Abstract
Background and aims
Heart rate variability (HRV) has previously been assessed as a biomarker for brain injury and prognosis in neonates. The aim of this cohort study was to use HRV to predict the electroencephalography (EEG) grade in neonatal hypoxic-ischaemic encephalopathy (HIE) within the first 12 h.
Methods
We included 120 infants with HIE recruited as part of two European multi-centre studies, with electrocardiography (ECG) and EEG monitoring performed before 12 h of age. HRV features and EEG background were assessed using the earliest 1 h epoch of ECG-EEG monitoring. HRV was expressed in time, frequency and complexity features. EEG background was graded from 0-normal, 1-mild, 2-moderate, 3-major abnormalities to 4-inactive. Clinical parameters known within 6 h of birth were collected (intrapartum complications, foetal distress, gestational age, mode of delivery, gender, birth weight, Apgar at 1 and 5, assisted ventilation at 10 min). Using logistic regression analysis, prediction models for EEG severity were developed for HRV features and clinical parameters, separately and combined. Multivariable model analysis included 101 infants without missing data.
Results
Of 120 infants included, 54 (45%) had normal-mild and 66 (55%) had moderate-severe EEG grade. The performance of HRV model was AUROC 0.837 (95% CI: 0.759–0.914) and clinical model was AUROC 0.836 (95% CI: 0.759–0.914). The HRV and clinical model combined had an AUROC of 0.895 (95% CI: 0.832–0.958). Therapeutic hypothermia and anti-seizure medication did not affect the model performance.
Conclusions
Early HRV and clinical information accurately predicted EEG grade in HIE within the first 12 h of birth. This might be beneficial when EEG monitoring is not available in the early postnatal period and for referral centres who may want some objective information on HIE severity.
Keywords: newborn, neonatal encephalopathy, heart rate variability, electroencephalography, electrocardiogram
Introduction
Despite developments in feto-maternal monitoring and care, hypoxia-ischaemia (HIE) remains the main cause of neonatal encephalopathy with an incidence of up to 1.5–3 per 1000 live births in high-income countries and significantly higher in low-middle income countries (1, 2). Therapeutic hypothermia is the only available treatment which has been shown to improve outcomes for moderate and severe HIE (3–6). To optimise the beneficial effects of hypothermia, treatment has to start as soon as possible after birth (7).
Sometimes, the early clinical picture alone is insufficient to decide if a newborn will benefit from therapeutic hypothermia and many neonatal centres use electroencephalography (EEG) to help assess the severity of encephalopathy. The use of EEG monitoring in neonatal units for diagnosis, treatment monitoring and prognosis is increasing worldwide (8, 9). International guidelines recognise the benefit of EEG for improved neonatal outcomes and recommend EEG for accurate diagnosis and monitoring of high risk infants (10, 11). However, introducing neonatal EEG monitoring is challenging; equipment is expensive and specialised personnel are required 24/7 to interpret the EEG. This is not readily available for most neonatal units, especially in low-income countries, thus the need of other methods to help identify those most at risk (12, 13).
Due to the widespread use of electrocardiography (ECG) monitoring, heart rate variability (HRV) has been assessed as a possible biomarker for brain injury and prognosis in neonatal populations (14–17). The naturally occurring variation in heartbeat can be described by HRV analysis. The autonomic nervous system, through sympathetic and parasympathetic control regulates HRV. Changes in beat-to-beat heart rate have been correlated with stress levels. A decrease in HRV was associated with stress elevation and studies suggest that HRV could predict HIE severity and outcome (18–21). In this study we investigated if HRV can predict EEG grade in infants with HIE in the first 12 h after birth. We hypothesised that HRV analysis may be a useful, objective tool to indicate EEG encephalopathy grade in HIE, which could be beneficial when EEG is not yet available. The aim of this study was to assess the use of early HRV analysis for the prediction of EEG encephalopathy grade in newborn infants.
Methods
Study setting and participants
The current study presents the results of a secondary analysis of data from two multi-centre cohort studies (ClinicalTrials.gov Identifier: NCT02160171 and NCT02431780) (22, 23) which recruited newborn infants requiring EEG monitoring across eight European tertiary Neonatal Units. Both studies had ethical approval granted by national and local ethics committees and parental/legal guardian written consent was obtained.
Newborn infants with the following were included: 36 weeks gestation or greater with presumed HIE and EEG-ECG monitoring for at least one hour before 12 h of age. The diagnosis of HIE was initially established based on signs of perinatal asphyxia and encephalopathy on neurological examination (modified Sarnat score within 24 h of age) and retrospectively corroborated with abnormalities suggestive of HIE on EEG and brain MRI. The clinical grade of HIE was based on the most severe score of modified Sarnat score. Infants with sepsis, meningitis, stroke, metabolic or genetic encephalopathy were excluded. Infants were also excluded if seizures were detected before, during or at least one hour after the EEG-ECG epoch selected for analysis.
EEG and ECG monitoring
EEG monitoring commenced as soon as possible after birth, using disposable electrodes according to the 10:20 electrode placement system for neonates (F3, F4, C3, C4, Cz, T3, T4, O1/P3 and O2/P4). EEG recording sampling rate was 250 Hz or 256 Hz and filter bandwidth was 0.5 to 70 Hz. Separate electrodes on each shoulder of the newborn were used for single channel ECG monitoring synchronised with EEG. The EEG machines used were: NicoletOne ICU Monitor (Natus, United States), Nihon Kohden EEG (Neurofax EEG-1200, Japan) or XLTek EEG (Natus, United States).
The earliest one-hour epoch of EEG-ECG was extracted for each infant, within 12 h of age.
EEG analysis and grading of HIE
EEG background was graded using a system described previously by our group: 0-normal EEG background, 1-mild abnormalities, 2-moderate abnormalities, 3-major abnormalities and 4-inactive EEG background (24).
HRV analysis
In-house software (HRV Analysis, Beta Version 1.12, ©University College Cork 2008–2012) was used to automatically identify R-peaks on ECG. A manual correction was applied after visual inspection to ensure all R-peaks were correctly identified and artefacts were annotated and removed from analysis. The RR interval was generated as the time difference between each R peak. HRV features were calculated for five-minute segments with 50% overlap, using the same procedure described in previous studies (19, 25–27). If more than 50% of a five-minute segment was artefact, this was removed. Across all 1 h epochs included in the analysis a median (IQR) of 1.2% (0.2% to 4.4%) was removed from the analysis. Features were summarised by median value over all segments. HRV was expressed in time, frequency and complexity features. The time domain features were mean NN-interval (the normalised RR interval), standard deviation of the NN-interval (SDNN), triangular interpolation of NN-interval histogram (TINN). The frequency domain features were high frequency power (HF, 0.2 to 2 Hz), low frequency power (LF, 0.04 to 0.2 Hz), very low frequency power (VLF, 0.01 to 0.04 Hz) and the low frequency/high frequency (LF/HF) ratio. The complexity features were calculated from multiscale entropy. The moving-average filter was used to generate the HRV at different scales, then entropy was calculated for each of these multiscale HRV signals (25, 26). Entropy was estimated using sample entropy with an embedding dimension of 2 and a tolerance of 0.15 (25). Four features were calculated from the plot of scale vs. entropy: 1) complexity index, estimated as the total area under the multiscale entropy curve, 2) maximum entropy, 3) slope of multiscale entropy over short-duration scale factors, by fitting a line to the entropy curve from scales 1 to 5 then calculating the slope of this line, and 4) slope of long-duration scale factors, the slope of a line fitted to the 6 to 20 scale factors (25, 26).
