Highlights
-
•
Stable mainstream anammox is demonstrated at pilot scale over one year.
-
•
High level of nitrogen removal is maintained with effluent total nitrogen < 10 mg N/L.
-
•
NOB suppression is robust, attributed to integration of multiple control strategies.
-
•
Anammox retained in anoxic and oxic zones jointly contribute to nitrogen removal.
Keywords: Mainstream anammox, Autotrophic nitrogen removal from wastewater, Bioenergy recovery, Effluent quality, NOB suppression
Abstract
Mainstream nitrogen removal via anammox is widely recognized as a promising wastewater treatment process. However, its application is challenging at large scale due to unstable suppression of nitrite-oxidizing bacteria (NOB). In this study, a pilot-scale mainstream anammox process was implemented in an Integrated Fixed-film Activated Sludge (IFAS) configuration. Stable operation with robust NOB suppression was maintained for over one year. This was achieved through integration of three key control strategies: i) low dissolved oxygen (DO = 0.4 ± 0.2 mg O2/L), ii) regular free nitrous acid (FNA)-based sludge treatment, and iii) residual ammonium concentration control (NH4+ with a setpoint of ∼8 mg N/L). Activity tests and FISH demonstrated that NOB barely survived in sludge flocs and were inhibited in biofilms. Despite receiving organic-deficient wastewater from a pilot-scale High-Rate Activated Sludge (HRAS) system as the feed, the system maintained a stable effluent total nitrogen concentration mostly below 10 mg N/L, which was attributed to the successful retention of anammox bacteria. This study successfully demonstrated large-scale long-term mainstream anammox application and generated new practical knowledge for NOB control and anammox retention.
Graphical abstract
Introduction
The microbial process of anaerobic ammonium oxidation (anammox) was discovered in late 1990s (Mulder et al., 1995), and since then researchers have proposed an innovative technology – Partial Nitritation and Anammox (PN/A) – for highly efficient nitrogen removal from wastewater, as a cost-effective alternative to the conventional nitrification and denitrification processes (Kuenen 2008). With significant efforts made, water engineers successfully installed PN/A in the sidestream line treating high-strength wastewater (Lackner et al., 2014), and are exploring PN/A in the main line of wastewater treatment plants (WWTPs). The extension to mainstream nitrogen removal can multiply the economic benefits, as the mainstream nitrogen load is about five times greater than the sidestream (Wang et al., 2022). The application of mainstream PN/A can also maximize the capture of organic carbon for bioenergy (i.e., methane) production, offsetting energy consumption in a WWTP and hence can potentially transform WWTPs from energy-consumers to energy-exporters (Kartal et al., 2010; McCarty et al., 2011). In the path to mainstream PN/A application, however, the critical challenge is the suppression of nitrite-oxidizing bacteria (NOB). NOB compete for the nitrite substrate with anammox bacteria, and its suppression is essential, but challenging under the conditions of low influent ammonium (NH4+) concentration and low operational temperature (Agrawal et al., 2018; Cao et al., 2017; Qiu et al., 2021; Wang et al., 2022).
The NOB control strategies developed to date can be divided into two major categories: in-situ NOB suppression and ex-situ NOB inactivation. The in-situ control comprises the use of low dissolved oxygen (DO) (Blackburne et al., 2008), real-time controlled intermittent aeration (Ma et al., 2017b; Miao et al., 2022; Regmi et al., 2014), step feed (Duan et al., 2022), short sludge retention time (SRT) (Laureni et al., 2019), residual NH4+ control (Poot et al., 2016), or creating acidic conditions (Meng et al., 2022; Wang et al., 2021b). The ex-situ control includes the use of harsh physical/chemical treatments such as free nitrous acid (FNA) (Wang et al., 2014), free ammonia (FA) (Wang et al., 2017), sulfide (Seuntjens et al., 2018), heat/ultrasonic shock (Chen et al., 2016), and light irradiation (Yang et al., 2022; Zheng et al., 2019), among others. It should be noted that most of these strategies were only tested in laboratories under well-controlled conditions. As the essential step prior to full-scale application, successful demonstration of mainstream PN/A process at a pilot scale remains sparse (Hausherr et al., 2022, Trojanowicz et al., 2016, Wu et al., 2021).
The level of NOB suppression is subject to the diversity of NOB members, across four genera Nitrobacter, Nitrospira, Candidatus (Ca.) Nitrotoga, and Nitrolancea that are commonly found in wastewater treatment systems (Daims et al., 2016). The NOB invasion from wastewater (Duan et al., 2019b) further adds to the complexity, meaning that some NOB genera or species can adapt to the aforementioned control strategies, leading to failure of NOB suppression during a long-term operation (Duan et al., 2019a; Liu and Wang 2013; Wang et al., 2021a). For example, Ca. Nitrotoga fabula, a newly isolated NOB from an activated sludge sample (Kitzinger et al., 2018), was found to possess strong resistance to ex-situ exposure of FNA above one parts per million (> 1 mg HNO2—N/L) (Zheng et al., 2020). These recent studies illustrate the importance of suppressing diverse NOB through the integration of multiple strategies. Therefore, this study aims to develop a combination of operational strategies to suppress the growth of NOB in a mainstream pilot system. Over one year of operation, this system successfully demonstrated the robustness of the mainstream PN/A process, thus opening a path to full-scale implementation.
Results
Long-term system operation and performance
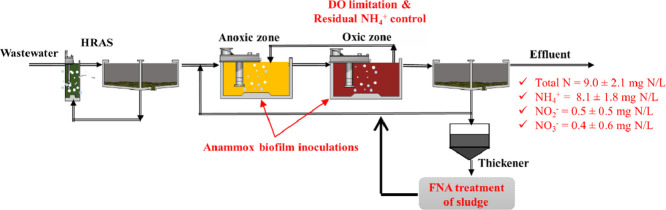
The pilot system comprised an integrated fixed film activated sludge (IFAS) process with the classical continuous-flow anoxic (A) and oxic (O) configuration and inoculation of anammox-contained carriers in both the A and O zones (Fig. S1). The system was fed with domestic wastewater pre-treated with a High Rate Activated Sludge (HRAS) process, which usually captured ∼60% of chemical oxygen demand (COD) from raw sewage (Table S1). The designed SRT of 12 d and hydraulic retention time (HRT) of 3.2 h in the A zone and 6.7 h in the O zone are representative parameters of the conventional activated sludge processes installed across the globe (Rittmann and McCarty 2001). Two NOB control strategies of low DO at 0.4 ± 0.2 mg O2/L in the O zone and external sludge treatment using FNA at ∼2 mg HNO2—N/L, which were optimized in our previous laboratory study (Wang et al., 2016), were initially integrated in the pilot system.
After start-up, the system successfully retained anammox bacteria in both the A- and O-biofilms (Fig. S2a). The TN and NH4+ removal efficiencies gradually increased to over 80% in two months (Fig. S2b, S2c). We monitored the maximum activity of AOB and NOB in sludge flocs using ex-situ batch tests on a weekly basis, which showed that at around 100th day, the NOB activity unexpectedly increased and reached 3 mg NO2−-N/(g volatile suspended solids (VSS)·h) in two weeks (Fig. S2d). This posed a risk of failed NOB control, and thus immediately, the in-situ DO setpoint was lowered to ∼0.2 mg O2/L. As expected, the NOB activity of sludge flocs decreased rapidly to below 0.2 mg NO2−-N/(g VSS·h) following this change. However, this also caused a significant deterioration in the NH4+ removal efficiency, reaching as low as ∼30%, indicating that the AOB activity was also negatively affected by the low DO concentration. Consequently, the DO setpoint was elevated back to 0.4 mg O2/L, and a residual NH4+ concentration control (∼8 mg N/L) was implemented. Maintaining a residual NH4+ level was hypothesized to suppress NOB activity by promoting anammox activity for nitrite competition and decreasing oxygen penetration in biofilms, which will be further elaborated in section 2.4.
