Estimates of how much energy a female needs to reproduce successfully are required to understand the impact of environmental perturbations on marine mammal populations. We review the state of knowledge and data availability on reproductive energetics, highlighting key data gaps to help refine future estimates from bioenergetic models.
Abstract
Reproductive costs represent a significant proportion of a mammalian female's energy budget. Estimates of reproductive costs are needed for understanding how alterations to energy budgets, such as those from environmental variation or human activities, impact maternal body condition, vital rates and population dynamics. Such questions are increasingly important for marine mammals, as many populations are faced with rapidly changing and increasingly disturbed environments. Here we review the different energetic costs that marine mammals incur during gestation and lactation and how those costs are typically estimated in bioenergetic models. We compiled data availability on key model parameters for each species across all six marine mammal taxonomic groups (mysticetes, odontocetes, pinnipeds, sirenians, mustelids and ursids). Pinnipeds were the best-represented group regarding data availability, including estimates of milk intake, milk composition, lactation duration, birth mass, body composition at birth and growth. There were still considerable data gaps, particularly for polar species, and good data were only available across all parameters in 45% of pinniped species. Cetaceans and sirenians were comparatively data-poor, with some species having little or no data for any parameters, particularly beaked whales. Even for species with moderate data coverage, many parameter estimates were tentative or based on indirect approaches, necessitating reevaluation of these estimates. We discuss mechanisms and factors that affect maternal energy investment or prey requirements during reproduction, such as prey supplementation by offspring, metabolic compensation, environmental conditions and maternal characteristics. Filling the existing data gaps highlighted in this review, particularly for parameters that are influential on bioenergetic model outputs, will help refine reproductive costs estimated from bioenergetic models and better address how and when energy imbalances are likely to affect marine mammal populations.
Introduction
Bioenergetic models provide valuable insight into conservation concerns facing many marine mammal populations, such as climate change, prey availability and disturbance from human activities (e.g. Villegas-Amtmann et al., 2015; Reimer et al., 2019; Silva et al., 2020; Gallagher et al., 2021). They also have broader ecosystem applications since results can be used to inform the integration of marine mammals into ecosystem-based fisheries management (Chasco et al., 2017; Goedegebuure et al., 2017; McHuron et al., 2020). Modeling approaches range from static “accounting” models, where the primary goal is often to estimate individual- and population-level prey consumption, to more dynamic approaches that simulate the allocation of energy to different processes (e.g. metabolism, growth, reproduction) to examine population-level processes, often within an individual-based modeling framework (Pirotta, 2022).
Reproductive females are a focal group of many bioenergetic approaches. This is largely because survival of reproductive females and reproductive rates are key drivers of marine mammal population dynamics (Blázquez et al., 2020; Fruet et al., 2021; Luck et al., 2022). Reproduction is also energetically expensive, meaning that females (and their offspring) may be more susceptible to perturbations in energy balance during this time. An inability to obtain sufficient prey or energy reserves may result in abortion or changes in lactation duration (e.g. Trillmich and Limberger, 1985; Pitcher et al., 1998; Wasser et al., 2017), skipped reproductive events (e.g. Marsh and Kwan, 2008; Gailey et al., 2020), or in extreme cases, female mortality (Chinn et al., 2016). For example, end-lactation syndrome, mortality due to depletion of energy reserves during lactation, has been observed more frequently in southern sea otters (Enhydra lutris) from resource limited populations compared with nonresource limited ones (Chinn et al., 2016). In addition, females often comprise a large proportion of a population’s total prey consumption, in part due to the high cost of lactation (McHuron et al., 2020), making them an important group to model in terms of their potential impacts on food web dynamics.
Accurate characterization of the role of reproductive females in food webs and the impacts of natural and anthropogenic stressors on this demographic group requires an understanding of the energetic costs of reproduction at various temporal scales. The reproductive energetics of pinnipeds has been extensively studied, albeit in a limited number of species. Their ease of handling and ability to house them in human care has led to comprehensive studies on energy expenditure, mass dynamics, and milk composition and intake (see Gestation and Lactation below). Knowledge on reproductive energetics for the less specious marine mammal groups (ursids, sirenians, and mustelids) is variable, ranging from little knowledge in sirenians to more extensive knowledge in polar bears (Ursus maritimus; Derocher et al., 1993; Arnould and Ramsay, 1994; Hedberg et al., 2011). Comparatively little is known of the reproductive energetics of cetaceans, the most specious group of marine mammals. This is mostly due to their large body size and fully aquatic nature, since many studies on reproductive energetics require some form of animal handling. While there are numerous cetacean bioenergetic models that have estimated the costs of reproduction, it has been some time since there has been a review of cetacean reproductive energetics or the parameters that influence the cost of reproduction (Perrin and Reilly, 1984; Oftedal, 1997). There is a need to revisit available data given the accumulation of data from new studies, many of which use recent technological advancements to collect data from free-ranging animals, and the recent identification of lactation energetics as a key unanswered question for bioenergetic applications to marine mammal management and conservation (McHuron et al., 2022a).
Here we provide a review of the current state of knowledge on the reproductive energetics of marine mammals, focusing on the specific costs of gestation and lactation and how these costs are estimated in traditional bioenergetic models (i.e. not dynamic energy budget [DEB] models). We also review data on food consumption during reproduction because, although it is the manifestation of the cumulative sum of an individual's current (and potentially future) energy needs, food consumption can provide useful information about reproductive costs and the existence of potential energetic compensatory mechanisms. A primary goal of this review was to compile existing data on the parameters used to estimate reproductive costs in marine mammals to not only highlight data gaps, but also provide a useful resource for researchers in parameterizing bioenergetic models. While we covered all taxonomic groups, we focused particular effort toward cetaceans given previous reviews on pinnipeds (Lavigne et al., 1982; Oftedal et al., 1987a; Costa, 1991; Costa and Valenzuala-Toro, 2021; Costa and Maresh, 2022) and the clear need to better understand the reproductive energetics of cetaceans.
Data Availability
To compile data availability on common parameters used in marine mammal bioenergetic models, we performed literature searches using Google Scholar and keywords relevant to each parameter and species. We also searched review articles on reproductive energetics and common marine mammal reference material (e.g. Encyclopedia for Marine Mammals) for values and their original sources. We traced the literature back to the source where the original estimate was obtained; in many cases, this proved to be a highly circuitous route as many papers cited other papers that were also not original sources. It was not always possible to obtain a copy of what was assumed to be the original source; when this occurred, we note this in the supplemental tables and provide both the citing research and source citation. We recommend caution in applying these values without accessing the original data sources. The exception to this was when the citation was to a known review, such as a book chapter, because in our experience these often yielded uncited values or reference to other sources. To avoid perpetuating erroneous or poorly informed parameters and overstating data availability, we did not include parameter values for which no citation was given. We also did not include every single reference for a given parameter and species, as this was not the goal of the review. While this effort was thorough, there is a vast amount of gray literature on marine mammals, and we recognize that it is possible that we missed data. We used the Society for Marine Mammalogy’s Taxonomy List of Marine Mammal Species and Subspecies, updated in June 2021, for species classification (https://marinemammalscience.org/science-and-publications/list-marine-mammal-species-subspecies/). On this list there were 15 extant species of mysticetes, 77 odontocetes (with one included as possibly extinct), 18 phocids, 14 otariids, 1 odobenid, 2 mustelids, 4 sirenians and 1 ursid.
Gestation
Energetic costs during gestation are incurred because the female must invest energy in creating and maintaining the fetus and other tissues associated with pregnancy. Physiological changes during pregnancy are not well documented for marine mammals, but in other mammals include changes in the circulatory (e.g. increased blood volume, changes in cell counts and platelet production), musculoskeletal (e.g. increased bone turnover) and renal systems (Soma-Pillay et al., 2016; Torres et al., 2018), in addition to the necessary changes to the reproductive system (e.g. development of the placenta, mammary tissue). The metabolic cost of pregnancy is referred to as the heat increment of gestation (HIG), defined as the amount of extra heat produced during gestation above nongestation levels. The gestation costs discussed in this section, the HIG and the energy stored in the fetus and associated tissues, do not include the energy needed by the female for her own metabolic needs (outside of the HIG), as while they are incurred during pregnancy, they are not a cost of gestation per se.
Pinnipeds, polar bears and sea otters exhibit embryonic diapause (delayed implantation) that occurs following conception, which considerably slows embryonic growth (Daniel, 1971). Diapause has not yet been documented in marine otters (Lontra felina) but is likely given its prevalence in other mustelids, including the closely related North American river otter (L. canadensis) and sea otter (Sinha et al., 1966; Thom et al., 2004). Diapause lasts anywhere from 1.5 to 8 months, depending on species (Sinha et al., 1966; Boyd, 1991; Atkinson, 1997). Most bioenergetic models do not consider this time of “nonactive” gestation when estimating energy budgets since any costs are likely negligible.
The most common approach for estimating the HIG in marine mammal bioenergetic models is the formula of Brody, 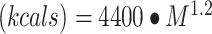 , where
, where 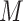 is the mass at birth in kg (Brody, 1938). This equation was derived using data from domesticated animals (rats, chickens, rabbits, pigs, cows, goats and horses) and humans, and represents the total HIG. Despite its widespread use, Brody (1938) noted that the numerical constants of his equation were tentative and subject to further revision given the limited number of individuals the equation was derived from (all but one species had data from one to six individuals). To our knowledge, such a revision was never undertaken. Other equations, as noted by Yunker et al. (2005), include those of Moen (1973) for ruminants and Robbins (1983) for ungulates. We do not provide any discussion or elaboration of these two equations given they are rarely used in the marine mammal literature, and we were unable to access the original sources. One alternate approach that has been used to estimate the HIG includes incorporating estimates of fetal mass into maternal mass, which is then used to estimate maternal metabolic demands (Hin et al., 2019).
is the mass at birth in kg (Brody, 1938). This equation was derived using data from domesticated animals (rats, chickens, rabbits, pigs, cows, goats and horses) and humans, and represents the total HIG. Despite its widespread use, Brody (1938) noted that the numerical constants of his equation were tentative and subject to further revision given the limited number of individuals the equation was derived from (all but one species had data from one to six individuals). To our knowledge, such a revision was never undertaken. Other equations, as noted by Yunker et al. (2005), include those of Moen (1973) for ruminants and Robbins (1983) for ungulates. We do not provide any discussion or elaboration of these two equations given they are rarely used in the marine mammal literature, and we were unable to access the original sources. One alternate approach that has been used to estimate the HIG includes incorporating estimates of fetal mass into maternal mass, which is then used to estimate maternal metabolic demands (Hin et al., 2019).
Body mass at birth is the only parameter required to estimate the HIG from Brody's equation. Estimates of body mass at birth (or length that can be converted to body mass) exist for 100% (odobenids, otariids, ursids), 94% (phocids), 75% (sirenians), 66% (mysticetes), 64% (odontocetes) and 50% (mustelids) of marine mammal species (Supplementary Tables S1-S5). Since the resulting value represents costs across the entire gestation period, many studies convert this to a daily cost using fetal growth curves (Pirotta et al., 2018; McHuron et al., 2021) or gestation duration (Gallagher et al., 2018), the latter of which assumes linear increases in the HIG. Fetal growth curves are available for 100% (odobenids), 53% (mysticetes), 39% (phocids), 18% (odontocetes), 14% (otariids) and 0% (sirenians, mustelids, ursids) of marine mammal species (Supplementary Tables S6-S10). Increases in fetal size with age typically follows an exponential pattern (e.g. Trites, 1991; Robeck et al., 2015; Lanzetti et al., 2020), resulting in relatively small energetic costs throughout much of pregnancy, only increasing in the later stages of gestation. For example, estimated gestation costs for southern right whales (Eubalaena australis) were 0 MJ day−1 at conception and 41 MJ day−1 at the end of second trimester, but increased to 725 MJ day−1 by the end of gestation (Christiansen et al., 2022b).
The energy stored in fetal tissue is typically calculated based on mass at birth and estimates of the chemical composition of the fetus. As above, these costs can be extrapolated to daily costs using fetal growth curves to calculate the amount of new mass added daily. In cetaceans, fat deposition increases as gestation progresses, both in terms of relative blubber deposition and the lipid content of blubber and muscle (Lockyer, 1993; Borrell et al., 1995; Struntz et al., 2004). For example, the percent blubber content of long-finned pilot whale (Globicephala melas) fetuses increased from zero during early gestation to an estimated 71% at birth (Borrell et al., 1995). Bottlenose dolphin (Tursiops truncatus) fetuses close to parturition had one of the thickest blubber layers (relative to body size) across all life history categories, which may help newborn dolphins thermoregulate and maintain buoyancy (Struntz et al., 2004). The energetic content of harp seal (Pagophilus groenlandica) pups increased rapidly in the last two months of gestation (Stewart et al., 1989), indicating that pinnipeds also exhibit increases in fat deposition toward the end of gestation despite additional thermoregulatory tissues (fur) and their semi-aquatic nature. In support of this, the lipid content in blubber of ringed seal (Pusa hispida) and California sea lion (Zalophus californianus) fetuses increased with fetal age (Greig et al., 2007; Brown et al., 2016). Similar patterns in fat deposition have been detected in some other mammals, such as guinea pigs, pigs and humans (Engle and Lemons, 1986; McPherson et al., 2004; Toro-Ramos et al., 2015).
Since there are very few estimates of fetal or neonatal body composition for marine mammals, most bioenergetic models do not account for the change in body composition during fetal growth, instead using body composition at birth (or near birth) to estimate energy storage. Estimates of fat and protein content of full-term fetuses or newborn phocid pups range from 3.0% to 14.0% and from 18.6% to 23.1%, respectively (Supplementary Table S11). Newborn or late-term cetacean fetuses are comprised of 22.0% to 43.4% blubber, 13.1% to 17.6% muscle and 12.0% to 14.5% viscera, although these ranges are based on a very limited number of species and individuals (Supplementary Table S11). Lipid composition of blubber ranges from 42.3% to 79.2% (Gauthier et al., 1998; Struntz et al., 2004; Dunkin et al., 2005; Pedro et al., 2017).
The inclusion of energy storage in other pregnancy-related tissues in bioenergetic models is variable, with some including these costs and others omitting it. Data from pinnipeds indicate that the vast majority (90–95%) of energy storage during gestation is in the fetus itself (Anderson and Fedak, 1987; Lydersen, 1995). Lavigne and Stewart (1979) noted that the energy density of harp seal placenta was 1.13 kcal g−1 (4.73 MJ kg−1) wet weight which, when combined with the mass of the placenta, resulted in a total energy content of 1430 kcal (5.98 MJ), roughly 5.8% of the energy stored in the fetus. The energy density of ringed seal placenta was similar at 3.75 MJ kg−1, for a total cost of 1.3 MJ (Lydersen, 1995). Boyd (1990) and Yunker et al. (2005) both provide equations for the relationship between placental mass and fetal mass, although they are for a single species (gray seals Halichoerus grypus), and it is unknown whether this relationship would hold outside the mass range of their study.