Clinical parameters
Several clinical features known within 6 h of birth were collected: presence of intrapartum complications, suspected foetal distress predelivery, gestational age, delivery mode, gender, birth weight, Apgar scores (1 and 5 min), need for assisted ventilation at 10 min of age, cord pH, first postnatal lactate, first postnatal base deficit. We considered intrapartum complications and their consequences as per the following: placental abruption, ruptured uterus, vasa praevia, intrapartum haemorrhage, cord accident or prolapse, shoulder dystocia, meconium-stained liquor or other (poor progression, HELLP syndrome/Eclampsia, breech presentation, reduced foetal movements, foetal bradycardia, prolonged rupture of membranes). These clinical parameters were used to predict the EEG grade of encephalopathy. Mode of delivery was combined into emergency (assisted vaginal delivery and emergency caesarean section) and non-emergency (unassisted vaginal delivery and elective caesarean section). The clinical management of each infant (including the need for therapeutic hypothermia and anti-seizure medication) was decided by the local clinical teams at each study site.
Statistical analysis
Categorical variables were described using frequencies and percentages and continuous variables using means and standard deviations (SDs), when data was normally distributed or medians and inter-quartile ranges (IQRs) otherwise. We investigated the ability of HRV features and clinical parameters (alone and in combination) to predict EEG encephalopathy grade using univariable and multivariable logistic regression analysis. The EEG grade was classified as a dichotomous variable, normal-mild group (grades 0 to 1, outcome = 0) and moderate-severe group (grades 2 to 4, outcome = 1). Univariable logistic regression analysis investigated the prediction of each variable for outcome (moderate-severe EEG grade). Using multivariable logistic regression analysis, we combined the variables with the best prediction for outcome and developed a HRV prediction model, a clinical prediction model and a combined model (HRV and clinical model). Variables with p < 0.25 in univariable analyses were eligible for inclusion in multivariable models. If variables were highly correlated, the variable with the highest area under receiver operating characteristic curve (AUROC) in the univariable analysis was included in the multivariable model. The Hosmer-Lemeshow goodness of fit test was used to determine the fit of the multivariable models with p < 0.05 indicating lack of fit. The following clinical parameters had missing data: suspected foetal distress in labour (14 (11.7%) infants), Apgar score at 1 min (4 (3.3%) infants), 5 min (4 (3.3%) infants) and 10 min 15 (12.5% infants), pH (18 (15%) infants), lactate (35 (29.2%) infants), base deficit (31 (25.8%) infants). For accurate comparison between models, the multivariable analysis included 101 infants with no missing clinical data. Subgroup analyses were performed exclusively with infants who received therapeutic hypothermia (n = 97 infants) and with infants not on anti-seizure medication prior to selected epoch (n = 71 infants). Due to missing data, cord pH, lactate and base deficit could not be included in the main multivariable analysis. However, the subgroup analyses also included the information on blood gases. Prior to performing logistic regression analyses, positively skewed HRV features were first log transformed and then all features were standardised to make the regression coefficients comparable. The AUROC and its corresponding 95% confidence interval (CI) were calculated from the univariable and multivariable models to assess their ability to predict EEG grade. An AUROC can range from 0.5 (discrimination no better than chance) to 1 (perfect discrimination). For multivariable models, Youden's index (index = sensitivity + specificity-1) was used to find the optimal sensitivity-specificity cut-off point on the AUROC curve and the corresponding sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) were estimated.
All tests were two-sided and a p < 0.05 was considered statistically significant. IBM SPSS Statistics (version 25.0, IBM Corp., Armonk, NY, United States) was used for the statistical analysis AUC curves were drawn using Stata (version 17.0, StataCorp, LP College Station, TX, USA).
Results
Out of the two original cohorts, 120 infants with HIE had at least one hour of EEG-ECG monitoring and were included in this analysis: 54 (45%) had a normal-mild EEG grade and 66 (55%) had a moderate-severe EEG grade on earliest EEG epoch. The two groups were similar in terms of gestational age, birth weight, gender, age at start of EEG monitoring and age when EEG epoch was analysed. Compared with the normal-mild EEG group, the moderate-severe EEG group had lower Apgar scores at 1 min (median (IQR) 2 (1 to 4) vs. 1 (0 to 2)), at 5 min (median (IQR) 5 (4 to 7) vs. 3 (1 to 4)) and at 10 min (median (IQR) 7 (5 to 9) vs. 4 (3 to 5)), had lower pH (mean (SD) 7.05 (0.15) vs. 6.95 (0.20)), higher lactate (mean (SD) 10.9 (3.2) vs. 13.0 (5.2)), higher base deficit (mean (SD) 14.6 (4.5) vs. 17.0 (6.4)), higher need for ventilation (22 (41.5%) vs. 58 (89.2%)), more infants were cooled (33 (61.1%) vs. 64 (97%)), and more infants had electrographic seizures (6 (11.1%) vs. 34 (51.5%)). Demographic data is presented in Table 1.
Table 1.
Study cohort demographics.
