Abstract
Data regarding the occurrence of venous thromboembolic events (VTE), including acute pulmonary embolism (PE) and deep vein thrombosis (DVT) in recovered COVID-19 patients are scant. We performed a systematic review and meta-analysis to assess the risk of acute PE and DVT in COVID-19 recovered subject. Following the PRIMSA guidelines, we searched Medline and Scopus to locate all articles published up to September 1st, 2022, reporting the risk of acute PE and/or DVT in patients recovered from COVID-19 infection compared to non-infected patients who developed VTE over the same follow-up period. PE and DVT risk were evaluated using the Mantel–Haenszel random effects models with Hazard ratio (HR) as the effect measure with 95% confidence interval (CI) while heterogeneity was assessed using Higgins I2 statistic. Overall, 29.078.950 patients (mean age 50.2 years, 63.9% males), of which 2.060.496 had COVID-19 infection, were included. Over a mean follow-up of 8.5 months, the cumulative incidence of PE and DVT in COVID-19 recovered patients were 1.2% (95% CI:0.9–1.4, I2: 99.8%) and 2.3% (95% CI:1.7-3.0, I2: 99.7%), respectively. Recovered COVID-19 patients presented a higher risk of incident PE (HR: 3.16, 95% CI: 2.63–3.79, I2 = 90.1%) and DVT (HR: 2.55, 95% CI: 2.09–3.11, I2: 92.6%) compared to non-infected patients from the general population over the same follow-up period. Meta-regression showed a higher risk of PE and DVT with age and with female gender, and lower risk with longer follow-up. Recovered COVID-19 patients have a higher risk of VTE events, which increase with aging and among females.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11239-022-02766-7.
Keywords: COVID-19, Venous thromboembolism, Pulmonary embolism, Deep vein thrombosis
Introduction
Recent data suggest that patients who have recovered from COVID-19 may experience late clinical sequelae including a broad spectrum of cardiovascular complications [1–4]. Previous analyses have reported a higher risk of venous thromboembolism (VTE) in patients with COVID-19 infection [5, 6]. Moreover, some recent randomized clinical trials (RCTs) have analyzed the benefit-risk of anticoagulation for these subjects, providing the basis for clinical recommendations regarding the use of thromboprophylaxis during and after COVID-19 infection [7–9]. In contrast, data regarding the occurrence of VTE events, including acute pulmonary embolism (PE) and deep vein thrombosis (DVT), as clinical sequelae of COVID-19 are scant [10]. This study aimed to assess the risk of acute pulmonary embolism (PE) and deep vein thrombosis (DVT) in COVID-19 recovered patients performing a systematic review and meta-analysis of the available data.
Materials and methods
Study design and eligibility criteria
This study followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline (Supplementary file 1) [11]. Data were obtained searching MEDLINE and Scopus for all studies published at any time up to September 1, 2022 and reporting the risk of VTE events in COVID-19 recovered patients, defined as those were alive after experiencing negativization for COVID-19 test. In the revised manuscripts, this group of patients were compared to contemporary cohorts (control group), defined as subjects who did not experience the infection and developed VTE over the same follow-up period.
Outcomes
The pooled incidence and risk of acute symptomatic PE after COVID-19 infection were chosen as the primary outcome and defined as events diagnosed within a maximum of 12 months post discharge (maximum follow-up length of revised studies) after index hospitalization. The secondary outcomes were the pooled incidence and risk of DVT in the same period.
Data extraction and quality assessment
The selection of studies to be included in our analysis was independently conducted by two authors (M.Z., C.B.) in a blinded fashion. Any discrepancies in study selection were resolved by consulting a third author (G.R.). The following MeSH terms were used for the search: “Venous thromboembolism after COVID-19” OR “COVID-19 sequelae”. Moreover, we searched the reference lists of target studies for additional references. Specific inclusion criteria were studies (i) having enrolled subjects with previous confirmed COVID-19 infection and (ii) providing the hazard ratio (HR) for the risk of PE and/or DVT in the long-term period. Conversely, case reports, review articles, abstracts, editorials/letters, and case series with less than 10 participants were excluded. Data extraction was independently conducted by two authors (M.Z., G.R). For all studies reviewed, we extracted the number of patients enrolled, the mean age, male gender, prevalence of cardiovascular comorbidities such as arterial hypertension (HT), diabetes mellitus (DM), chronic obstructive pulmonary disease (COPD), chronic kidney disease (CKD), obesity, heart failure (HF), cerebrovascular disease and length of follow-up. The quality of included studies was graded using the Newcastle-Ottawa quality assessment scale [12].
Data synthesis and analysis
The cumulative incidence of acute PE and DVT (n/N), defined as the ratio between patients experiencing the event during the follow-up period (n) and the number of COVID-19 patients (either hospitalized or treated as outpatients) enrolled in each study (N), were pooled using a random-effects model and presented with the corresponding 95% confidence interval (CI). When the incidence rate was not directly presented into the manuscript or supplementary material, the incidence rates was derived from the hazard ratios (HRs) presented in the included studies [13–16].