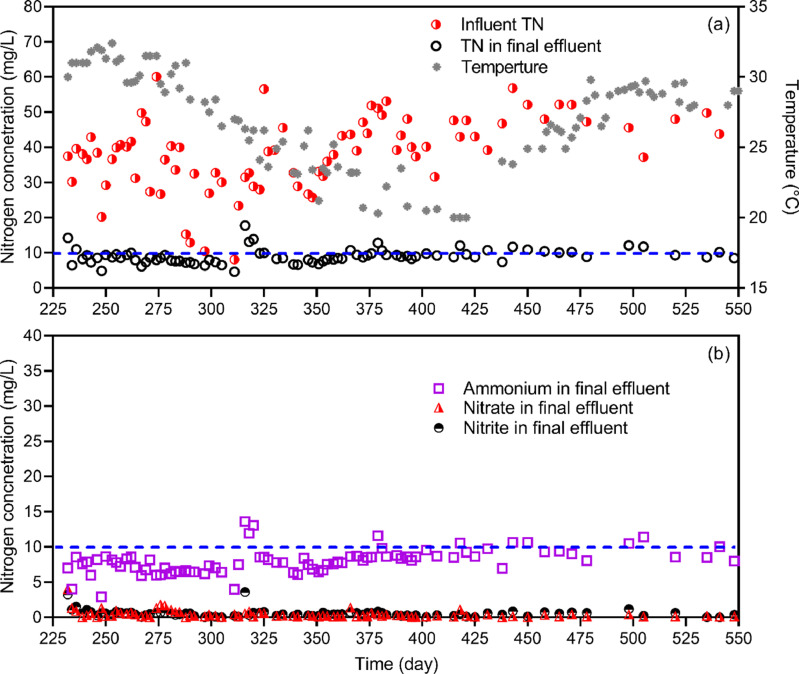
The improved operational strategy consisted of three key controls: the external FNA sludge treatment, in-situ low DO concentration (0.4 ± 0.2 mg O2/L), and a relatively high residual NH4+ concentration (∼8 mg N/L). With a fluctuating TN concentration in real wastewater and seasonally varying temperature, the system effluent had mostly maintained a TN concentration below 10 mg N/L, comprising of NH4+ (∼8 mg N/L), NO2− (∼1 mg N/L) and NO3− (∼1 mg N/L) (Fig. 1). This resulted in averaged TN and NH4+ removal efficiencies of > 80% over a one-year period, and therefore demonstrated a successful and robust operation of efficient mainstream nitrogen removal process.
Fig. 1.
Profiles of influent and effluent TN concentrations, temperature (a) and ammonium, nitrite and nitrate nitrogen concentrations in the final effluent (b) throughout the one-year stable operation of the pilot A/O system. The dashed line represents a nitrogen concentration of 10 mg N/L.
Retention of anammox bacteria in the A- and O-biofilms
The efficient nitrogen removal performance that was achieved during the pilot trial was largely attributed to anammox, because the HRAS pretreated wastewater only supported limited nitrate- and nitrite-dependent heterotrophic denitrification rates, measured at 0.8 and 1.5 mg N/(g VSS·h), respectively. These rates are comparable to the sludge denitrification rates in the absence of soluble carbon sources (e.g., 0.7–1.4 mg NO3−-N/(g VSS·h) (Zheng et al., 2013)), and were much lower than the denitrification rate measured in domestic wastewater with readily biodegradable organic matters (e.g., 6.6 mg NO3−-N/(g VSS·h) (Kujawa and Klapwijk 1999)). The low organic degradability in wastewater with HRAS pretreatment was also reflected by a low TN removal efficiency of only 10.4% ± 4.6% in our laboratory control reactor that used the same influent as the pilot system (Wang et al., 2021a).
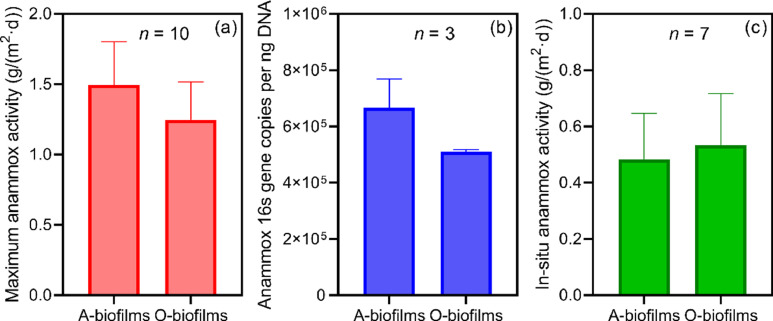
The 16S rRNA gene amplicon sequencing analysis revealed that anammox bacteria dominated in the A- and O-biofilms were Candidatus Brocadia, showing no difference at the genus level (Fig. S3). The abundance of Candidatus Brocadia within the A-biofilms was relatively higher at 0.74% ± 0.21% compared to the O-biofilms (0.30% ± 0.19%), suggesting a slightly higher capacity of A-biofilms to retain anammox bacteria. The use of ex-situ batch tests and q-PCR analyses also showed that the maximum activity and population of anammox bacteria within the A-biofilms were 1.5 ± 0.3 g N/(m2-carrier·d) and 6.6 ± 1.0 × 105 16S gene copies per ng DNA, respectively, which were comparable but slightly higher than those measured with the O-biofilms (Fig. 2a-2b). These differences in anammox abundance and activity may be related to differences in environmental conditions such as DO, nitrite concentration and shear force between the A and O zones. Together, these results indicate the successful retention of anammox bacteria in both the anoxic and oxic tanks, thus expanding the application of mainstream anammox technology.
Fig. 2.
Maximum activities (a), abundance (b), and in-situ activities (c) of anammox bacteria in the A- and O-biofilms during the stable operation of the pilot system.