| n | All infants | Normal-mild EEG background group | Moderate-severe EEG background group | |
|---|---|---|---|---|
| n = 120a | n = 54a | n = 66a | ||
| Gestational age at birth (weeks), median (IQR) | 40.1 (39.2 to 41.0) | 40.6 (39.4 to 41.1) | 40.0 (39.0 to 40.9) | |
| Mode of delivery, n (%) | ||||
| Unassisted vaginal delivery | 39 (32.5) | 13 (24.1) | 26 (39.4) | |
| Assisted vaginal delivery | 43 (35.8) | 25 (46.3) | 18 (27.3) | |
| Elective caesarean section | 6 (5.0) | 1 (1.9) | 5 (7.6) | |
| Emergency caesarean section | 32 (26.7) | 15 (27.8) | 17 (25.8) | |
| Birth weight (g), mean (SD) | 3,475 (607) | 3,525 (492) | 3,434 (689) | |
| Gender, male n (%) | 75 (62.5) | 32 (59.3) | 43 (65.2) | |
| Apgar score at 1 min, median (IQR) | 116 | 1 (0 to 3) | 2 (1 to 4) | 1 (0 to 2) |
| Apgar score at 5 min, median (IQR) | 116 | 4 (2 to 6) | 5 (4 to 7) | 3 (1 to 4) |
| Apgar score at 10 min, median (IQR) | 105 | 5 (4 to 8) | 7 (5 to 9) | 4 (3 to 5) |
| Adrenaline during resuscitation, yes n (%) | 20 (16.6) | 3 (5.6) | 17 (25.8) | |
| Assisted ventilation at 10 min of age, yes n (%) | 118 | 80 (67.8) | 22 (41.5) | 58 (89.2) |
| Lowest cord pH, mean (SD) | 102 | 7.0 (0.2) | 7.1 (0.2) | 7.0 (0.2) |
| First postnatal lactate, mean (SD) | 85 | 11.90 (4.35) | 10.9 (3.2) | 13 (5.2) |
| First postnatal base deficit, mean (SD) | 89 | 15.8 (5.6) | 14.6 (4.5) | 17 (6.4) |
| HIE clinical grade at discharge | ||||
| Mild, n (%) | 51 (42.5) | 40 (74.1) | 11 (16.7) | |
| Moderate, n (%) | 44 (36.7) | 13 (24.1) | 31 (47.0) | |
| Severe, n (%) | 25 (20.8) | 1 (1.9) | 24 (36.4) | |
| Therapeutic hypothermia (yes), n (%) | 97 (80.8) | 33 (61.1) | 64 (97.0) | |
| Age at start of therapeutic hypothermia (hours), median (IQR) | 97 | 1 (1 to 3) | 2 (1 to 3) | 1 (1 to 4) |
| Age at start of EEG monitoring (hours), median (IQR) | 4.4 (3.2 to 7.4) | 4.3 (3.2 to 7.4) | 4.7 (3.3 to 7.7) | |
| Age at start of EEG epoch (hours), median (IQR) | 5.0 (4.1 to 8.3) | 5 (4.1 to 8.2) | 6.5 (4.2 to 8.6) | |
| Electrographic seizures (yes), n (%) | 40 (33.3) | 6 (11.1) | 34 (51.5) | |
| Any anti-seizure medication given before EEG epoch analysed (yes), n (%) | 19 (15.8) | 0 | 19 (28.8) |
EEG, electroencephalography.
unless otherwise stated.
Univariable analysis
Out of the HRV features, a higher mean NN and lower LF/HF ratio, TINN, MSE complexity index, MSE short-scale slope and maximum MSE were associated with moderate-severe EEG grade (analysis presented in Table 2).
Table 2.
HRV univariable analysis.
| HRV Features | Normal-mild EEG group (median, IQR) | Moderate-severe EEG group (median, IQR) | ORa (95% CI) | p value | AUROC (95% CI) |
|---|---|---|---|---|---|
| n = 54 | n = 66 | ||||
| Mean NN (ms)b | 523.3 (481.9 to 603.6) | 591.6 (532.6 to 692.6) | 1.97 (1.30–2.97) | 0.001 | 0.675 (0.579–0.772) |
| SDNN (ms)c | 18.0 (13.6 to 34.0) | 19.8 (10.6 to 33.8) | 0.93 (0.65–1.34) | 0.712 | 0.504 (0.400–0.609) |
| VLF power (ms2)c | 1772.5 (678.3 to 4452.1) | 1835.8 (495.9 to 4308.9) | 0.79 (0.54–1.15) | 0.211 | 0.544 (0.441–0.648) |
| LF power (ms2)c | 271.0 (65.3 to 719.9) | 194.6 (47.4 to 958.3) | 0.89 (0.62–1.28) | 0.529 | 0.526 (0.422–0.629) |
| HF power (ms2)c | 3.8 (1.1 to 18.6) | 8.0 (2.3 to 43.2) | 1.38 (0.95–2.00) | 0.091 | 0.585 (0.483–0.688) |
| LF/HF ratioc | 48.9 (24.6 to 73.6) | 18.4 (7.8 to 37.1) | 0.40 (0.25–0.64) | <0.001 | 0.721 (0.629–0.813) |
| TINN (ms)c | 62.5 (46.9 to 95.7) | 56.6 (31.3 to 78.1) | 0.66 (0.45–0.97) | 0.035 | 0.597 (0.495–0.698) |
| MSE Complexity Indexb | 26.5 (23.4 to 29.6) | 23.5 (18.9 to 25.8) | 0.46 (0.29–0.73) | 0.001 | 0.695 (0.600–0.790) |
| MSE short-scale slopeb | 0.11 (0.05 to 0.14) | 0.04 (-0.01 to 0.07) | 0.26 (0.15–0.47) | <0.001 | 0.776 (0.692–0.860) |
| MSE long-scale slopeb | -0.01 (-0.03 to 0.03) | 0.00 (-0.02 to 0.03) | 1.14 (0.79–1.64) | 0.477 | 0.555 (0.447–0.663) |
| MSE maximumb | 2.4 (2.2 to 2.6) | 2.2 (1.9 to 2.5) | 0.39 (0.22–0.69) | 0.001 | 0.690 (0.596–0.783) |
For the non-transformed variables the odds ratio represents the change in odds for a one-standard deviation increase in the HRV variable. For the transformed variables the odds ratio represents the change in odds for a one-standard deviation increase in the log HRV variable.
HRV variable was standardised prior to conducting the logistic regression.
HRV variable was log transformed (log base 10) and then standardised prior to conducting the logistic regression.
HRV, Heart Rate Variability; EEG, electroencephalography; NN interval, normalised RR interval; SDNN, standard deviation of the NN interval; VLF power, very low frequency power (≤ 0.04 Hz); LF power, low frequency power (0.04–0.15 Hz); HF power, high frequency power (0.15–0.4 Hz), LF/HF ratio, low frequency/high frequency ratio; TINN, triangular interpolation of the NN interval histogram; MSE, multiscale entropy.
Out of the clinical parameters, non-emergency delivery mode, lower Apgar scores, higher need for ventilation at 10 min, lower pH and higher lactate and base deficit were associated with moderate-severe EEG grade (analysis presented in Table 3).
Table 3.
Clinical univariable analysis.
| Clinical Parameters | Normal-mild EEG group | Moderate-severe EEG group | OR (95% CI) | p value | AUROC (95% CI) |
|---|---|---|---|---|---|
| n = 54a | n = 66a | ||||
| Any Intrapartum complications, yes n (%) | 46 (85.2) | 57 (86.4) | 1.10 (0.39–3.08) | 0.854 | 0.506 (0.402–0.610) |
| Suspected foetal distress in labour, yes n (%)b | 40 (81.6) | 39 (68.4) | 0.49 (0.20–1.22) | 0.123 | 0.566 (0.457–0.675) |
| Gestational age at birth (weeks), median (IQR) | 40.6 (39.4 to 41.1) | 40.0 (39.0 to 40.9) | 0.78 (0.59–1.05) | 0.099 | 0.591 (0.489–0.694) |
| Mode of delivery, emergency n (%) | 40 (74.1) | 35 (53.0) | 0.40 (0.18–0.86) | 0.019 | 0.605 (0.504–0.707) |
| Gender, male n (%) | 32 (59.3) | 43 (65.2) | 1.29 (0.61–2.70) | 0.507 | 0.529 (0.425–0.634) |
| Birth weight (g), mean (SD) | 3,525 (492) | 3,434 (689) | 0.98 (0.92–1.04)h | 0.410 | 0.570 (0.468–0.673) |
| Apgar scores at 1 min, median (IQR)c | 2 (1 to 4) | 1 (0 to 2) | 0.71 (0.57–0.88) | 0.002 | 0.708 (0.614–0.802) |
| Apgar scores at 5 min, median (IQR)c | 5 (4 to 7) | 3 (1 to 4) | 0.67 (0.55–0.81) | <0.001 | 0.756 (0.667–0.846) |
| Assisted ventilation at 10 min of age, yes n (%)d | 22 (41.5) | 58 (89.2) | 11.68 (4.49–30.36) | <0.001 | 0.739 (0.644–0.833) |
| Lowest cord pH, mean (SD)e | 7.05 (0.15) | 6.95 (0.20) | 0.97 (0.95–0.99)i | 0.008 | 0.666 (0.562–0.770) |
| First postnatal lactate, mean (SD)f | 10.9 (3.2) | 13.0 (5.2) | 1.13 (1.01–1.25) | 0.029 | 0.644 (0.519–0.768) |
| First postnatal base deficit mean (SD)h | 14.6 (4.5) | 17.0 (6.4) | 1.08 (1.00–1.17) | 0.050 | 0.658 (0.539–0.777) |
unless otherwise stated.
