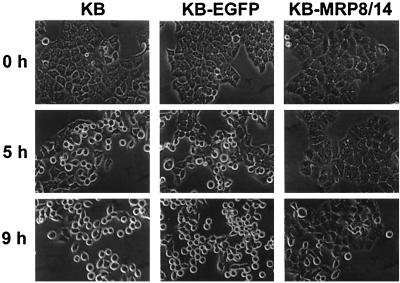
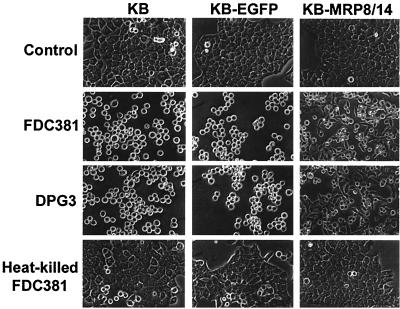
Prolonged exposure to P. gingivalis has been shown to induce epithelial cell rounding (1), suggesting compromise of the gingival epithelial barrier in periodontitis. To determine whether calprotectin expression affects epithelial cell responses to prolonged exposure to P. gingivalis, we incubated KB parental cells, KB-EGFP, and KB-MRP8/14 with P. gingivalis FDC381 and observed the morphological changes over time. KB-MRP8/14 showed a delay in cell rounding (Fig. 3). KB and KB-EGFP cells changed morphology from a flattened polygonal to round shape after 5 h of incubation. No change was observed in KB-MRP8/14 until 9 h of exposure to P. gingivalis. By 24 h, KB and KB-EGFP cells incubated with strain FDC381 were round, and some were detached from the culture plates (Fig. 4). In contrast, some KB-MRP8/14 cells did not become round and retained their polygonal morphology or appeared to be in the process of retraction. DPG3, a fimbria-deficient mutant that is unable to adhere to or invade KB cells (34), induced changes similar to those observed by the parental wild-type strain FDC381. Heat-killed P. gingivalis FDC381 had no effect on cell morphology (Fig. 4). Using trypan blue dye exclusion, more than 95% of detached cells were viable and able to attach and grow after subculture into fresh media (data not shown).
FIG. 3.
Changes in epithelial cell morphology after exposure to P. gingivalis FDC381 for 0, 5, and 9 h. KB, KB-EGFP, or KB-MRP8/14 was incubated with P. gingivalis FDC381 at an MOI of 100. Changes in epithelial cell morphology over time were observed by phase-contrast microscopy. Magnification, ×850.
FIG. 4.
Changes in epithelial cell morphology after 24 h of exposure to P. gingivalis FDC381, DPG3, or heat-killed FDC381. Epithelial cells that were not exposed to P. gingivalis served as controls. Magnification, ×800.
Arg-gingipain and Lys-gingipain induced epithelial cell detachment.
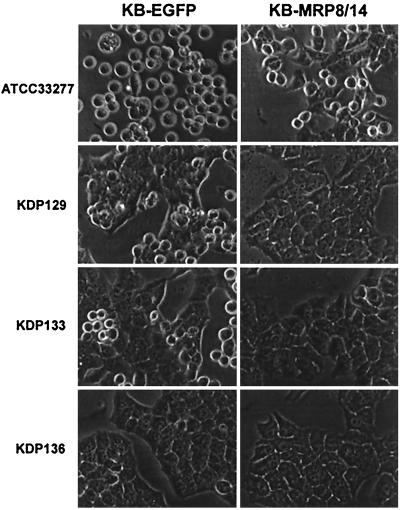
P. gingivalis proteases have been shown to induce epithelial cell detachment from monolayers (21). To identify specific proteases involved in this process, we incubated epithelial cell lines with protease-deficient mutants. After 24 h, the Rgp-deficient mutant (KDP129) and the Kgp-deficient mutant (KDP133) induced KB-EGFP cell rounding, but the effect was less than that observed with the wild-type parental strain ATCC 33277 (Fig. 5). Similar results were observed with KB cells (data not shown). Neither Rgp-deficient nor Kgp-deficient mutants induced rounding of KB-MRP8/14 cells. The Rgp and Kgp double mutant (KDP136) was unable to induce cell rounding of the epithelial cell lines.
FIG. 5.