Conversely for the estimation of long-term risk of PE and DVT, the hazard ratio (HR) with the related 95% confidence interval (CI) was pooled using a random-effect. Predefined sensitivity analyses (leave-one-out analysis) were performed removing one study at the time, to evaluate the stability of our results regarding the pooled incidence and the risk of PE or DVT. Statistical heterogeneity between groups was measured using the Higgins I2 statistic. The presence of potential publication bias was verified by visual inspection of the funnel plot. Due to the low number of the included studies (< 10), small-study bias was not examined as our analysis was underpowered to detect such bias. To further appraise the impact of potential baseline confounders, a meta-regression analysis was also performed. All meta-analyses were conducted using Comprehensive Meta-Analysis software, version 3 (Biostat, USA).
Results
Search results and included studies
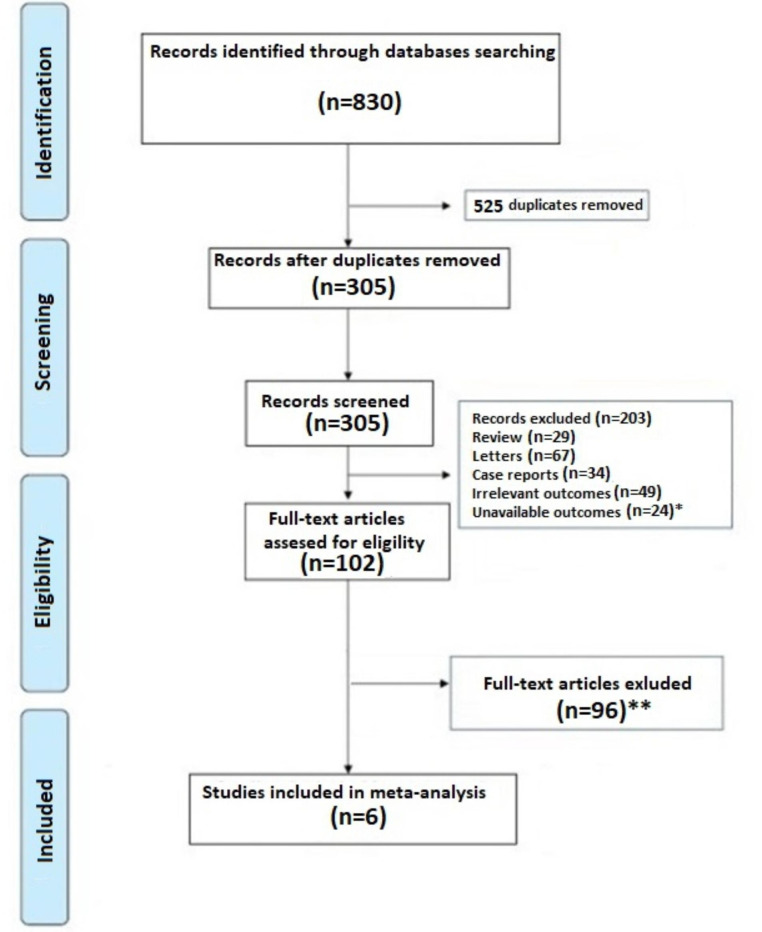
A total of 830 articles were obtained using our search strategy. After excluding duplicates and preliminary screening, 102 full-text articles were assessed for eligibility, 96 studies were excluded for not meeting the inclusion criteria, 2 records were identified using other sources and one investigation was excluded because presented a partial overlapping cohort [17], leaving 6 investigations fulfilling the inclusion criteria (Fig. 1) [18–23].
Fig. 1.
PRISMA flowchart. * Articles excluded because not provided data on VTE event; ** Articles excluded because not provided Hazard ratio for VTE events
Characteristics of the population and quality assessment
Overall, 29.078.950 patients (mean age 50.2 years, 63.9% males), of which 2.060–496 had COVID-19 infection, were included in this analysis [18–23]. The general characteristics of the studies included are showed in Table 1. Although the demographic characteristics and concomitant comorbidities were not systematically recorded in all investigations, the cohorts mainly consisted of middle-aged patients. The mean length of follow-up was 8.6 months ranging between 4 and 12 months. Quality assessment showed that all studies were of moderate-high quality according to the NOS scale [12].
Table 1.