n = 49 in normal-mild group and n = 57 in moderate-severe group.
n = 52 in normal-mild group and n = 64 in moderate-severe group.
n = 53 in normal-mild group and n = 65 in moderate-severe group.
n = 45 in normal-mild group and n = 57 in moderate-severe group.
n = 44 in normal-mild group and n = 41 in moderate-severe group.
n = 46 in normal-mild group and n = 43 in moderate-severe group.
The odds ratio represents the change in odds for a 100 g increase in birthweight.
The odds ratio represents the change in odds for a 0.01 increase in cord pH.
Mode of delivery was defined as emergency (assisted vaginal delivery and emergency caesarean section) and non-emergency (unassisted vaginal delivery and elective caesarean section).
Multivariable analysis
For comparability of the multivariable models, we report the results of the multivariable analysis restricted to 101 infants with complete clinical information, which were included in the clinical prediction model. The AUROC for the multivariable HRV prediction model was 0.837 (95% CI:0.759–0.914, p < 0.001), Table 4. Based on Youden's index, the optimal cut-off was p = 0.66, giving sensitivity 63.6%, specificity 91.3%, PPV 89.7% and NPV 67.7%. The AUROC for the multivariable clinical prediction model was 0.836 (95% CI:0.759–0.914, p < 0.001), with sensitivity 87.3%, specificity 69.6%, PPV 77.4% and NPV 82.1% using the optimal cut-off p = 0.57, Table 5. The combined HRV and clinical model, had the best performance with an AUROC of 0.895 (95% CI:0.832–0.958, p < 0.001) (Table 6), with sensitivity 74.6%, specificity 95.7%, PPV 95.3% and NPV 75.9% (optimal cut-off p = 0.66). Performance of prediction models is shown in Figure 1.
Table 4.
HRV multivariable analysis (HRV prediction model), n = 101 infants.
| ORa (95% CI) | p value | AUROC (95% CI) | |
|---|---|---|---|
| Mean NN (ms)b | 2.73 (1.34–5.53) | 0.005 | 0.837 (0.759–0.914) |
| HF power (ms2)c | 1.45 (0.44–4.81) | 0.544 | |
| LF/HF ratioc | 1.48 (0.51–4.34) | 0.471 | |
| TINN (ms)c | 0.61 (0.19–1.93) | 0.398 | |
| MSE Complexity Indexb | 0.60 (0.32–1.13) | 0.115 | |
| MSE slope shortb | 0.31 (0.12–0.79) | 0.014 |
For the non-transformed variables the odds ratio represents the change in odds for a one-standard deviation increase in the HRV variable. For the transformed variables the odds ratio represents the change in odds for a one-standard deviation increase in the log HRV variable.
HRV variable was standardised prior to conducting the logistic regression.
HRV variable was log transformed (log base 10) and then standardised prior to conducting the logistic regression.
The multivariable model is: log (p/(1 − p)) = 0.49 + 1.00 (standardized Mean NN) + 0.37 (standardised log 10 HF power) + 0.40 (standardised log 10 LF HF ratio) −0.50 (standardised log 10 TINN) − 0.51 (standardised MSE c index) −1.16 (standardised MSE slope short) p = 0.480 for Hosmer-Lemeshow goodness of fit.
HRV, Heart Rate Variability; NN interval, normalised RR interval; HF power, high frequency power (0.15–0.4 Hz), LF/HF ratio, low frequency/high frequency ratio; TINN, triangular interpolation of the NN interval histogram; MSE, multiscale entropy.
Table 5.
Clinical multivariable analysis (clinical prediction model), n = 101 infants.
| OR (95% CI) | p value | AUROC (95% CI) | |
|---|---|---|---|
| Suspected foetal distress in labour (yes) | 0.28 (0.06–1.39) | 0.120 | 0.836 (0.759–0.914) |
| Gestational age (weeks) | 0.78 (0.52–1.17) | 0.233 | |
| Mode of delivery (emergency) | 0.73 (0.20–2.58) | 0.619 | |
| Apgar scores at 5 min | 0.93 (0.74–1.17) | 0.532 | |
| Assisted ventilation at 10 min of age (yes) | 14.92 (3.81–58.35) | <0.001 |
The multivariable model is: log[p/(1-p)] = 9.71-1.26 (suspected foetal distress in labour)-0.25 (GA at delivery) −0.32 (emergency delivery) – 0.07 (Apgar score at 5 min) + 2.70 (assisted ventilation) p = 0.325 for Hosmer-Lemeshow goodness of fit.
Table 6.
HRV and clinical prediction model, n = 101 infants.
| OR (95% CI) | p value | AUROC (95% CI) | |
|---|---|---|---|
| Mean NN (ms)a | 2.34 (1.07–5.10) | 0.032 | 0.895 (0.832–0.958) |
| HF power (ms2)a | 1.94 (0.47–7.99) | 0.361 | |
| LF/HF ratiob | 1.51 (0.47–4.89) | 0.488 | |
| TINN (ms)b | 0.70 (0.19–2.58) | 0.595 | |
| MSE Complexity Indexa | 0.84 (0.38–1.84) | 0.661 | |
| MSE short-scale slopea | 0.35 (0.13–0.98) | 0.045 | |
| Suspected foetal distress in labour (yes) | 0.30 (0.04–2.20) | 0.237 | |
| Gestational age (weeks) | 0.76 (0.48–1.22) | 0.251 | |
| Mode of delivery (emergency) | 0.63 (0.12–3.29) | 0.583 | |
| Apgar scores at 5 min | 0.94 (0.72–1.23) | 0.654 | |
| Assisted ventilation at 10 min of age (yes) | 8.94 (1.86–43.00) | 0.006 |
HRV variable was standardised prior to conducting the logistic regression.
HRV variable was log transformed (log base 10) and then standardised prior to conducting the logistic regression.