Changes in epithelial cell morphology after exposure to P. gingivalis ATCC 33277 wild-type parental strain and protease-deficient mutants for 24 h. KDP129 is a Kgp-deficient mutant; KDP133 is an Rgp-deficient mutant; and KDP136 is a Kgp- and Rgp-deficient mutant. Magnification, ×1,000.
DISCUSSION
Periodontal diseases are infections of tooth-supporting tissues. P. gingivalis, a key putative periodontal pathogen, can invade the gingival tissue in advanced periodontitis (19, 39) and invade cultured epithelial cells in vitro (25, 41). P. gingivalis also produces enzymes that degrade a broad spectrum of host proteins, including epithelial adhesion molecules (22, 48) and extracellular matrix proteins (44). The ability of P. gingivalis to invade epithelial cells, disrupt the epithelial barrier, and penetrate deeper in connective tissue may contribute to tissue destruction and chronicity of periodontal diseases.
We previously reported that calprotectin expression is associated with reduced invasion and binding of intracellular pathogens, L. monocytogenes and salmonellae, to epithelial cells (32; Nisapakultorn et al., submitted). Since calprotectin expression is increased markedly in periodontal diseases (43), we tested whether epithelial calprotectin might promote resistance to P. gingivalis infection. To determine if calprotectin affects invasion and binding of the periodontal pathogen, P. gingivalis, the antibiotic protection assay was used. We recovered twofold fewer viable bacteria from epithelial cells that expressed calprotectin. However, the number of intracellular bacteria recovered after antibiotic treatment of extracellular bacteria is not absolute. In this study, extracellular P. gingivalis was treated with a combination of metronidazole (200 μg/ml) and gentamicin (300 μg/ml) for 1 h. This protocol has been widely used in studying P. gingivalis invasion (10, 11, 18, 25). To ensure that the antibiotic protocol actually killed all of the extracellular P. gingivalis, such that the viable counts could be attributed to only invaded intracellular bacteria, we determined the efficiency of killing in a broth assay. In this assay we carefully washed bacteria before plating to eliminate residual antibiotics and found that this amount of antibiotic killed 90 to 95% of the initial inoculum (108 CFU) after 1 h (data not shown). If we simply diluted the culture without washing the bacteria, we observed complete killing. Without washing the bacteria, the concentration of antibiotic present may still be sufficient to inhibit growth or kill bacteria on the plate. The findings suggested that drug carryover (33) can cause overestimation of killing and hence invasion in the antibiotic protection assay. This difficulty notwithstanding, the relative differences attributable to calprotectin are reliable.
The reduction in bacterial invasion may result from the well-known antibacterial effects of calprotectin. Bacterial invasion, however, is a multistep process involving binding of bacteria to the host cell surfaces and signaling between bacteria and host cells to induce cytoskeletal rearrangements which lead to translocation of the bacteria into host cells (15). Interference in any of these steps could reduce bacterial invasion. We observed twofold fewer P. gingivalis cells bound to cells expressing calprotectin. Reduced bacterial binding may, in part, explain reduced invasion into these cells. Host cell receptors that bind P. gingivalis to initiate internalization have not yet been identified. However, calprotectin-expressing cells bound fewer of each of three different bacterial species, L. monocytogenes, salmonellae, and now P. gingivalis, suggesting that common mechanisms probably exist. Calprotectin appeared to promote resistance to invasion by modulating cellular functions in addition to direct antibacterial activity.
We previously showed that calprotectin expression is associated with increased long actin filament formation, increased α3 integrin expression, and spreading cell morphology (Nisapakultorn et al., submitted). Increased expression of calprotectin and α3 integrin is also observed in oral and pocket epithelia of periodontitis tissue specimens (9, 16, 43), suggesting that changes observed in calprotectin transfected cells probably occur in vivo as a response to infection. Indeed, these changes may contribute to reduced P. gingivalis invasion into calprotectin expressing cells. If host receptors for P. gingivalis are localized on basolateral surfaces of epithelial cells, as is the case for L. monocytogenes (27), increased cell-cell and cell-substrate adhesion mediated by α3β1 integrin (5) may limit access to the receptors. Invasion by P. gingivalis is actin dependent (25). An alteration in actin cytoskeletal organization may also affect bacterial uptake. Finally, since calprotectin is a broad-spectrum antimicrobial, internalized P. gingivalis may be killed or its growth may be inhibited within epithelial cells.