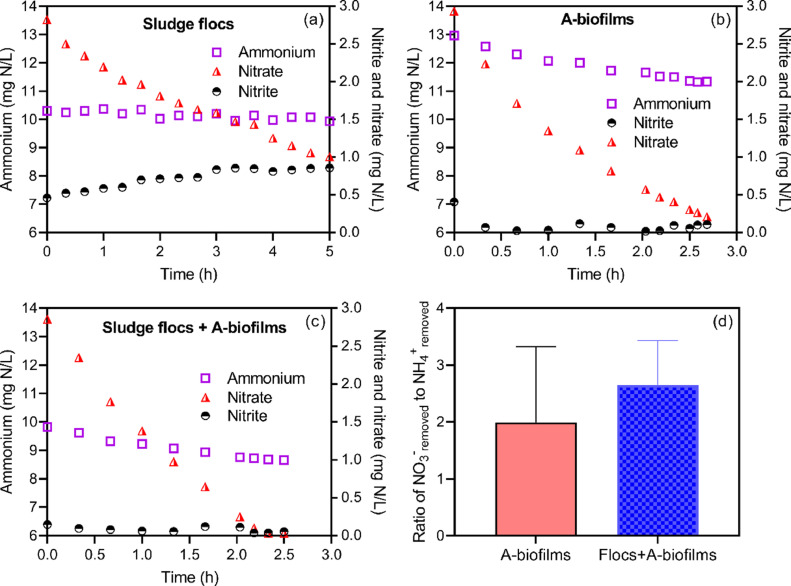
Some studies have recently revealed that the A-biofilms are the ideal location for retaining anammox bacteria in a continuous-flow nitrogen removal process, as anoxic environments can promote anammox enrichment which obtain nitrite from partial denitrification (Li et al., 2021; Li et al., 2019). Herein, we examined whether nitrate-dependent denitrification with HRAS effluent could support the A-biofilm anammox, i.e., partial denitrification and anammox (PD/A). The removal of NH4+ and NO3− without NO2− supply was tested in a series of anoxic batch assays. NH4+ and NO3− simultaneously decreased in the groups with the A-biofilms, while in the group without A-biofilms (i.e., sole sludge flocs), only the NO3− concentration decreased (Fig. 3a-c). This suggests that the NH4+ removal in the anoxic tank was driven by anammox bacteria in the A-biofilms, which obtained NO2− from partial denitrification occurring in both flocs and biofilms. The molar ratios of NO3− removed to NH4+ removed in batch tests with A-biofilms were calculated to be 2.0–2.6 on average (Fig. 3d). This suggests that more than 50% of NO3− could be partially reduced to NO2− and supplied to the anammox bacteria.
Fig. 3.
Profiles of ammonium, nitrite and nitrate in anoxic batch tests using sludge flocs (a), A-biofilms (b) and combined biofilms and flocs (c). (d) The ratios of NO3− removed to NH4+ removed calculated in the tests of (b) and (c).
Contribution of anammox to nitrogen removal
We developed a kinetic method to estimate the in-situ anammox activity based on the measured maximum rate multiplied by a Monod equation incorporating substrates and inhibitors, as described in the Activated Sludge Models (Henze et al., 2000). This was because NO2−, with measured concentrations averaging at 0.24 mg N/L in the A zone and 0.53 mg N/L in the O zone (Fig. S4), were comparable to the apparent nitrite affinity constant of 0.42 mg NO2−-N/L for the A-biofilm and 0.38 mg NO2−-N/L for the O-biofilm (Fig. S5). Thus, the in-situ anammox activity was limited by the in-situ nitrite concentration.
To this end, short-term batch tests were first carried out to measure the maximum anammox activity in the A- and O-biofilms in conditions with sufficient nitrite (Fig. S5). Together with the measured apparent nitrite affinity constant and the in-situ nitrite concentration, the in-situ anammox activity of the A-biofilms was estimated to be 0.48 ± 0.16 g N/(m2-carrier·d), comparable to that of the O-biofilm (0.53 ± 0.18 g N/(m2-carrier·d)) (Fig. 2c). The in-situ anammox activities, together with the HRT and carrier filling ratios applied to the A and O zones of the pilot system, revealed that anammox in the O- and A-biofilms contributed 60% and 40% of total nitrogen removal via anammox, respectively. Of note, the relative contribution may be influenced by the availability of organic carbon in the HRAS effluent, offering a certain level of flexibility to maintain the overall stable nitrogen removal, i.e., the A-biofilms should contribute more if more organic carbon is available and vice versa.
While the PN/A process contributed to the majority of nitrogen removal, a key role of the PD/A process is to remove NO3− generated by anammox bacteria and the O-biofilm NOB (see details in Section 2.4). This is evidenced by the extremely low residual NO3− concentration (< 1 mg N/L) in the final effluent, and it appeared that the denitrification process based on limited organic carbon in the HRAS effluent was adequate to consume almost all NO3− in the pilot system. Therefore, partial denitrification also played a critical role in achieving the high-level TN removal.
Mechanisms of stable NOB suppression
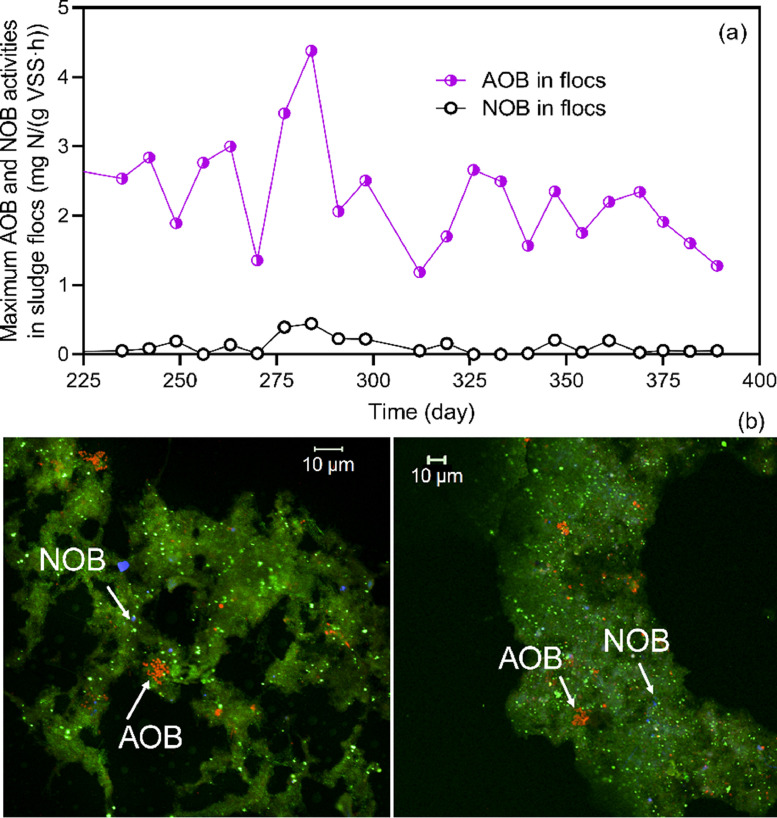
Restricting NOB activity in the O zone enabled the NO2− produced by AOB to be supplied to anammox bacteria, which represent the typical PN/A pathways for nitrogen removal. NOB in sludge flocs were satisfactorily washed out in the pilot system where the maximum NOB activity in sludge flocs was only 0.1 ± 0.1 mg NO2−-N/(gVSS·h), compared to 2.3 ± 0.8 mg NH4+-N/(gVSS·h) for AOB (Fig. 4a). This was corroborated by the Fluorescence in situ hybridization (FISH) analysis, which showed that AOB considerably outnumbered NOB (Fig. 4b). The strong suppression of NOB can be largely attributed to regular sludge treatment using FNA. Taking the inoculated sludge flocs as an example, the maximum NOB activity substantially decreased from 1.2 mg NO2−-N/(gVSS·h) to less than 0.1 mg NO2−-N/(gVSS·h) after 24-h of FNA treatment. Nevertheless, it should be noted that some NOB could adapt to the sole FNA treatment (Duan et al., 2019a; Ma et al., 2017a; Zheng et al., 2020). This indicated that the use of in-situ low DO conditions and the nitrite competition by anammox bacteria were also critical for the long term suppression of NOB (Wang et al., 2021a).