Gestation may affect energy demands through other pathways, but these costs have not been well quantified for marine mammals. For example, pregnant bottlenose dolphins experienced increased drag due to changes in body morphology that affected frontal surface area (Noren et al., 2011). Using fluid dynamics modeling, Nousek McGregor (2010) predicted that pregnant North Atlantic right whales (Eubalaena glacialis) have increased locomotor costs due to a 3% to 4% increase in drag caused by changes in body morphology. In deep-diving northern elephant seals (Mirounga angustirostris), pregnant females exhibited shorter dive durations after the 3rd month of pregnancy, potentially because of increased fetal oxygen needs. Such changes in diving behavior have the potential to affect energy balance since they limit the amount of time seals can spend at depth foraging (Hückstädt et al., 2018).
Lactation
Total and daily costs of lactation vary drastically among marine mammals. Energetic investment in offspring is driven in part by differences in body size, which ranges from < 5 kg for a female marine otter (Soto-Azat et al., 2011) to upward of 150 000 kg for an adult female blue whale (Balaenoptera musculus, Lockyer, 1976). Other factors, such as lactation duration and lactation strategy (i.e. where the animal falls on the capital-income spectrum), also drive interspecific variation in lactation costs. For example, costs of lactation in hooded seals (Cystophora cristata), which have the shortest lactation duration of any mammal at four days, were on average 756 MJ or 14 MJ day−1 kg-0.83 (Oftedal et al., 1993). This contrasts with otariid seals, such as Antarctic fur seals (Arctocephalus gazella) where total lactation costs were 813 to 1064 MJ or about 1.6 MJ day−1 kg-0.83 across the roughly 4-month lactation period (Arnould, 1997). Delivering high daily amounts of energy to offspring is more efficient than less energy delivered over a prolonged time because a female has to cover the metabolic overhead of her offspring throughout lactation (Boness and Bowen, 1996; Costa and Maresh, 2022). Many species do not, however, have the reserve capacity to achieve such high rates of energy transfer.
The cost of lactation is largely comprised of the energy contained within the milk itself. A female may also expend metabolic energy to produce the milk, but these costs appear to be negligible in many marine mammals. Using doubly labeled water (DLW), Costa and Trillmich (1988) and Costa and Gentry (1986) found no difference in onshore field metabolic rates of lactating and nonlactating Antarctic and northern fur seals (Callorhinus ursinus), suggesting negligible costs associated with milk production. While it is possible that any increased costs were obscured by measurement error or because milk production costs were incurred outside the measurement interval (i.e. at sea), similar results were found between resting metabolic rates of lactating and nonlactating female California sea lions in human care (Williams et al., 2007). In contrast, sea otters do exhibit a metabolic cost of milk production, as evidenced by increases in resting metabolic rate above nonreproductive levels in the second month following parturition that remained elevated throughout lactation (Thometz et al., 2016a). Disparities between sea otters and pinnipeds may be due to an increased need by sea otters for de novo synthesis of fatty acids and because of their limited capacity to store energy reserves, since the fatty acids present in blood lipids of fasting seals are nearly identical to those in milk (Riedman and Ortiz, 1979; Costa and Trillmich, 1988; Thometz et al., 2016a). As with gestation, we do not include further discussion of the female's metabolic overhead during lactation since it is not a cost of lactation per se (but see Maternal food intake during gestation and lactation).
The cost of lactation depends on the energy density of milk (determined by milk composition), the rate of milk consumption by offspring, and the duration of lactation. There are a variety of ways that lactation costs are estimated in bioenergetic models, largely due to data availability and model complexity. Some studies take a ‘top-down’ approach, where lactation costs are estimated based on the female’s output, such as milk output (or milk intake by offspring), body mass changes (only in capital breeders), or differences in food intake between reproductive and nonreproductive animals (e.g. Christiansen et al., 2016; Bejarano et al., 2017; Gallagher et al., 2018; McHuron et al., 2020). The alternate ‘bottom-up’ approach sums the costs experienced by offspring to determine maternal investment, such as metabolic rates, growth, and waste production (e.g. Lockyer, 1981; Fortune et al., 2013; Villegas-Amtmann et al., 2015). In the following sections, we provide an overview of the data availability and general patterns for the key parameters used to estimate lactation costs in bioenergetic models. We do not review offspring metabolic rates; this is a large topic unto itself, and metabolic rates are covered in a separate review in this special issue (Noren in review). Additional discussion of many of the parameters described below can be found in various reviews by Olav Oftedal (Oftedal et al., 1987a; Oftedal, 1993; Oftedal et al., 1996; Oftedal, 1997, 2000).
Milk composition and energy density
Proximate composition of milk has been determined in 100% (otariids, odobenids, ursids), 55% (phocids), 53% (mysticetes), 50% (mustelids), 26% (odontocetes) and 25% (sirenians) of species (Supplementary Tables S12-S15). Fat composition of marine mammal milk is extremely variable, ranging from < 10% to nearly 60% (Fig. 1, Supplementary Tables S12–S15). While phocids have the highest mean milk fat content of any marine mammal, the range of values almost entirely overlaps with that of otariids. The average milk fat composition of females otariids with newborn pups (28.2–38.6%) is within the range observed for phocids (16.1–40.8%), excluding hooded seals that have milk fat contents above 50% (Oftedal et al., 1988; Werner, 1996; Arnould and Hindell, 1999; Mellish et al., 1999b; Georges et al., 2001). It is difficult to draw comparisons for most other groups given small samples sizes and the general lack of good temporal coverage in sample collection, which has a considerable effect on milk fat composition (e.g. Derocher et al., 1993; Mellish et al., 1999a; Donohue et al., 2002; Lang et al., 2005; West et al., 2007). In otariids, milk composition changes not only across the lactation interval but also across a nursing visit (Costa and Gentry, 1986; Georges et al., 2001). In the only cetacean study to examine temporal variation, West et al. (2007) found that milk fat estimates from bottlenose dolphins never exceeded 25.2%, suggesting that at least some odontocetes have lower milk fat compositions than pinnipeds and mysticetes. Milk protein composition is much less variable, with most values between 8% and 11% across all marine mammal taxonomic groups.
Figure 1.
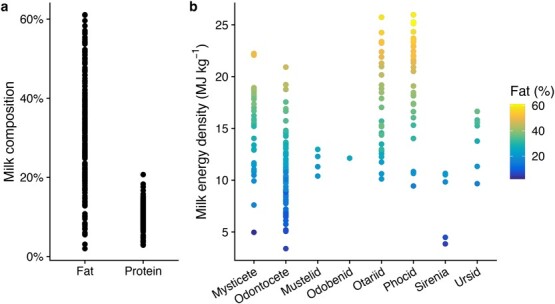
Fat and protein composition (a) and energy density (b) of marine mammal milk. In b, milk energy density was calculated using fat and protein compositions and conversion factors of 39.3 MJ kg−1 (fat) and 24.5 MJ kg−1 (protein). See Supplementary Tables S12-S15 for values and sources.
Milk energy density can be measured directly using bomb calorimetry or calculated based on the proximate composition of milk and the energy density of protein and fat. Other components of milk, such as carbohydrates, are often not included in energy density estimates since they comprise a relatively small proportion (often < 1%) of marine mammal milk and thus are not always measured (Oftedal, 1993; West et al., 2007; Eisert et al., 2013). Energy density values derived using proximate composition are highly correlated with those from bomb calorimetry (Oftedal et al., 2014). Based on conversion factors (39.3 MJ kg−1 for fat and 24.5 MJ kg−1 for protein), energy densities of marine mammal milk typically range from 3.4 to 26.0 MJ kg−1 (Fig. 1). Energy density of individual samples may in some cases exceed this range, as we primarily used study averages to calculate these values. Since protein composition is broadly similar across all marine mammals, variation in milk energy density is primarily driven by fat composition.
Milk intake rates
Milk intake has been measured in 100% (ursids), 43% (otariids) and 39% (phocids) of species using labeled water (Supplementary Table S16). Zhang et al. (2014) report milk intake in a captive spotted seal (Phoca largha) fed expressed milk, bringing the total for phocids to 44%. Milk intake rates vary from an average of 0.23 to 0.68 kg day−1 in polar bears, 0.35 (reported in mL day−1) to 2.1 kg day−1 in otariids, and 1.2 to 10.4 kg day−1 in phocids. Pinniped milk intake rates (kg day−1 and MJ day−1) typically increase as the pup grows, although the relationship between milk intake rates and body mass may break down at different pup developmental states (Oftedal et al., 1987b; McDonald et al., 2012). Milk intake rates do not always increase linearly until the end of lactation, at least in otariids where weaning is a gradual process. For example, Antarctic and Australian fur seals (Arctocephalus pusillus doriferus) exhibited peak milk intake rates at roughly 70% and 67% of the lactation interval, respectively (Arnould et al., 1996; Arnould and Hindell, 2002). It is unclear if this is a general pattern for otariids, since northern fur seal pups exhibited increased milk intake rates until at least 95 days of age, roughly 70% to 80% of the lactation interval (Donohue et al., 2002). Sex differences in milk intake rates have been detected in some studies (Costa and Gentry, 1986; Oftedal et al., 1987b) but not others (Kretzmann et al., 1993; Lunn and Arnould, 1997; Arnould and Hindell, 2002; Donohue et al., 2002). Across 2 years, Donohue et al. (2002) found that female northern fur seal pups consumed less milk per day in one year but not the other. When present, such differences appear to be largely driven by differences in body mass and not because of differential maternal investment. Male pups may be heavier than female ones because of how they invest that energy in growth, with female pups accumulating more fat mass and male pups more lean tissue mass (Arnould et al., 1996; Arnould and Hindell, 2002).
There are no empirical measurements of milk intake for cetaceans, mustelids or sirenians. Estimates of cetacean milk output are provided in Table 9 of Oftedal (1997); however, these values were derived using either (1) estimates of calf growth rates and the assumed relationship between kg of milk ingested per kg of body mass gained, or (2) assumptions about milk production based on the mass of mammary tissue. Assumptions about milk ingestion to growth and milk production based on mammary tissue mass were both derived from phocid seals. Resulting estimates of milk output ranged from 22 to 220 kg day−1 (330–4000 MJ day−1) in mysticetes and from 0.42 to 9.0 kg day−1 (6.0–110 MJ day−1) in odontocetes. Sumich (1986) estimated gray whale calves required 17.9 kg day−1 of milk (1430 kg of milk across an 80 day stay in a breeding lagoon) based on a bioenergetic model of calf energy needs, a value that is considerably different than the 60 to 150 kg day−1 estimated by Oftedal (1997) from mammary gland mass. Differences in predicted milk outputs may be due to the reliance on phocid data to estimate the amount of milk produced by each gram of mammary tissue. Because of the lack of information on milk output/intake, most recent bioenergetic models on species from these groups have used other approaches to estimate lactation costs.
Lactation duration
Lactation durations have been estimated for 100% (ursids, odobenids, mustelids, otariids), 89% (phocids), 60% (mysticetes), 45% (odontocetes) and 25% (sirenians) of marine mammal species (Fig. 2; Supplementary Tables S17–S21). There are estimates in the literature for two additional mysticetes (Bryde's Balaenoptera edeni and pygmy right whales Caperea marginata), but these estimates are speculative at best (Ross et al., 1975; Best, 1977) and were not included in the above percentages. In compiling lactation durations, we also generally excluded literature that did not explicitly provide a formal estimate of lactation duration, such as when anecdotal observations of weaning time were reported (e.g. Clark and Odell, 1999). The abundance of lactation duration data for cetaceans is somewhat misleading. Many of these estimates are not based on actual observations but assumed from other approaches, such as the presence of milk (or prey) in stomach contents of young animals, or the relative occurrence of different reproductive stages in harvested animals. In some cases, this can result in lactation durations that are not well resolved, particularly if the composition of fisheries catches is nonrandom (Kasuya, 1978). Even when direct observations are available, they are often based on associations between an adult female and her presumed calf, which could result in erroneous estimates if they remain associated for reasons other than nursing, are not always together (a common occurrence), or are in fact not a mother-calf pair (Hamilton et al., 2022).
Figure 2.
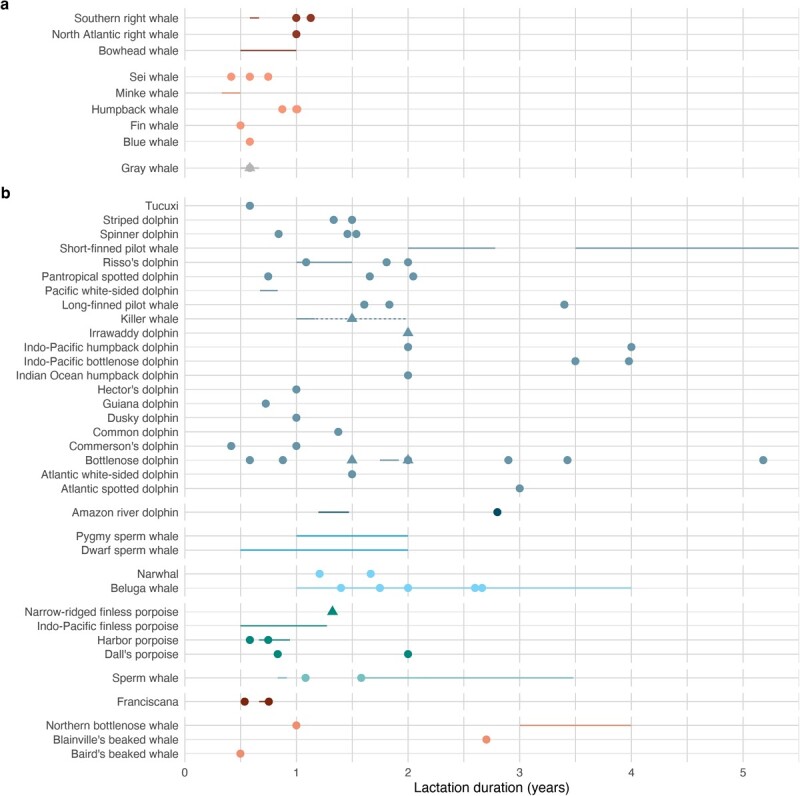
Mean lactation durations of mysticetes (a) and odontocetes (b), ordered and colored by family. Point estimates represent means from individual studies, with shapes indicating whether data were collected from animals in the wild (circle) or human care (triangle). Line ranges are provided when a range of values was given instead of a single mean, with dashed lines indicating data collected from animals in human care. See Supplementary Tables S17 and S18 for values and sources.