In periodontitis, pocket epithelia are constantly exposed to a large number of bacteria in dental plaque, including P. gingivalis. Resistance to infection of the connective tissue depends in part on maintaining the integrity of the pocket mucosal epithelium. In vitro, prolonged exposure of gingival epithelial cells to P. gingivalis induced epithelial cell rounding (1). In this study, we showed that cells expressing calprotectin are resistant to cell rounding and detachment after prolonged exposure to P. gingivalis. To clarify whether cell rounding is mediated by P. gingivalis invasion (1) or by bacterial factors such as proteases (21) we compared changes in epithelial morphology after exposure to the wild type, FDC381, or the fimbria-deficient mutant, DPG3. The fimbria-deficient mutant, DPG3, shows no detectable adherence and invasion into KB cells (34). DPG3, however, induces cell detachment similar to the wild type, FDC381, suggesting that P. gingivalis invasion does not mediate cell detachment. In contrast, heat-killed P. gingivalis did not induce change in epithelial cell morphology, suggesting that heat-labile proteases may be responsible for detachment.
Cysteine proteases are major extracellular and cell-associated proteases of P. gingivalis and include an arginine-specific cysteine protease (Arg-gingipain, or Rgp) and lysine-specific cysteine protease (Lys-gingipain, or Kgp) (7). The Rgp has two isoforms, RgpA and RgpB, encoded by two genes, rgpA and rgpB, respectively. Kgp is encoded by the single gene kgp. P. gingivalis cysteine proteases have been implicated in induction of epithelial cell rounding and detachment from the substratum (21). Cell rounding and detachment appear to be mediated by proteolysis of cellular adhesion molecules, including occludin, E-cadherin, β1 integrin (22), intercellular adhesion molecule-1, vascular cell adhesion molecule-1, and very late antigen-4 (48, 51).
Using protease-deficient mutants, we demonstrated that epithelial cell detachment after prolonged exposure to P. gingivalis is due to the activity of Kgp and Rgp. P. gingivalis mutants that do not express both Rgp and Kgp (KDP136 [ΔrgpA ΔrgpB Δkgp]) were unable to induce cell rounding and detachment. The mutants that lack either Kgp (KDP129 [Δkgp]) or Rgp (KDP133 [ΔrgpA ΔrgpB]) induced rounding in untransfected and sham-transfected cells but to a lesser extent than did the wild-type strain (ATCC 33277). Up to 24 h of incubation with these mutants was insufficient to affect transfected cells expressing calprotectin. Together, the data showed that cells expressing calprotectin are more resistant to P. gingivalis Kgp- and Rgp-mediated cell detachment. Increased α3 integrin in calprotectin expressing cells may enhance cell-cell and cell-matrix adhesion, therefore limiting access and the time necessary for protease digestion and cell detachment from the monolayer.
In conclusion, we demonstrated that epithelial calprotectin expression may have a protective role in periodontal diseases. Reduced P. gingivalis binding and invasion into epithelial cells could reduce bacterial colonization and persistence during the infections. Calprotectin-mediated resistance to cysteine proteases appears to fortify the physical barrier role of epithelia and deter bacterial invasion into connective tissue matrix. Therefore, increased calprotectin expression during periodontitis may reflect complimentary innate mucosal host defense mechanisms in response to P. gingivalis infection.
ACKNOWLEDGMENTS
This project was supported by NIH/NIDCR grants R01DE11831 and P30DE09737. K. Nisapakultorn also was supported by a scholarship from the Royal Thai Government.
We thank Caroline A. Genco, Department of Medicine, Boston University, and Koji Nakayama, Department of Microbiology, Kyushu University, who kindly provided the P. gingivalis mutant strains used in this study. We also thank Ashu Sharma, Department of Microbiology and Oral Biology, State University of New York at Buffalo, who provided rabbit anti-P. gingivalis sera. We appreciate the assistance with statistical analysis by James Hodges.
