Abstract
There are no specific and sensitive biomarkers for arteritis, and the occurrence of arteritis in nonclinical toxicological studies of a candidate drug makes development of the drug very difficult. However, we showed in a previous study that the high signal intensity region around the artery on magnetic resonance imaging (MRI) could be a candidate biomarker for detection of arteritis. The present study was conducted to clarify the details of midodrine hydrochloride (MH)-induced arteritis lesions and whether arteritis induced by a mechanism other than the vasodilatory effect, which was evaluated in a previous study, could be detected by MRI. MH is a selective peripherally acting alpha-1 adrenergic receptor agonist, known to induce arteritis due to its vasoconstrictor action, but there is not enough information about MH-induced arteritis. Based on the data obtained under multiple dosing conditions, MH was administered subcutaneously to each rat once daily for 2 days at a dose level of 40 mg/kg/day for MRI assessment. The mesenteric arteries were examined using in vivo MRI at 1 day or 7 days after administration of the final dose and examined histopathologically. On the day after the final dose, high signal intensity region around the artery was observed in animals with minimal perivascular lesions confirmed by histopathology and not observed in an animal without histological changes. On the 7th day after the final dose, no abnormality was observed in histopathological examinations and no high signal intensity regions were observed by MRI in any animal. In conclusion, although further investigation is needed to confirm that high signal intensity is a reliable biomarker for humans, it is suggested that high signal intensity around the artery could be a versatile candidate biomarker with high specificity and sensitivity.
Abbreviations: MH, midodrine hydrochloride; MRI, magnetic resonance imaging; FM, fenoldopam mesylate; FLASH, fast low-angle shot; TR, repetition time; TE, echo time; FOV, field of view; RARE, rapid acquisition with relaxation enhancement; 0.5% CMC, 0.5 w/v% carboxymethyl cellulose solution
Keywords: Arteritis, Biomarker, Drug, Midodrine hydrochloride, Magnetic resonance imaging, Perivascular edema
Graphical Abstract
Highlights
-
•
High signal intensity could be a versatile biomarker in drug-induced arteritis.
-
•
Even a minimal lesion which could fully recovered was detected by MRI.
-
•
High signal intensity could be a highly specific and sensitive candidate biomarker.
-
•
The detail of the lesion in arteritis induced by midodrine was clarified.
-
•
Arteries with no periarterial hard tissue may be a predominant site of arteritis.
1. Introduction
Arteritis is observed in nonclinical toxicological studies of several drugs [8]. Vasculitis including arteritis is also known to be induced by several drug classes including antimicrobials and antithyroid medications in humans [4]. Arteritis is a severe toxicity because the blood shortage due to the artery’s decreased ability to carry blood can result in damage of systemic organ and tissue; on the other hand, the lesion is completely recovered if the offending drug is discontinued or treatment is initiated at an early phase [12]. If arteritis can be detected in an early phase by using appropriate monitoring with a specific and sensitive biomarker, clinical trials of candidate drugs, which arteritis is observed in nonclinical toxicological studies, can be conducted safely. However, there are no specific and sensitive biomarkers [9], [10], and without these biomarkers the occurrence of arteritis in nonclinical toxicological studies of a candidate drug makes development of the drug very difficult. Therefore, developing a biomarker capable of detecting drug-induced arteritis in an early phase is desirable [10]. Appropriate monitoring with biomarkers will alleviate the difficulty of conducting clinical trials of candidate drugs associated with drug-induced arteritis.
Recently, we investigated whether fenoldopam mesylate (FM)-induced arteritis in rats could be detected by magnetic resonance imaging (MRI) [5]. FM is a dopamine agonist, and induces arteritis by vasodilatory effect in rats [3], [16]. In that study, the ex vivo MRI showed low-intensity areas in the arterial wall and a high signal intensity region around the artery; these findings were considered to be due to erythrocytes infiltrating the arterial wall and perivascular edema, respectively. In addition, we also showed that FM-induced arteritis could also be detected by the in vivo MRI and that perivascular edema observed in histopathology could be recognized as a high signal intensity region around the artery on the images of MRI. Based on the results, we consider the high signal intensity region around the artery to be useful for identifying arteritis. However, no other reports have used MRI to evaluate drug-induced arteritis, and it is unknown whether this method could detect arteritis induced by another drug with different pathogenic mechanism. Accordingly, further investigation is needed to verify the versatility and reliability of high signal intensity as a biomarker for arteritis. We thus conducted a study using midodrine hydrochloride (MH), which causes arteritis in rats by a different mechanism from the one causing FM-induced arteritis.