Lactation durations of marine mammals vary greatly among species, from a minimum of 4 days in the hooded seal to an average of 3 to 4 years in some odontocetes. Within pinnipeds, phocids have much shorter lactation durations (4 days to 5 months) than otariids (4 months to several years), which is consistent with observations that phocids have a greater reliance on energy reserves (capital) stored prior to the breeding season than otariids that support costs by foraging during lactation (income). Reviews of lactation durations of pinnipeds and analysis of the factors that may have led to differing lactation strategies between phocids and otariids can be found elsewhere (Schulz and Bowen, 2004, 2005; Ferguson, 2006; Costa and Valenzuala-Toro, 2021; Costa and Maresh, 2022). Mysticetes, which are capital breeders (or mostly), tend to have shorter lactation durations than odontocetes, with average durations of 1 year or less. In contrast, odontocetes typically nurse their calves for 1.5 to 4 years, with maximum durations up to 13+ years. Porpoises and some river dolphins are exceptions that nurse their calves for less than one year on average (Supplementary Table S18), although there are conflicting estimates for Dall's porpoise (Phocoenoides dalli) that make it unclear whether they follow this same pattern (Kasuya, 1978; Newby, 1982).
New data on lactation durations continue to refine earlier estimates. For example, a recent study by Feyrer et al. (2020) using stable isotope analysis of teeth showed that lactation durations of northern bottlenose whales (Hyperoodon ampullatus) were 3 to 4 years instead of the previous best estimate of at least 1 year, which was a minimum estimate based on the presence of milk and prey in the stomach of a one-year old calf (Benjaminsen and Christensen, 1979). Satellite telemetry data from a single leopard seal (Hydrurga leptonyx), a species where lactation durations are unconfirmed but speculations range from 10 days to 8 weeks (Southwell et al., 2003), provides preliminary support for a 2-week lactation duration (Kienle et al., 2022). In North Atlantic right whales, the use of genetic identification revealed that at least two calves never observed on the foraging grounds with their mothers survived, indicating successful weaning as early as 7.5 to 8.0 months of age on the northbound migration (Hamilton et al., 2022).
A particular challenge in estimating total lactation costs is that there is a high degree of intraspecific variation in lactation durations in some species. Bioenergetic models and associated outputs, such as dynamic models estimating reproductive parameters, can be sensitive to lactation durations (New et al., 2013). Variation is minimal in phocids (on the order of days) given short lactation durations, but even small absolute fluctuations can have significant impacts on individual lactation costs (Mellish et al., 1999a). Otariids can extend lactation durations by months or even years, typically in response to environmental conditions; such extensions often come at the expense of the reproductive effort in following years (Pitcher et al., 1998; Trillmich and Wolf, 2008) and can considerably increase the total cost of lactation (McHuron, unpubl. data). Variation in lactation duration in odontocetes is large, both in terms of mean and maximum durations. For example, wild bottlenose dolphins from Ireland had mean lactation durations of 2.9 years (Baker et al., 2018), whereas dolphins in Shark Bay weaned at over 3 years of age (Mann et al., 2000, Tursiops sp.), and those in Brazil around the age of 2 years (Fruet et al., 2015). Lactation durations of individuals ranged from 2.0 to 8.59 years (Mann et al., 2000; Baker et al., 2018). Bottlenose dolphin calves managed in human care weaned between 1 and 3 years of age (Peddemors et al., 1992; Reddy et al., 1993; Kastelein et al., 2002). Population differences may largely be driven by resource availability, while within-population differences have been associated with maternal age (Best et al., 1984; Kasuya and Marsh, 1984; Martin and Rothery, 1993; Karniski et al., 2018), body size (Mellish et al., 1999a) and body condition (Cordes and Thompson, 2013). When lactation durations drastically exceed mean durations, it is assumed, although technically unknown, that continued lactation is more about comfort and sociality and that offspring gain little in terms of nutrition.
Milk efficiency
The digestive efficiency of milk in marine mammals has not been directly measured but is typically assumed to be high given the fat content of milk and measurements of prey digestive efficiency. Ortiz et al. (1984) suggested that it must at least be as high as 90% to 95% in northern elephant seal pups, since predicted mass gain based on milk energy intake was very close to actual mass gain. In sheep, digestive and metabolizable energy efficiencies, which account for fecal and fecal + urinary energy losses, were 98.4% and 95.6% of gross energy intake, respectively (Jagusch and Mitchell, 1971). Proximate composition of sheep milk was 5.74% to 6.76% protein and 5.90% to 8.00% fat, which is considerably different from marine mammal milk that is higher in both fat and protein composition. While higher fat content should increase digestive efficiency, higher protein content could increase urinary energy losses if the protein is catabolized. It is likely, however, that protein in a growing pup or calf is used to create structural tissue (Condit and Ortiz, 1987), so it is unclear how transferable these values are to marine mammals.
Offspring growth
Growth costs in bioenergetic models are often calculated based on published growth curves and data on the chemical composition of growth (see also Adamczak et al. in review). Growth curves (length or mass at age) have been published for 100% (odobenids, ursids, phocids), 79% (otariids), 75% (sirenians), 67% (mysticetes), 51% (odontocetes) and 50% (mustelids) of marine mammal species (Supplementary Tables S6–S10). For cetaceans, dependent calf measurements are largely lacking from growth curves, in large part because many of these curves were generated using harvested animals, a data source biased toward larger individuals. This is changing, however, with the ability to use photogrammetry to estimate body size, in addition to the accumulation of measurements collected from stranded individuals or those sampled as part of long-term research programs (Cheney et al., 2018; Venuto et al., 2020; Fortune et al., 2021). Multiphase growth models indicate that calf growth is rapid in the first few months to a year following parturition (Cockcroft and Ross, 1990; Ferrero and Walker, 1996; Gol’din, 2004; McFee et al., 2010; Agbayani et al., 2020; Fortune et al., 2021), highlighting the need for multiphase growth curves to avoid miscalculations of growth costs. For example, gray whale calves reached one-third of their total expected body mass during their first year (Agbayani et al., 2020), while North Atlantic right whale calves had attained 47% of the mass of a sexually mature animal by 9.6 months (Fortune et al., 2021). The inability to accurately model growth using a single curve has also been noted for pinnipeds (McLaren, 1993). There is evidence from a limited number of species that growth rates and asymptotic lengths may in some cases be population-specific, although this is not well resolved for most species (Galatius and Gol’din, 2011; Baker et al., 2014; Durban et al., 2021).
The energy stored in tissues is typically estimated using either the protein and lipid composition of growth or the allocation of growth to different tissue types (e.g. blubber, muscle, viscera) in conjunction with the lipid and protein composition of each tissue type. Because tissues inherently differ in their energy density, the allocation of energy to different tissue types influences the estimated cost of growth. For example, total estimated growth costs of southern right whale calves varied between 458 000 and 995 000 MJ, depending on the assumed lipid and protein concentration of blubber, muscle, viscera and bone (Christiansen et al., 2022a). Data on growth composition for cetaceans are lacking, but corresponding data from pinnipeds suggests that life-history strategies play a role in energy allocation. Phocid pups typically deposit large amounts of lipid (as blubber) during the relatively short lactation period (Kretzmann et al., 1993; Oftedal et al., 1993), whereas otariid pups appear to prioritize lean tissue growth (and physiological development) across a much longer lactation period (Oftedal et al., 1993; Donohue et al., 2002). Cockcroft and Ross (1990) found a similar pattern of lean tissue prioritization in young bottlenose dolphins, with the ratio of composition diverging from 1:1 when animals exceeded approximately 44 kg in body mass. This does not mean that lipid deposition cannot be significant in otariids, as about 50% of the growth of young-of-the-year Steller sea lions (Eumetopias jubatus) aged 5 to 10 months came from lipid deposition (Rehberg et al., 2018). In southern right whale calves, blubber thickness at different sites on the body increased between 31% and 59% from neonates to young calves and 48% to 100% from young to old calves (Marón et al., 2021), although the contribution of lean tissue growth is unknown. Tissue-specific growth may also vary with sex (Arnould et al., 1996; Arnould and Hindell, 2002), but sex-specific growth strategies largely do not appear to impact maternal energy investment (see Milk intake rates).
Allonursing
Allonursing, where milk is provided to nonfilial offspring, has the potential to affect maternal energy budgets during lactation, primarily by reducing the amount of energy a female allocates to offspring while still maintaining offspring growth. In pinnipeds, observations of allonursing behavior are relatively widespread, having been observed in at least 5 phocids, odobenids and 11 otariids (e.g. Bartholomew, 1959; Riedman and Le Boeuf, 1982; Childerhouse and Gales, 2001; de Bruyn et al., 2010; Pitcher et al., 2011; Arso Civil et al., 2021). This behavior is often attributed to misdirected parental care (e.g. misidentification by the mother) or milk theft by the pup, although there are isolated examples of extended fostering, such as when a female has lost her own pup. Fostering has also been observed in polar bears (Malenfant et al., 2016) and sea otters (Staedler and Riedman, 1989), while allonursing and fostering have been observed in manatees (Bonde, 2009). Allonursing is less common in cetaceans, having been observed in sperm whales (Physeter macrocephalus) and captive beluga whales (Delphinapterus leucas) (Leung et al., 2010; Konrad et al., 2019), although this may be because it is difficult to observe in the wild and not because it does not occur in other species. There are also several isolated reports of adoption in wild bottlenose dolphins (Howells et al., 2009; Sakai et al., 2016) and one report of calf-switching in North Atlantic right whales (Frasier et al., 2010). In contrast to pinnipeds, allonursing in sperm whales appears to be best explained by kin selection rather than maternal mistakes or deliberate milk stealing attempts (Konrad et al., 2019).
It is largely unknown how much energy is transferred to offspring when they allosuckle nor how this impacts the energy budget or allocation decisions of the mother. The captive beluga whale calf from Leung et al. (2010) primarily suckled from her mother during the first 13 months, with allonursing events increasing in frequency between 15 months and 34 months of age. Overall nursing frequency decreased as the calf aged, with food consumption beginning around 12 months, suggesting that the energy gain from allonursing was likely minimal. Because of the general lack of knowledge around the frequency of allonursing and indications that energy transfer may be minimal, this behavior has not been considered when constructing energy budgets of marine mammals. One thing to consider moving forward is whether, in odontocetes where kin selection may be the driving cause, allonursing may become more energetically important with environmental change and potential reductions in prey availability. In primates, environmental conditions (high seasonality and high variability) were positively correlated with the frequency of allonursing, potentially because it increases fitness during periods of low food availability (Louppova, 2019).
Prey supplementation
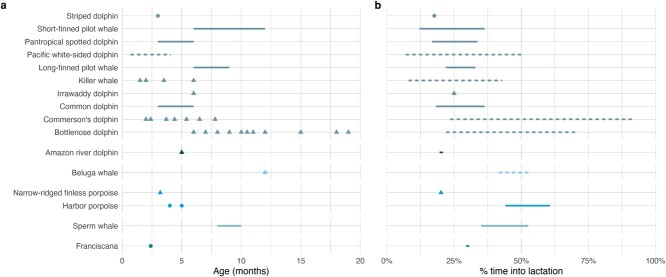
During lactation, offspring may supplement energy gained from milk with prey, either because of an actual energy need or to obtain valuable foraging and diving skills prior to independence. The occurrence of this behavior varies widely among marine mammals. In phocids, there is little indication that much (if any) foraging occurs, even for species where pups accompany mothers to sea. Prey ingestion of harbor seal (Phoca vitulina) pups, one of the most aquatic phocid species during pup development (Bowen et al., 1999), only comprised an average of 0.7% of ingestion events (Sauvé et al., 2014). In otariids, lactation duration, and potentially sex (see Piedrahita et al., 2014), appears to play a role in the extent of prey supplementation; no foraging occurs in northern or Antarctic fur seal pups that suckle for roughly 4 months, whereas frequent at-sea trips and supplemental foraging have been documented for species where pups do not wean until the end of their first or second year of life (Fowler et al., 2007; Lowther and Goldsworthy, 2016). Even though walrus (Odobenus rosmarus) calves, sea otter pups, and polar bear cubs all accompany their mothers while they are foraging, they do not independently forage with any frequency until they are in their second year of life (walrus and polar bears; Stirling and Latour, 1978; Fay, 1982) or near weaning at 20 to 24 weeks of age (sea otters; Payne and Jameson, 1984). There is at least one record of foraging by a dependent manatee (Trichechus manatus; Reynolds, 1981), but no mention of at what age this behavior begins or how frequently it might occur. Supplemental feeding by mysticete calves has been observed in at least one species, humpback whales (Clapham and Mayo, 1987; Szabo and Duffus, 2008), but no foraging was observed in North Atlantic right whale calves across the first 9 months of their lives, even when on the foraging grounds (Cusano et al., 2019). In odontocetes, supplemental feeding appears to be widespread and occurs as early as the first month or two of age (Fig. 3, Supplementary Table S22), although the age at which it starts is highly variable among individuals. Many of these observations were based on animals in human care, which could be biased toward earlier ages, but stomach contents and behavioral data from free-ranging odontocetes generally support such findings (Fig. 3, Supplementary Table S22).
Figure 3.
The age (a) or percent time into lactation (b) at which supplemental foraging has been documented in odontocete calves. Shapes and line types indicate whether data are from animals in the wild (circle, solid line) or managed in human care (triangle, dashed line). In b, the percent time into lactation was calculated using average lactation durations (see Fig. 2). When mean lactation duration was a range, we calculated the minimum (earliest age/longest lactation duration) and maximum (oldest age/shortest lactation duration) percent time that feeding likely occurred, with resulting values presented as a range. See Supplementary Table S22 for values and sources.
For most species, the contribution of prey to a dependent offspring’s energy budget is likely small given supplementation typically occurs late in lactation and young animals may be inefficient foragers. For mustelids and polar bears, this excludes prey provided by the mother, which occurs much earlier than independent foraging and likely contributes significantly to offspring energy needs (Payne and Jameson, 1984; Derocher and Stirling, 1998). Odontocetes appear to be an exception, where detailed records of calf food intake indicate rapid increases in the amount of prey consumed once feeding begins, with many individuals consuming similar quantities of prey to that observed at weaning in the months or even years prior to weaning (Kastelein et al., 2002, 2003). Data from wild odontocete calves support these findings that maternal investment declines as the calf ages (Fruet et al., 2015; Matthews and Ferguson, 2015). This indicates that prey supplementation by many odontocete calves likely plays a large role in meeting their energy needs, significantly reducing the energy demands on the female during much of lactation. It is unknown if this would hold true for deep-diving odontocetes, like sperm whales and beaked whales, where physiological constraints may restrict the ability of calves to forage. The diving capabilities of three first-year sperm whale calves were well-developed, with at least one calf reaching adult diving depths (Tønnesen et al., 2018), but it is largely unknown how much foraging actually occurs on such dives.