REFERENCES
- 1.Belton C M, Izutsu K T, Goodwin P C, Park Y, Lamont R J. Fluorescence image analysis of the association between Porphyromonas gingivalis and gingival epithelial cells. Cell Microbiol. 1999;1:215–233. doi: 10.1046/j.1462-5822.1999.00022.x. [DOI] [PubMed] [Google Scholar]
- 2.Bhardwaj R S, Zotz C, Zwadlo-Klarwasser G, Roth J, Goebeler M, Mahnke K, Falk M, Meinardus-Hager G, Sorg C. The calcium-binding proteins MRP8 and MRP14 form a membrane-associated heterodimer in a subset of monocytes/macrophages present in acute but absent in chronic inflammatory lesions. Eur J Immunol. 1992;22:1891–1897. doi: 10.1002/eji.1830220732. [DOI] [PubMed] [Google Scholar]
- 3.Brandtzaeg P, Dale I, Fagerhol M K. Distribution of a formalin-resistant myelomonocytic antigen (L1) in human tissues. IL. Normal and aberrant occurrence in various epithelia. Am J Clin Pathol. 1987;87:700–707. doi: 10.1093/ajcp/87.6.700. [DOI] [PubMed] [Google Scholar]
- 4.Brandtzaeg P, Gabrielsen T O, Dale I, Muller F, Steinbakk M, Fagerhol M K. The leucocyte protein L1 (calprotectin): a putative nonspecific defence factor at epithelial surfaces. Adv Exp Med Biol. 1995;371A:201–206. doi: 10.1007/978-1-4615-1941-6_41. [DOI] [PubMed] [Google Scholar]
- 5.Carter W G, Wayner E A, Bouchard T S, Kaur P. The role of integrins α2 β1 and α3 β1 in cell-cell and cell-substrate adhesion of human epidermal cells. J Cell Biol. 1990;110:1387–1404. doi: 10.1083/jcb.110.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cuida M, Halse A K, Johannessen A C, Tynning T, Jonsson R. Indicators of salivary gland inflammation in primary Sjogren's syndrome. Eur J Oral Sci. 1997;105:228–233. doi: 10.1111/j.1600-0722.1997.tb00205.x. [DOI] [PubMed] [Google Scholar]
- 7.Curtis M A, Kuramitsu H K, Lantz M, Macrina F L, Nakayama K, Potempa J, Reynolds E C, Aduse-Opoku J. Molecular genetics and nomenclature of proteases of Porphyromonas gingivalis. J Periodontal Res. 1999;34:464–472. doi: 10.1111/j.1600-0765.1999.tb02282.x. [DOI] [PubMed] [Google Scholar]
- 8.Dale I, Brandtzaeg P, Fagerhol M K, Scott H. Distribution of a new myelomonocytic antigen (L1) in human peripheral blood leukocytes. Immunofluorescence and immunoperoxidase staining features in comparison with lysozyme and lactoferrin. Am J Clin Pathol. 1985;84:24–34. doi: 10.1093/ajcp/84.1.24. [DOI] [PubMed] [Google Scholar]
- 9.Del Castillo L F, Schlegel Gomez R, Pelka M, Hornstein O P, Johannessen A C, von den Driesch P. Immunohistochemical localization of very late activation integrins in healthy and diseased human gingiva. J Periodontal Res. 1996;31:36–42. doi: 10.1111/j.1600-0765.1996.tb00461.x. [DOI] [PubMed] [Google Scholar]
- 10.Dorn B R, Burks J N, Seifert K N, Progulske-Fox A. Invasion of endothelial and epithelial cells by strains of Porphyromonas gingivalis. FEMS Microbiol Lett. 2000;187:139–144. doi: 10.1111/j.1574-6968.2000.tb09150.x. [DOI] [PubMed] [Google Scholar]
- 11.Dorn B R, Dunn W A, Jr, Progulske-Fox A. Invasion of human coronary artery cells by periodontal pathogens. Infect Immun. 1999;67:5792–5798. doi: 10.1128/iai.67.11.5792-5798.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elsinghorst E A. Measurement of invasion by gentamicin resistance. Methods Enzymol. 1994;236:405–420. doi: 10.1016/0076-6879(94)36030-8. [DOI] [PubMed] [Google Scholar]
- 13.Eversole L R, Miyasaki K T, Christensen R E. Keratinocyte expression of calprotectin in oral inflammatory mucosal diseases. J Oral Pathol Med. 1993;22:303–307. doi: 10.1111/j.1600-0714.1993.tb01077.x. [DOI] [PubMed] [Google Scholar]