The multivariable model is: log[p/(1-p)] = 11.49 + 0.85 (standardised Mean NN) + 0.66 (standardised log 10 HF power) + 0.42 (standardised log 10 LF/HF ratio) – 0.35 (standardised log 10 TINN)-0.18 (standardised MSE c index) −1.04 (standardised MSE slope short) −1.20 (suspected foetal distress in labour)-0.28 (GA at delivery) −0.46 (emergency delivery) – 0.06 (Apgar score at 5 min) + 2.19 (assisted ventilation) p = 0.548 for Hosmer-Lemeshow goodness of fit.
HRV, Heart Rate Variability; NN interval, normalised RR interval; HF power, high frequency power (0.15–0.4 Hz), LF/HF ratio, low frequency/high frequency ratio; TINN, triangular interpolation of the NN interval histogram; MSE, multiscale entropy.
Figure 1.
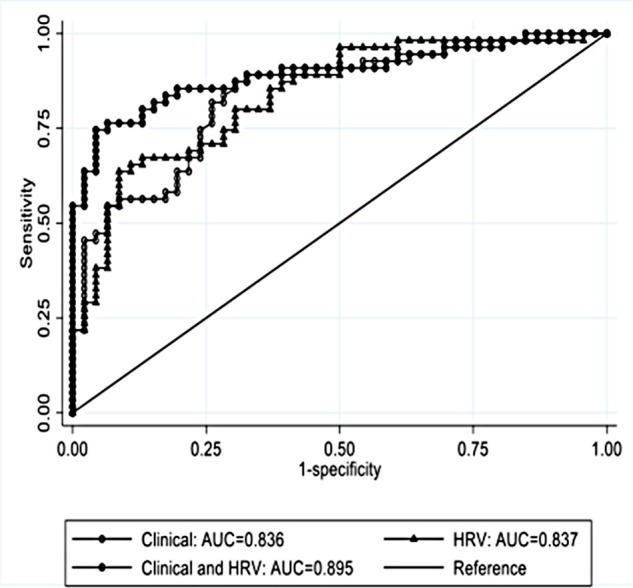
The AUROC of the multivariable analysis for HRV and clinical prediction model.
To account for the confounding effect of therapeutic hypothermia and anti-seizure medication, we analysed data exclusively from infants undergoing therapeutic hypothermia and from infants which did not receive anti-seizure medication prior to the selected epoch. For these subgroup analyses we also included data on blood gases (pH, lactate and base deficit). Predictive performance of the models including only infants that received therapeutic hypothermia (n = 97 infants) was: HRV model AUROC 0.812 (95% CI:0.726–0.898), clinical model AUROC 0.781 (95% CI:0.658–0.905), HRV and clinical model AUROC 0.864 (95% CI:0.766–0.963) (Table 7). Models from the subgroup of infants without anti-seizure medication given prior to selected ECG-EEG epoch (n = 71 infants) were: HRV model AUROC 0.821 (95% CI:0.721–0.922), clinical model AUROC 0.829 (95% CI:0.736–0.923), HRV and clinical model AUROC 0.883 (95% CI:0.800–0.965) (Table 8).
Table 7.
Subgroup analysis of infants undergoing therapeutic hypothermia - HRV and clinical prediction model, n = 52 infants.
| OR (95% CI) | p value | AUROC (95% CI) | |
|---|---|---|---|
| Mean NN (ms)a | 1.72 (0.50–5.86) | 0.386 | 0.864 (0.766–0.963) |
| LF/HF rateb | 1.70 (0.49–5.90) | 0.401 | |
| TINN (ms)b | 1.16 (0.43–3.12) | 0.769 | |
| MSE short-scale slopea | 0.27 (0.06–1.30) | 0.102 | |
| MSE maximuma | 1.88 (0.44–8.01) | 0.395 | |
| Suspected foetal distress in labour (yes) | 0.11 (0.00–2.77) | 0.178 | |
| Gestational age (weeks) | 0.80 (0.38–1.69) | 0.562 | |
| Mode of delivery (emergency) | 3.19 (0.17–58.73) | 0.434 | |
| Gender (male) | 1.54 (0.29–8.06) | 0.608 | |
| Apgar scores at 5 min | 0.79 (0.51–1.22) | 0.289 | |
| Assisted ventilation at 10 min of age (yes) | 4.03 (0.28–58.46) | 0.307 | |
| Cord pH | 0.01 (0.00–2.37) | 0.103 | |
| Base deficit | 0.97 (0.79–1.19) | 0.741 |
HRV variable was standardised prior to conducting the logistic regression.
HRV variable was log transformed (log base 10) and then standardised prior to conducting the logistic regression.
HRV, Heart Rate Variability; NN interval, normalised RR interval; LF/HF ratio, low frequency/high frequency ratio; TINN, triangular interpolation of the NN interval histogram; MSE, multiscale entropy.
Table 8.
Subgroup analysis of infants without anti-seizure medication prior to EEG epoch analysed - HRV and clinical prediction model, n = 56 infants.
| OR (95% CI) | p | AUROC (95% CI) | |
|---|---|---|---|
| Mean NN (ms)a | 2.23 (0.58–8.58) | 0.242 | 0.892 (0.793-0.990) |
| HF power (ms2)b | 1.75 (0.18–16.98) | 0.629 | |
| LF/HF ratiob | 1.40 (0.18–10.89) | 0.751 | |
| TINN (ms)b | 1.18 (0.19–7.42) | 0.858 | |
| MSE Complexity Indexa | 1.97 (0.41–9.56) | 0.400 | |
| MSE short-scale slopea | 0.31 (0.05–1.89) | 0.202 | |
| MSE long-scale slopea | 1.07 (0.26–4.41) | 0.922 | |
| Gestational age (weeks) | 0.76 (0.34–1.69) | 0.499 | |
| Birth weight (g)c | 1.09 (0.91–1.29) | 0.346 | |
| Apgar scores at 5 minutes | 0.72 (0.44–1.17) | 0.185 | |
| Cord pH | 0.01 (0.00–6.60) | 0.165 | |
| Lactate | 1.36 (0.98–1.89) | 0.062 | |
| Base deficit | 0.89 (0.69–1.14) | 0.351 | |
| Mode of delivery (emergency) | 0.79 (0.03-23.89) | 0.894 | |
| Suspected foetal distress in labour (yes) | 0.16 (0.00–5.54) | 0.314 | |
| Assisted ventilation at 10 minutes of age (yes) | 12.38 (1.02–149.88) | 0.048 |
HRV variable was standardised prior to conducting the logistic regression.
HRV variable was log transformed (log base 10) and then standardised prior to conducting the logistic regression.
The odds ratio represents the change in odds for a 100g increase in birthweight.
HRV, Heart Rate Variability; EEG, electroencephalography; NN interval, normalised RR interval; HF power, high frequency power (0.15-0.4 Hz), LF/HF ratio, low frequency/high frequency ratio; MSE, multiscale entropy.