Supplemental foraging by dependent offspring is not considered in most bioenergetic models. It has been included in some odontocete bioenergetic models (New et al., 2013; Bejarano et al., 2017; Gallagher et al., 2018; Hin et al., 2019), typically by assuming some linear reduction in maternal investment. In some models, prey supplementation may be accounted for even it is not explicitly considered by shortening the lactation duration to the time across which most calf energy demands are presumed to be met by milk. Exclusion of prey supplementation from bioenergetic models may result in overestimates of maternal investment in offspring and maternal energy requirements. This could be problematic in dynamic models where behavioral decisions are dependent on maternal energy gain and maternal and offspring body condition. It is unlikely to affect population-level prey consumption estimates from accounting models since it does not change the amount of prey extracted from the ecosystem, just who is extracting it. There may be some differences though, as dependent offspring may not forage on the same prey as adult animals that are larger in size and more experienced (Field et al., 2007; Lundström et al., 2010; Jeglinski et al., 2012). While most odontocete studies have used a linear relationship to describe the decrease in energy investment by the mother through time, this may not be the most appropriate functional form for this relationship since prey consumption of captive individuals often increases rapidly and then plateaus well before weaning (see references in Supplementary Table S22). For mustelids and polar bears, supplemental prey provided by the mother should be treated separately in bioenergetic models from that consumed by the mother to produce milk (or used for her own energy needs), since it is going directly to the offspring. Failure to separate this prey from that being ingested would result in an overestimate of prey energy needs, since there are no fecal or urinary energy losses incurred by the female.
Multiple offspring
With the exception of polar bears and marine otters, marine mammals typically give birth to a single offspring. Twins have been reported in all taxonomic groups of marine mammals (Kawamura, 1969; Spotte, 1982; Jameson and Bodkin, 1986; Fay et al., 1991; Osborn et al., 2012; Davison et al., 2016; Gutiérrez et al., 2021; Drinkwater and Branch, 2022), but these reports are uncommon to rare. In his review on the incidence of twins in pinnipeds, Spotte (1982) noted that twins are typically underweight for their age, with no reports of both twins surviving to weaning in the wild. In polar bears, 4-month old singleton cubs weighed 25% to 35% more than those from twin litters (Derocher and Stirling, 1998), with higher survival to 6 months for singleton cubs compared with those from twin and triplet litters (Derocher and Stirling, 1996). Olesiuk et al. (1990) reported two occurrences of twins in killer whales (Orcinus orca), with one pair surviving to at least 7.5 years of age. In Hawaiian monk seals (Neomonachus schauinslandi), 0.14% of births resulted in twins, with a 57.1% pooled survival rate from birth to weaning. Almost all surviving twins had lower axillary girth at weaning than singleton pups born in the same year (Schultz et al., 2011). These patterns of lower birth mass and the difficultly in rearing multiples are consistent with measurements from other mammals suggesting that parental cost is higher with twins than singletons (Link et al., 2006; Huck et al., 2014).
Most marine mammals wean their current offspring before the subsequent one is born, but there are some pinnipeds, namely the Galapagos fur seal (Arctocephalus galapagoensis) and Galapagos sea lion (Zalophus wollebaeki), where a female may concurrently nurse a new pup and an older sibling. Milk allocation to each offspring has not been measured but presumably is reduced, at least to the new pup, given growth rates and survival probability are considerably lower in pups with an older sibling compared to those without (Trillmich and Wolf, 2008). Overlap between current and future reproductive efforts (simultaneous pregnancy and lactation) commonly occurs in otariids, some odontocetes, and even some mysticetes (Pallin et al., 2018). Comparisons of fetal masses between lactating and nonlactating otariids indicated that fetuses from lactating females were smaller, suggesting that simultaneous pregnancy and lactation may reduce energy investment in fetal growth (Lima and Paez, 1995; Trillmich and Wolf, 2008). Given the rare occurrence of supporting multiple offspring during one reproductive period, almost no bioenergetic models estimate costs associated with concurrently rearing more than one offspring, except of course for species where it is a common occurrence (Reimer et al., 2019) and when the costs of gestation and lactation are experienced simultaneously.
Maternal Food Intake during Gestation and Lactation
Bioenergetic models typically model the energetic costs of reproduction as additive. As a result, predicted energy needs or food intake during reproduction are almost always higher than during a nonreproductive state (assuming all else is equal). Animals do not experience the costs of reproduction in isolation though, and there may be mechanisms to help offset reproductive costs and minimize the need to increase prey intake or use of stored energy reserves. Metabolic depression, which could occur through physiological or behavioral mechanisms, has been detected in some mammals during gestation and lactation (Krockenberger, 2003; Korine et al., 2004), including several pinniped species (Mellish et al., 2000; Sparling et al., 2006; Shuert et al., 2020). While it has not been studied in marine mammals, an alternative mechanism for minimizing the need to increase food intake during reproduction is through an increase in digestive efficiency (Pereira et al., 2020).
The potential mismatch that any compensation mechanisms could create between actual and predicted energy needs from a bioenergetic model will depend on how likely the estimated metabolic costs are to incorporate such mechanisms. For example, this issue may largely be irrelevant for pinniped lactation costs where metabolic costs are estimated from DLW, since most of these measurements are derived from lactating females. The challenge in incorporating such compensatory mechanisms in bioenergetic models lies primarily in limitations associated with collecting data to determine the occurrence and magnitude of compensation. In wild populations, there also may be mismatches between energy needs and prey intake due to heterogeneity in prey resources and life history patterns. These potential mismatches could result in inaccurate estimates of prey intake from bioenergetic models, particularly in accounting models where estimates of prey consumption are typically calculated from energy requirements (i.e. they do not take prey availability into account).
Changes in food intake of gestating marine mammals in human care are inconsistent, with conclusions typically drawn from a small number of individuals across only part of gestation. Some studies report no change or even decreases in food intake (in kg prey) during gestation (Joseph et al., 1987; Cheal and Gales, 1991; Kastelein et al., 1993, 2002, 2003), while others report increases, particularly later in gestation (Asper et al., 1988; Kastelein et al., 1990; Reddy et al., 1993; Kastelein et al., 2000a; McHuron et al., 2022b). There have also been conflicting findings within a species, such as for killer whales where Kastelein et al. (2003) and Williams et al. (2011) found no change in food intake while Asper et al. (1988) documented increases beginning in the 13th month of gestation. In one of the few studies on the cost of pregnancy in free-ranging marine mammals, Shero et al. (2018) documented an increase in diving effort by pregnant Weddell seals (Leptonychotes weddelli) compared with nonreproductive females, particularly during the last trimester, which was attributed to an increase in gestational energy demand. There are fairly consistent observations across species that food intake decreases in the days to weeks leading up to birth, regardless of whether it fluctuates at other time periods (Ronald and Thomson, 1981; Joseph et al., 1987; Kastelein et al., 1990, 2000b; Robeck et al., 2005; McHuron et al., 2022b). For some species, increases in food intake may not be related to the costs of gestation but rather due to an increase energy storage during gestation for the subsequent lactation interval (Noren et al., 2014).
During lactation, most studies of marine mammals in human care report increases in food intake above pre-lactation levels (e.g. Kastelein et al., 2000b, Kastelein et al., 2000a, 2002, 2003; Williams et al., 2007; McHuron et al., 2022b). Species that rely more heavily on energy reserves during lactation may be an exception, since much of the energy for lactation is stored prior to parturition (Kastelein et al., 1990). Robeck et al. (2005) did not report an increase in food intake above pre-lactation demands in beluga whales, with food intake returning to pre-parturient levels within 15 days. Whether this represents a true lack of food increase remains unknown, since there was considerable variation in both pre- and post-parturient intake, food intake was only reported for 30 days, and animals where food intake did not return to pre-parturient values within 15 days were excluded from the study.
The duration and magnitude of increase in food intake during lactation varies considerably, both within and across studies. For example, Kastelein et al. (2002) reported that food intake of bottlenose dolphins peaked 1 to 5 months after parturition, with total food intake during lactation between 48% and 98% higher than during nonreproductive periods. Asper et al. (1988) found that consumption of a single lactating killer whale peaked around 110 to 118 kg day−1, approximately 80% higher than intake during early gestation, with consumption remaining elevated (at 95 kg day−1) for six months past the time of weaning at 1.5 years of age. Reports from other species also show peak consumption estimates close to or in excess of 100% higher than nonreproductive periods (Kastelein et al., 2000b, 2003; McHuron et al., 2022b). Few comparative studies of food intake in wild marine mammals exist. Estimates derived from stomach contents of female northern fur seals shot at sea indicated that lactating females consumed 60% more food than nonlactating ones (Perez and Mooney, 1986). Similar data from harbor porpoises (Phocoena phocoena) in the Bay of Fundy indicated that lactating females consumed more than twice the prey mass and caloric content of nonlactating females (Recchia and Read, 1989). While not direct evidence, increases in diving effort throughout much of lactation from sea otters and several pinniped species are consistent with the increased food consumption documented in captive animals (Bowen et al., 2001b; Hoskins and Arnould, 2013; Thometz et al., 2016b).
Factors that Affect Reproductive Energetics
Prey availability is the most influential extrinsic factor influencing reproductive investment in marine mammals. Poor environmental conditions (or poor maternal condition) reduces energetic investment in reproduction as evidenced by changes in offspring production, smaller offspring mass and reduced growth, and in extreme cases, offspring mortality (Costa et al., 1989; Trites, 1992; Soto et al., 2004; Christiansen et al., 2014; McClatchie et al., 2016; McMahon et al., 2017; Gailey et al., 2020; Páez-Rosas et al., 2021; Smith, 2021). While it has not been studied in marine mammals, there is some evidence from other mammals that glucocorticoids in milk (indicative of maternal stress) are associated with offspring growth, potentially through changes in milk production (Stead et al., 2022). Stress is a potential concern for some marine mammal populations given associations between increased glucocorticoids and ocean noise (Rolland et al., 2012), warranting further investigation of how stress might affect reproductive energetics.
Maternal characteristics, such as mass and age, are well documented as influencing aspects of pinniped reproductive energetics. Maternal body size is often, although not always, positively correlated with birth mass (Boyd and McCann, 1989; Bowen et al., 1994; Boltnev and York, 2001; Wheatley et al., 2006). Larger females, regardless of whether they are capital or income-breeding species, often allocate more energy to their pups, either because of increased milk output, longer lactation durations, or both (Iverson et al., 1993; Arnbom et al., 1997; Mellish et al., 1999a; Bowen et al., 2001a; Crocker et al., 2001; McDonald et al., 2012). Changes in body volume of female southern right whales during the first three months of lactation follow a similar pattern, with longer, robust females losing volume at a faster rate than smaller, leaner ones (Christiansen et al., 2018). Maternal age, irrespective of body mass, can affect factors such as milk production and milk fat content, although these differences do not necessarily translate to higher total reproductive investment (McDonald and Crocker, 2006; Lang et al., 2011a, 2011b, 2012). For example, although primiparous gray seals had a reduced physiological capacity for milk production and storage compared with multiparous females, primiparous females compensated by increasing nursing frequency (Lang et al., 2011a, Lang et al., 2011b, Lang et al., 2012). Maternal age may have other effects on offspring growth unrelated to variation in maternal energy investment, as has been shown for northern elephant seals where pups of older mothers exhibited more energy-saving behaviors (Hooper et al., 2019) and gray seals where older mothers selected for birth sites that did not experience flooding (Allen et al., 2022). While still a developing field, there does appear to be an individual-based component to reproductive expenditure not explained by other factors, such that some females consistently have lower or higher reproductive energy costs than others (Lang et al., 2009; Paterson et al., 2016).
In general, the influences of intrinsic factors on reproductive effort are not explicitly incorporated in accounting or dynamic bioenergetic models. They are, however, often implicitly included since most models incorporate variation in reproductive parameters when such data are available. The effects of environmental variation on reproductive costs may be incorporated in a similar way, or explicitly modeled using year-specific values for parameters influencing reproductive costs, such as offspring growth rates and maternal activity budgets (e.g. McHuron et al., 2020). In dynamic bioenergetic models, the effects of environmental variation on reproductive output may be explicitly modeled through allocation rules or functional relationships between maternal body condition and reproductive effort (e.g. Hin et al., 2019; McHuron et al., 2021). These attempts should be viewed as a first effort to incorporate the effect of energy deficiency on reproductive costs, as the specific mechanisms and thresholds in marine mammals that result in a reduced rate of energy delivery to offspring are largely unknown. As the focus of most accounting models is not on the individual, it is likely not overly important how intrinsic factors are accounted for when estimating reproductive costs. This may not be the case for dynamic bioenergetic models, since these are typically individual-based models and often focus on linkages between energy reserves, vital rates and population dynamics. Thus, the fact that not all individuals invest the same in reproduction could affect predicted population trajectories, particularly if environmental stressors or disturbances disproportionately impact some individuals and not others, or if there are shifts in the age structure of a population. In practice, it remains difficult to accurately incorporate these effects given limited data availability.
Conclusions
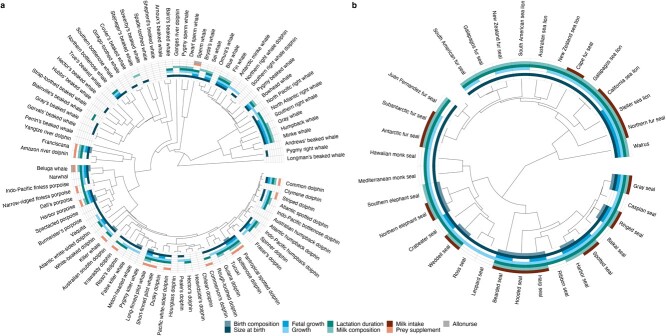
Many pressing management and conservation questions, such as those revolving around the impacts of climate change and anthropogenic disturbance on marine mammal populations, are difficult to fully address without an understanding of how much energy a female must invest to successfully gestate and wean her offspring. Bioenergetic modeling approaches use a variety of input parameters to estimate these costs, such as offspring growth, body mass and composition, and milk composition and intake rates. Data availability for these parameters are comparatively sparse in cetaceans relative to pinnipeds, particularly for beaked whales, although data gaps remain for some polar phocids (Fig. 4). There was also extremely limited information on these parameters for sirenians and the marine otter. On the one hand, our estimates for data availability represent a minimum since we excluded references to values with an estimate but no citation. On the other hand, many estimates we included are based on observations from just a few individuals, anecdotal observations, or inferred from other data sources and are thus tentative at best.
Figure 4.
Data availability for parameters typically used to estimate reproductive costs in marine mammal bioenergetic models in cetaceans (a) and pinnipeds (b), overlaid on a phylogenetic tree. Colors correspond to different data types, with the presence of color indicative of published data (body composition at birth, length or mass at birth, fetal growth curves, milk composition, milk intake rates) or confirmation of specific behaviors (prey supplementation, allonursing; cetaceans only). Missing species labels correspond to species currently recognized as subspecies by the Society for Marine Mammalogy Taxonomic Committee in the June 2021 species list. Currently recognized species not included on the tree are: Rice’s whale (Balaenoptera ricei), Sato’s beaked whale (Berardius minimus), Deraniyagala’s beaked whale (Mesoplodon hotaula), the Indus river dolphin (Platanista minor), and the Indian Ocean humpback dolphin (Sousa plumbea). Phylogenetic tree created using R packages treeio (Wang et al., 2020), ggtree (Yu, 2020), and ggtreeExtra (Xu et al., 2021) using data from the VertLife Project (www.vertlife.org).