Fig. 4.
(a) Measured maximum AOB and NOB activities in sludge flocs using ex-situ batch tests under non-limited substrate conditions. (b) Representative FISH images showing significant dominance of AOB over NOB in sludge flocs. EUB mix counterstaining is in green, probes specific for Betaproteobacterial AOB (Nso1225) in red), Nitrobacter (Nit3) in blue) and Nitrospira (Ntspa662 and Ntspa712) in blue).
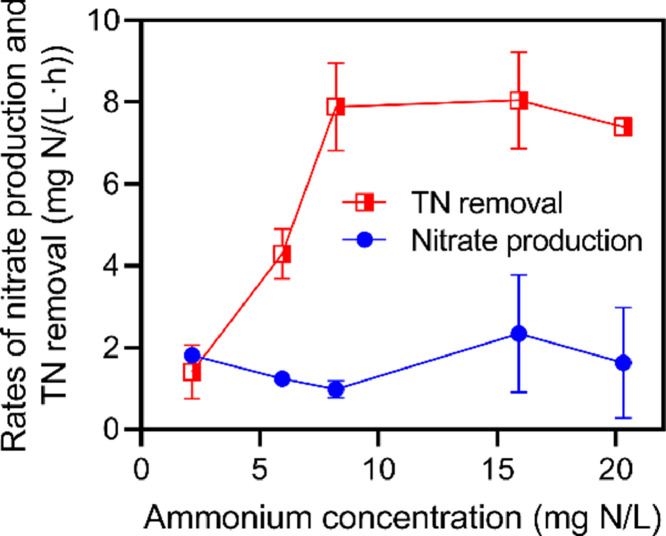
In contrast to flocs, the O-biofilms contained NOB (Nitrospira) according to the 16S rRNA gene amplicon sequencing analysis (Fig. S3b). The presence and abundance of NOB in the O-biofilms were also reflected by a maximum NOB activity to maximum AOB activity ratio of about 1 (Fig. S6), which is 2–3 times higher than that for biofilms from our laboratory PN/A system reported with stable NOB suppression (Meng et al., 2021). However, under the in-situ conditions of low DO and relatively high residual NH4+, the ratio of NO3− production to TN removal by the O-biofilms was only ∼20% (Fig. 5). This ratio was slightly higher than the stoichiometry of anammox reaction (i.e., 13%) and close to the ratios observed in previous laboratory PN/A systems (Laureni et al., 2016; Meng et al., 2021). This result indicated the suppression of the NOB activity in the O-biofilms by in-situ factors, rather than NOB elimination.
Fig. 5.
Measured TN removal and nitrate production rates of the O-biofilms under five different residual ammonium concentrations using ex-situ batch tests. The DO concentration was fixed at 0.4 mg O2/L during the tests.
Indeed, the NO3− production rate by the O-biofilms was measured as ∼2 mg NO3−-N/(L·h) when DO was controlled at 0.4 mg/L (Fig. 5). This was significantly lower than the maximum NOB activity rate of 4.7 mg NO2−-N/(L·h) measured at a high DO (> 8 mg O2/L) (Fig. S6), demonstrating the role of low DO in the suppression of the in-situ NOB activity. In PN/A biofilms, NOB are predominantly found within deep layers of the O-biofilms together with anammox bacteria (Zhao et al., 2023). Thus, the NOB and anammox activity should be essentially controlled by oxygen penetrating into biofilms, which could be lower than the monitored DO concentrations within the bulk liquid. Oxygen penetration in biofilms is affected by the AOB activity, meaning that the residual NH4+ concentration, which controls AOB activity, should be another important factor driving NOB suppression and anammox activity (Wang et al., 2022). By measuring the TN removal rates under different residual NH4+ concentrations in batch tests at DO of 0.4 mg O2/L, we found that a residual NH4+ higher than 8 mg N/L was essential to achieving a high TN removal rate by the O-biofilms (Fig. 5). In contrast, the decrease in residual NH4+ concentrations to ∼2 mg N/L dramatically reduced the nitrogen removal performance, which highlights the sensitivity of anammox activity to the residual NH4+ concentration. This result provides critical evidence to support a setpoint of residual NH4+ as high as ∼8 mg N/L in suppressing O-biofilm NOB for achieving efficient nitrogen removal in the pilot system.
Discussion
This study successfully demonstrated stable and long-term application of anammox bacteria for mainstream nitrogen removal at pilot scale. Efficient nitrogen removal (with the effluent TN less than 10 mg N/L), was achieved using industrially relevant operating conditions over one year, demonstrating the potential for real-world application of the mainstream anammox process. A high-level contribution of anammox to mainstream nitrogen removal was achieved by integrating multiple control strategies, which included in-situ low DO control together with regular FNA sludge treatment for effective elimination of NOB in sludge flocs, and low DO with residual NH4+ control for suppression of NOB activity in biofilms. These results demonstrated that multiple strategies were essential to overcome critical issues that result in NOB adaptation which has previously been documented in laboratory studies (Duan et al., 2019a; Wang et al., 2021a). The pilot system also employed the HRAS process to harvest organic carbon from wastewater to maximize bioenergy recovery in support of the ongoing paradigm shift for municipal WWTPs to maximize energy recovery from wastewater.
The pilot-scale demonstration of the mainstream PN/A process has been reported in a few studies (Table S2), but not all were successful. A general challenge mentioned by most previous studies is the NOB control, leading to a relatively poor effluent quality despite achieving comparable nitrogen removal rates. For example, a nitrogen removal rate of ∼0.2 kg N/m3/d was achieved in a pilot PN/A system with granular sludge, whereas the total nitrogen in the effluent was mostly above 15 mg N/L (Lotti et al., 2015). Likewise, another pilot trial that employed anammox carriers for mainstream wastewater treatment was also limited by high effluent total nitrogen concentration greater than 20 mg N/L, despite achieving a peak nitrogen removal rate of 0.13 kg N/m3/d (Gustavsson et al., 2020). A notable exemption was Hausherr et al. (2022), where the total nitrogen was maintained below 3 mg N/L in a two-stage PN/A system. To the best of our knowledge, this is the only pilot-scale trial employing a two-stage configuration. The researchers argued that the two-stage configuration is preferable if a low effluent TN is required. In comparison, a relatively high NH4+ concentration is usually needed in an one-stage configuration, to support anammox activity and inhibit NOB activity (Wang et al., 2022), which is also demonstrated in our study. In comparison to previous trials, the extremely low nitrate in the final effluent of our system should be highlighted, which was attributed to the denitrification/partial denitrification in the anoxic tank, as well as the stable NOB elimination in flocs and NOB activity suppression in biofilms in the oxic tank.
Despite the benefits given by the residual ammonium control strategy, the effluent of the proposed mainstream anammox process needs polishing before discharging into natural water bodies as it contains NH4+ of ∼8 mg N/L. The residual NH4+ control was thought to be an important strategy for NOB suppression in mainstream PN/A processes (Laureni et al., 2016; Yang et al., 2023), while a minimal NH4+ set-point remains elusive. A model-based study suggested a residual NH4+ concentration of ∼1 mg N/L for maintaining NOB repression in oxygen-limited PN/A (Pérez et al., 2014), yet our study indicated that a higher residual NH4+ level was required.