MH is a selective peripherally acting alpha-1 adrenergic receptor agonist that is indicated for the treatment of symptomatic essential hypotension and orthostatic hypotension [2]. Although no relationship between MH and arteritis has been reported in humans, MH is known to induce arteritis of the mesenteric artery in rats through its vasoconstrictor action [15], [14]. Although MH-induced arteritis has been reported in the mesenteric artery, the distribution of lesions in other arteries has not been reported.
In the present study, we aimed to clarify the details of MH-induced arteritis lesions and whether detection of perivascular edema by MRI could be useful for detecting arteritis induced by MH. In other words, the purpose of the present study is to clarify whether arteritis induced by a non-vasodilatory mechanism, which was evaluated previously, could be detected by MRI. First, a study for the dose-finding and evaluation for lesion distribution was performed to determine which dosing routes, dosing periods, and dose levels cause arteritis and which arteries are affected by MH. Then, choosing the dosing regimen based on the results of the dose-finding study, we performed the in vivo MRI assessment to clarify whether the arteritis-related change can be detected by MRI.
2. Materials and methods
2.1. Compounds
MH (the purity was 99.8%) was purchased from Sigma-Aldrich (St. Louis, MO, USA). MH was dissolved in 0.5 w/v% carboxymethyl cellulose solution (0.5% CMC) to reach a concentration of 5, 8, and 10 mg/ml for administration to the 25, 40, and 50 mg/kg/day groups, respectively. Chlorpromazine hydrochloride (the purity was 99% or more), which was used to suppress intestinal peristalsis, was purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan) and dissolved in 0.5 w/v% methyl cellulose solution to reach a concentration of 5 mg/ml.
2.2. Animals and husbandry
All animal studies were approved by the Committee for the Ethical Usage of Experimental Animals of Sumitomo Pharma Co., Ltd., and the Animal Welfare Committee of Osaka University.
Female Sprague-Dawley (Crl:CD) rats were purchased from Charles River Laboratories Japan, Inc. (currently Charles River Laboratories Japan G.K.; Kanagawa, Japan) and allowed an acclimation period of more than 1 week. These rats were housed individually in a barrier-sustained room with controlled temperature of 24 °C ± 2 °C, relative humidity of 55% ± 10%, and a 12-h light (8 a.m. to 8 p.m.)/dark cycle. They were fed a commercial pellet diet (CE-2, CLEA Japan, Ltd., Tokyo, Japan) and tap water ad libitum.
2.3. Animal model and experimental design
2.3.1. Study to find MH dose and evaluate the details of MH-induced arteritis
MH in 0.5% CMC (5 ml/kg) was administered orally, the most common dosing route, to each rat (6 weeks of age at the start of administration) once daily for 2 days at dose levels of 25 or 50 mg/kg/day or 4 days at a dose level of 50 mg/kg/day. We used rat for this study because rat is one of the most important animal models in toxicology and the most frequently used rodent in toxicological study for a candidate drug.
Regarding the induction of arteritis, the systemic route of exposure is considered to be important. The state of the induced lesion can depend on the method of systemic delivery; therefore, in addition to oral administration, we performed subcutaneous administration, which has different systemic consequences. Therefore, MH in 0.5% CMC (5 ml/kg) was administered subcutaneously to each rat (6 weeks of age at the start of administration) once daily for 2 days at a dose level of 25 mg/kg/day or 4 days at a dose level of 40 mg/kg/day.
Animals were divided into five groups (3 animals per group) as shown in Table 1.
Table 1.
Group composition in Study to find MH dose and evaluate the details of MH-induced arteritis.
Scheduled euthanasia was conducted on the day after the final dose by exsanguination under anesthesia and necropsied. In the scheduled euthanized animals, mesenteric, pancreatic, gastrointestinal, renal, and femoral arteries and arteries in the heart were collected from necropsied animals. All collected arteries were histopathologically examined and evaluated in all groups.
2.3.2. MRI assessment study
2.3.2.1. Experiment 1 (in vivo MRI on the day after the final dosing and histopathology)
Based on the results of the dose-finding study, MH in 0.5% CMC (5 ml/kg) was administered subcutaneously to each rat (6 weeks of age at the start of administration) once daily for 2 days at a dose level of 40 mg/kg/day. As a vehicle control, 0.5% CMC only was administered to animals as described above.
Rats, whose weight was 155–170 g at the first dose, were assigned to the MH group (N = 10) and 0.5% CMC group (vehicle control group; N = 5). Variation of body weights were kept as small as possible, considering the possibility that the size of the animal may affect the imaging quality. The MRI examination was conducted on the day after the final dose. The results of study to find MH dose and evaluate the details of MH-induced arteritis (Section 3.1) showed that arteritis-related lesions were most frequently observed in mesenteric arteries. Therefore, mesenteric arteries in all animals of both groups were evaluated by in vivo MRI. On the same day after the MRI examination, these rats were euthanized by exsanguination and necropsied. The mesenteric arteries were collected and evaluated histopathologically.