Concerted efforts to help refine existing estimates will improve the accuracy of bioenergetic model outputs, especially those involved in calculating lactation costs. Lactation duration is one example of a parameter that is not only influential on bioenergetic model output (New et al., 2013) but also one that can be estimated from free-ranging animals across all taxonomic groups. The recent work of Feyrer et al. (2020) and Hamilton et al. (2022) highlight how new data can alter our perceptions of lactation durations and relationships between lactation duration and offspring survival. Prey supplementation by odontocetes calves is another example of a parameter that deserves further attention, both in terms of understanding temporal dynamics in free-ranging animals and in how important that contribution is to a calf's energy budget. The widespread, and often early, occurrence of prey supplementation across odontocetes suggests that calf costs may rapidly exceed a female’s physiological capability to support the entirety of its energetic needs by milk. If this is true, disruptions that alter successful foraging (either environmental or anthropogenic) may be magnified because both the mother and the calf are contributing to the calf's energy needs.
Reproductive costs occur in concert with a female’s own energy needs (e.g. maintenance, locomotion, thermoregulation), but total energy budgets are not always additive since marine mammals (like other mammals) appear to have some compensatory mechanisms to cope with added reproductive costs. At present, it is difficult to incorporate such mechanisms into bioenergetic models given a general lack of understanding of how widespread these mechanisms are, their timing and magnitude, and whether there are any downstream effects on fitness (e.g. reduced immune function). Compensatory mechanisms may, however, become increasingly important in mitigating high reproductive costs since many marine mammal populations face increasingly altered prey landscapes.
Funding
This review was funded by the Office of Naval Research (N000142012392), with support from the Marine Mammal Commission (MMC 19-173).
Data availability
All data are incorporated into the article and its online supplementary material.
Supplementary Material
Acknowledgements
This work benefited from discussions with colleagues at a Marine Mammal Bioenergetics Workshop hosted by ONR, and comments on earlier manuscript drafts by E. Pirotta and R. Holser.
Contributor Information
Elizabeth A McHuron, Cooperative Institute for Climate, Ocean, and Ecosystem Studies, University of Washington, Seattle, WA, 98105, USA.
Stephanie Adamczak, Ecology and Evolutionary Biology Department, University of California Santa Cruz, Santa Cruz, CA, 95064, USA.
Daniel P Costa, Ecology and Evolutionary Biology Department, University of California Santa Cruz, Santa Cruz, CA, 95064, USA.
Cormac Booth, SMRU Consulting, Scottish Oceans Institute, St Andrews, UK.
Supplementary material
Supplementary material is available at Conservation Physiology online.
References
- Agbayani S, Fortune SME, Trites AW (2020) Growth and development of North Pacific gray whales (Eschrichtius robustus). J Mammal 101: 742–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen SJJ, Bowen WD, Heyer CE (2022) Birth-site habitat selection in gray seals (Halichoerus grypus): effects of maternal age and parity and association with offspring weaning mass. Mar Mamm Sci 38: 349–363. [Google Scholar]
- Anderson SS, Fedak MA (1987) Grey seal, Halichoerus grypus, energetics: females invest more in male offspring. J Zool London 211: 667–679. [Google Scholar]
- Arnbom T, Fedak MA, Boyd IL (1997) Factors affecting maternal expenditure in southern elephant seals during lactation. Ecology 78: 471–483. [Google Scholar]
- Arnould J, Boyd IL, Socha DG (1996) Milk consumption and growth efficiency in Antarctic fur seal (Arctocephalus gazella) pups. Can J Zool 74: 254–266. [Google Scholar]
- Arnould JP, Hindell MA (1999) The composition of Australian fur seal (Arctocephalus pusillus doriferus) milk throughout lactation. Physiol Biochem Zool 72: 605–612. [DOI] [PubMed] [Google Scholar]
- Arnould JPY (1997) Lactation and the cost of pup-rearing in Antarctic fur seals. Mar Mamm Sci 13: 516–526. [Google Scholar]
- Arnould JPY, Hindell MA (2002) Milk consumption, body composition and pre-weaning growth rates of Australian fur seal (Arctocephalus pusillus doriferus) pups. J Zool 256: 351–359. [Google Scholar]
- Arnould JPY, Ramsay MA (1994) Milk production and milk consumption in polar bears during the ice-free period in western Hudson Bay. Can J Zool 72: 1365–1370. [Google Scholar]
- Arso Civil M, Hague E, Langley I, Scott-Hayward L (2021) Allo-suckling occurrence and its effect on lactation and nursing duration in harbour seals (Phoca vitulina) in Orkney, Scotland. Behav Ecol Sociobiol 75: 121. [Google Scholar]
- Asper ED, Young WG, Walsh MT (1988) Observations on the birth and development of a captive-born killer whale Orcinus orca. Int Zoo Yearb 27: 295–304. [Google Scholar]
- Atkinson S (1997) Reproductive biology of seals. Rev Reprod 2: 175–194. [DOI] [PubMed] [Google Scholar]
- Baker I, O’Brien J, McHugh K, Berrow S (2018) Female reproductive parameters and population demographics of bottlenose dolphins (Tursiops truncatus) in the Shannon estuary, Ireland. Mar Biol 165: 1–19. [Google Scholar]
- Baker JD, Johanos TC, Wurth TA, Littnan CL (2014) Body growth in Hawaiian monk seals. Mar Mamm Sci 30: 259–271. [Google Scholar]
- Bartholomew GA (1959) Mother-young relations and the maturation of pup behaviour in the Alaska fur seal. Anim Behav 7: 163–171. [Google Scholar]
- Bejarano AC, Wells RS, Costa DP (2017) Development of a bioenergetic model for estimating energy requirements and prey biomass consumption of the bottlenose dolphin Tursiops truncatus. Ecol Model 356: 162–172. [Google Scholar]
- Benjaminsen T, Christensen I (1979) The natural history of the bottlenose whale, Hyperoodon ampullatus (Forster). In Winn HE, Olla BL, eds, Behavior of Marine Animals. Springer, pp. 143–164 [Google Scholar]
- Best P, Canham P, MacLeod N (1984) Patterns of reproduction in sperm whales, Physeter macrocephalus. Rep Int Whal Comimssion Spec Issue 6: 51–79. [Google Scholar]
- Best PB (1977) Two allopatric forms of Bryde’s whale off South Africa. Rep Int Whal Com Spec 1–9. [Google Scholar]
- Blázquez M, Baker I, O’Brien JM, Berrow SD (2020) Population viability analysis and comparison of two monitoring strategies for bottlenose dolphins (Tursiops truncatus) in the Shannon estuary, Ireland, to inform management. Aquat Mamm 46: 307–325. [Google Scholar]
- Boltnev AI, York AE (2001) Maternal investment in northern fur seals (Callorhinus ursinus): interrelationships among mothers’ age, size, parturition date, offspring size and sex ratios. J Zool 254: 219–228. [Google Scholar]
- Bonde RK (2009) Population Genetics and Conservation of the Florida Manatee: Past, Present, and Future. University of Florida [Google Scholar]
- Boness DJ, Bowen WD (1996) The evolution of in maternal care in pinnipeds. Bioscience 9: 645–654. [Google Scholar]
- Borrell A, Bloch D, Desportes G (1995) Age trends and reproductive transfer of organochlorine compounds in long-finned pilot whales from the Faroe Islands. Environ Pollut 88: 283–292. [DOI] [PubMed] [Google Scholar]
- Bowen WD, Boness DJ, Iverson SJ (1999) Diving behaviour of lactating harbour seals and their pups during maternal foraging trips. Can J Zool 77: 978–988. [Google Scholar]
- Bowen WD, Ellis SL, Iverson SJ, Boness DJ (2001a) Maternal effects on offspring growth rate and weaning mass in harbour seals. Can J Zool 79: 1088–1101. [Google Scholar]
- Bowen WD, Iverson SJ, Boness DJ, Oftedal OT (2001b) Foraging effort, food intake and lactation performance depend on maternal mass in a small phocid seal. Funct Ecol 15: 325–334. [Google Scholar]
- Bowen WD, Oftedal OT, Boness DJ, Iverson SJ (1994) The effect of maternal age and other factors on birth mass in the harbour seal. Can J Zool 72: 8–14. [Google Scholar]
- Boyd I (1990) Mass and hormone content of gray seal placentae related to fetal sex. J Mammal 71: 101–103. [Google Scholar]
- Boyd I, McCann T (1989) Pre-natal investment in reproduction by female Antarctic fur seals. Behav Ecol Sociobiol 24: 377–385. [Google Scholar]
- Boyd IL (1991) Environmental and physiological factors controlling the reproductive cycles of pinnipeds. Can J Zool 69: 1135–1148. [Google Scholar]
- Brody S (1938) Growth and development with special reference to domestic animals. XLVI. Relation between heat increment of gestation and birth weight, Agric Exp Stn Res Bull, p. 283 [Google Scholar]
- Brown TM, Ross PS, Reimer KJ (2016) Transplacental transfer of polychlorinated biphenyls, polybrominated diphenylethers, and organochlorine pesticides in ringed seals (Pusa hispida). Arch Environ Contam Toxicol 70: 20–27. [DOI] [PubMed] [Google Scholar]
- Bruyn PJN, Cameron EZ, Tosh CA, Oosthuizen WC, Reisinger RR, Mufanadzo NT, Phalanndwa MV, Postma M, Wege M, Merwe DSet al. (2010) Prevalence of allosuckling behaviour in Subantarctic fur seal pups. Mamm Biol 75: 555–560. [Google Scholar]
- Chasco BE, Kaplan IC, Thomas AC, Acevedo-Gutiérrez A, Noren DP, Ford MJ, Hanson MB, Scordino JJ, Jeffries SJ, Marshall KNet al. (2017) Competing tradeoffs between increasing marine mammal predation and fisheries harvest of Chinook salmon. Sci Rep 7: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheal AJ, Gales NJ (1991) Body mass and food intake in captive, breeding bottlenose dolphins, Tursiops truncatus. Zoo Biol 10: 451–456. [Google Scholar]
- Cheney B, Wells RS, Barton TR, Thompson PM (2018) Laser photogrammetry reveals variation in growth and early survival in free-ranging bottlenose dolphins. Anim Conserv 21: 252–261. [Google Scholar]
- Childerhouse S, Gales N (2001) Fostering behaviour in New Zealand Sea lions Phocarctos hookeri. New Zeal J Zool 28: 189–195. [Google Scholar]
- Chinn SM, Miller MA, Tinker MT, Staedler MM, Batac FI, Dodd EM, Henkel LA (2016) The high cost of motherhood: end-lactation syndrome in southern sea otters (Enhydra lutris nereis) on the Central California coast, USA. J Wildl Dis 52: 307–318. [DOI] [PubMed] [Google Scholar]
- Christiansen F, Bejder L, Burnell S, Ward R, Charlton C (2022a) Estimating the cost of growth in southern right whales from drone photogrammetry data and long-term sighting histories. Mar Ecol Prog Ser 687: 173–194. [Google Scholar]
- Christiansen F, Dujon AM, Sprogis KR, Arnould JPY, Bejder L (2016) Non-invasive unmanned aerial vehicle provides estimates of the energetic cost of reproduction in humpback whales. Ecosphere 7: e01468. [Google Scholar]
- Christiansen F, Uhart M, Bejder L, Ivashchenko Y, Tormosov D, Lewin N, Sironi M (2022b) Foetal growth, birth size and energetic cost of gestation in southern right whales. J Physiol 600: 2245–2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen F, Víkingsson GA, Rasmussen MH, Lusseau D (2014) Female body condition affects foetal growth in a capital breeding mysticete. Funct Ecol 28: 579–588. [Google Scholar]
- Christiansen F, Vivier F, Charlton C, Ward R, Amerson A, Burnell S, Bejder L (2018) Maternal body size and condition determine calf growth rates in southern right whales. Mar Ecol Prog Ser 592: 267–281. [Google Scholar]
- Clapham PJ, Mayo CA (1987) Reproduction and recruitment of individually identified humpback whales, Megaptera novaeangliae, observed in Massachusetts Bay, 1979-1985. Can J Zool 65: 2853–2863. [Google Scholar]
- Clark ST, Odell DK (1999) Nursing behavior in captive false killer whales (Pseudorca crassidens). Aquat Mamm 253: 183–191. [Google Scholar]
- Cockcroft VG, Ross GJB (1990) Observations on the early development of a captive bottlenose dolphin calf. In Leatherwood S, Reeves RR, eds, The Bottlenose Dolphin. Academic Press, pp. 461–478 [Google Scholar]
- Condit RS, Ortiz CL (1987) The physiological transition from fasting to feeding in weaned elephant seal pups. Mar Mamm Sci 3: 207–219. [Google Scholar]
- Cordes LS, Thompson PM (2013) Variation in breeding phenology provides insights into drivers of long-term population change in harbour seals. Proc R Soc B Biol Sci 280. 10.1098/rspb.2013.0847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa DP (1991) Reproductive and foraging energetics of pinnipeds: implications for life history patterns. In Renouf D, ed, The Behaviour of Pinnipeds. Springer, Dordrecht, pp. 300–344 [Google Scholar]
- Costa DP, Croxall JP, Duck CD (1989) Foraging energetics of Antarctic fur seals in relation to changes in prey availability. Ecology 70: 596–606. [Google Scholar]
- Costa DP, Gentry RL (1986) Reproductive energetics of northern fur seals. In Gentry RL, Kooyman GL, eds, Fur Seals: Maternal Strategies on Land and at Sea. Princeton University Press, Princeton, New Jersey, USA, pp. 79–101 [Google Scholar]
- Costa DP, Maresh JL (2022) Reproductive energetics of phocids. In Costa DP, McHuron EA, eds, Ethology and Behavioral Ecology of Phocids. Springer International Publishing, Cham, pp. 281–309 [Google Scholar]
- Costa DP, Trillmich F (1988) Mass changes and metabolism during the perinatal fast: a comparison between Antarctic (Arctocephalus gazella) and Galapagos fur seals (Arctocephalus galapagoensis). Physiol Zool 61: 160–169. [Google Scholar]
- Costa DP, Valenzuala-Toro AM (2021) When physiology and ecology meet: the interdependency between foraging ecology and reproduction in otariids. In Campagna C, Harcourt R, eds, The Ethology and Behavioral Ecology of Otariids and the Odobenid. Springer, Cham, pp. 21–50 [Google Scholar]