General characteristics of the population reviewed. HT: Arterial Hypertension; DM: Diabetes Mellitus; COPD: chronic obstructive pulmonary disease; CKD: Chronic Kidney disease; HF: Heart failure; FW: Follow-up; NR: Not reported. *Defined as Chronic Pulmonary disease; **Only DM type 2. R: Retrospective
| Authors | Study Design | Sample size n |
Cases n |
Controls n |
Age (years) |
Males n (%) |
HT n (%) |
DM n (%) |
COPD n (%) |
CKD n (%) |
Obesity n (%) |
HF n (%) |
Cancer n (%) |
Cerebrovascular disease n (%) |
FW-length (months) | NOS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cohen et al. [14] | R | 2.895.943 | 133,366 | 2,762,577 | 75.7 |
1.227.545 (42.0) |
2.081.772 (72.0) |
938.043 (32.7) |
578.650 (20)* |
528.314 (14.0) |
478.902 (17.0) |
334,654 (12) |
418.700 (14.4) |
364.782 (13.0) |
4 | 8 |
| Wang et al. [15] | R | 2.940.988 | 691,455 | 2,249,533 | 43.8 |
1.241.483 (42.2) |
440.998 (14.9) |
188.488 (6.4)** |
51,592 (1.7) |
59,177 (2.0) |
286,338 (9.7)** |
NR | NR | NR | 12 | 7 |
| Xie et al. [16] | R | 5.791.407 | 153,760 | 5,637,647 | 62.5 |
5.228.431 (90.2) |
1.525.944 (26.3) |
1.321.907 (22.8) |
633.000 (10.9) |
970.057 (16.7) |
2.462.44 (42.5) |
NR |
357.192 (6.1) |
NR | 12 | 8 |
| Daugherty et al. [17] | R | 9.247.505 | 266,586 | 8,980,919 | 42.4 |
4.640.393 (50.2) |
NR |
521.699 (5.6) |
NR | NR | NR | NR | NR | NR | 6 | 6 |
| Kompaniyets et al. [18] | R | 3.125.676 | 781,419 | 2,344,257 | 12 |
1.709.108 (54.6) |
NR | NR | NR | NR | NR | NR | NR | NR | 12 | 5 |
| Al-Aly et al. [19] | R | 5.017.431 | 33,940 | 4,983,491 | 64.9 |
4.512.549 (89.9) |
NR | 1.275.370 | NR |
423.329 (8.4) |
NR | NR |
371.526 (7.4) |
288.266 (5.7) |
6 | 7 |
Pooled incidence of pulmonary embolism in COVID-19 recovered patients
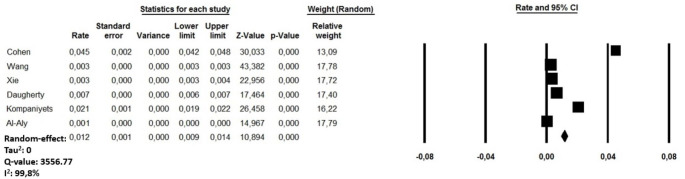
The cumulative rate of PE in COVID-19 recovered patients ranged between 0.1 and 4.5% among the reviewed studies [18–23]. A random-effect model revealed a pooled incidence of post COVID-19 PE in 1.2% of cases (95% CI:0.9–1.4, I2: 99.8%) (Fig. 2). Sensitivity analysis showed a combined incidence rate which remained statistically significant across a range from 0.6% (95% CI:0.4–0.8, I2:99.8%) to 1.5% of cases (95% CI: 1.1 to 1.8; I2 99.7%), suggesting that no single investigation had an undue impact on the study outcome. Another sub-analysis performed excluding a cohort of children and adolescent [22] revealed a pooled post COVID-19 PE in 0.9% of cases (95% CI: 0.7 to 1.2, I2: 97.3%). The visual inspection of the funnel plot is presented in Supplementary file 2, Panel A.
Fig. 2.
Forest plots investigating the pooled incidence of acute pulmonary embolism after 8.5 months from COVID-19 infection
Pooled incidence of deep vein thrombosis in COVID-19 recovered patients
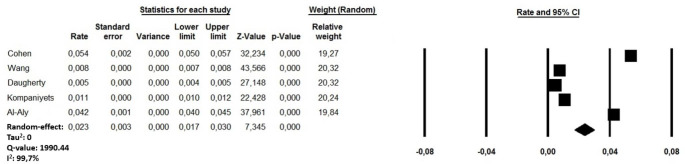
The cumulative rate of DVT in COVID-19 recovered patients ranged between 0.5 and 5.4% [18, 19, 21–23]. A random-effect model revealed a pooled incidence of post COVID-19 DVT in 2.3% of cases (95% CI:1.7-3.0, I2: 99.7%) (Fig. 3). Sensitivity analysis showed a combined incidence which remained statistically significant across a range from 1.6% (95% CI:1.1–2.1, I2:99.7%) to 2.8% of cases (95% CI: 1.5 to 4.1; I2 99.8%), suggesting that no single investigation had an undue impact on the study outcome. A further sub-analysis performed excluding a cohort of children and adolescent [22] revealed a pooled post COVID-19 DVT in 1.7% of cases (95% CI: 1.23 to 2.2, I2: 99.8%). The visual inspection of the funnel plot is presented in Supplementary file 2, Panel B.
Fig. 3.