Conclusions
This study demonstrated the robustness of mainstream anammox technology at pilot scale. The main conclusions from this study were:
-
•
Integration of three control strategies, including low DO, FNA sludge treatment and residual NH4+ control, was effective in the elimination of NOB in sludge flocs and suppression of NOB in biofilms;
-
•
Effluent quality of the mainstream anammox process was satisfactory in maintaining TN concentration generally less than 10 mg N/L, while a polishing process to remove the residual NH4+ of ∼8 mg N/L would be required in practice;
-
•
Both carriers in anoxic and oxic zones were effective in retaining anammox activity, with comparable treatment capacity and contribution to nitrogen removal.
Material and methods
Pilot system setup, operation, and monitoring
The pilot-scale treatment system consisted of an HRAS process for capturing organic carbon from wastewater followed by a continuous-flow A/O process for nitrogen removal (Fig. S1). The whole system was installed at the Innovation center, located in the Luggage Point Sewage Treatment Plant, Brisbane, Australia. The system was operated for one and a half years and continuously fed with screened real domestic wastewater. The HRAS system had a working volume of 0.25 m3, with a set HRT of 1.4 h. The HRAS was connected to a clarifier, where the HRAS sludge settled and was wasted or returned the HRAS reactor. The effluent was fed to the A/O system (0.47 m3 in the A zone and 0.98 m3 in the O zone). The anammox-containing K5 carriers, collected from a 5-m3 moving bed biofilm reactor (MBBR) that treated real anaerobic digestion liquor via the PN/A pathway, were added to the A and O zones with volumetric filling ratios of 48% and 33%, respectively. The HRAS pre-treated wastewater was pumped (Mono, CP11) into the A zone at a flow rate of 3.5 m3/d, resulting in HRT of 3.2 h in the A zone and 6.7 h in the O zone. The mixed liquor recirculation rate from the O to the A zone was set at ∼7 times the influent flow rate, i.e., 24.7 m3/d. The A and O zones were mixed by coarse bubbling at an air flow rate of 200 L/day. pH in the system was monitored by using a pH probe (miniCHEM, Labtek) and a transmitter (multiparameter transmitter M800, Mettler Toledo), but not controlled. DO concentration in the O zone was controlled between 0.2 and 0.8 mg O2/L (0.4 ± 0.2 mg O2/L on average) by using an on/off control of micro-bubbled air supply pump. An online NH4+ probe was installed in the O zone, which also controlled the air supply, i.e., the aeration pump was switched off when a residual NH4+ concentration was lower than a set level (i.e., 8 mg NH4+-N/L from day 225) and vice versa. These controls were merged into a programmable logic controller (PLC). SRT of the A/O system was 12 d by semi-continuously discharging mixed liquor from the O zone. Following the A/O system, a secondary settler was established to retain biomass and return it to the A zone with a sludge return rate of 3.5 m3/d. Both the HRAS and A/O systems were inoculated with conventional activated sludge from the full-scale Luggage Point plant.
An external unit was set up in the sidestream line of the A/O system to implement treatment of sludge from the A/O system with FNA. Activated sludge of 400 L was collected daily from the O zone and subsequently concentrated to 4 − 5 g total suspended solids (TSS)/L by using a centrifuge at 1000 rpm for 5 min. Afterwards, the thickened sludge was transferred to a 100 L treatment tank and exposed to ∼2 mg HNO2—N/L (i.e., pH = 5.6 − 5.8, NO2− = 500 mg N/L, Temperature = 25 °C). After ∼24 h exposure, the treated sludge was returned to the A zone.
The influent and HRAS effluent COD concentrations were measured 2 − 3 times per week. NH4+-N, NO2−-N, NO3−-N and PO43−-P concentrations in the influent and effluent of the A/O system were also measured 2 − 3 times per week. The maximum anammox activity of the A- and O-biofilms, and the maximum AOB and NOB activities of the floccular sludge were analysed weekly. The mixed liquor suspended solids (MLSS) and VSS concentrations of the sludge were monitored once per week. Other chemical and microbial analyses, as well as batch tests, were carried out when the whole system reached stable operation, as detailed in 5.5.
Chemical analysis
Concentrations of MLSS, VSS and COD were measured according to the standard methods (APHA 2005). Mixed liquor samples were filtered through 0.45 μm Millipore filters for the determination of NH4+-N, NO2−-N, NO3−-N and PO43−-P concentrations with a Lachat QuikChem8000 Flow Injection Analyzer (Lachat Instrument, USA).
Microbial community analysis
On day 450, floccular sludge, and the A- and O-biofilms were collected in triplicate and submitted to Australian center for Ecogenomics at The University of Queensland (https://ecogenomic.org/) for the analysis of microbial communities. DNA of the collected samples was extracted from 50 to 200 mg of each sample using Qiagen DNeasy Powersoil Pro-Kit (cat #7016) according to the manufacturer's protocol, and its quality was checked with gel electrophoresis. The 16S rRNA genes encompassing the V6 to V8 regions were targeted using the 926F (5′- AAA CTY AAA KGA ATT GRC GG −3′) and 1392wR (5′- ACG GGC GGT GWG TRC −3′) primers modified to contain Illumina specific adapter sequence (926F: 5′- TCG GCA GCG TCA GAT GTG TAT AAG AGA CAG AAA CTY AAA KGA ATT GRC GG −3′ and 1392wR: 5′- GTC TCG TGG GCT CGG AGA TGT GTA TAA GAG ACA GAC GGG CGG TGW GTR C −3′). The universal primer pair 926F-1392wR amplifies the small submit (SSU) ribosomal RNA of eukaryotes (18S) and prokaryotes (16S) specifically the V6, V7 and V8 regions. Raw sequencing data was processed by Quantitative Insights Microbial Ecology Ⅱ (QIIME Ⅱ) in multiple steps, including poor-sequences removal. The sequences were clustered into operational taxonomic units (OTUs) at 97% identify threshold.
qPCR and FISH analyses
Real-time qPCR was conducted to quantify anammox 16S rRNA genes in the A- and O-biofilms. The qPCR amplification reaction was performed with 25 µL solution, consisting of 1 µL (10−20 ng/µL) DNA, 12.5 µL Platinum Green Hot Start PCR Master Mix (2X, ThermoFisher Scientific), 10.5 µL nuclease-free water, and 1 µL primers (20 µM), in an Applied Biosystems Veriti™ 96-Well Thermal Cycler (Model 9902). The used primer set was Amx694F (5′−3′ GGGGAGAGTGGAACTTCGG)/Amx960R (5′−3′ GCTCGCACAAGCGGTGGAGC), developed in literature (Ni et al., 2010). The thermal profile was 95 °C for 3 min × 1 cycle, 95 °C for 30 s, 56 °C for 30 s, and then 72 °C for 40 s× 35 cycles. The amplification efficiency was estimated to be 105.54%.
FISH analysis was carried out to verify the presence of AOB and NOB in sludge flocs. The sludge samples were fixed in 4% paraformaldehyde stock solution and then hybridized with designed oligonucleotide probes, including EUB mix (338, 338II, and 338III), Nso1225, Nit3, Ntspa662 and Ntspa712. The detailed probes, hybridization, and visualization can be found in our previous reports (Meng et al., 2021; Zheng et al., 2021).