- 14.Eversole L R, Reichart P A, Ficarra G, Schmidt-Westhausen A, Romagnoli P, Pimpinelli N. Oral keratinocyte immune responses in HIV-associated candidiasis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;84:372–380. doi: 10.1016/s1079-2104(97)90035-4. [DOI] [PubMed] [Google Scholar]
- 15.Finlay B B, Falkow S. Common themes in microbial pathogenicity revisited. Microbiol Mol Biol Rev. 1997;61:136–169. doi: 10.1128/mmbr.61.2.136-169.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gurses N, Thorup A K, Reibel J, Carter W G, Holmstrup P. Expression of VLA-integrins and their related basement membrane ligands in gingiva from patients of various periodontitis categories. J Clin Periodontol. 1999;26:217–224. doi: 10.1034/j.1600-051x.1999.260404.x. [DOI] [PubMed] [Google Scholar]
- 17.Hammer H B, Kvien T K, Glennas A, Melby K. A longitudinal study of calprotectin as an inflammatory marker in patients with reactive arthritis. Clin Exp Rheumatol. 1995;13:59–64. [PubMed] [Google Scholar]
- 18.Han Y W, Shi W, Huang G T, Kinder Haake S, Park N H, Kuramitsu H, Genco R J. Interactions between periodontal bacteria and human oral epithelial cells: Fusobacterium nucleatum adheres to and invades epithelial cells. Infect Immun. 2000;68:3140–3146. doi: 10.1128/iai.68.6.3140-3146.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hillmann G, Dogan S, Geurtsen W. Histopathological investigation of gingival tissue from patients with rapidly progressive periodontitis. J Periodontol. 1998;69:195–208. doi: 10.1902/jop.1998.69.2.195. [DOI] [PubMed] [Google Scholar]
- 20.Hunter M J, Chazin W J. High level expression and dimer characterization of the S100 EF-hand proteins, migration inhibitory factor-related proteins 8 and 14. J Biol Chem. 1998;273:12427–12435. doi: 10.1074/jbc.273.20.12427. [DOI] [PubMed] [Google Scholar]
- 21.Johansson A, Kalfas S. Characterization of the proteinase-dependent cytotoxicity of Porphyromonas gingivalis. Eur J Oral Sci. 1998;106:863–871. doi: 10.1046/j.0909-8836.1998.eos106405.x. [DOI] [PubMed] [Google Scholar]
- 22.Katz J, Sambandam V, Wu J H, Michalek S M, Balkovetz D F. Characterization of Porphyromonas gingivalis-induced degradation of epithelial cell junctional complexes. Infect Immun. 2000;68:1441–1449. doi: 10.1128/iai.68.3.1441-1449.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kido J, Nakamura T, Kido R, Ohishi K, Yamauchi N, Kataoka M, Nagata T. Calprotectin in gingival crevicular fluid correlates with clinical and biochemical markers of periodontal disease. J Clin Periodontol. 1999;26:653–657. doi: 10.1034/j.1600-051x.1999.261004.x. [DOI] [PubMed] [Google Scholar]
- 24.Lagasse E, Clerc R G. Cloning and expression of two human genes encoding calcium-binding proteins that are regulated during myeloid differentiation. Mol Cell Biol. 1988;8:2402–2410. doi: 10.1128/mcb.8.6.2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lamont R J, Chan A, Belton C M, Izutsu K T, Vasel D, Weinberg A. Porphyromonas gingivalis invasion of gingival epithelial cells. Infect Immun. 1995;63:3878–3885. doi: 10.1128/iai.63.10.3878-3885.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malek R, Fisher J G, Caleca A, Stinson M, van Oss C J, Lee J Y, Cho M I, Genco R J, Evans R T, Dyer D W. Inactivation of the Porphyromonas gingivalis fimA gene blocks periodontal damage in gnotobiotic rats. J Bacteriol. 1994;176:1052–1059. doi: 10.1128/jb.176.4.1052-1059.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mengaud J, Ohayon H, Gounon P, Mege R M, Cossart P. E-cadherin is the receptor for internalin, a surface protein required for entry of L. monocytogenes into epithelial cells. Cell. 1996;84:923–932. doi: 10.1016/s0092-8674(00)81070-3. [DOI] [PubMed] [Google Scholar]