Forest plots investigating the pooled incidence of deep vein thrombosis after 8.5 months from COVID-19 infection
Risk of acute pulmonary embolism
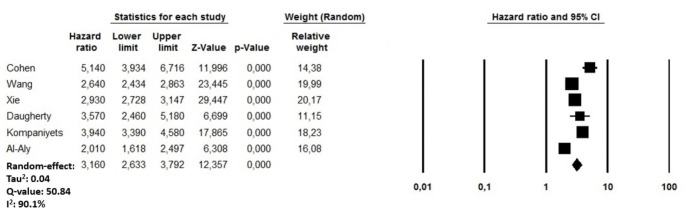
After a mean follow-up of 8.5 months, recovered COVID-19 patients presented a higher risk of incident PE (HR: 3.16, 95% CI: 2.63–3.79, p < 0.0001, I2 = 90.1%) compared to non-infected subjects from the general population over the same period (Fig. 4). A visual assessment of the funnel plot cannot reassure about the presence of an asymmetry with studies characterized by higher PE rate being missing at the basis of the triangle (Supplementary file 3, Panel A) while the sensitivity analysis confirmed yielded results reporting an HR ranging between 2.90 (95% CI: 2.45–3.43, p < 0.0001; I2: 87.9%) and 3.43 (95% CI; 2.85–4.13, p < 0.0001, I2:89.7%), indicating that the obtained results were not driven by any single study. A meta-regression analysis showed a significant direct relationship between for the risk of incident PE using age (p < 0.0001)) as moderator, while an indirect association was observed when male gender (p = 0.02) and the follow-up length (p = 0.005) were adopted as moderating variables (Table 2).
Fig. 4.
Forest plots investigating the long-term risk of incident acute pulmonary embolism after 8.5 months from COVID-19 infection
Table 2.
Meta regression analysis for the risk of acute pulmonary embolism after COVID-19 infection. CI: Confidence interval
| Item | Coeff | 95% CI | p |
|---|---|---|---|
| Age (years) | 0.019 | 0.010–0.029 | < 0.0001 |
| Males (%) | -0.006 | -0.001 to -0.0001 | 0.02 |
| Diabetes mellitus (%) | 0.007 | -0.016 to 0.031 | 0.52 |
| Follow-up lenght | -0.032 | -0.076 to -0.028 | 0.005 |
Risk for deep vein thrombosis
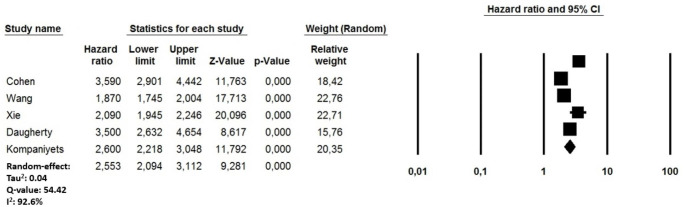
During the same follow-up period, a random-effect model also demonstrated that COVID-19 recovered subjects had an increased risk of DVT (HR: 2.55, 95% CI: 2.09–3.11, p < 0.0001, I2: 92.6%) (Fig. 5) compared to non-infected patients from the general population during the same follow-up period. Also in this case, the visual estimation of the funnel plot cannot reassure regarding the presence of potential bias, with studies characterized by higher DVT rate being missing at the basis of the triangle did not show evident asymmetries (Supplementary file 3, Panel B). One-by-one exclusion of the studies from the analysis slightly changed the combined HR, which remained statistically significant between 2.32 (95% CI: 1.95–2.76, p < 0.0001; I2: 88.7%) and 2.82 (95% CI: 2.14–3.70, p < 0.0001; I2: 91.2%) suggesting also in this case that no single study had an undue impact on the combined HR. Consistent with PE, the long-term DVT risk showed a significant direct relationship with age (p = 0.03) and an indirect relationship with male gender (p = 0.03) and the length of the follow-up period (p = 0.002) (Table 3).
Fig. 5.
Forest plots investigating the long-term risk of incident deep vein thrombosis after 8.5 months from COVID-19 infection
Table 3.
Meta regression analysis for the risk of deep vein thrombosis after COVID-19 infection. SE: standard error; CI: Confidence interval
| Item | Coeff | 95% CI | p |
|---|---|---|---|
| Age (years) | 0.013 | 0.007 to 0.027 | 0.01 |
| Males (%) | -0.033 | -0.226 to -0.011 | 0.03 |
| Diabetes mellitus (%) | 0.008 | -0.019 to 0.03 | 0.44 |
| Follow-up lenght | -0.060 | -0.107 to 0.032 | 0.002 |
Discussion
In this study, we performed an analysis of the cumulative incidence and risk of acute PE and DVT among COVID-19 recovered patients. Over 8.5 months, acute PE and DVT were observed in 1.2% and 2.3% of cases. Moreover, an increased PE and DVT risk was also observed in the same period. To the best of our knowledge, this is the first meta-analysis summarizing the growing data regarding the risk of VTE events after COVID-19 infection. Notably, our findings resulted from very heterogeneous rates described in individual studies, which may be attributed to the different characteristics of the included cohorts.