Discussion
Using a large multicentre European dataset, the current study is the first to develop HRV and clinical models that accurately predict EEG grade in neonatal HIE. Early HRV analysis at median age of 5.9 h showed that high mean NN and low LF/HF rate, TINN, MSE complexity index, MSE short-scale slope and maximum MSE were associated with a worse encephalopathic EEG grade. When these HRV features were combined into a prediction model, the performance improved beyond individual features. The clinical model had a similar predictive value to the HRV model. However, the model with the best prediction for EEG background severity was the combined HRV and clinical model, with a AUROC 0.895 (95% CI:0.832–0.958). Although therapeutic hypothermia and anti-seizure medication have been demonstrated to impact EEG background and HRV, when the analysis was adjusted for these confounders, the model performance did not change (28–31).
It is well known that EEG background is an excellent predictor of brain injury and long-term outcomes in neonatal HIE (32–34). Neonatal EEG monitoring was also used as screening tool for initiation of therapeutic hypothermia in neonatal encephalopathy (35). Although EEG monitoring can be a relatively accessible neuromonitoring tool in developed countries, due to the high costs associated with specialised equipment and neurophysiological expertise, it is not as readily available in low-income countries (10, 12, 13, 36). On the other hand, ECG monitoring is less expensive, widely used and can be performed with good accuracy antepartum, during labour and very early in the delivery room. Therefore, HRV analysis might be very useful as an early tool to assess severity in neonatal HIE (37, 38).
Since the 1980′s, HRV has been investigated in different conditions as a non-invasive assessment tool for autonomic function (39, 40). Specifically for neonatal HIE, individual HRV features have been shown to be good predictors of encephalopathy severity, abnormal EEG background and outcome (14–16, 19). The current study has shown that a high mean NN (lower heart rate) was associated with moderate-severe EEG grade (OR 1.97 (95% CI:1.30–2.97), p = 0.001). However, when the analysis was adjusted for cooling status the difference did not reach statistical significance (data not presented). TINN was significantly lower in the moderate-severe group compared to the normal-mild EEG group (OR 0.66 (95% CI:0.45–0.97), p = 0.035), regardless of cooling status. A lower LF/HF ratio was associated with a more severe EEG grade (OR 0.40 (95% CI:0.25–0.64), p < 0.001). These findings, also confirmed previously by Goulding et al.0 (19, 28) in infants from a single centre, demonstrated a reduction in autonomic nervous system activity with an increase in HIE severity.
Furthermore, MSE complexity index, MSE short-scale slope and MSE maximum were significantly lower in the moderate-severe group compared with normal-mild EEG group, suggesting a decrease in HRV complexity with an increase in HIE severity, likely due to suppression in autonomic control.
Consistent with the literature (41, 42), this study showed that some clinical parameters were associated with worse EEG grade (low Apgar scores, need for assisted ventilation at birth), but the combination of clinical parameters increased the predictive value (clinical model AUROC 0.836 (95% CI:0.759–0.914)). The HRV prediction model performance was similar to the clinical model (AUROC 0.837 (95% CI:0.759–0.914)) in predicting severity of encephalopathy. However, the model combining early HRV features and readily available clinical information within 6 h of age had the best performance, with AUROC 0.895 (95% CI:0.832–0.958). Performance was similar when the analysis was performed using exclusively infants undergoing therapeutic hypothermia and infants without anti-seizure medication given prior to EEG epoch. The presented models could be very useful for settings where EEG is not readily available. These could have a greater impact especially in community hospitals and in low-income countries where neonatal EEG monitoring and expertise is scarce.
Several limitations must be considered. This was a secondary analysis of data collected for studies focused on neonatal seizures. To ensure only good quality recording was used for this analysis, all epochs were visually inspected and artefacts were removed before performing the HRV analysis. However, artefacts could be problematic if ECG recordings are used in real-time, at the bedside. Real-time monitoring of HRV would require the integration of an artefact detection algorithm. At the time of the selected epoch, some infants were already under hypothermia treatment (n = 97 infants) and some had already received anti-seizure treatment for clinically suspected seizures (n = 19 infants). To account for the confounding effect of hypothermia and anti-seizure medication, we also performed the analysis including only those infants that were cooled and which did not receive anti-seizure medication prior to selected epoch. These subgroup analyses showed no change in the model performance. However, we did not collect data on inotropic treatment. Due to missing data for pH, lactate and base deficit we did not include these parameters in the main analysis. However, we did develop the same models in infants which had this information (71 infants) and the prediction did not improve.
Despite these limitations, this study included early EEG and ECG data from a large neonatal population with all grades of HIE. Continuous EEG monitoring which is the gold standard recommended by current guidelines was used in this study for background EEG assessment (10). We are aware that most Neonatal Units do not have the resources to perform conventional EEG monitoring and are using aEEG monitoring for infants with HIE undertaking therapeutic hypothermia. Although this study analysed conventional EEG monitoring, current aEEG monitors can display two or three raw EEG channels from which the background analysis can be extracted.
We have shown that HRV and clinical data combined in a model using logistic regression analysis is an excellent predictor of EEG grade in the early newborn period and may be a very useful additional tool for neonatologists who are often faced with challenging decisions about therapeutic hypothermia, where EEG monitoring is not available or feasible.
In summary, our results are promising and suggest that an early combined HRV and clinical model could be useful to assess the severity of newborn encephalopathy in the early postnatal period. This might be helpful as a proxy marker for injury severity in settings where EEG monitoring is not available or not easily accessible; on the other hand, clinical information is readily available and ECG monitoring is non-invasive, easily available and unexpensive. Future work will require for this model to be validated in real time at the cot side and in all settings.
Acknowledgments
The authors would like to thank all families for their participation and to the clinical and research teams at each recruiting centre for all their hard work and support.
Funding
This study was supported by a Strategic Translational Award and an Innovator Award from the Wellcome Trust (098983 & 209325); Science Foundation Ireland (15/RI/3239); Health Research Board, Ireland (NEPTuNE CDA-2018-008). Funders had no role in the design and conduct of the study, nor in this analysis.
Data availability statement
The datasets presented in this article are not readily available because the clinical data is collected under a written proxy consent from the participants' guardians/parents, which did not include permission for sharing or open data. To be allowed to share this data under Irish Health Research Regulations we are required to re-consent families or to obtain approval by the Health Regulation Consent Declaration Committee. Requests to access the datasets should be directed to corresponding author.
Ethics statement
The original studies were approved by national competent authorities and local ethics committees of participating centres and adhered to all applicable local and national regulations. Written informed consent to participate in these studies was provided by the participants’ legal guardian/next of kin.
ANSeR consortium
INFANT Research Centre and Department of Paediatrics and Child Health, University College Cork, Cork, Ireland: Andreea M Pavel, Eugene Dempsey, Vicki Livingstone, Elena Pavlidis, Liudmila Kharoshankaya, Sean R Mathieson, Liam Marnane, Gordon Lightbody, Jackie O'Leary, Mairead Murray, Jean Conway, Denis Dwyer, Andrey Temko, Taragh Kiely, Anthony C Ryan, Geraldine B Boylan. Institute for Women's Health, University College London, London, UK: Janet M Rennie, Subhabrata Mitra. Utrecht Brain Center, University Medical Center Utrecht, Utrecht University, The Netherlands: Linda S de Vries, Lauren C Weeke, Mona C Toet. Department of Neonatal Medicine and Division of Paediatrics, Department CLINTEC, Karolinska University Hospital, Karolinska Institutet, Stockholm, Sweden: Mikael Finder, Mats Blennow, Ingela Edqvist. Rotunda Hospital, Dublin, Ireland: Adrienne Foran, Raga Mallika Pinnamaneni, Jessica Colby-Milley. Royal London Hospital and Queen Mary University of London, London, UK: Divyen K Shah, Nicola Openshaw-Lawrence. Department of Clinical Neurophysiology, Great Ormond Street Hospital for Children NHS Trust, London, UK: Ronit M Pressler. Homerton University Hospital NHS Foundation Trust, London, UK: Olga Kapellou. Clinical Neurophysiology, University Medical Center Utrecht, Utrecht, The Netherlands: Alexander C van Huffelen.