- 28.Miyasaki K T, Bodeau A L, Murthy A R, Lehrer R I. In vitro antimicrobial activity of the human neutrophil cytosolic S-100 protein complex, calprotectin, against Capnocytophaga sputigena. J Dent Res. 1993;72:517–523. doi: 10.1177/00220345930720020801. [DOI] [PubMed] [Google Scholar]
- 29.Murthy A R, Lehrer R I, Harwig S S, Miyasaki K T. In vitro candidastatic properties of the human neutrophil calprotectin complex. J Immunol. 1993;151:6291–6301. [PubMed] [Google Scholar]
- 30.Nakamura T, Kido J, Kido R, Ohishi K, Yamauchi N, Kataoka M, Nagata T. The association of calprotectin level in gingival crevicular fluid with gingival index and the activities of collagenase and aspartate aminotransferase in adult periodontitis patients. J Periodontol. 2000;71:361–367. doi: 10.1902/jop.2000.71.3.361. [DOI] [PubMed] [Google Scholar]
- 31.Nakayama K, Kadowaki T, Okamoto K, Yamamoto K. Construction and characterization of arginine-specific cysteine proteinase (Arg-gingipain)-deficient mutants of Porphyromonas gingivalis. Evidence for significant contribution of Arg-gingipain to virulence. J Biol Chem. 1995;270:23619–23626. doi: 10.1074/jbc.270.40.23619. [DOI] [PubMed] [Google Scholar]
- 32.Nisapakultorn K, Ross K F, Herzberg M C. Calprotecin expression inhibits bacterial binding to mucosal epithelial cells. Infect Immun. 2001;69:3692–3696. doi: 10.1128/IAI.69.6.3692-3696.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nix D E, Tyrrell R, Muller M. Pharmacodynamics of metronidazole determined by a time-kill assay for Trichomonas vaginalis. Antimicrob Agents Chemother. 1995;39:1848–1852. doi: 10.1128/aac.39.8.1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Njoroge T, Genco R J, Sojar H T, Hamada N, Genco C A. A role for fimbriae in Porphyromonas gingivalis invasion of oral epithelial cells. Infect Immun. 1997;65:1980–1984. doi: 10.1128/iai.65.5.1980-1984.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Odink K, Cerletti N, Bruggen J, Clerc R G, Tarcsay L, Zwadlo G, Gerhards G, Schlegel R, Sorg C. Two calcium-binding proteins in infiltrate macrophages of rheumatoid arthritis. Nature. 1987;330:80–82. doi: 10.1038/330080a0. [DOI] [PubMed] [Google Scholar]
- 36.Okamoto K, Nakayama K, Kadowaki T, Abe N, Ratnayake D B, Yamamoto K. Involvement of a lysine-specific cysteine proteinase in hemoglobin adsorption and heme accumulation by Porphyromonas gingivalis. J Biol Chem. 1998;273:21225–21231. doi: 10.1074/jbc.273.33.21225. [DOI] [PubMed] [Google Scholar]
- 37.Propper C, Huang X, Roth J, Sorg C, Nacken W. Analysis of the MRP8-MRP14 protein-protein interaction by the two-hybrid system suggests a prominent role of the C-terminal domain of S100 proteins in dimer formation. J Biol Chem. 1999;274:183–188. doi: 10.1074/jbc.274.1.183. [DOI] [PubMed] [Google Scholar]
- 38.Roseth A G, Schmidt P N, Fagerhol M K. Correlation between faecal excretion of indium-111-labelled granulocytes and calprotectin, a granulocyte marker protein, in patients with inflammatory bowel disease. Scand J Gastroenterol. 1999;34:50–54. doi: 10.1080/00365529950172835. [DOI] [PubMed] [Google Scholar]
- 39.Saglie F R, Smith C T, Newman M G, Carranza F A, Jr, Pertuiset J H, Cheng L, Auil E, Nisengard R J. The presence of bacteria in the oral epithelium in periodontal disease. II. Immunohistochemical identification of bacteria. J Periodontol. 1986;57:492–500. doi: 10.1902/jop.1986.57.8.492. [DOI] [PubMed] [Google Scholar]