Our results demonstrate that the risk of VTE events after hospital discharge is much lower compared to the in-hospital incidence of the same events during the acute phase of COVID-19 infection [20]. Moreover, as suggested by the meta-regression analyses, the risk of PE and DVT was higher in the early phase after COVID-19 recovery (time to event). These findings are in accordance with our previous results regarding the pooled incidence of VTE events in the early phase after COVID-19 recovery [10]. The studies that we reviewed did not report the prevalence of specific VTE risk factors among the population enrolled, not allowing further subgroup analyses. Similarly, no specific data were reported regarding the administration of thromboprophylaxis either during the acute phase and after discharge, thus limiting any conclusions in this regard.
Meta-regression analyses have highlighted a high degree of heterogeneity, showing that the risk of VTE events increased with aging, as previously reported in COVID-19 patients during the acute phase of the disease as well as in general population [24–28]. In addition, the risk of PE and DVT were higher in women, in contrast to the higher risk described in men during the acute phase of the infection [29]. However, it is also true that the comparison of rates between sexes strictly depends on the age range of the population under study. Furthermore, when the death rate is high from causes other than the disease of interest, the incidence rates of the illness are generally overestimated in traditional Kaplan–Meier survival analysis due to existence of competing risks [30].
Our data based on a large cohort, may be useful for designing strategies to minimize the risk of thromboembolic events during the post-acute phase after COVID-19 infection, although our results must be considered preliminary and cannot be directly translated into clinical practice on the type and regimen of thrombophylactic regimens. Indeed, due the limited data provided in the reviewed manuscripts, which were not mainly focused on the risk of VTE but assessed the risk of general clinical consequences as a part of post-acute sequelae of SARS-CoV-2 infection (PACSs), we cannot provide any results regarding the potential benefit of extended anticoagulation. It is likely that several contributing risk factors directly related to the SARS-CoV-2 infection, such as cardiac or respiratory failure, prolonged immobility, presence of central venous lines, sepsis or acute kidney failure, may have triggered VTE events during the early phase of follow-up period [31–35].
Nevertheless, the results of our study may have some implications for clinical practice. First, the higher risk of VTE events in the early phase after recovery underlines the need to establish an optimal antithrombotic regimen to minimize the risk of thromboembolic events in these patients. In this regard, recent publications have proposed different therapeutic and prophylactic regimens, but the optimal therapeutic strategies have not yet identified. Available data evidence that thromboprophylaxis [36, 37] has no role in reducing the hospitalization rate or death in COVID-19 outpatients, although anticoagulant treatment improves the patient’s outcome after hospitalisation [9]. Second, it appears that the risk of VTE events me ay not just limited to the acute phase of COVID-19 infection but also extend to the early phase after recovery. However, the contribution derived from local “immunothrombosis” triggered by the SARS-CoV-2 infection and the traditional VTE risk factors remained to be established. Finally, the main strength of our study is the analysis of large, unselected cohorts, which reduced the risks of selection and attrition bias that might occur in more traditional cohort studies.
Limitations
Our study has several limitations related to the observational nature of the studies reviewed and their own limitations with all inherited bias. Potential underestimation could derive from detection bias considering that patients were not systematically screened for PE or DVT and per se, these conditions may be clinically silent. Moreover, sampling bias by the competing risk of death may also have led to underestimation of the real cumulative incidence of thromboembolic events. Furthermore, our data must be carefully interpreted considering that the reviewed studies mainly based their search strategy on the revision of hospital admission and screening of ICD-10 codes in the long-term period. In this perspective, only highly symptomatic events could have been captured. we could not assess if an adequate prophylactic anticoagulation was consistently administered in each study because these data were not provided in the reviewed investigations. Large administrative data provide multiple advantages, being readily available and able to provide large samples of unselected patients over extended periods; however, the utility of such data largely depends on their accuracy and reliability. We can neither exclude that geographical differences for the quality of care may have influenced our findings nor that the real impact of VTE events after COVID-19 was underestimated, especially during the early phase of the pandemic. Moreover, reviewed studies did not provide data regarding the severity of COVID-19 infection, not even using indirect measures, such as the need for intensive care treatment. Similarly, no data regarding the vaccination status of patients enrolled as Well as information on variants of the SARS-CoV-2 virus were provided, limiting the possibility to conduct further sub-analyses. Finally, as our analysis was based on death certificate data and relative ICD-10 codes, we cannot exclude that miscoding may have biased our results.
Conclusion
During the first months after COVID-19 infection, recovered patients have a higher risk of VTE events compared to subjects from the general population, which increase with aging and among females. The highest risk was observed for acute PE. Due to the absence of systematic screening for thromboembolic events in most studies, these data possibly represent the lowest estimate of what one would expect in this patient group.
Electronic supplementary material
Below is the link to the electronic supplementary material.