2.3.2.2. Experiment 2 (in vivo MRI on 7 days after the final dosing and histopathology)
As in Experiment 1, MH was administered subcutaneously to each rat once daily for 2 days at a dose level of 40 mg/kg/day in 0.5% CMC.
Rats, whose weight was 157–173 g at the first dosing, were assigned to the MH group (N = 5). MRI examination was conducted on 7 days after the administration of the final dose. As in Experiment 1, mesenteric arteries in all animals were evaluated by in vivo MRI. On the same day after the MRI examination, these animals were euthanized by exsanguination and necropsied. The mesenteric arteries were collected and evaluated histopathologically.
2.4. MRI
All MRI examinations were conducted using an 11.7 T vertical-bore Bruker Avance II imaging system (Bruker BioSpin, Ettlingen, Germany) and 33 mm volume radiofrequency coil for transmission and reception (Bruker BioSpin). This method is the same as used in the previous study [5].
Rats were fasted more than 12 h and given oral chlorpromazine hydrochloride (25 mg/kg, 5 ml/kg) just before the MRI examination for suppressing intestinal peristalsis. During the in vivo MRI, rats were given 0.5–3% isoflurane for general anesthesia (Wako, Osaka, Japan) administered via a mask covering the nose and mouth. Respiratory signals were monitored using a physiological monitoring system (SA Instruments, Stony Brook, NY, USA), and body temperature was continuously maintained at 36.0 ± 0.5 °C by circulating water through heating pads throughout all experiments.
Anatomical images used for slice positioning were acquired with IntraGate fast low-angle shot (FLASH; Bruker BioSpin) and the following scanning parameters: repetition time/echo time (TR/TE) 62/1.5 msec, flip angle 15°, 60 mm field of view (FOV), matrix of 256 × 256 pixels, and 5 slices with thickness = 1.1 mm. For evaluation the arteritis, images were acquired using rapid acquisition with relaxation enhancement (RARE) with the following scanning parameters: RARE Factor 8, TR/TE 5000/24.8 msec, FOV 35 × 35 mm, matrix of 256 × 256 pixels, 20 slices with thickness = 0.5 mm, and number of excitations = 1. For reducing the signal intensity of fat and minimizing the effects of respiration on the image of MRI, fat suppression and respiratory gating techniques were used.
2.5. Histopathology
All of the collected arteries were fixed in 10% neutral-buffered formalin, embedded in paraffin, sectioned, stained with hematoxylin and eosin, and examined by light microscopy.
2.6. Analytical method
2.6.1. Quantitative evaluation of signal intensity around the arteries on the images of the MRI assessment study in Experiment 1
On the images of MRI, signal intensity around the mesenteric arteries was measured using ImageJ (NIH, Bethesda, MD, USA) in samples obtained in Experiment 1 of the MRI assessment study. The signal intensity around the arteries was defined as a value derived from the formula: signal intensity = intensity around the arteries / intensity of the skeletal muscle around the spinal cord. The intensities of 5 mesenteric arteries were measured for each animal and the mean value of 5 arteries were calculated as the signal intensity of each animal. Since the signal intensities around the arteries in the MH-administered group were not uniform because the lesions were limited to part of artery, the higher signal intensity regions, which were considered to be affected areas, were selected for analysis of the signal intensity of the area.
2.6.2. Statistics
The signal intensity data for each group are expressed as the mean ± standard deviation. A two-tailed non-paired t-test was used to analyze the difference in data between two groups. We considered p-value less than 0.05 as statistically significant.
3. Results
3.1. Study to find MH dose and evaluate the details of MH-induced arteritis
3.1.1. Clinical signs and necropsy
In the group 2 (50 mg/kg/day orally for 2 days) and the group 3 (50 mg/kg/day orally for 4 days), one of three animals was found dead before administration on the second day of dosing.
No deaths were found in the other three groups: group 1 (the 25 mg/kg/day orally for 2 days), group 4 (25 mg/kg/day subcutaneously for 2 days), and group 5 (40 mg/kg/day subcutaneously for 4 days).
Throughout the dosing period, no animal showed clinical signs except for the deaths mentioned above.
At necropsy, mottled white lesions in the kidneys were observed in all animals in the group 5 (40 mg/kg/day subcutaneously for 4 days). In the other groups, no abnormality was observed in any animal.