- Crocker DE, Williams JD, Costa DP, Le Boeuf BJ (2001) Maternal traits and reproductive effort in northern elephant seals. Ecology 82: 3541–3555. [Google Scholar]
- Cusano DA, Conger LA, Van Parijs SM, Parks SE (2019) Implementing conservation measures for the North Atlantic right whale: considering the behavioral ontogeny of mother-calf pairs. Anim Conserv 22: 228–237. [Google Scholar]
- Daniel JC (1971) Growth of the preimplantation embryo and its correlation with changes of the northern fur seal in uterine protein. Dev Biol 26: 316–322. [DOI] [PubMed] [Google Scholar]
- Davison NJ, Ten Doeschate MTI, Dagleish MP, Read FL, Reid RJ, Foster G, Brownlow A, Barley J (2016) Twin foetuses in an Atlantic white-sided dolphin (Lagenorhynchus acutus) stranded on the coast of Scotland, UK. J Mar Biol Assoc United Kingdom 96: 841–844. [Google Scholar]
- Derocher AE, Andriashek D, Arnould JPY (1993) Aspects of milk composition and lactation in polar bears. Can J Zool 71: 561–567. [Google Scholar]
- Derocher AE, Stirling I (1996) Aspects of survival in juvenile polar bears. Can J Zool 74: 1246–1252. [Google Scholar]
- Derocher AE, Stirling I (1998) Maternal investment and factors affecting offspring size in polar bears (Ursus maritimus). J Zool 245: 253–260. [Google Scholar]
- Donohue MJ, Costa DP, Goebel E, Antonelis GA, Baker JD (2002) Milk intake and energy expenditure of free-ranging northern fur seal, Callorhinus ursinus, pups. Physiol Biochem Zool 75: 3–18. [DOI] [PubMed] [Google Scholar]
- Drinkwater RW, Branch TA (2022) Estimating proportions of identical twins and twin survival rates in cetaceans using fetal data. Mar Mamm Sci 38: 1398–1408. [Google Scholar]
- Dunkin RC, McLellan WA, Blum JE, Pabst DA (2005) The ontogenetic changes in the thermal properties of blubber from Atlantic bottlenose dolphin Tursiops truncatus. J Exp Biol 208: 1469–1480. [DOI] [PubMed] [Google Scholar]
- Durban JW, Fearnbach H, Paredes A, Hickmott LS, LeRoi DJ (2021) Size and body condition of sympatric killer whale ecotypes around the Antarctic peninsula. Mar Ecol Prog Ser 677: 209–217. [Google Scholar]
- Eisert R, Oftedal OT, Barrell GK (2013) Milk composition in the Weddell seal Leptonychotes weddellii: evidence for a functional role of milk carbohydrates in pinnipeds. Physiol Biochem Zool 86: 159–175. [DOI] [PubMed] [Google Scholar]
- Engle WA, Lemons JA (1986) Composition of the fetal and maternal Guinea pig throughout gestation. Pediatr Res 20: 1156–1160. [DOI] [PubMed] [Google Scholar]
- Fay FH (1982) Ecology and biology of the Pacific walrus, Odobenus rosmarus divergens illiger. North Am Fauna 74: 1–279. [Google Scholar]
- Fay FH, Burns JJ, Kibal’chich A, Hills S (1991) Incidence of twin fetuses in walruses. Northwest Nat 72: 110–113. [Google Scholar]
- Ferguson SH (2006) The influences of environment, mating habitat, and predation on evolution of pinniped lactation strategies. J Mamm Evol 13: 63–82. [Google Scholar]
- Ferrero RC, Walker WA (1996) Age, growth, and reproductive patterns of the Pacific white-sided dolphin (Lagenorhynchus obliquidens) taken in high seas drift nets in the central North Pacific Ocean. Can J Zool 74: 1673–1687. [Google Scholar]
- Feyrer LJ, Zhao ST, Whitehead H, Matthews CJD (2020) Prolonged maternal investment in northern bottlenose whales alters our understanding of beaked whale reproductive life history. PLoS One 15: e0235114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field IC, Bradshaw CJA, Van Den Hoff J, Burton HR, Hindell MA (2007) Age-related shifts in the diet composition of southern elephant seals expand overall foraging niche. Mar Biol 150: 1441–1452. [Google Scholar]
- Fortune S, Trites A, Mayo C, Rosen D, Hamilton P (2013) Energetic requirements of North Atlantic right whales and the implications for species recovery. Mar Ecol Prog Ser 478: 253–272. [Google Scholar]
- Fortune SME, Moore MJ, Perryman WL, Trites AW (2021) Body growth of North Atlantic right whales (Eubalaena glacialis) revisited. Mar Mamm Sci 37: 433–447. [Google Scholar]
- Fowler SL, Costa DP, Arnould JPY (2007) Ontogeny of movements and foraging ranges in the Australian sea lion. Mar Mamm Sci 23: 598–614. [Google Scholar]
- Frasier TR, Hamilton PK, Brown MW, Kraus SD, White BN (2010) Reciprocal exchange and subsequent adoption of calves by two North Atlantic right whales (Eubalaena glacialis). Aquat Mamm 36: 115–120. [Google Scholar]
- Fruet PF, Genoves RC, Möller LM, Botta S, Secchi ER (2015) Using mark-recapture and stranding data to estimate reproductive traits in female bottlenose dolphins (Tursiops truncatus) of the southwestern Atlantic Ocean. Mar Biol 162: 661–673. [Google Scholar]
- Fruet PF, Möller LM, Secchi ER (2021) Dynamics and viability of a small, estuarine-resident population of Lahille’s bottlenose dolphins from southern Brazil. Front Mar Sci 7: 1–13. [Google Scholar]
- Gailey G, Sychenko O, Tyurneva O, Yakovlev Y, Vertyankin V, Der V (2020) Effects of sea ice on growth rates of an endangered population of gray whales. Sci Rep 10: 1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galatius A, Gol’din PE (2011) Geographic variation of skeletal ontogeny and skull shape in the harbour porpoise (Phocoena phocoena). Can J Zool 89: 869–879. [Google Scholar]
- Gallagher CA, Grimm V, Kyhn LA, Kinze CC, Nabe-Nielsen J (2021) Movement and seasonal energetics mediate vulnerability to disturbance in marine mammal populations. Am Nat 197: 296–311. [DOI] [PubMed] [Google Scholar]
- Gallagher CA, Stern SJ, Hines E (2018) The metabolic cost of swimming and reproduction in harbor porpoises (Phocoena phocoena) as predicted by a bioenergetic model. Mar Mamm Sci 34: 875–900. [Google Scholar]
- Gauthier JMI, Brochu C, Moore S, Metcalfe CD, Beland P (1998) Environmental contaminants in tissues of a neonate St Lawrence beluga whale (Delphinapterus leucas). Mar Pollut Bull 36: 102–108. [Google Scholar]
- Georges JY, Groscolas R, Guinet C, Robin JP (2001) Milking strategy in subantarctic fur seals Arctocephalus tropicalis breeding on Amsterdam Island: evidence from changes in milk composition. Physiol Biochem Zool 74: 548–559. [DOI] [PubMed] [Google Scholar]
- Goedegebuure M, Melbourne-Thomas J, Corney SP, Hindell MA, Constable AJ (2017) Beyond big fish: the case for more detailed representations of top predators in marine ecosystem models. Ecol Model 359: 182–192. [Google Scholar]
- Gol’din PE (2004) Growth and body size of the harbour porpoise, Phocoena phocoena (Cetacea, Phocoenidae), in the sea of Azov and the Black Sea. Vestn Zool 38: 59–73. [Google Scholar]
- Greig DJ, Ylitalo GM, Hall AJ, Fauquier DA, Gulland FMD (2007) Transplacental transfer of organochlorines in California Sea lions (Zalophus californianus). Environ Toxicol Chem 26: 37–44. [DOI] [PubMed] [Google Scholar]
- Gutiérrez J, Seguel M, Saenz-Agudelo P, Acosta-Jamett G, Verdugo C (2021) Genetic diversity and kinship relationships in one of the largest south American fur seal (Arctocephalus australis) populations of the Pacific Ocean. Ecol Evol 11: 8743–8753. [Google Scholar]
- Hamilton PK, Frasier BA, Conger LA, George RC, Jackson KA, Frasier TR (2022) Genetic identifications challenge our assumptions of physical development and mother–calf associations and separation times: a case study of the North Atlantic right whale (Eubalaena glacialis). Mamm Biol . 10.1007/s42991-021-00177-4. [DOI] [Google Scholar]
- Hedberg GE, Derocher AE, Andersen M, Rogers QR, Depeters EJ, Lönnerdal B, Mazzaro L, Chesney RW, Hollis B (2011) Milk composition in free-ranging polar bears (Ursus maritimus) as a model for captive rearing milk formula. Zoo Biol 30: 550–565. [DOI] [PubMed] [Google Scholar]
- Hin V, Harwood J, Roos AM (2019) Bio-energetic modeling of medium-sized cetaceans shows high sensitivity to disturbance in seasons of low resource supply. Ecol Appl 29: 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper AW, Berger RW, Rubin LS, McDonald BI, Crocker DE (2019) Maternal age influences offspring behaviour and growth efficiency during provisioning in northern elephant seals. Anim Behav 151: 121–130. [Google Scholar]
- Hoskins AJ, Arnould JPY (2013) Temporal allocation of foraging effort in female Australian fur seals (Arctocephalus pusillus doriferus). PLoS One 8: e79484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howells EM, Reif JS, Bechdel SE, Murdoch ME, Bossart GD, McCulloch SD, Mazzoil MS (2009) A novel case of non-offspring adoption in a free-ranging Atlantic bottlenose dolphin (Tursiops truncatus) inhabiting the Indian River lagoon, Florida. Aquat Mamm 35: 43–47. [Google Scholar]
- Huck M, Van Lunenburg M, Dávalos V, Rotundo M, Di Fiore A, Fernandez-Duque E (2014) Double effort: parental behavior of wild Azara’s owl monkeys in the face of twins. Am J Primatol 76: 629–639. [DOI] [PubMed] [Google Scholar]
- Hückstädt LA, Holser RR, Tift MS, Costa DP (2018) The extra burden of motherhood: reduced dive duration associated with pregnancy status in a deep-diving mammal, the northern elephant seal. Biol Lett 14: 9–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iverson SJ, Bowen W, Boness DJ, Oftedal OT (1993) The effect of maternal size and milk energy output on pup growth in grey seals (Halichoerus grypus). Physiol Zool 66: 61–88. [Google Scholar]
- Jagusch KT, Mitchell RM (1971) Utilisation of the metabolisable energy of ewe’s milk by the lamb. New Zeal J Agric Res 14: 434–441. [Google Scholar]
- Jameson R, Bodkin JL (1986) An incidence of twinning in the sea otter (Enhydra lutris). Mar Mamm Sci 2: 305–309. [Google Scholar]
- Jeglinski JWE, Werner C, Robinson PW, Costa DP, Trillmich F (2012) Age, body mass and environmental variation shape the foraging ontogeny of Galapagos Sea lions. Mar Ecol Prog Ser 453: 279–296. [Google Scholar]
- Joseph BE, Antrim JE, Cornell LH (1987) Commerson’s dolphin (Cephalorhynchus commersonii): a discussion of the first live birth within a marine zoological park. Zoo Biol 6: 69–77. [Google Scholar]
- Karniski C, Krzyszczyk E, Mann J (2018) Senescence impacts reproduction and maternal investment in bottlenose dolphins. Proc R Soc B Biol Sci 285. 10.1098/rspb.2018.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastelein RA, Kershaw J, Berghout E, Wiepkema PR (2003) Food consumption and suckling in killer whales Orcinus orca at Marineland Antibes. Int Zoo Yearb 38: 204–218. [Google Scholar]
- Kastelein RA, McBain J, Neurohr B, Mohri M, Saijo S, Wakabayashi I, Wiepkema PR (1993) The food consumption of Commerson’s dolphins (Cephalorhynchus commersonii). Aquat Mamm 19: 99–121. [Google Scholar]
- Kastelein RA, Schooneman NM, Vaughan N, Wiepkema PR (2000a) Food consumption and growth of California Sea lions (Zalophus californianus californianus). Zoo Biol 19: 143–159. [Google Scholar]
- Kastelein RA, Schooneman NM, Wiepkema PR (2000b) Food consumption and body weight of captive Pacific walruses (Odobenus rosmarus divergens). Aquat Mamm 26: 175–190. [Google Scholar]
- Kastelein RA, Vaughan N, Walton S, Wiepkema PR (2002) Food intake and body measurements of Atlantic bottlenose dolphins (Tursiops truncatus) in captivity. Mar Environ Res 53: 199–218. [DOI] [PubMed] [Google Scholar]
- Kastelein RA, Wiepkema PR, Vaughan N (1990) The food consumption of grey seals (Halichoerus grypus) in human care. Aquat Mamm 15: 171–180. [Google Scholar]
- Kasuya T (1978) The life history of Dall’s porpoise with special reference to the stock off the Pacific coast of Japan. Sci Rep from Whales Res Inst 30: 1–63. [Google Scholar]
- Kasuya T, Marsh H (1984) Life history and reproductive biology of the short-finned pilot whale, Globicephala macrorhynchus, off the Pacific coast of Japan. Reports Int Whal Comm Spec Issue 6: 259–310. [Google Scholar]
- Kawamura A (1969) Siamese twins in the sei whale Balaenoptera borealis lesson. Nature 221: 490–491. [Google Scholar]
- Kienle SS, Goebel ME, LaBrecque E, Borras-Chavez R, Trumble SJ, Kanatous SB, Crocker DE, Costa DP (2022) Plasticity in the morphometrics and movements of an Antarctic apex predator, the leopard seal. Front Mar Sci 9. 10.3389/fmars.2022.976019. [DOI] [Google Scholar]
- Konrad CM, Frasier TR, Whitehead H, Gero S (2019) Kin selection and allocare in sperm whales. Behav Ecol 30: 194–201. [Google Scholar]
- Korine C, Speakman J, Arad Z (2004) Reproductive energetics of captive and free-ranging Egyptian fruit bats (Rousettus aegyptiacus). Ecology 85: 220–230. [Google Scholar]
- Kretzmann MB, Costa DP, Le Boeuf BJ (1993) Maternal energy investment in elephant seal pups: evidence for sexual equality? Am Nat 141: 466–480. [DOI] [PubMed] [Google Scholar]
- Krockenberger A (2003) Meeting the energy demands of reproduction in female koalas, Phascolarctos cinereus: evidence for energetic compensation. J Comp Physiol B Biochem Syst Environ Physiol 173: 531–540. [DOI] [PubMed] [Google Scholar]
- Lang SLC, Boness DJ, Bowen WD, Iverson SJ (2011a) Primiparous females do not exhibit reduced maternal care in gray seals (Halichoerus grypus). Mar Mamm Sci 27: 153–164. [Google Scholar]
- Lang SLC, Iverson SJ, Bowen WD (2005) Individual variation in milk composition over lactation in harbour seals (Phoca vitulina) and the potential consequences of intermittent attendance. Can J Zool 83: 1525–1531. [Google Scholar]
- Lang SLC, Iverson SJ, Bowen WD (2009) Repeatability in lactation performance and the consequences for maternal reproductive success in gray seals. Ecology 90: 2513–2523. [DOI] [PubMed] [Google Scholar]
- Lang SLC, Iverson SJ, Bowen WD (2011b) The influence of reproductive experience on milk energy output and lactation performance in the grey seal (Halichoerus grypus). PLoS One 6: e19487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang SLC, Iverson SJ, Bowen WD (2012) Primiparous and multiparous females differ in mammary gland alveolar development: implications for milk production. J Exp Biol 215: 2904–2911. [DOI] [PubMed] [Google Scholar]