PRISMA checklist
Funnel plots for the pooled incidence of (A) acute pulmonary embolism and deep vein thrombosis (DVT) over 8.5 months after COVID-19 infection
Supplementary file 3: Funnel plots for the risk of (A) acute pulmonary embolism and deep vein thrombosis (DVT) over 8.5 months after COVID-19 infection
Funding
None.
Declarations
Conflict of interest
SB received lecture/consultant fees from Bayer HealthCare, Concept Medical, BTG Pharmaceuticals, INARI, Boston Scientific, and LeoPharma; institutional grants from Boston Scientific, Bentley, Bayer HealthCare, INARI, Medtronic, Concept Medical, Bard, and Sanofi; and economic support for travel/congress costs from Daiichi Sankyo, BTG Pharmaceuticals, and Bayer HealthCare, outside the submitted work.SK reports lecture honoraria and advisory fees from Boston Scientific, Bayer AG, Daiichi-Sankyo, Pfizer – Brisol Myers Squibb; and institutional research support by Bostonm Scientific, Bayer AG and Daiichi Sankyo. GG reports lecture/consultant fees from Bayer HealthCare, Pfizer and LeoPharma. TV reports lecture/consultancy fees from Bayer, Boehringer Ingelheim, Daiichi Sankyo, BMS/Pfizer, Sanofi, and LeoPharma.LH received lecture Honoria from Johnson&Johson and MSD; outside to the submitted work.The other authors have no conflicts of interest to declare.
Ethical approval
not applicable.
Informed consent
not applicable.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Alkodaymi MS, Omrani OA, Fawzy NA, Shaar BA, Almamlouk R, Riaz M, Obeidat M, Obeidat Y, Gerberi D, Taha RM, Kashour Z, Kashour T, Berbari EF, Alkattan K, Tleyjeh IM. Prevalence of post-acute COVID-19 syndrome symptoms at different follow-up periods: a systematic review and meta-analysis. Clin Microbiol Infect. 2022;28:657–666. doi: 10.1016/j.cmi.2022.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tleyjeh IM, Saddik B, AlSwaidan N, AlAnazi A, Ramakrishnan RK, Alhazmi D, Aloufi A, AlSumait F, Berbari E, Halwani R. Prevalence and predictors of Post-Acute COVID-19 Syndrome (PACS) after hospital discharge: a cohort study with 4 months median follow-up. PLoS One. 2021;16:e0260568. doi: 10.1371/journal.pone.0260568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dixit NM, Churchill A, Nsair A, Hsu JJ. Post-Acute COVID-19 syndrome and the cardiovascular system: what is known? Am Heart J Plus. 2021;5:100025. doi: 10.1016/j.ahjo.2021.100025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sidik SM. Heart disease after COVID: what the data say. Nature. 2022;608:26–28. doi: 10.1038/d41586-022-02074-3. [DOI] [PubMed] [Google Scholar]
- 5.Tan BK M, S F, A, et al. Arterial and venous thromboembolism in COVID-19: a study-level meta-analysis.  Thorax. 2021;76:970–979. doi: 10.1136/thoraxjnl-2020-215383. [DOI] [PubMed] [Google Scholar]
- 6.Miró Ò, Jiménez S, Mebazaa A, Freund Y, Burillo-Putze G, Martín A, Martín-Sánchez FJ, García-Lamberechts EJ, Alquézar-Arbé A, Jacob J, Llorens P, Piñera P, Gil V, Guardiola J, Cardozo C, Mòdol Deltell JM, Tost J, Aguirre Tejedo A, Palau-Vendrell A, LLauger García L, Agudo Villa T, López-Laguna N, López Díez MP, Beddar Chaib F, Quero Motto E, González Tejera M, Ponce MC (2021) González Del Castillo J; Spanish Investigators on Emergency Situations TeAm (SIESTA) network Pulmonary embolism in patients with COVID-19: incidence, risk factors, clinical characteristics, and outcome. Eur Heart J. 42:3127–3142 [DOI] [PMC free article] [PubMed]
- 7.Spyropoulos AC G, M G, D, HEP-COVID Investigators et al. Efficacy and safety of therapeutic-dose heparin vs standard prophylactic or intermediate-dose heparins for thromboprophylaxis in high-risk hospitalized patients with COVID-19: the HEP-COVID randomized clinical trial.  JAMA Intern Med. 2021;181:1612–1620. doi: 10.1001/jamainternmed.2021.6203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ortega-Paz L G, M C, D, et al. Safety and efficacy of different prophylactic anticoagulation dosing regimens in critically and non-critically ill patients with COVID-19: a systematic review and meta-analysis of randomized controlled trials.  Eur Heart J Cardiovasc Pharmacother. 