3.1.2. Histopathology
In all groups, minimal to mild perivascular infiltration of inflammatory cells, proliferation of fibroblasts, and edema were observed in the mesenteric arteries. These changes were also observed in the pancreatic arteries, gastrointestinal arteries, and renal arteries in the group 4 (25 mg/kg/day subcutaneously for 2 days; minimal to mild). In addition, these changes were observed in the pancreatic arteries of the group 3 (50 mg/kg/day orally for 4 days; minimal), gastrointestinal arteries of the group 2 (50 mg/kg/day orally for 2 days; minimal) and renal arteries of the group 1 (25 mg/kg/day orally for 2 days; minimal). In regard to the gastrointestinal arteries, these lesions were mainly observed in arteries in serosa and adipose tissue around the gastrointestinal tract. On the other hand, no abnormality was observed in the femoral arteries and arteries in the heart including the coronary arteries in all groups.
Perivascular infiltration of inflammatory cell, proliferation of fibroblasts, and edema were observed in the mesenteric arteries of 2 of 3 animals in the group 1 (25 mg/kg/day orally for 2 days; minimal), 2 of 2 animals in the group 2 (50 mg/kg/day orally for 2 days; minimal to mild), 2 of 2 animals in the group 3 (50 mg/kg/day orally for 4 days; minimal), 3 of 3 animals in the group 4 (25 mg/kg/day subcutaneously for 2 days; minimal to mild), and 1 of 3 animals in the group 5 (40 mg/kg/day subcutaneously for 4 days; minimal). Details of the histopathology of samples from the study to find MH dose and evaluate the details of MH-induced arteritis are shown in Table 2 and the lesions observed in these arteries are shown in Fig. 1.
Table 2.
Histopathological findings of the study for dose finding and evaluating the description of arteritis induced by midodrine hydrochloride.
 |
* : Number of animals which were examinded histopathologically
-:No abnormality, ± :minimal, + :mild
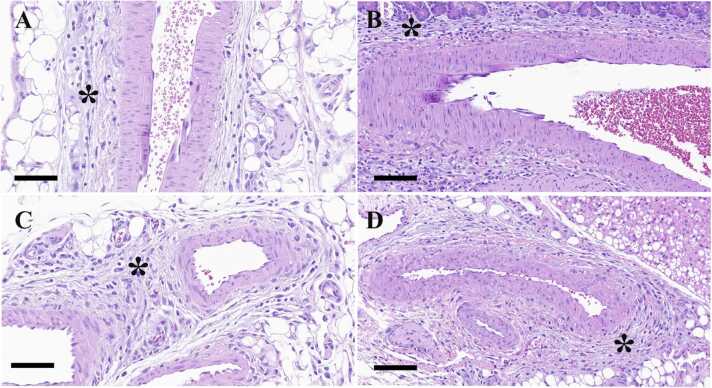
Fig. 1.
Representative histopathological images of affected lesions of the mesenteric, pancreatic, gastrointestinal, and renal arteries stained with hematoxylin and eosin from the study to find MH dose and evaluate the details of MH-induced arteritis. Edema, infiltration of inflammatory cells, and proliferation of fibroblasts were observed in the perivascular area. (A-D) Histopathological images of the arteries after MH administration. (A) Mesenteric artery; Bar, 70 µm. (B) Pancreatic artery; Bar, 100 µm. (C) Gastrointestinal artery; Bar, 70 µm. (D) Renal artery; Bar, 100 µm. Perivascular edema is indicated with asterisks.
Based on the above results, we considered that subcutaneously administered once daily 40 mg/kg/day for 2 days is an optimal dosing regimen and decided to use this regimen for MRI assessment.
3.2. MRI assessment study
3.2.1. Clinical signs and necropsy
Throughout the dosing period, no animal died or showed clinical signs. At necropsy, mottled white lesions in the kidneys were observed in 2 of 10 animals necropsied on the day after the final dose and 2 of 5 animals necropsied on 7 days after administration of the final dose.
3.2.2. Histopathology
3.2.2.1. Experiment 1 (in vivo MRI on the day after the final dosing and histopathology)
In the mesenteric arteries of the MH-administered group, there was minimal degeneration/necrosis of medial smooth muscle cells accompanied by minimal intramural hemorrhage and/or minimal perivascular infiltration of inflammatory cell, proliferation of fibroblasts, and edema. These lesions in the arterial wall and perivascular area were observed in 7 of 10 animals and 9 of 10 animals, respectively. There were no abnormal changes in 1 of 10 animals. Although they were multifocal, these lesions were observed only in a limited area. In the animals with mottled white renal lesions as gross lesions, acute tubular necrosis was observed. In the vehicle control group, no abnormality was observed in any animal. Table 3 describes the histopathology of samples used in the MRI assessment study and the lesions observed in the mesenteric arteries are shown in Fig. 2 (A-D).
Table 3.
Histopathological findings of the MRI assessment study.