Monitoring of maximal activity for AOB, NOB and anammox bacteria in long-term operation
Carriers in the A and O zones (random collection of 130 pieces) and sludge flocs (0.5 L) were collected and transferred to batch reactors for the analysis of maximum anammox and AOB/NOB activities, respectively. In brief, all assays were conducted in 1-L glass bottle. Initially, NH4Cl and NaNO2 stock solutions were added to increase NH4+-N and NO2−-N concentrations to about 20–30 mg N/L, ensuring that these substrates were not rate-limiting. A magnetic stirrer was used to mix with a speed of 200 rpm. Each assay lasted for ∼3 h at room temperature (22 ± 1 °C), during which pH was maintained between 7.0 and 7.5 by manually adding 0.1 M HCL or 0.1 M NaOH. To measure the maximum anammox activity, compressed pure dinitrogen (N2) gas was continually flushed at 1.0 L/min. To estimate the maximum AOB and NOB activities, air was supplied at 1.0 L/min. Liquid samples were taken every 0.5 h and filtered with 0.45 μm disposable sterile Millipore filters (Merck) for the measurement of NH4+-N, NO2−-N and NO3−-N concentrations. The maximum anammox activity was determined by dividing the volumetric TN reduction rate (linear regression of TN versus time) to the K5 carrier biofilm surface area (800 m2/m3-packed volume). The maximum AOB and NOB activities were calculated to be slopes of NH4+ reduction and NO3− production versus time divided by the VSS concentration, respectively.
Experimental design of other ex-situ batch tests
More ex-situ batch tests were conducted in the same experimental set-up as that for the measurement of maximum activity (section 5.5), while the experimental conditions were designed according to the actual in-situ environments.
In-situ anammox activity of the A- and O-biofilms. The tests were performed with the initial NH4+ concentration of ∼10 mg N/L, close to that in the A and O zones. Tests using the O-biofilms were carried out at controlled DO concentration of ∼0.4 mg O2/L, the same as that in the O zone of the pilot A/O system. In each test, the initial NO2− concentration was raised to ∼2 mg N/L via adding a nitrite stock solution. The test was carried out until the NO2− concentration decreased to zero, and the liquid samples were taken every 5 min for 1–2 h. After that, the NO2 concentration was raised to 15 mg N/L via re-addition of the nitrite stock solution, and the experiments was continued for a further 1 h, with the liquor samples collected every 15 min. The two tests enabled the calculation of the TN removal rate (i.e., r) under varied NO2− concentrations (i.e., S) from 0 to ∼5 mg N/L and the maximum TN removal rate (i.e., rmax) separately. The data was fitted into the Monod equation (r = rmax·(S/(K + S)). Through a non-linear regression of r versus S, the apparent K-values with respect to NO2− of anammox in the A- and O-biofilms were obtained. Then, the in-situ anammox activity was calculated with the measured maximum anammox activity (see section 5.5), the apparent K-value, and the in-situ NO2− concentrations in the A zone by using the Monod equation. For the O-biofilms, the inhibition of DO should also be considered for the calculation of in-situ anammox activity. This factor was normalized to be 0.74 in this work according to a ratio of the measured maximum anammox activity at DO of 0.4 mg O2/L to that at DO of 0 mg O2/L.
Effect of residual NH4+ concentration on TN removal and NO3− production rates of the O-biofilms. This group of tests were carried out at DO of 0.4 mg O2/L under different residual NH4+ concentrations using the O-biofilms. Initially, 25 mg N/L of NO2− was added to ensure the non-limited NO2− condition.
Simultaneous removal of NH4+and NO3− in the A zone. To examine the anammox activity supported by partial denitrification in the A zone, three batch tests were carried out with sludge flocs, A-biofilms, and their combination. Initially, NH4+ of ∼10 mg N/L and NO3− of ∼3 mg N/L were provided as substrates without NO2− addition. The HRAS effluent (volumetric ratio of 1:3) was also added to support denitrification with the limited organic carbon. The operational conditions including the provision of compressed dinitrogen gas, magnetic mixing, pH control, liquor sampling and analysis were identical to those described in section 5.5.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the UQ Vice-Chancellor and Deputy Vice-Chancellor Research Strategic Initiatives Fund, Urban Utilities (Queensland), Melbourne Water, and SA Water. Professor Zhiguo Yuan acknowledges the Australian Research Council (ARC) Laureate Fellowship support (FL170100086). Dr Tao Liu is an ARC Discovery Early Career Researcher Award (DECRA) Fellow (DE220101310). Professor Jianhua Guo, Associate Professor Shihu Hu, and Professor Zhiguo Yuan were also supported by the Australian Research Council Linkage Project LP180100772.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.wroa.2023.100166.
Contributor Information
Min Zheng, Email: mzheng@uq.edu.au.
Shihu Hu, Email: s.hu@uq.edu.au.
Zhiguo Yuan, Email: z.yuan@uq.edu.au.
Appendix. Supplementary materials
Data availability
Data will be made available on request
References
- Agrawal S., Seuntjens D., Cocker P.D., Lackner S., Vlaeminck S.E. Success of mainstream partial nitritation/anammox demands integration of engineering, microbiome and modeling insights. Curr. Opin. Biotechnol. 2018;50:214–221. doi: 10.1016/j.copbio.2018.01.013. [DOI] [PubMed] [Google Scholar]
- APHA . 2005. Standard Methods for the Examination of Water and Wastewater. [Google Scholar]
- Blackburne R., Yuan Z., Keller J. Partial nitrification to nitrite using low dissolved oxygen concentration as the main selection factor. Biodegradation. 2008;19(2):303–312. doi: 10.1007/s10532-007-9136-4. [DOI] [PubMed] [Google Scholar]
- Cao Y., van Loosdrecht M.C., Daigger G.T. Mainstream partial nitritation-anammox in municipal wastewater treatment: status, bottlenecks, and further studies. Appl. Microbiol. Biotechnol. 2017;101(4):1365–1383. doi: 10.1007/s00253-016-8058-7. [DOI] [PubMed] [Google Scholar]
- Chen G., Li J., Wei J., Zeng J., Zhang Y., Bian W., Deng H. Nitritation via heat shock using immobilized active sludge aggregates. Desalinat. Water Treat. 2016;57(48–49):22779–22787. [Google Scholar]