- Lanzetti A, Berta A, Ekdale EG (2020) Prenatal development of the humpback whale: growth rate, tooth loss and skull shape changes in an evolutionary framework. Anat Rec 303: 180–204. [DOI] [PubMed] [Google Scholar]
- Lavigne DM, Barchard W, Innes S, Oritsland NA (1982) Pinniped bioenergetics. In Mammals in the Seas - Small Cetaceans, Seals, Sirenians, and Otters. Food and Agriculture Organization of the United Nations, Fisheries Series 5, Rome, pp. 191–235 [Google Scholar]
- Lavigne DM, Stewart REA (1979) Energy content of harp seal placentas. J Mammal 60: 854–856. [Google Scholar]
- Leung ES, Vergara V, BarLancerett-Lennard LG (2010) Allonursing in captive belugas (Delphinapterus leucas). Zoo Biol 29: 633–637. [DOI] [PubMed] [Google Scholar]
- Lima M, Paez E (1995) Growth and reproductive patterns in the south American fur seal. J Mammal 76: 1249–1255. [Google Scholar]
- Link A, Palma AC, Velez A, Luna AG (2006) Costs of twins in free-ranging white-bellied spider monkeys (Ateles belzebuth belzebuth) at Tinigua National Park, Colombia. Primates 47: 131–139. [DOI] [PubMed] [Google Scholar]
- Lockyer C (1976) Body weights of some species of large whales. ICES J Mar Sci 36: 259–273. [Google Scholar]
- Lockyer C (1981) Estimation of the energy costs of growth, maintenance and reproduction of the female minke whale, (Balaenoptera acutorostrata), from the southern hemisphere. Rep Int Whal Comm 31: 337–343. [Google Scholar]
- Lockyer C (1993) Seasonal changes in body fat condition of Northeast Atlantic pilot whales, and their biological significance. Rep Int Whal Comm Spec Issue 14: 325–350. [Google Scholar]
- Louppova A (2019) Climatic Variables Are Strong Predictors of Allonursing and Communal Nesting in Primates. City University of New York [Google Scholar]
- Lowther AD, Goldsworthy SD (2016) When were the weaners weaned? Identifying the onset of Australian sea lion nutritional independence. J Mammal 97: 1304–1311. [Google Scholar]
- Luck C, Jessopp M, Cronin M, Rogan E (2022) Using population viability analysis to examine the potential long-term impact of fisheries bycatch on protected species. J Nat Conserv 67: 126157. [Google Scholar]
- Lundström K, Hjerne O, Lunneryd S, Karlsson O (2010) Understanding the diet composition of marine mammals: grey seals (Halichoerus grypus) in the Baltic Sea. ICES J Mar Sci 1230–1239. [Google Scholar]
- Lunn NJ, Arnould JPY (1997) Maternal investment in Antarctic fur seals: evidence for equality in the sexes? Behav Ecol Sociobiol 40: 351–362. [Google Scholar]
- Lydersen C (1995) Energetics of pregnancy, lactation and neonatal development in ringed seals (Phoca hispida). Dev Mar Biol 4: 319–327. [Google Scholar]
- Malenfant R, Coltman DW, Richardson E, Lunn N, Stirling I, Adamowicz E, Davis CS (2016) Evidence of adoption , monozygotic twinning , and low inbreeding rates in a large genetic pedigree of polar bears. Polar Biol 39: 1455–1465. [Google Scholar]
- Mann J, Connor RC, Barre LM, Heithaus MR (2000) Female reproductive success in bottlenose dolphins (Tursiops sp.): life history, habitat, provisioning, and group-size effects. Behav Ecol 11: 210–219. [Google Scholar]
- Marón CF, Lábaque MC, Beltramino L, Di Martino M, Alzugaray L, Ricciardi M, Fernández Ajó AA, Adler FR, Seger J, Sironi Met al. (2021) Patterns of blubber fat deposition and evaluation of body condition in growing southern right whale calves (Eubalaena australis). Mar Mamm Sci 37: 1309–1329. [Google Scholar]
- Marsh H, Kwan D (2008) Temporal variability in the life history and reproductive biology of female dugongs in Torres Strait: the likely role of sea grass dieback. Cont Shelf Res 28: 2152–2159. [Google Scholar]
- Martin AR, Rothery P (1993) Reproductive parameters of female long-finned pilot whales (Globicephala melas) around the Faroe Islands. Rep Int Whal Comimssion Spec Issue 14: 263–304. [Google Scholar]
- Matthews CJD, Ferguson SH (2015) Weaning age variation in beluga whales (Delphinapterus leucas). J Mammal 96: 425–437. [Google Scholar]
- McClatchie S, Field J, Thompson AR, Gerrodette T, Lowry M, Fiedler PC, Nieto KM, Vetter RD (2016) Food limitation of sea lion pups and the decline of forage off central and southern California. R Soc Open Sci 3: 150628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald BI, Crocker DE (2006) Physiology and behavior influence lactation efficiency in northern elephant seals (Mirounga angustirostris). Physiol Biochem Zool 79: 484–496. [DOI] [PubMed] [Google Scholar]
- McDonald BI, Goebel ME, Crocker DE, Costa DP (2012) Dynamic influence of maternal and pup traits on maternal care during lactation in an income breeder, the Antarctic fur seal. Physiol Biochem Zool 85: 243–254. [DOI] [PubMed] [Google Scholar]
- McFee WE, Schwacke JH, Stolen MK, Mullin KD, Schwacke LH (2010) Investigation of growth phases for bottlenose dolphins using a Bayesian modeling approach. Mar Mamm Sci 26: 67–85. [Google Scholar]
- McHuron EA, Adamczak S, Arnould JPY, Ashe E, Booth C, Don Bowen W, Christiansen F, Chudzinska M, Costa DP, Fahlman Aet al. (2022a) Key questions in marine mammal bioenergetics. Conserv Physiol 10: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHuron EA, Aerts L, Gailey G, Sychenko O, Costa DP, Mangel M, Schwarz LK (2021) Predicting the population consequences of acoustic disturbance, with application to an endangered gray whale population. Ecol Appl 31: e02440. [DOI] [PubMed] [Google Scholar]
- McHuron EA, Leonard P, Rosen DAS, Carpenter J, Sirpenski G, Sterling JT (2022b) Variation in body mass and food intake of northern fur seals (Callorhinus ursinus). Mar Mamm Sci 38: 1160–1181. [Google Scholar]
- McHuron EA, Luxa K, Pelland NA, Holsman K, Ream R, Zeppelin T, Sterling JT (2020) Practical application of a bioenergetic model to inform management of a declining fur seal population and their commercially important prey. Front Mar Sci 7: 597973. [Google Scholar]
- McLaren IA (1993) Growth in pinnipeds. Biol Rev Camb Philos Soc 68: 1–79. [DOI] [PubMed] [Google Scholar]
- McMahon CR, Harcourt RG, Burton HR, Daniel O, Hindell MA (2017) Seal mothers expend more on offspring under favourable conditions and less when resources are limited. J Anim Ecol 86: 359–370. [DOI] [PubMed] [Google Scholar]
- McPherson RL, Ji F, Wu G, Blanton JR, Kim SW (2004) Growth and compositional changes of fetal tissues in pigs. J Anim Sci 82: 2534–2540. [DOI] [PubMed] [Google Scholar]
- Mellish JAE, Iverson SJ, Bowen WD (2000) Metabolic compensation during high energy output in fasting, lactating grey seals (Halichoerus grypus): metabolic ceilings revisited. Proc R Soc B Biol Sci 267: 1245–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellish JE, Iverson SJ, Bowen WD (1999a) Variation in milk production and lactation performance in grey seals and consequences for pup growth and weaning characteristics. Physiol Biochem Zool 72: 677–690. [DOI] [PubMed] [Google Scholar]
- Mellish JE, Iverson SJ, Bowen WD, Hammill MO (1999b) Fat transfer and energetics during lactation in the hooded seal: the roles of tissue lipoprotein lipase in milk fat secretion and pup blubber deposition. J Comp Physiol B 169: 377–390. [DOI] [PubMed] [Google Scholar]
- Moen AN (1973) An Analytical Approach. In Wildlife Ecology. W.H, Freeman and Company, San Francisco, CA [Google Scholar]
- New LF, Moretti DJ, Hooker SK, Costa DP, Simmons SE (2013) Using energetic models to investigate the survival and reproduction of beaked whales (family Ziphiidae). PLoS One 8: e68725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newby TC (1982) Life History of Dall Porpoise (Phocoenoides Dalli, True 1885) Incidentally Taken by the Japanase High Seas Salmon Mothership Fishery in the Northwestern North Pacific and Western Bering Sea, 1978 and 1980. University of Washington [Google Scholar]
- Noren SR, Redfern JV, Edwards EF (2011) Pregnancy is a drag: hydrodynamics, kinematics and performance in pre-and postparturition bottlenose dolphins (Tursiops truncatus). J Exp Biol 214: 4151–4159. [DOI] [PubMed] [Google Scholar]
- Noren SR, Udevitz MS, Jay CV (2014) Energy demands for maintenance, growth, pregnancy, and lactation of female Pacific walruses (Odobenus rosmarus divergens). Physiol Biochem Zool 87: 837–854. [DOI] [PubMed] [Google Scholar]
- Nousek McGregor AE (2010) The Cost of Locomotion in North Atlantic Right Whales Eubalaena Glacialis. Duke University [Google Scholar]
- Oftedal OT (1993) The adaptation of milk secretion to the constraints of fasting in bears, seals, and baleen whales. J Dairy Sci 76: 3234–3246. [DOI] [PubMed] [Google Scholar]
- Oftedal OT (1997) Lactation in whales and dolphins: evidence of divergence between baleen- and toothed-species. J Mammary Gland Biol Neoplasia 2: 205–230. [DOI] [PubMed] [Google Scholar]
- Oftedal OT (2000) Use of maternal reserves as a lactation strategy in large mammals. Proc Nutr Soc 59: 99–106. [DOI] [PubMed] [Google Scholar]
- Oftedal OT, Boness DJ, Bowen WD (1988) The composition of hooded seal (Cystophora cristata) milk: an adaptation for postnatal fattening. Can J Zool 66: 318–322. [Google Scholar]
- Oftedal OT, Boness DJ, Tedman RA (1987a) The behavior, physiology, and anatomy of lactation in the pinnipedia. In Geneoways HH, ed, Current Mammology. Springer, US, pp. 175–245 [Google Scholar]
- Oftedal OT, Bowen WD, Boness DJ (1993) Energy transfer by lactating hooded seals and nutrient deposition in their pups during the four days from birth to weaning.Physiol Biochem Zool Ecol Evol Approaches 66: 412–436. [Google Scholar]
- Oftedal OT, Bowen WD, Boness DJ (1996) Lactation performance and nutrient deposition in pups of the harp seal, Phoca groenlandica, on ice floes off Southeast Labrador. Physiol Zool 69: 635–657. [Google Scholar]
- Oftedal OT, Eisert R, Barrell GK (2014) Comparison of analytical and predictive methods for water, protein, fat, sugar, and gross energy in marine mammal milk. J Dairy Sci 97: 4713–4732. [DOI] [PubMed] [Google Scholar]
- Oftedal OT, Iverson SJ, Boness DJ (1987b) Milk and energy intakes of suckling California Sea lion Zalophus californianus pups in relation to sex, growth, and predicted maintenance requirements. Physiol Zool 5: 560–575. [Google Scholar]
- Olesiuk PF, Bigg M, Ellis G (1990) Life history and population dynamics of resident killer whales (Orcinus orca) in the coastal waters of British Columbia and Washington state. Rep - Int Whal Comm Spec Issue 12: 209–243. [Google Scholar]
- Ortiz CL, Le Boeuf BJ, Costa DP (1984) Milk intake of elephant seal pups: an index of parental investment. Am Nat 124: 416–422. [Google Scholar]
- Osborn S, Dalton L, Dold C, Robeck T (2012) Management of twin pregnancy and perinatal concerns in a beluga (Delphinapterus leucas). J Zoo Wildl Med 43: 193–196. [DOI] [PubMed] [Google Scholar]
- Páez-Rosas D, Torres J, Espinoza E, Marchetti A, Seim H, Riofrío-Lazo M (2021) Declines and recovery in endangered Galapagos pinnipeds during the El Niño event. Sci Rep 11: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallin LJ, Baker CS, Steel D, Kellar NM, Robbins J, Johnston DW, Nowacek DP, Read AJ, Friedlaender AS (2018) High pregnancy rates in humpback whales (Megaptera novaeangliae) around the Western Antarctic peninsula, evidence of a rapidly growing population. R Soc Open Sci 5: 180017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson JT, Rotella JJ, Mannas JM, Garrott RA (2016) Patterns of age-related change in reproductive effort differ in the pre-natal and post-natal periods in a long-lived mammal. J Anim Ecol 85: 1540–1551. [DOI] [PubMed] [Google Scholar]
- Payne SF, Jameson RJ (1984) Early behavioral development of the sea otter, Enhydra lutris. J Mammal 65: 527–531. [Google Scholar]
- Peddemors VM, Fothergill M, Cockcroft VG (1992) Feeding and growth in a captive-born bottlenose dolphin Tursiops truncatus. South African J Zool 27: 74–80. [Google Scholar]
- Pedro S, Boba C, Dietz R, Sonne C, Rosing-Asvid A, Hansen M, Provatas A, McKinney MA (2017) Blubber-depth distribution and bioaccumulation of PCBs and organochlorine pesticides in Arctic-invading killer whales. Sci Total Environ 601–602: 237–246. [DOI] [PubMed] [Google Scholar]
- Perez MA, Mooney EE (1986) Increased food and energy consumption of lactating northern fur seals, Callorhinus ursinus. Fish Bull 84: 371–381. [Google Scholar]
- Pereira JVM, Marcondes MI, Filho SCV, Caton J, Sguizzato ALL, Silva AL, da Silva JT, Moraes VCL, Gomes LF, Rotta PP (1979) Digestive parameters during gestation of Holstein heifers: Digestion during pregnancy. Livest Sci 242: 104325. [Google Scholar]
- Perrin WF, Reilly SB (1984) Reproductive parameters of dolphins and small whales of the family Delphinidae. Rep Int Whal Comimssion Spec Issue 6: 97–133. [Google Scholar]
- Piedrahita P, Meise K, Werner C, Krüger O, Trillmich F (2014) Lazy sons, self-sufficient daughters: are sons more demanding? Anim Behav 98: 69–78. [Google Scholar]
- Pirotta E (2022) A review of bioenergetic modelling for marine mammal populations. Conserv Physiol 10: coac036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirotta E, Mangel M, Costa DP, Mate BR, Goldbogen JA, Palacios DM, Hückstädt LA, McHuron E, Schwarz LK, New LF (2018) A dynamic state model of migratory behavior and physiology to assess the consequences of environmental variation and anthropogenic disturbance on marine vertebrates. Am Nat 191: E40–E56. [DOI] [PubMed] [Google Scholar]
- Pitcher BJ, Ahonen H, Charrier I, Harcourt RG (2011) Allosuckling behavior in the Australian sea lion (Neophoca cinerea): an updated understanding. Mar Mamm Sci 27: 881–888. [Google Scholar]