-:No abnormality, ±:minimal
No abnormality was observed in 0.5% CMC group
* : Number of animals in each experiment
Fig. 2.
Representative histopathological images of the hematoxylin- and eosin-stained mesenteric arteries from the MRI assessment study. (A-D) Histopathological images of the mesenteric arteries in Experiment 1 (in vivo MRI on the day after the final dosing and histopathology). (A and B) Mesenteric artery in the vehicle control group; Bar, 500 µm in A and 70 µm in B. (C and D) Mesenteric artery in the MH group on the day after the final dose; Bar, 500 µm in C and 70 µm in D. Minimal edema, infiltration of inflammatory cells, and proliferation of fibroblasts can be seen in the perivascular area. In addition, minimal medial smooth muscle cell degeneration and necrosis accompanied by intramural hemorrhage can be seen. Perivascular edema is indicated with asterisks and erythrocytes infiltrating the arterial wall are indicated with arrowheads. (E and F) Histopathological images of the mesenteric arteries in Experiment 2 (in vivo MRI on 7 days after the final dosing and histopathology). Mesenteric artery in the MH group on 7 days after the administration of the final dose; Bar, 500 µm in E and 70 µm in F. There were no findings.
3.2.2.2. Experiment 2 (in vivo MRI on 7 days after the final dosing and histopathology)
Although regeneration of renal tubules, hyaline casts, and dilation of renal tubules were observed in the kidneys with grossly visible mottled white lesions, no abnormality in the mesenteric arteries was observed in any animal on 7 days after the administration of the final dose. Histopathological images of mesenteric arteries are shown in Fig. 2 (E, F).
3.2.3. MRI
3.2.3.1. Experiment 1 (in vivo MRI on the day after the final dosing and histopathology)
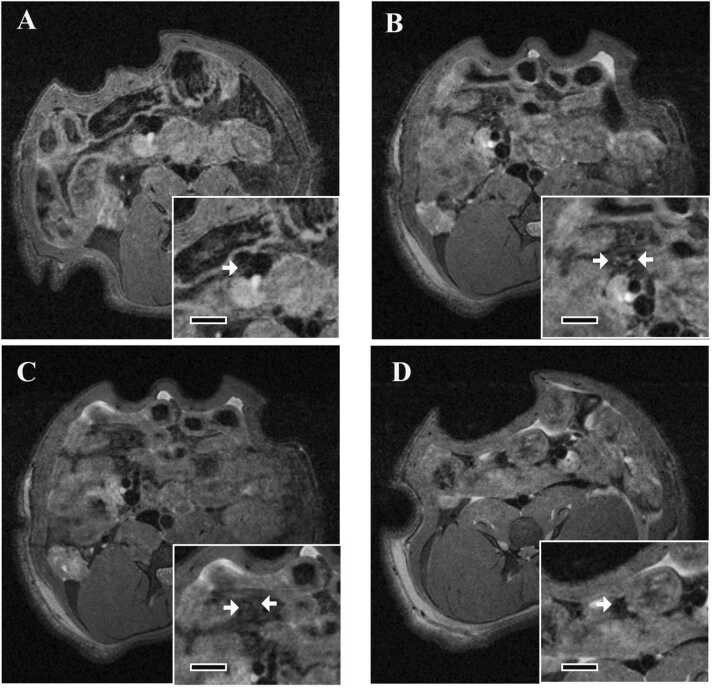
In animals in the vehicle control group, a few mesenteric arteries with low signal intensity were observed (Fig. 3A). In the MH-administered group, high signal intensity region around the mesenteric artery was observed in animals with perivascular lesions confirmed by histopathology and in limited areas (Fig. 3B-C). On the other hand, in one animal without histological changes, the image of MRI was similar to that in the vehicle control group (Fig. 3D).
Fig. 3.
Typical images of MRI of mesenteric arteries in Experiment 1 (in vivo MRI on the day after the final dosing and histopathology) of MRI assessment study. All images are axial images. (A) Image in the vehicle control group. Inserts show cross section of the mesenteric artery at higher magnification. Bar in insert, 3 mm. (B-D) Images in the MH group. Inserts show cross section of the mesenteric artery at higher magnification. Bar in insert, 3 mm. (B and C) These images are acquired from same animal with perivascular lesions in a limited area confirmed by histopathology. C shows a site that is 1 mm caudal to the site in B. High signal intensity region around the mesenteric artery is seen in the limited area, as observed in the image of B but not in the image of C. (D) The image showing the lack of a high signal intensity region is from an animal without histological change. Arteries are indicated by arrows.