Author contributions
AM P recruited participants and collected data, analysed the data, drafted the initial manuscript and revised the manuscript. SR M recruited participants and collected data, graded all the EEG epochs. VL was involved in conceptualisation and design of the original studies, analysed the data and drafted the initial manuscript and revised the manuscript. JM O carried out the HRV analysis and drafted the initial manuscript and revised the manuscript. RM P, LS d, JM R, SM, EM D, VL, DM M, WP M, GB B conceptualised, designed and coordinated the original studies, designed the EEG analysis protocol. All authors reviewed and revised the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2022.1016211/full#supplementary-material.
References
- 1.Lee AC, Kozuki N, Blencowe H, Vos T, Bahalim A, Darmstadt GL, et al. Intrapartum-related neonatal encephalopathy incidence and impairment at regional and global levels for 2010 with trends from 1990. Pediatr Res. (2013) 74(Suppl 1):50–72. 10.1038/pr.2013.206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gale C, Statnikov Y, Jawad S, Uthaya SN, Modi N. Neonatal brain injuries in England: population-based incidence derived from routinely recorded clinical data held in the national neonatal research database. Arch Dis Child Fetal Neonatal Ed. (2018) 103(4):F301–f6. 10.1136/archdischild-2017-313707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. (2005) 353(15):1574–84. 10.1056/NEJMcps050929 [DOI] [PubMed] [Google Scholar]
- 4.Azzopardi DV, Strohm B, Edwards AD, Dyet L, Halliday HL, Juszczak E, et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med. (2009) 361(14):1349–58. 10.1056/NEJMoa0900854 [DOI] [PubMed] [Google Scholar]
- 5.Simbruner G, Mittal RA, Rohlmann F, Muche R. Systemic hypothermia after neonatal encephalopathy: outcomes of neo.nEURO.network RCT. Pediatr. (2010) 126(4):e771–8. 10.1542/peds.2009-2441 [DOI] [PubMed] [Google Scholar]
- 6.Gluckman PD, Wyatt JS, Azzopardi D, Ballard R, Edwards AD, Ferriero DM, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet. (2005) 365(9460):663–70. 10.1016/S0140-6736(05)17946-X [DOI] [PubMed] [Google Scholar]
- 7.Groenendaal F. Time to start hypothermia after perinatal asphyxia: does it matter? BMJ paediatr Open. (2019) 3(1):e000494. 10.1136/bmjpo-2019-000494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sandoval Karamian AG, Wusthoff CJ. Current and future uses of continuous EEG in the NICU. Front in Pediatr. (2021) 9:1–11. 10.3389/fped.2021.768670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bourel-Ponchel E, Querne L, Flamein F, Ghostine-Ramadan G, Wallois F, Lamblin MD. The prognostic value of neonatal conventional-EEG monitoring in hypoxic-ischemic encephalopathy during therapeutic hypothermia. Dev Med Child Neurol. (2022) 65:58–66. 10.1111/dmcn.15302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shellhaas RA, Chang T, Tsuchida T, Scher MS, Riviello JJ, Abend NS, et al. The American clinical neurophysiology Society's Guideline on continuous electroencephalography monitoring in neonates. J Clin Neurophysiol. (2011) 28(6):611–7. 10.1097/WNP.0b013e31823e96d7 [DOI] [PubMed] [Google Scholar]
- 11.Pressler RM, Cilio MR, Mizrahi EM, Moshé SL, Nunes ML, Plouin P, et al. The ILAE classification of seizures and the epilepsies: modification for seizures in the neonate. Position paper by the ILAE task force on neonatal seizures. Epilepsia. (2021) 62(3):615–28. 10.1111/epi.16815 [DOI] [PubMed] [Google Scholar]
- 12.Wang Z, Zhang P, Zhou W, Zhou X, Shi Y, Cheng X, et al. Electroencephalography monitoring in the neonatal intensive care unit: a Chinese perspective. Transl Pediatr. (2021) 10(3):552–9. 10.21037/tp-20-340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krishnan V, Kumar V, Variane GFT, Carlo WA, Bhutta ZA, Sizonenko S, et al. Need for more evidence in the prevention and management of perinatal asphyxia and neonatal encephalopathy in low and middle-income countries: a call for action. Semin in Fetal and Neonatal Med. (2021) 26(5):101271. 10.1016/j.siny.2021.101271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andersen M, Andelius TCK, Pedersen MV, Kyng KJ, Henriksen TB. Severity of hypoxic ischemic encephalopathy and heart rate variability in neonates: a systematic review. BMC Pediatr. (2019) 19(1):242. 10.1186/s12887-019-1603-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oliveira V, Martins R, Liow N, Teiserskas J, von Rosenberg W, Adjei T, et al. Prognostic accuracy of heart rate variability analysis in neonatal encephalopathy: a systematic review. Neonatol. (2019) 115(1):59–67. 10.1159/000493002 [DOI] [PubMed] [Google Scholar]
- 16.Bersani I, Piersigilli F, Gazzolo D, Campi F, Savarese I, Dotta A, et al. Heart rate variability as possible marker of brain damage in neonates with hypoxic ischemic encephalopathy: a systematic review. Eur J Pediatr. (2021) 180(5):1335–45. 10.1007/s00431-020-03882-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Latremouille S, Lam J, Shalish W, Sant, Anna G. Neonatal heart rate variability: a contemporary scoping review of analysis methods and clinical applications. BMJ Open. (2021) 11(12):e055209. 10.1136/bmjopen-2021-055209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aliefendioğlu D, Doğru T, Albayrak M, Dibekmısırlıoğlu E, Sanlı C. Heart rate variability in neonates with hypoxic ischemic encephalopathy. Indian J Pediatr. (2012) 79(11):1468–72. 10.1007/s12098-012-0703-2 [DOI] [PubMed] [Google Scholar]
- 19.Goulding RM, Stevenson NJ, Murray DM, Livingstone V, Filan PM, Boylan GB. Heart rate variability in hypoxic ischemic encephalopathy: correlation with EEG grade and 2-y neurodevelopmental outcome. Pediatr Res. (2015) 77(5):681–7. 10.1038/pr.2015.28 [DOI] [PubMed] [Google Scholar]