- Daims H., Lücker S., Wagner M. A new perspective on microbes formerly known as nitrite-oxidizing bacteria. Trends Microbiol. 2016;24(9):699–712. doi: 10.1016/j.tim.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan H., Watts S., Zheng M., Wang Z., Zhao J., Li H., Liu P., Dwyer J., McPhee P., Rattier M. Achieving robust mainstream nitrite shunt at pilot-scale with integrated sidestream sludge treatment and step-feed. Water Res. 2022;223 doi: 10.1016/j.watres.2022.119034. [DOI] [PubMed] [Google Scholar]
- Duan H., Ye L., Lu X., Yuan Z. Overcoming nitrite oxidizing bacteria adaptation through alternating sludge treatment with free nitrous acid and free ammonia. Environ. Sci. Technol. 2019;53(4):1937–1946. doi: 10.1021/acs.est.8b06148. [DOI] [PubMed] [Google Scholar]
- Duan H., Ye L., Wang Q., Zheng M., Lu X., Wang Z., Yuan Z. Nitrite oxidizing bacteria (NOB) contained in influent deteriorate mainstream NOB suppression by sidestream inactivation. Water Res. 2019;162:331–338. doi: 10.1016/j.watres.2019.07.002. [DOI] [PubMed] [Google Scholar]
- Gustavsson D.J., Suarez C., Wilén B.-.M., Hermansson M., Persson F. Long-term stability of partial nitritation-anammox for treatment of municipal wastewater in a moving bed biofilm reactor pilot system. Sci. Total Environ. 2020;714 doi: 10.1016/j.scitotenv.2019.136342. [DOI] [PubMed] [Google Scholar]
- Hausherr D., Niederdorfer R., Bürgmann H., Lehmann M., Magyar P., Mohn J., Morgenroth E., Joss A. Successful year-round mainstream partial nitritation anammox: assessment of effluent quality, performance and N2O emissions. Water Res. X. 2022;16 doi: 10.1016/j.wroa.2022.100145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henze M., Gujer W., Mino T., van Loosdrecht M.C. IWA Publishing; 2000. Activated Sludge Models ASM1, ASM2, ASM2d and ASM3. [Google Scholar]
- Kartal B., Kuenen J.G., van Loosdrecht M.C.M. Sewage treatment with anammox. Science. 2010;328(5979):702. doi: 10.1126/science.1185941. [DOI] [PubMed] [Google Scholar]
- Kitzinger K., Koch H., Lücker S., Sedlacek C.J., Herbold C., Schwarz J., Daebeler A., Mueller A.J., Lukumbuzya M., Romano S., Leisch N., Karst S.M., Kirkegaard R., Albertsen M., Nielsen P.H., Wagner M., Daims H. Characterization of the first "Candidatus Nitrotoga" isolate reveals metabolic versatility and separate evolution of widespread nitrite-oxidizing bacteria. MBio. 2018;9(4) doi: 10.1128/mBio.01186-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuenen J.G. Anammox bacteria: from discovery to application. Nat. Rev. Microbiol. 2008;6(4):320–326. doi: 10.1038/nrmicro1857. [DOI] [PubMed] [Google Scholar]
- Kujawa K., Klapwijk B. A method to estimate denitrification potential for predenitrification systems using NUR batch test. Water Res. 1999;33(10):2291–2300. [Google Scholar]
- Lackner S., Gilbert E.M., Vlaeminck S.E., Joss A., Horn H., van Loosdrecht M.C.M. Full-scale partial nitritation/anammox experiences – an application survey. Water Res. 2014;55:292–303. doi: 10.1016/j.watres.2014.02.032. [DOI] [PubMed] [Google Scholar]
- Laureni M., Falås P., Robin O., Wick A., Weissbrodt D.G., Nielsen J.L., Ternes T.A., Morgenroth E., Joss A. Mainstream partial nitritation and anammox: long-term process stability and effluent quality at low temperatures. Water Res. 2016;101:628–639. doi: 10.1016/j.watres.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laureni M., Weissbrodt D.G., Villez K., Robin O., de Jonge N., Rosenthal A., Wells G., Nielsen J.L., Morgenroth E., Joss A. Biomass segregation between biofilm and flocs improves the control of nitrite-oxidizing bacteria in mainstream partial nitritation and anammox processes. Water Res. 2019;154:104–116. doi: 10.1016/j.watres.2018.12.051. [DOI] [PubMed] [Google Scholar]
- Li J., Peng Y., Gao R., Yang L., Deng L., Zhao Q., Liu Q., Li X., Zhang Q., Zhang L. Highly enriched anammox within anoxic biofilms by reducing suspended sludge biomass in a real-sewage A2/O process. Water Res. 2021;194 doi: 10.1016/j.watres.2021.116906. [DOI] [PubMed] [Google Scholar]
- Li J., Peng Y., Zhang L., Liu J., Wang X., Gao R., Pang L., Zhou Y. Quantify the contribution of anammox for enhanced nitrogen removal through metagenomic analysis and mass balance in an anoxic moving bed biofilm reactor. Water Res. 2019;160:178–187. doi: 10.1016/j.watres.2019.05.070. [DOI] [PubMed] [Google Scholar]
- Liu G., Wang J. Long-term low DO enriches and shifts nitrifier community in activated sludge. Environ. Sci. Technol. 2013;47(10):5109–5117. doi: 10.1021/es304647y. [DOI] [PubMed] [Google Scholar]
- Lotti T., Kleerebezem R., Hu Z., Kartal B., de Kreuk M.K., van Erp Taalman Kip C., Kruit J., Hendrickx T.L., van Loosdrecht M.C.M. Pilot-scale evaluation of anammox-based mainstream nitrogen removal from municipal wastewater. Environ. Technol. 2015;36(9–12):1167–1177. doi: 10.1080/09593330.2014.982722. [DOI] [PubMed] [Google Scholar]
- Ma B., Yang L., Wang Q., Yuan Z., Wang Y., Peng Y. Inactivation and adaptation of ammonia-oxidizing bacteria and nitrite-oxidizing bacteria when exposed to free nitrous acid. Bioresour. Technol. 2017;245(Pt A):1266–1270. doi: 10.1016/j.biortech.2017.08.074. [DOI] [PubMed] [Google Scholar]
- Ma Y., Domingo-Felez C., Plósz B.G., Smets B.F. Intermittent aeration suppresses nitrite-oxidizing bacteria in membrane-aerated biofilms: a model-based explanation. Environ. Sci. Technol. 2017;51(11):6146–6155. doi: 10.1021/acs.est.7b00463. [DOI] [PubMed] [Google Scholar]
- McCarty P.L., Bae J., Kim J. Domestic wastewater treatment as a net energy producer–can this be achieved? Environ. Sci. Technol. 2011;45(17):7100–7106. doi: 10.1021/es2014264. [DOI] [PubMed] [Google Scholar]
- Meng J., Hu Z., Wang Z., Hu S., Liu Y., Guo H., Li J., Yuan Z., Zheng M. Determining factors for nitrite accumulation in an acidic nitrifying system: influent ammonium concentration, operational ph, and ammonia-oxidizing community. Environ. Sci. Technol. 2022;56(16):11578–11588. doi: 10.1021/acs.est.1c07522. [DOI] [PubMed] [Google Scholar]
- Meng J., Liu T., Zhao J., Lu X., Li J., Zheng M. Assessing the stability of one-stage PN/A process through experimental and modelling investigations. Sci. Total Environ. 2021;801 doi: 10.1016/j.scitotenv.2021.149740. [DOI] [PubMed] [Google Scholar]
- Miao Y., Zhang L., Yu D., Zhang J., Zhang W., Ma G., Zhao X., Peng Y. Application of intermittent aeration in nitrogen removal process: development, advantages and mechanisms. Chem. Eng. J. 2022;430 [Google Scholar]