2021 doi: 10.1093/ehjcvp/pvab070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramacciotti E, Barile Agati L, Calderaro D, Aguiar VCR, Spyropoulos AC, de Oliveira CCC, Lins Dos Santos J, Volpiani GG, Sobreira ML, Joviliano EE, Bohatch Júnior MS, da Fonseca BAL, Ribeiro MS, Dusilek C, Itinose K, Sanches SMV, de Araujo Ramos A, de Moraes K, Tierno NF, de Oliveira PFGMM, Tachibana ALML, Chate A, Santos RC, de Menezes Cavalcante MVB, Moreira BB, Chang RCR, Tafur C, Fareed A, Lopes J, MICHELLE investigators Rivaroxaban versus no anticoagulation for post-discharge thromboprophylaxis after hospitalisation for COVID-19 (MICHELLE): an open-label, multicentre, randomised, controlled trial. Lancet. 2022;399:50–59. doi: 10.1016/S0140-6736(21)02392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zuin M, Engelen MM, Barco S, Spyropoulos AC, Vanassche T, Hunt BJ, Vandenbriele C, Verhamme P, Kucher N, Rashidi F, Zuliani G, Konstantinides SV, Roncon L. Incidence of venous thromboembolic events in COVID-19 patients after hospital discharge: a systematic review and meta-analysis. Thromb Res. 2022;209:94–98. doi: 10.1016/j.thromres.2021.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P (2012) The Newcastle-Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. http://www.ohrica/programs/clinical_epidemiology/oxfordasp, Accessed August 29, 2022
- 13.Li Z, Wang X, Wu Y, Owzar K (2018) Sample size calculation for studies with grouped survival data. Stat Med. Nov 30;37(27):3904–3917. 10.1002/sim.7847. Epub 2018 Jun 10. PMID: 29888484; PMCID: PMC6262878 [DOI] [PMC free article] [PubMed]
- 14.Halabi S, Singh B (2004) Sample size determination for comparing several survival curves with unequal allocations. Stat Med. Jun 15;23(11):1793 – 815. 10.1002/sim.1771. PMID: 15160409 [DOI] [PubMed]
- 15.Wang S, Zhang J, Lu W (2012) Sample size calculation for the proportional hazards cure model. Stat Med. Dec 20;31(29):3959–71. 10.1002/sim.5465. Epub 2012 Jul 11. PMID: 22786805; PMCID: PMC3505258 [DOI] [PMC free article] [PubMed]
- 16.Schoenfeld DA (1983 Jun) Sample-size formula for the proportional-hazards regression model. Biometrics 39(2):499–503 PMID: 6354290 [PubMed]
- 17.Xie Y, Bowe B, Al-Aly Z. Burdens of post-acute sequelae of COVID-19 by severity of acute infection, demographics and health status. Nat Commun. 2021;12:6571. doi: 10.1038/s41467-021-26513-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen K, Ren S, Heath K, Dasmariñas MC, Jubilo KG, Guo Y, Lipsitch M, Daugherty SE. Risk of persistent and new clinical sequelae among adults aged 65 years and older during the post-acute phase of SARS-CoV-2 infection: retrospective cohort study. BMJ. 2022;376:e068414. doi: 10.1136/bmj-2021-068414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang W, Wang CY, Wang SI, Wei JC. Long-term cardiovascular outcomes in COVID-19 survivors among non-vaccinated population: a retrospective cohort study from the TriNetX US collaborative networks. EClinicalMedicine. 2022;53:101619. doi: 10.1016/j.eclinm.2022.101619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xie Y, Xu E, Bowe B, Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nat Med. 2022;28:583–590. doi: 10.1038/s41591-022-01689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daugherty SE, Guo Y, Heath K, Dasmariñas MC, Jubilo KG, Samranvedhya J, Lipsitch M, Cohen K. Risk of clinical sequelae after the acute phase of SARS-CoV-2 infection: retrospective cohort study. BMJ. 2021;373:n1098. doi: 10.1136/bmj.n1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kompaniyets L, Bull-Otterson L, Boehmer TK, Baca S, Alvarez P, Hong K, Hsu J, Harris AM, Gundlapalli AV, Saydah S. Post-COVID-19 symptoms and conditions among children and adolescents - United States, March 1, 2020-January 31, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:993–999. doi: 10.15585/mmwr.mm7131a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Al-Aly Z, Bowe B, Xie Y. Long COVID after breakthrough SARS-CoV-2 infection. Nat Med. 2022;28:1461–1467. doi: 10.1038/s41591-022-01840-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roncon L, Zuin M, Barco S, Valerio L, Zuliani G, Zonzin P, Konstantinides SV. Incidence of acute pulmonary embolism in COVID-19 patients: systematic review and meta-analysis. Eur J Intern Med. 2020;82:29–37. doi: 10.1016/j.ejim.2020.