3.2.3.2. Experiment 2 (in vivo MRI on 7 days after the final dosing and histopathology)
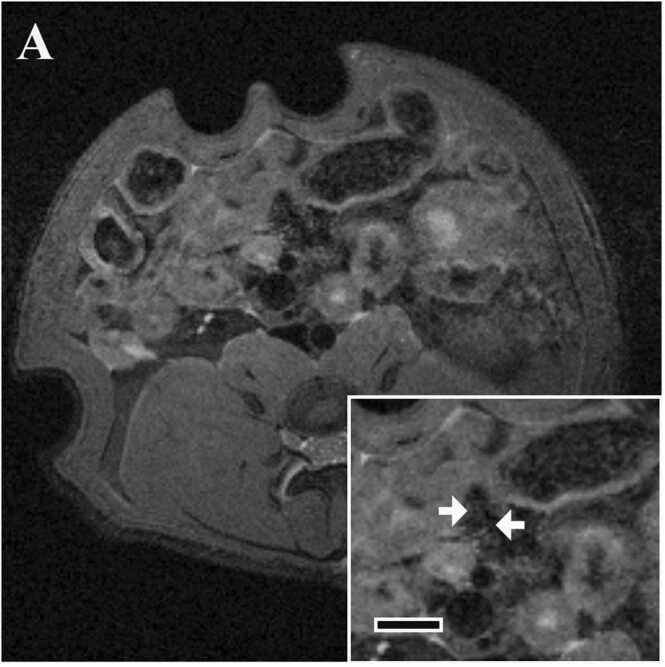
No high signal intensity region was observed around the mesenteric artery in any animal (Fig. 4).
Fig. 4.
Typical images of MRI of mesenteric arteries in Experiment 2 (in vivo MRI 7 days after the final dose and histopathology). Axial image in the MH group 7 days after administration of the final dose. Inserts show cross section of the mesenteric artery at higher magnification. Bar in insert, 3 mm. There is no high signal intensity region. Arteries are indicated by arrows.
3.3. Quantitative evaluation of signal intensity around the arteries on MRI images in Experiment 1
The mean value was 0.62 ± 0.08 and 1.03 ± 0.23 in the vehicle-control and MH-administered groups, respectively, and the difference was statistically significant. The maximum value in the vehicle control group was 0.73, whereas the minimum value in the MH-administered group was 0.85, which revealed that the signal intensity was clearly higher in the MH-administered group.
4. Discussion
To the best of our knowledge, this is the first report of an evaluation of arteritis caused by a vasoconstrictor using MRI. In addition, in the present study, MH-induced arteritis was comprehensively examined. This is also the first reported detailed evaluation of MH-induced arteritis for arteries other than the mesenteric artery.
In the histopathological examination, MH induced arteritis. In MRI, higher signal intensity around the artery was observed in the MH-administered group than the control group. The quantitative analysis of signal intensity also showed significant differences in signal intensity between the two groups. Although the histopathological lesion induced by MH was minimal, high signal intensity regions were observed by MRI in all animals with perivascular edema. On the other hand, the one animal without perivascular edema did not show high signal intensity. Given that perivascular edema is one of the characteristic lesions of arteritis [9], high signal intensity region in MRI could be a versatile biomarker for detecting the arteritis with high specificity and high sensitivity.
Arteritis caused by the vasoconstrictor action of MH, which is different from that caused by the vasodilatory action of FM, could also be detected by MRI by finding a high signal intensity region. Although the histopathological severity of MH-induced arteritis, including perivascular edema, was less than that of FM-induced arteritis and the MH-induced changes were fully resolved, MH-induced arteritis could be detected by MRI. In addition, the high signal intensity by MRI correlated with histopathologic changes, even minimal changes. The results of our previous and present studies suggest that, regardless of pathogenic mechanism and degree of changes, MRI can detect perivascular edema as a sign of arteritis and be used to detecting arteritis without performing histopathological examination.
In all animals evaluated on 7 days after the administration of the final dose, no lesion was observed in the histopathological examination, suggesting that the lesion had fully resolved during the 6-day recovery period. When the histopathological lesion disappeared, the high signal intensity region was no longer observed by MRI. This suggests that the presence or absence of a high signal intensity region in MRI can be a useful criterion to confirm the arteritis is recovered or not as an indicator of perivascular edema.
Arteritis is known to be fully recovered if the lesion was minimal [12] and changes were also fully resolved in this study. Even a minimal lesion, which disappeared over time, was detected in rats by MRI by finding a high signal intensity region, suggesting that minimal lesions could be detected in the clinical study. In other words, it could be possible to judge the discontinuation of administration of a drug in the phase of minimal lesion, which can be completely resolved. This is extremely useful for conducting clinical trials of drugs that may cause arteritis.