- Mulder A., van de Graaf A.A., Robertson L.A., Kuenen J.G. Anaerobic ammonium oxidation discovered in a denitrifying fluidized bed reactor. FEMS Microbiol. Ecol. 1995;16(3):177–183. [Google Scholar]
- Ni B.-.J., Hu B.-.L., Fang F., Xie W.-.M., Kartal B., Liu X.-.W., Sheng G.-.P., Jetten M., Zheng P., Yu H.-.Q. Microbial and physicochemical characteristics of compact anaerobic ammonium-oxidizing granules in an upflow anaerobic sludge blanket reactor. Appl. Environ. Microbiol. 2010;76(8):2652–2656. doi: 10.1128/AEM.02271-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez J., Lotti T., Kleerebezem R., Picioreanu C., van Loosdrecht M.C.M. Outcompeting nitrite-oxidizing bacteria in single-stage nitrogen removal in sewage treatment plants: a model-based study. Water Res. 2014;66:208–218. doi: 10.1016/j.watres.2014.08.028. [DOI] [PubMed] [Google Scholar]
- Poot V., Hoekstra M., Geleijnse M.A.A., van Loosdrecht M.C.M., Pérez J. Effects of the residual ammonium concentration on NOB repression during partial nitritation with granular sludge. Water Res. 2016;106:518–530. doi: 10.1016/j.watres.2016.10.028. [DOI] [PubMed] [Google Scholar]
- Qiu S., Li Z., Hu Y., Shi L., Liu R., Shi L., Chen L., Zhan X., Technology What's the best way to achieve successful mainstream partial nitritation-anammox application? Crit. Rev. Environ. Sci. Technol. 2021;51(10):1045–1077. [Google Scholar]
- Regmi P., Miller M.W., Holgate B., Bunce R., Park H., Chandran K., Wett B., Murthy S., Bott C.B. Control of aeration, aerobic SRT and COD input for mainstream nitritation/denitritation. Water Res. 2014;57:162–171. doi: 10.1016/j.watres.2014.03.035. [DOI] [PubMed] [Google Scholar]
- Rittmann B.E., McCarty P.L. McGraw-Hill Education; 2001. Environmental Biotechnology: Principles and Applications. [Google Scholar]
- Seuntjens D., Van Tendeloo M., Chatzigiannidou I., Carvajal-Arroyo J.M., Vandendriessche S., Vlaeminck S.E., Boon N. Synergistic exposure of return-sludge to anaerobic starvation, sulfide, and free ammonia to suppress nitrite oxidizing bacteria. Environ. Sci. Technol. 2018;52(15):8725–8732. doi: 10.1021/acs.est.7b06591. [DOI] [PubMed] [Google Scholar]
- Trojanowicz K., Plaza E., Trela J. Pilot scale studies on nitritation-anammox process for mainstream wastewater at low temperature. Water Sci. Technol. 2016;73(4):761–768. doi: 10.2166/wst.2015.551. [DOI] [PubMed] [Google Scholar]
- Wang D., Wang Q., Laloo A., Xu Y., Bond P.L., Yuan Z. Achieving stable nitritation for mainstream deammonification by combining free nitrous acid-based sludge treatment and oxygen limitation. Sci. Rep. 2016;6(1):25547. doi: 10.1038/srep25547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Duan H., Wei W., Ni B.-.J., Laloo A., Yuan Z. Achieving stable mainstream nitrogen removal via the nitrite pathway by sludge treatment using free ammonia. Environ. Sci. Technol. 2017;51(17):9800–9807. doi: 10.1021/acs.est.7b02776. [DOI] [PubMed] [Google Scholar]
- Wang Q., Ye L., Jiang G., Hu S., Yuan Z. Side-stream sludge treatment using free nitrous acid selectively eliminates nitrite oxidizing bacteria and achieves the nitrite pathway. Water Res.. 2014;55:245–255. doi: 10.1016/j.watres.2014.02.029. [DOI] [PubMed] [Google Scholar]
- Wang Z., Zheng M., Duan H., Yuan Z., Hu S. A 20-year journey of partial nitritation and anammox (PN/A): from sidestream toward mainstream. Environ. Sci. Technol. 2022;56(12):7522–7531. doi: 10.1021/acs.est.1c06107. [DOI] [PubMed] [Google Scholar]
- Wang Z., Zheng M., Hu Z., Duan H., De Clippeleir H., Al-Omari A., Hu S., Yuan Z. Unravelling adaptation of nitrite-oxidizing bacteria in mainstream PN/A process: mechanisms and counter-strategies. Water Res. 2021;200 doi: 10.1016/j.watres.2021.117239. [DOI] [PubMed] [Google Scholar]
- Wang Z., Zheng M., Meng J., Hu Z., Ni G., Guerrero Calderon A., Li H., De Clippeleir H., Al-Omari A., Hu S., Yuan Z. Robust nitritation sustained by acid-tolerant ammonia-oxidizing bacteria. Environ. Sci. Technol. 2021;55(3):2048–2056. doi: 10.1021/acs.est.0c05181. [DOI] [PubMed] [Google Scholar]
- Wu J., Kong Z., Luo Z., Qin Y., Rong C., Wang T., Hanaoka T., Sakemi S., Ito M., Kobayashi S. A successful start-up of an anaerobic membrane bioreactor (AnMBR) coupled mainstream partial nitritation-anammox (PN/A) system: a pilot-scale study on in-situ NOB elimination, AnAOB growth kinetics, and mainstream treatment performance. Water Res. 2021;207 doi: 10.1016/j.watres.2021.117783. [DOI] [PubMed] [Google Scholar]
- Yang M., Qiu S., Wang L., Chen Z., Hu Y., Guo J., Ge S. Effect of short-term light irradiation with varying energy densities on the activities of nitrifiers in wastewater. Water Res. 2022;216 doi: 10.1016/j.watres.2022.118291. [DOI] [PubMed] [Google Scholar]
- Yang Y., Jiang Y., Long Y., Xu J., Liu C., Zhang L., Peng Y. Insights into the mechanism of the deterioration of mainstream partial nitritation/anammox under low residual ammonium. J. Environ. Sci. 2023;126:29–39. doi: 10.1016/j.jes.2022.04.005. [DOI] [PubMed] [Google Scholar]
- Zhao J., Liu T., Meng J., Hu Z., Lu X., Hu S., Yuan Z., Zheng M. Ammonium concentration determines oxygen penetration depth to impact the suppression of nitrite-oxidizing bacteria inside Partial Nitritation and Anammox biofilms. Chem. Eng. J. 2023 doi: 10.1016/j.cej.2022.140. [DOI] [Google Scholar]
- Zheng M., Li S., Ni G., Xia J., Hu S., Yuan Z., Liu Y., Huang X. Critical factors facilitating candidatus nitrotoga to be prevalent nitrite-oxidizing bacteria in activated sludge. Environ. Sci. Technol. 2020;54(23):15414–15423. doi: 10.1021/acs.est.0c04192. [DOI] [PubMed] [Google Scholar]
- Zheng M., Liu Y.-.C., Wang C.-.W., Xu K.N. Study on enhanced denitrification using particulate organic matter in membrane bioreactor by mechanism modeling. Chemosphere. 2013;93(11):2669–2674. doi: 10.1016/j.chemosphere.2013.08.045. [DOI] [PubMed] [Google Scholar]
- Zheng M., Wang Z., Meng J., Hu Z., Liu Y., Yuan Z., Hu S. Inactivation kinetics of nitrite-oxidizing bacteria by free nitrous acid. Sci. Total Environ. 2021;752 doi: 10.1016/j.scitotenv.2020.141876. [DOI] [PubMed] [Google Scholar]
- Zheng M., Wu S., Dong Q., Huang X., Yuan Z., Liu Y. Achieving mainstream nitrogen removal via the nitrite pathway from real municipal wastewater using intermittent ultrasonic treatment. Ultrason. Sonochem. 2019;51:406–411. doi: 10.1016/j.ultsonch.2018.07.033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request