- 20.Metzler M, Govindan R, Al-Shargabi T, Vezina G, Andescavage N, Wang Y, et al. Pattern of brain injury and depressed heart rate variability in newborns with hypoxic ischemic encephalopathy. Pediatr Res. (2017) 82(3):438–43. 10.1038/pr.2017.94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamaguchi K, Lear CA, Beacom MJ, Ikeda T, Gunn AJ, Bennet L. Evolving changes in fetal heart rate variability and brain injury after hypoxia-ischaemia in preterm fetal sheep. J Physiol. (2018) 596(23):6093–104. 10.1113/JP275434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rennie JM, de Vries LS, Blennow M, Foran A, Shah DK, Livingstone V, et al. Characterisation of neonatal seizures and their treatment using continuous EEG monitoring: a multicentre experience. Arch Dis Child Fetal Neonatal Ed. (2018) 104:F493–F501. 10.1136/archdischild-2018-315624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pavel AM, Rennie JM, de Vries LS, Blennow M, Foran A, Shah DK, et al. A machine-learning algorithm for neonatal seizure recognition: a multicentre, randomised, controlled trial. Lancet Child Adolesc Health. (2020) 4(10):740–9. 10.1016/S2352-4642(20)30239-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murray DM, O'Connor CM, Ryan CA, Korotchikova I, Boylan GB. Early EEG grade and outcome at 5 years after mild neonatal hypoxic ischemic encephalopathy. Pediatrics. (2016) 138(4). 10.1542/peds.2016-0659 [DOI] [PubMed] [Google Scholar]
- 25.Costa M, Goldberger AL, Peng CK. Multiscale entropy analysis of biological signals. Phys Rev E Stat Nonlin Soft Matter Phys. (2005) 71(2 Pt 1):021906. 10.1103/PhysRevE.71.021906 [DOI] [PubMed] [Google Scholar]
- 26.De Wel O, Lavanga M, Dorado AC, Jansen K, Dereymaeker A, Naulaers G, et al. Complexity analysis of neonatal EEG using multiscale entropy: applications in brain maturation and sleep stage classification. Entropy. (2017) 19(10):516. 10.3390/e19100516 [DOI] [Google Scholar]
- 27.Kasai M, Lear CA, Davidson JO, Beacom MJ, Drury PP, Maeda Y, et al. Early sinusoidal heart rate patterns and heart rate variability to assess hypoxia-ischaemia in near-term fetal sheep. J Physiol. (2019) 597(23):5535–48. 10.1113/JP278523 [DOI] [PubMed] [Google Scholar]
- 28.Goulding RM, Stevenson NJ, Murray DM, Livingstone V, Filan PM, Boylan GB. Heart rate variability in hypoxic ischemic encephalopathy during therapeutic hypothermia. Pediatr Res. (2017) 81(4):609–15. 10.1038/pr.2016.245 [DOI] [PubMed] [Google Scholar]
- 29.Vesoulis ZA, Rao R, Trivedi SB, Mathur AM. The effect of therapeutic hypothermia on heart rate variability. J Perinatol. (2017) 37(6):679–83. 10.1038/jp.2017.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hallioglu O, Okuyaz C, Mert E, Makharoblidze K. Effects of antiepileptic drug therapy on heart rate variability in children with epilepsy. Epilepsy Res. (2008) 79(1):49–54. 10.1016/j.eplepsyres.2007.12.020 [DOI] [PubMed] [Google Scholar]
- 31.Obeid R, Tsuchida TN. Treatment effects on neonatal EEG. J Clin Neurophysiol. (2016) 33(5):376–81. 10.1097/WNP.0000000000000300 [DOI] [PubMed] [Google Scholar]
- 32.Koskela T, Kendall GS, Memon S, Sokolska M, Mabuza T, Huertas-Ceballos A, et al. Prognostic value of neonatal EEG following therapeutic hypothermia in survivors of hypoxic-ischemic encephalopathy. Clinical Neurophysiol. (2021) 132(9):2091–100. 10.1016/j.clinph.2021.05.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu W, Yang Q, Wei H, Dong W, Fan Y, Hua Z. Prognostic value of clinical tests in neonates with hypoxic-ischemic encephalopathy treated with therapeutic hypothermia: a systematic review and meta-analysis. Front Neurol. (2020) 11:1–11. 10.3389/fneur.2020.00133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murray DM, Boylan GB, Ryan CA, Connolly S. Early EEG findings in hypoxic-ischemic encephalopathy predict outcomes at 2 years. Pediatr. (2009) 124(3):e459–67. 10.1542/peds.2008-2190 [DOI] [PubMed] [Google Scholar]
- 35.Holmes G, Rowe J, Hafford J, Schmidt R, Testa M, Zimmerman A. Prognostic value of the electroencephalogram in neonatal asphyxia. Electroencephalogr Clin Neurophysiol. (1982) 53(1):60–72. 10.1016/0013-4694(82)90106-7 [DOI] [PubMed] [Google Scholar]
- 36.Toet MC, Lemmers PM. Brain monitoring in neonates. Early Hum Dev. (2009) 85(2):77–84. 10.1016/j.earlhumdev.2008.11.007 [DOI] [PubMed] [Google Scholar]
- 37.Shah BA, Wlodaver AG, Escobedo MB, Ahmed ST, Blunt MH, Anderson MP, et al. Impact of electronic cardiac (ECG) monitoring on delivery room resuscitation and neonatal outcomes. Resuscitation. (2019) 143:10–6. 10.1016/j.resuscitation.2019.07.031 [DOI] [PubMed] [Google Scholar]
- 38.Neilson JP. Fetal electrocardiogram (ECG) for fetal monitoring during labour. The Cochrane Database of Syst rev. (2015) 2015(12):CD000116-CD. 10.1002/14651858.CD000116.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jenkins JG, Reid MM, McClure BG. Study of heart rate variability in sick newborn infants. Acta Paediatr Scand. (1980) 69(3):393–6. 10.1111/j.1651-2227.1980.tb07098.x [DOI] [PubMed] [Google Scholar]
- 40.Cabal LA, Siassi B, Zanini B, Hodgman JE, Hon EE. Factors affecting heart rate variability in preterm infants. Pediatr. (1980) 65(1):50–6. 10.1542/peds.65.1.50 [DOI] [PubMed] [Google Scholar]
- 41.Horn AR, Swingler GH, Myer L, Linley LL, Raban MS, Joolay Y, et al. Early clinical signs in neonates with hypoxic ischemic encephalopathy predict an abnormal amplitude-integrated electroencephalogram at age 6 h. BMC Pediatr. (2013) 13:52. 10.1186/1471-2431-13-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Horn AR, Swingler GH, Myer L, Linley LL, Chandrasekaran M, Robertson NJ. Early clinical predictors of a severely abnormal amplitude-integrated electroencephalogram at 48 h in cooled neonates. Acta Paediatr. (2013) 102(8):e378–84. 10.1111/apa.12306 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets presented in this article are not readily available because the clinical data is collected under a written proxy consent from the participants' guardians/parents, which did not include permission for sharing or open data. To be allowed to share this data under Irish Health Research Regulations we are required to re-consent families or to obtain approval by the Health Regulation Consent Declaration Committee. Requests to access the datasets should be directed to corresponding author.