Histopathological examination revealed that MH induced arteritis in the mesenteric, pancreatic, gastrointestinal, and renal arteries in rats, most frequently in mesenteric arteries. On the other hand, no abnormality was observed in the femoral arteries and arteries in the heart.
In the previous study, administration of FM, which is a dopamine (D1 receptor) agonist and causes arteritis due to its vasodilatory action, also induced arteritis in the mesenteric, pancreatic, and gastrointestinal arteries in rats [5]. Although arteritis in the renal arteries was not induced by subcutaneous administration in the previous study, it was induced by intravenous administration of FM for 24 h [7], [16]). Since D1 receptor is expressed on the renal artery [1], the presence or absence of renal artery lesions may depend on the duration of vasodilatory effects by FM. In addition, alpha 1 receptor, on which midodrine acts, is also known to be expressed on the renal artery [13] and arteritis induced by MH was observed in renal arteries of some animals in this study.
In our studies, arteritis was commonly induced in the mesenteric, pancreatic, and gastrointestinal arteries by MH and FM, suggesting that these three arteries may be the predominant site of arteritis in rats. Although alpha 1 receptor is also expressed in femoral and coronary arteries, and D1 receptor is also expressed in coronary and other arteries in the heart [6], [11], [17], no lesions were observed in these arteries in our studies using MH- and FM-administered animals (Supplemental Table 1 shows the data of FM-administered animals). Considering that the main lesion in the gastrointestinal arteries was observed in arteries in serosa and adipose tissue around the gastrointestinal tract, a common point of these affected arteries is that the surrounding tissues, such as adipose tissue and pancreatic tissue, are soft. Therefore, we consider that arteries without hard surrounding tissues are vulnerable to excessive vasodilatory/vasoconstrictor action and therefore becomes predominant sites. No hard tissue around the renal artery, where arteritis was induced, is also consistent with this consideration.
Given that the LD50 of subcutaneously injected MH in female rats is 51 mg/kg in the interview form of Metligine (trade name of midodrine hydrochloride), 40 mg/kg used for MRI assessment is close to the maximum tolerated dose. However, the lesion induced by MH was less severe than that induced by FM; the extent of the lesions including perivascular edema in MH-administered animals was minimal, while that in FM-administered animals ranged from minimal to moderate [5]. As for the perivascular edema, the extent was moderate in most FM-administered animals. As regard to the mesenteric artery lesions, histopathological changes in the arterial wall such as intramural hemorrhage were observed in all FM-administered animals, but in only 7 out of 10 MH-administered animals, and no lesions including periarterial lesions were observed in 1 of 10 MH-administered animals. It has also been reported that hemorrhage is rare in MH-administered animals [15]. Therefore, we consider that the frequency of arteritis-associated hemorrhage occurrence may be higher with vasodilators. As a histopathological difference other than arteries, no lesions were observed in the kidney in FM-administered animals, but renal acute tubular necrosis related to ischemia due to vasoconstriction was observed in some MH-administered animals.
In conclusion, our results indicated that arteritis induced by vasoconstrictor action, in addition to arteritis induced by vasodilatory action as previously reported, can be detected using in vivo MRI. Furthermore, we also showed that high signal intensity around the artery could be a highly specific and sensitive candidate biomarker. However, further investigation is needed to confirm that high signal intensity is a reliable biomarker in humans. As part of that, we currently are considering assessing this method using dogs or monkeys with drug-induced arteritis.
Funding
This work was supported by Sumitomo Pharma Co., Ltd.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We wish to thank Yumi Tateishi for her histotechnical assistance to this work.
Handling Editor: Prof. L.H. Lash
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.toxrep.2023.01.001.
Appendix A. Supplementary material
Supplementary material.
.
Supplementary material.
.
Data Availability
Data will be made available on request.