- 40.Sander J, Fagerhol M K, Bakken J S, Dale I. Plasma levels of the leucocyte L1 protein in febrile conditions: relation to aetiology, number of leucocytes in blood, blood sedimentation reaction and C-reactive protein. Scand J Clin Lab Investig. 1984;44:357–362. doi: 10.3109/00365518409083820. [DOI] [PubMed] [Google Scholar]
- 41.Sandros J, Papapanou P, Dahlen G. Porphyromonas gingivalis invades oral epithelial cells in vitro. J Periodontal Res. 1993;28:219–226. doi: 10.1111/j.1600-0765.1993.tb01072.x. [DOI] [PubMed] [Google Scholar]
- 42.Sandros J, Papapanou P N, Nannmark U, Dahlen G. Porphyromonas gingivalis invades human pocket epithelium in vitro. J Periodontal Res. 1994;29:62–69. doi: 10.1111/j.1600-0765.1994.tb01092.x. [DOI] [PubMed] [Google Scholar]
- 43.Schlegel Gomez R, Langer P, Pelka M, von den Driesch P, Johannessen A C, Simon M., Jr Variational expression of functionally different macrophage markers (27E10, 25F9, RM3/1) in normal gingiva and inflammatory periodontal disease. J Clin Periodontol. 1995;22:341–346. doi: 10.1111/j.1600-051x.1995.tb00159.x. [DOI] [PubMed] [Google Scholar]
- 44.Scragg M A, Cannon S J, Rangarajan M, Williams D M, Curtis M A. Targeted disruption of fibronectin-integrin interactions in human gingival fibroblasts by the RI protease of Porphyromonas gingivalis W50. Infect Immun. 1999;67:1837–1843. doi: 10.1128/iai.67.4.1837-1843.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shi Y, Ratnayake D B, Okamoto K, Abe N, Yamamoto K, Nakayama K. Genetic analyses of proteolysis, hemoglobin binding, and hemagglutination of Porphyromonas gingivalis. Construction of mutants with a combination of rgpA, rgpB, kgp, and hagA. J Biol Chem. 1999;274:17955–17960. doi: 10.1074/jbc.274.25.17955. [DOI] [PubMed] [Google Scholar]
- 46.Sohnle P G, Collins-Lech C, Wiessner J H. Antimicrobial activity of an abundant calcium-binding protein in the cytoplasm of human neutrophils. J Infect Dis. 1991;163:187–192. doi: 10.1093/infdis/163.1.187. [DOI] [PubMed] [Google Scholar]
- 47.Steinbakk M, Naess-Andresen C F, Lingaas E, Dale I, Brandtzaeg P, Fagerhol M K. Antimicrobial actions of calcium binding leucocyte L1 protein, calprotectin. Lancet. 1990;336:763–765. doi: 10.1016/0140-6736(90)93237-j. [DOI] [PubMed] [Google Scholar]
- 48.Wang P L, Shinohara M, Murakawa N, Endo M, Sakata S, Okamura M, Ohura K. Effect of cysteine protease of Porphyromonas gingivalis on adhesion molecules in gingival epithelial cells. Jpn J Pharmacol. 1999;80:75–79. doi: 10.1254/jjp.80.75. [DOI] [PubMed] [Google Scholar]
- 49.Weinberg A, Belton C M, Park Y, Lamont R J. Role of fimbriae in Porphyromonas gingivalis invasion of gingival epithelial cells. Infect Immun. 1997;65:313–316. doi: 10.1128/iai.65.1.313-316.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wilkinson M M, Busuttil A, Hayward C, Brock D J, Dorin J R, Van Heyningen V. Expression pattern of two related cystic fibrosis-associated calcium-binding proteins in normal and abnormal tissues. J Cell Sci. 1988;91:221–230. doi: 10.1242/jcs.91.2.221. [DOI] [PubMed] [Google Scholar]
- 51.Yoshioka M, Wang P L, Ohura K. Effect of proteases from Porphyromonas gingivalis on adhesion molecules of human periodontal ligament fibroblast cells. Nippon Yakurigaku Zasshi. 1997;110:347–355. doi: 10.1254/fpj.110.347. [DOI] [PubMed] [Google Scholar]
- 52.Zambon J J. Periodontal diseases: microbial factors. Ann Periodontol. 1996;1:879–925. doi: 10.1902/annals.1996.1.1.879. [DOI] [PubMed] [Google Scholar]