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xie J, Prats-Uribe A, Feng Q, Wang Y, Gill D, Paredes R, Prieto-Alhambra D. Clinical and genetic risk factors for Acute Incident venous thromboembolism in ambulatory patients with COVID-19. JAMA Intern Med. 2022 doi: 10.1001/jamainternmed.2022.3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Douillet D, Riou J, Penaloza A, Moumneh T, Soulie C, Savary D, Morin F, Mahieu R, Roy PM. Risk of symptomatic venous thromboembolism in mild and moderate COVID-19: a comparison of two prospective european cohorts. Thromb Res. 2021;208:4–10. doi: 10.1016/j.thromres.2021.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burn E, Duarte-Salles T, Fernandez-Bertolin S, Reyes C, Kostka K, Delmestri A, Rijnbeek P, Verhamme K, Prieto-Alhambra D. Venous or arterial thrombosis and deaths among COVID-19 cases: a european network cohort study. Lancet Infect Dis. 2022;22:1142–1152. doi: 10.1016/S1473-3099(22)00223-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stein PD, Hull RD, Kayali F, Ghali WA, Alshab AK, Olson RE. Venous thromboembolism according to age: the impact of an aging population. Arch Intern Med. 2004;164:2260–2265. doi: 10.1001/archinte.164.20.2260. [DOI] [PubMed] [Google Scholar]
- 29.Gómez CA, Sun CK, Tsai IT, Chang YP, Lin MC, Hung IY, Chang YJ, Wang LK, Lin YT, Hung KC. Mortality and risk factors associated with pulmonary embolism in coronavirus disease 2019 patients: a systematic review and meta-analysis. Sci Rep. 2021;11:16025. doi: 10.1038/s41598-021-95512-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ay C, Posch F, Kaider A, Zielinski C, Pabinger I. Estimating risk of venous thromboembolism in patients with cancer in the presence of competing mortality. J Thromb Haemost. 2015;13:390–397. doi: 10.1111/jth.12825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vakili K, Fathi M, Pezeshgi A, Mohamadkhani A, Hajiesmaeili M, Rezaei-Tavirani M, Sayehmiri F. Critical complications of COVID-19: a descriptive meta-analysis study. Rev Cardiovasc Med. 2020;21:433–442. doi: 10.31083/j.rcm.2020.03.129. [DOI] [PubMed] [Google Scholar]
- 32.Zuin M, Rigatelli G, Bilato C, Zuliani G, Roncon L. Heart failure as a complication of COVID-19 infection: systematic review and meta-analysis. Acta Cardiol. 2022;77:107–113. doi: 10.1080/00015385.2021.1890925. [DOI] [PubMed] [Google Scholar]
- 33.Anderson FA, Jr, Spencer FA. Risk factors for venous thromboembolism. Circulation. 2003;107:I9–16. doi: 10.1161/01.CIR.0000078469.07362.E6. [DOI] [PubMed] [Google Scholar]
- 34.Yeh YT, Tsai SE, Chen YC, Yang SF, Yeh HW, Wang BY, Yeh LT, Shih NC, Wang YH, Chen YY, Yeh CB. Deep venous thrombosis and risk of consequent Sepsis event: a Retrospective Nationwide Population-Based Cohort Study. Int J Environ Res Public Health. 2021;18:7879. doi: 10.3390/ijerph18157879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuo TH, Li HY, Lin SH. Acute kidney injury and risk of deep vein thrombosis and pulmonary embolism in Taiwan: a nationwide retrospective cohort study. Thromb Res. 2017;151:29–35. doi: 10.1016/j.thromres.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 36.Barco S, Voci D, Held U, Sebastian T, Bingisser R, Colucci G, Duerschmied D, Frenk A, Gerber B, Götschi A, Konstantinides SV, Mach F, Robert-Ebadi H, Rosemann T, Simon NR, Spechbach H, Spirk D, Stortecky S, Vaisnora L, Righini M, Kucher N, OVID investigators Enoxaparin for primary thromboprophylaxis in symptomatic outpatients with COVID-19 (OVID): a XXXandomized, open-label, parallel-group, multicentre, phase 3 trial. Lancet Haematol. 2022;9:e585–e593. doi: 10.1016/S2352-3026(22)00175-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cools F, Virdone S, Sawhney J, Lopes RD, Jacobson B, Arcelus JI, Hobbs FDR, Gibbs H, Himmelreich JCL, MacCallum P, Schellong S, Haas S, Turpie AGG, Ageno W, Rocha AT, Kayani G, Pieper K, Kakkar AK, ETHIC investigators Thromboprophylactic low-molecular-weight heparin versus standard of care in unvaccinated, at-risk outpatients with COVID-19 (ETHIC): an open-label, multicentre, randomised, controlled, phase 3b trial. Lancet Haematol. 2022;9:e594–e604. doi: 10.1016/S2352-3026(22)00173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA checklist
Funnel plots for the pooled incidence of (A) acute pulmonary embolism and deep vein thrombosis (DVT) over 8.5 months after COVID-19 infection
Supplementary file 3: Funnel plots for the risk of (A) acute pulmonary embolism and deep vein thrombosis (DVT) over 8.5 months after COVID-19 infection