References
- 1.Amenta F., Barili P., Bronzetti E., Felici L., Mignini F., Ricci A. Localization of dopamine receptor subtypes in systemic arteries. Clin. Exp. Hypertens. 2000;22:277–288. doi: 10.1081/ceh-100100077. [DOI] [PubMed] [Google Scholar]
- 2.Cruz D.N. Midodrine: a selective alpha-adrenergic agonist for orthostatic hypotension and dialysis hypotension. Expert Opin. Pharmacother. 2000;1:835–840. doi: 10.1517/14656566.1.4.835. [DOI] [PubMed] [Google Scholar]
- 3.Dalmas D.A., Scicchitano M.S., Mullins D., Hughes-Earle A., Tatsuoka K., Magid-Slav M., Frazier K.S., Thomas H.C. Potential candidate genomic biomarkers of drug induced vascular injury in the rat. Toxicol. Appl. Pharmacol. 2011;257:284–300. doi: 10.1016/j.taap.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 4.Doyle M.K., Cuellar M.L. Drug-induced vasculitis. Expert Opin. Drug Saf. 2003;2:401–409. doi: 10.1517/14740338.2.4.401. [DOI] [PubMed] [Google Scholar]
- 5.Fujii Y., Yoshino Y., Chihara K., Nakae A., Enmi J., Yoshioka Y., Miyawaki I. Detection of fenoldopam-induced arteritis in rats using ex vivo / in vivo MRI. Toxicol. Rep. 2022;9:1595–1602. doi: 10.1016/j.toxrep.2022.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kayki-Mutlu G., Papazisi O., Palmen M., Danser A.H.J., Michel M.C., Arioglu-Inan E. Cardiac and vascular alpha1-adrenoceptors in congestive heart failure: a systematic review. Cells. 2020:9. doi: 10.3390/cells9112412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kerns W.D., Arena E., Macia R.A., Bugelski P.J., Matthews W.D., Morgan D.G. Pathogenesis of arterial lesions induced by dopaminergic compounds in the rat. Toxicol. Pathol. 1989;17:203–213. doi: 10.1177/019262338901700116. [DOI] [PubMed] [Google Scholar]
- 8.Louden C., Brott D., Amuzie C.J., Bennet B., Chamanza R. In: Toxicologic pathology: Nonclinical Safety Assessment, Second Edition. Sahota P.S., Popp J.A., Hardisty J.F., Gopinath C., Bouchard P.R., editors. CRC Press; Florida: 2019. The Cardiovascular System; pp. 743–825. [Google Scholar]
- 9.Louden C., Brott D., Katein A., Kelly T., Gould S., Jones H., Betton G., Valetin J.P., Richardson R.J. Biomarkers and mechanisms of drug-induced vascular injury in non-rodents. Toxicol. Pathol. 2006;34:19–26. doi: 10.1080/01926230500512076. [DOI] [PubMed] [Google Scholar]
- 10.Mikaelian I., Cameron M., Dalmas D.A., Enerson B.E., Gonzalez R.J., Guionaud S., Hoffmann P.K., King N.M., Lawton M.P., Scicchitano M.S., Smith H.W., Thomas R.A., Weaver J.L., Zabka T.S. Nonclinical safety biomarkers of drug-induced vascular injury: current status and blueprint for the future. Toxicol. Pathol. 2014;42:635–657. doi: 10.1177/0192623314525686. [DOI] [PubMed] [Google Scholar]
- 11.Ozono R., O'Connell D.P., Vaughan C., Botkin S.J., Walk S.F., Felder R.A., Carey R.M. Expression of the subtype 1A dopamine receptor in the rat heart. Hypertension (Dallas. Tex.: 1979) 1996;27:693–703. doi: 10.1161/01.hyp.27.3.693. [DOI] [PubMed] [Google Scholar]
- 12.Radić M., Martinović Kaliterna D., Radić J. Drug-induced vasculitis: a clinical and pathological review. Netherlands J. Med. 2012;70:12–17. [PubMed] [Google Scholar]
- 13.Schmitz J.M., Graham R.M., Sagalowsky A., Pettinger W.A. Renal alpha-1 and alpha-2 adrenergic receptors: biochemical and pharmacological correlations. J. Pharmacol. Exp. Therap. 1981;219:400–406. [PubMed] [Google Scholar]
- 14.Thomas R.A., Scicchitano M.S., Mirabile R.C., Chau N.T., Frazier K.S., Thomas H.C. MicroRNA changes in rat mesentery and serum associated with drug-induced vascular injury. Toxicol. Appl. Pharmacol. 2012;262:310–320. doi: 10.1016/j.taap.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 15.Tobin G.A., Zhang J., Goodwin D., Stewart S., Xu L., Knapton A., Gonzalez C., Bancos S., Zhang L., Lawton M.P., Enerson B.E., Weaver J.L. The role of eNOS phosphorylation in causing drug-induced vascular injury. Toxicol. Pathol. 2014;42:709–724. doi: 10.1177/0192623314522885. [DOI] [PubMed] [Google Scholar]
- 16.U.S. Food and Drug Administration. Drug Approval Package: CORLOPAM (fenoldopam mesylate). Drugs@FDA. Silver Spring, MD. Available from: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=019922.
- 17.Zacharia J., Hillier C., Tanoue A., Tsujimoto G., Daly C.J., McGrath J.C., MacDonald A. Evidence for involvement of alpha1D-adrenoceptors in contraction of femoral resistance arteries using knockout mice. Br. J. Pharmacol. 2005;146:942–951. doi: 10.1038/sj.bjp.0706395. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.
Supplementary material.
Data Availability Statement
Data will be made available on request.