- Pitcher K, Calkins D, Pendleton G (1998) Reproductive performance of female Steller Sea lions: an energetics-based reproductive strategy? Can J Zool 76: 2075–2083. [Google Scholar]
- Recchia CA, Read AJ (1989) Stomach contents of harbour porpoises, Phocoena phocoena (L.), from the bay of Fundy. Can J Zool 67: 2140–2146. [Google Scholar]
- Reddy M, Kamolnick T, Skaar D, Curry C, Ridgway S (1993) Bottlenose dolphins: energy consumption during pregnancy, lactation, and growth. Int Mar Mammal Trainers Assoc Annu Conf 1–37. [Google Scholar]
- Rehberg MJ, Rea LD, Eischens CA (2018) Overwintering Steller Sea lion (Eumetopias jubatus) pup growth and behavior prior to weaning. Can J Zool 96: 97–106. [Google Scholar]
- Reimer JR, Mangel M, Derocher AE, Lewis MA (2019) Modeling optimal responses and fitness consequences in a changing Arctic. Glob Chang Biol 25: 3450–3461. [DOI] [PubMed] [Google Scholar]
- Reynolds JE (1981) Behavior patterns in the West Indian manatee, with emphasis on feeding and diving. Florida Sci 44: 233–242. [Google Scholar]
- Riedman M, Ortiz CL (1979) Changes in milk composition during lactation in the northern elephant seal. Physiol Zool 52: 240–249. [Google Scholar]
- Riedman ML, Le Boeuf BJ (1982) Mother-pup separation and adoption in northern elephant seals. Behav Ecol Sociobiol 11: 203–215. [Google Scholar]
- Robbins CT (1983) Wildlife Feeding and Nutrition. Academic Press [Google Scholar]
- Robeck TR, Monfort SL, Calle PP, Dunn JL, Jensen E, Boehm JR, Young S, Clark ST (2005) Reproduction, growth and development in captive beluga (Delphinapterus leucas). Zoo Biol 49: 29–49. [Google Scholar]
- Robeck TR, Schmitt TL, Osborn S (2015) Development of predictive models for determining fetal age-at-length in belugas (Delphinapterus leucas) and their application toward in situ and ex situ population management. Mar Mamm Sci 31: 591–611. [Google Scholar]
- Rolland RM, Parks SE, Hunt KE, Castellote M, Corkeron PJ, Nowacek DP, Wasser SK, Kraus SD (2012) Evidence that ship noise increases stress in right whales. Proc R Soc B Biol Sci 279: 2363–2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronald K, Thomson C (1981) Parturition and postpartum behaviour of a captive harbour seal, Phoca vitulina. Aquat Mamm 8: 79–90. [Google Scholar]
- Ross GJB, Best PB, Donnelly BG (1975) New records of the pygmy right whale (Caperea marginata) from South Africa, with comments on distribution, migration, appearance, and behavior. J Fish Res Board Canada 32: 1005–1017. [Google Scholar]
- Sakai M, Kita YF, Kogi K, Shinohara M, Morisaka T, Shiina T, Inoue-Murayama M (2016) A wild indo-Pacific bottlenose dolphin adopts a socially and genetically distant neonate. Sci Rep 6: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauvé CC, Van de Walle J, Hammill MO, Arnould JPY, Beauplet G (2014) Stomach temperature records reveal nursing behaviour and transition to solid food consumption in an unweaned mammal, the harbour seal pup (Phoca vitulina). PLoS One 9: e90329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz JK, Becker BL, Johanos TC, Lopez JU, Kashinsky L (2011) Dizygotic twinning in the Hawaiian monk seal. J Mammal 92: 336–341. [Google Scholar]
- Schulz TM, Bowen WD (2004) Pinniped lactation strategies: evaluation of data on maternal and offspring life history traits. Mar Mamm Sci 20: 86–114. [Google Scholar]
- Schulz TM, Bowen WD (2005) The evolution of lactation strategies in pinnipeds: a phylogenetic analysis. Ecol Monogr 75: 159–177. [Google Scholar]
- Shero MR, Goetz KT, Costa DP, Burns JM (2018) Temporal changes in Weddell seal dive behavior over winter: are females increasing foraging effort to support gestation? Ecol Evol 8: 11857–11874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuert CR, Halsey LG, Pomeroy PP, Twiss SD (2020) Energetic limits: defining the bounds and trade-offs of successful energy management in a capital breeder. J Anim Ecol 89: 2461–2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva WTAF, Harding KC, Marques GM, Bäcklin BM, Sonne C, Dietz R, Kauhala K, Desforges JP (2020) Life cycle bioenergetics of the gray seal (Halichoerus grypus) in the Baltic Sea: population response to environmental stress. Environ Int 145: 106145. [DOI] [PubMed] [Google Scholar]
- Sinha A, Conaway C, Kenyon K (1966) Reproduction in the female sea otter. J Wildl Manage 30: 121–130. [Google Scholar]
- Smith C (2021) A warming Southern Ocean may compromise Antarctic blue whale foetus growth. J Vertebr Biol 70: 1–11. [Google Scholar]
- Soma-Pillay P, Nelson-Piercy C, Tolppanen H, Mebazaa A (2016) Physiological changes in pregnancy. Cardiovasc J Afr 27: 89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto KH, Trites AW, Arias-Schreiber M (2004) The effects of prey availability on pup mortality and the timing of birth of south American sea lions (Otaria flavescens) in Peru. J Zool 264: 419–428. [Google Scholar]
- Soto-Azat C, Boher F, Flores G, Monsalve R, Santibáñez A, Vianna J, Medina-Vogel G (2011) Marine otters (Lontra felina) in ecological studies in Chile. In proceedings of Xth international otter colloquium, IUCN otter spec. Group bull Vol 28A, pp. 114–126 [Google Scholar]
- Southwell C, Kerry K, Ensor P, Woehler EJ, Rogers T (2003) The timing of pupping by pack-ice seals in East Antarctica. Polar Biol 26: 648–652. [Google Scholar]
- Sparling CE, Speakman JR, Fedak MA (2006) Seasonal variation in the metabolic rate and body composition of female grey seals: fat conservation prior to high-cost reproduction in a capital breeder? J Comp Physiol B 176: 505–512. [DOI] [PubMed] [Google Scholar]
- Spotte S (1982) The incidence of twins in pinnipeds. Can J Zool 60: 2226–2233. [Google Scholar]
- Staedler MM, Riedman ML (1989) A case of adoption in the California Sea otter. Mar Mamm Sci 5: 391–394. [Google Scholar]
- Stead SM, Bădescu I, Boonstra R (2022) Of mammals and milk: how maternal stress affects nursing offspring. Mamm Rev 52: 129–147. [Google Scholar]
- Stewart REA, Stewart BE, Lavigne DM, Miller GW (1989) Fetal growth of Northwest Atlantic harp seals, Phoca groenlandica. Can J Zool 67: 2147–2157. [Google Scholar]
- Stirling I, Latour PB (1978) Comparative hunting abilities of polar bear cubs of different ages. Can J Zool 56: 1768–1772. [Google Scholar]
- Struntz DJ, Mclellan WA, Dillaman RM, Blum JE, Kucklick JR, Pabst DA (2004) Blubber development in bottlenose dolphins (Tursiops truncatus). J Morphol 259: 7–20. [DOI] [PubMed] [Google Scholar]
- Sumich JL (1986) Latitudinal Distribution, Calf Growth and Metabolism, and Reproductive Energetics of Gray Whales. Oregon State University, Eschrichtius Robustus [Google Scholar]
- Szabo A, Duffus D (2008) Mother-offspring association in the humpback whale, Megaptera novaeangliae: following behaviour in an aquatic mammal. Anim Behav 75: 1085–1092. [Google Scholar]
- Thom MD, Johnson DDP, Macdonald DW (2004) The evolution and maintenance of delayed implantation in the Mustelidae (Mammalia: Carnivora). Evolution (N Y) 58: 175–183. [DOI] [PubMed] [Google Scholar]
- Thometz NM, Kendall TL, Richter BP, Williams TM (2016a) The high cost of reproduction in sea otters necessitates unique physiological adaptations. J Exp Biol 219: 2260–2264. [DOI] [PubMed] [Google Scholar]
- Thometz NM, Staedler MM, Tomoleoni JA, Bodkin JL, Bentall GB, Tinker MT (2016b) Trade-offs between energy maximization and parental care in a central place forager, the sea otter. Behav Ecol 27: 1552–1566. [Google Scholar]
- Tønnesen P, Gero S, Ladegaard M, Johnson M, Madsen PT (2018) First-year sperm whale calves echolocate and perform long, deep dives. Behav Ecol Sociobiol 72. 10.1007/s00265-018-2570-y. [DOI] [Google Scholar]
- Toro-Ramos T, Paley C, Pi-Sunyer FX, Gallagher D (2015) Body composition during fetal development and infancy through the age of 5 years. Eur J Clin Nutr 69: 1279–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres DA, Freitas MB, Gonçalves RV (2018) Changes in bone turnover and calcium homeostasis during pregnancy and lactation in mammals: a meta-analysis. Reprod Fertil Dev 30: 681–688. [DOI] [PubMed] [Google Scholar]
- Trillmich F, Limberger D (1985) Drastic effects of El Niño on Galapagos pinnipeds. Oecologia 67: 19–22. [DOI] [PubMed] [Google Scholar]
- Trillmich F, Wolf JBW (2008) Parent – offspring and sibling conflict in Galápagos fur seals and sea lions. Behav Ecol Sociobiol 62: 363–375. [Google Scholar]
- Trites AW (1991) Fetal growth of northern fur seals: life-history strategy and sources of variation. Can J Zool 69: 2608–2617. [Google Scholar]
- Trites AW (1992) Fetal growth and the condition of pregnant northern fur seals off western North America from 1958 to 1972. Can J Zool 70: 2125–2131. [Google Scholar]
- Venuto R, Botta S, Barreto AS, Secchi ER, Fruet PF (2020) Age structure of strandings and growth of Lahille’s bottlenose dolphin (Tursiops truncatus gephyreus). Mar Mamm Sci 36: 813–827. [Google Scholar]
- Villegas-Amtmann S, Schwarz LK, Sumich JL, Costa DP (2015) A bioenergetics model to evaluate demographic consequences of disturbance in marine mammals applied to gray whales. Ecosphere 6: 183. [Google Scholar]
- Wang L, Lam T, Xu S, Dai Z, Zhou L, Feng T, Guo P, Dunn C, Jones B, Bradley Tet al. (2020) Treeio: an R package for phylogenetic tree input and output with richly annotated and associated data. Mol Biol Evol 37: 599–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasser SK, Lundin JI, Ayres K, Seely E, Giles D, Balcomb K, Hempelmann J, Parsons K, Booth R (2017) Population growth is limited by nutritional impacts on pregnancy success in endangered southern resident killer whales (Orcinus orca). PLoS One 12: e0179824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner R (1996) Composition and energy content of milk from southern sea lions (Otaria flavescens). Mar Mamm Sci 12: 313–317. [Google Scholar]
- West KL, Oftedal OT, Carpenter JR, Krames B, Campbell M, Sweeney J (2007) Effect of lactation stage and concurrent pregnancy on milk composition in the bottlenose dolphin. J Zool 273: 148–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheatley KE, Bradshaw CJA, Davis LS, Harcourt RG, Hindell MA (2006) Influence of maternal mass and condition on energy transfer in Weddell seals. J Anim Ecol 75: 724–733. [DOI] [PubMed] [Google Scholar]
- Williams R, Krkošek M, Ashe E, Branch TA, Clark S, Hammond PS, Hoyt E, Noren DP, Rosen D, Winship A (2011) Competing conservation objectives for predators and prey: estimating killer whale prey requirements for Chinook salmon. PLoS One 6: e26736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams TM, Rutishauser M, Long B, Fink T, Gafney J, Mostman-Liwanag H, Casper D (2007) Seasonal variability in otariid energetics: implications for the effects of predators on localized prey resources. Physiol Biochem Zool 80: 433–443. [DOI] [PubMed] [Google Scholar]
- Xu S, Dai Z, Guo P, Fu X, Liu S, Zhou L, Tang W, Feng T, Chen M, Zhan Let al. (2021) ggtreeExtra: compact visualization of richly annotated phylogenetic data. Mol Biol Evol 38: 4039–4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu G (2020) Using ggtree to visualize data on tree-like structures. Curr Protoc Bioinforma 69: e96. [DOI] [PubMed] [Google Scholar]
- Yunker GB, Hammill MO, Gosselin JF, Dion DM, Schreer JF (2005) Foetal growth in north-West Atlantic grey seals (Halichoerus grypus). J Zool 265: 411–419. [Google Scholar]
- Zhang PJ, Song XR, Han JB, Wang LM, Yang Y (2014) Milk composition, milk consumption, and growth rate of a captive spotted seal (Phoca largha) pup from Liaodong Bay, China. Can J Zool 92: 449–452. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
To compile data availability on common parameters used in marine mammal bioenergetic models, we performed literature searches using Google Scholar and keywords relevant to each parameter and species. We also searched review articles on reproductive energetics and common marine mammal reference material (e.g. Encyclopedia for Marine Mammals) for values and their original sources. We traced the literature back to the source where the original estimate was obtained; in many cases, this proved to be a highly circuitous route as many papers cited other papers that were also not original sources. It was not always possible to obtain a copy of what was assumed to be the original source; when this occurred, we note this in the supplemental tables and provide both the citing research and source citation. We recommend caution in applying these values without accessing the original data sources. The exception to this was when the citation was to a known review, such as a book chapter, because in our experience these often yielded uncited values or reference to other sources. To avoid perpetuating erroneous or poorly informed parameters and overstating data availability, we did not include parameter values for which no citation was given. We also did not include every single reference for a given parameter and species, as this was not the goal of the review. While this effort was thorough, there is a vast amount of gray literature on marine mammals, and we recognize that it is possible that we missed data. We used the Society for Marine Mammalogy’s Taxonomy List of Marine Mammal Species and Subspecies, updated in June 2021, for species classification (https://marinemammalscience.org/science-and-publications/list-marine-mammal-species-subspecies/). On this list there were 15 extant species of mysticetes, 77 odontocetes (with one included as possibly extinct), 18 phocids, 14 otariids, 1 odobenid, 2 mustelids, 4 sirenians and 1 ursid.
All data are incorporated into the article and its online supplementary material.